Abstract
Background/purpose
The association between dysregulated innate immune responses seen in Kawasaki disease (KD) with predisposition to Kawasaki-like multisystem inflammatory syndrome in children (MIS-C) remains unclear. We aimed to compare the innate immunity transcriptome signature between COVID-19 and KD, and to analyze the interactions of these molecules with genes known to predispose to KD.
Methods
Transcriptome datasets of COVID-19 and KD cohorts (E-MTAB-9357, GSE-63881, GSE-68004) were downloaded from ArrayExpress for innate immune response analyses. Network analysis was used to determine enriched pathways of interactions.
Results
Upregulations of IRAK4, IFI16, STING, STAT3, PYCARD, CASP1, IFNAR1 and CD14 genes were observed in blood cells of acute SARS-CoV-2 infections with moderate severity. In the same patient group, increased expressions of TLR2, TLR7, IRF3, and CD36 were also noted in blood drawn a few days after COVID-19 diagnosis. Elevated blood PYCARD level was associated with severe COVID-19 in adults. Similar gene expression signature except differences in TLR8, NLRP3, STING and IRF3 levels was detected in KD samples. Network analysis on innate immune genes and genes associated with KD susceptibility identified enriched pathways of interactions. Furthermore, higher expression levels of KD susceptibility genes HLA-DOB, PELI1 and FCGR2A correlated with COVID-19 of different severities.
Conclusion
Our findings suggest that most enriched innate immune response pathways were shared between transcriptomes of KD and COVID-19 with moderate severity. Genetic polymorphisms associated with innate immune dysregulation and KD susceptibility, together with variants in STING and STAT3, might predict COVID-19 severity and potentially susceptibility to COVID-19 related MIS-C.
Keywords: Kawasaki disease, COVID-19, Innate immunity, Multisystem inflammatory syndrome
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is widespread globally. Compared with adults, children were less frequently diagnosed with COVID-19, and had milder symptoms.1 , 2 However, Kawasaki-like multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 has emerged recently in a small subset of pediatric cases in Europe and the US.3, 4, 5, 6, 7 Most of the reported MIS-C subjects were positive for SARS-CoV-2 by real-time PCR (RT-PCR) or serology testing.1 , 4 , 8 Compared with classical Kawasaki disease (KD), MIS-C temporally associated with COVID-19 occurred more frequently in children more than 5 years old, and patients were more often presented with previous gastrointestinal symptoms, shock, and IVIG resistance requiring second-line immunosuppressant or biologics.1 , 3 , 9 , 10 Risk factors predisposing children to COVID-19 related MIS-C, however, are still not clear.
KD, also known as mucocutaneous lymph node syndrome, is a febrile (fever more than 5 days) childhood vasculitis characterized by bilateral conjunctivitis, skin rash, lesions in oral mucosa and extremities, and cervical lymphadenopathy.11 Although the exact etiology of KD is unknown, evidence suggests that common respiratory viruses, such as adenoviruses, enteroviruses, rhinoviruses, and coronaviruses might be the triggers of KD in genetically predisposed individual.12 , 13 Pathogen-associated molecular patterns (PAMPs)/Danger-associated molecular patterns (DAMPs) induced innate immune responses, including toll-like receptor (TLR) signaling and inflammasome activation, have been shown to play the important roles in KD pathogenesis.11 , 14
Similarly, components of the severe acute respiratory syndrome coronaviruses (SARS-CoV/SARS-CoV-2) could trigger the host innate immune sensor responses.15 , 16 While the spike protein was reported to activate TLR2, single-stranded viral RNA and double-stranded RNA (appears during viral replication) could induce TLR7/8, and TLR3 signaling, respectively.17 , 18 TLR adaptor myeloid differentiation primary response 88 (MyD88) signaling leads to nuclear factor kappa B (NF-kB) activation and thus the production of pro-inflammatory cytokines and priming of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome.19 TLR-Interferon regulatory factor (IRF) signaling induces type I interferons (IFNs) which are essential antiviral cytokines.20 Furthermore, the membrane (M) protein of SARS-CoV could activate NF-kB and type I IFN cascades via TLR related signaling.21 In addition, it has been reported that STING (stimulator of interferon genes), a cytosolic DNA sensor adaptor, could sense the fusion of RNA virus with target cells, and could be stimulated by IFI16 (γ-interferon-inducible protein 16) inflammasome triggered by virus cytopathic effect-related host DNA damage.22, 23, 24 STING/TBK1 (TANK-binding kinase 1)/IRF pathway further increases type I IFN production, and has been suggested to be involved in COVID-19 pathology.22 Since abnormal STING activation has been proposed to underlie vascular inflammation and aneurysm formation, Berthelot et al. suggested that delayed over-activation of the STING pathway might contribute to Kawasaki-like disease and coagulopathy in COVID-19.25 However, a DNA microarray study failed to elicit a robust type I IFN response in a small cohort of acute KD, suggesting STING hyper-activation might not be the only factor for Kawasaki-like MIS-C related to COVID-19.26
Despite the similarities in innate immune responses observed between KD and COVID-19, differences in clinical manifestations exist. Cheung EW et al. reported in a New York cohort of children/adolescents with MIS-C associated with COVID-19 that while the proinflammatory cytokines IL-6 and IL-18 were elevated in MIS-C, the levels of TNF-α(tumor necrosis factor-α) and IL-1β, which are frequently elevated in KD, were not increased.5 Although the incidence of KD is the highest in East Asian countries, the majority of reported cases of MIS-C related to COVID-19 were from European countries and the US.3 , 4 , 7 These differences suggest that multiple genetic variations involving the regulation of innate immune responses might contribute to susceptibility to MIS-C temporally associated with the COVID-19 pandemic. In this study, we analyzed the innate sensor signaling in COVID-19 and KD transcriptome datasets, and investigated the potential roles of known KD susceptibility genetic variants on dysregulated innate immune responses predisposing to MIS-C related to COVID-19.
Methods
COVID-19 and KD transcriptome dataset analysis
The transcriptome dataset of whole blood derived from COVID-19 cases (E-MTAB-9357) was downloaded from ArrayExpress Archive of Functional Genomic Data (ebi.ac.uk/arrayexpress/experiments/E-MTAB-9357),27 age 56.1 ± 18.5 years, n = 129 blood draw near COVID-19 diagnosis (BL, male n = 55, female n = 74), n = 125 blood draw a few days later after the COVID-19 diagnosis (AC, male n = 52, female n = 73); among all the patient samples, 125 were paired; 16 healthy controls, age 42.6 ± 16.3 years, male n = 5, female n = 11. The transcriptome dataset of whole blood derived from KD patients (GSE-68004, GSE-63881) were downloaded from Gene Expression Omnibus/GEO).28 , 29 GSE-68004: healthy control, age 8.3 ± 4.7 years, male n = 9, female n = 14; complete KD (cKD) patients, age 4.2 ± 2.3 years, male n = 6, female n = 13. GSE-63881: KD patients, age 3.3 ± 2.8 years, n = 171 at acute phase (male n = 102, female n = 69) and n = 170 at convalescent phase (male n = 101, female n = 69); among all the patient samples, 131 were paired. The RNA expression value of each gene in the transcriptome dataset was normalized to z score (sample value minus data mean and then divided by data standard deviation). Unpaired t tests were performed to calculate the differences of gene expressions between two groups. Statistical tests were analyzed by using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA), and the levels of significance were considered as p < 0.05. For multiple group comparisons, the false discovery rate (FDR) was controlled by using the Benjamini-Hochberg (BH) correction method using R software.
Functional interaction network analysis
Network analysis was performed using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) v.11. The protein–protein interaction network was generated based on IDs of KD-associated genes and genes involving innate immune responses in COVID-19. KD-associated genes were identified by searching KD-associated SNPs across 15 GWAS studies with trait “mucocutaneous lymph node syndrome” using NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/),30 downloaded on 2020.07.07 and summarized on an association plot using LocusZoom v0.5.6. Gene Ontology (GO) functional enrichment analysis was also performed by using STRING v.11 (https://string-db.org/).
Results
Innate immune responses in acute SARS-CoV-2 infection
The transcriptome dataset of whole blood derived from COVID-19 cases (E-MTAB-9357) was derived from 129 COVID-19 adult patients (age 56.1 ± 18.5 years) and 16 healthy controls (HC, age 42.6 ± 16.3 years). Of the COVID-19 patients, 129 had blood drawn near the diagnosis of COVID-19 (BL group), 125 had blood drawn again a few days later (AC group). The HC vs. BL and HC vs. AC cohorts were each randomly divided into a test dataset (70% of samples) and a validation dataset (30% of samples). A confusion matrix using the z score of innate immune genes TLR2, TLR7, TLR8, IRAK4, IRF3, IFI16, STING, IFNAR1, STAT3, NLRP3, PYCARD, CASP1, CD36, and CD14 as variables was built to evaluate the performance of these variables on classifying the COVID-19 patient groups. The classification algorithm accuracies of test dataset and validation dataset for differentiating COVID-19-BL group from HC were 91.1% and 85.0%, respectively. The accuracies of test dataset and validation dataset for differentiating COVID-19-AC group from HC were 90.9% and 90.5%, respectively.
Association of innate immune gene expression with COVID-19 severity
In E-MTAB-9357 COVID-19 cohort, the severity of COVID-19 patients was stratified according to the WHO Ordinal Scale (WOS): mild (WOS 1–2), moderate (WOS 3–4), severe (WOS 5–7).27 We therefore further compared the levels of innate immune gene expressions in patients with different severity scales, and the p values of multiple t tests and adjusted p values corrected by BH method were listed in supplementary Table 1.
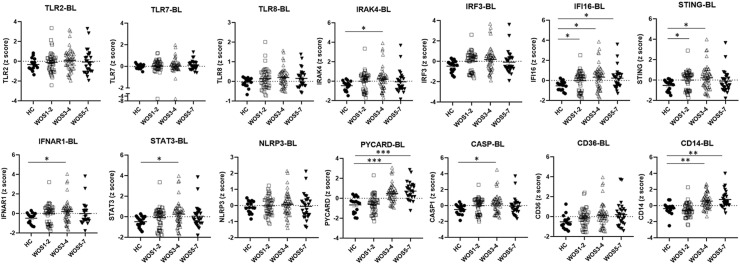
In the BL group, we found increased expression level of IFI16 in COVID-19 patients of all severity scales (FDR<0.05, Fig. 1 ). Moreover, as compared with healthy controls, significantly elevated amounts of IRAK4, STING, IFNAR1, STAT3, PYCARD, CASP1 and CD14 (FDR<0.05) were observed in the group of moderate disease severity already at the time around COVID-19 diagnosis (Fig. 1). Of note, higher expression levels of CD14 and PYCARD, a key adaptor of inflammasome, were also associated with severe diseases (WOS 5–7, Fig. 1).
Figure 1.
Innate immune responses in blood draw at the time around COVID-19 diagnosis. Data was derived from E-MTAB-9357 blood transcriptome dataset. The severity of COVID-19 patients in E-MTAB-9357 was stratified according to the WHO Ordinal Scale (WOS): mild (WOS 1–2), moderate (WOS 3–4), severe (WOS 5–7). HC: healthy controls; BL: blood draw at the time around COVID-19 diagnosis. Sample gene expression z scores were compared between the HC and the BL group. Lines and bars represent mean ± SEM.∗FDR (adjusted P) < 0.05, ∗∗FDR<0.01, ∗∗∗FDR<0.001, by BH correction of multiple unpaired t tests.
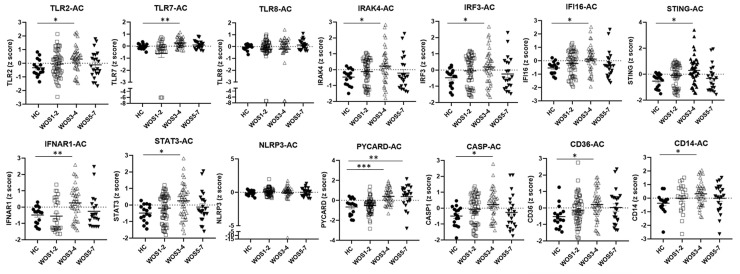
In the AC group, similar innate immune gene expression patterns with the addition of TLR2, TLR7, IRF3 and CD36 activations were noted in COVID-19 patients of moderate severity as compared with controls (Fig. 2 ). Interestingly, markedly increased expression level of PYCARD was detected in moderate to severe COVID-19 patients (WOS 3–4, WOS 5–7) both at the time of diagnosis and in blood drawn a few days after COVID-19 diagnosis (Fig. 2).
Figure 2.
Innate immune responses in blood draw a few days after COVID-19 diagnosis. HC: healthy controls; AC: blood draw a few days after COVID-19 diagnosis. Sample gene expression z scores were compared between the HC and the AC group. Lines and bars represent mean ± SEM. ∗FDR (adjusted P) < 0.05, ∗∗FDR<0.01, ∗∗∗FDR<0.001, by BH correction of multiple unpaired t tests.
Innate immune responses in kawasaki disease
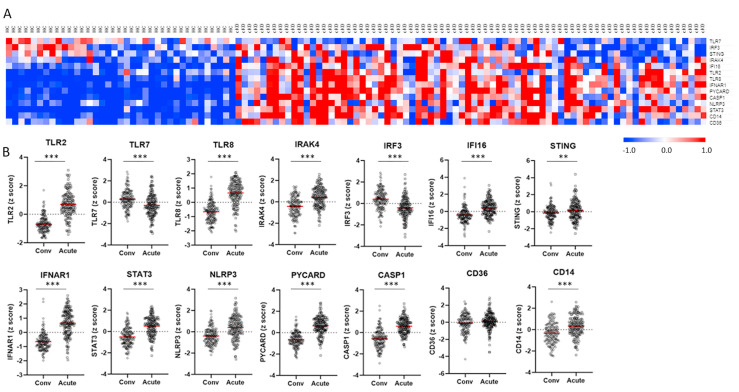
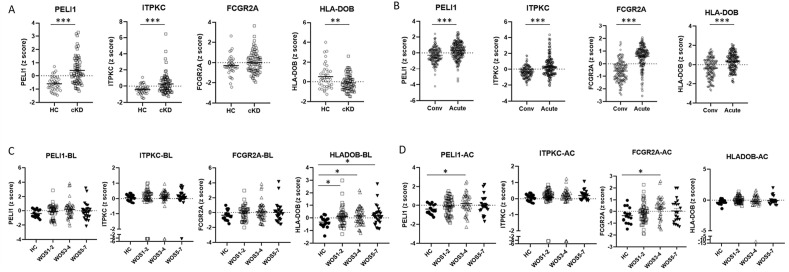
The transcriptome dataset of whole blood derived from KD patients (GSE-68004, GSE-63881) were downloaded from Gene Expression Omnibus/GEO).28 , 29 We then randomly divided the GSE-68004 cohort into a test dataset (70% of samples) and a validation dataset (30% of samples). A confusion matrix using the z score of innate immune genes TLR2, TLR7, TLR8, IRAK4, IRF3, IFI16, STING, IFNAR1, STAT3, NLRP3, PYCARD, CASP1, CD36, and CD14 as variables was built and the classification accuracies of test dataset and validation dataset for differentiating cKD from HC were 100% and 92.9%, respectively. Furthermore, we compared the innate immune gene expression signature between cKD patients (n = 19, age 4.2 ± 2.3 years) and healthy controls (HC, n = 23, age 8.3 ± 4.7 years) in GSE-68004. KD patients showed similar gene expression patterns as those observed in COVID-19 patients with moderate severity, except that as compared with HC, TLR7 was downregulated in cKD, and there were no significant differences in IRF3 and STING expressions between cKD and HC (Fig. 3 A, supplementary Table 2).
Figure 3.
Innate immune responses in Kawasaki disease (KD). Data were derived from GSE-68004 (A) and GSE-63881 (B) blood transcriptomes. A, Blood gene expression levels (z scores) in complete KD (cKD) were compared with levels in pediatric healthy controls (HC). In the heatmap, colors represent z score levels as explained by the scale bar at the right lower corner. B, Changes of blood gene expression levels (z scores) in acute KD were compared with levels in convalescent phase (Conv). Lines and bars represent mean ± SEM. ∗∗FDR<0.01, ∗∗∗FDR<0.001, by BH correction of multiple unpaired t tests.
In GSE-63881, transcriptome data of KD patients at acute phase (n = 171) and convalescent phase (n = 170), age 3.3 ± 2.8 years, were collected. As compared with blood taken at the convalescent phase, blood collected at acute KD showed elevated expression levels of genes involved in TLR-signaling, STING-IFN signaling, inflammasome related signaling, and innate immune cell signaling (Fig. 3B, supplementary Table 2). Of note, downregulation of TLR7 and IRF3 together with upregulation of STING were observed in acute KD (Fig. 3B).
Network analysis of potential interactions between genes related to innate immune responses in COVID-19 and genes known to confer susceptibility to KD
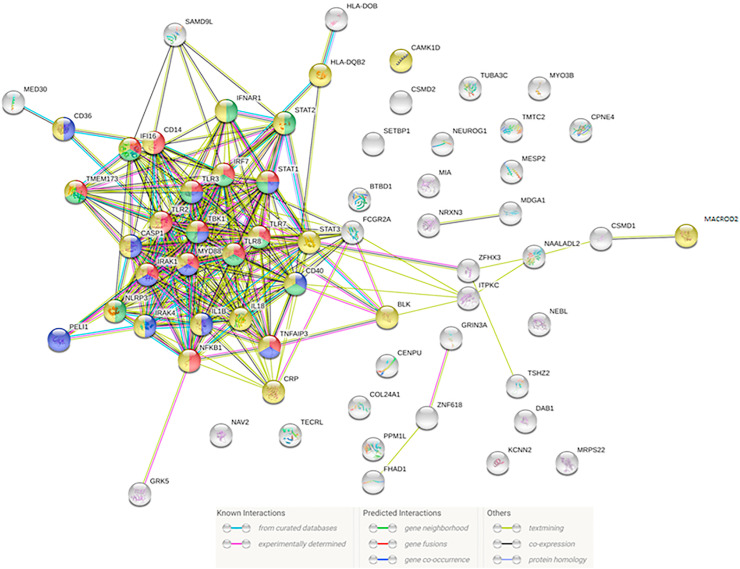
Since TLR-inflammasome and STING-IFN pathway activations were detected in both COVID-19 and acute KD cases, we further investigated if genetic variants associated with KD susceptibility might have potential interactions with genes involving innate immune responses to SARS-CoV-2 infection. Associations of SNPs across 15 GWAS studies with trait “mucocutaneous lymph node syndrome” were downloaded from the NHGRI-EBI GWAS Catalog30 on 2020.07.07 and summarized on an association plot using LocusZoom v0.5.6 (see Supplementary Fig E1). We then performed network analysis on genes mapped to the KD-associated SNPs and genes involving innate immune responses in COVID-19 using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) v.11.31 As shown in Fig. 4 , we detected strong evidence of functional associations between molecules contributing to KD susceptibility and molecules related to viral/stress innate immune responses. Enriched functional pathways include regulation of type I interferon production (GO:0032,479), regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043,122), response to virus (GO:0009615), response to stress (GO:0006950) (Fig. 4). Known experimentally determined protein–protein interactions were found between Pellino E3 ubiquitin protein ligase 1 (PELI1) and IRAK4 (STRING functional link combined score 0.971), PELI1 and MyD88 (combined score 0.738), BLK proto-oncogene, Src family tyrosine kinase (BLK) and STAT3 (combined score 0.940). Known interactions determined by curated databases were noted between molecules mediator complex subunit 30 (MED30) and CD36 (combined score 0.900), HLA-DQB2 and IRF7 (combined score 0.904), CD40 and nuclear factor kappa B subunit 1 (NFKB1) (combined score 0.948). A functional link between Fc fragment of IgG receptor IIa (FCGR2A) and TLR8 was also predicted by STRING, with a combined score of 0.744.
Figure 4.
Network analysis of potential interactions between genes related to innate immune responses in COVID-19 and genes known to confer susceptibility to KD by using STRING v.11. Enriched functional pathways include regulation of type I interferon production (red nodes, GO:0032,479), regulation of I-kappaB kinase/NF-kappaB signaling (blue nodes, GO:0043,122), response to virus (green nodes, GO:0009615), response to stress (yellow nodes, GO:0006950).
Expression of KD susceptibility genes in KD and COVID-19
We further analyzed the expression signature of KD susceptibility genes PELI1, ITPKC (Inositol-trisphosphate 3-kinase C), FCGR2A and HLA-DOB in COVID-19 and KD cohorts. In the KD cohort GSE-68004, the blood expression levels of PELI1 and ITPKC were markedly elevated, while the expression level of HLA-DOB was significantly downregulated in cKD patients as compared with HC (Fig. 5 A). In GSE-63881, however, acute phase KD showed upregulation of all four KD susceptibility genes as compared with convalescent phase (Fig. 5B). In the COVID-19 cohort (E-MTAB-9357), HLA-DOB level was elevated in patients with all severity scales at the time around COVID-19 diagnosis, as compared with healthy controls (Fig. 5C). Furthermore, higher expression levels of PELI1 and FCGR2A were associated with moderate COVID-19 at a few days after COVID-19 diagnosis (Fig. 5D).
Figure 5.
Expression levels of KD susceptibility genes in KD and COVID-19 cohorts. A, Expression z scores of PELI1, ITPKC, FCGR2A and HLA-DOB in KD (GSE-68004). Sample gene expression z scores were compared between the HC and the cKD group. B, Expression z scores of PELI1, ITPKC, FCGR2A and HLA-DOB in KD (GSE-63881). Sample gene expression z scores were compared between Acute KD and KD at convalescent phase (Conv). C, Expression z scores of PELI1, ITPKC, FCGR2A and HLA-DOB in COVID-19 (E-MTAB-9357)-BL group (blood draw at initial COVID-19 diagnosis). Sample gene expression z scores were compared between HC and BL. D, Expression z scores of PELI1, ITPKC, FCGR2A and HLA-DOB in COVID-19 (E-MTAB-9357)-AC group (blood draw a few days after COVID-19 infection). Sample gene expression z scores were compared between HC and AC. The severity of COVID-19 patients in E-MTAB-9357 was stratified according to the WHO Ordinal Scale (WOS): mild (WOS 1–2), moderate (WOS 3–4), severe (WOS 5–7). Lines and bars represent mean ± SEM. ∗FDR (adjusted P) < 0.05, ∗∗FDR<0.01, ∗∗∗FDR<0.001, by BH correction of multiple unpaired t tests.
Expression of innate immune genes and KD susceptibility genes in other viral infections
Since activation of the innate immune pathway could be a general phenomenon in viral infections, we analyzed the expression signature of our gene list using integrated meta-analysis data of multi-cohort transcriptomes of respiratory virus infections (including human rhinovirus, influenza virus, and respiratory syncytial virus) downloaded from MetaSignature (https://metasignature.stanford.edu/).32 Similar and distinctive innate immune responses were observed in the meta-virus transcriptomes. Unlike the upregulation of STING noted in COVID-19 and KD patients, the change of STING expression was not significant in the meta-virus infection data (supplementary Fig E2). Furthermore, while increased expression of HLA-DOB was observed in COVID-19 patients of all severity scales at the time of diagnosis, decreased HLA-DOB expression was noted in the meta-virus multi-transcriptomic data (Supplementary Fig E2). Elevated PELI1 and FCGR2A levels seen in moderate COVID-19 patients (AC group) were not observed in the meta-virus data, while significant downregulation of IRF3 was noted in the meta-virus meta-analysis (Supplementary Fig E2).
Expression of other innate immune genes in MIS-C, KD and COVID-19
Due to the unavailability of public transcriptome data of MIS-C in ArrayExpress/GEO, we could not analyze the expression signature of our selected genes in MIS-C. However, other innate immune genes including CD177, GPR84, VNN1, S100A12, and a gene related to platelet aggregation, ITGA2B, were reported to be upregulated in blood of MIS-C and pediatric COVID-19 as compared with adult healthy controls.33 Analysis of the expression of these genes in our transcriptome datasets revealed similar upregulation pattern in KD (GSE-68004, Supplementary Fig E3A). In E-MTAB-9357, we found markedly increased S100A12 levels in patients’ blood drawn a few days later after the diagnosis of moderate and severe COVID-19 (p < 0.001 by t tests as compared with healthy controls, Supplementary Fig E3B). Significant ITGA2B upregulation was also detected in severe COVID-19 patients (AC group, WOS 5–7 vs HC, p < 0.001 by t test, Supplementary Fig E3B).
Discussion
In this study, we compared the innate immune responses between blood transcriptome datasets of COVID-19 and KD. Consistent with previous reports, extensive activations of viral – stimulated innate sensors TLRs and STING, and upregulation of the downstream inflammasome pathways were identified in the COVID-19 patients with moderate severity (WOS 3–4).15 , 22 , 34 Furthermore, increased expression of IFI16, PYCARD and CD14 at the time around diagnosis, and elevated PYCARD level found a few days after diagnosis, were associated with severe COVID-19 (WOS 5–7). As compared with pediatric healthy controls, similar innate immune activation responses were also noted in the KD transcriptome, except the expression of TLR7, IRF3 and STING. Elevated level of PYCARD, an important adaptor molecule in the inflammasome complex, was found to be associated with moderated to severe COVID-19 patients and KD patients. Our results are consistent with previous findings suggesting inflammasome hyperactivation in severe pulmonary/systemic COVID-19 and in MIS-C.35 , 36 Interestingly, we observed elevated IRF3 level in patients of moderate severity a few days after COVID-19 diagnosis. Increased IFN signaling component, along with IL-6 and IL-10 productions, and lack of TNF-αelevation were reported in a New York MIS-C cohort related to COVID-19 5. Whether dysregulated IRF3 responses in children contributes to COVID-19 associated MIS-C requires further investigations.
Our study showed elevated STING level in COVID-19 patients of moderate severity and in acute KD patients as compared with the level in convalescent phase. Although not being reported to be associated with KD susceptibility, genetic polymorphism in STING has been suggested to underlie differential host immune response to SARS-CoV-2 infection, including MIS-C temporally associated with COVID-19 25. Moreover, upregulation of STAT3, a central molecule of the pro-inflammatory cytokine amplification loop, was detected in both the COVID-19 (moderate severity) and KD transcriptomes in our study. Variant in STAT3 has been shown to confer susceptibility to inflammatory bowel disease in children.37 It is reported that most children diagnosed with MIS-C showed gastrointestinal symptoms at the early phases of SARS-CoV-2 infection.7 Further research needs to be done to answer the question that whether functional STAT3 polymorphism involving aberrant intestinal immune response could contribute to MIS-C related to COVID-19.
In the aspect of innate immune cell activations, upregulation of both monocyte marker CD14 and scavenger receptor CD36 were detected in the cKD blood transcriptome. Similarly, in the COVID-19 dataset, increased expression of the two cell surface markers was found a few days after diagnosis in patients of moderate severity. Indeed, recent analyses of COVID-19 patients revealed involvement of monocytes, macrophages, and activated granulocytes.16 , 38 Besides its role in phagocytosis, CD36 binding to cell-derived microparticles has been reported to induce platelet activation, a phenomenon found in KD.39 Similarly, increased platelet activation and formation of platelet-monocyte aggregate were detected in severe, not mild COVID-19 patients.40 Furthermore, we found that neutrophil and platelet activation-related genes reported to be upregulated in MIS-C33 were upregulated in the KD transcriptome; and increased S100A12 and ITGA2B expression were also observed in delayed severe response to COVID-19 infection (E-MTAB-9357 cohort), not in mild patients. Taken together, our results implicate potential roles of CD14 and CD36 expressing myeloid cells and neutrophil/platelet activation in MIS-C.
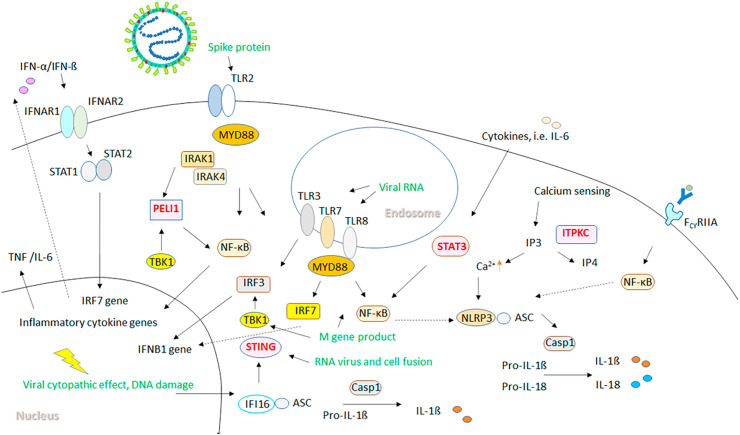
The identified shared innate immune responses, including the TLR-IRAK, STING, STAT3 and the inflammasome pathways between KD and moderate to severe COVID-19 suggest possible roles of these genes in the development of KD-like disease related to COVID-19 in susceptible children. Our network analysis of potential interactions between genes related to innate immune responses in COVID-19 and genes known to confer susceptibility to KD revealed evidence of functional associations. ITPKC has been reported to regulate IP3 (inositol triphosphate)-mediated calcium release and subsequent NLRP inflammasome activation resulting in production of IL-1β and IL-18.41 The KD susceptibility gene, PELI1, has been reported as a negative regulator of NF-κB, and showed close interactions with MYD88, IRAK4 and TBK1.42 , 43 Furthermore, FcγRIIA is known to mediate IgG-immune complex induced NF-κB signaling and priming of NLRP3 inflammasome activation.44 Functional polymorphism in FCGR2A (rs1801274) has been reported to influence the treatment response to IVIG in KD.45 Interestingly, it has been shown that adult COVID-19 subjects produce IgG antibodies with reduced Fc domain fucosylation when compared with SARS-CoV-2-seropositive children.46 The reduced fucosylation was proposed to enhance interactions with the activating FcγR, leading to development of COVID-19 symptoms.46 In our study, we found elevated HLA-DOB expression in COVID-19 patients with all severity scales at the time around diagnosis. However, higher expression levels of both PELI1 and FCGR2A detected a few days after COVID-19 infection were associated with more severe diseases. We therefore postulate that genetic variants associated with KD, including polymorphisms in PELI1 and FCGR2A, might predispose children to MIS-C related to COVID-19 through TLR/NF-κB/NLRP3 inflammasome dysregulations (Fig. 6 ). Of note, no significant change in PELI1, FCGR2A and STING was found in meta-analysis of transcriptomes of other viral infections, suggesting that the activation of these genes in COVID-19 patients might be a response more specific to coronavirus, and hyperactivation of these genes might lead to MIS-C related to COVID19.
Figure 6.
Model of potential impacts of KD associated genetic variants PELI1, ITPKC, FCGR2A, and genetic polymorphisms in STING and STAT3 on TLR/inflammasome/IFN dysregulation, which might lead to predisposition to COVID-19 related MIS-C. Components of SARS-CoV-2 and viral associated PAMPs/DAMPs are shown in green. The spike protein can activate TLR2/MyD88/NF-κB pathway, leading to the production of proinflammatory cytokines, and priming of the NLRP3 inflammasome. The single-strand viral RNA can stimulate TLR7/TLR8, and double-strand RNA produced during viral replication can stimulate TLR3 in the endosome, which activate IRF and NF-κB signaling. The M gene product (membrane protein) can stimulate TBK1/IRF3/IFNB1 pathway and TLR-related NF-κB signaling. STING can be activated by viral related host DNA damage via IFI16, or can be stimulated directly by product of RNA virus and cell fusion, leading to type I IFN production. PELI1 is a negative regulator of NF-κB, while STAT3 is a positive regulator of NF-κB. FcγRIIA mediates IgG-immune complex induced NF-κB signaling and priming of NLRP3 inflammasome activation. ITPKC regulates IP3-mediated calcium release and subsequent NLRP inflammasome activation.
Our study is limited that the COVID-19 cohort is of adult population due to the current unavailability of pediatric COVID-19 and MIS-C transcriptome datasets in open database repositories. Extensive comparisons of potential mechanisms underlying pulmonary COVID-19, MIS-C and KD have revealed both differences and similarities in immunological processes. It has been reported that lower expression levels of angiotensin converting enzyme II (ACE2) in children respiratory tract is associated with milder COVID-19 pulmonary symptoms, in contrast to the hallmark of hyperinflammation related acute respiratory distress syndrome (ARDS) seen in severe adult COVID-19 patients.47 Furthermore, differential adaptive immune responses were observed in adult COVID-19 ARDS and MIS-C. Adult ARDS usually occurred within 2 weeks of SARS-CoV-2 infection, while MIS-C generally developed much later after COVID-19 diagnosis.47 The terminally differentiated effector CD57+CD4+T cells were reduced in adult, but not in pediatric COVID-19 patients.48 Moreover, IgG antibodies to the common cold coronavirus was found to be lacking only in MIS-C patients.48 These findings suggest that age-dependent factors affecting the efficiency of adaptive immune response could modulate different immuno-pathologies in SARS-CoV-2 infection.
However, in the aspect of innate immunity, it is reported that adult levels of pulmonary macrophages are reached within days after birth, and newborn myeloid dendritic cells (mDCs) stimulated with TLR ligands express adult-like concentrations of pro-inflammatory cytokines.49 Similarities of innate immune responses have been observed among MIS-C, adult severe COVID-19 diseases, and KD.16 , 28 , 33 , 35 , 36 , 40 For example, infiltration of monocytes/granulocytes, and activation of inflammasomes have been reported in all these severe diseases,16 , 28 , 33 , 35 , 36 , 40 and were also observed in moderate to severe adult COVID-19 patients and in KD patients of our study.
In conclusion, we provide a model of potential impacts of STING and STAT3 polymorphisms, and genetic variants previously known to be associated with KD, such as PELI1 and FCGR2A, on TLR/inflammasome/IFN dysregulation. This model, together with enhanced myeloid cell and platelet activation, might lead to predisposition of MIS-C related to COVID-19. Direct DNA sequencing of genetic polymorphisms associated with KD susceptibility and functions of STING and STAT3 in large cohorts of MIS-C are required to validate our hypothesis.
Funding
This work was partially supported by China Medical University Hsinchu Hospital [grant number CMUHCH-DMR-110-022].
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
The authors thank Mr. Fong-Ming Yang for technical consultations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfma.2021.06.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 9.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020;38(11):2492.e5–2492.e6. doi: 10.1016/j.ajem.2020.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebina-Shibuya R., Namkoong H., Shibuya Y., Horita N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T., Nakashima Y., Sakai Y., Nishio H., Motomura Y., Yamasaki S. Kawasaki disease: a matter of innate immunity. Clin Exp Immunol. 2016;186(2):134–143. doi: 10.1111/cei.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L.Y., Lu C.Y., Shao P.L., Lee P.I., Lin M.T., Fan T.Y., et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113(3):148–154. doi: 10.1016/j.jfma.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnier J.L., Anderson M.S., Heizer H.R., Jone P.N., Glode M.P., Dominguez S.R. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. 2015;136(3):e609–e614. doi: 10.1542/peds.2015-0950. [DOI] [PubMed] [Google Scholar]
- 14.Chen K.Y.H., Messina N., Germano S., Bonnici R., Freyne B., Cheung M., et al. Innate immune responses following Kawasaki disease and toxic shock syndrome. PloS One. 2018;13(2) doi: 10.1371/journal.pone.0191830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Bruggen M.C., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1–2):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 19.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuri T., Zhang X., Habjan M., Martinez-Sobrido L., Garcia-Sastre A., Yuan Z., et al. Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation. J Gen Virol. 2009;90(Pt 11):2686–2694. doi: 10.1099/vir.0.013599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Liu L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a toll-like-receptor-related TRAF3-independent mechanism. mBio. 2016;7(1) doi: 10.1128/mBio.01872-15. e01872-01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthelot J.M., Liote F. COVID-19 as a STING disorder with delayed over-secretion of interferon-beta. EBioMedicine. 2020;56:102801. doi: 10.1016/j.ebiom.2020.102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunphy G., Flannery S.M., Almine J.F., Connolly D.J., Paulus C., Jonsson K.L., et al. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-kappaB signaling after nuclear DNA damage. Mol Cell. 2018;71(5):745–760 e745. doi: 10.1016/j.molcel.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao T.S. The nucleic acid-sensing inflammasomes. Immunol Rev. 2015;265(1):103–111. doi: 10.1111/imr.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthelot J.M., Drouet L., Liote F. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway? Emerg Microb Infect. 2020;9(1):1514–1522. doi: 10.1080/22221751.2020.1785336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popper S.J., Watson V.E., Shimizu C., Kanegaye J.T., Burns J.C., Relman D.A. Gene transcript abundance profiles distinguish Kawasaki disease from adenovirus infection. J Infect Dis. 2009;200(4):657–666. doi: 10.1086/603538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183(6):1479–1495.e20. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang L.T., Shimizu C., Ling L., Naim A.N., Khor C.C., Tremoulet A.H., et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 2014;6(11):541. doi: 10.1186/s13073-014-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaggi P., Mejias A., Xu Z., Yin H., Moore-Clingenpeel M., Smith B., et al. Whole blood transcriptional profiles as a prognostic tool in complete and incomplete Kawasaki Disease. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0197858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andres-Terre M., McGuire H.M., Pouliot Y., Bongen E., Sweeney T.E., Tato C.M., et al. Integrated, multi-cohort analysis identifies conserved transcriptional signatures across multiple respiratory viruses. Immunity. 2015;43(6):1199–1211. doi: 10.1016/j.immuni.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckmann N.D., Comella P.H., Cheng E., Lepow L., Beckmann A.G., Mouskas K., et al. medRxiv; 2020. Cytotoxic lymphocytes are dysregulated in multisystem inflammatory syndrome in children. [Google Scholar]
- 34.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Rivero Vaccari J.C., Dietrich W.D., Keane R.W., de Rivero Vaccari J.P. The inflammasome in times of COVID-19. Front Immunol. 2020;11:583373. doi: 10.3389/fimmu.2020.583373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C.A., Chiang B.L. Inflammasomes and childhood autoimmune diseases: a review of current knowledge. Clin Rev Allergy Immunol. Nov 25, 2020:1–15. doi: 10.1007/s12016-020-08825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amre D.K., Mack D.R., Morgan K., Israel D., Deslandres C., Seidman E.G., et al. Susceptibility loci reported in genome-wide association studies are associated with Crohn's disease in Canadian children. Aliment Pharmacol Ther. 2010;31(11):1186–1191. doi: 10.1111/j.1365-2036.2010.04294.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martinez-Colon G.J., McKechnie J.L., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora K., Guleria S., Jindal A.K., Rawat A., Singh S. Platelets in Kawasaki disease: is this only a numbers game or something beyond? Genes Dis. 2020;7(1):62–66. doi: 10.1016/j.gendis.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pao C.R.R., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeter D., Deth R. ITPKC susceptibility in Kawasaki syndrome as a sensitizing factor for autoimmunity and coronary arterial wall relaxation induced by thimerosal's effects on calcium signaling via IP3. Autoimmun Rev. 2012;11(12):903–908. doi: 10.1016/j.autrev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Chang M., Jin W., Chang J.H., Xiao Y., Brittain G.C., Yu J., et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat Immunol. 2011;12(10):1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin W., Chang M., Sun S.C. Peli: a family of signal-responsive E3 ubiquitin ligases mediating TLR signaling and T-cell tolerance. Cell Mol Immunol. 2012;9(2):113–122. doi: 10.1038/cmi.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galeotti C., Kaveri S.V., Bayry J. Molecular and immunological biomarkers to predict IVIg response. Trends Mol Med. 2015;21(3):145–147. doi: 10.1016/j.molmed.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Khor C.C., Davila S., Breunis W.B., Lee Y.C., Shimizu C., Wright V.J., et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43(12):1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 46.Chakraborty S., Edwards K., Buzzanco A.S., Memoli M.J., Sherwood R., Mallajosyula V., et al. medRxiv; 2020. Symptomatic SARS-CoV-2 infections display specific IgG Fc structures. [Google Scholar]
- 47.Kabeerdoss J., Pilania R.K., Karkhele R., Kumar T.S., Danda D., Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41(1):19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968–981. doi: 10.1016/j.cell.2020.09.016. e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.