Abstract
The study investigated the antioxidant effect of Ruzu herbal bitters (RHB) on alloxan-induced diabetic rats, the pH and the bioactive components of RHB using gas chromatography and mass spectroscopy (GC-MS). Fifty-four adult albino rats were divided into nine groups of six rats each. Group 1 was the normal control. Groups 2–6 were diabetic. Group 2 was untreated positive control, while groups 3–6 were respectively treated with 5 mg/kg b. w of glibenclamide, 0.14, 0.29 and 0.57 ml/kg b. w of RHB for 21 days. Groups 7–9 were not diabetic but treated as in groups 4–6 respectively. The results showed significant (p < 0.05) increase in the blood glucose level and significant (p < 0.05) decrease in weight in diabetic untreated group compared to the normal control. The oxidative stress parameters showed significant (p < 0.05) increases in the serum activities of superoxide dismutase (SOD) and catalase (CAT), with significant (p < 0.05) decrease in glutathione peroxidase (GPx); while there were significant (p < 0.05) increases in the levels of vitamin C (VIT C), vitamin E (VIT E), C-reactive protein (CRP) and malondialdehyde (MDA), with significant (p < 0.05) decrease in the level of glutathione (GSH) in the diabetic untreated group compared to the normal control group. However, treatment of the diabetic groups with different doses of RHB resulted in the reversal of the effects to near-normal levels in a dose-dependent manner. The pH of RHB was found to be 3.45. The GC-MS result of RHB revealed the presence of 10 bioactive compounds, out of which four are pharmacologically important antioxidants: 11-Octadecenioc acid -Methyl esther, 2,7-Dioxatricyclodeca-4, 9-diene, Cis-Z-α- Bisabolene epoxide, and Tetradecanoic acid (lauric acid). Thus, the study revealed that Ruzu herbal bitters possesses antidiabetic and antioxidant activities due to the bioactive antioxidant compounds it contains.
Keywords: Diabetes, Ruzu herbal bitters, Antioxidant, pH, Bioactive compounds
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder affecting a great number of world population, leading to both morbidity and mortality. The two most common effects of DM are impaired insulin secretion as a result of damage to the insulin-secreting β-cells of the pancreas, and defective insulin action, otherwise known as insulin insensitivity, whereby the presence of insulin is not able to stimulate the peripheral tissues for glucose uptake. The consequence of the effects is abnormally high blood glucose level, called hyperglycemia, resulting from abnormal glucose, lipid and protein metabolism. Impaired insulin secretion and defective insulin action are the primary causes of the two most common forms of DM - type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) respectively. Common to both forms of DM are hyperglycemia, excessive urine production (polyuria), compensatory thirst, increased water intake (polydipsia), blurred vision, sudden weight loss, lethargy, and changes in energy metabolism [1].
Chronic hyperglycemia caused by diabetes mellitus increases the production of free radicals that are associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [2,3]. Free radical damage is one of the most prominent causes of devastating diseases that are responsible for killing many people in the world, such as cardiovascular disease, which can manifest as heart attacks, and cancer [4]. Reactive oxygen species can directly damage lipids, proteins or DNA and modulate intracellular signaling pathways [5].
Over the years, natural medicinal products comprising of different blended plants or their extracts, known as “poly-herbal mixtures” have been used in the treatment of ailments with better therapeutic effects for a particular disease or multiple diseases than that of a single plant. This is because the individual plant contains different therapeutic agents in which when combined together will give a better result than that of a single plant; believed to be due to the synergistic effect of the component plants’ phytochemicals. One of such polyherbal products is Ruzu herbal bitters [6].
Ruzu herbal bitters (RHB) (Fig. 1) is a polyherbal mixture, produced by Ruzu Natural Health Product and Services, Nigeria, with a NAFDAC Registration Number: A7-1102 L. The polyherbal mixture is made up of three different plants: 20% Uvaria chamae (bush banana), 40% Citrullus colocynthis (bitter apple) and 40% Curculigo pilosa (squirrel groundnut). RHB is commercially available and the manufacturers infer that the product has the following medicinal functions amongst others, as indicated in the leaflet of the product: management/treatment of diabetes, typhoid and malaria, high blood pressure, waist and back pains, etc.
Fig. 1.
Ruzu herbal bitters - Dosage: Adults – 2–4 tablespoons 1 or 2 times daily. Children – 1 tablespoon (5 ml) once in 3 days or as directed by physician (Producer) (Obasi and Ogugua, 2020) [6].
However, there have been reports that the extracts of two component plants of RHB, Citrullus colocynthis and Uvaria chamae, caused significant decrease in the concentration of blood glucose and ameliorated kidney damage in alloxan-induced diabetic rats [[7], [8], [9]].
Based on the medicinal claims of the manufacturer of RHB with scanty scientific proofs, there was need to carry out scientific investigations to ascertain the efficacy of RHB in the management of diabetes mellitus and its antioxidant property using alloxan-induced diabetic rats. Also, the determination of its pH and the bioactive compounds contained in the product using gas chromatography and mass spectroscopy (GC-MS).
2. Materials and methods
2.1. Materials: ruzu herbal bitters (RHB) was purchased from the producer and used directly
Equipment:
Spectrophotometer (Spectro 21D PEC MEDICALS USA), centrifuge (Techmel and techmel U.S.A), electrothermal incubator (Model: DNP), refrigerator (Haier thermocool; HTF-429H), weighing balance (Golden-mettler U.S.A), glucometer (Accu-Check Active, Model-GC, Roche, Germany), pH paper, pH meter (PHS – 25 PEC Medical USA), measuring cylinder, glass wares (pyrex), sample containers.
2.1.1. Chemicals/reagents
Alloxan monohydrate (Sigma Aldrich, St. Louis, MO, USA), kits. All the chemicals and reagents used in this research were of the purest analytical grade commercially available.
2.1.2. Methods
2.1.2.1. Animal management
Adult inbred male Wister albino rats (weighing between 96 and 121 g) were purchased from the animal house of the Department of Zoology, University of Nigeria, Nsukka and acclimatized for one week prior to commencement of the experiment. They were kept at room temperature and maintained ad libitum on water and feed; weighed prior to commencement of experiment and weekly till the end of the experiment.
2.1.2.2. Induction of diabetes
Rats were fasted overnight (24 h) and experimental diabetes was induced by intraperitoneal injection of freshly prepared alloxan (3 g of alloxan monohydrate was dissolved in 30 ml of 0.9% w. v. normal saline to get 0.1 g/ml of alloxan) with a single dose of 120 mg/kg body weight. After three days, rats with blood glucose level greater than 200 mg/dl (hyperglycemia) were selected for the experiment [10]. Only few rats showed insufficient glycemia and there were not used for the experiment. However, they showed normoglycemia after some days.
2.1.2.3. Experimental design
Dosages of 0.14, 0.29 and 0.57 ml/kg body weight of RHB, equivalent to 10, 20 and 40 ml of RHB/70 kg body weight adult daily, as prescribed by the producer, were chosen for the study. Fifty-four (54) albino rats were divided into nine groups of six rats each as follows:
-
(a)
Group 1: Normal control rats (non-diabetic).
-
(b)
Group 2: Positive control rats (diabetic untreated).
-
(c)
Group 3: Standard control rats (diabetic) treated with 5 mg/kg body weight of glibenclamide [11].
-
(d)
Group 4: Diabetic rats treated with 0.14 ml/kg body weight of RHB.
-
(e)
Group 5: Diabetic rats treated with 0.29 ml/kg body weight of RHB.
-
(f)
Group 6: Diabetic rats treated with 0.57 ml/kg body weight of RHB.
-
(g)
Group 7: Non-diabetic rats treated with 0.14 ml/kg body weight of RHB
-
(h)
Group 8: Non-diabetic rats treated with 0.29 ml/kg body weight of RHB
-
(i)
Group 9: Non-diabetic rats treated with 0.57 ml/kg body weight of RHB.
The baseline blood glucose levels of the rats were determined before the induction of diabetes. The treatment, weight and blood glucose determinations (7 days’ intervals) lasted for 21 days. The polyherbal mixture (RHB) and glibenclamide were given to the diabetic rats as indicated every morning (about 9:00 a.m.). At the end of the experiment, blood samples were collected from the rats via ocular and cardiac puncture on the first day of post-treatment. The blood samples were collected in anticoagulant free bottles, allowed to clot and centrifuged at 3000 rpm for 15 min. The serum collected was used for the biochemical assays.
2.1.2.4. Determination of blood glucose level
The blood glucose levels of the rats were determined using a one-touch blood glucose monitoring meter (glucometer). The Accu-Check strip was inserted into the indicated area of the glucometer and allowed to show light at the point where a drop of blood would be placed. A lancet was used to puncture the tail end of the rat. The tail was pressed to bring out blood which was then placed on the indicated portion of the test strip in the glucometer. The blood glucose level which showed on the screen of the glucometer (in mg/dl) was recorded.
2.1.3. Oxidative stress parameters
2.1.3.1. Assay of superoxide dismutase activity
This was determined using the method of Arthur and Boyne [12].
Principle: Superoxide dismutases (SOD) are enzymes that catalyze the conversion of two superoxides into hydrogen peroxide and oxygen. The principle of SOD assay was based on the inhibition of adrenaline oxidation.

Procedure: Adrenalin (0.01 g) was dissolved in 17 ml of distilled water and 0.1 ml of serum and 0.9 ml of phosphate buffer (pH 7.8) were taken in triplicates in 2.5 ml buffer. A volume (0.3 ml) of adrenaline solution was added and mixed inside the cuvette. The absorbance was taken at 480 nm at 30 s interval for five (5) times. The changing rate of absorbance was used to determine superoxide dismutase activity.
2.1.3.2. Assay of catalase activity
The activity of catalase (CAT) was assayed by the method of Sinha [13].
Principle: Dichromate in acetic acid was reduced to chromic acetate when heated in the presence of hydrogen peroxide with the formation of per chromic acid as an unstable intermediate. The chromic acetate formed was measured at 590 nm. Catalase was allowed to split H2O2 for different periods of time. The reaction was stopped at different time intervals by the addition of dichromate acetic acid mixture and the remaining H2O2 was determined by measuring chromic acetate colorimetrically after heating the reaction mixture.
Procedure: To 0.9 ml of phosphate, 0.1 ml of plasma and 0.4 ml of H2O2 were added. The reaction was stopped after 15, 30, 45 and 60 s by adding 2 ml of dichromate acetic acid mixture. The tubes were kept in a boiling water bath for 10 min, cooled and the colour developed was read at 530 nm. Standards in the concentration range of 20–100 μmol were processed for the test. The activity of catalase was expressed as U/ml for plasma (U- micro moles of H2O2 utilised/second).
2.2. Assay of glutathione peroxidase activity
Gluthathione peroxidase (GPx) activity was assayed based on the method of Paglia and Valentine [14].
Principle: Gluthathione peroxidase catalyses the oxidation of glutathione (GSH) by colourless liquid hydrocarbon hydroperoxide. In the presence of glutathione reductase (GR) and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm is measured.
GPx
| 2 GSH + ROOH → GSSG + ROH + H2O |
GR
| GSSG + NADPH + H+ → NADP+ + 2 GSH |
Procedure: A known volume of the sample (0.1 ml) was added into a test tube. Then, 0.1 ml of diluted reagent 1 (R1) was mixed with 0.1 ml of reagent 2 (R2) and was added into the test tube and mixed. The initial absorbance was taken after 30 s and the final absorbance was taken after 1 min at 340 nm. Glutathione peroxidase activity was calculated from the formula:
| GPx (U/L) = 8412 × A 340 nm/min |
2.3. Determination of reduced glutathione concentration
The reduced glutathione (GSH) level was determined by the method of Exner et al. [15].
Principle: This method was based on the development of yellow colour when 5,5′- dithio-bis-2-nitrobenzoate (DTNB) is added to compound containing sulphydryl groups. The colour developed was read at 412 nm in spectrophotometer.
Procedure: A volume of 0.2 ml of sample was mixed with 1.8 ml of EDTA solution. To this, 3.0 ml of precipitating reagent was added, mixed thoroughly and kept for 5 min before centrifugation. To 2 ml of the filtrate, 4 ml of 0.3 M disodium hydrogen phosphate solution and 1 ml of DTNB reagent were added and the colour developed was read at 412 nm in spectrophotometer. A set of standard solutions containing 20–100 μg of reduced glutathione was treated similarly. The values were expressed in mg/dl for plasma.
2.4. Determination of vitamin C concentration
Vitamin C (ascorbic acid) concentration was determined by the method described by Angirekula et al. [16]. Determination was made after devitalisation with 2, 4 dinitrophenylhydrazine.
Principle: Ascorbic acid is oxidized by copper to form dihydroascorbic acid and diketoguloric acid. These products are treated with 2, 4 DNP to form a derivative of bis 2,4 dinitrophenylhydrazine. This compound in conc. H2SO4 undergoes a rearrangement to form a product with an absorption band that is measured at 520 nm. This reaction is seen in the presence of thiourea to provide an unduly reducing reaction which helps to produce an inter substance from non-ascorbic acid chromogens.
Reagents
-
1.
TCA (trichloroacetic Acid) 5% and 10% in distilled water
-
2.
2,4 dinitrophenylhydrazine/thiourea/copper (DTC) solution (add 0.4 g thiourea, 0.05 g CuSO4 5H2O and 30 g 2, 4 dinitrophenylhydrazine and bring to a total volume of 100 ml with 9 NH2SO4.
-
3.
A 65% H2SO4
Procedure: Serum (1 ml) of and 1 ml of ice cold 10% TCA were mixed well and centrifuged for 20 min at 3500 rpm. Then, 0.5 ml of supernatant and 1 ml of DTC were taken and incubated for 3 h at 37 °C. To convert this into an inter arrange product, 0.75 ml of ice cold 65% H2SO4 was added. Then mixed well and cooled. The final product was read at 520 nm.
2.5. Determination of vitamin E concentration
Vitamin E (Alpha Tocopherol) content was determined by the method of Pearson [17].
Principle: This method involves the conversion of ferric ions to ferrous ions by α-tocopherol and the formation of red coloured complex with α, α-dipyridyl. Absorbance of chromophore was measured at 520 nm in the spectrophotometer.
Procedure: To 0.5 ml of serum, 1.5 ml of ethanol was added and mixed. To this was added 1.0 ml of α, α-dipyridyl solution and 1.0 ml of ferric chloride solution and mixed. The colour developed was read at 520 nm in the spectrophotometer. Values were read as mg/dl of serum.
2.6. Determination of C-Reactive protein concentration
C-reactive protein (CRP) was determined by turbidmetric immunoassay method [18].
Principle: TURBILYTE-CRP is a turbidmetric immunoassay for the determination of C-reactive protein in human serum and is based on the principle of agglutination reaction. The test specimen is mixed with activation buffer (R1), TURBILYTE-CRP latex reagent (R2) and allowed to react. The presence of CRP in the test specimen results in the formation of an insoluble complex producing a turbidity, which is measured at 630 nm wavelength. The increase in turbidity correspond to the concentration of CRP in the specimen.
Procedure: Into two cuvettes were respectively pipetted 450 μl of R1 and 50 μl of R2 for calibration (standard) and sample, mixed and incubated for 5 min. Next, 5 μl of calibrator and sample were added into their respective cuvettes and mixed well. The absorbance A1 at 10 s and A2 at 2 min were read at 630 nm using a spectrophotometer.
Calculation
2.7. Determination of malondialdehyde concentration
Lipid peroxidation was estimated by measuring spectrophotometrically the level of the lipid peroxidation product, malondialdehyde (MDA) as described by Wallin et al. [19].
Principle: Lipid degradation occurs forming such products as malondialdehyde (from fatty acids with three or more double bonds), ethane and pentane (from the n-terminal carbons of 3 and 6 fatty acids, respectively). MDA reacts with thiobarbituric acid to form a red or pink coloured complex which in acid solution absorbs maximally at 532 nm.
Procedure: A volume, 0.1 ml of the serum was mixed with 0.9 ml of H2O in a test tube. A volume, 0.5 ml of 25% TCA (trichloroacetic acid) and 0.5 ml of 1% TBA (thiobarbituric acid) in 0.3% NaOH were also added to the mixture. The mixture was boiled for 40 min in water-bath and then cooled in cold water. Then 0.1 ml of 20% sodium dodecyl sulfate (SDS) was added to the cooled solution and mixed properly. The absorbance was taken at wavelength 532 nm and 600 nm against a blank.
Calculation
Statistical Analysis: The data obtained from the laboratory tests was subjected to one-way analysis of variance (ANOVA). Significant differences were obtained at P ≤ 0.05 and the results were expressed as mean ± standard error of mean (SEM). The SPSS version 20 and Microsoft excel 2007 software were used.
3. Results
3.1. Blood glucose level
The effect of RHB on the blood glucose levels (mg/dl) of the rats is presented in Table 1. Administration of alloxan caused a significant (p < 0.05) increase in the blood glucose levels (BGL) of the rats when compared to their baseline BGL. There was a significant (p < 0.05) increase in the BGL of the diabetic-untreated group from day 0 of the baseline (70.00 ± 66.97) to diabetes induction (241.25 ± 36.42), and progressively to the day 21 (514.3 ± 8.06); unlike the normal control group where there was a gradual increase in the BGL from day 0 (78.50 ± 4.80) to the day 21 (88.25 ± 2.06) of the experiment. However, treatment of the diabetic groups 4, 5 and 6 with 0.14, 0.29 and 0.57 ml/kg b. w of RHB respectively, resulted in significant (p < 0.05) decreases in their BGL in a dose dependent manner. Also, for the non-diabetic groups 7, 8 and 9 treated with 0.14, 0.29 and 0.57 ml/kg b. w of RHB respectively; there was no significant (p < 0.05) decrease in the blood glucose levels in group 7 (79.25 ± 9.11 to 75.50 ± 4.93) but there were significant (p < 0.05) decreases in groups 8 (84.33 ± 8.02 to 70.33 ± 4.16) and 9 (81.33 ± 5.03 to 66.33 ± 1.15) within the 21 days of treatment.
Table 1.
Effects of Ruzu herbal bitters (RHB) on blood glucose levels of alloxan-induced diabetic rats.
|
Blood glucose levels in mg/dl expressed in mean ± S.D | |||||
|---|---|---|---|---|---|
| GROUPS | DAY 0 | DIABETES (DAY 0) | DAY 7 | DAY 14 | DAY 21 |
| GROUP 1 | 78.50 ± 4.80a | 86.25 ± 5.19ab | 83.69 ± 9.00ab | 88.25 ± 2.06b | |
| GROUP 2 | 70.00 ± 66.97a | 241.25 ± 36.42b | 294.75 ± 7.14c | 379.70 ± 3.39d | 514.3 ± 8.06e |
| GROUP 3 | 76.00 ± 11.79a | 384.00 ± 6.00c | 159.67 ± 10.60b | 91.33 ± 17.92a | 72.33 ± 14.57a |
| GROUP 4 | 70.33 ± 2.08a | 296.67 ± 147.55b | 134.00 ± 34.51a | 96.00 ± 6.00a | 79.67 ± 15.04a |
| GROUP 5 | 84.00 ± 6.08a | 304.67 ± 21.08c | 157.66 ± 8.96b | 103.33 ± 8.08a | 81.00 ± 14.52a |
| GROUP 6 | 68.00 ± 5.20a | 330.67 ± 167.16c | 183.67 ± 6.42b | 103.33 ± 10.97a | 62.67 ± 9.71a |
| GROUP 7 | 79.25 ± 9.11a | 84.75 ± 5.68a | 81.75 ± 4.79a | 75.50 ± 4.93a | |
| GROUP 8 | 84.33 ± 8.02b | 82.00 ± 3.46ab | 76.00 ± 7.94ab | 70.33 ± 4.16a | |
| GROUP 9 | 81.33 ± 5.03c | 77.33 ± 4.73bc | 71.33 ± 6.85ab | 66.33 ± 1.15a | |
Mean values with different letters as superscripts across the rows are considered significant (p < 0.05).
(a-c Within a row, least square means without a common superscript differ (p < 0.05) due to variations in the blood glucose levels).
Group 1: normal control; Group 2: diabetic - untreated; Group 3: diabetic treated with 0.5 mg/kg b.w. of glibenclamide.
Group 4: diabetic treated with 0.14 ml/kg b.w. of RHB; Group 5: diabetic treated with 0.29 ml/kg b.w. of RHB.
Group 6: diabetic treated with 0.57 ml/kg b.w. of RHB; Group 7: non-diabetic treated with 0.14 ml/kg b.w. of RHB.
Group 8: non-diabetic treated with 0.29 ml/kg b.w. of RHB; Group 9: non-diabetic treated with 0.57 ml/kg b.w. of RHB.
DAY 0 = baseline blood glucose level; DIABETES (DAY 0) = blood glucose level after induction of diabetes.
3.1.1. Body weight
Table 2 shows the effect of Ruzu herbal bitters (RHB) on the body weight (g) of alloxan-induced diabetic rats. There was a significant (p < 0.05) increase in the body weight of the normal control group from day 0 (117.03 ± 15.14) to the day 21 (139.27 ± 14.38) of the experiment. The body weight of the diabetic-untreated group showed significant (p < 0.05) decrease from day 0 before induction of diabetes (104.57 ± 28.25), after induction of diabetes (99.00 ± 23.19 to the day 21 (75.10 ± 5.66) of the experiment. For the diabetic groups 4, 5 and 6 treated with 0.14, 0.29 and 0.57 ml/kg b. w of RHB respectively, there were significant (p < 0.05) increases in the body weight from day 0 to day 21 in a dose dependent manner; which is comparable to that observed in the diabetic group 3 treated with 0.5 mg/kg b. w. of glibenclamide. Also, for the non-diabetic groups 7, 8 and 9 treated with 0.14, 0.29 and 0.57 ml/kg b. w of RHB respectively, there was significant (p < 0.05) increases in the body weight from day 0 to the day 21 of treatment comparable to that of the normal control.
Table 2.
Effects of Ruzu herbal bitters (RHB) on the body weights of alloxan-induced diabetic rats.
| Body weights in g expressed in mean ± S.D | |||||
|---|---|---|---|---|---|
| GROUPS | DAY 0 | DIABETES (DAY 0) | DAY 7 | DAY 14 | DAY 21 |
| GROUP 1 | 117.03 ± 15.14a | 122.10 ± 12.83a | 128 35 ± 15.10b | 139.27 ± 14.38c | |
| GROUP 2 | 104.57 ± 28.25d | 99.00 ± 23.19cd | 94.97 ± 18.93c | 83.67 ± 12.34b | 75.10 ± 5.66a |
| GROUP 3 | 119.37 ± 9.26a | 121.80 ± 9.31a | 125.86 ± 4.30a | 140.53 ± 11.09b | 151.60 ± 10.53c |
| GROUP 4 | 101.20 ± 0.31a | 108.20 ± 9.49a | 114.67 ± 13.55ab | 121.73 ± 5.27bc | 132.73 ± 21.07c |
| GROUP 5 | 113.87 ± 7.24a | 116.40 ± 8.13a | 122.67 ± 6.81ab | 129.33 ± 1.53bc | 134.00 ± 1.48c |
| GROUP 6 | 121.07 ± 9.62a | 131.57 ± 3.38b | 135.17 ± 2.58bc | 141.17 ± 2.05c | 144.97 ± 2.97d |
| GROUP 7 | 111.55 ± 14.16a | 117.90 ± 14.26a | 127.05 ± 9.30ab | 137.15 ± 11 .43b | |
| GROUP 8 | 100.64 ± 15.13a | 112.88 ± 11.12a | 128.93 ± 13.04b | 131.10 ± 12.58b | |
| GROUP 9 | 96.44 ± 18.25a | 116.85 ± 9.29b | 129.50 ± 13.56b | 136.23 ± 9.80b | |
Mean values with different letters as superscripts across the rows are considered significant (p < 0.05).
(a-c Within a row, least square means without a common superscript differ (p < 0.05) due to changes in body weights of the rats).
Group 1: normal control; Group 2: diabetic - untreated; Group 3: diabetic treated with 0.5 mg/kg b.w. of glibenclamide; Group 4: diabetic treated with 0.14 ml/kg b.w. of RHB; Group 5: diabetic treated with 0.29 ml/kg b.w. of RHB.
Group 6: diabetic treated with 0.57 ml/kg b.w. of RHB; Group 7: non-diabetic treated with 0.14 ml/kg b.w. of RHB.
Group 8: non-diabetic treated with 0.29 ml/kg b.w. of RHB; Group 9: non-diabetic treated with 0.57 ml/kg b.w. of RHB.
DAY 0 = initial body weight; DIABETES (DAY 0) = body weight after induction of diabetes.
3.1.2. Oxidative stress markers
The result of the effect of Ruzu herbal bitters (RHB) on some serum oxidative stress markers of alloxan-induced diabetic rats is presented in Table 3.
Table 3.
Effect of Ruzu herbal bitters (RHB) on some serum oxidative stress markers of alloxan-induced diabetic rats.
| Oxidative stress markers expressed in mean ± S.D | ||||||||
|---|---|---|---|---|---|---|---|---|
| GROUPS | SOD (U/L) | CAT (U/L) | GPx (U/L) | GSH (mg/dl) | MDA (mg/mL) | VIT C (mg/dl) | VIT E (mg/dl) | CRP (mg/dl) |
| GROUP 1 | 11.08 ± 0.44a | 10.83 ± 0.60a | 67.52 ± 0.75e | 2.50 ± 0.16ab | 4.77 ± 0.19a | 0.60 ± 0.04a | 1.95 ± 0.07a | 1.30 ± 0.11a |
| GROUP 2 | 11.72 ± 0.25b | 17.70 ± 0.1 6e | 56.34 ± 1.28a | 2.19 ± 0.22a | 5.59 ± 0.11d | 5.59 ± 0.11d | 2.14 ± 0.06c | 1.70 ± 0.02c |
| GROUP 3 | 11.22 ± 0.11a | 11.62 ± 0.36b | 62.06 ± 0.98c | 2.34 ± 0.12ab | 4.99 ± 0.28ab | 0.61 ± 0.05a | 2.00 ± 0.06ab | 1.40 ± 0.05ab |
| GROUP 4 | 11.40 ± 0.21a | 15.18 ± 0.32d | 59.59 ± 0.66b | 2.33 ± 0.29ab | 5.28 ± 0.20c | 0.64 ± 0.07a | 2.10 ± 0.03bc | 1.45 ± 0.04b |
| GROUP 5 | 11.26 ± 0.13a | 14.05 ± 0.46c | 61.22 ± 1.73bc | 2.35 ± 0.11ab | 5.05 ± 0.14bc | 0.62 ± 0.03a | 2.02 ± 0.06abc | 1.38 ± 0.23ab |
| GROUP 6 | 11.18 ± 0.20a | 11.83 ± 0.43b | 64.02 ± 2.91d | 2.41 ± 0.56ab | 4.84 ± 0.31ab | 0.59 ± 0.08a | 1.98 ± 0.11ab | 1.38 ± 0.23ab |
| GROUP 7 | 11.12 ± 0.1 4a | 11.08 ± 0.38a | 65.97 ± 1.08e | 2.42 ± 0.79ab | 4.76 ± 0.23a | 0.59 ± 0.06a | 1.95 ± 0.16a | 1.29 ± 0.10a |
| GROUP 8 | 11.13 ± 0.17a | 11.06 ± 0.04a | 65.82 ± 0.96e | 2.45 ± 0.38ab | 4.73 ± 0.15a | 0.60 ± 0.04a | 1.97 ± 0.18ab | 1.31 ± 0.04a |
| GROUP 9 | 11.15 ± 0.30a | 11.02 ± 0.31a | 66.60 ± 1.02e | 2.77 ± 0.51b | 4.72 ± 0.17a | 0.59 ± 0.03a | 1.97 ± 0.13ab | 1.32 ± 0.03a |
Mean values with different letters as superscripts down the columns are considered significant (p < 0.05).
(a-e Within a column, least square means without a common superscript differ (p < 0.05) due to variation of the tested parameter).
Group 1: normal control; Group 2: diabetic - untreated; Group 3: diabetic treated with 0.5 mg/kg b.w. of glibenclamide.
Group 4: diabetic treated with 0.14 ml/kg b.w. of RHB; Group 5: diabetic treated with 0.29 ml/kg b.w. of RHB.
Group 6: diabetic treated with 0.57 ml/kg b.w. of RHB; Group 7: non-diabetic treated with 0.14 ml/kg b.w. of RHB.
Group 8: non-diabetic treated with 0.29 ml/kg b.w. of RHB; Group 9: non-diabetic treated with 0.57 ml/kg b.w. of RHB.
SOD = superoxide dismutase, CAT = catalase, GPx = glutathione peroxidase, GSH = glutathione, MDA = malondialdehyde, VIT C = vitamin C, VIT E = vitamin E, CRP = C-reactive protein.
3.1.3. Superoxide dismutase (SOD) activity (U/L)
The activity of superoxide dismutase (SOD) increased significantly (p < 0.05) in the diabetic untreated group (11.72 ± 0.25) compared to that of the normal control group (11.08 ± 0.44). There was no significant (p < 0.05) difference but gradual decrease in SOD activity in the RHB treated diabetic groups 4 (11.40 ± 0.21), 5 (11.26 ± 0.13) and 6 (11.18 ± 0.20) in a dose dependent manner, which are not significantly different from that of diabetic group 3 treated with 0.5 mg/kg b. w. of glibenclamide (11.22 ± 0.11) and the normal control group. Likewise, there was no significant (p < 0.05) difference in SOD activities of the RHB treated non-diabetic groups 7 (11.12 ± 0.14), 8 (11.13 ± 0.17) and 9 (11.15 ± 0.30), when compared with that of the normal control group.
3.1.4. Catalase (CAT) activity (U/L)
Catalase (CAT) activity increased significantly (p < 0.05) in the diabetic untreated group (17.70 ± 0.1 6) compared to that of the normal control group (10.83 ± 0.60). The RHB-treated diabetic groups 4 (15.18 ± 0.32), 5 (14.05 ± 0.46) and 6 (11.83 ± 0.43) showed significantly (p < 0.05) decreased catalase activity in a dose dependent manner. There was no significant (p < 0.05) difference in the catalase activities of group 6 treated with 0.57 ml/kg b. w of RHB and that of diabetic group 3 treated with 0.5 mg/kg b. w. of glibenclamide (11.62 ± 0.36). Also, there was no significant (p < 0.05) difference in catalase activity observed in the RHB treated non-diabetic groups 7 (11.08 ± 0.38), 8 (11.06 ± 0.04) and 9 (11.02 ± 0.31), when compared with that of the normal control group.
3.1.5. Glutathione peroxidase (GPx) activity (U/L)
Glutathione peroxidase (GPx) activity decreased significantly (p < 0.05) in the diabetic untreated group (56.34 ± 1.28) compared to that of the normal control group (67.52 ± 0.75). However, the GPx activity increased significantly (p < 0.05) in the RHB treated diabetic groups 4 (59.59 ± 0.66), 5 (61.22 ± 1.73) and 6 (64.02 ± 2.91) in a dose dependent manner. There was no significant (p < 0.05) difference in the GPx activities of the RHB treated non-diabetic groups 7 (65.97 ± 1.08), 8 (65.82 ± 0.96) and 9 (66.60 ± 1.02); when compared with that of the normal control group.
3.1.6. Glutathione (GSH) concentration (mg/dl)
There was a gradual decrease with no significant (p < 0.05) difference in glutathione (GSH) concentration observed in the diabetic untreated group (2.19 ± 0.22) compared to that of the normal control group (2.50 ± 0.16). Similarly, there were no significant (p < 0.05) differences in GSH concentrations in both the RHB-treated and non-treated groups when compared with that of the normal control group.
3.1.7. Vitamin C (VIT C) concentration (mg/dl)
Vitamin C concentration increased significantly (p < 0.05) in the diabetic untreated group (0.74 ± 0.07) compared to that of the normal control group (0.60 ± 0.04). However, there were no significant (p < 0.05) differences in the vitamin C concentrations of both the RHB-treated and non-treated groups compared to that of the normal control group.
3.1.8. Vitamin E (VIT E) concentration (mg/dl)
Vitamin E (VIT E) concentration showed a significant (p < 0.05) increase in the diabetic untreated group (2.14 ± 0.06) compared to that of the normal control group (1.95 ± 0.07). There were no significant (p < 0.05) differences in the vitamin E concentrations, though gradual decreases were observed, in the RHB treated diabetic groups 4 (2.10 ± 0.03), 5 (2.02 ± 0.06) and 6 (1.98 ± 0.11) and non-diabetic groups 7 (1.95 ± 0.16), 8 (1.97 ± 0.18) and 9 (1.97 ± 0.13) treated with different doses of RHB; including the diabetic group 3 (2.00 ± 0.06) treated with glibenclamide, when compared with that of the normal control group.
3.1.9. Malondialdehyde (MDA) concentration (mg/mL)
There was a significant (p < 0.05) increase in the malondialdehyde (MDA) concentration observed in the diabetic untreated group (5.59 ± 0.1L) compared to that of the normal control group (4.77 ± 0.19). There was a gradual decrease in the MDA concentrations in the RHB treated diabetic groups 4 (5.28 ± 0.20), 5 (5.05 ± 0.14) and 6 (4.84 ± 0.31) in a dose dependent manner. However, there was no significant (p < 0.05) difference in MDA concentrations of group 6 treated with 0.57 ml/kg b. w of RHB and that of diabetic group 3 treated with 0.5 mg/kg b. w. of glibenclamide (4.99 ± 0.28). Likewise, there were no significant (p < 0.05) differences in MDA concentrations in the RHB treated non-diabetic groups 7 (4.76 ± 0.23), 8 (4.73 ± 0.15) and 9 (4.72 ± 0.17), which are comparable with that of the normal control group.
3.1.10. C-reactive protein (CRP) concentration (mg/dl)
There was a significant (p < 0.05) increase in the C-reactive protein (CRP) concentration observed in the diabetic untreated group (1.70 ± 0.02) compared to that of the normal control group (1.30 ± 0.11). There was a gradual decrease but no significant (p < 0.05) difference in CRP concentrations were observed in the RHB treated diabetic groups 4 (1.45 ± 0.04), 5 (1.42 ± 0.04) and 6 (1.38 ± 0.23). Whereas there were no significant (p < 0.05) differences in CRP concentrations of groups 5, 6 and 3 (1.40 ± 0.05); their CRP concentrations were higher than that of the normal control group. There were no significant (p < 0.05) differences in the CRP concentrations of the RHB treated non-diabetic groups 7 (1.29 ± 0.10), 8 (1.31 ± 0.04) and 9 (1.32 ± 0.03) when compared with that of the normal control group.
3.1.11. pH of ruzu herbal bitters
The pH of Ruzu herbal bitters was found to be 3.00 with pH paper and 3.45 with pH meter.
3.1.12. Gas chromatography and mass spectroscopy (GC-MS) of ruzu herbal bitters
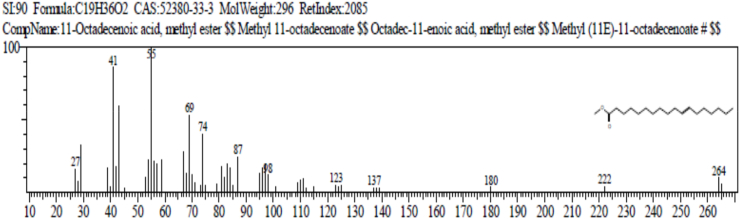
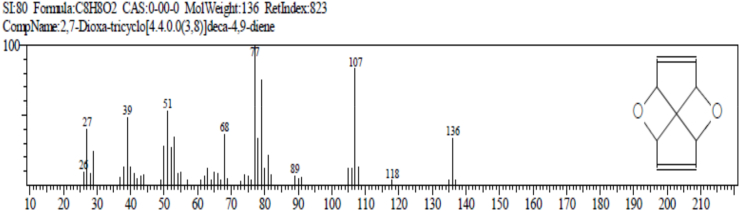
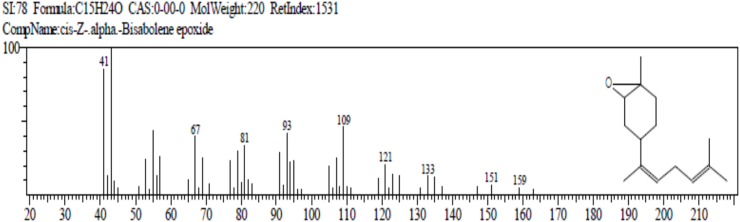
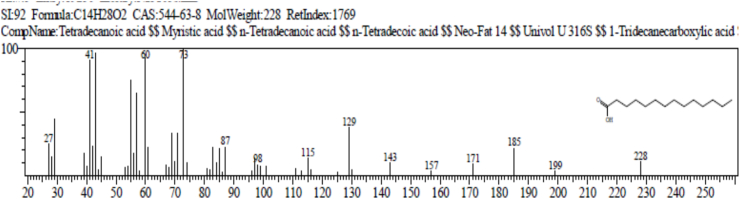
The result of gas chromatography and mass spectroscopy (GC-MS) of Ruzu herbal bitters (RHB) is presented in Table 4. The result revealed the presence of 10 bioactive compounds: 2, 7 - dioxatricyclodeca-4, 9-diene (1.38%), nonanoic acid (pelargic/pelargonic acid) (1.68%), dodecanoic acid (lauric acid) (7.87%), tetradecanoic acid (myristic acid) (16.47%), methyl - 6 – methylheptanoate (1.40%), n-hexadecanoic acid (palmitic acid) (5.64%), 11-octadecenoic acid, methyl ester (4.05%), 13-methyloxacyclotetradecane-2-one (4.88%), Cis-Z-α-bisabolene epoxide (2.66%) and 9-octadecanamide, (Z) (crodamide) (3.07%).
Table 4.
Identified bioactive compounds present in Ruzu herbal bitters using GC-MS.
| Name of Compound | Retention |
Molecular |
Molecular |
% Peak |
|---|---|---|---|---|
| Time | Formula | Weight | Area | |
| 2,7 - Dioxatricyclodeca-4, 9-diene | 9.375 | C8H8O2 | 136 | 1.38 |
| Nonanoic acid (Pelargic/pelargonic acid) | 10.100 | C9H18O2 | 158 | 1.68 |
| Dodecanoic acid (Lauric acid) | 12.508 | C12H24O2 | 200 | 7.87 |
| Tetradecanoic acid (Myristic acid) | 14.900 | C14H28O2 | 228 | 16.47 |
| Methyl - 6 – methylheptanoat | 17.033 | C9H18O | 2 158 | 1.40 |
| n-Hexadecanoic acid (palmitic acid) | 18.467 | C16H32O2 | 256 | 5.64 |
| 11-Octadecenoic acid, methyl ester | 20.100 | C19H36O | 296 | 4.05 |
| 13-Methyloxacyclotetradecane-2-one | 21.167 | C14H26O2 | 226 | 4.88 |
| Cis-Z-α-Bisabolene epoxide | 22.800 | C15H24O | 220 | 2.66 |
| 9-Octadecanamide, (Z) (Crodamide) | 23.725 | C18H35NO | 281 | 3.07 |
4. Discussion
4.1. Effect of RHB on the blood glucose level
There was a significant increase (p < 0.05) in the blood glucose level of the rats after alloxan injection. The increase in glucose level could be as a result of alloxan-induced reactive oxygen species, in addition to a simultaneous massive increase in cytosolic calcium concentrations that led to a rapid destruction of pancreatic islet cells and a concomitant reduction in synthesis/release of insulin [20,21].
In the study, there was a significant (p < 0.05) increase in the blood glucose level of the diabetic-untreated group after diabetes induction up to the last day of the experiment, unlike in the normal control group. However, treatment of the diabetic rats with different doses of Ruzu herbal bitters (RHB) resulted in a significant (p < 0.05) reduction in their blood glucose levels in a dose-dependent manner (Table 1). The reduction in the blood glucose levels in diabetic rats treated with RHB showed that RHB contains various bioactive compounds with the ability to arrest and reverse oxidative stress-induced destruction of the pancreatic β-islet cells, enhancing β-islet cells regeneration, insulin secretion and consequently the transport of blood glucose to peripheral tissues. It was reported in our previous work that Ruzu herbal bitters contains some medicinally important phytochemicals such as flavonoids, alkaloids, steroids, phenols, tannins and saponins [22]. Some studies have shown that the extracts of two component plants of RHB, Citrullus colocynthis and Uvaria chamae, caused significant decrease in the concentration of blood glucose in alloxan-induced diabetic rats [7,8]; Emordi et al., 2018). Our findings are in agreement with previous studies on the antidiabetic activities of polyherbal formulations on alloxan-induced diabetic rats [23,24].
4.2. Effect of RHB on the body weight
The result of the body weights of the rats in Table 2 showed a steady body weight gain in the normal control group and the non-diabetic groups treated with RHB throughout the period of the experiment, whereas the diabetic-untreated group showed significant (p < 0.05) weight loss. Treatment of diabetic rat groups with different doses of RHB led to significant (p < 0.05) increase in body weights, comparable to that of the glibenclamide-treated group. The weight gain observed in the rats treated with RHB showed that the product contains essential and non-essential nutrients that can boost the body's nutritional requirements for growth [22] affected by diabetes mellitus. Similar observation was reported by Otunola and Afolayan [23] who used combined spices for the treatment of diabetes. Also, Lakshmi et al. [8] reported that Citrullus colocynthis seeds extract caused weight gain in alloxan-induced diabetic rats. Similar observations were reported by Otunola and Afolayan [23] who used combined spices in the treatment of diabetes mellitus.
4.3. Effect of RHB on oxidative stress markers
In this study, diabetic untreated group showed significant (p < 0.05) increases in serum activities of superoxide dismutase (SOD) and catalase (CAT), with significant (p < 0.05) decrease in glutathione peroxidase (GPx) activity compared to the normal control group. Also, there were significant (p < 0.05) increases in the levels of vitamin C (VIT C), vitamin E (VIT E), C-reactive protein (CRP) and malondialdehyde (MDA), with significant (p < 0.05) decrease in the level of glutathione (GSH) in the diabetic untreated group compared to the normal control group (Table 3). Treatment of diabetic groups with different doses of Ruzu herbal bitters resulted in significant reductions in the serum activities of SOD and CAT; elevation in GPx activity; reductions in VIT C, VIT E, CRP and MDA levels; and elevation in GSH levels. There was no significant difference in the results of the RHB treated non-diabetic groups 7, 8 and 9, which are comparable with that of the normal control group. Furthermore, there were no significant (p < 0.05) differences between the results of group 6 treated with 0.57 ml/kg b. w of RHB and that of diabetic group 3 treated with 0.5 mg/kg b. w. of glibenclamide, Hence, 0.57 ml/kg b. w of Ruzu herbal bitters was found to be equipotent with 0.5 mg/kg b. w. of glibenclamide in the restoration of free radical-induced oxidative stress, indicating that RHB has antioxidant properties. Our findings are in agreement with other findings on the antidiabetic activities of polyherbal formulations on alloxan-induced diabetic rats [23,24].
The enzymatic defense against free radicals in tissues is primarily carried out by superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), together with glutathione (GSH). SOD converts superoxide anion (O2•−) to hydrogen peroxide (H2O2), which is then detoxified by conversion to water by catalase and GPx (Mahmoud et al., 2014). Reduced glutathione (GSH), is involved in the non-enzymatic removal of ROS and also serves as the hydrogen donor in GPx-mediated reaction. The oxidized glutathione (GSSG) is either reduced back to GSH by glutathione reductase, or exported out of the cells (Mahmoud et al., 2014).
Some authors have reported decreased serum activities of SOD and catalase in diabetic rats [25,26]; Kumawat et al., 2013), unlike in our study in which we observed increased serum activities of SOD and catalase in the diabetic rats. However, in agreement with our study, Ahmed et al. [27] and Zarei et al. [28] reported that the activities of catalase and SOD were significantly higher in the diabetic rat group than the normal group. Whereas in their study, Zarei et al. [28] reported higher activity of SOD in diabetic uncontrolled rats and no much difference in catalase activities between the diabetic and the normal control; the reverse is the case in our study - increased catalase activity in the diabetic untreated group and no much difference in SOD activities between both the diabetic and the normal control. This suggests that high level of free radicals in diabetes may increase the activities of the antioxidant enzymes that are activated to arrest them. Also, high blood glucose can lead to the combination of glucose with the enzymes, which are proteins, so that in patients with diabetes, extracellular SOD is highly glycosylated comparing to healthy subjects [29], thereby reducing its activity. Another study that evaluated the antioxidant status and SOD activity in diabetic patients did not show any significant differences between the tested groups [30], as observed in the present study. However, higher catalase and SOD activities in untreated diabetic group than the normal control in this study were indications that oxidative stress occurred in the diabetic group. Reduction in their activities after treatment with Ruzu herbal bitters indicated that the polyherbal mixture has antioxidant activity.
In hyperglycemia, increased intracellular glucose leads to activation of polyol pathways leading to its increased enzymatic conversion in sorbitol utilizing nicotinic acid adenine dinucleotide phosphate (NADPH). With concomitant decrease in NADPH level and the regeneration of reduced glutathione (GSH), the antioxidant mechanism is impaired. Since glutathione is a scavenger of reactive oxygen species, this can induce or exacerbate oxidative stress [31]. The reduction in erythrocyte glutathione peroxidase (GPx) activity in the diabetic groups when compared to the normal control in the present study may be attributed to excessive metabolism of glucose by polyol pathway. This pathway utilizes NADPH as a hydrogen donor and decreases the NADPH/NADP+ ratio. Increased sorbitol pathway utilizes the NADPH leading to decreased regeneration of reduced glutathione (GSH). Failure in regeneration of glutathione (GSH) weakens the antioxidant defense by glutathione peroxidase and decreases its activity [32], as observed in our study. Loven et al. [33] suggested that the decrease in tissue GSH could be the result of decreased synthesis or increased degradation of GSH by oxidative stress in diabetes. Hence, the increase in the level of GSH, including an increase in GPx activity in the RHB treated diabetic groups in our study is suggestive that RHB can enhance GSH biosynthesis and/or reduce oxidative stress.
Free radicals change lipid/protein ratio of membranes by affecting polyunsaturated fatty acids and lipid peroxidation, thereby causing functional irregularities of several cellular organelles [34]. Lipid peroxides are broken down quickly to form reactive carbon compounds, of which malondialdehyde (MDA) is an important reactive carbon compound used commonly as an indicator of lipid peroxidation [35]. The presence of higher MDA in the serum is an indication of lipid peroxidation and oxidative stress of which has been reported as one of the underlying causes of diabetes mellitus [36]. Therefore, the higher MDA level in the diabetic rats observed in our study was an indication of lipid peroxidation caused by free radicals. Some studies have also reported higher MDA in diabetic untreated rats than in the normal control [28,37].
Vitamins A, C and E are among the important nonenzymatic antioxidants [38,39]. Sinha and Grosh [40] proposed that the absence of antioxidant vitamins in diabetic patients could lead to the development of several abnormalities related with absorption. Vitamin C is a physiological antioxidant of major importance for protection against diseases and degenerative processes caused by oxidative stress and is associated with better scavenging properties in vivo than the other antioxidants, because of its presence both in intracellular and the extracellular fluid [41]. Plasma ascorbic acid is the only endogenous antioxidant that can completely protect the lipids from the peroxidative damage induced by aqueous peroxyl radicals [42]. Vitamin C also acts as a co-antioxidant by regenerating alpha-tocopherol from alpha-tocopheroxyl radical produced during scavenging of oxygen free radicals [16]. While several studies have reported decreased blood levels of vitamin E in diabetic patients [25,43,44], other studies reported that there are increases in vitamin E levels of the diabetic group compared to controls [[45], [46], [47]]. Our result is in agreement with the later report for both vitamins E and C as observed in Table 3; although there is no significant difference between the RHB treated diabetic groups and the normal control group. The increases observed in the diabetic groups could be due to increased need for these vitamins in order to arrest oxidative stress.
C-reactive protein (CRP) is a plasma protein which is not normally present in the body but its advent is marked by its elevated levels in the blood during certain specific condition, especially marked when inflammatory events occurred as a result of any kind of injury. Due to its involvement in inflammation, which is also seen as one of the mediators of progression of type 2 diabetes mellitus and its various complications, it was suspected to be engrossed in their pathogenesis [48]. It is well known that CRP is a common inflammatory biomarker that is elevated in the blood of subjects with severe inflammation and diseases including T2DM and cardiovascular diseases (CVD) [49]. Hence, the elevated level of CRP in the diabetic rats observed in our study could be due to inflammation and CVD, which was reduced by treatment with Ruzu herbal bitters. Our result is in agreement with other reports [48,49].
4.4. pH of ruzu herbal bitters
The pH of Ruzu herbal bitters was found to be 3.00 and 3.45 with pH paper and pH meter respectively. pH is a measure of hydrogen ion concentration in a medium. The pH of RHB is acidic, indicating the presence of hydrogen ions in it. The decrease in pH of RHB is advantageous as low pH inhibits growth of pathogenic microorganisms and the acidic environment created acts as a preservative [50], enhancing the efficacy of the product (RHB). Research in gastric health suggests that the pH environment of simple-stomached vertebrates serves a more prominent function as an ecological filter, capable, through its acidity, of killing microbial taxa that would otherwise colonize the intestines [51]. Carnivores need more acidic stomachs in order to lyse the protein in their meat-based diets. For example, secretion of pepsinogen and its activation to pepsin in the stomach is modulated by an acid pH (2–4) [52]. Also, the activities of proteases in a simple acid stomach depends on an acidic environment (pH 2–4) [53]. The major inhibitory regulator of gastric acid secretion is an increase in intra-gastric acidity. A decrease of luminal pH below 3.0 has a concentration-dependent inhibitory influence on HCl and gastrin secretion, and at pH 1.0 further acid output is abolished [54]. Therefore, the pH of RHB (3.45) is within the range of normal activity of gastric enzymes.
4.5. Gas chromatography and mass spectroscopy (GC-MS) of RHB
The result of gas chromatography and mass spectroscopy (GC-MS) of RHB presented in Table 4 revealed the presence of 10 bioactive compounds: 2, 7-Dioxatricyclodeca-4, 9-diene (1.38%), Nonanoic acid (Pelargic/pelargonic acid) (1.68%), Dodecanoic acid (Lauric acid) (7.87%), Tetradecanoic acid (Myristic acid) (16.47%), Methyl - 6 – methylheptanoate (1.40%), n-Hexadecanoic acid (palmitic acid) (5.64%), 11-Octadecenoic acid, methyl ester (4.05%), 13-Methyloxacyclotetradecane-2-one (4.88%), Cis-Z-α-Bisabolene epoxide (2.66%) and 9-Octadecanamide, (Z) (Crodamide) (3.07%).
Below are some literature reports on the functions of these compounds.
-
1.
2, 7-Dioxatricyclodeca-4, 9-diene: antioxidant, cytotoxic and antimalarial effects [55].
-
2.
Nonanoic acid (Pelargic/Perlargonic acid): used as herbicides, flavouring constituents, food additives, also used as a blossom thinner for apple and pear tree [56].
-
3.
Tetradecanoic acid (lauric acid): acts as a lipid anchor in bio-membranes, acts as an antioxidant [57,58].
-
4.
Dodecanoic acid (Lauric acid): It acts as an antibacterial and algal metabolite, antifungal and antiviral agent. It is used for the treatment of cancer and heart diseases [59]. It is used mainly for the production of soaps and cosmetics, also used to investigate the molar mass of an unknown substance via the freezing-point depression [60].
-
5.
Methyl 6-methyl heptanoate: acts as a flavouring agents, also used as food additives [61].
-
6.
N-hexadecanoic acid (Palmatic acid): plays a role in the presentation of a definite tissue concentration and repartition in different lipids classes which requires a fine regulation of its metabolism. It is also used to produce soup, cosmetic and industrial mold release agents [62].
-
7.
11-Octadecenioc acid, Methyl ester: acts as an antioxidant, anti-inflammatory, antimicrobial, antiviral, anti-mutagenic and anti-carcinogenic activities and gene expression. It also contributes towards lowering the risks of diverse disease, especially for coronary heart and pulmonary diseases as well as cancer [[63], [64], [65]].
-
8.
Cis-Z-α- Bisabolene epoxide: It acts as an antioxidant and antimicrobial [66].
-
9.
9-octadecanamide, (z) (crodamide): It is used as chemical additives which must be added to the low-density polyethylene (LDPE) film material. It is also used as the modifying agent of plastic. It is used as the lubricants such as polypropylene (PP), polystyrene (GPPS), phenol (PF) resin, antistatic agents and anti-caking additives [67].
Therefore, from our result, we infer that the antidiabetic and antioxidant activities of Ruzu herbal bitters are as a result of the presence of four pharmacologically important bioactive compounds: 11-Octadecenioc acid, Methyl esther; 2,7-Dioxatricyclodeca-4, 9-diene; Cis-Z-α-Bisabolene epoxide and Tetradecanoic acid (lauric acid), which have antioxidant activities, amongst others.
The 4 bioactive antioxidant compounds in Ruzu herbal bitters:
(a) 11-Octadecenoic acid, Methyl ester (FATTY ACID AND ITS ESTER).
Functions: antioxidant, anti-inflammatory, antimicrobial, antiviral, anti-mutagenic and anti-carcinogenic activities.
(b) 2,7-Dioxa-tricyclo [4.4.0.0 (3,8)]deca-4,9-diene (STEROID).
Functions: antioxidant, cytotoxic and antimalarial effects.
(c) Cis-Z-α- Bisabolene epoxide (ESSENTIAL OIL).
Functions: antioxidant and antimicrobial agent.
(d) Tetradecanoic acid (lauric acid) (FATTY ACID).
Function: antioxidant.
5. Conclusion
Ruzu herbal bitters (RHB) used in the treatment of various ailments possesses antidiabetic and antioxidant properties. This is as a result of its constituent phytochemicals and bioactive compounds that have different medicinal effects. However, further studies should be carried out to confirm the exact mechanism of action of Ruzu herbal bitters and its possible side effects.
Declaration of competing interest
This research was solely sponsored by the author. Hence, there was no financial support from any individual or group of individuals for the research. Therefore, there is no conflict of interest.
Acknowledgement
We appreciate the management of Ruzu Natural Health Product and Services, Nigeria, for permitting us to use their product for this research.
References
- 1.Mathur S., Mehta D.K., Kapoor S., Yadav S. Liver function in type-2 diabetes mellitus patients. Int. J. Sci. Stud. 2016;3(10):43–47. [Google Scholar]
- 2.Oboh G. Prevention of garlic induced haemolytic aneamia by some commonly con- sumed green leafy vegetables in Nigeria. J. Med. Food. 2004;7(4):500–503. doi: 10.1089/jmf.2004.7.498. [DOI] [PubMed] [Google Scholar]
- 3.Oboh G. Effect of blanching on the antioxidant property of some tropical green leafy vegetables. Lebensmittel-Wissenschaft und-Technologie. 2005;38(5):513–517. [Google Scholar]
- 4.Amic D., Davidovic-Amic D., Beslo D., Trinajstic N. Structure-radical scavenging activity-relationship of flavonoids. Croat. Chem. Acta. 2003;76(1):55–61. [Google Scholar]
- 5.Nia R., Paper D.H., Essien E.E., Iyadi K.C., Bassey A.I.L., Anti A.B., Franz G. Evaluation of the anti-oxidant and anti-angiogenic effects of Sphenocentrum jollyanum Pierre. Afr. J. Biomed. Res. 2004;7:129–132. [Google Scholar]
- 6.Obasi D.C., Ogugua V.N. Effect of Ruzu herbal bitters on the kidney function and hematological parameters of alloxan-induced diabetic rats. Int. J. Sci. Eng. Res. 2020;11(5):63–86. [Google Scholar]
- 7.Agarwal V., Sharma A.K., Upadhyay A., Singh G., Gupta R. Hypoglycemic effects of Citrullus colocynthis roots. Acta Pol. Pharm. 2012;69(1):75–79. [PubMed] [Google Scholar]
- 8.Lakshmi B., Sendrayaperumal V., Subramanian S. Beneficial effects of Citrullus colocynthis seeds extract studied in alloxan-induced diabetic rats. Int. J. Pharmaceut. Sci. Rev. Res. 2013;19(1):47–55. [Google Scholar]
- 9.Emordi J.E., Agbaje E.O., Oreagba I.A., Iribhogbe O.I. Antidiabetic effects of the ethanolic root extract of Uvaria chamae P. Beauv (Annonaceae) in alloxan-induced diabetic rats: a potential alternative treatment for diabetes mellitus. Advances in Pharmacological Sciences. 2018;(2):1–13. doi: 10.1155/2018/1314941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbarzadeh A., Norouzian D., Mehrabi M.R., Jamshidi S., Farhangi A., Allah A.V., Mofidian S.M.A., Lame B.R. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007;22(2):60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lav V.K., Gupta P.P., Tripathi P., Pandey A. Interaction of aqueous extract of Trigonellafoenum graecum seeds with glibenclamide in streptozotocin induced diabetic rats. American Medical Journal of Pharmaceutical Toxicology. 2011;6(4):102–106. [Google Scholar]
- 12.Arthur J.R., Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophil from selenium deficient and copper deficient cattle. Life Sci. 1985;36:1569–1575. doi: 10.1016/0024-3205(85)90381-9. [DOI] [PubMed] [Google Scholar]
- 13.Sinha K.A. Colorimetric assay of catalase. Annals of Biochemistry. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 14.Paglia D.E., Valentine W.N. Qualitative and quantitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–163. [PubMed] [Google Scholar]
- 15.Exner C., Hebebrand J., Remschmidt H., Wewetzer C., Ziegler A., Herpertz S. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol. Psychiatr. 2000;5:476–481. doi: 10.1038/sj.mp.4000771. [DOI] [PubMed] [Google Scholar]
- 16.Angirekula S., Atti L., Atti S. Estimation of serum ascorbic acid (vitamin C) in various morphological types and clinical stages of age related (senile cataract) International Journal of Research in Medical Sciences. 2018;6(3):893–898. [Google Scholar]
- 17.Pearson D.A. seventh ed. Volume 1. Church Hill Livingstone; New York: 1976. pp. 422–511. (Chemical Analysis of Foods). [Google Scholar]
- 18.Aderson H.C., McCarthy M. Determination of C-reactive protein in blood as a measure of the activity of the disease process in acute rheumatic fever. Am. J. Med. 1960;(8):445–455. doi: 10.1016/0002-9343(50)90226-9. [DOI] [PubMed] [Google Scholar]
- 19.Wallin B., Rosengren B., Shertzer H.G., Camejo G. Lipoprotein oxidation and measurement of TBARS formation in single microlitre plate; it's use for evaluation of antioxidants. Anal. Biochem. 1993;208:10–15. doi: 10.1006/abio.1993.1002. [DOI] [PubMed] [Google Scholar]
- 20.Etuk E., Muhammed B. Evidence-based analysis of chemical method of induction of diabetes mellitus in experimental animals. Asian Journal of Experimental Biology. 2010;1:331–336. [Google Scholar]
- 21.Adeyi A., Idowu B., Mafiana C., Oluwalana S., Ajayi O. Rat model of food-induced non-obese-type 2 diabetes mellitus: comparative pathophysiology and histopathology. International Journal of Physiology, Pathophysiology and Pharmacology. 2012;4(1):51–58. [PMC free article] [PubMed] [Google Scholar]
- 22.Obasi D.C., Ogugua V.N., Obasi J.N., Okagu I.U. Phytochemical, nutritional and anti-nutritional analyses of Ruzu herbal bitters. IOSR J. Pharm. Biol. Sci. 2020;15(1):4–17. [Google Scholar]
- 23.Otunola G.A., Afolayan A.J. Antidiabetic effects of combined spices of Allium sativvum, Zingiberofficinaleand Capsicum frutescens in alloxan-induced diabetic rats. Front. Life Sci. 2015;8(4):314–323. [Google Scholar]
- 24.Isitua C.C., Akinyemi A.J., Akharaiyi F.C., Olubiyi O.O., Anadozie S.O., Olayide I.I. Antihyperglycemic and anti-hyperlipidemic effect of herbamed, a herbal formulation in alloxan-induced diabetic rats. Intervention in Obesity and Diabetes. 2018;1(4):1–5. [Google Scholar]
- 25.Sundaram R.K., Bhaskar A., Vijayalingam S., Vswanathan M., Mohan R., Shanmuga Sundaram K.R. Antioxidants status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin. Sci. (Lond.) 1996;90:255–260. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S., Shukla R., Vonkata M.S., Kaur G.J., Madhava P.K. Antioxidant status, lipid peroxidation and nitric oxide and products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003;36:557–562. doi: 10.1016/s0009-9120(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed F.N., Naqvi F.N., Shafiq F. Lipid peroxidation and serum antioxidant enzymes in patients with type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2006;1084:481–489. doi: 10.1196/annals.1372.022. [DOI] [PubMed] [Google Scholar]
- 28.Zarei M., Farahnak Z., Hosseinzadeh-Attar M.J., Javanbakht M.H., Hosseinzadeh P., Derakhshanian H., Farahbakhsh-Farsi P., Mahmoud Djalali M. Lipid peroxidation and antioxidant enzymes activity in controlled and uncontrolled Type 2 diabetic patients. ARYA Atherosclerosis. 2016;12(3):118–123. [PMC free article] [PubMed] [Google Scholar]
- 29.Arai K., Maguchi S., Fujii S., Ishibashi H., Oikawa K., Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J. Biol. Chem. 1987;262(35):16969–16972. [PubMed] [Google Scholar]
- 30.Colak E., Majkic-Singh N., Stankovic S., Sreckovic-Dimitrijevic V., Djordjevic P.B., Lalic K. Parameters of antioxidative defense in type 2 diabetic patients with cardiovascular complications. Ann. Med. 2005;37(8):613–620. doi: 10.1080/07853890500330193. [DOI] [PubMed] [Google Scholar]
- 31.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;29(107):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palekar A.V., Ray K.S. Oxidative stress, antioxidative enzymes and dietary antioxidant intake in patients with diabetes mellitus with and without nephropathy. SM Journal of Diabetes and Metabolism. 2017;2(1):1006–1012. [Google Scholar]
- 33.Loven D., Schedl H., Wilson H., Diekus M. Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes. 1986;35:503–511. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- 34.Cheesman K.H., Slater T.F. Introduction to free radical biochemistry. Br. Med. Bull. 1993;49(3):481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 35.Jacob R.A., Burri B.J. Oxidative damage and defense. Am. J. Clin. Nutr. 1996;63:985–990. doi: 10.1093/ajcn/63.6.985. [DOI] [PubMed] [Google Scholar]
- 36.Akinosun O.M., Bolajoko E.B. Total antioxidant status in type II diabetic patients. Niger. J. Clin. Pract. 2007;10(2):126–129. [PubMed] [Google Scholar]
- 37.Ceriello A., Bortolotti N., Motz E., Crescentini A., Lizzio S., Russo A., Tonutti L., Taboga C. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care. 1998;21(9):1529–1533. doi: 10.2337/diacare.21.9.1529. [DOI] [PubMed] [Google Scholar]
- 38.Dıplock A.T. Antioxdant nutrients and disease prevention: an overview. Am. J. Clin. Nutr. 1991;53:1895–1935. doi: 10.1093/ajcn/53.1.189Sb. [DOI] [PubMed] [Google Scholar]
- 39.Halliwell B. Free radical antioxidants in human disease. Curiosity, cause or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 40.Sinha M., Grosh A.K. Influence of dietary vitamin A deficiency on rat digestive & absorptive functions during diabetes mellitus. Indian J. Med. Res. 1994;100:196–200. [PubMed] [Google Scholar]
- 41.Karthikeyan J., Rani P. Enzymatic and non-enzymatic antioxidants in selected piper species. Indian J. Exp. Biol. 2003;41:135–140. [PubMed] [Google Scholar]
- 42.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am. J. Med. 1994;97(3):5–13. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell S.R., Thomason H., Sandler D., Leguen C., Baxter M.A., Thorpe G.H., Jones A.F., Barnett A.H. Antioxidant status in patients with uncomplicated insülin-dependent and noninsülin- dependent diabetes mellitus. Eur. J. Clin. Invest. 1997;27(6):484–490. doi: 10.1046/j.1365-2362.1997.1390687.x. [DOI] [PubMed] [Google Scholar]
- 44.Nourooz-Zadeh J., Rahimi A., Tajaddini-Sarmadi J., Tritschler H., Rosen P., Halliwell B., Betteridge D.J. Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia. 1997;40(6):647–653. doi: 10.1007/s001250050729. [DOI] [PubMed] [Google Scholar]
- 45.Ceriello A., Bortolotti N., Falleti E., Taboga C., Tonutti L., Crescentini A., Motz E., Lizzio S., Russo A., Bartoli E. Total radical-trapping antioxidant parameter in NIDDM patients. Diabetes Care. 1997;20(2):194–197. doi: 10.2337/diacare.20.2.194. [DOI] [PubMed] [Google Scholar]
- 46.Krempf M., Ranganathan S., Ritz P., Morin M., Charbonnel B. Plasma vitamin A and E in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) adult diabetic patients. Int. J. Vitam. Nutr. Res. 1991;61(1):38–42. [PubMed] [Google Scholar]
- 47.Caye-Vaugien C., Krempf M., Lamarche P., Charbonnel B., Pieri J. Determination of alpha-tocopherol in plasma, platelets and erythrocytes of type I and type II diabetic patients by high-performance liquid chromatography. Int. J. Vitam. Nutr. Res. 1990;60(4):324–330. [PubMed] [Google Scholar]
- 48.Behl T., Goel H., Kaur I., Sudan P., Sharma M., WanchooMisri R.W., Sihag S.R., Patyal P., Medapati S. Role of C Reactive protein in diabetes mellitus and its associated complications. Indo American Journal of Pharmaceutical Research. 2014;4(11):5315–5320. [Google Scholar]
- 49.Kanmani S., Kwon M., Shin M.K., Mi Kyung Kim M.K. Association of C-reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci. Rep. 2019;9(1–8):4573–4581. doi: 10.1038/s41598-019-40987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav R., Tripathi D.A., Jha A. Effects of storage time on the physical chemical properties and sensory attributes of Aloe vera ready-to-serve (RTS) beverage. International Journal of food, nutrition and public health. 2013;6(2):173–192. [Google Scholar]
- 51.Martinsen T.C., Bergh K., Waldrum H.L. Gastric juice: a barrier against infectious diseases. Basic Clin. Pharmacol. Toxicol. 2005;96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- 52.Stevens C.E., Hume I.D. second ed. Cambridge University Press; New York: 1995. Comparative Physiology of the Vertebrate Digestive System; pp. 329–390. [Google Scholar]
- 53.Sangild P.T. Transitions in the life of the gut at birth. In: Lindberg J.E., Ogle B., editors. Digestive Physiology of Pigs. CABI Publishers; New York: 2001. pp. 3–16. [Google Scholar]
- 54.Shulkes A., Baldwin G.S., Giraud A.S. Regulation of gastric acid secretion. In: Johnson L.R., editor. Physiology of the Gastrointestinal Tract. fourth ed. Academic Press; San Diego: 2006. pp. 1223–1258. [Google Scholar]
- 55.Shenvi R.A., Schnermann M.J. The scripps institute; chemistry national cancer institute. Journal Product Report. 2014;32:543–577. [Google Scholar]
- 56.Biswas S.M., Jana A. Bioactivity of 2-amino-9-(4-oxoazetidin-2-yl) nonanoic acid from the root exudates of Cleome viscosa L. Biol. Res. 2010;8:1–5. [Google Scholar]
- 57.Naik R.R., Shakya A.K., Khalaf N.A., Abuhamdah S., Oriquat G.A., Maraqa A. GC-MS analysis and biological evaluation of essential oil of ZanthoxylumRheta (roxb) DC pericarp. Jordan Journal of Pharmaceutical Sciences. 2015;8:181–193. [Google Scholar]
- 58.Naik R.R. GC-FD analysis of fatty acids and biological activity of ZanthoxylumRheta seed oil. Orient. J. Chem. 2015;31:1929–1935. [Google Scholar]
- 59.Ninamian S., Ninamian S. Dodecanoic acid extra virgin coconut oil, may reduce the incidence of heart disease and cancer in humans. Int. J. Sci. Res. 2015;5(11):792–797. [Google Scholar]
- 60.David J.A., Sabine B., Ralf C., Geroge F., Udo S., Alfred W. Vol. 10. 2006. pp. 1002–1435. (Fatty Acids in Ullmann's Encyclopedia of Industrial Chemistry). [Google Scholar]
- 61.Mosciano G.P. Food Chem. Toxicol. 1990;26:381. [Google Scholar]
- 62.Gianfranca C., Elisabetta M., Sebastiano B., Claudia M. Palmatic acid; physicological role, metabolism and nutrition implication. Front. Physiol. 2017;8:32–39. [Google Scholar]
- 63.Queiroz R.F., Jordao A.K., Cunha A.C. Nitroxides attenuate carrageenan-induced inflammation in rat paws by reducing neutrophil infiltration and the resulting myeloperioxidase- mediated damage. Free Radic. Biol. Med. 2017;53:1942–1953. doi: 10.1016/j.freeradbiomed.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Huang W.Y., Cai Y.Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants; potential use for cancer prevention. Nutr. Canc. 2017;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 65.Villanueva R.G., Abarca-Vargas R., Vera L.P. Chemical compounds and biological activity of an extract from bougainvilleax buttiana (Vineyard rose) holttum and standl. Int. J. Pharm. Pharmaceut. Sci. 2017;9(3):42–46. [Google Scholar]
- 66.Rouka H., Hadj M.M., El-Hadj O.M. Chemical composition and antioxidant and antimicrobial activities of the essential oil from Teucrium poliumgeyrii (labiatae) J. Med. Plants Res. 2013:1506–1510. [Google Scholar]
- 67.CAS Database . (USP); 2018. U. S. Pharmacopeia. [Google Scholar]