Abstract
Background
The coronavirus disease (COVID-19) has afflicted large populations worldwide. Although vaccines aroused great expectations, their side effects on Japanese people and the antibody titer transition after vaccination are unclear.
Methods
The side effects of the BNT162b2 mRNA COVID-19 vaccine in participants who received vaccination at our center were investigated. Some participants were also surveyed for the antibody titer transition.
Results
In this study, 983 and 798 Japanese participants responded to the first and second doses, respectively. Side effects occurred in 757 (77.0%) and 715 participants (90.0%) after the first and second doses, respectively. No Grade 4 side effects occurred. The second dose had significantly more side effects than the first dose (p < 0.001). Side effects occurred after the second dose in 571 female (92.1%) and 178 male participants (80.1%). Female participants had a higher incidence of side effects than the male participants (p < 0.001). A comparison among the age groups showed significant differences (p = 0.018), and the frequency of side effects decreased with age. Twenty-three individuals participated in the survey of antibody titer transition. After the second vaccine dose, the median antibody titers for IgG and IgM were 3.76 and 0.07 AU/mL, respectively. Both IgG and IgM titers showed a significant increase over the study period (p < 0.001).
Conclusions
The BNT162b2 mRNA COVID-19 vaccine might be safe for Japanese people, and the antibody titer increased with two doses of vaccination. Larger nationwide studies are warranted to verify these findings.
Keywords: Coronavirus disease, Side effect, Vaccine, Antibody titer, IgG/IgM antibody
Abbreviations: COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction; IQR, interquartile range; OD, odds ratio; CI, confidence interval
1. Introduction
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and continues to spread worldwide [1]. In Japan, the number of cases has also been increasing rapidly since the national and local governments declared a state of emergency. Elderly people, patients with comorbidities, and healthcare workers are at high risk of SARS-CoV-2 infection [2]. Recently, vaccines against SARS-CoV-2 have been developed mainly in Western countries, and vaccination has begun [3]. Reports from Europe and the United States suggest that the SARS-CoV-2 vaccine has high efficacy and minor but serious side effects [4]. In Japan, healthcare workers have been vaccinated with the BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine (Comirnaty, BioNTech, and Pfizer, USA). The mRNA vaccine is administered by intramuscular injection, and proteins are then produced based on the mRNA in immunocompetent cells, such as muscle and dendritic cells; some of these proteins are presented to lymphocytes for immunity [5]. For SARS-CoV-2 to invade human cells, the spike protein on the surface of virus particles needs to bind to the angiotensin-converting enzyme 2 in human cells; however, the BNT162b2 vaccine uses the entire gene of the spike protein [5]. BNT162b2 vaccine has shown excellent results with an efficacy rate of approximately 90% [4]. Furthermore, reports have suggested that it can also prevent severe diseases [6]. Regarding the safety profile, the frequency of pain following the administration of the BNT162b2 vaccine was found to be as high as 70–80% [4]. Approximately 30% and 15% of individuals who received the first and second vaccination doses, respectively reported moderate or high pain that interfered with daily activities [4]. However, vaccination against SARS-CoV-2 for healthcare professionals and a few elderly individuals has just initiated in Japan, and no side effects have been reported. Regarding vaccination, details such as the difference in side effects between the first and second vaccinations, sex-, and age-related differences are unknown in Japan.
Additionally, the antibody titer transition after SARS-CoV-2 vaccination has not been reported in Japan, and reports about dynamic changes and the prevalence of SARS-CoV-2 antibodies are few worldwide [7].
Therefore, the present prospective cohort study aimed to investigate the side effects of SARS-CoV-2 vaccination and survey the antibody titer transition after vaccination in Japanese healthcare workers.
2. Materials and methods
2.1. Ethics statement, eligibility criteria, and clinical analyses
This study was approved by the Ethics Committee for Clinical Studies of the Japanese Red Cross Medical Center (nos. 1224 and 1225; February 28, 2021). Written informed consent was obtained via the institution's website that created the electronic medical records provided by the participants as per the principles of the Declaration of Helsinki. Additionally, participants who agreed to undergo measurement of antibody titer transition provided a separate written consent. The BNT162b2 mRNA COVID-19 vaccine was used as the SARS-CoV-2 vaccine. Two 30-μg doses of BNT162b2 were administered intramuscularly 21 days apart. Vaccination was started on March 10, 2021. The cutoff deadline for this study was April 30, 2021. The study data were collected by administering an anonymous survey via the institution's website that created electronic medical records. The period for the information collection regarding side effects after vaccination was 1 week. The contents of the questionnaire included age; sex; symptoms after vaccination such as fever, general fatigue, muscle pain, injection site pain, injection site swelling, diarrhea, joint pain, nausea, headache, allergic reactions; and others. Side effects were reported as per the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
2.2. Antibody titer transition after COVID-19 vaccination
Some people participated in the survey of antibody titer transition of the BNT162b2 mRNA COVID-19 vaccine. All the participants had no clinical history of COVID-19. All the participants measured antibody titers at baseline and after the first and second vaccinations. An immunofluorescence analyzer (Mokosensor-Q100, Mokobio Biotechnology R&D) was used to measure the antibody titer [8]. The following is a summary of the measurement methods used in this kit: Immunoglobulin (Ig)M and IgG targeting the spike protein of the virus were detected using a lateral flow immunofluorescence assay kit (SARS-CoV-2 IgM and IgG Quantum Dot Immunoassay, Mokobio Biotechnology R&D, USA), with 20 μL of undiluted sera applied to the assay cassette, followed by 100 μL of the running buffer provided in the kit. The fluorescence signal was semi quantified using an immunofluorescence analyzer (Mokosensor-Q100, Mokobio Biotechnology R&D, USA). To increase the precision of the diagnosis, IgG seroconversion was confirmed orthogonally using another serological method, targeting the nucleocapsid protein (anti-SARS-CoV-2 NCP ELISA [IgG], Euroimmun AG). Participant sera were diluted at 1:100 and assessed in duplicates at the wavelength of 450 nm measured using Varioskan LUX (Thermo Fisher Scientific). The seropositivity for both assays was determined as per the cutoff values provided by the manufacturers. The positive cutoff antibody titer was defined as >0.10 AU/mL for IgG and 0.20 AU/mL for IgM [9].
2.3. Statistical analyses
Data are presented as numbers (percentages). Differences between the groups were compared using Fisher exact tests for categorical variables. The associations between the groups were presented as odds ratios (ODs) and 95% confidence intervals (CIs). The Friedman test for paired data was used to evaluate the significance of the differences in antibody titers at baseline and after the first and second doses of the time course of the COVID-19 vaccination. The correlation between antibody titer and age was analyzed using Spearman's rank correlation. All the reported P values were two-sided, and P values < 0.05 were considered indicative of statistical significance. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [10].
3. Results
3.1. Participant characteristics
The demographic characteristics are shown in Table 1 . A total of 983 and 798 Japanese participants responded to the first and second doses, respectively. Of the participants, there were 237 men and 746 women, and 23.6% were aged ≥50 years at the first dose vaccination, while 178 were men and 620 were women, and 27.3% were aged ≥50 years at the second dose vaccination. No significant differences were found between the two groups.
Table 1.
Demographic characteristics of the participants.
| Characteristic | First dose n = 983 | Second dose n = 798 | P value |
|---|---|---|---|
| Sex | 0.398 | ||
| Male, n (%) | 237 (24.1) | 178 (22.3) | |
| Female, n (%) | 746 (75.9) | 620 (77.7) | |
| Age group, n (%) | 0.212 | ||
| 20–29 years | 246 (26.4) | 179 (22.4) | |
| 30–39 years | 263 (26.8) | 189 (23.7) | |
| 40–49 years | 228 (23.2) | 212 (26.6) | |
| 50–59 years | 179 (18.2) | 157 (19.7) | |
| ≥60 years | 67 (5.4) | 61 (7.6) | |
Data are presented as numbers (percentages).
Differences between the groups were compared using Fisher exact tests.
3.2. Local or systemic side effects of COVID-19 vaccination
The local or systemic side effects of the first- and second-dose COVID-19 vaccinations reported by the participants in the questionnaires are presented in Table 2 . All side effects occurred in 757 participants (77.0%) after the first dose and 715 participants (90.0%) after the second dose. No anaphylaxis occurred after both the first and second vaccinations. The second dose had significantly more side effects than the first dose (p < 0.001; OD, 2.57; 95% CI, 1.96–3.37). Grade 3 side effects (fever with a body temperature ≥ 38 °C) according to the CTCAE were reported in 128 participants (16.9%) after the first dose and 393 participants (55.0%) after the second dose (p < 0.001; OD, 6.48; 95% CI, 5.14–8.17). After the first vaccine dose, side effects reported were as follows: fever in 29 (3.0%) participants; general fatigue in 251 (25.5%); muscle pain in 413 (42.0%); joint pain in 92 (9.4%); allergic reaction (including urticaria) in 13 (1.3%); injection site pain in 550 (56.0%); injection site swelling in 184 (18.7%); headache in 149 (15.2%); nausea in 44 (4.5%); diarrhea in 32 (3.3%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in 86 (8.7%) participants. After the second vaccine dose, fever occurred in 358 (44.9%) participants; general fatigue in 543 (68.0%); muscle pain in 415 (52.0%); joint pain in 332 (41.6%); allergic reaction (including urticaria) in 18 (2.3%); injection site pain in 536 (67.2%); injection site swelling in 226 (28.3%); headache in 400 (50.1%); nausea in 110 (13.8%); diarrhea in 76 (9.5%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in 156 (19.5%) participants. A comparison between the first- and second-dose vaccinations showed that every second dose had a significantly higher incidence of side effects except for allergic reaction than the first dose vaccination (p < 0.001).
Table 2.
Local or systemic side effects in the participants after COVID-19 vaccination (Coronavirus Modified Uridine RNA Vaccine, BNT162b2, Comirnaty).
| Side effect | First dose, n (%) n = 983 | Second dose, n (%) n = 798 | P value | Odds ratio (95% CI) |
|---|---|---|---|---|
| All | 757 (77.0) | 715 (90.0) | <0.001 | 2.57 (1.96–3.37) |
| Grade 3 | 128 (16.9) | 393 (55.0) | <0.001 | 6.48 (5.14–8.17) |
| Fever (>37.5 °C) | 29 (3.0) | 358 (44.9) | <0.001 | 26.77 (18.03–39.73) |
| General fatigue | 251 (25.5) | 543 (68.0) | <0.001 | 6.21 (5.05–7.64) |
| Muscle pain | 413 (42.0) | 415 (52.0) | <0.001 | 1.50 (1.24–1.80) |
| Joint pain | 92 (9.4) | 332 (41.6) | <0.001 | 6.90 (5.34–8.92) |
| Allergic reaction | 13 (1.3) | 18 (2.3) | 0.148 | 1.72 (0.84–3.54) |
| Injection site pain | 550 (56.0) | 536 (67.2) | <0.001 | 1.70 (1.40–2.07) |
| Injection site swelling | 184 (18.7) | 226 (28.3) | <0.001 | 1.72 (1.37–2.41) |
| Headache | 149 (15.2) | 400 (50.1) | <0.001 | 5.63 (4.50–7.03) |
| Nausea | 44 (4.5) | 110 (13.8) | <0.001 | 3.41 (2.37–4.91) |
| Diarrhea | 32 (3.3) | 76 (9.5) | <0.001 | 3.13 (2.05–4.78) |
| Others | 86 (8.7) | 156 (19.5) | <0.001 | 2.53 (1.91–3.36) |
Others: hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness.
Data are presented as numbers (percentages).
Differences between the groups were compared using Fisher exact tests.
CI, confidence interval; COVID-19, coronavirus disease.
3.3. Sex-related differences in side effects of COVID-19 vaccine
Side effects occurred in 596 female (79.9%) and 161 male participants (67.9%) following the first dose (Table 3 ). A comparison between female and male participants showed that female participants had a significantly higher incidence of side effects than the male participants (p < 0.001; OD, 1.88; 95% CI, 1.35–2.60). Grade 3 side effects (fever with a body temperature of ≥38 °C) according to the CTCAE were reported in 108 female participants (18.1%) and 20 male participants (12.4%; p = 0.015; OD, 1.84; 95% CI, 1.11–3.03).
Table 3.
Sex-related differences in local or systemic side effects in participants following the first dose of the COVID-19 vaccine (Coronavirus Modified Uridine RNA Vaccine, BNT162b2, Comirnaty).
| Side effect | Female, n (%) n = 746 | Male, n (%) n = 237 | P value | Odds ratio (95% CI) |
|---|---|---|---|---|
| All | 596 (79.9) | 161 (67.9) | <0.001 | 1.88 (1.35–2.60) |
| Grade 3 | 108 (18.1) | 20 (12.4) | 0.015 | 1.84 (1.11–3.03) |
| Fever (>37.5 °C) | 23 (3.1) | 6 (2.5) | 0.827 | 1.23 (0.49–3.05) |
| General fatigue | 219 (29.4) | 32 (13.5) | <0.001 | 2.66 (1.78–3.99) |
| Muscle pain | 375 (50.3) | 38 (16.0) | <0.001 | 5.29 (3.64–7.71) |
| Joint pain | 82 (11.0) | 10 (4.2) | 0.001 | 2.80 (1.43–5.50) |
| Allergic reaction | 9 (1.2) | 4 (1.7) | 0.526 | 0.71 (0.22–2.33) |
| Injection site pain | 491 (65.8) | 59 (24.9) | <0.001 | 5.81 (4.17–8.09) |
| Injection site swelling | 175 (23.5) | 9 (3.8) | <0.001 | 7.76 (3.90–15.44) |
| Headache | 129 (17.3) | 20 (8.4) | <0.001 | 2.25 (1.37–3.69) |
| Nausea | 42 (5.6) | 2 (0.8) | <0.001 | 7.01 (1.68–29.19) |
| Diarrhea | 28 (3.8) | 4 (1.7) | 0.143 | 2.27 (0.79–6.55) |
| Others | 78 (10.5) | 8 (3.4) | <0.001 | 3.34 (1.59–7.03) |
Others: hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness.
Data are presented as numbers (percentages).
Differences between the groups were compared using Fisher exact tests.
CI, confidence interval; COVID-19, coronavirus disease.
Fever occurred in 23 female (3.1%) participants; general fatigue in 219 (29.4%); muscle pain in 375 (50.3%); joint pain in 82 (11.0%); allergic reaction (including urticaria) in nine (1.2%); injection site pain in 491 (65.8%); injection site swelling in 175 (23.5%); headache in 129 (17.3%); nausea in 42 (5.6%); diarrhea in 28 (3.8%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in 78 (10.5%) participants.
Fever was observed in six male (2.5%) participants; general fatigue in 32 (13.5%); muscle pain in 38 (16.0%); joint pain in 10 (4.2%); allergic reaction (including urticaria) in four (1.7%); injection site pain in 59 (24.9%); injection site swelling in nine (3.8%); headache in 20 (8.4%); nausea in two (0.8%); diarrhea in four (1.7%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in eight (3.4%) participants. A comparison revealed that female participants had a significantly higher incidence for all side effects except fever, allergic reaction, and diarrhea than male participants.
All the side effects occurred in 571 female (92.1%) and 178 male (80.1%) participants after the second dose (Table 4 ). A comparison between the female and male participants showed that the female participants had a significantly higher incidence of side effects than the male participants (p < 0.001; OD, 2.75; 95% CI, 1.71–4.42). Grade 3 side effects (fever with a body temperature of ≥38 °C) according to the CTCAE were reported in 322 female (51.9%) and 71 male participants (39.9%; p = 0.005; OD, 1.63; 95% CI, 1.16–2.29).
Table 4.
Sex-related differences in local or systemic side effects in the participants after the second dose of the COVID-19 vaccine (Coronavirus Modified Uridine RNA Vaccine, BNT162b2, Comirnaty).
| Side effect | Female, n (%) n = 620 | Male, n (%) n = 178 | P value | Odds ratio (95% CI) |
|---|---|---|---|---|
| All | 571 (92.1) | 144 (80.1) | <0.001 | 2.75 (1.71–4.42) |
| Grade 3 | 322 (51.9) | 71 (39.9) | 0.005 | 1.63 (1.16–2.29) |
| Fever (>37.5 °C) | 291 (46.9) | 67 (37.6) | 0.032 | 1.47 (1.04–2.06) |
| General fatigue | 441 (71.1) | 102 (57.3) | <0.001 | 1.84 (1.30–2.59) |
| Muscle pain | 331 (53.4) | 84 (47.2) | 0.149 | 1.28 (0.92–1.79) |
| Joint pain | 286 (46.1) | 46 (25.8) | <0.001 | 2.46 (1.70–3.56) |
| Allergic reaction | 8 (1.3) | 3 (1.7) | 0.716 | 0.76 (0.20–2.91) |
| Injection site pain | 429 (69.2) | 107 (60.1) | 0.030 | 1.49 (1.06–2.11) |
| Injection site swelling | 188 (30.3) | 38 (21.3) | 0.019 | 1.60 (1.08–2.39) |
| Headache | 319 (51.5) | 81 (45.5) | 0.174 | 1.27 (0.91–1.77) |
| Nausea | 92 (14.8) | 18 (10.1) | 0.111 | 1.55 (0.91–2.65) |
| Diarrhea | 64 (10.3) | 12 (6.7) | 0.196 | 1.59 (0.84–3.02) |
| Others | 133 (21.0) | 23 (12.9) | 0.010 | 1.84 (1.14–2.97) |
Others: hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness.
Data are presented as numbers (percentages).
Differences between the groups were compared using Fisher exact tests.
CI, confidence interval; COVID-19, coronavirus disease.
Fever occurred in 291 female (46.9%) participants; general fatigue in 441 (71.1%); muscle pain in 331 (53.4%); joint pain in 286 (46.1%); allergic reaction (including urticaria) in eight (1.3%); injection site pain in 429 (69.2%); injection site swelling in 188 (30.3%); headache in 319 (51.5%); nausea in 92 (14.8%); diarrhea in 64 (10.3%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in 133 (21.0%) participants.
Fever was observed in 67 male (37.6%) participants; general fatigue in 102 (57.3%); muscle pain in 84 (47.2%); joint pain in 46 (25.8%); allergic reaction (including urticaria) in three (1.7%); injection site pain in 107 (60.1%); injection site swelling in 38 (21.3%); headache in 81 (45.5%); nausea in 18 (10.1%); diarrhea in 12 (6.7%); and others (including hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness) in 23 (12.9%) participants. A comparison revealed that the female participants had a significantly higher incidence of all side effects except for muscle pain, allergic reaction, headache, and nausea than the male participants.
3.4. Age-related differences in side effects after the second dose COVID-19 vaccine
The side effects after the second dose of vaccine were examined and compared according to age (Table 5 ). All side effects occurred in 168 (93.9%) participants aged 20–29 years, 175 (92.6%) aged 30–39 years, 188 (88.7%) aged 40–49 years, 133 (84.7%) aged 50–59 years, and 51 (83.6%) participants aged ≥60 years. A comparison among the age groups revealed significant differences (p = 0.018), and the incidence of side effects decreased with age. Grade 3 side effects (fever with a body temperature of ≥38 °C) according to the CTCAE were reported in 111 (62.0%) participants aged 20–29 years, 99 (52.4%) aged 30–39 years, 111 (52.3%) aged 40–49 years, 53 (33.8%) aged 50–59 years, and 19 (31.1%) participants aged ≥60 years (p < 0.001). In particular, the comparison between the age groups revealed that fever, general fatigue, joint pain, and headache showed significant differences.
Table 5.
Age-related differences in local or systemic side effects in the participants after the second dose of the COVID-19 vaccine (Coronavirus Modified Uridine RNA Vaccine, BNT162b2, Comirnaty).
| Side effect | 20–29 years, n (%) n = 179 | 30–39 years, n (%) n = 189 | 40–49 years, n (%) n = 212 | 50–59 years, n (%) n = 157 | ≥60 years, n (%) n = 61 | P value |
|---|---|---|---|---|---|---|
| All | 168 (93.9) | 175 (92.6) | 188 (88.7) | 133 (84.7) | 51 (83.6) | 0.018 |
| Grade 3 | 111 (62.0) | 99 (52.4) | 111 (52.3) | 53 (33.8) | 19 (31.1) | <0.001 |
| Fever (>37.5 °C) | 101 (56.4) | 88 (46.6) | 94 (44.3) | 52 (33.1) | 23 (37.7) | <0.001 |
| General fatigue | 136 (76.0) | 130 (68.8) | 145 (68.4) | 97 (61.8) | 35 (57.4) | 0.005 |
| Muscle pain | 99 (55.3) | 107 (56.6) | 107 (50.5) | 72 (45.9) | 30 (49.2) | 0.191 |
| Joint pain | 83 (46.4) | 83 (43.9) | 88 (41.5) | 65 (41.4) | 13 (21.3) | 0.014 |
| Allergic reaction | 4 (2.2) | 6 (3.2) | 3 (1.4) | 4 (2.5) | 1 (1.6) | 0.814 |
| Injection site pain | 132 (73.7) | 127 (67.2) | 146 (68.9) | 94 (59.9) | 37 (60.7) | 0.068 |
| Injection site swelling | 64 (35.8) | 50 (26.5) | 50 (23.6) | 42 (26.8) | 20 (32.8) | 0.080 |
| Headache | 110 (61.5) | 100 (52.9) | 108 (50.9) | 61 (38.9) | 21 (34.4) | <0.001 |
| Nausea | 33 (18.4) | 25 (13.2) | 30 (14.2) | 17 (10.8) | 5 (8.2) | 0.193 |
| Diarrhea | 17 (9.5) | 15 (7.9) | 24 (11.3) | 19 (12.1) | 1 (1.6) | 0.138 |
| Others | 32 (17.9) | 37 (19.6) | 41 (19.3) | 35 (22.3) | 11 (18.0) | 0.884 |
Others: hypertension, oropharyngeal pain, dizziness, lymph node swelling, and drowsiness.
Data are presented as numbers (percentages).
Differences between the groups were compared using Fisher exact tests.
COVID-19, coronavirus disease.
3.5. Antibody titer transition after COVID-19 vaccination
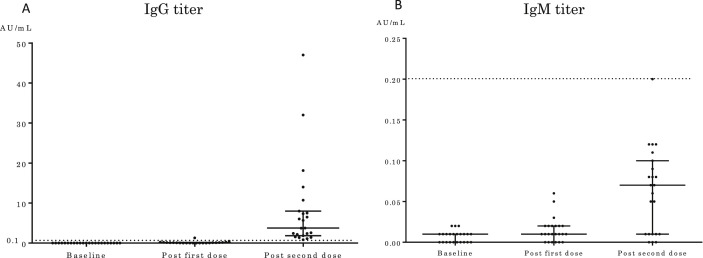
Twenty-three Japanese people participated in the survey of the antibody titer transition of the BNT162b2 mRNA COVID-19 vaccine. The median age of the participants was 42 years (interquartile range [IQR], 34–46 years), with 13 female (57%) participants. At baseline, the median antibody titers for IgG and IgM before vaccination were 0.01 AU/mL (IQR, 0.005–0.01 AU/mL) and 0.01 AU/mL (IQR, 0.00–0.01 AU/mL), respectively. The median time of measurement of the first and second antibody titers was 19 days (IQR, 17–20 days) and 15 days (IQR, 15–15 days) after vaccination, respectively. After the first dose of vaccine, the median antibody titers for IgG and IgM were 0.09 AU/mL (IQR, 0.02–0.135 AU/mL) and 0.01 AU/mL (IQR, 0.005–0.02 AU/mL), respectively. Next, after the second dose of vaccine, the median antibody titers for IgG and IgM were 3.76 AU/mL (IQR, 1.85–7.98 AU/mL) and 0.07 AU/mL (IQR, 0.01–0.10 AU/mL), respectively (Fig. 1 ). Both the IgG and IgM titers showed a significant increase over the study period (p < 0.001).
Fig. 1.
Antibody titer transition after COVID-19 vaccination (Coronavirus Modified Uridine RNA Vaccine, BNT162b2, Comirnaty). The dotted line shows the cutoff value. The cutoff IgG and IgM titers are 0.1 AU/mL and 0.2 AU/mL, respectively. A, IgG titer transition. B, IgM titer transition. Ig, Immunoglobulin; COVID-19, coronavirus disease.
The positive cutoff antibody titer was defined as >0.10 AU/mL for IgG and 0.20 AU/mL for IgM. Of the 23 participants, eight (35%) became positive for IgG and none for IgM after the first dose. All the participants became positive for IgG and none for IgM after the second dose.
For the first dose, there was no significant correlation between antibody titer and age. However, there was a significant correlation between age and antibody titers for IgG and IgM after the second dose (rs: Spearman's rank correlation coefficient = −0.427, p = 0.042 for IgG; rs = −0.442, p = 0.035 for IgM).
During the study period, one (0.1%) of 798 participants who had the second vaccination dose and responded to the questionnaire tested positive for SARS-CoV-2 in a polymerase chain reaction test. However, this participant, who was infected by a family member from the same household, was completely asymptomatic during the disease course.
4. Discussion
This is the first report on the side effects of the BNT162b2 mRNA COVID-19 vaccine in Japanese healthcare workers and a survey of the antibody titer transition after vaccination for Japanese population.
In a multinational clinical trial of the BNT162b2 vaccine, the incidence of serious adverse events was low and similar across vaccine and placebo groups [4]. In localized reactions, the frequency of pain following administration of the BNT162b2 vaccine was found to be as high as 70–80%. Approximately 30% and 15% of individuals who received the first and second vaccination doses, respectively reported moderate or high pain that interfered with daily activities [4]. Furthermore, adverse systemic reactions were frequently observed with the frequency of fatigue, headache, nausea, and general fatigue being particularly high. The following side effects were reported after the second dose in the clinical trial of BNT162b2: fever in 16% of participants, general fatigue in 23%, muscle pain in 37%, joint pain in 22%, injection site pain in 78%, injection site swelling in 6%, headache in 52%, and nausea in 2% of participants. Fever (≥38 °C) was low following the first dose; however, was also observed in 10–17% following the second dose. The frequency of fever tended to be higher in the younger group than in the older adult group [4]. Compared with the side effects reported in previous overseas studies about the BNT162b2 mRNA COVID-19 vaccine [4], similar side effects were observed for the Japanese participants in this study. The side effects were more frequent after the second vaccination, and Grade 3 side effects were also more frequent after the second vaccination than the first vaccination [11]. This result is the same as that in the overseas report [4]. However, 55% of the participants experienced Grade 3 side effects after the second vaccination in this study. This suggests that people in the same workplace and family members may have been affected by vaccination with the same vaccine on the same day, and the vaccination date should be adjusted, whenever feasible. Moreover, one of the side effects, fever, was observed in only 3.0% of the participants after the first vaccination; however, the incidence rate significantly increased to 44.9% after the second vaccination. After the second vaccination, many participants had fever, which is an important indicator of SARS-CoV-2 infection. During this study period, no typical anaphylaxis occurred after both the first and second vaccinations.
Side effects according to sex were examined. The difference in side effects according to sex was also significantly greater in the women than in men, as was the case with overseas reports [4]. Between the male and female participants, the latter were significantly more likely to have side reactions. Although the cause is not yet known, polyethylene glycol (PEG) is cited as one of the possible causes of anaphylaxis [12]. PEG is also used in shampoo and toothpaste and often in cosmetics; therefore, females might be more prone to anaphylaxis as they generally use more cosmetics than males. Although anaphylaxis did not occur in any of the participants in this study, this idea might apply to all side reactions. Significantly more female participants had Grade 3 side effects than the male participants. General fatigue and joint pain were more common in women than men; therefore, caution is required. A rest day might be necessary after the second vaccination.
We also examined the side effects of age-based vaccination. As shown in Table 5, the incidence of side effects was significantly higher in the young participants than in the elderly participants. Additionally, the incidence of Grade 3 side effects was higher in the young participants than in the elderly participants. Young participants are more likely to have systemic reactions such as fever and general fatigue than elderly participants. However, as the incidence of the side effects of the vaccine was lower in the elderly than in the young participants, it will be advantageous to recommend the vaccine to the elderly. In severe COVID-19, being elderly is considered a significant risk factor [4]. Vaccination of elderly people is scheduled to start after the vaccination of medical staff in Japan. If the elderly experience many side effects of the vaccine, they might hesitate to be vaccinated, which could hinder their vaccinatio. Therefore, this study result is important.
We also investigated the antibody titer transition after the COVID-19 vaccination. Only 35% of the participants had positive antibody titers after the first dose. In contrast, all the participants had a positive antibody titer after the second dose. This result might support the need for two cycles of vaccination to obtain a preventive effect against COVID-19 in clinical practice [7]. However, among those with a positive antibody titer, some showed extremely high and others showed low antibody titers. The cause of the differences was unclear owing to the small number of participants examined for antibody titer transition in this study. For the second dose, antibody titer was significantly negatively correlated with age. This indicated that older people had lower antibody titers than younger people, which may be related to the fact that older adults have a lower incidence of side effects owing to being vaccinated in this study. However, in this study, the correlation between antibody titer and side effects could not be investigated due to the limited specifications of the database; thus, further studies are required. Furthermore, the duration of the positive antibody titer is also unclear. The 23 participants who underwent evaluation for antibody titer transition at our facility should be followed up at 6 months and 1 year after vaccination to survey the antibody titer transition.
Several study limitations should be considered. First, this was a one-time survey of the first and second doses of the BNT162b2 mRNA COVID-19 vaccine; thus, longitudinal data were lacking. Second, this study involved healthcare workers from a single institution; therefore, the results may not be representative of all institutions in Japan. Side effects of this vaccine vaccinated for approximately 4 million Japanese people are shown on the website of the Japanese Ministry of Health, Labor and Welfare [13]. The safety of vaccines should be considered with a large number of participants, particularly side effects with low frequency such as anaphylactic shock (0.017%) and possibly fatal conditions. Larger nationwide studies are warranted to verify these findings.
5. Conclusions
In conclusion, the BNT162b2 mRNA COVID-19 vaccine might be safe for the Japanese people in this study, and the antibody titer increased after two vaccinations. Larger nationwide studies are warranted to verify these findings.
Ethics approval and consent to participate
This study was approved by the Ethics Committee for Clinical Studies of the Japanese Red Cross Medical Center (nos. 1224 and 1225; February 28, 2021).
Funding source
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for this research.
Authors’ contributions
Study concept and design: TI. Acquisition of data: all authors. Analysis and interpretation of data: TI and MI. Writing of the manuscript: TI. Statistical analysis: TI. All authors approved the final version of the manuscript for publication.
Conflict of Interest
The authors have no conflicts of interest to declare regarding this study.
Acknowledgments
We would like to thank all of the participants and staff members involved in this study. We also express our gratitude to Yasutoshi Kido, Kotaro Fujii, Akihiro Ueda, Osamu Hosoya, Junko Kawakami, Akito Miyauchi, Hitoshi Koyama, Hiroyuki Miyakawa, Keiko Kanno, and Shizuko Yamabe for their outstanding support. The authors also thank Enago for the English-language editing. The corresponding author had full access to all of the study data and had final responsibility for the decision to submit the findings for publication.
References
- 1.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q., Xu Q., Chen Y.Y., Lou L.X., Che L.H., Li X.H. Clinical characteristics of 41 patients with pneumonia due to 2019 novel coronavirus disease (COVID-19) in Jilin, China. BMC Infect Dis. 2020;20:961. doi: 10.1186/s12879-020-05677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo S.P. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2021 doi: 10.1177/08971900211009650. [DOI] [PubMed] [Google Scholar]
- 6.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo C., Liu M., Li Q., Zheng X., Ai W., Gong F. Dynamic changes and prevalence of SARS-CoV-2 IgG/IgM antibodies: multiple factors-based analysis. Int J Infect Dis. 2021;108:57–62. doi: 10.1016/j.ijid.2021.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchida T., Nitahara Y., Suzuki S., Komase Y., Candray K., Kido Y. Back to normal; serological testing for COVID-19 diagnosis unveils missed infections. J Med Virol. 2021;93:4549–4552. doi: 10.1002/jmv.26949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshiyama T., Saito Y., Masuda K., Nakanishi Y., Kido Y., Uchimura K. Prevalence of SARS-CoV-2-specific antibodies, Japan, June 2020. Emerg Infect Dis. 2021;27:628–631. doi: 10.3201/eid2702.204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimek L., Eckrich J., Hagemann J., Casper I., Huppertz J. Allergic reactions to COVID-19 vaccines: evidence and practice-oriented approach. Internist. 2021;62:326–332. doi: 10.1007/s00108-021-00959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Japanese Ministry of Health, labor and Welfare. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_hukuhannou-utagai-houkoku.html