Abstract
Ten to 15% of school-age children have reading difficulties (RD, or dyslexia), defined by deficits in phonological processing, fluency, and executive functions (EFs). Although RD is referred to as a genetic disorder, reading ability may also be affected by environmental factors such as inadequate exposure to literacy and a lack of parental involvement. These environmental components are a part of the socioeconomic status (SES) measure, which is defined by parental occupation, educational attainment, and household income and are positively correlated to reading ability. The goal of the current study was to relate maternal education, a construct of SES to executive functions (EFs) that relate to reading in children with RD compared to typical readers (TRs) using behavioral and neurobiological resting-state fMRI data. The results show that higher maternal education is negatively correlated to inhibitory control for TRs and not for children with RD. Higher maternal education was also associated with negative functional connectivity of the frontal-parietal network to the left central opercular cortex and left occipital gyrus for children with RD compared to TRs. These results suggest that higher maternal education has contrasting roles on the behavioral and neurobiological correlates of EFs for children with RD compared to TRs. We conclude that higher education levels for mothers may provide their children with a structured environment and educational resources that may assist their children with RD and TRs with cognitive development based on their reading profile.
Keywords: Children, Executive functions networks, Functional connectivity, Maternal education, Reading difficulties, Resting-state
Graphical Abstract

1. Introduction
1.1. Reading relies on networks related to executive functions
Reading difficulty (RD, or dyslexia) is a common learning disability amongst school-age children affecting 10 to 15% with lower reading ability in one or more reading domains such as phonemic awareness, word reading, and reading comprehension [1]. Although a core deficit of RD is phonological processing [1], previous work examining children with RD show decreased executive functions (EFs) which may contribute to their impaired reading ability [2]. EFs is an umbrella term for cognitive abilities that include, working memory, set-shifting, planning behavior, and inhibition [1–3]. Slow and inaccurate reading abilities are associated with deficits in EFs for children with RD [2] and poorer reading fluency compared to typical readers (TRs) [4]. However, studies also point to the importance of brain networks related to EFs, attention, and cognitive control for children with RD and (TRs) during reading acquisition [2, 4, 5].
The brain is organized into large-scale networks, with neuroimaging research pointing to the role of orienting and executive control networks related to EFs and cognitive control known as the attention system networks [5, 6]. The orienting system consists of the dorsal attention network (DAN) and the ventral attention network (VAN) [5]. DAN is involved in top-down visuospatial orienting of attention and the VAN is involved in reorienting attention to unexpected stimuli [5]. Executive control within the attention system networks is attributed to two networks: fronto-parietal (FP) and the cingulo-opercular (CO) [5]. The FP network is involved in rapid adaptive control (speed of processing) with task switching and initiation during a task in real-time, while the CO network is involved in slower processes such as error monitoring and maintenance of task performance [5, 7].
Interestingly, the literature suggests there are differences in the involvement of neural circuits associated with the attention system networks for children with RD and TRs. 8-12-year-old TRs that completed an event-related fMRI paradigm examining silent word reading and checkerboards had increased activation in the VAN and DAN (orienting), as well as the FP and CO (executive control) for remembered vs. forgotten words [8]. This suggests that TRs rely on the attention system networks for processes such as monitoring and information processing (EFs) during word reading and their encoding in memory [8]. On the other hand, children with RD have shown decreased functional connectivity of the DAN, VAN, CO, and FP networks during a reading comprehension task compared to TRs suggesting this may be a contributor to their impaired reading abilities [9]. Taken together, these studies point to the importance of the synchronization of the attention system networks to regions related to language and EFs in order to carry out reading-related processes for children. However, are these networks exhibiting differences in functional connectivity at rest in children with varying reading profiles when accounting for their mother’s education level? This is one of the questions of the current study.
1.2. Maternal education is a predictor of cognitive development
Although many cases of RD are attributed to an organic dyslexic etiology (i.e. a genetic source), many children have RD due to inadequate resources, motivation, and stimulation in the home reading environment [10]. Socioeconomic Status (SES) is an environmental construct (i.e. education, occupation, income) that is strongly associated with cognitive ability [11] and neurobiological constructs [12]. Neuroimaging data has shown that children from low SES backgrounds had reduced activation in the left inferior frontal gyrus during a phonological processing task showing poorer language skills [12]. EFs are correlated with SES and children with mothers who had lower education levels showing reduced selective attention on neural processing during an EEG paradigm [13]. SES disparities in abilities associated with the attention system networks (alerting, orienting, and EFs) have also been reported in children as early as 6 years old, showing that the environment has a role in cognitive development [14].
It has been suggested that parental education and household income should be measured separately as they have different contributions to child cognitive development [15]. Household income examines the resources available to a child, whereas parental education has a role in shaping cognitive development through parent-child interactions [16]. Parental education levels were associated with total brain surface area of regions related to language, reading, and EFs suggesting this construct of SES influences structural brain development [15]. More specifically, maternal variables have strong associations to child development with maternal education showing positive associations with children’s academic outcomes and cognitively stimulating parental practices [17]. Higher education levels tend to be an advantage not only for the mothers but for children through knowledge and resources that are important for academic success [17]. Mothers with higher educational attainment used child-directed speech that mediated the relationship between SES and child vocabulary knowledge due to the lexical richness and sentence complexity [18]. These data show that maternal education levels have a strong role in child development and academic outcomes through resources and knowledge about advantageous opportunities. Based on these data, we chose to examine maternal education as a covariate of interest.
Although there is evidence that maternal education has associations with child cognitive development [17], it is still unclear how it’s associated with the development of EFs and its neurobiological correlates in children with varying reading profiles, which is the goal of the current study. Therefore, we aimed to explore the mechanism for which maternal education contributes to neurocognitive functions related to EFs that support reading for school-age children. We hypothesized that higher maternal education will be associated with better EFs in 8-12-year-old children with RD and TRs. Due to previous literature showing negative correlations between functional connectivity and maternal variables during resting-state [19] and differences in the functional connectivity of the attention system networks in typical vs. atypical readers [2], we hypothesized that maternal education will show different functional patterns in both reading groups. Therefore, higher maternal education will be negatively associated with functional connectivity between the attention system networks (i.e. DAN, VAN, FP, and CO) and other regions supporting reading ability in children with RD and positively associated for TRs during a resting-state fMRI condition.
2. Results
2.1. Behavioral measures
2.1.1. Reading measures for group assignment
Children with RD had decreased reading abilities compared to TRs (phonological processing, automatic decoding and orthographical processing, reading fluency, non-timed decoding and orthographical processing, reading comprehension, and oral reading ability (See Table 2). Significant differences in attention ability between the two reading groups were observed, as defined by the Conner’s self-report test [RD: X=50.29±7.12, TR: X=46.14±5.56, t(43)=2.155, p<.05].
Table 2.
Correlations between maternal education and executive functions measures.
| Executive Functions Test | RD | TR | Correlation Coefficient |
|---|---|---|---|
| Maternal Education | |||
| Inattentiveness: CPT | r=−.417* p=.048 |
r=−.110 p=.645 |
Z=−1.039(P=.149) |
| Sustain/Divided Attention: TEACH | r=.423* p=.044 |
r=−.442 p=.051 |
Z=2.883(P=.002) |
| Inhibition switching: D-KEFS | r=.251 p=.249 |
r=−.172 p=.469 |
Z=1.339(P=.09) |
| Phonemic fluency: D-KEFS | r=.452* p=.030 |
r=.078 p=.745 |
Z=1.273(P=.101) |
| Impulse control: BRIEF | r=−.020 p=.929 |
r=−.646** p=.002 |
Z=2.455(P=.007) |
2.1.2. Executive functions measures
There were significant differences in EFs measures: children with RD showed greater inattentiveness as measured by the CPT: Detectability and poorer attention abilities as measured by the Conner’s Self-Report (although it did not reach the clinical threshold). There was a trend towards significant differences in inhibition/switching performance as measured by the D-KEFS Color Naming inhibition/switching condition with children with RD performing more poorly. There were no significant differences between the groups in sustained/divided attention, inhibition, or phonemic fluency (See Figure 1).
Fig. 1. Mean differences in EFs measures for children with RD and TRs.
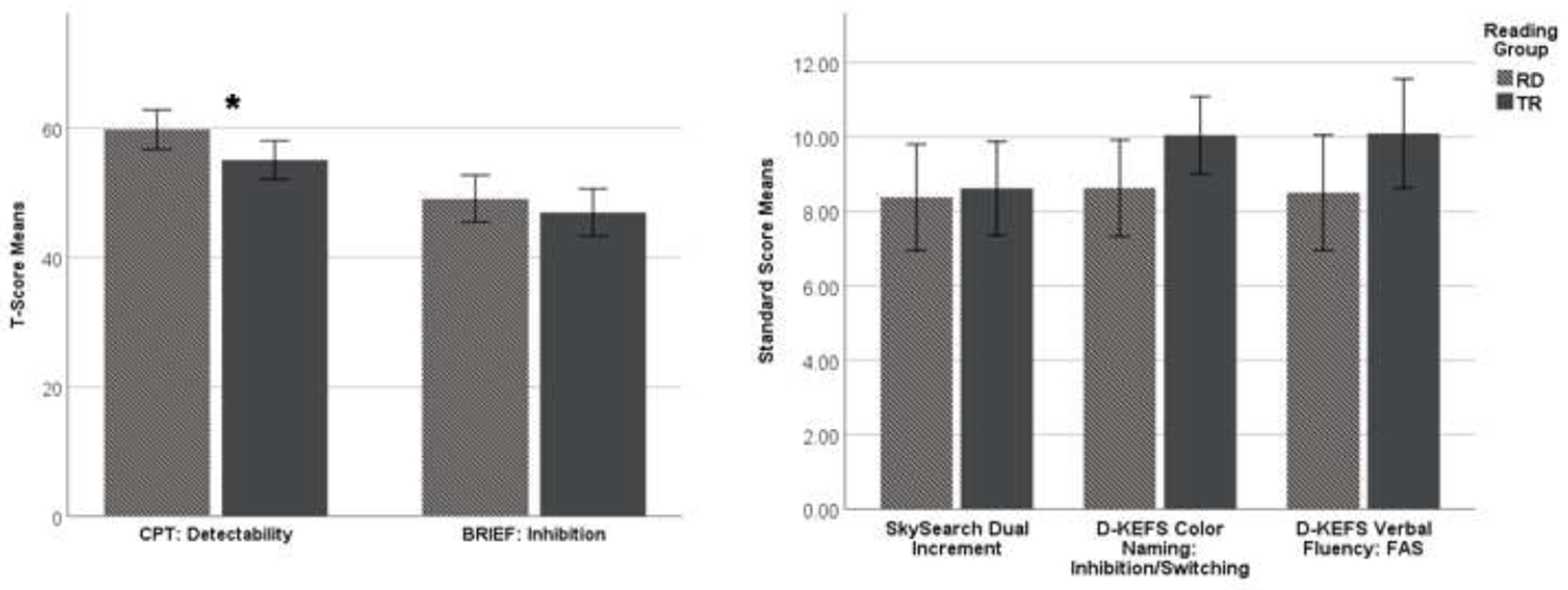
Conner’s Continuous Performance Test: Detectability, Behavior Rating Inventory of Executive Function: Inhibition, Sky Search Dual Increment Task, Delis-Kaplan Executive Function System Color Naming: Inhibition Switching, Delis-Kaplan Executive Function System FAS: Verbal Fluency) for children with RD (Light Grey) and TRs (Dark Grey). Standard deviation ±2 included in graphs. *p < 0.05.
2.1.3. Maternal education
For children with RD, 33% of their mothers reported having less than or equal to a doctoral degree, 54% reported having less than or equal to a bachelor’s degree, and 13% reported having less than or equal to a high school degree. For TRs, 48% of mothers reported having less than or equal to a doctoral degree, 52% reported having less than or equal to a bachelor’s degree. There were no significant differences in maternal education for children with RD and TRs [RD: X=17.38±3.09, TR: X=18.10±1.61, t(43)=−.959, p>.05].
2.1.4. Correlations between maternal education and EFs measures
When controlling for non-verbal IQ, higher maternal education was positively associated with phonemic fluency (r=.452, p<.05) and sustained/divided attention (r=.423, p<.05), and negatively associated with inattentiveness (r=−.417, p<.05) for children with RD. Higher maternal education was also negatively associated with inhibition for TRs (r=−.646, p<.01). There were no significant associations between maternal education and inhibition/switching or inhibitory control for children with RD and no significant associations between maternal education and phonemic fluency, sustained/divided attention, inattentiveness, or inhibition/switching for TRs. After correcting for multiple comparisons, higher maternal education was negatively associated with inhibition for TRs (p<.01). A scatterplot was created in SPSS by regressing non-verbal intelligence onto BRIEF: Inhibition T-scores (See Figure 2). Regression lines were added to the scatterplot for each group. Comparison of correlation coefficients showed that TRs had greater correlations between maternal education and inhibition compared to children with RD (Z=2.455, p<.01). In addition, children with RD had greater correlations between maternal education and sustained/divided attention compared to TRs (Z=2.883, p<.01) (See Table 3).
Figure 2. Scatterplot of the relationship between maternal education and inhibition for children with RD and TRs.

Higher maternal education scores were associated with decreased impulsivity for TRs, controlling for non-verbal IQ. Non-verbal IQ was regressed onto BRIEF: Inhibition as an unstandardized residual to plot the Pearson Correlation in SPSS. Data were non-significant for children with RD. Circles represent children with RD and diamonds represent TRs. A regression line was added to the scatter plot. Data presented in a significance level of *p<0.01.
Table 3.
Networks and coordinates for the attention system networks created by WFU PickAtlas Toolbox (spherical size 10 mm radius) [2, 7, 75].
| Network | Regions | X | Y | Z |
|---|---|---|---|---|
| Cingulo-Opercular | left anterior prefrontal cortex | −28 | 51 | 15 |
| right anterior prefrontal cortex | 27 | 50 | 23 | |
| left lateral anterior insula / frontal operculum | −51 | 18 | 13 | |
| right lateral anterior insula / frontal operculum | 45 | 23 | −4 | |
| left medial anterior insula / frontal operculum | −33 | 24 | 1 | |
| right medial anterior insula / frontal operculum | 33 | 25 | −1 | |
| left anterior insula / frontal operculum | −35 | 14 | 5 | |
| right anterior insula / frontal operculum | 36 | 16 | 4 | |
| dorsal anterior cingulate / medial superior frontal cortex | −1 | 10 | 46 | |
| Fronto-Parietal | left dorso-lateral prefrontal cortex | −43 | 22 | 34 |
| right dorso-lateral prefrontal cortex | 43 | 22 | 34 | |
| left inferior parietal lobule | −51 | −51 | 36 | |
| right inferior parietal lobule | 51 | 47 | 42 | |
| left intraparietal sulcus | −31 | 59 | 42 | |
| right intraparietal sulcus | 30 | −61 | 39 | |
| left precuneus | −9 | −72 | 37 | |
| right precuneus | 10 | −69 | 39 | |
| mid cingulate cortex | 0 | −29 | 30 | |
| DA Network | Right FEF | 28 | −10 | 53 |
| Right Posterior IPS | 20 | −67 | 51 | |
| Right Anterior IPS | 35 | −47 | 45 | |
| Left FEF | −25 | −12 | 55 | |
| , Left Posterior IPS | −22 | −68 | 46 | |
| Left Anterior IPS | −42 | −41 | 43 | |
| Left SMA/Pre-SMA | −4 | −1 | 53 | |
| Right Inferior Frontal Gyrus | 52 | 6 | 27 | |
| Right MT+ | 51 | −63 | −7 | |
| Right Middle Frontal Gyrus | 37 | 38 | 20 | |
| Right Insula | 30 | 17 | 9 | |
| Left MT+ | −46 | −68 | −7 | |
| VA Network | Right Insula | 28 | 12 | 2 |
| Right Supramarginal Gyrus (TPJ) | 57 | −43 | 34 | |
| Left Insula | −41 | 11 | 1 | |
| Right Superior Frontal Gyrus | 29 | 44 | 29 | |
| Right Inferior Frontal Gyrus | 41 | 41 | 5 | |
| Right Inferior/Middle Frontal Gyrus | 47 | 14 | 32 | |
| Right Superior Frontal Gyrus | 4 | 20 | 49 | |
| Right Middle Temporal Gyrus | 52 | −32 | −7 | |
| Left Superior Temporal Gyrus | −60 | −49 | 19 | |
| Right Precuneus | 2 | −53 | 51 | |
| Right Medial Frontal Gyrus | 8 | 3 | 62 | |
| Right Middle Temporal Gyrus | 41 | −13 | −7 | |
| Right Sulcus Callosomarginalis | 6 | −28 | 43 |
2.2. Neuroimaging data results
2.2.1. Functional connectivity of attention system networks to the whole brain
Children with RD:
FP network had positive functional connectivity with the right frontal pole and right superior division of the lateral occipital cortex and negative functional connectivity to the thalamus. The CO network had positive functional connectivity with the right frontal pole and right occipital lobe. The DAN had positive functional connectivity with the right frontal pole and right cerebellum Crus 2, and negative functional connectivity with the posterior division of the cingulate gyrus. The VAN had positive functional connectivity with the right frontal pole and negative functional connectivity with the right occipital lobe.
TRs:
FP network had positive functional connectivity with the right frontal pole, right cerebellum, and negative functional connectivity to the hippocampus. CO network had positive functional connectivity with the right frontal pole and left occipital pole, and negative functional connectivity to the precuneus cortex, and left and right cerebellum. DAN had positive functional connectivity with the left precentral gyrus, right cerebellum, and left frontal orbital cortex and negative functional connectivity with the posterior division of the cingulate gyrus. VAN had positive functional connectivity with the right frontal pole, left cerebellum crus2, left occipital pole, and thalamus, and negative functional connectivity with the hippocampus, right cerebellum crus1, and subcallosal cortex.
No significant differences in functional connections for DAN, VAN, FP, or CO networks were found when comparing the two groups.
2.2.2. Correlation between maternal education and functional connectivity between attention system networks and the whole brain (seed to voxel analyses)
Children with RD and TRs:
There were no significant associations between maternal education and the functional connectivity of the DAN, VAN, FP, or CO networks with the whole brain when examining children with RD and TRs separately.
Children with RD greater than TRs:
Seed to voxel correlation analysis revealed that maternal education was negatively associated with functional connectivity between the FP network and the left central opercular cortex and left occipital fusiform gyrus in children with RD greater than TRs. In contrast, maternal education was positively associated with the functional connectivity between the FP network and the left central opercular cortex and left occipital fusiform gyrus in TRs greater than children with RD (See Figure 3 and Figure 4). See Supplementary Table 2 for peak intensity. There were no significant results for the CO network, VAN, or DAN for children with RD or TRs.
Figure 3. Maternal education and its association to the functional connectivity between the FP network and all voxels for RD and TRs.

A) Maternal education was related to negative functional connectivity between the FP network and a cluster of voxels within the left central opercular cortex and left occipital fusiform gyrus for RD greater than TRs (Slices are represented from Z(−20) to Z(10)). B) Maternal education was related to positive functional connectivity between the FP network and left central opercular cortex and left occipital fusiform gyrus for TRs greater than children with RD (Slices are represented from Z(−20) to Z(10)). Each Fig. is presented in neurological orientation (left on left, right on right). Cooler colors represent negative functional connectivity values. Hotter colors represent positive functional connectivity values. *p<0.05, FDR-corrected.
Figure 4. Scatterplot of the relationship between maternal education and functional connectivity of FP network and all voxels for children with RD and TRs.

A) Maternal education was associated with negative functional connectivity between the FP network to the left central opercular cortex for children with RD and positive for TRs. B) Maternal education was associated with negative functional connectivity between the FP network to the left occipital fusiform gyrus for children with RD and positive for TRs. FD was regressed onto functional connectivity beta correlation values as unstandardized residuals in SPSS. Circles represent children with RD and diamonds represent TRs. A regression line was added for each group. Data presented in a significance level of *p<0.05
3. Discussion
The goal of the current study was to examine the behavioral and neurobiological correlates between maternal education (as a construct of SES) and EFs in 8-12 years old children with RD and TRs. Behavioral data showed that higher maternal education was associated with better phonemic fluency, sustained/divided attention, and decreased inattentiveness for children with RD; however, this data did not survive multiple comparisons. TRs with mothers that had higher maternal education had more inhibitory control as measured by lower inhibition T-scores compared to children with RD. The imaging results revealed that maternal education was negatively associated with the functional connectivity between the FP network and the left central opercular cortex and left occipital fusiform gyrus for children with RD (when comparing them to TRs) during a resting-state condition, as postulated. Our results suggest that maternal education is associated with different roles for the behavioral and neurobiological correlates of EFs for children with RD and TRs.
3.1. Maternal education and executive function abilities
Our results show an association between higher maternal education and better phonemic fluency, sustained divided attention, and decreased inattentiveness in children with RD. Fluent reading relies on automatic word recognition and phonological processing which are common deficits for children with RD [20]. Children with RD often have decreased EFs abilities which impair their ability to focus on a complex task such as reading [9]. We suggest that higher maternal education acts as a “cushion” for children that have difficulties in reading by possibly providing them with a more structured environment through more child-directed speech, tutors, better schools, and/or additional educational experiences which improves their EFs that support reading. This may result in children with RD performing better on tests that probe EFs for better performance than their low SES peers. Specifically, the variable we chose to assess, maternal education, is a strong indicator of parenting behaviors, cognitive development, and academic achievement [17, 18]. There were also significant differences observed for the correlations between maternal education and sustained/divided attention for children with RD compared to TRs. Although these results did not survive multiple comparisons, possibly due to low sample size and more children having mothers with higher education, these maternal parenting practices may provide children with RD with better attention abilities.
We also found associations between higher maternal education and more inhibitory control in TRs. The ability to inhibit external stimuli for goal-directed behaviors is essential for self-regulation [21, 22] and predicts academic success and school readiness [23, 24]. Children ages 5-7 years of age have shown substantial age-related improvements in inhibition with a leveling in performance by age 8, suggesting that inhibition develops early and error awareness becomes more apparent during cognitive development [25]. Maternal education is positively associated with maternal sensitive parenting styles and home environment [26, 27], which are related to better self-regulation abilities in early childhood [28–30]. It is plausible that TRs with mothers that have higher educational attainment may be exposed to a structured environment that contributes to better self-regulation, the ability to accomplish goal-directed behaviors and control impulses. Ultimately this relationship for TRs could be driven by mothers that reported “some-college or no degree” which was related to higher inhibition T-scores which requires diversifying our sample. Additionally, more information needs to be collected about school/home environment and maternal sensitive parenting styles to understand these contributions to inhibition. In contrast, there was not a significant association between maternal education and inhibition for children with RD. Impairments in inhibition have been reported in children with RD [31] which can contribute to the poorer letter and word identification [32, 33]. In our sample, there were no significant differences between children with RD and TRs in inhibition as reported by the parental BRIEF assessment. There may be no significant association between maternal education and inhibition for children with RD due to lack of variability in inhibition T-scores. It could also be due to the literature suggesting task-based assessments are more reliable for documenting non-linear changes than questionnaires [34, 35] which could be influenced by parental biases. In the future, including a task-based assessment of inhibition may be beneficial to better understand the relationship between maternal education and inhibition for children with RD and TRs.
3.2. Maternal Education is negatively associated with the functional connectivity of the FP network and left central opercular cortex.
Here, we pointed at negative associations between maternal education and the functional connectivity between an executive control network (FP network) and the left central opercular cortex for children with RD greater than TRs. Teenager and young adults with RD have displayed decreased connectivity in the bilateral FP network comprising bilateral dorsolateral prefrontal and posterior parietal regions and increased connectivity in hippocampal and thalamic regions showing they rely more on classical reading and memory pathways as a compensatory mechanism [36]. The left frontal operculum is an ROI within the CO network involved in speed of processing, error monitoring, and planning behavior [2]. Due to the involvement of this region in error monitoring, it is active when individuals are reading pronounceable non-words compared to real words [37, 38]. In regards to SES, children from higher income levels exhibit greater activation of brain regions comprising the FP network compared to lower-income children due to growing working memory demands [39]. Based on these data, our findings suggest higher maternal education for children with RD is related to less sustained attention for error monitoring compared to higher maternal education for TRs at rest. Individuals with RD display errors during reading due to slow and inaccurate decoding abilities, which can impact their ability to read unfamiliar words [40, 41]. Based on these data, it is relevant to determine if having a mother with higher education levels has a significant effect on pseudoword reading through the mediation of the functional connectivity between the FP network and the left central opercular cortex for children with RD compared to TRs. This will explain the underlying mechanism of maternal education’s influence on decoding non-words through the neural correlates of sustained attention and error monitoring for children with varying reading profiles.
3.3. Maternal education is negatively associated with the functional connectivity between the FP network and left occipital fusiform gyrus
In our sample, we observed a negative association between maternal education and the functional connectivity between the FP network and the left occipital fusiform gyrus for children with RD compared to TRs. Studies of reading acquisition in children highlight the importance of the left fusiform gyrus in object [42] and word recognition [43–45]. Kindergarteners with a familial risk of RD displayed hypoactivation in the fusiform gyrus in responses to letters and false fonts which was subsequently related to reading impairments by second grade [46]. A signature of RD is impairments in grapheme-phoneme correspondence reflected by underactivation in the left fusiform gyrus. An fMRI study examining children with variations of phonological ability found that higher SES children had attenuated activation in the fusiform gyrus during pseudoword reading compared to lower SES children that had increased activation [47]. The authors suggest that among children with more resources and access to literacy materials due to higher SES, the relationship between phonological reading skill and fusiform gyrus activity concerning reading may contribute to this atypical-brain behavior relationship [47]. Based on these data, higher maternal education levels could relate to less sustained attention during visual word recognition for children with RD compared to TRs. It has been proposed that mothers with higher education levels have social capital through networks that build skills and knowledge that can improve their children’s academic achievement [17]. Mothers with higher education levels may be engaged in alleviating their children’s RD through tutors and access to educational programs, which should be investigated in the future. Exploring the nature of this mechanism could determine if functional connectivity between the FP network and the left central opercular cortex mediates the relationship between maternal education and site-word reading efficiency for children with RD compared to TRs.
3.4. Limitations and future directions
This study has several limitations. First, SES is an environmental construct that consists of parental educational attainment, household income, and occupation. For this study, we focused on maternal education which is only one piece of this large variable. Therefore, we may not be accounting for other key environmental variables of the model for EFs supporting reading acquisition such as school/home environment and maternal sensitive parenting styles related to maternal education. Secondly, we did not have a very diverse sample of maternal education as the average for both groups is a Bachelor’s degree. This is mostly due to the recruitment and retention of low SES participants during our study which is a major limitation. We also did not see many significant differences in EFs between groups which could be due to participants coming from similar socioeconomic backgrounds where the levels of resources being provided may be the same. In the future, more low SES participants should be included to gain a better understanding of the role of maternal education on EFs important for reading ability. Resting-state fMRI has statistically significant reliability with a subject’s eyes fixated on an object such as a cross [48]. Although this paradigm has been used in past studies [4, 49, 50], there is a chance that there may be difficulties in fixation for our children with RD. Future directions include testing for differences between eyes open and fixation during resting-state fMRI for our children with RD compared to TRs. In our sample, we did not exclude any participants for head motion but controlled for FD. The Friston 24-parameter model is a richer set of motion parameters that regress out head motion effects from realigned data (6 head motion parameters, 6 head motion parameters one time-point before, and the 12 corresponding squared items) [51, 52]. After running the Friston 24-parameter model, results showed that maternal education was associated with negative functional connectivity between the DAN and the left occipital fusiform gyrus and left central opercular cortex for children with RD greater than TRs. These data suggest that there may be some overlap in network functional connectivity between the DAN and FP network, which should be explored using graph theory [53].
4. Conclusions
Maternal education is strongly associated with academic and cognitive abilities for developing children. The current study provides evidence for the role of maternal education on inhibitory control for TRs and differences in functional connectivity between the FP network and left central opercular cortex and left occipital fusiform gyrus for children with RD compared to TRs at rest. Our findings suggest that higher maternal education levels have contrasting roles on sustained attention for error monitoring and visual word recognition for children with RD compared to TRs, which should be explored further in relation to site-word and pseudoword reading outcomes. This study is the first to describe a relationship between maternal education and the behavioral and neurobiological correlates of EFs that support reading for school-age children with RD and TRs. Our results add to the literature the important role of maternal educational attainment on EFs and cognitive control in relation to reading for children with varying reading profiles.
5. Methods
5.1. Participants
Forty-five school-age children were recruited for this study [(24 children with RD: mean age= 9.83±1.40 years, 12 females, 22 right-handed; 21 TRs mean age=10.05±1.47, 6 females, 19 right-handed)] all matched for age (t(43)=−.500, p>.05). All participants had intact non-verbal intelligence (IQ) as measured by the TONI-IV (≥85) [54] [RD: X=100.67±14.65, TR: X=111.10±10.97, t(39.7)=−2.671, p<.05], with children with RD having lower scores. None of the participants had a history of neurological disorders, head trauma, or psychiatric conditions. Participants were recruited using study flyers, website ads, and communication on the news. Written consents were provided for all participants and written assents for children age 11 and above. All participants were compensated for their time and travel. The study protocol was approved by the institutional review board.
5.2. Reading measures
Children with RD:
Parents of the participants in the RD group reported if their child had an Individualized Educational Plan (IEP) or 504 in relation to their RD. Reading ability was verified using normative subtests in English, where children had to demonstrate a standard score of −1 and below in at least two reading tests listed below (following [55]).
The reading battery included: a) phonological processing: Elision and Blending Words subtests from the Comprehensive Test of Phonological Processing, Second Edition (CTOPP-2) [56], b) Automatic decoding and orthographical processing: Site word efficiency and pseudoword efficiency tests from the Test of Words Reading Efficiency (TOWRE) [57], c) Reading fluency: Test of Silent Reading Efficiency and Comprehension (TOSREC) [58], d) non-timed decoding and orthographical processing: letter word identification, and word attack subtests from the Woodcock-Johnson (WJ-IV) [59], e) reading comprehension: WJ-IV passage comprehension [59], f) oral reading ability (fluency and comprehension combined scaled scores) Gray Oral Reading Test (GORT-V) [60]. Attention was measured using the Conner’s self-report test and scores of less than or equal to 4 were inclusion criteria [61]. Typical readers had average or above-average reading abilities in all examined reading tests.
5.3. Behavioral Measures
5.3.1. Maternal education
A Reading Demographics 2.0 form (following [62]) containing questions relating to race, household income, as well as the highest level of education attained were acquired during this study. This form is a modified version of the Barratt Simplified Measure of Socioeconomic Status (BSMSS) [63], where we ranked education level from never attended school/kindergarten to a doctoral degree resulting in scores from 0 to 22 (See Supplementary Table 1). Maternal education was assessed as a continuous variable due to the majority of mothers having higher maternal education for both groups.
5.3.2. Executive functions measures
Each child completed neuropsychological assessments that examined the components of the attention system networks including EFs: a) inattentiveness: Continuous Performance Test (CPT): Detectability [64], b) sustained/divided attention: The Test of Everyday Attention for Children (TEA-Ch): Sky Search DT [65], c) inhibition/switching: Delis-Kaplan Executive Function System (D-KEFS) Color Naming, d) phonemic fluency: D-KEFS Verbal Fluency-FAS [66], and e) inhibitory control or impulsivity: Parental Behavioral Rating Inventory of Executive Function (BRIEF, Inhibition subscale) [67].
5.4. Functional MRI-resting-state condition
Approximately 30-60 minutes after the completion of the behavioral session, children were escorted to the MRI facilities. Each child completed two resting-state scans where they were instructed to look at across for five minutes for each session. All participants were instructed to avoid excessive motion, as well as avoid falling asleep. They were also desensitized to scanner conditions to ensure that they were comfortable during the paradigm (following our previous procedures [68]). Head motion was controlled using elastic straps that were attached to either side of the head coil apparatus.
5.5. MRI data acquisition
All participants were scanned using a 3T Phillips Ingenia MRI scanner at the Cincinnati Children’s Hospital Medical Center. A gradient echo-planar multi-band sequence was used for T2*-weighted resting-state fMRI scans with the following fMRI parameters: TR/TE= 700/30 msec, FOV= 200x200 mm, matrix size = 68 x 67, slice thickness= 3 mm, and an anisotropic voxel size of = 2.5 x 2.5 x 3 mm3 with 48 slices covering one volume. 430 whole-brain scans were acquired during the resting-state condition for a total time of five minutes, which was completed twice resulting in 860 whole-brain scans. The first 6 volumes for each of the scans were not included in the analyses. A high-resolution T1*-weighted 3D anatomical image was acquired for each subject using an inversion recovery (IR)-prepared turbo-gradient-echo acquisition protocol with a spatial resolution of 1 x 1 x 1 mm3 and the following parameters: TR/TE= 8.1/3.7, flip angle= 8
5.6. Data analyses
5.6.1. Behavioral data analyses
The Statistical Package for the Social Sciences (SPSS) software version 26 was used for conducting all the behavioral analyses (IBM, 2020). Two sample t-tests were used to examine RD and TRs group differences in relation to maternal education. Additionally, group differences in reading and EFs were examined for children with RD and TRs using two-sample t-tests. Partial correlations were performed to assess the relations between maternal education and EFs in children with RD and TRs. Non-verbal IQ was assessed as a control variable as this was an inclusion criterion for our study due to the RD definition [1, 69, 70], and children with RD having lower scores compared to TRs. Data were corrected for multiple comparisons using a Bonferroni correction. All behavioral data analyses were conducted with two-tailed parametric tests. Comparison of correlation coefficients between maternal education and EFs measures for children with RD and TRs were conducted in Psychometrica (https://www.psychometrica.de/correlation.html) using a one-tailed parametric test.
5.7. Neuroimaging data preprocessing
Imaging data were preprocessed using SPM12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) implemented into the CONN toolbox v.20.b [71]. Preprocessing steps included a functional realignment and unwarping, slice timing correction, outlier detection, and scrubbing, simultaneous segmentation of grey and white matter, and cerebrospinal fluid and normalization to Montreal Neurological Institute (MNI) space. The data were spatially smoothed at an 8 mm full width at half-maximum (FWHM) Gaussian Kernel following previous resting-state fMRI studies [4, 49]. The data were motion-corrected with SPM-12 using a rigid-body registration approach with three rotational and three translational to yield 6 motion parameters [72]. Functional detection outliers were set to subject motion threshold of 0.9 mm and global signal z-value threshold of 5. Artifact Detection Tools (ART) software package were used to calculate the average framewise composite motion and average framewise change in global mean signal intensity for all subjects which is integrated into the CONN functional connectivity toolbox v.20.b. [71].
After the completion of the spatial preprocessing, the data were further processed using the CONN functional connectivity toolbox v.20.b supported by MATLAB R2019b (The Mathworks, Natick, MA) [71]. The data were denoised using the anatomical component-based noise correction framework (aCompCor) [73] which included a linear regression of five principles eigenvariate confounding effects such as white matter and cerebrospinal fluid (CSF) from the BOLD time-courses to remove signal variation from these non-cortical regions. The six motion parameters for each resting-state session and their first-order derivatives were regressed out of the voxel wide time series. The voxel time series data were filtered through a band-pass between 0.008 to 0.09 Hz which were the standard settings in CONN [74]. Due to excessive motion often observed in pediatric populations, we evaluated the average frame-wise displacement (FD) for all participants and found it to be .36±0.23 mm following [49]. An independent t-test analysis showed that there were no significant differences in FD between groups [RD: mean=.40±.24, TR: mean=.31±.22, t(43)=1.277, p=.208]. After the completion of the denoising step, the two resting-state fMRI scans were concatenated for computation of first-level connectivity measures.
5.8. Target regions of interest
Regions of interest masks were created for the four attention system networks (ie. CO, FP, DAN, and VAN) using the WFU PickAtlas Toolbox in SPM12 based on coordinates listed in previous studies (spherical seed of 10 mm radius) [2, 75]. Reference frame and scaling biases were accounted for by best fit-transformation of the MNI coordinates to Talairach space (icbm_spm2tal; http://brainmap.org/icbm2tal/). The coordinates for each network are listed in Table 1.
Table 1.
Mean differences in language and reading measures for children with RD and TRs.
| Measure | Test | RD(M,SD) | TR(M,SD) | Contrast RD>TR | T(P) |
|---|---|---|---|---|---|
| Phonological processing | CTOPP Blending Word | 8.83±2.75 | 10.81±2.64 | −1.976 | T(−2.453)P=.018 |
| Phonological processing | CTOPP Elision | 7.71±3.13 | 11.33±2.01 | −3.625 | T(−4.68)P=.000 |
| Automatic decoding and orthographical processing: site-word efficiency | TOWRE SWE | 77.89±14.10 | 103.62±9.69 | −25.744 | T(−7.03)P=.000 |
| Automatic decoding and orthographical processing: pseudoword efficiency | TOWRE PWE | 78.08±12.77 | 101.43±10.17 | −23.345 | T(−6.72)P=.000 |
| Reading fluency | TOSREC | 82.92±14.44 | 102.86±12.69 | −19.940 | T(−4.89)P=.000 |
| Non-timed decoding and orthographical processing | WJ Letter Word Identification | 82.83±19.58 | 114.24±11.65 | −31.405 | T(−6.42)P=.000 |
| Reading comprehension | WJ Passage Comprehension | 83.38±17.79 | 104.33±12.23 | −20.958 | T(−4.54)P=.000 |
| Non-timed decoding and orthographical processing: pseudoword reading | Word Attack | 86.33±17.73 | 119.71±16.04 | −33.381 | T(−6.58)P=.000 |
| Oral reading ability: fluency and comprehension | GORT ORI | 86.13±13.16 | 107.81±13.20 | −21.685 | T(−5.51)P=.000 |
5.9. Seed-to-voxel analysis: Attention system networks (seeds) and the whole brain with maternal education
The attention system networks generated from the WFU PickAtlas Toolbox in section 5.8 were examined as networks of interest for the seed-to-voxel analysis. In the CONN toolbox, the attention system networks were examined as seeds for children with RD and TRs separately and then contrasted to assess group differences (RD>TRs). Then, maternal education was assessed as a covariate of interest for attention system networks to the whole brain for children with RD and TRs separately, as well as contrasted to assess group differences (maternal education RD>maternal education TRs). FD was controlled for due to previous reports showing that pediatric populations may have a high variance of motion which can impact the BOLD signal of the results [76, 77]. Data were corrected for multiple comparisons, using a p<.05 False Discovery Rate (FDR)-correction and analyzed with a two-tailed parametric test.
Supplementary Material
Highlights.
Maternal education was negatively associated with inhibitory control for typical readers.
Higher maternal education was associated with negative functional connectivity of the fronto-parietal network to brain regions involved in reading for children with reading difficulties compared to typical readers.
Acknowledgements
National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (PI: Horowitz-Kraus; 1R01HD086011-01A1); National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Health Development (NICHD) “Research Supplements to Promote Diversity in Health-Related Research” PA-18-586 (PI: Horowitz-Kraus; 1R01HD086011-01A1). This work was done in contribution to Paige Greenwood’s dissertation research. The authors would like to thank Ms. Elisha Scott for her assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statements
All authors confirm that there is not a conflict of interest.
References
- 1.Shaywitz SE, Morris R, & Shaywitz BA, The education of dyslexic children from childhood to young adulthood. Annual Review of Psychology 2008. 59: p. 451–475. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz-Kraus T, Toro-Serey C, & DiFrancesco M, Increased Resting-State Functional Connectivity in the Cingulo-Opercular Cognitive-Control Network after Intervention in Children with Reading Difficulties. PLoS One, 2015. 10(7): p. 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnan M, Demetre J, Hamill S, Robson K, Shepherd H, & Cody G , Executive functioning in adults and children with developmental dyslexia. Neuropsychologia, 2002. 40(12): p. 2144–2155. [DOI] [PubMed] [Google Scholar]
- 4.Freedman L, Zivan M, Farah R, & Horowitz-Kraus T, Greater functional connectivity within the cingulo-opercular and ventral attention networks is related to better fluent reading: A resting-state functional connectivity study. NeuroImage, 2020. 26: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen SE, & Posner MI, The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 2012. 45: p. 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reineberg AE, Gustavson DE, Benca C, Banich MT, & Friedman NP, The Relationship Between Resting State Network Connectivity and Individual Differences in Executive Functions. Frontiers in Psychology, 2018. 9(1600): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE, A dual-networks architecture of top-down control. Trends in Cognitive Science, 2008. 12(3): p. 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farah R, Coalson RS, Petersen SE, Schlaggar BL, & Horowitz-Kraus T, Children Use Regions in the Visual Processing and Executive Function Networks during a Subsequent Memory Reading Task. Cerebral Cortex, 2019: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meri R, Farah R, & Horowitz-Kraus T, Children with dyslexia utilize both top-down and bottom-up networks equally in contextual and isolated word reading. Neuropsychologia, 2020. 147: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackman DA, & Farah MJ, Socioeconomic status and the developing brain Trends in Cognitive Science 2009. 13(2): p. 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble KG, Houston SM, Kan E, & Sowell ER, Neural correlates of socioeconomic status in the developing human brain. Developmental Science 2012. 15(4): p. 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raizada RDS, Richards TL, Meltzoff A, & Kuhl PK, Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage, 2008. 40(3): p. 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens C, Lauinger B, & Neville H, Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science, 2009. 12: p. 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mezzacappa E, Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development, 2004. 75: p. 1373–1386. [DOI] [PubMed] [Google Scholar]
- 15.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, et al. , Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 2015. 18(5): p. 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan GJ, & Magnuson K, Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdisciplinary Reviews. Cognitive Science, 2012. 3(3): p. 377–386. [DOI] [PubMed] [Google Scholar]
- 17.Harding JF, Morris PA, & Hughes D , The relationship between maternal education and children’s academic outcomes: A theoretical framework. Journal of Marriage and Family, 2015. 771(1): p. 60–76. [Google Scholar]
- 18.Hoff E, The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development, 2003. 74(5): p. 1368–1378. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood P, Hutton J, Dudley J, & Horowitz-Kraus T, Maternal reading fluency is associated with functional connectivity between the child’s future reading network and regions related to executive functions and language processing in preschool-age children. Brain and Cognition, 2018. 131: p. 87–93. [DOI] [PubMed] [Google Scholar]
- 20.Elhassan Z, Crewther SG, & Bavin EL , The Contribution of Phonological Awareness to Reading Fluency and Its Individual Sub-skills in Readers Aged 9- to 12-years. Frontiers of Psychology 2017. 8(533): p. 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson SL, Sameroff AJ, Lunkenheimer ES, & Kerr D , Self-regulatory processes in development of disruptive behavior problems: the preschool-to school transition. Biopsychosocial regulatory processes in the development of childhood behavioral problems, ed. Olson SL, & Sameroff AJ 2009, New York: Cambridge University Press [Google Scholar]
- 22.Liu Q, Zhu X, Ziegler A, & Shi J , The effects of inhibitory control training for preschoolers on reasoning ability and neural activity. Scientific Reports, 2015. 5(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair CB, & Razza RP , Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development, 2007. 78(2): p. 647–663. [DOI] [PubMed] [Google Scholar]
- 24.Sektnan M, McClelland MM, Acock A, & Morrison FJ , Relations between early family risk, children’s behavioral regulation and academic achievement. Early Childhood Research Quarterly, 2010. 25(4): p. 464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald JA, Beauchamp MH, Crigan JA, & Anderson PJ, Age-related differences in inhibitory control in the early school years. Child Neuropsychology 2013. 20(5): p. 509–526. [DOI] [PubMed] [Google Scholar]
- 26.Tamis-Lemonda CS, Briggs RD, McClowry SG, & Snow DL , Maternal Control and Sensitivity, Child Gender, and Maternal Education in Relation to Children’s Behavioral Outcomes in African American Families. Journal of applied developmental psychology, 2009. 30(3): p. 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network NECCR, Do children’s attention processes mediate the link between family predictors and school readiness? Developmental Psychology, 2003. 39(3): p. 581–593. [DOI] [PubMed] [Google Scholar]
- 28.Blair CB, Granger DA, Willoughby M, Mills-Koonce WR, Cox M, Greenberg MT; FLP Investigators, Salivary cortisol mediates effect of poverty and parenting on executive functions in early childhood. Child Development, 2011. 82(6): p. 1970–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brophy-Herb HE, Stansbury K, Bocknek E, & Horodynski MA , Modeling maternal emotion-related socialization behaviors in a low-income sample: Relations with toddlers’ self-regulation. Early Childhood Research Quarterly, 2012. 27(3): p. 352–364. [Google Scholar]
- 30.Mistry RS, Brenner AD, Biesanz J, Clark S, & Howes C , Family and social risk, and parental investments during the early childhood years as predictors of low-income children’s school readiness outcomes. Early Childhood Research Quarterly, 2010. 25(4): p. 432–449. [Google Scholar]
- 31.de Jong CG, Van De Voorde S, Roeyers H, Raymaekers R, Oosterlaan J, & Sergeant JA , How distinctive are ADHD and RD? Results of a double dissociation study. Journal of abnormal child psychology, 2009. 37(7): p. 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid J, Labuhn AS, & Hasselhorn M, Response Inhibition and its Relationship to Phonological Processing in Children with and without Dyslexia. International Journal of Disability Development and Education, 2011. 58(1): p. 19–32. [Google Scholar]
- 33.Chiappe P, Hasher L, & Siegel LS , Working memory, inhibitory control, and reading disability. Memory & Cognition, 2000. 28(1): p. 8–17. [DOI] [PubMed] [Google Scholar]
- 34.Albert D, & Steinberg L , Age Differences in Strategic Planning as Indexed by the Tower of London. Child Development, 2011. 82(5): p. 1501–1517. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, & Woolard J, Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 2008. 44(6): p. 1764–1778. [DOI] [PubMed] [Google Scholar]
- 36.Wolf RC, Sambataro F, Lohr C, Steinbrink C, Martin C, Vasic N, Functional brain network abnormalities during verbal working memory performance in adolescents and young adults with dyslexia. Neuropsychologia, 2010. 48(1): p. 309–318. [DOI] [PubMed] [Google Scholar]
- 37.Fiez JA, Petersen SE , Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences of the United States of America 1998. 95(3): p. 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jobard G, Crivello F, Tzourio-Mazoyer N , Evaluation of the dual route theory of reading: A meta-analysis of 35 neuroimaging studies. NeuroImage, 2003. 20(2): p. 693–712. [DOI] [PubMed] [Google Scholar]
- 39.Finn AS, Minas JE, Leonard JA, Mackey AP, Salvatore J, Goetz C, West MR, Gabrieli CFO, & Gabrieli JDE , Functional brain organization of working memory in adolescents varies in relation to family income and academic achievement. Developmental Science, 2017. 20(5): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler JC, Perry C, Ma-Wyatt A, Ladner D, & Schulte-Koṙne G , Developmental dyslexia in different languages: Language-specific or universal? Journal of Experimental Child Psychology, 2003. 86(3): p. 169–193. [DOI] [PubMed] [Google Scholar]
- 41.Rack JP, Snowling MJ & Olson RK , The nonword reading deficit in developmental dyslexia: a review. Reading Research Quarterly, 1992. 27(1): p. 28–53. [Google Scholar]
- 42.Grill-Spector K, Kourtzi Z, & Kanwisher N , The lateral occipital complex and its role in object recognition. Vision Research, 2001. 41(10-11): p. 1409–1422. [DOI] [PubMed] [Google Scholar]
- 43.Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, &Gore JC., Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 2002. 52(2): p. 101–110. [DOI] [PubMed] [Google Scholar]
- 44.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, & Eden GF, Development of neural mechanisms for reading. Nature Neuroscience, 2003. 6(7): p. 767–773. [DOI] [PubMed] [Google Scholar]
- 45.Weinera KS, & Zilles K, The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia, 2016. 83: p. 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centanni TM, Norton ES, Ozernov-Palchik O, Park A, Beach SD, Halverson K, Gaab N, & Gabrieli JDE, Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. Neuroimage Clinical 2019. 22: p. 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble KG, Wolmetz ME, Ochs LG, Farah MJ, & McCandliss BD, Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 2006. 9(6): p. 642–654. [DOI] [PubMed] [Google Scholar]
- 48.Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, Birn RM, The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage, 2013. 78: p. 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz-Kraus T, Toro-Serey C, & DiFrancesco M, Increased Resting State Functional Connectivity in the Cingulo-Opercular Cognitive-Control Network after Intervention in Children with Reading Difficulties PLOS ONE, 2015. 10(7): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nachshon O, Farah R, & Horowitz-Kraus T, Decreased Functional Connectivity Between the Left Amygdala and Frontal Regions Interferes With Reading, Emotional, and Executive Functions in Children With Reading Difficulties. Frontiers in Human Neuroscience, 2020. 14: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friston KJ, Williams S, Howard R, Frackowiak RS, & Turner R, Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine, 1996. 35(3): p. 346–355. [DOI] [PubMed] [Google Scholar]
- 52.Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, & Milham MP , A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 2013. 76: p. 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sporns O, Graph theory methods: applications in brain networks. Dialogues of Clinical Neuroscience, 2018. 20(2): p. 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown L, Sherbenou RJ, & Johnsen SK , Test of Nonverbal Intelligence 4th Edition (TONI-4). 2010: Pearson Assessments. [Google Scholar]
- 55.Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Wolf M, Whitfield-Gabrieli S, & Gabrieli JD, Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex, 2012. 22(4): p. 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner RK, Torgesen JK, Rashotte CA, & Pearson NA, CTOPP-2: Comprehensive Test of Phonological Processing–Second Edition. 2013, Austin, TX: Pro-Ed. [Google Scholar]
- 57.Torgesen JK, Wagner RK, & Rashotte CA, TOWRE–2 Test of Word Reading Efficiency–Second Edition. 2012, Austin, TX: Pro-Ed. [Google Scholar]
- 58.Wagner RK, Torgesen JK, Rashotte CA, & Pearson NA, Test of Silent Reading Efficiency and Comprehension (TOSREC). 2010, Austin, TX: Pro-Ed. [Google Scholar]
- 59.Schrank FA, Mather N, & McGrew KS , Woodcock-Johnson IV Tests of Achievement. 2014, Rolling Meadows, IL: Riverside [Google Scholar]
- 60.Wiederholt JL, & Bryant BR, GORT-5: Gray Oral Reading Tests–Fifth Edition. 2012, Austin, TX: PRO-ED. [Google Scholar]
- 61.Conners CK, Conners Self-Report 3rd Edition (Conners 3). 2008: MHS Assessments [Google Scholar]
- 62.Romeo RR, Christodoulou JA, Halverson KK, Murtagh J, Cyr AB, Schimmel C, Chang P, Hook PE, & Gabrieli JDE, Socioeconomic Status and Reading Disability: Neuroanatomy and Plasticity in Response to Intervention. Cerebral Cortex 2018. 28(7): p. 2297–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barratt W, Barratt simplified measure of social status (BSMSS). Indiana State University; 2006. [Google Scholar]
- 64.Conners CK, Conners Continuous Performance Test 3rd Edition. 2014: MHS Assessments [Google Scholar]
- 65.Manly T, Anderson V, Crawford J, George M, & Robertson IH, Test of Everyday Attention for Children, Second Edition (TEA-Ch2). 2016: Pearson Assessments [Google Scholar]
- 66.Delis DC, Kaplan E, & Kramer JH, Delis-Kaplan Executive Function System (D-KEFS). 2001: Pearson Assessments [Google Scholar]
- 67.Gioia GA, Isquith PK, Guy SC, & Kenworthy L, Behavior Rating Inventory of Executive Function (BRIEF). 2015: PAR, Inc. [Google Scholar]
- 68.Byars AW, Holland SK, Strawsburg RH, Schmithorst VJ, Dunn RS, & Ball WS, Practical Aspects of Conducting Large-Scale fMRI Studies in Children. Journal of Child Neurology, 2002. 17(12): p. 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaywitz SE, Dyslexia. New England Journal of Medicine, 1998. 338(5): p. 307–312. [DOI] [PubMed] [Google Scholar]
- 70.Shaywitz SE, & Shaywitz BA , Dyslexia (specific reading disability). Biological Psychiatry, 2005. 57(11): p. 1301–1309. [DOI] [PubMed] [Google Scholar]
- 71.Whitfield-Gabrieli S, & Nieto-Castanon A, Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity 2012. 2(3): p. 125–141. [DOI] [PubMed] [Google Scholar]
- 72.Friston KJ, Frith CD, Frackowiak RS, & Turner R, Characterizing dynamic brain responses with fMRI: a multivariate approach. NeuroImage, 1995. 2(2): p. 166–172. [DOI] [PubMed] [Google Scholar]
- 73.Behzadi Y, Restoma K, Liau J, & Liua TT, A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 2007. 37(1): p. 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baria AT, Baliki MN, Parrish T, & Apkarian AV, Anatomical and Functional Assemblies of Brain BOLD Oscillations. Journal of Neuroscience 2011. 31(21): p. 7910–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox MD, Corbetta M, Snyder AZ, Vincent JL, & Raichle ME, Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. PNAS, 2006. 103(26): p. 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fassbender C, Mukherjee P, & Schweitzer JB , Minimizing Noise in Pediatric Task-Based functional MRI; Adolescents with Developmental Disabilities and Typical Development. Neuroimage, 2017. 149: p. 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fair D, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang Y-F, Mostofsky S, Castellanos FX, & Milham MP., Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience, 2012. 6: p. 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


