Abstract
Introduction:
For well over 100 years, meningococcal disease due to serogroup A Neisseria meningitidis (MenA) has caused severe epidemics globally, especially in the meningitis belt of sub-Saharan Africa.
Areas covered:
The article reviews the background and identification of MenA, the global and molecular epidemiology of MenA, and the outbreaks of MenA in the African meningitis belt. The implementation (2010) of an equitable MenA polysaccharide-protein conjugate vaccine (PsA-TT, MenAfriVac) and the strategy to control MenA in sub-Saharan Africa is described. The development of a novel multi-serogroup meningococcal conjugate vaccine (NmCV-5) that includes serogroup A is highlighted. The PubMed database (1996–2019) was searched for studies relating to MenA outbreaks, vaccine, and immunization strategies; and the Neisseria PubMLST database of 1755 MenA isolates (1915–2019) was reviewed.
Expert opinion:
Using strategies from the successful MenAfriVac campaign, expanded collaborative partnerships were built to develop a novel, low-cost multivalent component meningococcal vaccine that includes MenA. This vaccine promises greater sustainability and is directed toward global control of meningococcal disease in the African meningitidis belt and beyond. The new WHO global roadmap addresses the continuing problem of bacterial meningitis, including meningococcal vaccine prevention, and provides a framework for further reducing the devastation of MenA.
Keywords: Neisseria meningitidis, serogroup A, conjugate vaccine, immunization strategies
1. Introduction
1.1. N. meningitidis: background and discovery
Neisseria meningitidis is a fastidious, aerobic gram-negative diplococcus of the bacterial family Neisseriaceae, that can be encapsulated or not, and causes disease in the human host only [1]. Invasive meningococcal disease occurs worldwide and usually presents clinically as meningitis with or without septicemia (often with a petechial or purpuric rash) resulting in significant morbidity and mortality. Early symptoms of meningococcal disease can be mild and nonspecific, or can be fulminant (hours), making diagnosis and treatment challenging. Therefore, prophylaxis via vaccination is the key prevention strategy. The introduction of meningococcal vaccines, first as purified capsular polysaccharide vaccines and now as capsular polysaccharide-protein conjugate vaccines (MenC, MenA, or MenACWY) and outer membrane protein-based vaccines (MenB), has significantly controlled outbreaks and reduced the morbidity and mortality associated with meningococcal disease. However, challenges of defining meningococcal disease burden (surveillance limitations and natural variations in meningococcal disease epidemiology), vaccine affordability, vaccine introduction, and sustainable vaccine protection remain for many, especially in low- and middle-income countries. This review is focused on serogroup A N. meningitidis and vaccine prevention.
The first reports of meningococcal disease were from Geneva, Switzerland in 1805, and New Bedford, Massachusetts in 1806 [2,3]. Both reports described epidemic outbreaks, with rash, rapid onset of fever, and central nervous system (CNS) inflammation-causing high mortality. Epidemic meningococcal disease was subsequently reported throughout the United States and Europe after these first reports. Epidemics in the meningitis belt of sub-Saharan Africa were first noted ~1900; Gordon and Murray in 1915 developed the first meningococcal classification system (I, II, III, and IV); and in 1918, a similar system (A, B, C) was developed by Nicolle, Debains, and Jouan [4]. During WWI and in 1928 to 1930 and 1941, significant US and worldwide epidemics (serogroup A) occurred. In 1935, Henry Scherp and Geoffrey Rake first identified the meningococcal capsular material of serogroup A meningococci as a polysaccharide [5].
Meningococcal biology and pathogenesis can be defined by stain to strain differences in N. meningitidis virulence, transmission and colonization dynamics among humans, and differences in human susceptibility (e.g. bactericidal antibody, age, complement deficiencies) to invasive meningococcal disease. Each of these areas has implications for vaccine prevention strategies. Although the carriage rates are falling, meningococci historically have colonized the nasopharyngeal mucosa of about 3-25% of the worldwide population [6]. The rates of carriage are generally highest among adolescents and young adults. Transmission among individuals is by direct contact with respiratory secretions or by the inhalation of large respiratory droplet nuclei; the latter may increase in low humidity environments such as in the dry season of sub-Saharan Africa. Other factors influence meningococcal transmission and colonization including crowding and close contact, exposure through migration and travel, season, active and passive smoking, and respiratory co-infections [7]. Invasive disease usually occurs 1–10 days after acquisition. The relationship between nasopharyngeal colonization and infection of N. meningitidis is complex. While carriage does not necessarily increase the risk of disease and often does not directly correlate with epidemic outbreaks, the case-to-carrier ratio is a useful marker. For example, the ratio is much higher for serogroup A (ST-5) and serogroup C (ST-11) than for serogroup X meningococci, presumably reflecting differences in virulence.
Twelve serogroups of N. meningitidis have been identified, based on genetic analysis and structural differences of the capsular polysaccharides. However, only six of these serogroups, A, B, C, W, Y, X, are responsible for causing the majority of invasive meningococcal disease [8]. The polysaccharide capsule is the main virulence factor of N. meningitidis. Serogroups B and C capsular polysaccharides are sialic acid homopolymers of (2→8) and (2→9) linkages, respectively; while serogroups Y and W are alternating units of D-glucose or D-galactose and sialic acid, respectively. Serogroup X expresses (1→4) linked N-acetyl-D-glucosamine 1-phosphate capsules [9]. Serogroup A N. meningitidis uniquely expresses a homopolymer (1→6) N-acetyl mannosamine-1-phosphate capsule [10]. Unencapsulated strains have rarely been found to cause invasive infections, but are frequently recovered in the pharynx of asymptomatic carriers.
With advancements in molecular pathogenesis and whole genome sequencing, additional outer membrane surface structures and specific genotypes (clonal complexes such as ST-11, ST-5, ST-23) have also been linked to meningococcal virulence. The meningococcus has a single chromosome of 2.1–2.3 mega bases. Multilocus sequence typing (MLST), which involves DNA sequencing of portions of seven housekeeping genes and whole genome sequencing are now the tools utilized for molecular epidemiology and characterization of N. meningitidis [11] and are also used to identify molecular markers such as capsular switching. The first whole genome sequences were of serogroup B (MC58, ST-32) and serogroup A (Z2491, ST-4) strains and were reported in 2000 [i]. N. meningitidis has the capacity to acquire, at high frequency, DNA through horizontal gene transfer and homologous recombination. In addition, there are several mechanisms that have been defined (e.g. genetic slipped strand mispairing) that allow the pathogen to alter its antigenic profile. This variability does create challenges for meningococcal vaccine development, vaccine efficacy, and long-term effectiveness. For example, the allelic replacement of capsular biosynthesis genes, e.g. capsular switching [12,13], can reduce meningococcal vaccine effectiveness and influence vaccine strategies.
1.2. Epidemiology of serogroup A N. meningitidis and past epidemics
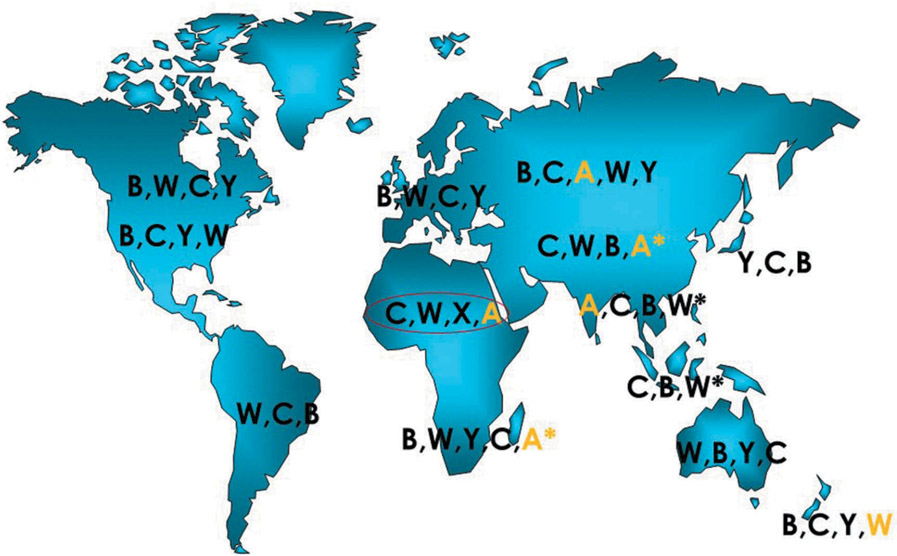
The current epidemiology of N. meningitidis by serogroup is shown in Figure 1. Serogroup A N. meningitidis has been associated with the highest incidence and the largest epidemics of meningococcal disease, especially meningococcal meningitis. In the last century, prior to and during WW II, large serogroup A outbreaks occurred in the US, Europe, and Asia, South America as well as Africa, were cyclical in nature, having peaks and troughs every 5–12 years. These large serogroup A outbreaks dominated meningococcal disease throughout the world in the first half of the twentieth century but disappeared from the US, Western Europe, and Japan after WW II for reasons that are not well understood. They continued in parts of Asia, South America, and sub-Saharan Africa in the second half of the twentieth century. For example, prior to and during WW II, the incidence of meningococcal disease in the US was ~3-4/100,000 with episodic serogroup A outbreaks raising the incidence to 8-17/100,000 [1]. Following WWII, incidence fell in the US below 2/100,000. In Japan, about 4000 cases of ‘epidemic cerebrospinal meningitis’ were reported at the end of WWII. However, by 1969, the incidence fell to less than 100 cases per year, in the absence of meningococcal vaccines [14].
Figure 1.
Global epidemiology of meningococcal disease by serogroup, including serogroup A. Meningococcal serogroups are listed in the order of the estimated incidence (2010–2019) causing disease; however, in some areas, (*) data are quite limited. Serogroups are linked to 12 major genetic clonal complexes currently causing almost invasive meningococcal disease (modified from Stephens, DS, Neisseria meningitidis in Mandell, Douglas, Bennett Principles & Practices of Infectious Diseases, Bennett, Dolin, Blaser, eds.).
In China, there were five epidemics of serogroup A meningococcal disease between 1938 and 1977. The peak incidence was reported as 403/100,000 in 1967 [15], with declining rates after the introduction of the MenA polysaccharide vaccine in the 1980 s. Mongolia and Nepal experienced similar epidemics in the 1960 s and 1970 s [16]. India has also had several epidemics of serogroup A disease, although the true burden and magnitude of meningococcal disease in India is difficult to fully characterize. Recent serogroup A outbreaks have been documented in India in 2005 and 2009 [17]. A significant outbreak in the Philippines attributable to serogroup A occurred in 2004 and 2005, with the WHO reporting 98 cases (33% mortality) [18]. In addition to sub-Saharan Africa (see below), cases of serogroup A meningococcal disease are reported from Russia, Kyrgyzstan, Slovenia, Malta, Turkey, and China in 2015, 2016, 2017, 2018, or 2019.
Molecular and genotypic analyses of prior pandemic strains of serogroup A demonstrated that only a few clonal lineages are responsible for global spread. There are over 1700 serogroup A strains in the current PubMLST database [19]. Serogroup A strains are genetically distinct from other meningococci and appear to have evolved from a common ancestor in the nineteenth century. Morelli et al. described nearly identical distinct chromosomal loci of subgroups III (ST-5) and IV (ST-4) from isolates from 1966 to 1917 [20]. Serogroup A global pandemics have been caused over the last century by three major ST clonal complexes (cc) (e.g., ST-1, ST-4, ST-5). For example, ST-1 and ST-4 cc strains are linked to serogroup A disease in the USA in 1915, 1917, 1930, 1937, and 1943, in the UK in 1941, and the African meningitis belt in the 1960’s. Three ST-5 pandemic waves arising in China spread to Russia, Middle East, Africa, and globally were recorded in the 1960 s-1970 s, 1980 s and into the 1990’s. Epidemiological surveillance has demonstrated that these serogroup A clonal complexes were distributed in specific geographic areas but could emerge as an outbreak or large epidemics [21].
Achtman’s group linked the epidemics of China in the mid-1960 s and epidemics in Moscow (1969–1971), Norway (1973), Finland (1975), and Brazil (1970 s) to the same lineage, ST-5 complex/subgroup III [22,23]. The same ST-5 complex/subgroup III bacteria then caused new outbreaks, a decade later, in China, Nepal, and among the Hajj pilgrims. In the mid-1990 s, the ST-5 complex/subgroup III was also linked to outbreaks in Sudan, Chad, Ethiopia, Kenya, Tanzania, Zambia, and the Central African Republic [24]. In the 1990 s, two ST-5 cc serogroup A MLSTs, ST-5, and ST-7, were responsible for the global serogroup A pandemics, African meningitis belt outbreaks, endemic serogroup A meningococcal disease in the meningitis belt, and the worldwide cases of serogroup A disease. However, there is continued evolution within these three major serogroup A clonal complexes. ST-7 replaced ST-5 within the ST-5 cc, 1994–2013, ST-2859 another member of the ST-5 cc emerged 2006–2017; and ST-75 and ST-3349 of cc ST-1 emerged to cause serogroup A disease and carriage in Russia 2006–2019 and 2002–2011, respectively.
2. The history of serogroup A meningococcal outbreaks and vaccine development
2.1. Serogroup A meningococcal outbreaks in the African meningitis belt
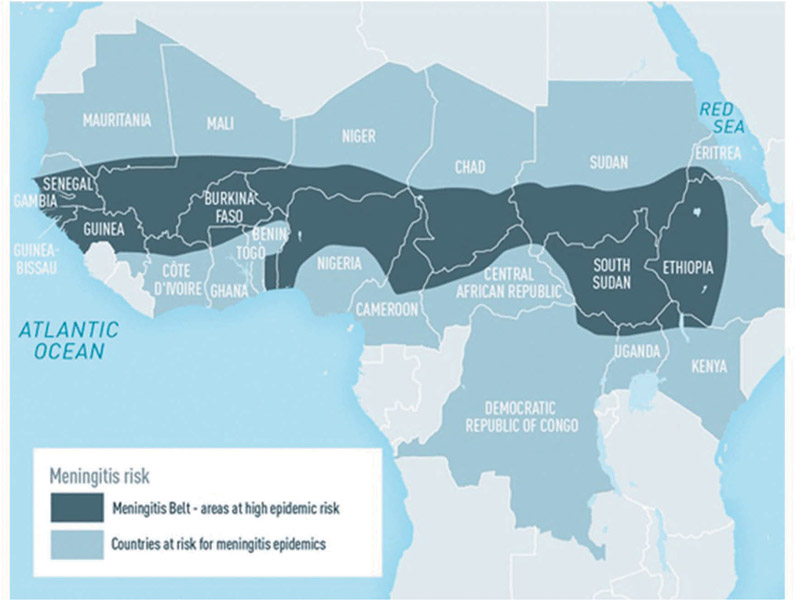
The ‘meningitis belt’ in Africa has been particularly affected by serogroup A meningococcal disease. In sub-Saharan countries of Africa, extending from Senegal and The Gambia in the west to Ethiopia, Eritrea, and northern Kenya in the east (Figure 2), large periodic epidemics of serogroup A meningococcal disease with high morbidity and mortality occurred during the dry season (December to June, with peak activity from February to March) and ended during the intervening rainy season, every 5–12 years since 1905 [1]. Virulent meningococci were likely first introduced into the region by returning Hajj pilgrims around the turn of the century.
Figure 2.
The meningitis belt of sub-Saharan Africa, from Meningococcal Disease, Chapter 4–2018 Yellow Book Centers for Disease Control and Prevention. Disease data source: World Health Organization. International Travel and Health. Geneva, Switzerland: 2012.
The epidemic patterns in the belt are linked to environmental factors, such as climatic changes (dry season, ‘dust’, and winds of the Harmattan), co-infections, poor sanitation, and living conditions, household crowding, travel, and population displacements and specific population immunological susceptibility [25-27]. Respiratory droplets that facilitate meningococcal transmission have higher density in the dry season. While serogroup W, X, and C also cause epidemic outbreaks in sub-Saharan populations, serogroup A epidemic meningococcal disease was a major public health threat in meningitis belt and other areas of the developing world. The size of serogroup A epidemics in the region was enormous. In major African epidemics, the attack rate ranged from 100 to 800 per 100,000/population during epidemics, and individual communities reported rates as high as 1 per 100 [1]. Between 1988 and 1997, 704,000 cases and more than 100,000 deaths were reported, in 1996–1997 > 300,000 cases and 30,000 deaths, the largest serogroup A epidemic year ever recorded with spread to countries south of the belt (e.g., Rwanda, Burundi, Tanzania, Zambia, and the Central African Republic) [28,29]; between 1998 and 2002, 224,000 cases, and in 2009, 88,199 meningococcal meningitis cases occurred in the belt [1]. However, the true disease burden in sub-Saharan Africa was likely to be greater because seasonally associated endemic rates of disease were often 5-10/100,000. Routine surveillance systems often break down during epidemics, and death may occur before reaching a health center.
Meningococcal outbreaks have been documented during Hajj pilgrimages such as the serogroup A ST-5 outbreak of 1987, and thought to be related to a high burden of meningococcal carriage in pilgrims, as well as increased transmission due to crowding, reduced hand hygiene, and other respiratory tract infections. In a surveillance study conducted in Mecca in 1992, the meningococcal carriage rate was documented at 86%, with a case–fatality ratio (CFR) of 14.7% among bacteriologically confirmed cases [30]. Hajj pilgrims are required to receive meningococcal ACWY vaccination prior to traveling, and those originating from the African meningitis belt must also receive ciprofloxacin chemoprophylaxis.
2.2. The history and development of the serogroup A polysaccharide-conjugate vaccine
The first effective Nm vaccine was developed by Gotschlich and colleagues in the 1960s. His group was able to develop a new approach to purify the high molecular weight polysaccharides of meningococcus, and demonstrated that the product was both safe and immunogenic when used as a vaccine [31]. However, the lack of T-cell-dependent immunity was a limitation of polysaccharide vaccines, as they do not produce long-term immunologic memory, and have less immunogenicity in younger children. The development of meningococcal conjugate vaccines in the 1990 s helped to overcome this limitation. A monovalent group C conjugate vaccine was the first to introduced into routine immunization schedules in 1999, in the UK. The incidence of group C disease fell by 80%, and the ages covered was quickly extended to include 20–24 year old individuals [32].
Meanwhile, in Sub-Saharan Africa, severe meningococcal epidemics (mostly caused by group A strains) continued to cause high mortality in the population. Initially, only polysaccharide vaccines were available: bivalent group A and C (MPSV2) and trivalent group A, C, and W (MPSV3). As described above, these polysaccharide vaccines are poorly immunogenic in infants and young children, and do not induce T-cell dependent immunity. However, since the early 2000s, great strides have been made in development of serogroup A containing meningococcal conjugate vaccines. The successful introduction beginning in 2010 of the serogroup A meningococcal conjugate vaccine, MenAfriVacR (Serum Institute of India Ltd., Pune, India), in sub-Saharan Africa has radically altered serogroup A meningococcal disease in this region. Pharmaceutical and commercial companies had little incentive to invest in the development of or to provide a group A meningococcal conjugate vaccine for this resource-limited area. In order to create solutions for development of a lower cost vaccine, the World Health Organization (WHO) initiated a feasibility study in 1999, which later served as the foundation for an application to the Bill and Melinda Gates Foundation [33]. Seventy million dollars were allocated from the Bill and Melinda Gates Foundation to the WHO and the Program for Appropriate Technology in Health (PATH) to create a meningococcal group A conjugate vaccine. Investigators at the Center for Biologics Evaluation and Research in Bethesda, MD developed and made available a novel conjugation method to create a highly immunogenic meningococcal serogroup A polysaccharide (Mn A PS) – tetanus toxoid (TT) conjugate vaccine (PsA-TT) [34], called MenAfriVac. Another novel aspect of this vaccine is that it was the first to be approved for use in a controlled-temperature chain [35]. Unlike traditional cold chain dependent vaccines, MenAfriVac can be stored at a broader range of temperatures (under monitored conditions). Manufacturing of this vaccine was then completed at the Serum Institute of India, Ltd [36]. Importantly, the final product was also very affordable, costing less than 0.50 USD US dollars per dose. In 2010, a mass vaccination campaign began in Burkina Faso, Mali, and Niger, among persons aged 1 to 29 years old [37], with the goal to expand to all countries in the African meningitis belt. Using the mass vaccination strategy that maximizes herd protection, the decline in group A meningitis cases was dramatic. By 2015, surveillance data from nine countries (Benin, Burkina Faso, Chad, Cote d’Ivoire, Ghana, Mali, Niger, Nigeria, and Togo) showed that the incidence of suspected meningitis cases had declined by 57% in vaccinated versus unvaccinated groups, and the incidence of confirmed group A disease in fully vaccinated populations was reduced by more than 99% [38]. By 2017, only two cases of serogroup A meningitis were detected in the region [39]. Importantly, follow-up studies have demonstrated sustained MenA serum bactericidal antibody (SBA) levels after a single dose of PsA-TT up to 4 years later, at a level that is considered protective against MenA disease [40]. Surveillance through programs such as MenAfriNet shows the virtual elimination of serogroup A meningococcal disease in these countries in the years after the mass campaigns.
As anticipated, based on the herd protection effects of polysaccharide-protein conjugate vaccines (via reduced human to human transmission), the dissemination of MenAfriVac in mass vaccination campaigns also was shown to reduce the carriage of serogroup A. Kristiansen et al. evaluated the carriage rates of serogroup A before and after the mass vaccination campaign with MenAfriVac in Burkina Faso. The authors described elimination of N. meningitidis group A carriage in both vaccinated and unvaccinated persons after the campaign, indicating a strong herd protection effect [41]. Similar findings were described in study conducted in Chad, which evaluated meningitis disease incidence and carriage rates from 2009 to 2012 [42]. The Bill and Melinda Gates Foundation and WHO continue to support routine MenAfriVac immunization and surveillance networks (MenAfriNet) in the meningitis belt.
3. New vaccines for serogroup A and goals for sustainability and global control
Although the MenAfriVac campaign with ~350 M doses administered across sub-Saharan Africa has been remarkably successful in virtually eliminating serogroup A in the meningitis belt, outbreaks of serogroups C, W, Y, and X continue in the region. Thus, new meningococcal vaccines are under development (summarized in Table 1) to retain serogroup A protection in the population and target these other important meningococcal serogroups in combination. A phase 1 study was recently conducted to evaluate the safety and immunogenicity of a pentavalent meningococcal protein conjugate vaccine that contained serogroups A, C, Y, W, and X conjugated to 5 ug TT (A, X) or 5 ug CRM197 (C, Y, W) and designated NmCV-5 [43]. Participants were randomly assigned to receive either the adjuvanted NmCV-5 (adjuvanted with aluminum-phosphate), non-adjuvanted NmCV-5, or the control, the quadrivalent meningococcal conjugate vaccine MenactraR (A, C, Y, W conjugated to diphtheria toxoid, Sanofi). The results showed that NmCV-5 adjuvanted and non-adjuvanted immunizations were well-tolerated and produced serum antibodies for all five serogroups that were predicted to confer individual protection. A phase 2 trial is being completed in Mali to evaluate the NmCV-5 vaccine (adjuvanted and non-adjuvanted) versus Menactra in toddlers, aged 12–16 months (ClinicalTrials.gov Identifier NCT03295318). A large phase 3 study, with a targeted goal of 1800 participants, is also underway in Mali and the Gambia (ClinicalTrials.gov Identifier: NCT03964012) to evaluate the immunogenicity of NmCV-5 versus Menactra. Additional phase III trials are planned in India and Africa in adults or infants and will compare NmCV-5 to other licensed meningococcal conjugate vaccines. The results of the clinical trials will assess the safety and efficacy of NmCV-5 in the population where it will be deployed and is the next important step in meningococcal disease control in the region. Other meningococcal vaccines in development containing serogroup A polysaccharide-protein conjugates include ABCWY pentavalent vaccines in clinical trials (ClinicalTrials.gov Identifiers: NCT03135834 and NCT02212457).
Table 1. New Vaccines Containing Serogroup A:
Novel vaccines that are currently in Phase II–III trials that provide MenA coverage. The trials target different age groups and different geographical regions.
| New Vaccines Containing Serogroup A | |||
|---|---|---|---|
| Vaccine Name | Meningococcal Serogroups Covered | Clinical Trial Phase | Targeted Age Group |
| NmCV-5 (pentavalent meningococcal protein conjugate vaccine, Serum Institute of India Pvt. Ltd.) | A, C, Y, W, X | Phase II: Mali | 12-16 months old |
| Phase III: Mali and the Gambia | 2-10 years old; 11–17 years old; 18–29 years old | ||
| Phase III: India, Africa | 2-10 years old; 11–17 years old; 18–29 years old | ||
| MenACWY (Oligosaccharide diptheria CRM-197 conjugate, GlaxoSmithKline) combined with meningococcal (group B) multicomponent recombinant (MenABCWY) | A, C, W, Y | Phase IIb: US, Finland, Poland | 10-18 years old |
| MenABCWY (bivalent RLP2086-containing pentavalent vaccine, Pfizer) | A, B, C, W, Y | Phase III: US, Czechia, Finland, Poland | 10-26 years old |
| Tritanrix™-HepB/Hib-MenAC (GlaxoSmithKline) | A, C | Phase III: Thailand | 15-24 months old |
There have also been recent research efforts to produce novel homogeneous glycoconjugate meningococcal vaccines. Instead of isolating carbohydrate fragments and carrier proteins from cultured meningococci, this method produces meningococcal antigens by chemical or chemoenzymatic synthesis, thus better controlling the carbohydrate produced [44]. This method was utilized by Harale et al. to create a monomer unit of the serogroup A capsular polysaccharide, which was then conjugated to a tetanus toxoid [45]. Future work will need to evaluate these novel products in human trials.
The World Health Organization organized a taskforce in July 2018 to address the continuing problem of global meningitis, with a roadmap planned to reduce cases and deaths from vaccine-preventable meningitis [46]. The revised global roadmap, ‘Defeating meningitis by 2030,’ was reviewed in April and October 2019 at the WHO SAGE meetings. Using the successful model of discovery and implementation of the MenAfriVac in the meningitis belt, WHO and partner organizations are now replicating the model to create other affordable vaccines (e.g. the meningococcal vaccine to cover serogroups A, C, W, X, Y, conjugate). Parties involved include WHO, PATH, the CDC, UNICEF, the London School of Hygiene and Tropical Medicine, the Meningitis Research Foundation, the Bill and Melinda Gates Foundation, and the Norwegian Institute of Public Health. The taskforce goals are focused on the main organisms responsible for global bacterial meningitis, which includes N. meningitidis (Nm). The plan highlights the overlapping pillars that will be essential to reducing the prevalence of meningitis: disease surveillance, creation of a global genome network for meningitis pathogens, prevention and epidemic control, appropriate and accurate diagnosis and treatment, advocacy and engagement, and support for survivors of meningitis. The plan includes appropriate and tailored immunization strategies to achieve and maintain high vaccination coverage against Nm (including serogroup A) in all countries, and reinforcing and complementing existing immunization strategies, including those targeting special risk groups.
The taskforce goals have been summarized in Figure 3, with a focus on Nm. Briefly, by 2021, the goal is to introduce vaccination against MenA in routine immunization programs completed in ≥18 meningitis belt countries. By 2022, a revised policy (if necessary) will be published on antibiotic prophylaxis during meningococcal meningitis outbreaks in the African meningitis belt, with possibly time-limited considerations (in coordination with introduction and availability of multivalent Nm conjugate vaccines). By 2022, modeling research studies on multivalent meningococcal conjugate vaccination strategy will be completed and the results disseminated with open access to support vaccine introduction strategies. By 2023, introduction of vaccination against Nm serogroups ACWY/ACWXY in routine immunization programs will be started in ≥5 meningitis belt countries and the evaluation of vaccination strategies for use of multivalent meningococcal conjugate vaccines to achieve herd protection will be targeted. By 2024, cluster-randomized studies and/or carriage studies on multivalent meningococcal conjugate to inform vaccination strategy will be completed and published. By 2026, locally relevant meningococcal disease vaccination strategies, including Nm multivalent conjugate vaccines and/or Nm serogroup B vaccines as relevant, will be introduced in all countries in alignment with epidemiological evidence and according to regional policy started in ≥10 countries. Finally, by 2030, vaccination against Nm serogroups ACWY/ACWXY implemented in countries in the meningitis belt as indicated and agreed with countries (target coverage as defined by Immunization Agenda 2030 and regional prioritization) will be achieved. This collaborative partnership between both private and public sectors will be essential to achieve the vision ‘towards a world free of meningitis.’
Figure 3.
A summary of the meningitis task force goals and timeline, with a focus on Nm.
4. Expert opinion
Historically, serogroup A N. meningitidis caused severe and devastating global epidemics. This was especially true in the meningitis belt of sub-Saharan Africa. In response to a significant need for a new affordable vaccine against MenA, in a setting with little financial incentives for industry, an alternative pathway for development and licensure was formed via new public and private partnerships. The creation of novel development pathways through these global collaborations with the strong support of the countries effected is a remarkable story that led to the creation of the PsA-TT (MenAfriVac). This vaccine has been successfully implemented in 22 countries of the meningitis belt as part of mass immunization campaigns, but only included in eight countries as part of the routine Expanded Programme on Immunization (EPI) schedule. The herd protection provided by this vaccine due to the mass vaccination implementation strategy has been a major component of its remarkable effectiveness.
The sustainability and additional building on this successful model are the next key challenges. Can the partnerships between the private and public sectors be sustainable to keep MenA essentially eliminated from sub-Saharan Africa? Although the incidence of MenA disease in the vaccinated countries of meningitis belt is now quite low and serogroup A outbreaks have currently ceased, sustained surveillance, full introduction of the vaccine in routine immunization programs and other efforts must be maintained to fully control MenA disease and prevent recurrence of outbreaks both in Africa and globally. Novel vaccines are now in the pipeline, developed at an affordable cost, to broaden protection against serogroup A and other serogroups causing N. meningitidis invasive disease and address the broader issues of bacterial meningitis. Leaders and experts in the most affected countries must be engaged in order to effectively guide and implement these plans. Continued surveillance programs, prevention, and educational will be essential to achieve the goal of the global control of N. meningitidis and the other bacterial meningitis pathogens.
Article highlights.
Significant and devastating outbreaks of serogroup A meningococcal disease caused high morbidity and mortality globally, especially in the meningitis belt of Africa from 1905-2010.
Through a unique partnership with both private and public sectors, a highly immunogenic serogroup A polysaccharide conjugate vaccine (PsA-TT, MenAfriVac) was created and implemented in the meningitis belt 2010-present.
Although the MenAfriVac campaign has been highly successful and has virtually eliminated serogroup A meningitis from sub-Saharan Africa, the challenge is to identify and prevent other serogroups of N. meningitidis in the belt, as well as to maintain immunity and protection against serogroup A.
A new low-cost multivalent (PsA-TT, PsC-CRM197, PsY-CRM197, PsW-CRM197, PsX-TT; NmCV-5) meningococcal conjugate vaccine is being developed and tested in clinical trials that not only cover serogroup A but also protects against other serogroups which continue to cause outbreaks in sub-Saharan Africa.
The WHO and partners have developed a roadmap ‘Defeating meningitis by 2030’ to further reduce the global burden of meningitis due to MenA and the acute bacterial meningitis pathogens.
Acknowledgments
The authors would like to thank Dianne Watson for her contributions to manuscript preparation and to the work of the WHO “Defeating Meningitis by 2030” Taskforce.
Funding
This work was supported by Emory University and NIH training grants (VTP-T32 T32AI074492)
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Stephens DS. Neisseria meningitidis. In: editor. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, updated edition,9th. Philadelphia, PA: Elsevier/Saunders; 2020. p. 2585–2607 [Google Scholar]
- 2.Viesseux M Memoire sur le maladie qui à regne à Genève au printemps de 1805. J Med Chir Pharmacol. 1805;11:243. [Google Scholar]
- 3.Danielson L, Mann E. Letter to medical and agricultural registrar. Boston. 1806. [Google Scholar]
- 4.Trotter CL, Gay NJ, Edmunds WJ. The natural history of meningococcal carriage and disease. Epidemiol Infect. 2006. June;134(3):556–566. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright KA, Stuart JM, Jones DM, et al. The Stonehouse Survey: nasopharyngeal Carriage of Meningococci and Neisseria Lactamica. Epidemiol Infect. 1987. December;99(3):591–601. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens DS. Uncloaking the Meningococcus: dynamics of Carriage and Disease. Lancet. 1999. March 20;353(9157):941–942. . [DOI] [PubMed] [Google Scholar]
- 7.Jones GR, Christodoulides M, Brooks JLA, et al. Dynamics of Carriage of Neisseria Meningitidis in a Group of Military Recruits: subtype Stability and Specificity of the Immune Response Following Colonization. J Infect Dis. 1998. August 1;178(2):451–459. . [DOI] [PubMed] [Google Scholar]
- 8.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic Meningitis, Meningococcaemia, and Neisseria Meningitidis. Lancet. 2007. June 30;369(9580):2196–2210. . [DOI] [PubMed] [Google Scholar]
- 9.Swartley JS, Liu L-J, Miller YK, et al. Characterization of the Gene Cassette Required for Biosynthesis of the (A1→6)-LinkedN-Acetyl-d-Mannosamine-1-Phosphate Capsule of Serogroup A Neisseria Meningitidis. J Bacteriol. 1998. March 15;180(6):1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzeng Y-L, Noble C, Stephens DS. Genetic Basis for Biosynthesis of the (A1→4)-Linked N-Acetyl-d-Glucosamine 1-Phosphate Capsule of Neisseria Meningitidis Serogroup X. Infect Immun. 2003. December;71(12):6712–6720. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiden MC, Bygraves JA, Feil E, et al. Multilocus Sequence Typing: A Portable Approach to the Identification of Clones within Populations of Pathogenic Microorganisms. Proc Natl Acad Sci U S A. 1998. March 17;95(6):3140–3145. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartley JS, Marfin AA, Edupuganti S, et al. Capsule Switching of Neisseria Meningitidis. Proc Natl Acad Sci U S A. 1997. January 7;94(1):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison LH, Shutt KA, Schmink SE, et al. “Population Structure and Capsular Switching of Invasive Neisseria Meningitidis Isolates in the Pre-Meningococcal Conjugate Vaccine Era–United States, 2000-2005. J Infect Dis. 2010. April 15;201(8):1208–1224. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infectious Agents Surveillance Report. Meningococcal meningitis, 1999-2004, Japan. IASR. 2005;26:33–34. [Google Scholar]
- 15.Li J, Shao Z, Liu G, et al. Meningococcal Disease and Control in China: findings and Updates from the Global Meningococcal Initiative (GMI). J Infect. 2018. May 1;76(5):429–437. . [DOI] [PubMed] [Google Scholar]
- 16.Harrison LH, Granoff DM, et al. Meningococcal Capsular Group A, C, W, and Y Conjugate Vaccines. In: Plotkin’s Vaccines. 7th. Philadelphia, PA: Elsevier; 2018. p. 619–643.e11. http://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323357616000389?scrollTo=%23hl0000897 [Google Scholar]
- 17.John TJ, Gupta S, Chitkara AJ, et al. An Overview of Meningococcal Disease in India: knowledge Gaps and Potential Solutions. Vaccine. 2013. June 7;31(25):2731–2737. . [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO ∣ meningococcal Disease in the Philippines - Update 2. cited 2019 Sep 5. https://www.who.int/csr/don/2005_01_28a/en/
- 19.Neisseria Profile/Sequence Definitions. cited 2019 Oct 19 https://pubmlst.org/bigsdb?db=pubmlst_neisseria_seqdef. This publication made use of the PubMLST website (https://pubmlst.org/) developed by Keith Jolley (Jolley et al. Wellcome Open Res 2018, 3:124 [version 1; referees: 2 approved]) and sited at the University of Oxford. The development of that website was funded by the Wellcome Trust.
- 20.Morelli G, Malorny B, Müller K, et al. Clonal Descent and Microevolution of Neisseria Meningitidis during 30 Years of Epidemic Spread. Mol Microbiol. 1997. September;25(6):1047–1064.. [DOI] [PubMed] [Google Scholar]
- 21.Harrison LH, Trotter CL, Ramsay ME. Global Epidemiology of Meningococcal Disease. Vaccine. 2009. June 24;27(Suppl 2):B51–63.. [DOI] [PubMed] [Google Scholar]
- 22.Olyhoek T, Crowe BA, Achtman M. Clonal Population Structure of Neisseria Meningitidis Serogroup A Isolated from Epidemics and Pandemics between 1915 and 1983. Rev Infect Dis. 1987. August;9(4):665–692. [DOI] [PubMed] [Google Scholar]
- 23.Wang JF, Caugant DA, Li X, et al. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People’s Republic of China.. Infect Immun. 1992. December;60(12):5267–5282. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorvatn B, Hassan-King M, Greenwood B, et al. DNA Fingerprinting in the Epidemiology of African Serogroup A Neisseria Meningitidis. Scand J Infect Dis. 1992;24(3):323–332. . [DOI] [PubMed] [Google Scholar]
- 25.Ghipponi P, Darrigol J, Skalova R, et al. Study of Bacterial Air Pollution in an Arid Region of Africa Affected by Cerebrospinal Meningitis. Bull World Health Organ. 1971;45(1):95–101. [PMC free article] [PubMed] [Google Scholar]
- 26.Molesworth AM, Cuevas LE, Connor SJ, et al. Environmental Risk and Meningitis Epidemics in Africa. Emerg Infect Dis. 2003. October;9(10):1287–1293. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens DS, Apicella MA. Neisseria Meningitidis. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Updated Edition. 8th. Philadelphia, PA: Elsevier/Saunders; 2015. p. 2425–2445.e6. http://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323401616002138 [Google Scholar]
- 28.Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal Disease. N Engl J Med. 2001. May 3;344(18):1378–1388. . [DOI] [PubMed] [Google Scholar]
- 29.Response to epidemic meningitis in Africa, 1997. Wkly Epidemiol Rec. 1997;42:313–318. [PubMed] [Google Scholar]
- 30.Al Gahtani YM, El Bushra HE, al-Qarawi SM, et al. Epidemiological Investigation of an Outbreak of Meningococcal Meningitis in Makkah (Mecca), Saudi Arabia, 1992. Epidemiol Infect. 1995. December;115(3):399–409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotschlich EC, Liu TY, Artenstein MS. Human Immunity to the Meningococcus. J Exp Med. 1969. June 1;129(6):1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: Coverage and initial impact of the campaign. Commun Dis Public Health. 2002. September;5(3):220–225. [PubMed] [Google Scholar]
- 33.Jódar L, Marc LaForce F, Ceccarini C, et al. Meningococcal conjugate vaccine for africa: a model for development of new vaccines for the poorest countries. Lancet. 2003. May 31;361(9372):1902–1904. .• The article describes the initial financial and technical barriers to development of a novel meningococcal vaccine in sub-Saharan Africa, and highlights the approaches utilized to develop a cost-effective vaccine.
- 34.Lee C-H, Kuo W-C, Beri S, et al. Preparation and characterization of an immunogenic meningococcal group a conjugate vaccine for use in Africa. Vaccine. 2009. January 29;27(5):726–732. . [DOI] [PubMed] [Google Scholar]
- 35.Zipursky S, Djingarey MH, Lodjo JC, et al. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine. 2014. March 14;32(13):1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaForce FM, Konde K, Viviani S, et al. The meningitis vaccine project. Vaccine. 2007. September 3;25(Suppl 1):A97–100. . [DOI] [PubMed] [Google Scholar]
- 37.LaForce FM, Okwo-Bele JM. Eliminating epidemic group A meningococcal meningitis in Africa through a new vaccine. Health Aff (Millwood). 2011;30:1049–1057. [DOI] [PubMed] [Google Scholar]
- 38.Trotter CL, Lingani C, Fernandez K, et al. Impact of menafrivac in nine countries of the African meningitis BELT, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017. August 1;17(8):867–872. . [DOI] [PubMed] [Google Scholar]
- 39.WHO. Meningitis weekly bulletin, epidemiological situation. World Health Organization. 2017. cited 2018 Mar 16. http://www.who.int.proxy.library.emory.edu/csr/disease/meningococcal/epidemiological/en/ [Google Scholar]
- 40.Diallo A, Sow SO, Idoko OT, et al. Antibody persistence at 1 and 4 years following a single dose of menafrivac or quadrivalent polysaccharide vaccine in healthy subjects aged 2-29 years. Clin Infect Dis. 2015. November 15;61(Suppl 5):S521–530. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kristiansen PA, Diomandé F, Ba AK, et al. Impact of the serogroup a meningococcal conjugate vaccine, menafrivac, on carriage and herd immunity. Clinl Infect Dis. 2013. February 1;56(3):354–363..• This article assessed the rates of serogroup A carriage in Burkina Faso before and up to 13 months after the vaccination campaign in 2010, and described the disappearance of serogroup A carriage in both vaccinated and unvaccinated individuals. The authors’ findings support a vaccine-induced herd immunity effect.
- 42.Daugla DM, Gami JP, Gamougam K, et al. Effect of a serogroup a meningococcal conjugate vaccine (PsA–TT) on serogroup a meningococcal meningitis and carriage in chad: a community study. Lancet. 2013. January 4;383(9911):40–47. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen WH, Neuzil KM, Rebecca Boyce C, et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect Dis. 2018. October 1;18(10):1088–1096. .•• The first-in-man, phase 1 study that assessed the safety and immunogenicity of a novel pentavalent meningococcal conjugate vaccine (NmCV-5) that targets serogroups A, C, Y, W, and X.
- 44.McCarthy PC, Sharyan A, Moghaddam LS. Meningococcal vaccines: current status and emerging strategies. Vaccines (Basel). 2018;6(1). DOI: 10.3390/vaccines6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harale KR, Rout JK, Chhikara MK, et al. Synthesis and immunochemical evaluation of a novel neisseria meningitidis serogroup a tetrasaccharide and its conjugate. Org Chem Front. 2017. November 21;4(12):2348–2357. . [Google Scholar]
- 46.World Health Organization. Defeating Meningitis by 2030. cited 2019 Sep 11. https://www.who.int/immunization/research/Defeating_meningitis_2030_TTFJuly2018_report.pdf•• The WHO article describes the roadmap developed by the Taskforce, with specific strategic goals to eliminate and control meningococcal disease by 2030.