Abstract
Objective:
Previous studies have demonstrated that HIV-exposed uninfected (HEU) infants and children experience morbidity and mortality at rates exceeding those of their HIV-unexposed uninfected (HUU) counterparts. We sought to summarize the association between HEU vs. HUU infants and children for the outcomes of diarrhea and pneumonia.
Design:
Meta-analysis.
Methods:
We reviewed studies comparing infants and children in the 2 groups for these infectious disease outcomes, in any setting, from 1993 to 2018 from 6 databases.
Results:
We included 12 studies, and 17,955 subjects total [n = 5074 (28.3%) HEU and n = 12,881 (71.7%) HUU]. Random-effects models showed HEU infants and children had a 20% increase in the relative risk of acute diarrhea and a 30% increase in the relative risk of pneumonia when compared with their HUU counterparts. When stratifying by time since birth, we showed that HEU vs. HUU children had a 50% and 70% increased risk of diarrhea and pneumonia, respectively, in the first 6 months of life.
Conclusions:
We show an increased risk of diarrhea and pneumonia for HEU vs. HUU infants and children. Although we acknowledge, and commend, the immense public health success of prevention of mother-to-child transmission, we now have an enlarging population of children that seem to be vulnerable to not only death, but increased morbidity. We need to turn our attention to understanding the underlying mechanism and designing effective public health solutions. Further longitudinal research is needed to elucidate possible underlying immunological and/or sociological mechanisms that explain these differences in morbidity.
Keywords: HIV-exposed uninfected, HIV-unexposed uninfected, morbidity, pneumonia, diarrhea meta-analysis
INTRODUCTION
Great progress has been made in efforts to prevent mother-to-child transmission (PMTCT) of HIV. The percentage of pregnant women receiving antiretroviral therapy (ART) for PMTCT increased from 47% in 2010 to 80% in 2016.1 The global scale-up of PMTCT of HIV through provision of maternal ART is credited with a 70% worldwide decline in new HIV infections among children between 2000 and 2015.2 Today, there is substantial evidence that under ideal conditions effective maternal ART use can almost eliminate mother-to-child transmission, and at a programmatic level, it may be possible to decrease transmission to >2% in low- and middle-income countries.3,4 Despite this significant progress, the number of children exposed to HIV remains unacceptably high, with an estimated 1.3 million children born (exposed) to HIV-positive mothers in 2015,1,5 most of whom reside in sub-Saharan Africa. Assuming continued high uptake of PMTCT under current World Health Organization (WHO) guidelines,6 HIV-exposed but uninfected infants and children are now the predominant pediatric population affected by HIV.
Infants and children perinatally infected with HIV experience higher rates of morbidity and mortality than uninfected infants and children.7 However, there is a growing body of literature reporting HIV-exposed uninfected (HEU) children also experience increased mortality8 and morbidity9–20 compared with HIV-unexposed uninfected (HUU) children. This increased mortality for HEU children has been noted in both the pre-PMTCT and post-PMTCT eras and across various study settings.21,22 While effective PMTCT programs have sharply reduced rates of HIV transmission, the effects of in utero exposure to an infected mother remain even if the child is not infected. Precise etiology of the disparity between exposed and unexposed infants is likely multifactorial in nature, encompassing immunological, biological, maternal, social, behavioral, and health systems factors.
Given the large numbers of children perinatally exposed to HIV each year, further analysis and examination of these trends in morbidity, in particular, is warranted. In a previous meta-analysis focused specifically on mortality among HEU compared with HUU children, we reported a 60% increase in risk of death.8 In this follow-on analysis, we focus on impacts on morbidity due to pneumonia and diarrhea in these 2 populations.
METHODS
Search Strategy and Selection Criteria
We searched the databases PubMed, Web of Science (including Medline), Excerpta Medica Abstract Journals (EMBASE), ProQuest Dissertation & Thesis Outline, International AIDS Society abstract archives, and AIDS Conference abstract archives for articles and abstracts published between January 1, 1993, and August 1, 2018. Our search key words can be found in Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B341. We limited the search to human studies published in English. Two reviewers (M.K. and R.B.) screened all titles and abstracts to capture potentially relevant studies and one reviewer (A.T.B.) resolved any discrepancies between them. We reviewed the references in the included articles and added any studies that met our inclusion criterion. We included studies published during those years from any geographic location that compared pneumonia and/or diarrhea among HEU infants or children to HUU infants or children. Because morbidity can be a challenging definition, all studies that reported on any pneumonia and/or diarrhea outcome were included, regardless of follow-up time for each study. We used each study’s definition of pneumonia and/or diarrhea (summarized in Table 1). Additional data were not obtained from study authors.
TABLE 1.
Characteristics of Included Studies and Overall Risk of Acute or Chronic Diarrhea and Pneumonia and Risk Ratios Comparing HEU and HUU for Each Study (n = 12)
| Author | Year Published | Region | Country | Followup Period (mo) | Overall Loss to Follow-up (%) | Outcome Definition | HEU Total, N | HEU events, n | HUU Total, N | HUU events, n | Crude RR % (95% CI) | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | ||||||||||||
| diarrhea | ||||||||||||
| O’Reilly et al10 | 2011 | SSA | Kenya | 0–60 mo | 10.0 | ≥3 loose stools in 24–48 h | 45 | 4 | 152 | 20 | 0.70 (0.25 to 1.94) | High |
| Landes et al11 | 2012 | SSA | Malawi | 0–20 mo | 53.8 | ≥3 loose stools in 24–48 h | 128 | 63 | 200 | 114 | 0.90 (0.73 to 1.12) | High |
| Koyanagi et al12 | 2011 | SSA | Zimbabwe | 0–12 mo | 0.4 | ≥3 loose stools in 24–48 h | 2661 | — | 9207 | — | 0.95 (0.35 to 2.58)* | High |
| Shapiro et al13 | 2007 | SSA | Botswana | 0–24 mo | 1.8 | ≥3 loose stools in 24–48 h | 534 | 171 | 137 | 46 | 1.00 (0.76 to 1.31) | High |
| Thea et al15 | 1993 | SSA | DRC | 0–24 mo | 20.0 | ≥3 loose stools in 24–48 h | 139 | — | 191 | — | 1.00 (0.50 to 2.01)* | High |
| Pavlinac et al18 | 2014 | SSA | Kenya | 6–60 mo | 17.0 | ≥3 loose stools in 24–48 h | 105 | 35 | 821 | 224 | 1.20 (0.90 to 1.60) | High |
| Rollins et al19 | 2013 | SSA | South Africa | 0–6 mo | 19.8 | ≥3 loose stools in 24–48 h | 936 | 1155 | 1.60 (0.77 to 3.34)* | High | ||
| Marquez et al20 | 2014 | SSA | Uganda | 6–36 mo | 13.8 | ≥3 loose stools in 24–48 h | 200 | 25 | 389 | 11 | 4.42 (2.22 to 8.79) | High |
| Chronic diarrhea | ||||||||||||
| Spira et al14 | 1999 | SSA | Rwanda | 0–60 mo | 11.0 | Chronic diarrhea (≥14 days) | 138 | — | 209 | — | 1.00 (0.50 to 2.01)* | High |
| Thea et al15 | 1993 | SSA | DRC | 0–24 mo | 20.0 | Chronic diarrhea (≥14 days) | 139 | — | 191 | — | 1.00 (0.50 to 2.01)* | High |
| Luabeya et al16 | 2007 | SSA | South Africa | 6–24 mo | 26.3 | Chronic diarrhea (≥14 days) | 142 | 123 | 165 | 132 | 1.08 (0.99 to 1.22) | High |
| Rollins et al18 | 2013 | SSA | South Africa | 0–6 mo | 19.8 | Chronic diarrhea (≥14 days) | 936 | 1155 | 0.82 (0.31 to 2.18)* | High | ||
| Pneumonia | ||||||||||||
| Luabeya et al16 | 2007 | SSA | South Africa | 6–24 mo | 26.3 | Children who had chest in-drawing with or without fast breathing | 142 | 19 | 165 | 21 | 1.05 (0.59 to 1.87) | High |
| Jeena et al17 | 2007 | SSA | South Africa | 3–59 mo | 13.9 | Children who had chest in-drawing with or without fast breathing | 40 | 16 | 244 | 84 | 1.16 (0.77 to 1.76) | High |
| Koyanagi et al12 | 2011 | SSA | Zimbabwe | 0–6 mo | 0.4 | Children who had chest in-drawing with or without fast breathing | 2661 | — | 9207 | — | 1.30 (1.05 to 1.61)* | High |
| Shapiro et al13 | 2007 | SSA | Botswana | 0–24 mo | 1.8 | Children who had chest in-drawing with or without fast breathing | 534 | 52 | 137 | 10 | 1.33 (0.69 to 2.55) | High |
| Spira et al14 | 1999 | SSA | Rwanda | 0–60 mo | 11.0 | Children who had chest in-drawing with or without fast breathing | 138 | — | 209 | — | 1.40 (0.98 to 1.99)* | High |
| Dimi triades et al9 | 2014 | SSA | South Africa | 0–6 mo | NR | Children who had chest in-drawing with or without fast breathing, with or without isolation of multidrug-resistant bacteria | 6 | 5 | 11 | 3 | 3.05 (1.09 to 8.54) | Moderate |
Note that each study can contribute to all outcomes.
Studies only reported ratio measures and not the number of morbidity events.
Data Extraction
The primary objective of this study was to compare pneumonia and diarrhea outcomes among HEU children to HUU children in any setting. For each study, when possible, we extracted year published, region, country, dates enrollment started and ended, length of follow-up, and total N in each cohort. For our outcomes (Table 1), we extracted data on the number of pneumonia and/or diarrhea events in the total population and/or simple proportions, ratio, and difference measures, in addition to corresponding 95% confidence intervals (CIs) from individual studies, if available.
Data Analysis
We conducted meta-analyses to estimate proportions of acute (defined as ≥3 loose stools in 24–48 hours) and chronic diarrhea (defined as loose stools lasting ≥14 days) and pneumonia (defined as chest in-drawing with or without fast breathing) comparing HEU and HUU children. We stratified by duration of follow-up time from birth (0) to 6, 0 to 12, 0 to >24, and 0 to >24 months and stratified by year enrollment ended in each study: pre 2002 vs. 2002 or after and pre 2004 vs. 2004 or after, when single-dose nevirapine (2002)21 and combination therapy (2004)22 for PMTCT commenced in most public-sector clinics in low-income and middle-income countries. We also used metaregression to assess the effect of year study enrollment ended (1993–2018) on diarrhea and pneumonia by regressing the natural log of the ratio measures for our desired outcomes on the year study enrollment ended as a continuous variable. We examined heterogeneity between the studies using Cochran’s Q and the I2 statistic.23 Random-effects models were used to estimate all infectious disease proportions and corresponding 95% CIs using standard methods because there was evidence of high heterogeneity between studies.24 We performed an analysis of publication bias for diarrhea and pneumonia using a funnel plot and Egger’s linear regression test.24 We assessed the quality of each study using the Newcastle-Ottawa scale.25 Those studies that scored above a 7 were defined as high quality, those with a score between 4 and 6 were considered moderate quality, while those <4 were considered low quality.
RESULTS
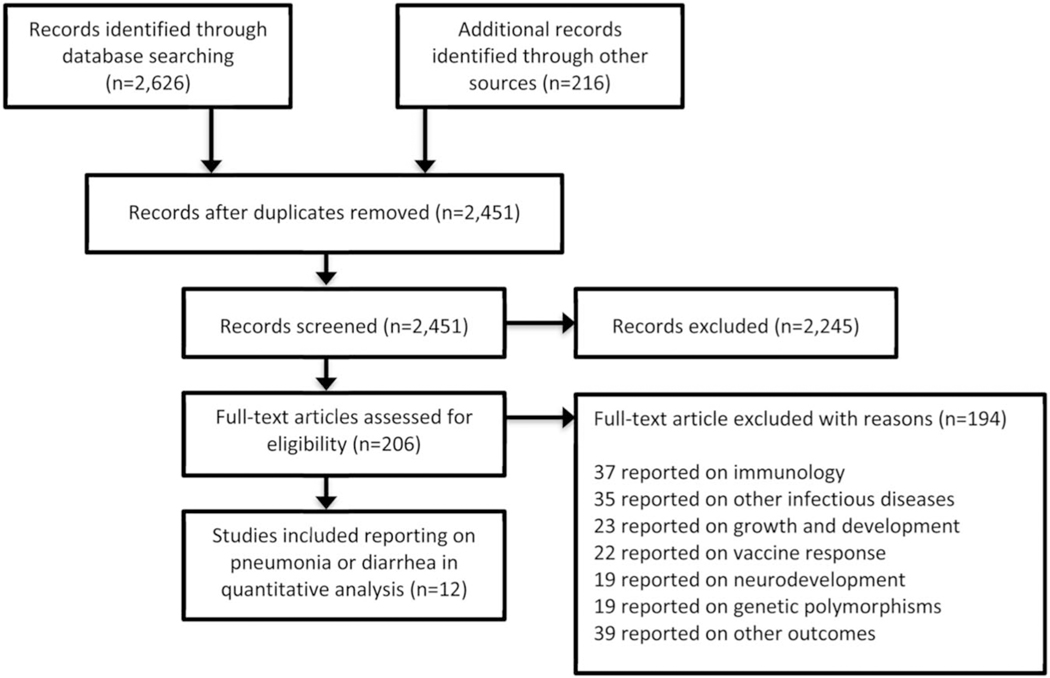
A total of 2842 potentially relevant citations were identified, and 2451 remained after de-duplication (Fig. 1). Of 2451 articles, 206 appeared relevant and merited full text review, of which 12 studies published between 1993 and 2018 reported on diarrhea and/or pneumonia and were included; all 12 were from sub-Saharan Africa (Table 1).9–20 The total population of HEU and HUU in our study was 17,955 subjects [n = 5074 (28.3%) HEU and n = 12,881 (71.7%) HUU]. Cohort size ranged from 179 to 11,86819 HEU and HUU children combined.
FIGURE 1.
Study selection process and reasons for exclusion of studies.
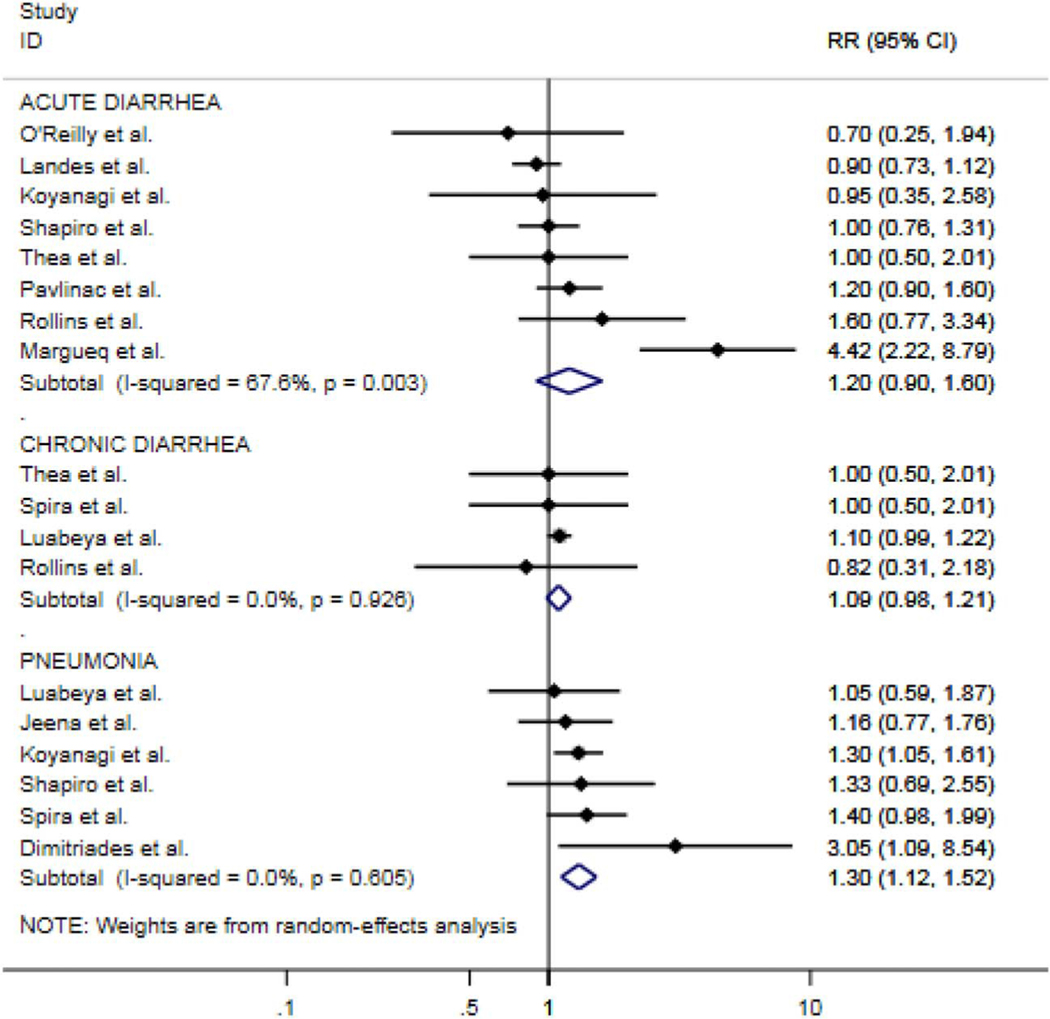
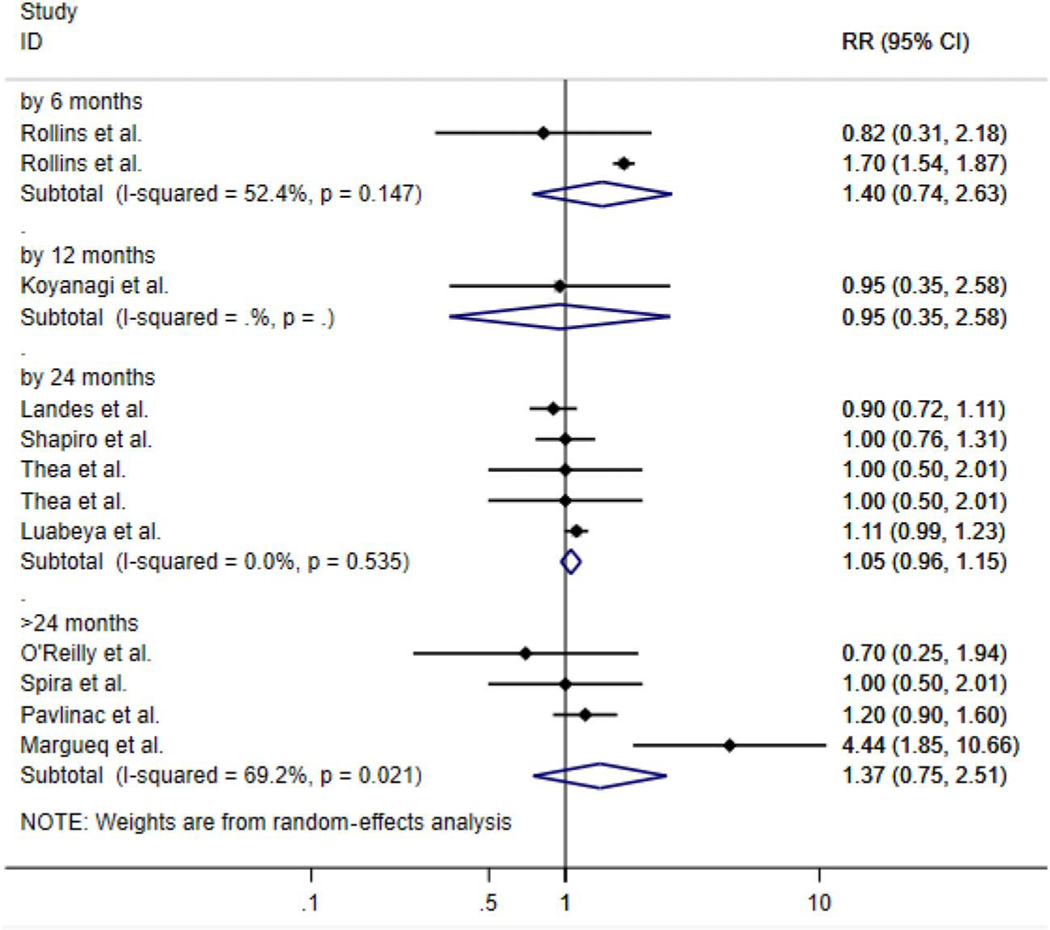
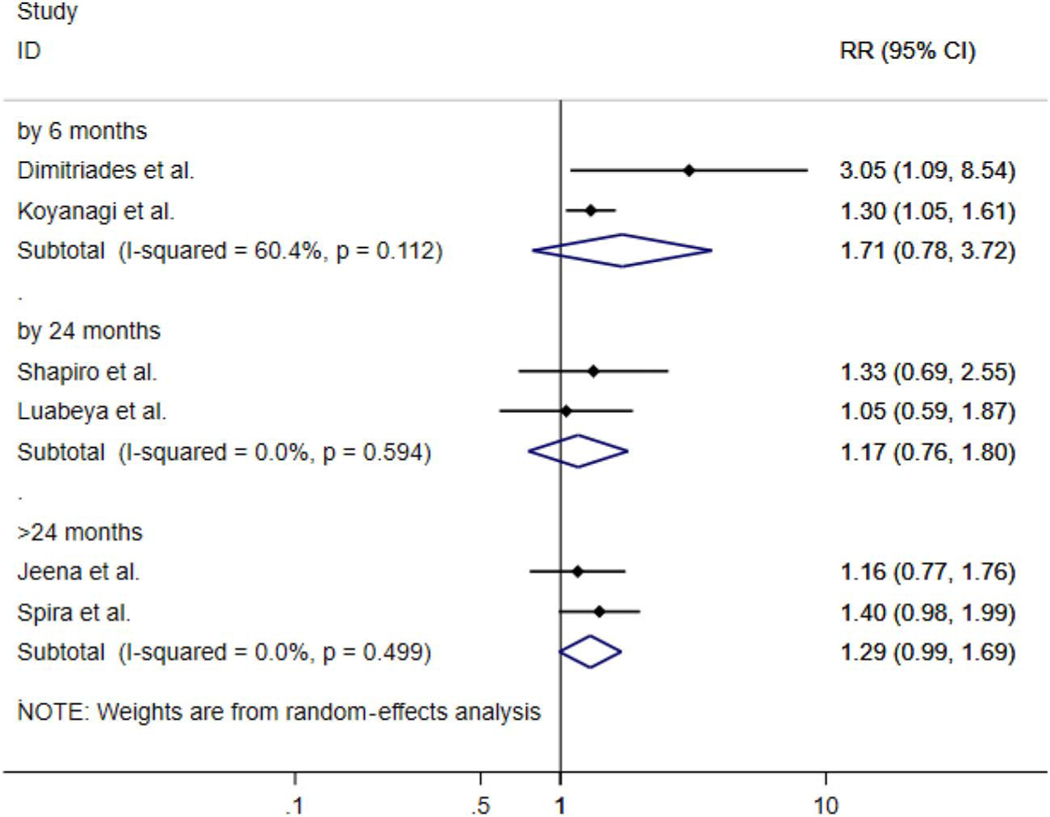
All estimates are summary pooled random-effects estimates of diarrhea (acute or chronic) and pneumonia. Although imprecise, the summary measures of association suggest that HEU children have over a 20% increased risk in acute diarrhea over 5 years [risk ratio (RR): 1.20; 95% CI: 0.90 to 1.60] and close to a 10% increased risk of chronic diarrhea (RR: 1.09; 95% CI: 0.98 to 1.99). In regard to pneumonia, there was a 30% increased risk (RR: 1.30; 95% CI: 1.12 to 1.52) over 5 years of follow-up (Fig. 2) when comparing the 2 groups. When stratifying by time since birth, we found that HEU infants and children had close to a 50% increased risk of diarrhea at 6 months (RR: 1.48; 95% CI: 1.03 to 2.12) and close to 40% increased risk at >24 months (RR: 1.37; 95% CI: 0.75 to 2.51) (Fig. 3) vs. HUU infants and children. For pneumonia, we saw the same trend at 6 (RR: 1.71; 95% CI: 0.78 to 3.72) and >24 months of life (RR: 1.29; 95% CI: 0.99 to 1.69) (Fig. 4).
FIGURE 2.
Forest plot of RRs for acute and chronic diarrhea and pneumonia comparing HEU with HUU children for all studies stratified by outcome (n = 12).
FIGURE 3.
Forest plot of RRs for diarrhea (acute and chronic combined) comparing HEU children with HUU children for all studies stratified by follow-up time from birth (0) to 6, 0 to 12, 0 to 24, and 0 to >24 months.
FIGURE 4.
Forest plot of RRs for pneumonia comparing HEU children with HUU children for all studies stratified by follow-up time from birth (0) to 6, 0 to 24, and 0 to >24 months.
When comparing HEU children with HUU children after implementation of PMTCT globally (pre 2002 vs. 2002 and after), we saw little difference in the relative risk of diarrhea or pneumonia before and after 2002 (see Figure 1 and 2, Supplemental Digital Content, http://links.lww.com/QAI/B341).When shifting the cutoff to 2004, our results remained the same. Metaregression analyses indicated no evidence of any trend from 1993 to 2018 (year study enrollment ended) in diarrhea or pneumonia among the HEU children vs. HIV-unexposed children. We did find evidence of asymmetry using Egger’s test for diarrhea suggesting some publication bias, while we found no evidence of asymmetry for pneumonia suggesting little to no publication bias (see Figure 3A and B, Supplemental Digital Content, http://links.lww.com/QAI/B341).
The overall quality of the evidence for the association between HIV exposure status and the outcomes of pneumonia, and diarrhea was moderate to high (see Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B341). All studies received a score of 7 points or above on the 9-point Newcastle-Ottawa scale25 for quality. Studies in our meta-analysis assessing the association between HIV exposure status, and diarrhea showed either no association or a slight increased risk when comparing HEU with HUU, while all studies included for the outcome of pneumonia showed an increased risk in pneumonia when comparing the 2 groups.
DISCUSSION
The overall benefits of PMTCT programs in low- and middle-income countries are undeniable: maternal ART in pregnancy has prevented millions of children from acquiring HIV-1 infection.5 Nevertheless, there is compelling evidence that uninfected children born to HIV-infected mothers, particularly in the low- and middle-income countries, can experience increased morbidity and mortality, predominantly from infectious diseases.8–20 In our meta-analysis of the literature, although imprecise, we found that HEU children had a 30% increase in the risk of pneumonia over 5 years and a 20% increased risk in acute diarrhea over 5 years compared with HUU children. Our results also suggest that HEU vs. HUU children have the highest risk of diarrhea and pneumonia within the first 6 months of life. There is also some suggestion that the risk of these infectious diseases seems to decrease as children aged beyond 24 months.
As we reported in our previous meta-analysis assessing mortality between HEU infants and children compared with their HIV-unexposed counterparts,8 we believe the precise reason for this difference in morbidity is likely multifactorial. Factors may include, but are not limited to (1) unrecognized or unreported co-infections in HEU children, (2) the impact of HIV on maternal health status during pregnancy, (3) poorer maternal health or maternal postnatal death impairing the quality of childcare, or (4) corresponding lower socioeconomic status and subsequent threat of food insecurity for children born into households with an HIV-infected mother.8 While unrecognized HIV transmission postnatally (either delayed diagnosis or through breastmilk transmission) may also be a contributing factor, the cohorts included in this meta-analysis repeatedly assessed infant/child HIV infection status, and it is unlikely that HIV-infected subjects were misclassified as exposed uninfected.9–20
It is important to note that even when neonates escape HIV infection, HIV exposure in utero may present an altered environment for fetal growth and development. As such, the dysregulation of transplacental antibody transfer could be another contributing factor to our results. Transfer of maternal immunoglobulin G (IgG) is an efficient process in HIV-negative mothers who have healthy pregnancies. Infant cord blood concentrations of maternal IgG can well exceed maternal IgG serum concentrations by delivery in full-term pregnancies.26 However, in HIV-positive mothers, several studies have demonstrated that HIV-exposed uninfected infants have lower levels of maternal antibodies than their unexposed counterparts,27–33 specifically to pathogens including Streptococcus pneumoniae, Haemophilus influenzae, group B Streptoccoccus, pertussis, poliomyelitis, and measles.34 In our study, we saw an increased risk of pneumonia and diarrhea for HEU children persisting up to 2 years postnatally, suggesting that poor antibody transfer cannot be the sole explanation for the difference observed, as passive maternal antibody transfer has most likely waned by 12 months of life.
The potential impact of both HIV exposure and maternal ART on the immune system of an infant without HIV is unclear. Increased immune activation factors have been observed in the infants of HIV-infected mothers, associated with high maternal viral load.35–37 Previous research has documented HIV-exposed uninfected infants, exposed to various maternal ART regimens, as having altered cell-mediated immunity. One study reported lower CD4 T-lymphocyte cell counts in HEU infants born to mothers who had a viral load >1000 copies/mL at the time of delivery compared with HEU infants whose mothers had a viral load <50 copies/mL at the time of delivery.38 Prevaccination vaccine-specific antibody levels are lower in HIV-exposed uninfected infants than HIV-unexposed uninfected infants, with some HIV-exposed uninfected infants mounting insufficient humoral immune response after vaccination with diphtheria toxoid, haemophilus influenza type B, pertussis, measles, hepatitis B, tetanus toxoid, and pneumococcal conjugate vaccines.39 However, t HIV-specific immune responses have not been thoroughly assessed in HEU infants born to mothers on ART with a suppressed HIV viral load.38 Given the persistence of increased risk for pneumonia beyond 2 years of age, early immunological differences noted in HIV-exposed infants and children, whether due to high maternal viral loads or the presence of maternal ART, likely does not account for our observed difference in morbidity between the 2 groups.
Decreased adaptive immunity to standard childhood immunizations has also been found in HEU children up to 2 years of age,40,41 although the data on this is conflicted. Some studies report that HIV-exposed uninfected infants’ humoral and cell-mediated responses to vaccines, including to the measles vaccine and bacille Calmette-Guerin, are similar to responses in unexposed infants.42–44 One study out of South Africa even demonstrated that HIV-exposed uninfected infants mounted higher increments of antibody responses to pertussis and pneumococcus vaccines.44 The same research group also studied the impact of maternal HIV infection and demonstrated that HIV-exposed, uninfected infants have normal responses to bacille Calmette-Guerin vaccination administered at birth.44 However, this likely does not account for differences in morbidity described in our study, as H. influenzae type B, and pneumococcal vaccines for pneumonia were not widely available in low-income countries before 2000 and 2008, respectively,45,46 and rotavirus for diarrhea before 2012.47
Very few studies included in this analysis reported clearly on breastfeeding practices, maternal ART, or use of cotrimoxazole prophylaxis in children and infants, in addition to social or environmental conditions that might contribute to this difference in infectious disease outcomes. One possibility is that HIV-infected mothers may be sicker or more likely to be deceased (along with their male partner) than non–HIV-infected mothers and therefore may be less able to provide care. Poor socioeconomic status resulting in food insecurity could also be a factor. A review of the literature found data to support food insecurity as a critical barrier to adherence to ART and to other health care recommendations among HIV-infected adults, HIV-infected pregnant women and their HIV-exposed infants, and child and adolescent populations of people living with HIV and AIDS.48 Such differences in maternal health status could also account for differences in breastfeeding practices between HIV-positive and HIV-negative mothers. It is well established that breastfeeding is protective against pneumonia and diarrhea for all infants49,50 and is the recommended feeding modality for all mothers, including those with HIV, in low- and middle-income countries, when feasible.50
In high-income countries, both monotherapy51 and combination ART52 have been used since the mid-1990’s in PMTCT efforts and to improve maternal health status, so women could live longer to care for their offspring.52,53 ART in the form of single-dose nevirapine for PMTCT use was introduced in resource-limited settings in 200221 and ART combination therapy in 2004.22 Our results show no difference in the risk of diarrhea or pneumonia before 2002 compared with during and after 2002 (or when we shifted the cutoff to 2004), in HEU vs. HUU children. While the World Health Organization recommended the use of cotrimoxazole prophylaxis for all HIV-exposed infants in 2000,54 very few studies included in this analysis clearly reported on cotrimoxazole use, and subanalyses based on a cutoff date of 2000 (cotrimoxazole guidelines) were not possible with these data.
It is important to note that, although imprecise, there has been a substantial, overall decrease in incidence of pneumonia and diarrhea globally since the mid-1990’s,53 synchronous with improved treatment guidelines, including Integrated Management of Childhood Illness55 and increased access to vaccines.56 As such, one may expect to see an overall smaller effect size over calendar time, with the assumption that childhood pneumonia morbidity would similarly decline in both HEU and HUU populations over the same period. Alternatively, if overall risk in the population is declining, then the relative effect may be increasing because the baseline risk is decreasing, potentially explaining why we do not see a decline in effect sizes over time in our study.
Our results should be considered alongside their limitations. First, as with any meta-analysis, there is the possibility of incomplete retrieval or abstraction of data, due to either human error, or that the primary outcomes of the studies were not infectious disease morbidity. However, we used the most comprehensive publicly available literature in which most major and well-conducted studies should be reported. Second, we did not obtain raw data from study investigators for pooled estimation of infectious disease outcomes. While all studies included happened to be from sub-Saharan Africa, there are a small number of studies reporting on other clinical outcomes than pneumonia and diarrhea in higher income countries, such as neonatal sepsis, with higher incidence in HIV-exposed infants and children compared with unexposed infants.57 Third, there was also substantial heterogeneity among the studies. With respect to the specific infectious disease morbidities, we did not have further specific data on etiology of illness (eg, pneumonia due to pneumocystis as opposed to S. pneumoniae). Despite this heterogeneity, we felt it was important to pool the existing data to estimate infectious disease probability as these data provide a more robust estimate than any single study alone. Fourth, as no studies included in our analysis controlled for nonbiological risk factors, we may have unmeasured confounding, potentially resulting in bias in our summary estimates. Fifth, time-varying rates of disease incidence were lacking in most of these studies. As discussed previously, whether in the presence of HIV exposure or not, infant mortality from these infectious diseases is greatest in the first month of life, and meta-analysis itself risks obscuring those events by averaging them out. Finally, given the smaller number of studies in the pre- and post-ART PMTCT eras, it is not possible for us to know how much of this effect on child morbidity is mediated by maternal ART (and, subsequently, improved maternal or paternal health status).
CONCLUSIONS
Our results suggest an increased risk of diarrhea and pneumonia for HIV-exposed, uninfected vs. HIV-unexposed, uninfected infants, and children. Our results may be confounded by a number of other factors not reported in the main studies, such as maternal health and functional care-taking status, duration of maternal antiretroviral treatment, infant feeding modality and nutritional status. Although we acknowledge, and commend, the immense public health success of prevention of mother-to-child transmission, we now have an enlarging population of children that seem to be vulnerable to not only death, but increased morbidity. We need to turn our attention to understanding the underlying mechanism and designing effective public health solutions. Further longitudinal research is needed to elucidate possible underlying immunological and/or sociological mechanisms that explain these differences in morbidity and mortality between these 2 groups.
Supplementary Material
Acknowledgments
Supported by the American People and the President’s Emergency Plan for AIDS Relief (PEPFAR) through USAID under the terms of Cooperative Agreements AID-674-A-12–00029 and 72067419CA00004 to HE2RO. The contents are the responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID, or the United States Government. The authors have no conflicts of interest to disclose.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
References
- 1.UNAIDS. 2017. Globcal HIV Statistics. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed September 2018.
- 2.UNAIDS. Children and HIV Fact Sheet. Available at: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf. Accessed September 2018.
- 3.Mandelbrot L, Tubiana R, Le Chenadec J, et al. ; ANRS-EPF Study Group. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015; 61:1715–1725. [DOI] [PubMed] [Google Scholar]
- 4.Chi BH, Stringer JS, Moodley D. Antiretroviral drug regimens to prevent mother-to-child transmission of HIV: a review of scientific, program, and policy advances for sub- Saharan Africa. Curr HIV/AIDS Rep. 2013;10: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. 2015. Progress Report on the Global Plan: towards the elimination of new HIV infections among children and keeping their mothers alive. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. Accessed September 2018.
- 6.WHO. Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. 2012. Available at: http://www.who.int/hiv/PMTCT_update.pdf. Accessed September 2018.
- 7.Newell ML, Coovadia H, Cortina-Borja M, et al. ; Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 8.Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS. 2016;30:2351–2360. [DOI] [PubMed] [Google Scholar]
- 9.Dimitriades K, Morrow BM, Jeena P. A retrospective study on the effects of colistin therapy in children with multidrug-resistant gram-negative bacterial pathogens: impact of HIV status on outcome. Arch Dis Child. 2014;99:262–266. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly CE, Jaron P, Ochieng B, et al. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med. 2012;9:e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landes M, van Lettow M, Chan AK, et al. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One. 2012;7:e47337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyanagi A, Humphrey JH, Ntozini R, et al. ; ZVITAMBO Study Group. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro RL, Lockman S, Kim S, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196:562–569. [DOI] [PubMed] [Google Scholar]
- 14.Spira R, Lepage P, Msellati P, et al. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Mother-to-Child HIV-1 Transmission Study Group. Pediatrics. 1999;104:e56. [DOI] [PubMed] [Google Scholar]
- 15.Thea DM, St Louis ME, Atido U, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med. 1993; 329:1696–1702. [DOI] [PubMed] [Google Scholar]
- 16.Luabeya KK, Mpontshane N, Mackay M, et al. Zinc or multiple micro-nutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PLoS One. 2007;2:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeena PM, Minkara AK, Corr P, et al. Impact of HIV-1 status on the radiological presentation and clinical outcome of children with WHO defined community-acquired severe pneumonia. Arch Dis Child. 2007;92:976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlinac PB, John-Stewart GC, Naulikha JM, et al. High-risk enteric pathogens associated with HIV infection and HIV exposure in Kenyan children with acute diarrhoea. AIDS. 2014;28:2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollins NC, Ndirangu J, Bland RM, et al. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One. 2013;8:e81307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez C, Okiring J, Chamie G, et al. Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J Trop Pediatr. 2014;60:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevention of mother-to-child transmission of HIV: use of Nevirapine among women of unknown serostatus. Report of a technical consultation. 5–6 December 2001, Geneva: 1. 2002. Available at: http://www.who.int/hiv/pub/mtct/en/isbn9241562129.pdf?ua=1. Accessed October 2018. [Google Scholar]
- 22.Antiretroviral drugs for treating pregnant women and preventing HIV-infection in infants. In: Guidelines on Care, Treatment and Support for Women Living With HIV/AIDS and Their Children in Resource-Constrained Settings. 2004. Available at: http://www.who.int/hiv/pub/mtct/guidelinesarv/en/. Accessed October 2018. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed October 2019.
- 26.Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–255. [DOI] [PubMed] [Google Scholar]
- 27.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–F205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dechavanne C, Cottrell G, Garcia A, et al. Placental malaria: decreased transfer of maternal antibodies directed to plasmodium falciparum and impact on the incidence of febrile infections in infants. PLoS One. 2015; 10:e0145464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordi J, Menendez C, Ismail MR, et al. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J Infect Dis. 2001;183:1100–1107. [DOI] [PubMed] [Google Scholar]
- 30.Scott S, Cumberland P, Shulman CE, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis. 2005;191:1854–1860.15871118 [Google Scholar]
- 31.Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–557. [DOI] [PubMed] [Google Scholar]
- 32.de Moraes-Pinto MI, Almeida AC, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–1084. [DOI] [PubMed] [Google Scholar]
- 33.de Moraes-Pinto MI, Farhat CK, Carbonare SB, et al. Maternally acquired immunity in newborns from women infected by the human immunodeficiency virus. Acta Paediatr. 1993;82:1034–1038. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Raya B, Kollmann TR, Marchant A, et al. The immune system of HIV-exposed uninfected infants. Front Immunol. 2016;7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 36.Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy JA, Hsueh F, Blackbourn DJ, et al. CD8 cell noncytotoxic antiviral activity in human immunodeficiency virus-infected and –uninfected children. J Infect Dis. 1998;177:470–472. [DOI] [PubMed] [Google Scholar]
- 38.Kakkar F, Lamarre V, Ducruet T, et al. Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: protective mechanism or immunodeficiency. BMC Infect Dis. 2014;14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidzeru EB, Hesseling AC, Passmore JA, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;28:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Center for Disease Control. Morbidity and mortality weekly report: mmwr: progress in introduction of pneumococcal conjugate vaccine- worldwide, 2000–2008. MMWR Morb Mortal Wkly Rep 2008;57;1148–1151. [PubMed] [Google Scholar]
- 41.Olayinka F, Ewald L, Steinglass R. Beyond new vaccine introduction: the uptake of pneumococcal conjugate vaccine in the African region. Pan Afr Med J. 2017;27(suppl 3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansoor N, Scriba TJ, de Kock M, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille CalmetteGuérin vaccine. J Infect Dis. 2009;199:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helfand RF, Witte D, Fowlkes A, et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis. 2008;198:1457–1465. [DOI] [PubMed] [Google Scholar]
- 44.Jones CE, Naidoo S, De Beer C, et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–584. [DOI] [PubMed] [Google Scholar]
- 45.Center for Disease Control. Morbidity and mortality weekly report: MMWR: progress toward introduction of Haemophilus influenza type b vaccine in low-income countries-worldwide, 2004–2007. MMWR Morb Mortal Wkly Rep 2008;57;148–151. [PubMed] [Google Scholar]
- 46.Ojo LR, O’Loughlin RE, Cohen AL, et al. Global use of Haemophilus influenza type b conjugate vaccine. Vaccine. 2010;28:7117–7122. [DOI] [PubMed] [Google Scholar]
- 47.Parashar UD, Johnson H, Steele AD, et al. Health impact of rotavirus vaccination in developing countries; progress and way forward. CID. 2016;62(suppl 2):S91–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young S, Wheeler AC, McCoy SI, et al. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav. 2014;18(suppl 5):S505–S515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamberti LM, Zakarija-Grković I, Fischer Walker CL, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13(suppl 3):S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO. Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and summary of evidence. 2010. Available at: http://apps.who.int/iris/bitstream/10665/44345/1/9789241599535_eng.pdf. Accessed October 2018. [PubMed]
- 51.The Petra Study Team. Efficacy of short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother-to- child in Tanzania, South Africa and Uganda (Petra study): a randomised double-blind, placebo controlled trial. Lancet. 2002;359:1178–1186. [DOI] [PubMed] [Google Scholar]
- 52.HIV-CAUSAL Collaboration, Ray M, Ray M, Logan R, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–2013, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. [DOI] [PubMed] [Google Scholar]
- 54.Evans C, Prendergast AJ. Co-trimoxazole for HIV-exposed uninfected infants. Lancet Glob Health. 2017;5: e468–469. [DOI] [PubMed] [Google Scholar]
- 55.Technical updates of the guidelines on Integrated management of childhood illness (IMCI): evidence and recommendations for further adaptations World health organization. Available at: http://www.who.int/child-adolescent-health/New_Publications/IMCI/ISBN_92_4_159348_2.pdf. Accessed June 29, 2005. [Google Scholar]
- 56.Vaccine preventable deaths and the global immunization vision and strategy, 2006–2015. MMWR Morb Mortal Wkly Rep. 2006;55:511–515. [PubMed] [Google Scholar]
- 57.Epalza C, Goetghebuer T, Hainaut M, et al. High incidence of invasive Group B Streptococcal infections in HIV-exposed uninfected infants. Pediatrics. 2010;126:e631–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.