Abstract
Introduction
Skin quality is an important component of human attractiveness. To date, there are no standardized criteria for good skin quality. To establish a consensus for good skin quality parameters and measurement and treatment options, a virtual skin quality advisory board consisting of a global panel of highly experienced aesthetic dermatologists/aesthetic physicians was convened.
Methods
A total of 10 dermatologists/aesthetic physicians served on the advisory board. A modified version of the Delphi method was used to arrive at consensus. Members accessed an online platform to review statements on skin quality criteria from their peers, including treatment and measurement options, and voted to indicate whether they agreed or disagreed. Statements that did not have agreement were modified and the members voted again. Consensus was defined as: strong consensus = greater than 95% agreement; consensus = 75% to 95% agreement; majority consent = 50% to 75% agreement; no consensus = less than 50% agreement.
Results
There was strong consensus that good skin quality is defined as healthy, youthful in appearance (appearing younger than a person’s chronological age), undamaged skin and that skin quality can be described across all ethnicities by four emergent perceptual categories (EPCs): skin tone evenness, skin surface evenness, skin firmness, and skin glow. The EPCs can be affected by multiple tissue layers (ie, skin surface quality can stem from and be impacted by deep structures or tissues). This means that topical approaches may not be sufficient. Instead, improving skin quality EPCs can require a multilayer treatment strategy.
Conclusion
This global advisory board established strong consensus that skin quality can be described by four EPCs, which can help clinicians determine the appropriate treatment option(s) and the tissue or skin layer(s) to address. Skin quality is important to human health and wellbeing and patients’ perception for the need for aesthetic treatment.
Keywords: aesthetic treatment, consensus, emergent perceptual skin quality categories, EPCs, skin quality
Introduction
Skin quality is important to human attractiveness1,2 and it helps determine the need for and choice of aesthetic treatment.3 Skin quality significantly influences perception of age, attractiveness, health and youth.2,4,5 Even small changes in skin surface and pigmentation pattern can have a strong impact on perceived facial attractiveness.2 This holds true across ethnicities and for both female and male faces.6–8
Age-related skin changes stem from aging-induced alterations that occur in all tissue layers. Skeletal aging leads primarily to a loss of bony support. This is visible as a decrease in volume, which decreases support and contributes to an aged appearance of the face.9 The changes in bony support also alter the position of the true retaining ligaments. Aging of retaining ligaments and muscles leads to an expansion of facial spaces, which accentuates increased laxity and the appearance of volume loss.10 Age-related changes in fat tissue in the face lead to volume loss and sagging.11 The elasticity of skin decreases with age due to collagen and elastin loss12,13 while there’s an increase in surface roughness possibly due to decreased water and sebum content in the skin, which contributes to the development of skin rhytids, including but not limited to periorbital wrinkles (crow’s feet) and forehead and glabellar lines.14
The age-related deterioration in viscoelastic properties are most pronounced after age 50 but insufficient skin hydration is more common in younger age groups, particularly between age 40 years to 50 years.13,15 This creates the need for a multimodal and age-dependent treatment algorithm to enhance skin quality.
However, to date, there is no standardized criteria for skin quality.16 To that end, a skin quality advisory board consisting of a global panel of dermatologists/aesthetic physicians was convened to establish a consensus for skin quality parameters, concepts, definitions, and measurement and treatment options.
Methods
The advisory board consisted of 10 dermatologists and aesthetic physicians from eight countries – Brazil, Germany, Israel, Malaysia, Republic of Korea, Russia, United Kingdom, and United States. Martina Kerscher and Kate Goldie co-chaired the advisory board. A modified version of the Delphi method was used to arrive at consensus.17 The advisory board members began with a blinded question and answer round to establish a general understanding of skin quality. This was followed by an open discussion among the members. Advisory board members prepared video lectures on specific topics, including skin quality attributes, measurements, perception, and the skin microbiome. Members had an opportunity to view the recorded lectures on an online platform and were asked to review and comment on specific questions linked to each presentation.
From the discussions, statements and theses on skin quality criteria were extracted. Members accessed the online platform to review and refine the statements and voted to indicate whether they agreed or disagreed. If they disagreed, they were asked to explain why. Subsequent voting rounds were held. Statements that did not have agreement were rephrased or modified, and the members voted again.
Consensus was defined as: strong consensus = greater than 95% agreement; consensus = 75% to 95% agreement; majority consent = 50% to 75% agreement; no consensus = less than 50% agreement. Statements and recommendations had to have consensus or strong consensus to be considered for inclusion in this consensus report.
The advisory board held its meetings virtually due to the COVID-19 global pandemic and the risk of face-to-face meeting. The virtual meetings, which spanned 6 weeks, conferred several advantages over a conventional in-person meeting that typically lasts for only a few hours. For example, the advisory board members could have more in-depth discussions in the virtual format and respond to inquiries at their convenience. Members also had time to more deeply consider their responses and review presentations or relevant research that they otherwise would not have been able to in a live face-to-face meeting. In addition, participants submitted written responses, which helped ensure clarification and reduced the risk of misinterpretation.
This research did not involve human or animal subjects so institutional review board approval was not required.
Consensus Results/Recommendations
Emergent Perceptual (Skin Quality) Categories
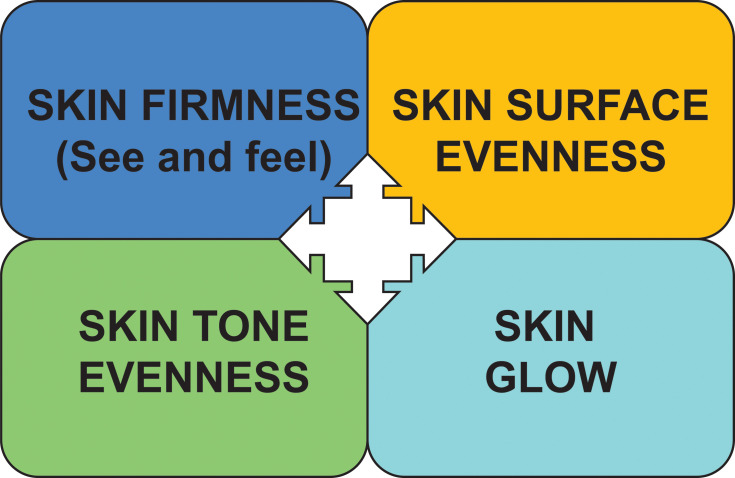
There was strong consensus that skin quality can be described across all ethnicities, age groups and gender by four emergent perceptual categories (EPCs) (Figure 1):
Skin tone evenness
Skin surface evenness
Skin firmness
Skin glow
Figure 1.
The four emergent perceptual skin quality categories (EPCs).
Good skin quality is defined as skin that is healthy, undamaged, and youthful in appearance (appearing younger than a person’s chronological age).
There was strong consensus that the EPCs are composed of individual parameters, each one associated with specific measurement methods and treatment options (Figures 2–5). The EPCs and their parameters are described in further detail in the following sections. See Supplementary Table 1 for a glossary of terms.
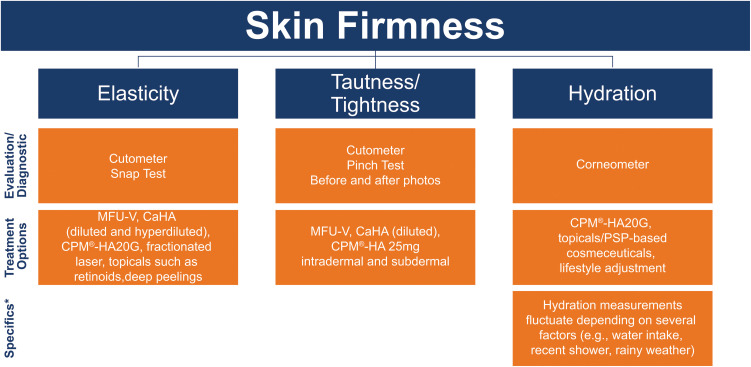
Figure 2.
Skin firmness parameters, measurement methods, and treatment options.
Note: *Factors to consider specific to each EPC parameter where applicable.
Abbreviations: CaHA, calcium hydroxylapatite; CPM®-HA, cohesive polydensified matrix-hyaluronic acid; CPM®-HA20G, cohesive polydensified matrix-hyaluronic acid + glycerol; HA, hyaluronic acid; MFU-V, microfocused ultrasound with visualization.
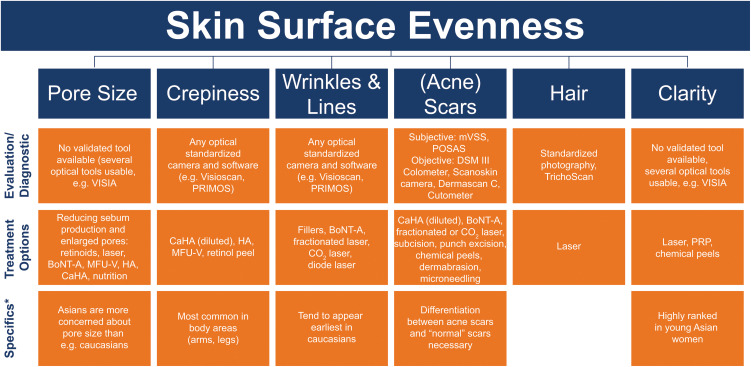
Figure 3.
Skin surface evenness parameters, measurement methods, and treatment options.
Note: *Factors to consider specific to each EPC parameter where applicable.
Abbreviations: BoNT‐A, botulinum toxin type A; CaHA, calcium hydroxylapatite; CO2, carbon dioxide; HA, hyaluronic acid; MFU-V, microfocused ultrasound with visualization; mVSS, Modified Vancouver Scar Scores; POSAS, Patient and Observer Scar Assessment Scale; PRIMOS, Phaseshift Rapid in vivo Measurement of Skin; PRP, platelet-rich plasma.
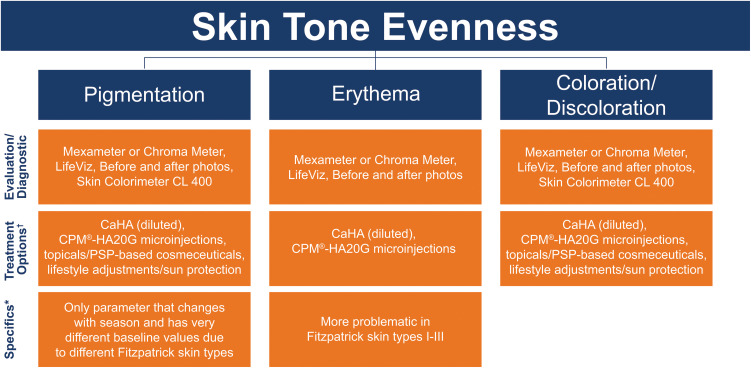
Figure 4.
Skin tone evenness parameters, measurement methods, and treatment options.
Notes: *Factors to consider specific to each EPC parameter where applicable. †Both CaHA (diluted) and CPM®-HA20G indirectly improve skin tone evenness.
Abbreviations: CaHA, calcium hydroxylapatite; CPM®-HA20G, cohesive polydensified matrix-hyaluronic acid + glycerol; HA, hyaluronic acid.
Figure 5.
Skin glow measurement methods and treatment options.
Abbreviations: BoNT‐A, botulinum toxin type A; CaHA, calcium hydroxylapatite; CPM®-HA20G, cohesive polydensified matrix-hyaluronic acid + glycerol; HA, hyaluronic acid; MFU-V, microfocused ultrasound with visualization.
Skin Firmness
Firmness comprises all viscoelastic properties of the skin and underlying tissues. The individual parameters that constitute skin firmness are (Figure 2):
Elasticity – The ability of the skin to return to its original position.
Tautness/Tightness – The resistance of skin against mechanical force.
Hydration – The water content of the epidermis.
Measurement methods and treatments to improve skin firmness vary depending on the targeted parameter as described below.
Elasticity
Measurement: The methods for measuring elasticity include a Cutometer (Courage + Khazaka, Cologne, Germany)18 and/or a snap test.19 The Cutometer is a validated option for measuring elasticity whereas the snap test can be useful for in-office assessment.
Treatment: Skin elasticity is mainly addressed with biostimulators, including calcium hydroxylapatite (CaHA; Radiesse®, Merz North America, Raleigh, NC, USA) – both diluted and hyperdiluted have been shown to have a biostimulating effect – and dermal/subdermal microinjections of hyaluronic acid (HA) with glycerol (cohesive polydensified matrix-HA + glycerol [CPM®-HA20G; Belotero® Revive, Merz Pharmaceuticals GmbH, Frankfurt, Germany]).20,21 Areas that benefit the most from CaHA treatment include cheeks, neck, chest, inner arms, inner thighs, and hands.22 Other treatment options for improving elasticity include microfocused ultrasound with visualization (MFU-V; Ultherapy®, Merz North America, Raleigh, NC, USA),23,24 fractional laser,25 deep peelings,26 and topicals, such as retinoids.27
Tautness/Tightness
Measurement: Tautness can be measured with a Cutometer.13,28–30 Other useful assessment methods include a pinch-test19 and comparison of before and after photos.31
Treatment: The treatment options to improve tautness include MFU-V,23 CaHA (diluted),32 and CPM®-HA (25 mg, intradermal and subdermal).20 MFU-V improves skin laxity, is safe in all skin types, and does not cause post-inflammatory hyperpigmentation (PIH).33
Hydration
Measurement: Hydration can be assessed with a Corneometer (Courage + Khazaka, Cologne, Germany).34
Treatment: Lifestyle adjustments, such as limiting sun exposure, avoiding excessive bathing, and regular use of moisturizers, may improve skin hydration.35,36 Other treatment options are CPM®-HA20G and topicals/PSP-based cosmeceuticals.20,37
Skin Surface Evenness
There was strong consensus that skin surface evenness consists of six parameters (Figure 3):
Pores – Visible, topographic feature of skin surface that are generally the enlarged opening of pilosebaceous follicles.
Crepiness – Skin that is thin, wrinkled, similar in appearance to crepe paper. More common on arms, legs, neck, and under eyes.
Wrinkles and lines – Lines in the skin caused by mechanical pressure, skin dehydration, or continuous muscle movement. Wrinkles and lines tend to appear earliest in Caucasians.
Scars – Area of fibrous tissue that replaces normal skin after an injury. Acne scars are distinct from other scar types.
Hair – No visible hair on the face.
Clarity – Clear skin without black and white heads, pimples, and spots (red or brown rough age spots). Very important to young Asian women.
For measuring overall surface evenness, VISIA (Canfield Scientific, Parsippany, New Jersey, USA) is a good choice. Treatment options for improving surface evenness in general include botulinum toxin type A (BoNT‐A), CaHA (diluted or hyperdiluted depending on the parameter being targeted as detailed below), CPM®-HA, and MFU-V.
Pores
Measurement: There is no validated tool for measurement of pore size, but one option is the LifeViz 3D imaging system (QuantifiCare S.A., Biot, France), which is currently used as a pore measurement tool.38
Treatment: BoNT‐A, often used in a hyperdiluted form, can be useful for pore tightening in patients with increased sebum production, such as acne and rosacea.39,40 HA can reduce pore size,41,42 and hyperdiluted CaHA43 can improve overall skin quality that encompasses pore size.
Crepiness
Measurement: An optical standardized camera and software, such as Visioscan (Courage + Khazaka Electronic, Cologne, Germany) and PRIMOS (Phaseshift Rapid In vivo Measurement Of Skin, LMI Technologies GmbH, Teltow/Berlin, Germany), can assess crepiness.44
Treatment: The treatment options for improving crepiness include CaHA (diluted),22 HA, MFU-V,45,46 retinol peel, lasers,46 and deep peelings.47,48
Wrinkles and Lines
Measurement: Optical standardized camera and software, such as Visioscan and PRIMOS, can measure wrinkles and lines.44,49
Treatment: CaHA, HA, BoNT‐A, fractionated laser, carbon dioxide (CO2) laser, and diode laser can reduce wrinkles and lines.50,51
Scars
Measurement: Acne scars can be measured subjectively with Modified Vancouver Scar Scores (mVSS) or Patient and Observer Scar Assessment Scale (POSAS).52,53 Objective measurement tools include DSM II Colormeter (Cortex Technology, Hadsund, Denmark [DSM III is the currently available model]),54–56 Scanoskin camera (Leniomed Ltd, London, United Kingdom),56 DermaScan C (Cortex Technology, Hadsund, Denmark),55,56 and Cutometer.55,56
Treatment: Acne scars can be reduced by treatment with CaHA (diluted), BoNT‐A (hyperdiluted), fractionated or CO2 laser, subcision, punch excision, chemical peels, dermabrasion, and microneedling.57
Hair
Measurement: Hair density, diameter, and growth rate can be measured with TrichoScan (Tricholog GmbH, Freiburg, Germany),58 included in TrichoScale AI (FotoFinder Systems GmbH, Bad Birnbach, Germany) and TrichoScan HD (DermoScan GmbH, Regensburg, Germany) systems, and standardized photography.
Treatment: The preferred treatment option for removing unwanted hair is laser.59
Clarity
Measurement: There is no validated measurement tool for clarity, but several optical tools, such as VISIA, can be used.60,61
Treatment: Laser,62 platelet-rich plasma (PRP),63,64 and chemical peels65 can improve clarity.
Skin Tone Evenness
The parameters that make up this EPC include (Figure 4):
Pigmentation – Determined by distribution of melanosomes within melanocytes. Evenness is defined as uniformity in skin pigmentation and even distribution of pigments. Pigmentation issues, such as PIH, are more prominent in Fitzpatrick skin types IV–VI. This is the only parameter that changes by season and has very different baseline values that vary by Fitzpatrick skin type.
Erythema – Redness due to increased blood flow. More prominent in Fitzpatrick skin types I–III.
Coloration/Discoloration – Consensus on a definition for this term could not be reached (see Discussion section). However, the panel did agree that patients generally do not want to change their skin color, but rather want it to be more even in tone.
Pigmentation
Measurement: Mexameter (Courage + Khazaka, Cologne, Germany)66,67 or Chroma Meter CR-400 (Konica Minolta, Ramsey, New Jersey, USA)66,68 can measure pigmentation. Other options include LifeViz,55 standardized before and after photos, and the Skin Colorimeter CL 400 (Courage + Khazaka, Cologne, Germany).69
Treatment: Treatments that can improve pigmentation include topicals/PSP-based cosmeceuticals (NEOCUTIS®, Merz North America, Raleigh, NC, USA),70 and lifestyle adjustments, such as sun protection.71 Lasers help with pigment62 and are the second line therapy after topicals. CaHA (diluted) and CPM®-HA20G microinjections can improve undereye hyperpigmentation.72
Erythema
Measurement: Assessing erythema can be done with Mexameter, Chroma Meter, LifeViz, or standardized before and after photos.73
Treatment: CaHA (diluted)43 and CPM®-HA20G microinjections20 both indirectly improve skin tone evenness. Lasers can also improve skin tone evenness. BoNT-A has also been shown to enhance skin tone evenness.74
Coloration/Discoloration
Measurement: This parameter can be measured with Mexameter,66,75 Chroma Meter,66 LifeViz, standardized before and after photos, or Skin Colorimeter CL 400.75
Treatment: The treatment options are the same as for pigmentation and include CaHA (diluted),43,72 CPM®-HA20G microinjections, topicals/PSP-based cosmeceuticals, lifestyle adjustments/sun protection.
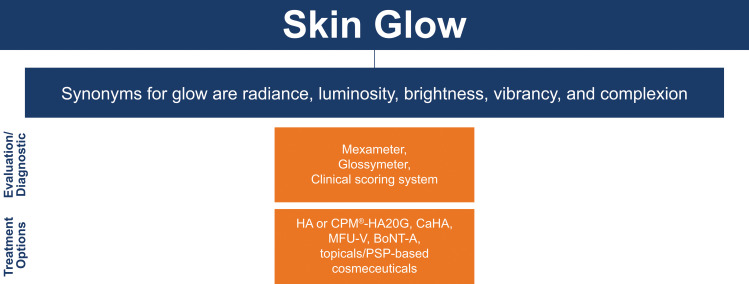
Skin Glow
Skin glow is the only EPC that does not have individual parameters (Figure 5). However, it can be described by several synonyms, including radiance, luminosity, brightness, vibrancy, and complexion.
Measurement: Glow can be measured using a Mexameter, Glossymeter (Courage + Khazaka, Cologne, Germany), or clinical scoring system.20
Treatment: The recommended treatments to improve glow include HA or CPM®-HA20G,20 CaHA,43 MFU-V, BoNT‐A, chemical peels,65 and topicals/PSP-based cosmeceuticals.70 Microinjections with HA can improve hydration and have biostimulatory properties, which improves glow.20 Brightening after MFU-V is mainly due to the decrease of scattered reflection with texture improvement rather than depigmentation. 20
Thinking Beyond the Skin
The EPCs can be affected by multiple tissue layers, so it’s important to note that surface appearance and quality can stem from and be impacted by deep structures or tissues (Table 1). This means that topical approaches targeting the surface skin level may not be sufficient. Instead, improving skin quality EPCs can require a holistic, multilayer treatment strategy, and deep tissue supporting treatments can affect skin surface.
Table 1.
Examples of Multilayer Origins of Emergent Perceptual Skin Quality Categories
| Tissue Origin | Cellular Changes | Skin Appearance Effect | EPC Affected |
|---|---|---|---|
| Epidermis | Slower cell turnover | Skin roughness/texture | Surface evenness |
| Epidermis | Cellular adhesion/compromise Disease/age/hormones |
Increased trans epidermal water loss | Firmness |
| Epidermis | Increased melanin | Uneven tone | Tone evenness |
| Dermis | Decreased HA | Dryness/loss of skin turgor | Firmness |
| Dermis | Elastin fragmentation | Laxity/crepiness | Firmness |
Treatment Options
To aid clinicians in the development of a treatment strategy, Table 2 provides treatment options for each EPC.
Table 2.
Treatment Options for Each Emergent Perceptual Skin Quality Category
| Skin Firmness | Skin Glow | Skin Tone Evenness | Skin Surface Evenness | |
|---|---|---|---|---|
| IncobotulinumtoxinA | ✓ | ✓ | ||
| CaHA | ✓ | ✓ | ✓* | ✓ |
| CPM®-HA | ✓†ǂ¥ | ✓¥ | ✓¥ | ✓†ǂ |
| MFU-V | ✓ | ✓* | ✓ | |
| Topicals (eg, NEOCUTIS®) | ✓ | ✓ | ✓ | ✓ |
| Lasers | ✓ | ✓ | ✓ | |
| Microneedling | ✓ | ✓ | ✓ | ✓ |
| Peelings | ✓ | ✓ | ✓ | ✓ |
Notes: *Indirectly effective. †CPM®-HA 25.5 Mg. ǂCPM®-HA 26 Mg.¥CPM®-HA20G.
Abbreviations: CaHA, calcium hydroxylapatite; CPM®-HA, cohesive polydensified matrix-hyaluronic acid; HA, hyaluronic acid; MFU-V, microfocused ultrasound with visualization.
Discussion
The main outcomes of the advisory board were a strong consensus that good skin quality is defined as skin that is healthy, undamaged, youthful in appearance and that skin quality can be described using the four EPCs. To our knowledge, this is the first comprehensive assessment of skin quality in aesthetic practice. This consensus definition forms the foundation of a universal understanding of skin quality. Further work is needed to develop tailored treatment algorithms based on the EPCs that incorporate gender and different ethnicities and ages; however, the EPCs and associated measurement and treatment options presented here can help guide clinicians in their daily practice to establish when aesthetic treatment is appropriate and which treatment modality can be used to ensure optimal results. The measurement options can be incorporated during patient consultation to establish a plan for treatment. Using this paradigm, clinicians can determine the EPC in which a patient has the most predominant treatment need and select a treatment from the options listed (Figures 2–5). In addition, patients can be educated about the EPCs to help them make informed decisions about the EPC they would most like to address (eg, skin tone evenness). The clinician can in turn determine the appropriate treatment option(s) and the tissue or skin layer(s) to address.
Skin quality as defined by the four EPCs also plays a critical role in patients’ decision to seek treatment. There was strong consensus that skin quality influences self-esteem and perception of attractiveness and age, which in turn influences patients’ desire for aesthetic treatments. For example, there was strong consensus that patients are mainly concerned about what is relevant for good skin quality in their age group, and a balance of the EPCs is beneficial to be perceived as younger and attractive.
EPC quality can be affected by multiple tissue layers. This implies that topical treatments might not be adequate to improve target parameters. The preferred approach to improve EPC quality is a multilayer treatment strategy. It’s important to note that this may require deep tissue supporting treatments.
The panel was unable to reach a consensus on the proposed definition of skin coloration (comprises redness, telangiectasias, erythema, melanin). Some members considered the term to be ambiguous, while some objected to the inclusion of telangiectasias because they regarded that term to be more descriptive of redness. Others considered coloration to comprise both melanin and vascular disorders.
This consensus is not intended to be a systematic review but the core treatment options recommended by the consensus advisory board are supported both by their clinical experience and research that demonstrates the therapies improve the target EPC or parameter. The treatments can be used in combination to target multiple issues and achieve a multilayer treatment strategy. The following details their mechanism of action, their advantages and proven uses, and the recommended target EPCs.
BoNT-A
BoNT‐A’s primary mechanism of action is inhibition of acetylcholine release, which leads to muscle paralysis.76 Intramuscular injections of BoNT‐A reduce wrinkles that are due to muscle contraction. Intradermal injections can decrease sebum production possibly by acetylcholine inhibition and improve skin laxity and enlarged facial pores possibly by inhibiting contraction of arrector pili muscles.40
IncobotulinumtoxinA (INCO; Xeomin®/Bocouture®, Merz Pharmaceuticals GmbH, Germany) may have advantages over other botulinum toxin formulations for improving skin quality because it is made by a unique two-step chromatographic purification process that yields only the pure 150 kDa active neurotoxin without unnecessary bacterial proteins and/or denatured BoNT protein, which may initiate an immune response and the production of neutralizing antibodies that can be associated with decreased effect over time or treatment non-response.40 Further studies are needed to support this hypothesis. Compared with other BoNT-A formulations (onabotulinumtoxin, abobotulinumtoxin, prabotulinumtoxinA, and letibotulinumtoxin A), INCO was the only one that shortened fibroblasts at all dilutions (ranging from 1:1 to 1:10), indicating it could induce near-immediate lifting effects with no apparent cytotoxic effect.77 The low potential for induction of immunogenicity of INCO makes it an ideal choice for intradermal injections, which may be more immunogenic than intramuscular because of the larger number of dendritic cells in the dermis that could facilitate antigen presentation, and evolving aesthetic procedures that require larger amounts of BoNT-A.40 Intradermal microdroplet injection of INCO was recently shown to improve facial laxity, sebum production, and pore count for up to 12 weeks after injection.40 There was consensus that BoNT‐A is also useful for improving glow and acne scars.
CaHA
CaHA can be used to immediately restore volume and contours and stimulate fibroblasts to produce collagen and elastin, allowing the skin’s natural processes to result in renewed skin structure and ongoing lift and contouring. CaHA has been increasingly used in a diluted or hyperdiluted form as a biostimulatory agent rather than a volumizing filler to improve skin quality and firmness for both the face and body.78 This makes CaHA a versatile biostimulator that can improve parameters across the EPCs, including wrinkles, elasticity, tightness, pores, crepiness, scars, pigmentation, erythema, coloration, and glow.
Hyaluronic Acid Fillers
HA can revitalize skin texture by increasing dermal hydration and production of collagen and elastin.79–82 CPM®-HA formulations (Belotero®, Merz Pharmaceuticals GmbH, Frankfurt, Germany) have also been shown to rejuvenate skin and are beneficial for enhancing all of the EPCs. In subjects with signs of facial skin aging, CPM®-HA20G, safely improved skin elasticity, firmness, tone, glow, and hydration.20 There was consensus that the CPM®-HA range of products can also improve tightness, enlarged pores, undereye pigmentation, skin tone evenness, coloration, and glow.
MFU-V
MFU-V is the only US Food and Drug Administration-cleared device for noninvasive lifting and tightening of the skin on the neck and brow, and under the chin and improvement in the appearance of lines and wrinkles on the décolleté.23 Previous consensus recommendations agreed that MFU-V can be incorporated as part of combination therapy to safely and effectively treat the aging face.3 The benefits of MFU-V include that it delivers ultrasound energy at precise, predefined depths with real-time visualization to target specific foundational tissues and trigger the natural healing process to result in gradual collagen and elastin production83,84 and that it induces thermal coagulation points of 65°C without damaging the epidermal surface and preserves the skin’s integrity.85 This makes MFU-V an effective treatment option for improving multiple EPCs and associated parameters, including glow, surface evenness, pore size, crepiness, elasticity, and tautness.
Lasers
Lasers are a necessary component of a comprehensive approach for improving skin quality. These devices primarily enhance skin surface evenness by addressing several parameters, including enlarged pores, wrinkles, unwanted hair, scars, and hyperpigmentation. Depending on the target EPC or parameter, certain lasers are preferred. CO2 lasers can reduce wrinkles86 and fractional lasers reduce acne scars.87 Several types of lasers are effective for hair removal88 and improving hyperpigmentation.89
Topicals
Lifestyle choices and topicals can serve as a preventative to maintain skin quality and a first-line option for improving skin quality.90 The consensus panel unanimously agreed that patients should be encouraged to adjust their lifestyle choices as appropriate and use topicals as necessary. As noted previously, lifestyle choices that can improve EPCs include limiting sun exposure and routine use of ultraviolet protection sunscreen and moisturizers. Beneficial topicals include PSP-based cosmeceuticals that contain growth factors, cytokines, and peptides that stimulate production of collagen, elastin, and hyaluronic acid and help rejuvenate skin.91 Daily application of PSP-based cosmeceuticals reduces facial wrinkles, roughness, and pores and improves skin firmness and elasticity.91,92
Conclusion
This global, multinational advisory board established strong consensus that skin quality is defined by the four EPCs—firmness, surface evenness, tone evenness, and glow—and associated parameters. The four EPCs can help clinicians determine the appropriate treatment option(s) and the tissue layer(s) to address. Good skin quality is important to human health, social and emotional wellbeing, and patients’ perception of attractiveness and need for aesthetic treatment.
Acknowledgments
Drs. Goldie and Kerscher share co-first authorship in this study. The authors gratefully acknowledge Simone Arnold, Birgit Bleßmann-Gurk, Janina Kolb, and Jeannette Simon who organized the advisory board, helped facilitate and moderate the advisory board members by summarizing discussions and extracting theses from our discussions, and contributed to the review of this manuscript. Steve Mitchell provided medical writing and editorial support. All are with Merz Aesthetics.
Funding Statement
This consensus and publication was sponsored by Merz Aesthetics, Raleigh, NC, USA.
Dedication
The authors dedicate this paper to the memory of Dr. Torsten Walker who unexpectedly passed away in 2020. In addition to being our dear friend and colleague, Dr. Walker made important contributions to the aesthetic community.
Author Contributions
Dr. Goldie focused on the categorization and creation of the four EPC definitions and the multilayer approach and treatment options for each EPC parameter. Dr. Kerscher focused on the measurements for each EPC parameter, the overall definition and the definition for each parameter. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Kate Goldie reports personal fees from Merz Aesthetics, during the conduct of the advisory board. Dr. Martina Kerscher reports grants from Merz Pharmaceuticals, served in the advisory board member and speaker bureau for Galderma/Qmed, outside the submitted work. Drs. Martina Kerscher and Kate Goldie served as co-chairs of the advisory board for Merz. Dr. Sabrina Guillen Fabi reports grants for supporting the advisory board from Merz, during the conduct of the study; received grants as speaker’s bureau investigator from Allergan, Galderma, Revance, and Merz, outside the submitted work. Dr. Cyro Hirano reports personal fees from Merz, during the conduct of the study. Dr. Heather Woolery-Lloyd reports personal fees from Merz, during the conduct of the advisory board. The authors report no other conflicts of interest in this work.
References
- 1.Fink B, Liebner K, Müller A-K, Hirn T, McKelvey G, Lankhof J. Hair colour and skin colour together influence perceptions of age, health and attractiveness in lightly pigmented young women. Int J Cosmet Sci. 2018;40(3):303–312. doi: 10.1111/ics.12467 [DOI] [PubMed] [Google Scholar]
- 2.Samson N, Fink B, Matts P. Interaction of skin color distribution and skin surface topography cues in the perception of female facial age and health: perception of skin color and topography. J Cosmet Dermatol. 2011;10(1):78–84. doi: 10.1111/j.1473-2165.2010.00538.x [DOI] [PubMed] [Google Scholar]
- 3.Carruthers J, Burgess C, Day D, et al. Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillers, and energy-based devices. Dermatol Surg. 2016;42(5):586–597. doi: 10.1097/DSS.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 4.Samson N, Fink B, Matts PJ. Visible skin condition and perception of human facial appearance. Int J Cosmet Sci. 2010;32(3):167–184. doi: 10.1111/j.1468-2494.2009.00535.x [DOI] [PubMed] [Google Scholar]
- 5.Fink B, Neave N. The biology of facial beauty. Int J Cosmet Sci. 2005;27(6):317–325. doi: 10.1111/j.1467-2494.2005.00286.x [DOI] [PubMed] [Google Scholar]
- 6.Fink B, Matts PJ, Brauckmann C, Gundlach S. The effect of skin surface topography and skin colouration cues on perception of male facial age, health and attractiveness. Int J Cosmet Sci. 2018;40(2):193–198. doi: 10.1111/ics.12451 [DOI] [PubMed] [Google Scholar]
- 7.Borelli C, Berneburg M. Beauty lies in the eye of the beholder? Aspects of beauty and attractiveness. J Dtsch Dermatol Ges. 2010;8(5):326–330. doi: 10.1111/j.1610-0387.2009.07318_supp.x [DOI] [PubMed] [Google Scholar]
- 8.Tan KW, Stephen ID. Skin color preferences in a Malaysian Chinese population. Front Psychol. 2019;10:1352. doi: 10.3389/fpsyg.2019.01352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw RB, Katzel EB, Koltz PF, et al. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;127(1):374–383. doi: 10.1097/PRS.0b013e3181f95b2d [DOI] [PubMed] [Google Scholar]
- 10.Gierloff M, Stöhring C, Buder T, Gassling V, Açil Y, Wiltfang J. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129(1):263–273. doi: 10.1097/PRS.0b013e3182362b96 [DOI] [PubMed] [Google Scholar]
- 11.Mendelson B, Wong C-H. Anatomy of the aging face. Plast Surg. 2013;2:78–92. [Google Scholar]
- 12.Krueger N, Luebberding S, Oltmer M, Streker M, Kerscher M. Age-related changes in skin mechanical properties: a quantitative evaluation of 120 female subjects. Skin Res Technol. 2011;17(2):141–148. doi: 10.1111/j.1600-0846.2010.00486.x [DOI] [PubMed] [Google Scholar]
- 13.Luebberding S, Krueger N, Kerscher M. Mechanical properties of human skin in vivo: a comparative evaluation in 300 men and women. Skin Res Technol. 2014;20(2):127–135. doi: 10.1111/srt.12094 [DOI] [PubMed] [Google Scholar]
- 14.Luebberding S, Krueger N, Kerscher M. Quantification of age-related facial wrinkles in men and women using a three-dimensional fringe projection method and validated assessment scales. Dermatol Surg. 2014;40(1):22–32. doi: 10.1111/dsu.12377 [DOI] [PubMed] [Google Scholar]
- 15.Pearce RH, Grimmer BJ. Age and the chemical constitution of normal human dermis. J Invest Dermatol. 1972;58(6):347–361. doi: 10.1111/1523-1747.ep12540531 [DOI] [PubMed] [Google Scholar]
- 16.Cavallini M, Papagni M, Ryder TJ, Patalano M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: objective results using digital analysis. Dermatol Surg. 2019;45(12):1598–1604. doi: 10.1097/DSS.0000000000001932 [DOI] [PubMed] [Google Scholar]
- 17.Kea B, Sun BC-A. Consensus development for healthcare professionals. Intern Emerg Med. 2015;10(3):373–383. doi: 10.1007/s11739-014-1156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn S, Kim S, Lee H, Moon S, Chang I. Correlation between a cutometer and quantitative evaluation using moire topography in age-related skin elasticity. Skin Res Technol. 2007;13(3):280–284. doi: 10.1111/j.1600-0846.2007.00224.x [DOI] [PubMed] [Google Scholar]
- 19.Hussain SH, Limthongkul B, Humphreys TR. The biomechanical properties of the skin. Dermatol Surg. 2013;39(2):193–203. doi: 10.1111/dsu.12095 [DOI] [PubMed] [Google Scholar]
- 20.Hertz-Kleptow D, Hanschmann A, Hofmann M, Reuther T, Kerscher M. Facial skin revitalization with CPM®-HA20G: an effective and safe early intervention treatment. Clin Cosmet Investig Dermatol. 2019;12:563–572. doi: 10.2147/CCID.S209256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JW, Kwon SH, Huh CH, Park KC, Youn SW. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19(1):e349–355. doi: 10.1111/j.1600-0846.2012.00650.x [DOI] [PubMed] [Google Scholar]
- 22.Goldie K, Peeters W, Alghoul M, et al. Global consensus guidelines for the injection of diluted and hyperdiluted calcium hydroxylapatite for skin tightening. Dermatol Surg. 2018;44(Suppl 1):S32–S41. doi: 10.1097/DSS.0000000000001685 [DOI] [PubMed] [Google Scholar]
- 23.Kerscher M, Nurrisyanti AT, Eiben-Nielson C, Hartmann S, Lambert-Baumann J. Skin physiology and safety of microfocused ultrasound with visualization for improving skin laxity. Clin Cosmet Investig Dermatol. 2019;12:71–79. doi: 10.2147/CCID.S188586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabi SG, Few JW, Moinuddin S. Practical guidance for optimizing patient comfort during microfocused ultrasound with visualization and improving patient satisfaction. Aesthet Surg J. 2020;40(2):208–216. doi: 10.1093/asj/sjz079 [DOI] [PubMed] [Google Scholar]
- 25.Kołodziejczak AM, Rotsztejn H. Mexametric and cutometric assessment of the signs of aging of the skin area around the eyes after the use of non-ablative fractional laser, non-ablative radiofrequency and intense pulsed light. Dermatol Ther. 2017;30(2). doi: 10.1111/dth.12470 [DOI] [PubMed] [Google Scholar]
- 26.Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25(5):329–336. doi: 10.1055/s-0029-1243082 [DOI] [PubMed] [Google Scholar]
- 27.Korolkova TN, Shepilova IA, Kharitonova EE. [Age-related factors in the impact of chemical peeling with retinol on the functional parameters of the skin]. Adv Gerontol. 2019;32(5):829–836. Russian. [PubMed] [Google Scholar]
- 28.Ohshima H, Kinoshita S, Oyobikawa M, et al. Use of Cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Res Technol. 2013;19(1):e238–242. doi: 10.1111/j.1600-0846.2012.00634.x [DOI] [PubMed] [Google Scholar]
- 29.Ryu HS, Joo YH, Kim SO, Park KC, Youn SW. Influence of age and regional differences on skin elasticity as measured by the cutometer. Skin Res Technol. 2008;14(3):354–358. doi: 10.1111/j.1600-0846.2008.00302.x [DOI] [PubMed] [Google Scholar]
- 30.Dobrev H. Application of cutometer area parameters for the study of human skin fatigue. Skin Res Technol. 2005;11(2):120–122. doi: 10.1111/j.1600-0846.2005.00090.x [DOI] [PubMed] [Google Scholar]
- 31.McBean JC, Katz BE. A pilot study of the efficacy of a 1064 and 1320 nm sequentially firing Nd: yAGlaser device for lipolysis and skin tightening. Lasers Surg Med. 2009;41(10):779–784. doi: 10.1002/lsm.20858 [DOI] [PubMed] [Google Scholar]
- 32.Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a Pilot Study. J Drugs Dermatol. 2017;16(1):68–74. [PubMed] [Google Scholar]
- 33.Fabi SG, Massaki A, Eimpunth S, Pogoda J, Goldman MP. Evaluation of microfocused ultrasound with visualization for lifting, tightening, and wrinkle reduction of the décolletage. J Am Acad Dermatol. 2013;69(6):965–971. doi: 10.1016/j.jaad.2013.06.045 [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto-Kumasaka K, Takahashi K, Tagami H. Electrical measurement of the water content of the stratum corneum in vivo and in vitro under various conditions: comparison between skin surface hygrometer and corneometer in evaluation of the skin surface hydration state. Acta Derm Venereol. 1993;73(5):335–339. doi: 10.2340/0001555573335339 [DOI] [PubMed] [Google Scholar]
- 35.Iizaka S. Skin hydration and lifestyle-related factors in community-dwelling older people. Arch Gerontol Geriatr. 2017;72:121–126. doi: 10.1016/j.archger.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 36.Rahrovan S, Fanian F, Mehryan P, Humbert P, Firooz A. Male versus female skin: what dermatologists and cosmeticians should know. Int J Womens Dermatol. 2018;4(3):122–130. doi: 10.1016/j.ijwd.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowther JM. Understanding effects of topical ingredients on electrical measurement of skin hydration. Int J Cosmet Sci. 2016;38(6):589–598. doi: 10.1111/ics.12324 [DOI] [PubMed] [Google Scholar]
- 38.Petit L, Zugaj D, Bettoli V, et al. Validation of 3D skin imaging for objective repeatable quantification of severity of atrophic acne scarring. Skin Res Technol. 2018;24(4):542–550. doi: 10.1111/srt.12464 [DOI] [PubMed] [Google Scholar]
- 39.Sayed KS, Hegazy R, Gawdat HI, et al. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: a Split Face-Controlled Study. J Dermatol Treat. 2020:1–7. doi: 10.1080/09546634.2019.1708241 [DOI] [PubMed] [Google Scholar]
- 40.Park J-Y, Cho SI, Hur K, Lee DH. Intradermal microdroplet injection of diluted incobotulinumtoxin-a for sebum control, face lifting, and pore size improvement. J Drugs Dermatol. 2021;20(1):49–54. doi: 10.36849/JDD.2021.5616 [DOI] [PubMed] [Google Scholar]
- 41.Qian W, Zhang Y-K, Hou Y, et al. Effect analysis of intradermal hyaluronic acid injection to treat enlarged facial pores. J Cosmet Dermatol. 2018;17(4):596–599. doi: 10.1111/jocd.12385 [DOI] [PubMed] [Google Scholar]
- 42.Cheng H-Y, Chen Y-X, Wang M-F, Zhao J-Y, Li L-F. Evaluation of changes in skin biophysical parameters and appearance after pneumatic injections of non-cross-linked hyaluronic acid in the face. J Cosmet Laser Ther. 2018;20(7–8):454–461. doi: 10.1080/14764172.2018.1427868 [DOI] [PubMed] [Google Scholar]
- 43.Chao YY, Kim JW, Kim J, Ko H, Goldie K. Hyperdilution of CaHA fillers for the improvement of age and hereditary volume deficits in East Asian patients. Clin Cosmet Investig Dermatol. 2018;11:357–363. doi: 10.2147/CCID.S159752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloemen MCT, van Gerven MS, van der Wal MBA, Verhaegen PDHM, Middelkoop E. An objective device for measuring surface roughness of skin and scars. J Am Acad Dermatol. 2011;64(4):706–715. doi: 10.1016/j.jaad.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 45.Wu DC, Green JB. Rejuvenation of the aging arm: multimodal combination therapy for optimal results. Dermatol Surg. 2016;42(Suppl 2):S119–123. doi: 10.1097/DSS.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 46.Vanaman M, Fabi SG, Cox SE. Neck rejuvenation using a combination approach: our experience and a review of the literature. Dermatol Surg. 2016;42(Suppl 2):S94–S100. doi: 10.1097/DSS.0000000000000699 [DOI] [PubMed] [Google Scholar]
- 47.Kligman DE, Draelos ZD. High-strength tretinoin for rapid retinization of photoaged facial skin. Dermatol Surg. 2004;30(6):864–866. doi: 10.1111/j.1524-4725.2004.30254.x [DOI] [PubMed] [Google Scholar]
- 48.Sadick N, Edison BL, John G, Bohnert KL, Green B. An advanced, physician-strength retinol peel improves signs of aging and acne across a range of skin types including melasma and skin of color. J Drugs Dermatol. 2019;18(9):918–923. [PubMed] [Google Scholar]
- 49.Dzwigałowska A, Sołyga-żurek A, Dębowska RM, Eris I. Preliminary study in the evaluation of anti-aging cosmetic treatment using two complementary methods for assessing skin surface. Skin Res Technol. 2013;19(2):155–161. doi: 10.1111/srt.12027 [DOI] [PubMed] [Google Scholar]
- 50.Archer KA, Carniol P. Diode laser and fractional laser innovations. Facial Plast Surg. 2019;35(3):248–255. doi: 10.1055/s-0039-1688846 [DOI] [PubMed] [Google Scholar]
- 51.Pavicic T, Few JW, Huber-Vorländer J. A novel, multistep, combination facial rejuvenation procedure for treatment of the whole face with incobotulinumtoxinA, and two dermal fillers- calcium hydroxylapatite and a monophasic, polydensified hyaluronic acid filler. J Drugs Dermatol. 2013;12(9):978–984. [PubMed] [Google Scholar]
- 52.Gankande TU, Wood FM, Edgar DW, et al. A modified vancouver scar scale linked with TBSA (mVSS-TBSA): inter-rater reliability of an innovative burn scar assessment method. Burns. 2013;39(6):1142–1149. doi: 10.1016/j.burns.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 53.Reinholz M, Schwaiger H, Heppt MV, et al. Comparison of two kinds of lasers in the treatment of acne scars. Facial Plast Surg. 2015;31(5):523–531. doi: 10.1055/s-0035-1567814 [DOI] [PubMed] [Google Scholar]
- 54.van der Wal M, Bloemen M, Verhaegen P, et al. Objective color measurements: clinimetric performance of three devices on normal skin and scar tissue. J Burn Care Res. 2013;34(3):e187–194. doi: 10.1097/BCR.0b013e318264bf7d [DOI] [PubMed] [Google Scholar]
- 55.Lee KC, Dretzke J, Grover L, Logan A, Moiemen N. A systematic review of objective burn scar measurements. Burns Trauma. 2016;4:14. doi: 10.1186/s41038-016-0036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee KC, Bamford A, Gardiner F, et al. Investigating the intra- and inter-rater reliability of a panel of subjective and objective burn scar measurement tools. Burns. 2019;45(6):1311–1324. doi: 10.1016/j.burns.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soliman YS, Horowitz R, Hashim PW, Nia JK, Farberg AS, Goldenberg G. Update on acne scar treatment. Cutis. 2018;102(1):21;25;47;48. [PubMed] [Google Scholar]
- 58.Hoffmann R. TrichoScan: a novel tool for the analysis of hair growth in vivo. J Investig Dermatol Symp Proc. 2003;8(1):109–115. doi: 10.1046/j.1523-1747.2003.12183.x [DOI] [PubMed] [Google Scholar]
- 59.Gan SD, Graber EM. Laser hair removal: a review. Dermatol Surg. 2013;39(6):823–838. doi: 10.1111/dsu.12116 [DOI] [PubMed] [Google Scholar]
- 60.Mekas M, Chwalek J, MacGregor J, Chapas A. An evaluation of efficacy and tolerability of novel enzyme exfoliation versus glycolic acid in photodamage treatment. J Drugs Dermatol. 2015;14(11):1306–1319. [PubMed] [Google Scholar]
- 61.Merati M, Woods C, Reznik N, Parker L. An assessment of microneedling with topical growth factors for facial skin rejuvenation: a randomized controlled trial. J Clin Aesthet Dermatol. 2020;13(11):22–27. [PMC free article] [PubMed] [Google Scholar]
- 62.Ungaksornpairote C, Manuskiatti W, Junsuwan N, Wanitphakdeedecha R. A prospective, split-face, randomized study comparing picosecond to Q-switched Nd: YAG laser for treatment of epidermal and dermal pigmented lesions in Asians. Dermatol Surg. 2020;46(12):1671–1675. doi: 10.1097/DSS.0000000000002486 [DOI] [PubMed] [Google Scholar]
- 63.Cameli N, Mariano M, Cordone I, Abril E, Masi S, Foddai ML. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826–835. doi: 10.1097/DSS.0000000000001083 [DOI] [PubMed] [Google Scholar]
- 64.Gawdat HI, Hegazy RA, Fawzy MM, Fathy M. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40(2):152–161. doi: 10.1111/dsu.12392 [DOI] [PubMed] [Google Scholar]
- 65.Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I, Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3(7):32–43. [PMC free article] [PubMed] [Google Scholar]
- 66.Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R). Skin Res Technol. 2000;6(4):230–238. doi: 10.1034/j.1600-0846.2000.006004230.x [DOI] [PubMed] [Google Scholar]
- 67.Feather JW, Ellis DJ, Leslie G. A portable reflectometer for the rapid quantification of cutaneous haemoglobin and melanin. Phys Med Biol. 1988;33(6):711–722. doi: 10.1088/0031-9155/33/6/005 [DOI] [PubMed] [Google Scholar]
- 68.Lee JA, Osmanovic S, Viana MAG, Kapur R, Meghpara B, Edward DP. Objective measurement of periocular pigmentation. Photodermatol Photoimmunol Photomed. 2008;24(6):285–290. doi: 10.1111/j.1600-0781.2008.00377.x [DOI] [PubMed] [Google Scholar]
- 69.Fossa Shirata MM, Alves GAD, Maia Campos PMBG. Photoageing-related skin changes in different age groups: a clinical evaluation by biophysical and imaging techniques. Int J Cosmet Sci. 2019;41(3):265–273. doi: 10.1111/ics.12531 [DOI] [PubMed] [Google Scholar]
- 70.Dreher F, Draelos ZD, Gold MH, Goldman MP, Fabi SG, Puissegur Lupo ML. Efficacy of hydroquinone-free skin-lightening cream for photoaging. J Cosmet Dermatol. 2013;12(1):12–17. doi: 10.1111/jocd.12025 [DOI] [PubMed] [Google Scholar]
- 71.Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi: 10.1016/j.jdermsci.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 72.Corduff N. An alternative periorbital treatment option using calcium hydroxyapatite for hyperpigmentation associated with the tear trough deformity. Plast Reconstr Surg Glob Open. 2020;8(2):e2633. doi: 10.1097/GOX.0000000000002633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fullerton A, Fischer T, Lahti A, Wilhelm KP, Takiwaki H, Serup J. Guidelines for measurement of skin colour and erythema. A report from the standardization group of the European society of contact dermatitis. Contact Dermatitis. 1996;35(1):1–10. doi: 10.1111/j.1600-0536.1996.tb02258.x [DOI] [PubMed] [Google Scholar]
- 74.Guida S, Farnetani F, Nisticò SP, et al. New trends in botulinum toxin use in dermatology. Dermatol Pract Concept. 2018:277–282. doi: 10.5826/dpc.0804a05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matias AR, Ferreira M, Costa P, Neto P. Skin colour, skin redness and melanin biometric measurements: comparison study between Antera(®) 3D, Mexameter(®) and Colorimeter(®). Skin Res Technol. 2015;21(3):346–362. doi: 10.1111/srt.12199 [DOI] [PubMed] [Google Scholar]
- 76.Giordano CN, Matarasso SL, Ozog DM. Injectable and topical neurotoxins in dermatology: basic science, anatomy, and therapeutic agents. J Am Acad Dermatol. 2017;76(6):1013–1024. doi: 10.1016/j.jaad.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 77.Wanitphakdeedecha R, Kaewkes A, Ungaksornpairote C, Limsaengurai S, Panich U, Manuskiatti W. The effect of botulinum toxin type A in different dilution on the contraction of fibroblast-In vitro Study. J Cosmet Dermatol. 2019;18(5):1215–1223. doi: 10.1111/jocd.13058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Almeida AT, Figueredo V, da Cunha ALG, et al. Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (radiesse) as a face and body biostimulatory agent. Plast Reconstr Surg Glob Open. 2019;7(3):e2160. doi: 10.1097/GOX.0000000000002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mammucari M, Gatti A, Maggiori S, Bartoletti CA, Sabato AF. Mesotherapy, definition, rationale and clinical role: a consensus report from the Italian society of mesotherapy. Eur Rev Med Pharmacol Sci. 2011;15(6):682–694. [PubMed] [Google Scholar]
- 80.Galadari H, Al Faresi F. Mesotherapy. Skinmed. 2011;9(6):342–343. [PubMed] [Google Scholar]
- 81.O’Mahony M. Skin rejuvenation using mesotherapy: indications, techniques and ingredients. J Aesthetic Nurs. 2012;1(6):292–297. doi: 10.12968/joan.2012.1.6.292 [DOI] [Google Scholar]
- 82.Fabi SG, Goldman MP. Hand rejuvenation: a review and our experience. Dermatol Surg. 2012;38(7 Pt 2):1112–1127. doi: 10.1111/j.1524-4725.2011.02291.x [DOI] [PubMed] [Google Scholar]
- 83.Laubach HJ, Makin IRS, Barthe PG, Slayton MH, Manstein D. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34(5):727–734. doi: 10.1111/j.1524-4725.2008.34196.x [DOI] [PubMed] [Google Scholar]
- 84.Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32(1):71–77. doi: 10.1016/j.det.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 85.White WM, Makin IRS, Slayton MH, Barthe PG, Gliklich R. Selective transcutaneous delivery of energy to porcine soft tissues using Intense Ultrasound (IUS). Lasers Surg Med. 2008;40(2):67–75. doi: 10.1002/lsm.20613 [DOI] [PubMed] [Google Scholar]
- 86.Ross EV, Miller C, Meehan K, et al. One-pass CO2 versus multiple-pass Er: yAGlaser resurfacing in the treatment of rhytides: a comparison side-by-side study of pulsed CO2 and Er: yAGlasers. Dermatol Surg. 2001;27(8):709–715. doi: 10.1046/j.1524-4725.2001.01015.x [DOI] [PubMed] [Google Scholar]
- 87.Qian H, Lu Z, Ding H, Yan S, Xiang L, Gold MH. Treatment of acne scarring with fractional CO2 laser. J Cosmet Laser Ther. 2012;14(4):162–165. doi: 10.3109/14764172.2012.699679 [DOI] [PubMed] [Google Scholar]
- 88.Ibrahimi OA, Avram MM, Hanke CW, Kilmer SL, Anderson RR. Laser hair removal. Dermatol Ther. 2011;24(1):94–107. doi: 10.1111/j.1529-8019.2010.01382.x [DOI] [PubMed] [Google Scholar]
- 89.Crispin MK, Hruza GJ, Kilmer SL. Lasers and energy-based devices in men. Dermatol Surg. 2017;43(Suppl 2):S176–S184. doi: 10.1097/DSS.0000000000001274 [DOI] [PubMed] [Google Scholar]
- 90.Carruthers J, Carruthers A. A multimodal approach to rejuvenation of the lower face. Dermatol Surg. 2016;42(Suppl 2):S89–93. doi: 10.1097/DSS.0000000000000749 [DOI] [PubMed] [Google Scholar]
- 91.Aldag C, Nogueira Teixeira D, Leventhal PS. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: a review of the literature. Clin Cosmet Investig Dermatol. 2016;9:411–419. doi: 10.2147/CCID.S116158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dreher F. A novel matrikine-like micro-protein complex (MPC) technology for topical skin rejuvenation. J Drugs Dermatol. 2016;15(4):457–464. [PubMed] [Google Scholar]