Abstract
Epidermal growth factor receptor (EGFR) fusions are rare genomic events in non-small-cell lung cancer (NSCLC). With advances in detection technology, some uncommon genomic mutation events, including EGFR fusions, have been detected. There are no standard treatment options for NSCLC patients harboring EGFR fusion. Herein, we report a case of KIF5B-EGFR fusion in NSCLC responding to tyrosine kinase inhibitors (TKIs). A 50-year-old male underwent left upper lobectomy followed by adjuvant chemotherapy for pathological stage IA3 lung adenocarcinoma. The tumor tissue was subjected to next-generation sequencing (NGS) and showed a KIF5B-EGFR fusion. When cancer recurrence occurred thirteen months later, the patient received afatinib (40 mg qd) as second-line treatment, and a partial response was observed, which resulted in an 11-month progression-free survival (PFS). This case provides valuable information on the response to afatinib in an NSCLC patient with a novel KIF5B-EGFR fusion. The NGS assay provides a powerful tool for identifying rare or atypical EGFR gene mutations in patients with NSCLC, which should be encouraged in clinical practice.
Keywords: KIF5B-EGFR, EGFR fusion, next-generation sequencing, afatinib, non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-associated death globally, and non-small-cell lung cancer (NSCLC) accounts for 80–85% of cases.1 NSCLC in nonsmokers tends to be driven by a single somatic mutation or a gene fusion.2 The identification of driver mutations in epidermal growth factor receptor (EGFR) and subsequent successful clinical development of tyrosine kinase inhibitors (TKIs) significantly improve clinical outcomes in NSCLC.3 To date, TKIs targeting EGFR mutation have become one of the standard treatment options for patients with advanced NSCLC harboring EGFR mutations.4 However, the molecular pathogenesis of >40% of NSCLCs is still unknown.5 Gene fusions are increasingly recognized as important cancer drivers. With advances in detection methods, such as next-generation sequencing (NGS), some rare or atypical gene fusions involving EGFR have been found in NSCLC patients, including EGFR-RAD51, EGFR-TNS3, EGFR-SEPTIN14, EGFR-ZCCHC6, EGFR-SHC1 and EGFR-PURB.6–11 To date, only a few reports have reported the effectiveness of TKIs in patients with these rare mutations in the literature.
Herein, we report a case of a novel kinesin family member 5B (KIF5B)-EGFR fusion and the efficacy of afatinib in this type of fusion in NSCLC patients. The current case provides important insights into the use of TKIs in NSCLC patients with rare EGFR fusions.
Case Report
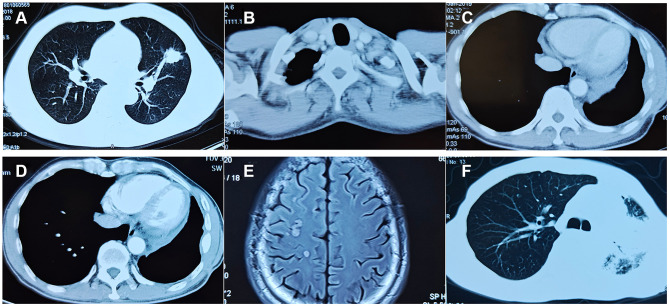
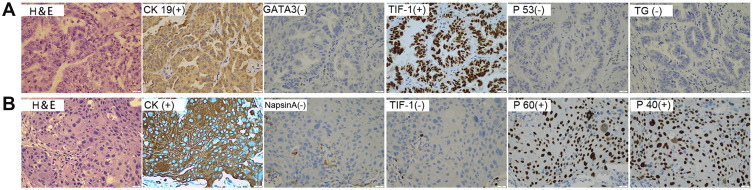
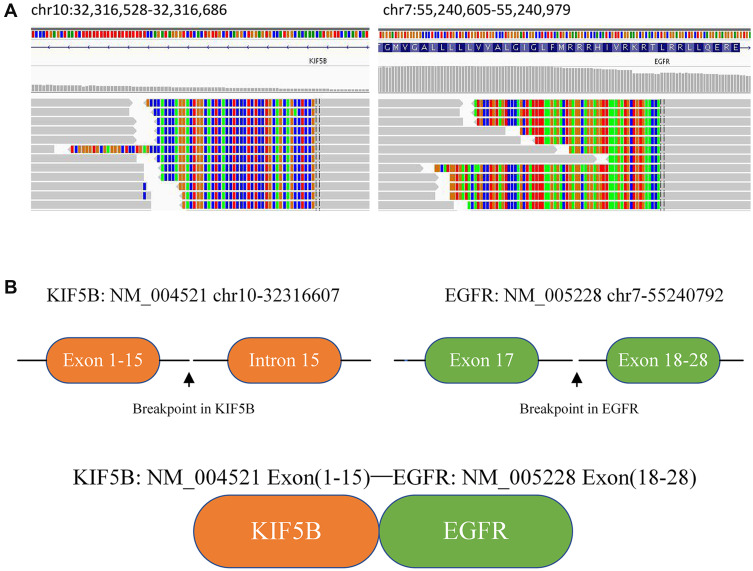
A 50-year-old male former smoker underwent left upper lobectomy in January 2018 after an irregular nodule was found in the left upper lobe during a screening computed tomography (CT) of the thorax (Figure 1A). Immunohistochemistry (IHC) was positive for cytokeratin 19, EGFR, Ki67 (10%) and TTF-1; and negative for ALK, GATA3, TG and P53 (Figure 2A). The tumor tissue was also subjected to NGS (Berry oncology, Fujian China) and showed a novel KIF5B-EGFR fusion (Figure 3A and B). The diagnosis of stage I A3 (pT1cN0M0) lung adenocarcinoma (ADC) and intraductal tumor thrombus was confirmed by pathological examination, and the patient received adjuvant chemotherapy with pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 every 21 days for 4 cycles from January 2018 to April 2018. The patient was treated with chemotherapy again (pemetrexed and cisplatin) starting in February 2019 due to recurrence of the cancer in the left supraclavicular lymph node (LN) and left rib at 13 months after surgery (Figure 1B and C). The patient was considered to have disease progression after 2 cycles of chemotherapy. Because of the detection of a KIF5B-EGFR fusion from a biopsy sample of metastatic supraclavicular LNs after disease progression, treatment with afatinib (40 mg qd) was initiated in April 2019. In addition, metastatic LNs showed components of both squamous cell carcinoma (SCC) and ADC. After 2 months of afatinib treatment, CT examinations showed that partial response was achieved (Figure 1D). He remained on afatinib for 11 months, after which he experienced only intracranial disease progression (Figure 1E). Subsequently, the patient discontinued afatinib treatment and was treated with whole brain radiation therapy. Due to the presence of T790M identified by NGS on plasma cell-free DNA, the patient received osimertinib one month after the completion of radiotherapy. Obstructive pneumonia developed with signs of disease progression after four months of osimertinib (Figure 1F). The patient was readmitted for dyspnea and progressive weakness. A repeat biopsy of pulmonary lesions was performed for NGS to explore the mechanism(s) of acquired resistance and showed components of only SCC with the KIF5B-EGFR fusion. IHC was positive for cytokeratin, P40, P60, PD-L1 (10%) and Ki67 (20%); and negative for ALK, TTF-1 and Napsin A (Figure 2B). After occurrence of obstructive pneumonia, his condition deteriorated resulting in antitumor treatment discontinuation, and subsequently treatment changed to best supportive care. Four months after stopping all antitumor therapy, the patient is still alive. The timeline of the clinical course is summarized in Figure S1.
Figure 1.
Imaging scans show: (A) a mass in the left upper lung lobe; (B) left supraclavicular lymph node metastasis; (C) left rib metastasis before afatinib therapy; (D) partial response after 2 months of afatinib treatment; (E) disease progression in brain; (F) obstructive pneumonia developed.
Figure 2.
Multibiomarker immunohistochemistry confirms pathological typing. (A) Pathologic examination revealed adenocarcinoma of the primary tumor. (B) The pathological result of recurrent pulmonary lesions by repeat biopsy was squamous cell carcinoma. (400×magnification).
Figure 3.
Next-generation sequencing analysis of KIF5B-EGFR fusion mutations in the patient’s postoperative tumor tissue. (A) Integrative Genomics Viewer snapshot of KIF5B-EGFR fusion; (B) a schematic map of the KIF5B-EGFR fusion protein domain structure.
Discussion
EGFR fusions are rare genomic events in NSCLC. Advances in detection technology, such as NGS, have created a new method for the detection of some rare variants of EGFR fusions.6–11 EGFR fusions were found at a frequency of 0.13% in the MSK-IMPACT NSCLC data.10 The investigators surveyed over 10,000 lung cancers using NGS and found five EGFR gene fusions (0.05%), most commonly EGFR-RAD51.7 In a recent report in 2019, a novel EGFR-SHC1 fusion occurred in 0.06% (1 of 1681 cases) of Chinese patients with lung ADC.8 Using data from OrigiMed, EGFR fusion was found at a frequency of 0.05% (5 of 989 cases) in Chinese patients with lung ADC.12
Kartik et al reported five patients with stage IV lung ADC harboring EGFR fusions, four of whom were treated with erlotinib.7 They also demonstrated that the EGFR-RAD51 fusion confers an oncogenic phenotype and is able to activate downstream signaling via the MAPK and PI3K/AKT pathways in preclinical studies.7 An in vitro model showed that the growth of Ba/F3 cells expressing EGFR-RAD51 was inhibited by both TKIs and the EGFR antibody cetuximab.7 These results supported the dependence of EGFR-fusion tumors on EGFR-mediated downstream signaling and suggested EGFR-targeted agents for patients whose tumors harbor these novel variants. Literature reports of the effectiveness of EGFR-TKIs in patients with EGFR fusion are scarce. Detailed characteristics of patients with NSCLC harboring EGFR fusions are summarized in Table 1. In addition to this case, fourteen EGFR fusion cases have been reported in NSCLC, most commonly RAD51 (8 of 15 cases). Eighty-six percent of cases (13/15) are ADCs. Among them, eleven patients received EGFR-TKIs. One patient showed disease stabilization to afatinib. The other ten patients achieved a partial response to first- or second-generation TKIs, including erlotinib (6 patients), icotinib (2 patients) and afatinib (2 patients). For advanced NSCLC patients harboring EGFR fusion, EGFR-TKIs may hold promise as the initial treatment, where they have demonstrated superiority over conventional chemotherapy in patients with sensitizing EGFR mutation.
Table 1.
Individual Clinicopathologic Data of Patients with Non-Small Cell Lung Cancer Harboring Novel EGFR Fusions
| Patient No. | Ethnicity | Age/Gender | Smoking Status | Diagnosis | EGFR Fusion | Drug | Response | PFS, mo |
|---|---|---|---|---|---|---|---|---|
| 17 | South Asian | 35/F | Never | ADC/IV | EGFR-RAD51 | Erlotinib | PR | >8 |
| 27 | Caucasian | 21/F | Prev/Curr | ADC/IV | EGFR-RAD51 | Erlotinib | PR | >5 |
| 37 | Caucasian | 43/F | Prev/Curr | ADC/IV | EGFR-PURB | Erlotinib | PR | >20 |
| 47 | Caucasian | 38/M | Prev/Curr | ADC/IV | EGFR-RAD51 | Erlotinib | PR | >6 |
| 57 | Caucasian | 60/F | Never | ADC/IV | EGFR-RAD51 | Pemetrexed | PR | NA |
| 66,11 | American | NA/F | Prev/Curr | ADC/NA | EGFR-TNS3 | NA | NA | NA |
| 76,11 | American | NA/M | Never | SCC/NA | EGFR-ZCCHC6 | NA | NA | NA |
| 86 | Chinese | 48/M | Prev/Curr | ADC/IV | EGFR-RAD51 | Erlotinib | PR | >5 |
| 912 | Chinese | 26/M | Never | ADC/IV | EGFR-RAD51 | Icotinib | PR | >15 |
| 1010 | American | 62/F | Never | ADC/IV | EGFR-RAD51 | Afatinib | SD | >6 |
| 119 | Chinese | 62/F | NA | ADC/NA | EGFR-SEPTIN14 | Icotinib | PR | NA |
| 128 | Chinese | 61/M | Prev/Curr | ADC/IIIA | EGFR-SHC1 | NA | NA | NA |
| 1326 | American | 36/M | NA | NSCLC/IV | EGFR-RAD51 | Erlotinib | PR | >24 |
| 1427 | Chinese | 45/F | NA | ADC/NA | KIF5B-EGFR | Afatinib/ | PR | >10 |
| Bevacizumab | ||||||||
| 15 | Chinese | 50/M | Prev/Curr | ADC/IV | KIF5B-EGFR | Afatinib | PR | 11 |
Abbreviations: PFS, progression-free survival; mo, months; F, female; M, male; NA, not available; Prev, previous; Curr, current; PR, partial remission; SD, stable disease.
In this study, we identified a case of NSCLC with a novel EGFR fusion who was sensitive to afatinib treatment. Afatinib is a highly potent, irreversible dual EGFR/HER2 tyrosine kinase inhibitor potentially efficacious in the treatment of cancers dependent on EGFR/HER2 signaling.13 Recent clinical data indicate that patients treated with afatinib have a better prognosis than first-generation TKIs in patients with NSCLC harboring uncommon and compound EGFR mutations.14–17 Moreover, afatinib also showed the significant antitumor efficacy for patient with lung ADC harboring the EGFR kinase domain duplication.18 These results indicate that afatinib may be an appropriate choice of treatment for this population with uncommon EGFR mutations including rare EGFR fusions.
While multiple mechanisms can lead to TKI resistance, the EGFR T790M mutation is a major mechanism of acquired resistance to afatinib, being present in 56–73% of cases.19,20 In this study, blood-based NGS testing revealed the acquisition of T790M. Osimertinib, a third-generation EGFR-TKI, has demonstrated high efficacy in patients with T790M-positive advanced NSCLC in whom disease had progressed during prior first- or second-generation TKI therapy.21,22 However, the patient obtained a limited PFS benefit from osimertinib in this case, the reasons may be the low abundance of T790M (0.17%). Previous study has shown that highly abundant EGFR mutations in pretreatment blood samples predicted longer PFS on EGFR-TKIs in advanced NSCLC patients.23 More studies are needed to establish the efficacy of osimertinib for NSCLC patients with EGFR fusions in either first- or second-line treatment. Interestingly, histology on biopsies revealed the gradual development of SCC transformation. Little is known about the underlying genomic mechanisms during the ADC to SCC transformation process and probably relate to driver genetic alterations under selective pressure or the heterogeneity of the tumor.24,25 In this case, the genotype-phenotype correlations related to the KIF5B-EGFR fusion are unclear.
Taken individually, these EGFR fusion events are rare. Fluorescence in situ hybridization (FISH) and quantitative polymerase chain reaction (qPCR) have various limitations for the accurate discovery and detection of uncommon but potentially oncogenic alterations. The gene detection sensitivity of targeted sequencing by NGS has been found to exceed that of FISH and qPCR. Developments in NGS have created a new method for the simultaneous detection of a large number of gene events with known and unknown genes and gene mutations, including novel gene fusions.7,8,11,26 Given the ever-increasing landscape of genomic events in NSCLC, NGS testing should be encouraged in clinical practice in NSCLC, which could identify uncommon gene alterations and gene fusions.
In conclusion, we detected a novel KIF5B-EGFR fusion in advanced NSCLC as an indicator of a good response to afatinib therapy. This study provides a meaningful reference for the treatment of NSCLC patients harboring EGFR fusion mutations. NGS technologies provide a reliable diagnostic tool for the identification of novel fusion partner genes for EGFR in patients with NSCLC.
Acknowledgments
We would like to thank the patient and his family for giving consent for publication.
Funding Statement
This work was supported by the National Nature Science Foundation [grant number 82060577, 82060547].
Abbreviations
EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; KIF5B, kinesin family member 5B; TKIs, tyrosine kinase inhibitors; NGS, next-generation sequencing; PFS, progression-free survival; CT, computed tomography; IHC, immunohistochemistry; ADC, adenocarcinoma; LN, lymph node; SCC, squamous cell carcinoma; FISH, fluorescence in situ hybridization; qPCR, quantitative polymerase chain reaction.
Data Sharing Statement
For patients’ privacy, the patient information is publicly inaccessible.
Ethics Approval and Consent to Participate
This study was approved by institutional ethics committee of Second Affiliated Hospital of Nanchang University, Nanchang, China. Written informed consent to participate was obtained from the patient.
Consent for Publication
Written informed consent for publication has been obtained from the patient stating that the details/images can be available on the Internet and may be seen by the general public.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors have no competing interests to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Chai S, Liang Z, et al. KIF5B-RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer. 2014;13(1):176. doi: 10.1186/1476-4598-13-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–122. doi: 10.1016/j.lungcan.2019.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22(3):436–445. doi: 10.1101/gr.133645.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu YC, Wang WX, Xu CW, et al. EGFR-RAD51 fusion variant in lung adenocarcinoma and response to erlotinib: a case report. Lung Cancer. 2018;115:131–134. doi: 10.1016/j.lungcan.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Kartik K, Gallant JN, Chae YK, et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov. 2016;6(6):601–611. doi: 10.1158/2159-8290.CD-16-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Zhang Y, Ye T, et al. Detection of novel NRG1, EGFR, and MET fusions in lung adenocarcinomas in the Chinese population. J Thorac Oncol. 2019;14(11):2003–2008. doi: 10.1016/j.jtho.2019.07.022 [DOI] [PubMed] [Google Scholar]
- 9.Zhu YC, Wang WX, Li XL, et al. Identification of a novel icotinib-sensitive EGFR-SEPTIN14 fusion variant in lung adenocarcinoma by next-generation sequencing. J Thorac Oncol. 2019;14(8):e181–e183. doi: 10.1016/j.jtho.2019.03.031 [DOI] [PubMed] [Google Scholar]
- 10.Raez LE, Pinto JA, Schrock AB, Ali SM. EGFR-RAD51 fusion: a targetable partnership originated from the tumor evolution? J Thorac Oncol. 2018;13(3):e33–e34. doi: 10.1016/j.jtho.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Song Z, Li Y, et al. Effectiveness of EGFR-TKIs in a patient with lung adenocarcinoma harboring an EGFR-RAD51 fusion. Oncologist. 2019;24(8):1027–1030. doi: 10.1634/theoncologist.2018-0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masood A, Kancha RK, Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: focus on afatinib. Semin Oncol. 2019;46(3):271–283. doi: 10.1053/j.seminoncol.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 15.Passaro A, Mok T, Peters S, Popat S, Ahn MJ, de Marinis F. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2020;16(5):764–773. doi: 10.1016/j.jtho.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Schuler M, Popat S, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: a database of 693 cases. J Thorac Oncol. 2020;15(5):803–815. doi: 10.1016/j.jtho.2019.12.126 [DOI] [PubMed] [Google Scholar]
- 17.Tanaka I, Morise M, Kodama Y, et al. Potential for afatinib as an optimal treatment for advanced non-small cell lung carcinoma in patients with uncommon EGFR mutations. Lung Cancer. 2019;127:169–171. doi: 10.1016/j.lungcan.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 18.Gallant JN, Sheehan JH, Shaver TM, et al. EGFR kinase domain duplication (EGFR-KDD) is a novel oncogenic driver in lung cancer that is clinically responsive to afatinib. Cancer Discov. 2015;5(11):1155–1163. doi: 10.1158/2159-8290.CD-15-0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochmair MJ, Buder A, Schwab S, et al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol. 2019;14(1):75–83. doi: 10.1007/s11523-018-0612-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai CS, Liam CK, Poh ME, et al. Predictors of acquired T790M mutation in patients failing first- or second-generation epidermal growth factor receptor-tyrosine kinase inhibitors. Cancer Manag Res. 2020;12:5439–5450. doi: 10.2147/CMAR.S253760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eide IJZ, Helland Å, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study). Lung Cancer. 2020;143:27–35. doi: 10.1016/j.lungcan.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Liu Y, Meng Z, et al. Plasma EGFR mutation abundance affects clinical response to first-line EGFR-TKIs in patients with advanced non-small cell lung cancer. Ann Transl Med. 2021;9(8):635. doi: 10.21037/atm-20-7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Shim JH, Lee B, et al. Paired genomic analysis of squamous cell carcinoma transformed from EGFR-mutated lung adenocarcinoma. Lung Cancer. 2019;134:7–15. doi: 10.1016/j.lungcan.2019.05.024 [DOI] [PubMed] [Google Scholar]
- 25.Shao Y, Zhong DS. Histological transformation after acquired resistance to epidermal growth factor tyrosine kinase inhibitors. Int J Clin Oncol. 2018;23(2):235–242. doi: 10.1007/s10147-017-1211-1 [DOI] [PubMed] [Google Scholar]
- 26.Farago AF, Azzoli CG. Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(5):550–559. doi: 10.21037/tlcr.2017.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Shao C. KIF5B-EGFR fusion: a novel EGFR mutation in lung adenocarcinoma. Onco Targets Ther. 2020;13:8317–8321. doi: 10.2147/OTT.S263994 [DOI] [PMC free article] [PubMed] [Google Scholar]