Abstract
Background
The efficacy of systemic chemotherapy for hepatocellular carcinoma (HCC) is predominantly hampered by low accumulation in tumor tissue and the high systemic toxicity of anticancer drugs. In this study, we designed an in situ drug-loaded injectable thermosensitive hydrogel system for the simultaneous delivery of norcantharidin-loaded nanoparticles (NCTD-NPs) and doxorubicin (Dox) via intratumoral administration to HCC tumors.
Methods
NCTD-NPs were prepared by the thin film dispersion method using PCEC polymers as the carrier. Then, NCTD-NPs and Dox were co-encapsulated in a thermosensitive hydrogel based on Pluronic F127 (PF127) to construct a dual drug-loaded hydrogel system. The rheological properties of the drug-loaded hydrogel were studied using a rheometer. Drug release of the drug-loaded hydrogel and cytotoxicity in HepG2 cells were evaluated in vitro. An H22 tumor-bearing mice model was used to assess the in vivo antitumor activity of the drug-loaded hydrogel via intratumoral administration.
Results
The prepared drug-loaded hydrogel exhibited good thermal-sensitive properties, which remained liquid at room temperature and rapidly transformed into a non-flowing gel at body temperature, and released the drugs in a sustained manner. In vitro studies revealed that the drug-loaded hydrogel exhibited remarkable antiproliferative activity in HepG2 cells compared to free drugs. In vivo antitumor efficacy experiments showed that the drug-loaded hydrogel significantly suppressed tumor growth, alleviated side effects, and prolonged the survival time of mice bearing H22 tumors compared to the other groups. Moreover, immunohistochemical staining revealed that the expression of Ki-67 and CD31 in the drug-loaded hydrogel group was significantly lower than that in the other groups (P < 0.05), indicating that the drug-loaded hydrogel effectively inhibited tumor proliferation and angiogenesis.
Conclusion
The formulated hybrid thermosensitive hydrogel system with sustained drug release and enhanced therapeutic efficacy was demonstrated to be a promising strategy for the local-regional treatment of HCC via intratumoral administration.
Keywords: Norcantharidin, Doxorubicin, Nanoparticles, Thermosensitive hydrogel, Hepatocellular carcinoma, Intratumoral injection
Introduction
Globally, hepatocellular carcinoma (HCC) ranks the sixth most frequently diagnosed malignancy and the fourth leading cause of cancer-associated mortality.1 Surgical resection, liver transplantation, and percutaneous ablation remain the potentially curative therapeutic approaches with a proven survival benefit for patients in the early stage of HCC according to the Barcelona Clinic Liver Cancer (BCLC) staging system.2 Regrettably, about one-third of patients with newly diagnosed HCC are suitable for these curative therapies. However, these therapies are associated with a recurrence rate of 60–70% at 5 years.3,4 Furthermore, over 40% of patients present with advanced-stage HCC, and systemic chemotherapy represents the mainstay palliative treatment option for patients with advanced HCC. However, the systemic intravenous administration of chemotherapeutic agents manifests severe adverse effects due to non-selective drug biodistribution in healthy tissues and organs. Besides, the relatively low bio-accessibility of these drugs to target tumor tissues and the rapid clearance from blood circulation can lead to inadequate therapy, which seriously affects therapeutic efficacy and leads to an increased incidence of drug resistance.5–7 Intratumoral administration directly delivers anticancer drugs to the tumor site via local injection and has demonstrated potential advantages over systemic chemotherapy, owing to the highly selective accumulation in tumors and active cellular uptake, thereby improving treatment efficacy while avoiding toxicity in normal cells.8,9 Thus, this targeted delivery is expected to improve both the efficacy and safety of cancer chemotherapy and has gained increasing attention in recent decades.
Doxorubicin (Dox), a representative anthracycline antibiotic and effective anticancer drug with broad-spectrum activity functions by inducing DNA damage in cancer cells through multiple mechanisms, including intercalation into DNA and the inhibition of topoisomerase II.10–12 Although Dox is widely used for treating a variety of solid tumors and hematological malignancies, its therapeutic value is hindered by its severe dose-dependent toxic side effects, which include cardiotoxicity, nephrotoxicity, and myelosuppression. Besides, the rapid metabolism of Dox to inactive derivatives mainly via the liver after intravenous injection is also a critical issue that remains unresolved.13,14 Norcantharidin (NCTD), a demethylation derivative of cantharidin, is a natural anticancer agent that has been shown to exhibit extensive antitumor effects toward diverse cancer types, including HCC, esophageal cancer, and gastric cancer.15,16 Moreover, NCTD is unique as it causes no bone marrow suppression and induces the production of leucocytes, which could enhance the immunity of patients.17 Owing to these characteristics, NCTD has been frequently used in combination with other anticancer agents to achieve improved treatment efficacy. However, NCTD has poor water solubility, short half-life after oral or intravenous administration, and low tumor-targeting efficiency, significantly affecting its anticancer effect.18,19 Therefore, many novel drug delivery systems, such as nanoparticles (NPs),20,21 liposomes,22 and microspheres23 have been developed to improve the efficacy and safety of NCTD treatment.
Recently, polymeric nanoparticles have gained increasing attention owing to their potential to deliver drugs efficiently. By virtue of nanoparticle formulations, the coupling of antitumor drugs with polymeric drug carriers can improve the pharmacological properties of drugs, particularly the water solubility of hydrophobic pharmaceuticals. Anticancer agents can also efficiently accumulate in tumor tissues through the enhanced permeability and retention (EPR) effect, thereby enhancing the therapeutic efficacy and reducing adverse effects.24–26 Among various polymers, poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) (PCL-PEG-PCL, PCEC) is a biocompatible chemically synthesized amphiphilic triblock copolymer with hydrophobic PCL chains which can encapsulate hydrophobic drugs, and with hydrophilic PEG chains which exhibit good water solubility.27,28 Because of its biocompatibility, biodegradability, and low toxicity, PCEC is extensively used as a nanocarrier for the tumor-targeted delivery of several chemotherapeutic drugs. Previously, PCEC nanoparticles were successfully prepared to deliver anticancer drugs, such as gefitinib, paclitaxel, and honokiol, and appeared to be a sustained drug release system.29–31 In this study, we prepared NCTD-loaded PCEC nanoparticles (NCTD-NPs) to improve the water solubility of NCTD and enhance its antitumor efficacy.
Injectable in-situ-forming hydrogels have received considerable attention due to their outstanding properties, such as excellent biocompatibility, facile preparation, minimal invasiveness, shape adaptability, and improved patient compliance, making them excellent candidates for biomedical applications.32 Among them, thermosensitive hydrogels, which exist as a free-flowing injectable liquid at room temperature and transform into a biodegradable semi-solid gel under physiological conditions, have been widely used for controlled drug delivery owing to their sustained releasing characteristics, enhanced drug activity, and low systemic toxicity.33,34 Pluronic F127 (PF127), a synthetic amphiphilic triblock copolymer, exhibits amphiphilic properties and undergoes a thermoreversible sol-gel transition.35,36 PF127 hydrogel self-assembles into micelles (a packed chain of molecules) and releases PF127 micelles during gel erosion like other thermosensitive polymers.34 Due to its low toxicity and compatibility with various bioactive agents, the PF127 in situ hydrogel system has been applied for the regional (including intratumoral, intraperitoneal, and subcutaneous) delivery of drugs.37–39
In this study, an in situ dual drug-loaded hydrogel system was constructed to simultaneously deliver NCTD-NPs and Dox via intratumoral administration for sustained and efficient antitumor therapy. The composite hydrogel system was prepared and optimized, and the physicochemical properties were characterized. HepG2 cells and H22 tumor-bearing mice model were used to evaluate in vitro and in vivo antitumor activity of the PF127 drug-loaded hydrogel via intratumoral administration.
Materials and Methods
Materials, Cell Lines, and Animals
Stannous octoate [Sn(Oct)2] was purchased from Sigma (USA), ε-caprolactone (ε-CL) was obtained from Alfa Aesar (USA), and poly (ethylene glycol; PEG, Mn = 2000) was procured from Fluka (USA). NCTD was purchased from Meilun Biological Technology Co. Ltd. (Dalian, China). Dox was provided by Southwest Medical University (Luzhou, China). Methanol and dimethyl sulfoxide (DMSO) were purchased from Chengdu Kelong Chemicals (Chengdu, China), and dichloromethane was obtained from Tianjin Fuyu Fine Chemical Co. Ltd (Tianjin, China). All reagents were analytical grade and used as received without further purification.
The human hepatoma cell line (HepG2) and murine hepatoma cell line (H22) were obtained from the Experimental Medicine Center, the Affiliated Hospital of Southwest Medical University (Luzhou, China). HepG2 cells were cultured in Dulbecco’s modified Eagle media (DMEM), supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. H22 cells were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS; Gibco) in a humidified 5% CO2 atmosphere at 37 °C. Prior to treatment, the cells were grown to 80% to 90% confluence. The use of the cell lines was approved by the Research Ethics Committee of Southwest Medical University, and the HepG2 cells were verified by short tandem repeat (STR) profiling.
Kunming mice (female, body weight 21 ± 2 g) were purchased from the Experimental Animal Center of Southwest Medical University (Luzhou, China). All mice were housed in a specific pathogen-free and temperature-controlled environment (20–22 °C), with 50–60% relative humidity and a 12-h-light/12-h-dark cycle. The animals were provided access to standard laboratory chow and tap water ad libitum. All mice were healthy and had no infection during the experimental period. All animal experimental procedures were approved by the Institutional Animal Care and Treatment Committee of Southwest Medical University (license no. 2020699). The mice were cared for in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Synthesis of PCEC Copolymers
PCEC copolymer was synthesized by the ring-opening polymerization of ε-CL using PEG as an initiator and Sn(Oct)2 as a catalyst. Briefly, the calculated amount of ε-CL and PEG (weight ratio =20:1) were introduced into a dry glass flask, and several drops of Sn(Oct)2 were added with mild agitation under a nitrogen atmosphere at 130 °C for 6 hours, and then the reaction mixture was rapidly heated to 140 °C for one hour under vacuum. After cooling to room temperature under a nitrogen atmosphere, the synthesized copolymer was dissolved in dichloromethane and reprecipitated from the filtrate with an excess of cold petroleum ether. Finally, the PCEC copolymers were vacuum dried to a constant weight and stored in a desiccator until use.
Preparation and Characterization of NCTD-NPs
Preparation of NCTD-NPs
The thin-film dispersion method was used to prepare NCTD-NPs as described previously.40 Briefly, 90 mg of PCEC copolymers and 10 mg of NCTD were completely dissolved in a round-bottomed flask with 2 mL of dichloromethane. Then, the organic solvent was removed through rotary evaporation at 37 °C. Next, the thin film deposited on the walls of the flask was re-dissolved in deionized water preheated to 60 °C and filtered through a 220-nm filter to obtain a clarified solution. Lastly, the filtrate was freeze-dried to yield NCTD-NPs powder. Similarly, blank nanoparticles were prepared using the above technique without the addition of NCTD.
Characterization of NCTD-NPs
The morphology of the NCTD-NPs was examined using transmission electron microscopy (TEM, Tecnai G2 F20, USA). Dynamic light scattering (DLS, NanoBrook 90Plus Zeta, USA) was used to measure the particle size distribution of the NCTD-NPs at 25 °C. Drug loading (DL) and encapsulation efficiency (EE) were determined by a reverse-phase high-performance liquid chromatography (HPLC, Agilent 1260, USA) system with a C18 column (4.6 × 150 mm, 5 um) at 30 °C. The mobile phase was comprised of methyl alcohol and monopotassium phosphate solution (17/83, v/v; the aqueous phase was adjusted to pH 3.1 with phosphoric acid). The flow rate was 0.8 mL/min, and the detection wavelength was 210 nm. DL and EE were calculated using the following equations:
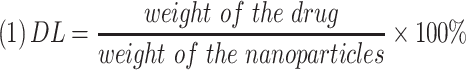 |
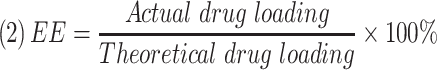 |
In vitro Cellular Uptake of Nanoparticles
To evaluate the cellular uptake of PCEC nanoparticles by HepG2 cells, coumarin-6 fluorescent-loaded PCEC nanoparticles were prepared by the thin-film dispersion method. Coumarin-6 loaded PCEC NPs were prepared by the same method as that used to prepare the NCTD-NPs. The HepG2 cells were seeded into a 24-well plate at a density of 1×105 cells per well and incubated for 24 h at 37 °C. Next, the cells were exposed to medium supplemented with 500 ug/mL blank nanoparticles, 100 ug/mL free coumarin-6, or coumarin-6-loaded nanoparticles (equivalent to 100 ug/mL free coumarin-6) and incubated for another 2 h. Subsequently, the cells were washed three times with phosphate-buffered saline (PBS, pH = 7.4), and observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Preparation of Blank and Drug-Loaded Hydrogels
The “cold” method was adopted for the preparation of the blank and drug-loaded hydrogels as described previously.41 Briefly, F127 (2 mg) was dissolved in 10 mL of normal saline with stirring at 4 °C until a clear solution was obtained. Then, 85 mg of NCTD-NPs and 30 mg of doxorubicin were added to the clear solution prepared above and gently mixed with a magnetic stirrer to form a uniform mixture. The drug-loaded hydrogel system (NCTD-NPs/Dox Gel) was prepared and stored at room temperature until further use. The blank hydrogel was fabricated using a similar procedure by adding only an appropriate amount of blank nanoparticles.
Rheological Study
The rheological properties were investigated using a rheometer (Physica MCR 92, Anton Paar, Germany). The temperature sweep test was performed at a fixed frequency of 1 Hz and a gap size of 0.5 mm, and the temperature was gradually increased from 10 °C to 45 °C at a rate of 1 °C/min. The storage modulus (G’) and loss modulus (G”) of the blank hydrogel and NCTD-NPs/Dox Gel were measured with changes in the temperature. The temperature at which the value of G’ was equal to G” was noted as the gelation temperature.42
In vitro Release Behavior
The in vitro release behavior of the drug from the nanoparticles and hydrogel was assessed using the dialysis membrane method. Briefly, 2 mL suspension of NCTD (2 mg/mL) or NCTD-NPs (equivalent to 4 mg NCTD) or NCTD-NPs/Dox Gel (equivalent to 4 mg NCTD and 16 mg Dox) were added to separate dialysis bags (molecular weight cut-off was 3.5 kDa), and then immersed in 40 mL of PBS (pH 7.4) containing Tween 80 (0.5%, w/v) at 37 °C with mild shaking at 100 rpm. At the scheduled time, 2 mL of the release medium was removed and replaced with an equivalent volume of prewarmed fresh medium. After centrifugation at 12,000 rpm for 15 min, 1 mL of supernatant was taken for further analysis. The concentrations of NCTD and Dox were measured by HPLC and an ultraviolet spectrophotometer, respectively. All measurements were performed in triplicate.
In vitro Cytotoxicity Assay
The cytotoxicity of blank PCEC nanoparticles, free NCTD/Dox, and NCTD-NPs/Dox Gel in HepG2 cells was assessed by the MTT assay in vitro. Briefly, HepG2 cells were seeded in 96-well plates at a density of 5×103 viable cells per well in 100 uL of DMEM medium and incubated for 24 h. Next, the medium was removed, and 100 μL of fresh culture medium or medium containing different concentrations of drug preparations were added and incubated up to another 48 h. Subsequently, 20 μL of MTT solution (5 mg/mL in PBS) was added to each well, and the cells were incubated at 37 °C for another 4 h. The precipitated formazan was dissolved in 150 μL of dimethyl sulfoxide (DMSO). Finally, the absorbance was measured at 490 nm using a microplate reader (Omega, Germany). The concentration of the prepared drugs producing 50% inhibition (IC50) was determined to evaluate cytotoxicity.
In vivo Antitumor Activity
To establish H22-bearing mice models, the right thighs of Kunming mice (female, 21 ± 2 g) were subcutaneously injected with 100 μL of an H22 cell suspension (1 × 107/mL). When the volume of the solid tumors reached 150–200 mm3, the tumor-bearing mice were randomly divided into four groups of ten mice each: (1) normal saline (NS), (2) blank hydrogel, (3) free NCTD (2.5 mg/kg) and Dox (10 mg/kg), and (4) NCTD-NPs/Dox Gel (equivalent to 2.5 mg/kg free NCTD and 10 mg/kg free Dox). The time was defined as day 0. Subsequently, the mice were injected intratumorally with 0.1 mL of the respective formulations once a week for a total of two times. The tumor volume and body weight were measured on alternative days from the start of treatment. The tumor size was measured using vernier calipers, and tumor volume was calculated by the following formula: volume = length × width2/2. Body weight changes of the mice were observed to assess the toxicity of the treatments. Half of the mice in each group were selected at random and sacrificed by cervical dislocation on day 13. The tumors and major organs (heart, liver, spleen, lung, and kidney) were immediately excised. Eventually, the remaining mice (five mice in each group) were used for survival analysis.
Histological and Immunohistochemical Analysis
The collected tumors and vital organ (heart, liver, spleens, lung, and kidney) tissue specimens were fixed in 10% neutral buffered formalin solution, embedded in paraffin, and sectioned into 4 μm thick sections for histopathological analysis. The tissue sections were stained with hematoxylin and eosin (H&E), examined, and photographed under optical microscopy to assess the pathological changes. Immunohistochemical (IHC) Ki-67 and CD31 staining were performed in accordance with the manufacturer’s protocol. Briefly, the formalin-fixed, paraffin-embedded tissue sections were cut into 4-μm sections, dewaxed in xylene, rinsed in graded ethanol, and rehydrated in double-distilled water. IHC staining was performed with anti-human Ki-67 and CD31 protein antibodies. The proportion of Ki-67+ cells relative to the total number of cells and the number of CD31+ micro-vessels were counted in five randomly selected fields in each tumor sample, and then the mean values were calculated.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.0 software (GraphPad Software, San Diego, CA, USA). The data were expressed as the mean value ± standard deviation. The Student’s t-test was used to analyze the differences between two groups, and one-way analysis of variance (ANOVA) was used to analyze multiple groups. The Kaplan-Meier method was used to plot the survival curve and compared using the log-rank test. A P-value of < 0.05 was considered statistically significant.
Results
Preparation and Characterization of Nanoparticles
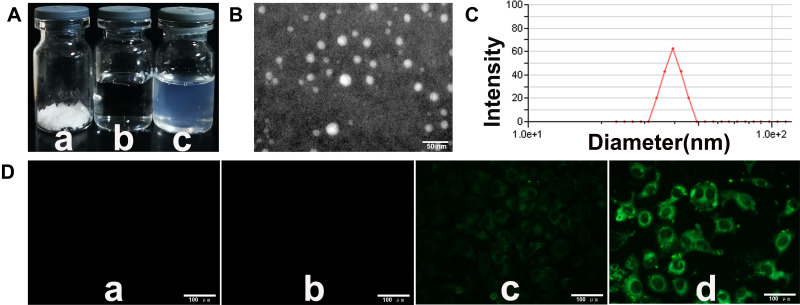
NCTD-NPs were successfully prepared using a thin-film dispersion method. As illustrated in Figure 1A, the NCTD-NPs were freeze-dried into a white powder (a). Compared to the NS (b), the re-dissolved aqueous NCTD-NPs solution had a blue opalescence (c). The morphology and particle size of the NCTD-NPs were characterized by TEM and DLS, as presented in Figure 1B and C. The image showed that the NCTD-NPs were uniform spherical particles with an average particle size of 88.5 ± 1.8 nm and a polydispersity index (PDI) of 0.3 ± 0.003. In this study, the theoretical DL of the nanoparticles was set to 10%. The results indicated that the actual DL and EE of the NCTD-NPs were 8.80 ± 0.42% and 88.03 ± 4.23%, respectively.
Figure 1.
The characterization of NCTD-NPs.
Notes: (A) Appearance of several solutions: (a) freeze-dried powder of NCTD-NPs, (b) NS, (c) re-dissolved NCTD-NPs. (B) TEM image of the NCTD-NPs. Scale bar, 50 nm. (C) DLS analysis of the NCTD-NPs. (D) Fluorescent images of HepG2 cells treated with (a) medium, (b) PCEC nanoparticles, (c) free C6, and (d) C6-NPs for 2 h. Scale bar, 100 μm.
Abbreviations: NCTD, norcantharidin; NPs, nanoparticles; NS, normal saline; TEM, transmission electron microscopy; DLS, dynamic light scattering; PCEC, PCL-PEG-PCL; C6, coumarin-6.
In vitro Cellular Uptake of Nanoparticles
After incubation with blank NPs, free coumarin-6, and coumarin-6-loaded nanoparticles for 2 h, images were obtained using a fluorescence microscope as presented in Figure 1D. The control (a) and blank nanoparticles (b) groups appeared completely black with no fluorescence. However, the other two groups showed significant fluorescence, and coumarin-6-loaded nanoparticles (d) exhibited a higher density of fluorescence than free coumarin-6 (c), suggesting that coumarin-6-loaded nanoparticles were taken up efficiently by the HepG2 cells.
Preparation and Rheological Investigation of F127 Hydrogel
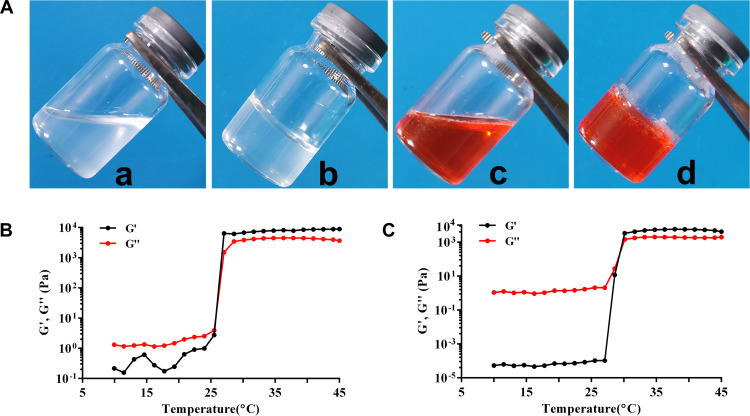
Rheological properties were investigated using a rheometer. As depicted in Figure 2A, both blank hydrogel and NCTD-NPs/Dox Gel appeared to be uniform and transparent, while the latter became reddish due to the original color of doxorubicin. Both gels were in free-flowing form at ambient temperature (a and c) and gradually transformed into solid gels at 37 °C (b and d). Furthermore, the storage modulus (G’) and loss modulus (G”) values of the two samples at varying temperatures (10–45 °C) were measured, as illustrated in Figure 2B and C. The gelation temperature was defined as the temperature at which the liquid transformed into an immobile gel when G’ was equal to G”.41 The gelation temperature of the blank hydrogel and NCTD-NPs/Dox Gel was 25.8 °C and 29.4 °C, respectively, suggesting that the gel-forming ability of the hydrogel was not significantly affected by the addition of NCTD-NPs and doxorubicin.
Figure 2.
Thermal-sensitive analysis of the PF127 hydrogel drugs.
Notes: (A) Morphology of the blank hydrogel (a) and drug-loaded hydrogel (c) at room temperature; the blank hydrogel (b) and drug-loaded hydrogel (d) at 37 °C. Rheological analysis as a function of temperature: (B) the blank hydrogel and (C) drug-loaded hydrogel.
Abbreviation: PF127, Pluronic F127.
In vitro Drug Release Behavior
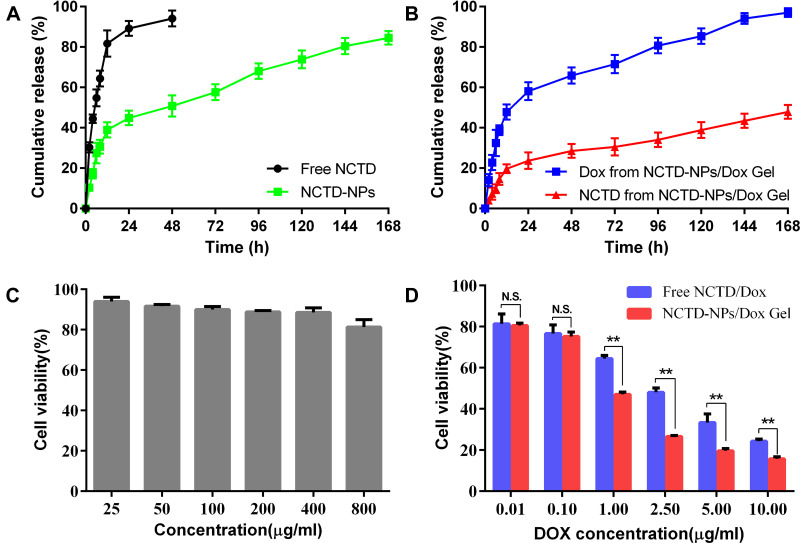
The in vitro release behavior of the free NCTD and NCTD-NPs was investigated by the dialysis membrane method to assess the feasibility of the sustained release of the encapsulated drugs. As presented in Figure 3A, free NCTD exhibited a rapid release behavior, and over 94.2% of the NCTD was released into the medium within 48 h. In contrast, the NCTD in the NCTD-NPs was released in a controlled and sustained manner, with approximately 84.5% of the NCTD released within 168 h. These results indicated that the NPs allowed sustained drug release. Furthermore, the in vitro release behavior of NCTD and Dox from the drug-loaded hydrogel was also investigated. As shown in Figure 3B, the cumulative release rate of Dox was approximately 97% within 168 h, whereas that of NCTD was much slower with a cumulative release rate of 47.8%. Taken together, these results implied that the drug-loaded hydrogel exhibited a sustained and extended-release behavior for the encapsulated drugs.
Figure 3.
In vitro drug release and cytotoxicity assessment.
Notes: (A) In vitro release profiles of free NCTD and NCTD-NPs. (B) In vitro release profiles of Dox and NCTD from the drug-loaded hydrogel. (C) In vitro cytotoxicity of the blank PCEC nanoparticles in HepG2 cells; (D) In vitro cytotoxic effects of free NCTD/Dox and the drug-loaded hydrogel in HepG2 cells. Data are presented as mean ± standard deviation (n = 3; ** P < 0.01).
Abbreviations: NCTD, norcantharidin; NPs, nanoparticles; Dox, doxorubicin; PCEC, PCL-PEG-PCL; N.S., no significance.
In vitro Cytotoxicity Assay
The cytotoxicity of the different drug preparations was evaluated by the MTT assay in HepG2 cells. As presented in Figure 3C, the blank PCEC nanoparticles did not exhibit any significant cytotoxicity because the cell viability was higher than 80% even when the concentration was increased to 800 μg/mL. This indicated that PCEC nanoparticles could be considered as a safe carrier for drug delivery systems. In contrast, both free NCTD/Dox and NCTD-NPs/Dox Gel inhibited cell proliferation in a dose-dependent manner (Figure 3D). Compared to free drugs, the drug-loaded hydrogel exhibited a more significant cytotoxic effect, mainly when the concentration of Dox was higher than 1 μg/mL (P < 0.05). Consistently, the IC50 value of Dox was lower in drug-loaded hydrogel (0.523 μg/mL) than that of free drugs (1.57 μg/mL), further confirming the enhanced cytotoxicity of drugs in encapsulated formulations against HepG2 cells.
In vivo Antitumor Activity
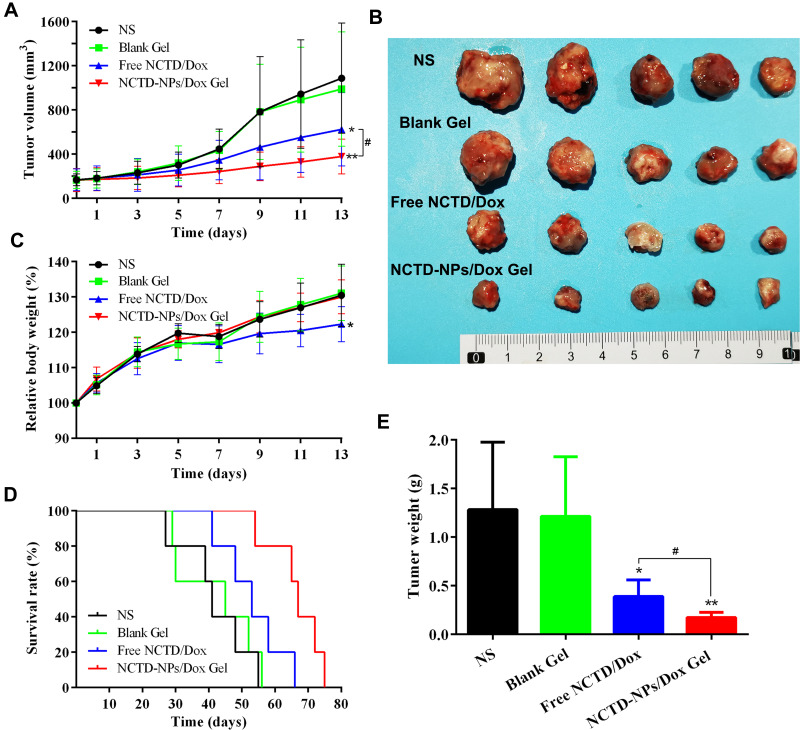
The antitumor effect of NCTD-NPs/Dox Gel was examined in H22 tumor-bearing mice via intratumoral administration. As depicted in Figure 4A, the NCTD-NPs/Dox Gel group mice showed significant inhibition of tumor growth, with only a slight increase in tumor volume throughout the observation period. Over 13 days of observation, the tumors treated with NCTD-NPs/Dox Gel grew to 2.49 ± 0.76-fold of the original tumor volume, whereas the tumors of mice treated with normal saline, blank hydrogel, and free NCTD/Dox exhibited 6.66 ± 2.64 (P < 0.01), 5.91 ± 1.31 (P < 0.01), and 3.85 ± 0.96-fold increases (P < 0.05) in tumor volumes, respectively, indicating a statistically significant difference. Gross photographs of the excised tumors are shown in Figure 4B, which also suggested that NCTD-NPs/Dox Gel had better tumor growth inhibition efficiency. As presented in Figure 4E, the NCTD-NPs/Dox Gel group had much smaller tumor weights compared to the other groups (P < 0.05), which further verified the excellent antitumor efficiency of the drug-loaded hydrogel. Furthermore, as shown in Figure 4D, the NCTD-NPs/Dox Gel group exhibited a significantly longer median survival time (67 days) compared to the NS group (41 days, P < 0.01), blank hydrogel group (45 days, P < 0.01), and free NCTD/Dox group (53 days, P < 0.05). Taken together, the NCTD-NPs/Dox Gel effectively inhibited the growth of the implanted tumors and prolonged the survival time of the tumor-bearing mice.
Figure 4.
In vivo anti-tumor efficacy of the drug-loaded hydrogel in H22 tumor-bearing mice.
Notes: (A) Tumor volume curves in each group (n = 10). (B) Photographs of excised tumors in each group. (C) Relative body weight of mice in each group (n = 10). (D) Survival curves in each group (n = 5). (E) Tumor weight of the different groups (n = 5). Data are presented as mean ± standard deviation (* P < 0.05, ** P < 0.01 of indicated groups vs NS group; # P < 0.05 of indicated NCTD-NPs/Dox Gel vs Free NCTD/Dox).
Abbreviations: NS, normal saline; NCTD, norcantharidin; Dox, doxorubicin; NPs, nanoparticles.
Toxicology Investigation
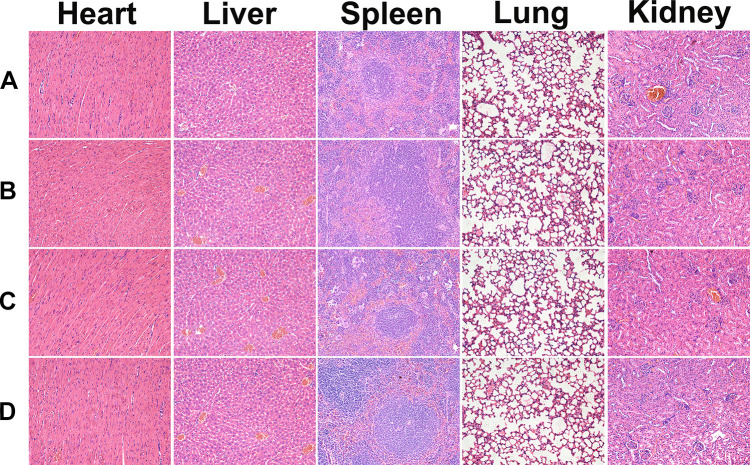
The systemic toxicity of the treatments was evaluated by body weight changes and H&E staining of the organ tissue sections. During the experimental periods, the body weight of the mice was monitored regularly, and the results are presented in Figure 4C. The mice receiving free NCTD/Dox had the least increase in body weight (P < 0.05), while the weight curves of the blank hydrogel group and NCTD-NPs/Dox Gel group were similar to those of the saline-treated mice, implying that the drug-loaded hydrogel was nontoxic and could alleviate the side effects of the free drugs. Histological analysis of the major organs showed no visible tissue damage, inflammation, or lesions (Figure 5).
Figure 5.
Representative images of H&E staining of heart, liver, spleen, lung, and kidney sections in the group of (a) NS, (b) Blank Gel, (c) Free NCTD/Dox, and (d) NCTD-NPs/Dox Gel (× 200).
Abbreviations: H&E, hematoxylin and eosin; NS, normal saline; NCTD, norcantharidin; Dox, doxorubicin; NPs, nanoparticles.
Histological and Immunohistochemical Analysis
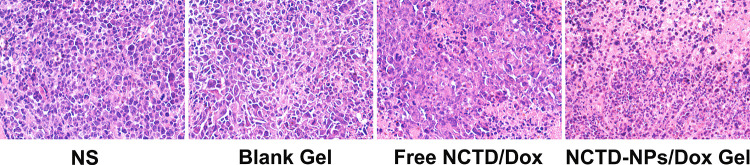
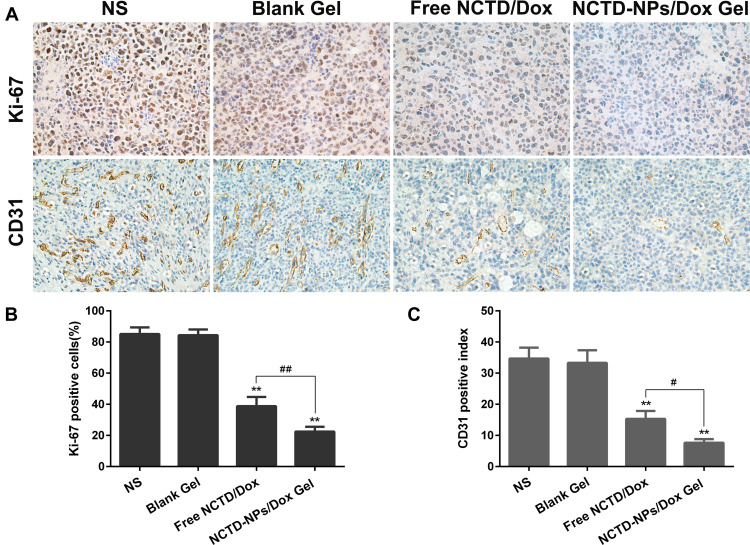
At the end of the experiment, the mice were sacrificed, and the tumors were collected for further histological and immunohistochemical analysis. As shown in Figure 6, the tumors treated with free drugs exhibited a lower proportion of necrotic cells than those treated with drug-loaded hydrogel, whereas the saline- and blank hydrogel-treated tumors exhibited no significant necrosis. As presented in Figure 7A, Ki-67 and CD31 immunostaining were investigated to assess tumor cell proliferation and tumor angiogenesis. Figure 7B shows that the proportion of Ki-67-positive cells in the NCTD-NPs/Dox Gel group (22.46 ± 2.51%) was significantly lower than that in the NS group (85.16 ± 3.41%, P < 0.01), the blank hydrogel group (84.37 ± 2.97%, P < 0.01), and the free NCTD/Dox group (38.77 ± 4.85%, P < 0.05). Figure 7C shows that the CD31-positive microvessel density (MVD) in the NCTD-NPs/Dox Gel group (7.67 ± 0.94) was significantly less than that in the free NCTD/Dox group (15.34 ± 2.05, P < 0.05), the blank hydrogel group (33.34 ± 3.30, P < 0.01), and the NS group (34.67 ± 2.87, P < 0.01). These findings indicated that the drug-loaded hydrogel more effectively inhibited tumor proliferation and angiogenesis, suggesting that drug-loaded hydrogel could exert enhanced antineoplastic activity.
Figure 6.
H&E-stained histological sections of tumors of the different groups (× 200).
Abbreviations: H&E, hematoxylin and eosin; NS, normal saline; NCTD, norcantharidin; Dox, doxorubicin; NPs, nanoparticles.
Figure 7.
(A) Representative images of immunohistochemical analysis of each group for the evaluation of Ki-67 and CD31 (×400). (B) The quantitative analysis of Ki-67 expression in each group. (C) The quantitative analysis of vascularization in each group.
Notes: Data are presented as mean ± standard deviation (n = 3; ** P < 0.01 of indicated groups vs NS group; # P < 0.05, ## P < 0.01 of indicated NCTD-NPs/Dox Gel vs Free NCTD/Dox).
Abbreviations: NS, normal saline; NCTD, norcantharidin; Dox, doxorubicin; NPs, nanoparticles.
Discussion
HCC, the most prevalent gastrointestinal malignancy, is characterized by high morbidity and mortality rates worldwide. Since most patients are diagnosed at an advanced stage and are not amenable to curative therapies, chemotherapy plays a vital role in the treatment of HCC. Compared to systemic administration, intratumoral administration has received increasing attention due to its higher regional drug concentration and lower systemic toxicity. Regrettably, its clinical application is still limited due to the rapid clearance of locally injected chemotherapeutic drugs.8,9,42 Accumulating studies have demonstrated that injectable drug-loaded hydrogels, particularly thermosensitive hydrogels, exhibited controlled and sustained drug release and enhanced drug activity, making them suitable for in situ intratumoral administration.34,43 However, many hydrophobic antitumor drugs, such as NCTD, have low aqueous solubility and cannot be well dispersed in the hydrogel. With the advances in nanotechnology, many polymeric systems have been developed as delivery carriers to improve the solubility of hydrophobic drugs. Among them, PCEC nanoparticles represent promising drug delivery systems with significantly increased drug solubility and sustained drug release profiles.30,40 Furthermore, the present study results indicated that blank PCEC nanoparticles did not exhibit noticeable cytotoxicity in HepG2 cells in vitro, demonstrating that PCEC nanocarriers were safe for biomedical application. Herein, we first developed NCTD-NPs using the thin-film dispersion method and then encapsulated them with Dox in a PF127-based thermosensitive hydrogel for regional chemotherapy.
In this study, the prepared NCTD-NPs with an average diameter of 88.5 nm exhibited a stable DL, high EE, and sustained drug release. Previous studies have shown that NPs smaller than 200 nm had the advantages of good stability, prolonged blood circulation, and favorable accumulation by the EPR effect.26,29 In addition, the drug-loaded hydrogel was successfully prepared according to the “cold method” and exhibited good thermal-sensitive properties, and could rapidly transform into a non-flowing gel at physiological temperature. For regional chemotherapy, the sustained release of antitumor drugs plays a crucial role in improving therapeutic drug efficacy and alleviating severe adverse effects. The findings of the in vitro release assay revealed that both NCTD and Dox could be released from the drug-loaded hydrogel in a controlled manner, and the release rate of NCTD was relatively slower, with a cumulative release of 47.88% within 14 days. This could be attributed to the dual slow-release mechanism of this hybrid hydrogel system. When the NCTD-NPs were introduced into the thermosensitive hydrogel complex, the drug encountered two barriers for release, resulting in a sustained release, and its prolonged release effect was better than that of nanoparticles or hydrogel alone.
Furthermore, the in vitro cytotoxicity assay indicated that the antitumor effect of drug-loaded hydrogel was superior to that of free drugs, which was attributed to the more effective uptake of the encapsulated drugs by HepG2 cells. Moreover, the in vivo studies also confirmed that the drug-loaded hydrogel exhibited an enhanced therapeutic effect, which could effectively inhibit tumor growth and prolong the survival time of tumor-bearing mice. These results may be explained by the controlled and sustained release and increased drug uptake of the hydrogel system, which released the incorporated drugs slowly at a controllable rate, thus increasing the accumulation of drugs in the tumor and inducing the extended and long-term suppression of tumor growth.44 Moreover, immunohistochemical analysis of the tumor tissues treated with drug-loaded hydrogel revealed a lower quantity of both Ki-67 and CD31-positive MVD compared to the other three groups, indicating a significant suppression of tumor proliferation and angiogenesis.
The biocompatibility and nontoxicity of the drug-loaded hydrogel system are essential parameters for its clinical application. Wen et al38 prepared a similar drug-loaded thermosensitive hydrogel comprised of a mixture of PCEC microspheres and PF127 for intraperitoneal chemotherapy. Extended in vivo studies revealed that this system exhibited good biocompatibility and presented low toxicity to major organs. Consistently, in our study, the drug-loaded hydrogel reduced the side effects of free drugs without apparent systemic toxicity, making it a promising drug delivery system for regional chemotherapy.
Similar drug delivery systems have been reported in some previous studies. Xie et al45 synthesized a novel poloxamer-based thermosensitive in situ gel system for the sustained delivery of NCTD via intratumoral administration to treat liver cancer. Consequently, the thermosensitive hydrogel exhibited good thermal sensitivity and the gelation temperature was 34 °C. The in vivo experiments indicated that the NCTD-thermosensitive in situ gel significantly inhibited the tumor growth of H22 tumor-bearing mice compared to free NCTD injection. These results suggest that the poloxamer-based thermosensitive hydrogel system is a promising approach for the localized delivery of drugs. In another study, Dox-loaded hydrogel based on the temperature-sensitive triblock polymer, poly(lactide-co-glycolide)-block-poly(ethylene glycol)-block-poly(lactide-co-glycolide) (PLGA-b-PEG-b-PLGA), was exploited as an in situ sustained drug delivery reservoir for regional chemotherapy for HCC.46 This formulation showed moderate mechanical properties, good biodegradability, and the sustained release profiles of Dox for up to 30 days. In addition, the Dox-loaded hydrogel exhibited significant inhibition of tumor growth in vivo and reduced side effects compared to those in the free Dox group. In the current study, the established nanoparticle-hydrogel drug delivery system had a dual slow-release mechanism, and could simultaneously load two drugs with different solubilities, which is conducive to the implementation of combined chemotherapy. Besides, the proposed hybrid drug-loaded system was easy to prepare and use, and can be produced on a large scale, so it has significant potential for clinical applications. Furthermore, the PF127-based thermosensitive hydrogel exhibited additional advantages such as good biodegradability, convenient drug encapsulation, local injectable administration, and minimized side effects, making it a safe and effective drug delivery system for regional chemotherapy. However, further studies are needed to investigate the tissue biodistribution and long-term toxicity and optimize hydrogel preparation and the DL of nanoparticles.
Conclusions
In this work, a localized codelivery system of NCTD and Dox was developed for regional chemotherapy. We prepared NCTD-NPs and incorporated them with Dox into the thermosensitive hydrogel to construct a nanoparticle-hydrogel drug-loaded delivery system with good thermal sensitivity, sustained release profile, and enhanced cytotoxicity in HepG2 cells. Moreover, in vivo experiments demonstrated that intratumoral administration of the drug-loaded hydrogel significantly inhibited tumor growth, alleviated side effects, and prolonged the survival time of mice bearing H22 tumors. Collectively, the hybrid injectable drug-loaded hydrogel with sustained drug release and enhanced therapeutic efficacy represents a promising drug delivery system for local chemotherapy.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 81802778), the Southwest Medical University Foundation (no. 2018-ZRZD-010), and the Doctoral Startup Fund of Affiliated Hospital of Southwest Medical University. Benjian Gao, Jia Luo, and Ying Liu are co-first authors.
Disclosure
The authors declare that they have no conflicts of interest related to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/s0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 3.Llovet J, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729. doi: 10.1016/j.jhep.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 5.Chari R. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 2008;41(1):98–107. doi: 10.1021/ar700108g [DOI] [PubMed] [Google Scholar]
- 6.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. doi: 10.3322/caac.21161 [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Fan J, Wang S, et al. Biodegradable drug-loaded hydroxyapatite nanotherapeutic agent for targeted drug release in tumors. ACS Appl Mater Interfaces. 2018;10(9):7832–7840. doi: 10.1021/acsami.7b19281 [DOI] [PubMed] [Google Scholar]
- 8.Wolinsky J, Colson Y, Grinstaff M. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159(1):14–26. doi: 10.1016/j.jconrel.2011.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhan H, Liu J, Chen Y, et al. Novel injectable thermosensitive hydrogels for delivering hyaluronic acid-doxorubicin nanocomplexes to locally treat tumors. Nanomedicine. 2015;10(8):1263–1274. doi: 10.2217/nnm.14.211 [DOI] [PubMed] [Google Scholar]
- 10.Tewey K, Rowe T, Yang L, et al. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226(4673):466–468. doi: 10.1126/science.6093249 [DOI] [PubMed] [Google Scholar]
- 11.Swift L, Rephaeli A, Nudelman A, et al. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006;66(9):4863–4871. doi: 10.1158/0008-5472.can-05-3410 [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Kang X, Wang Y, et al. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019;84:367–377. doi: 10.1016/j.actbio.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 13.Young R, Ozols R, Myers C. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305(3):139–153. doi: 10.1056/nejm198107163050305 [DOI] [PubMed] [Google Scholar]
- 14.Chun C, Lee S, Kim C, et al. Doxorubicin-polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials. 2009;30(27):4752–4762. doi: 10.1016/j.biomaterials.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 15.Wang G. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26(2):147–162. doi: 10.1016/0378-8741(89)90062-7 [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Li M, Hu Y, et al. Multifunctional self-assembled micelles of galactosamine-hyaluronic acid-vitamin E succinate for targeting delivery of norcantharidin to hepatic carcinoma. Carbohydr Polym. 2018;197:194–203. doi: 10.1016/j.carbpol.2018.05.090 [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Blazsek I, Comisso M, et al. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer. 1995;31(6):953–963. doi: 10.1016/0959-8049(95)00050-x [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Chi J, Han B, et al. Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohydr Polym. 2017;174:282–290. doi: 10.1016/j.carbpol.2017.06.072 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Sun X, Zhang Z. An investigation on liver-targeting microemulsions of norcantharidin. Drug Deliv. 2005;12(5):289–295. doi: 10.1080/10717540500176829 [DOI] [PubMed] [Google Scholar]
- 20.Guan M, Zhou Y, Zhu Q, et al. N-trimethyl chitosan nanoparticle-encapsulated lactosyl-norcantharidin for liver cancer therapy with high targeting efficacy. Nanomedicine. 2012;8(7):1172–1181. doi: 10.1016/j.nano.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Jiang Y, Ni X, et al. Glycyrrhetinic acid-modified norcantharidin nanoparticles for active targeted therapy of hepatocellular carcinoma. J Biomed Nanotechnol. 2018;14(1):114–126. doi: 10.1166/jbn.2018.2467 [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Liu L, Wang X, et al. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int J Nanomedicine. 2016;11:1395–1412. doi: 10.2147/ijn.s96862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lixin W, Haibing H, Xing T, et al. A less irritant norcantharidin lipid microspheres: formulation and drug distribution. Int J Pharm. 2006;323(1–2):161–167. doi: 10.1016/j.ijpharm.2006.05.060 [DOI] [PubMed] [Google Scholar]
- 24.Farokhzad O, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m [DOI] [PubMed] [Google Scholar]
- 25.Burgess P, Hutt P, Farokhzad O, et al. On firm ground: IP protection of therapeutic nanoparticles. Nat Biotechnol. 2010;28(12):1267–1270. doi: 10.1038/nbt.1725 [DOI] [PubMed] [Google Scholar]
- 26.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Deng X, Yang H. Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) block copolymers: characterization and their use as drug carriers for a controlled delivery system. Biomaterials. 2003;24(20):3563–3570. doi: 10.1016/s0142-9612(03)00207-2 [DOI] [PubMed] [Google Scholar]
- 28.Dai M, Xu X, Song J, et al. Preparation of camptothecin-loaded PCEC microspheres for the treatment of colorectal peritoneal carcinomatosis and tumor growth in mice. Cancer Lett. 2011;312(2):189–196. doi: 10.1016/j.canlet.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 29.Ni X, Chen L, Zhang H, et al. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017;24(1):1501–1512. doi: 10.1080/10717544.2017.1384862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1–2):313–322. doi: 10.1016/j.ijpharm.2016.11.047 [DOI] [PubMed] [Google Scholar]
- 31.Yang B, Ni X, Chen L, et al. Honokiol-loaded polymeric nanoparticles: an active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017;24(1):660–669. doi: 10.1080/10717544.2017.1303854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poustchi F, Amani H, Ahmadian Z, et al. Combination therapy of killing diseases by injectable hydrogels: from concept to medical applications. Adv Healthc Mater. 2021;10(3):e2001571. doi: 10.1002/adhm.202001571 [DOI] [PubMed] [Google Scholar]
- 33.Jeong B, Kim S, Bae Y. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54(1):37–51. doi: 10.1016/s0169-409x(01)00242-3 [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, Gao W, Hu H, et al. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: high drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J Control Release. 2014;174:161–170. doi: 10.1016/j.jconrel.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 35.Ci L, Huang Z, Liu Y, et al. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: preparation, characterization and application in a vaginal drug delivery system. Acta Pharm Sin B. 2017;7(5):593–602. doi: 10.1016/j.apsb.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akash M, Rehman K. Recent progress in biomedical applications of Pluronic (PF127): pharmaceutical perspectives. J Control Release. 2015;209:120–138. doi: 10.1016/j.jconrel.2015.04.032 [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Wang J, Zhang X, et al. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J Control Release. 2009;135(2):175–182. doi: 10.1016/j.jconrel.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 38.Wen Q, Zhang Y, Luo J, et al. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int J Pharm. 2020;582:119334. doi: 10.1016/j.ijpharm.2020.119334 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Lu W, Wang J, et al. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Control Release. 2007;117(3):387–395. doi: 10.1016/j.jconrel.2006.11.024 [DOI] [PubMed] [Google Scholar]
- 40.Jia W, Gu Y, Gou M, et al. Preparation of biodegradable polycaprolactone/poly (ethylene glycol)/polycaprolactone (PCEC) nanoparticles. Drug Deliv. 2008;15(7):409–416. doi: 10.1080/10717540802321727 [DOI] [PubMed] [Google Scholar]
- 41.Sellers D, Kim T, Mount C, et al. Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion. Biomaterials. 2014;35(31):8895–8902. doi: 10.1016/j.biomaterials.2014.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan J, Geng S, Zhao H, et al. Doxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administration. J Control Release. 2016;235:328–336. doi: 10.1016/j.jconrel.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 43.You J, Cao J, Zhao Y, et al. Improved mechanical properties and sustained release behavior of cationic cellulose nanocrystals reinforeced cationic cellulose injectable hydrogels. Biomacromolecules. 2016;17(9):2839–2848. doi: 10.1021/acs.biomac.6b00646 [DOI] [PubMed] [Google Scholar]
- 44.Ju C, Sun J, Zi P, et al. Thermosensitive micelles-hydrogel hybrid system based on poloxamer 407 for localized delivery of paclitaxel. J Pharm Sci. 2013;102(8):2707–2717. doi: 10.1002/jps.23649 [DOI] [PubMed] [Google Scholar]
- 45.Xie MH, Ge M, Peng JB, et al. In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharm Dev Technol. 2019;24(5):623–629. doi: 10.1080/10837450.2018.1550788 [DOI] [PubMed] [Google Scholar]
- 46.Zhang YB, Ding JX, Sun DK, et al. Thermogel-mediated sustained drug delivery for in situ malignancy chemotherapy. Mater Sci Eng C Mater Biol Appl. 2015;49:262–268. doi: 10.1016/j.msec.2015.01.026 [DOI] [PubMed] [Google Scholar]