Abstract
Critical illness is often painful, both from the underlying source of illness, as well as necessary procedures performed for the monitoring and care of these patients. Pain is often under-recognized in the critically ill, especially among those who cannot self-report, so accurate assessment and management continue to be major consideration in their care. Pain management in the intensive care unit (ICU) is an evolving practice, with a focus on accurate and frequent pain assessment, and targeted pharmacologic and non-pharmacologic treatment methods to maximize analgesia and minimize sedation. In this review, we will evaluate several validated methods of pain assessment in the ICU and present management options. We will review the evidence-based recommendations put forth by the largest critical care societies and several high-quality studies related to both the in-hospital approach to pain, as well as the short- and long-term consequences of untreated pain in ICU patients. We conclude with future directions.
Keywords: pain, critical illness, ICU liberation, PICS
Introduction
Critical illness, regardless of etiology, is a painful condition. Studies have noted that at least half of all critically ill patients would describe having moderate to severe pain at rest.1,2 This is despite recommendations from the Society of Critical Care Medicine (SCCM) to utilize analgosedation, wherein a patient’s pain is treated adequately before using sedatives.3 As more focus has been directed toward minimizing sedation and early mobility, renewed attention is being paid to the assessment and treatment of pain in the critically ill in order to achieve these goals. In a critical care setting where many patients are mechanically ventilated, and over 30–50% develop delirium4 this can make an accurate and timely pain assessment difficult, and requires standardized and objective measurements.5,6 The most common and reliable way to assess pain is subjectively using self-report numeric scales; however, even in patients who cannot self-report, there are several validated tools to assess pain.7 Accurate assessment and treatment of pain can have effects on both short- and long-term outcomes.8 Untreated pain is associated with added psychological and physiological stress that can worsen critical medical conditions.9 With our increasing understanding of the importance of multi-modal pain regimens, there are many novel options for treatment. The Society for Critical Care Medicine (SCCM) recently published guidelines in both the most recent edition of ICU Liberation,10 as well as the 2018 PADIS Guidelines,3 elements of which will be reviewed in this article, along with potential future directions for research in ICU pain management.
Epidemiology of Pain in the ICU
More than 5 million patients are admitted to ICUs in the United States annually, with an average length of stay of 3.8 days.11 Unfortunately, over half of these patients will experience moderate to severe pain at rest associated with their admission, and 80% will have pain during procedures.1,3,12–15 The rates of pain do not differ between the causes of admission – either medical or surgical – and this is likely related to the various etiologies of pain.1,2,16 In medical admissions, patient often have inflammatory or ischemic pain related to their underlying disease. There can also be components of neuropathic pain with many disease processes and post-procedural, incisional, and traumatic pain in surgical and trauma patients. All these contribute to pain at rest. In addition to that, procedures add discrete episodes of acute pain. Recent studies have demonstrated that patients rate ICU-related procedures such as arterial line insertion, chest tube and drain removal as the most painful for ICU survivors.14,17 Other procedures rated as uncomfortable include mechanical ventilation, endotracheal tube suctioning, and repositioning.14
To fully understand the etiology of pain in critically ill patients, one should consider the definition of pain established by the International Association for the Study of Pain (IASP). IASP has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”16 This definition allows that the experience of pain is multi-dimensional and not a purely physical entity, helping illuminate the experience of pain in the ICU. In the critical care setting, pain may be commonly augmented by the psychological distress of being hospitalized and the loss of a sense of control. In addition to the sudden dependence on others for survival that is inherent in the ICU, many patients must contend with pain while experiencing a limited ability to communicate, or in some cases, a near total loss of the ability to communicate (eg, while receiving mechanical ventilation). Evidence also suggests that “pain distress,” an emotional component of pain, may be experienced by patients undergoing procedures.18 Untreated pain in the ICU has even been associated with higher risk of death,19 and thus the prompt assessment, treatment and mitigation management of pain in the ICU is paramount.
Assessment of Pain in the ICU
The first element in the Society of Critical Care Medicine (SCCM) ICU Liberation Bundle10 and the 2018 Pain, Agitation/Sedation, Delirium, Immobility and Sleep Disruption (PADIS) Clinical Practice Guideline3 is the assessment and appropriate management of pain in ICU patients. Pain is a basic physiological pathway evolutionarily developed for the avoidance of tissue injury. What makes pain in the ICU unique, more complex, and potentially more harmful, is that critically ill patients typically cannot communicate, whether due to delirium, sedation, or mechanical ventilation, and cannot withdraw as they would normally do secondary to physical or chemical restraints. Pain is also a profoundly subjective experience such that no two individuals likely experience, or react to, the same painful event identically. These complex elements require a systematic and thoughtful approach to the assessment and management of pain in the ICU.
The appropriate assessment and management of pain in the ICU has been associated with improved patient outcomes such as fewer days of mechanical ventilation, decreased ICU length of stay, delirium and mortality rates.3,8,15,20 Appropriate analgesia can reduce sedation use,8,21 which might reduce delirium and its negative long-term effects.22
Assessment Methods
There are a variety of validated tools for the assessment of pain in hospitalized patients. Many of these are anchored to a subjective assessment that is typically a numeric or visual scale. As suggested in the 2018 PADIS Guidelines3 and the ICU Liberation Bundle,10 providers should elicit a self-report of pain first. If the patient’s ability to communicate is limited by either sedation, mechanical ventilation or altered mental status, then one can use one of the objective, validated measures of pain assessment.
Subjective or Self-Report Assessment Tools
Upon admission to the ICU, a thorough pain assessment including the character (dull vs lancinating, etc.,) duration, location and severity of pain should be attempted. The PQRSTUV mnemonic can be helpful in this initial intake.
PQRSTUV
Provocative/Palliative factors: Pain cause; pain-relieving strategies.10
Quality: Pain sensation.
Region: Pain location.
Severity: Pain intensity.
Time: Pain duration or temporality (constant, intermittent).
Understand: Previous pain experience and known problems.
Values: Values and preferences for pain treatment.
This assessment tool is recommended in the ICU Liberation Bundle10 for use on initial admission to the ICU, to be followed by numerical scale or objective pain assessment measures.
Subsequently, after the initial intake, pain should be assessed regularly. Pain can fluctuate over time, can be present at rest and escalate during procedures or movement. SCCM recommends pain be assessed every two to three hours at rest, and during procedures or other mobility events.3
Numeric Scales
The most common self-report method for pain assessments are numeric rating scales, typically from 0 to 10, with 0 representing no pain and 10 representing severe pain.23 A preferred scale for ICU patients who may not be able to verbalize but are interactive is the Numeric Pain Rating Scale-Visual Component (NRS-V), in which patients report their pain on a scale from 0 to 10. An example of the NRS-V is shown in Figure 1. The visual component shows the scale in large font, with “no pain” next to the number 0 on the left side of the scale and “extreme pain” next to the number 10 on the right side of the scale. Intubated patients can point to their corresponding pain level with the use of a large communication board including the NRS-V scale, or providers can hold up the scale and ask the patient to nod when they point at the accurate rating of their pain. Practical limitations to using these visual scales are patients who have active delirium, those with vision or hearing impairment, or those who otherwise cannot follow commands.
Figure 1.
Numeric pain rating scale – visual component.
Objective/Behavioral Assessment Tools
Vital Sign Assessment
Vital sign derangements should be considered sensitive, but not specific, for predicting pain in critically ill patients.3,24–27 Vital signs are not adequate for the assessment of pain and should never be used as the sole measurement. Non-painful ICU experiences and underlying pathology such as sepsis may also trigger vital sign changes such as tachycardia that are unrelated to pain but may be inappropriately attributed to pain by providers.3,24,25 The wide variation of physiologic derangements of critically ill patients limits the use of vital signs as the sole method for assessing pain.
Validated Behavioral Pain Assessments
Given the complexity of communicating with non-verbal ICU patients, several, now well-validated, behavioral assessment tools have been developed for pain.28 There are two primary tools that we will discuss here: the Behavioral Pain Scale (BPS)5 and the Critical-Care Pain Observation Tool (CPOT).7
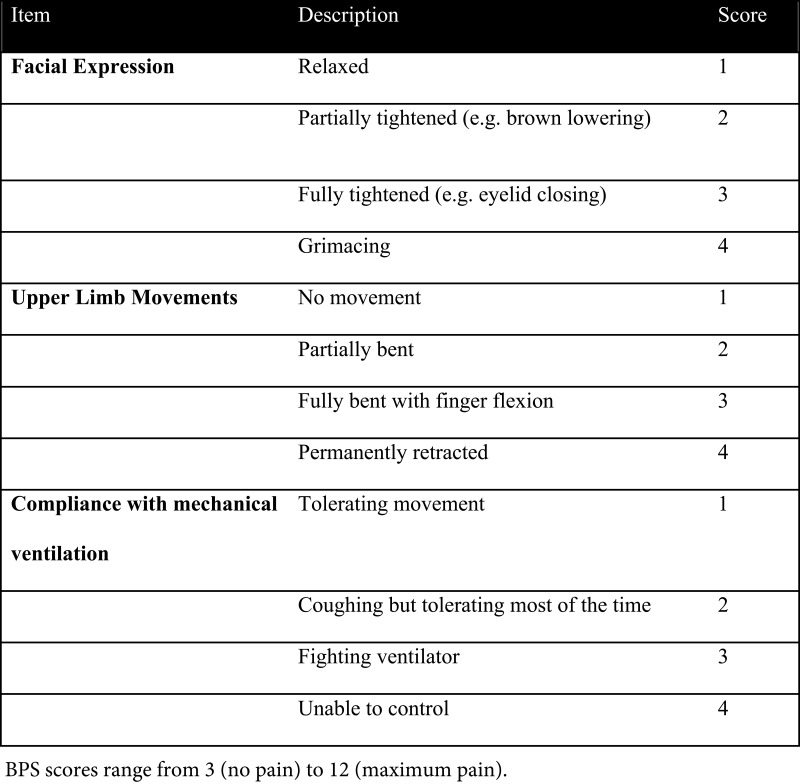
The Behavioral Pain Scale ranges from 3 to 12 and has 3 domains: facial expression, upper limb movement, and compliance with mechanical ventilation (see Figure 2). A BPS score ≥ 6 signifies pain that should be addressed with treatment.5
Figure 2.
Behavioral pain scale.
Behavioral pain scales have been validated in a variety of critically ill patient populations,6,29,30 but are not directly interchangeable with patient-reported scales, and should be used only when patient reported scales are unable to be assessed or obtained or as an adjunct to patient report.3,6
The CPOT scoring system is similar, including facial expression, body movements, compliance with the ventilator or, for extubated patients, vocalization, and muscle tension. This scoring system is on a scale from 0 to 8, with scores greater than 2 signifying an unacceptable level of pain that requires treatment.7
The adequate assessment of pain should be followed by appropriate titration of analgesic interventions to achieve the above-stated positive outcomes. However, the existing literature suggests that this is not as prevalent in practice. In one large multicenter prospective cohort study of medication administration in Canadian ICUs, over half of the ICUs studied had a standardized assessment and management protocol, but the assessment tool was used in only 19% and the management protocol in only 25%.31 In another large multicenter observational trial of over a thousand mechanically ventilated patients, limited to those who had pain assessments and analgesia administered on Day 2, those assessed for pain had less sedative use and more directed pain treatment surrounding painful procedures or interventions, and had improved patient outcomes.8 In this study, standardized protocols were more likely to be used in an academic university-affiliated setting. Thus, although having standardized protocols for the assessment and management of pain are critically important, the actual implementation and adherence to these protocols remains an area for improvement.
In summary, clinicians should assess critically ill patients for their level of pain regularly, using self-report if possible. If the patient cannot communicate, then one should use validated behavioral pain scales. Vital signs should not be used as surrogates for pain assessment. Pain should be reassessed every 2–3 hours and more frequently before painful procedures or mobilization.
ICU Pain Management
Appropriate assessment of pain must be partnered with an adequate, multi-modal, and evidence-based management strategy. This multi-modal strategy should incorporate both pharmacologic and non-pharmacologic modalities of pain control. The recommended approach is to employ an inclusive assessment and management protocol, which directs recommended pain management strategies based on pain scores.3 In this section, we will discuss currently recommended pharmacologic and non-pharmacologic pain management strategies.
Non-Pharmacologic Management
There have been several non-pharmacologic methods that have gained increasing evidence over the last several years. The SCCM ICU Liberation Bundle3 recommends four primary non-pharmacologic methods: massage therapy, cold therapy, music and sound, and relaxation therapy.
The goal of non-pharmacologic therapies is to address both physical sensory pain pathways (massage therapy, cold therapy) as well as the emotional, affective and cognitive elements of pain perception (music and sounds, relaxation therapy). A challenge in critically ill patients is in many cases they are unable to communicate their sensations, perceptions or emotions surrounding their pain. However, many of these methods have been shown to decrease both self-reported pain scores and behavioral pain assessments.3
Massage Therapy
Massage therapy for ICU patients typically involves massage on the back, feet and/or hands. Depending on the patient’s clinical status, hands-only massage is also acceptable.32 The ICU Liberation Bundle recommends at least 20 minutes of light pressure massage at least twice in a 24-hour period.3 Massage therapy, when done consistently, has been shown to reduce visual numeric pain scores by up to 2 points.33–35
Massage is typically paired with decreasing sensory stimuli such as dimming lights and either muting alarms or decreasing the volume and providing earplugs or an eye mask to the patient.3 This is often seen as a barrier to implementation of a massage protocol, given frequent disruptions in an ICU setting. There have been no feasibility studies performed on the implementation of a massage protocol. The ICU Liberation Bundle recommends engagement of family members to participate in massage care with guidance from the nursing staff.3,10
Cold Therapy
Cold therapy in ICU patients for pain management has been described by applying gauze-wrapped ice packs to procedural areas pre-procedure. This can be done with or without pharmacologic analgesia. In a randomized study of patients having chest tubes removed, this was done for a period of 10–20 minutes pre-procedure until the skin reached 15º C, and was associated with a 1 point drop on a 0–10 visual scale, with effects diminishing after 15 minutes.36–40
Music/Sound Therapy
Music or sound therapy has been associated with moderate decreases in pain scores in ICU patients.10,41–47 This intervention portends no physical risk to the patient so should be considered. The existing studies recommend at least 20–30 minutes,48 taking in the patient’s preferences into account. A randomized study of a music intervention compared to standard care or even noise reduction showed a decrease in self-reported pain scores as high as 2.6 points.45 This is another area that is ripe for patient family involvement, as family will likely know what music the patient would best enjoy. Familiar voices have not been as wellstudied as music interventions, but from the existing data and anecdotal recounts by ICU survivors, hearing a familiar voice, especially during procedures, is considered helpful in relieving anxiety symptoms or mental stress,49 and possibly pain.
Relaxation Therapy
Relaxation therapy includes techniques such as guided imagery, breathing exercises, biofeedback and self-hypnosis, with guided imagery and breathing exercises being the most frequently used in critically ill patients.3,10 These therapies have been shown to have an up to a 2.6-point reduction in visual scale pain scores (0–10), albeit from small sample size and limited study designs.3,10 Guided-image therapy which typically involves having the patient imagine a calm and relaxing location of their choice to take them psychologically out of the current painful environment and can sometimes utilize pre-recorded tapes instead of the bedside nurse, has been associated with decreased pain scores, less opioid use and shorter length of stay.50,51 In cardiac surgery patients, a study of breathing exercises led by a bedside nurse demonstrated significantly lower pain scores when combined with opioid therapy compared to opioid therapy alone for chest tube removal.52
We recommended using a combination of these non-pharmacologic therapies in conjunction with pharmacologic therapy as needed for ICU-related pain.10 These strategies can be compiled into a comprehensive pain assessment and management protocol that is standardized to ensure the highest quality of pain management in the ICU.
Pharmacologic Management
Pharmacologic management of pain has been the mainstay of treatment for critically ill patients. However, pharmacologic agents for pain are not without side effects, and can lead to unwanted issues such as opioid tolerance/withdrawal, and delirium. Pharmacologic management should be paired with protocolized pain assessments, and approached in a gradated fashion in response to pain scores.3,10 SCCM guidelines recommend opioids as first-line for non-neuropathic pain, being careful to use a protocol based on pain scores for titration.3,10 They also recommend the method of “analgosedation,” which treats pain before initiating sedation therapy, and only using sedation if needed. Multi-modal adjunct therapies are recommended, such as ketamine infusions, acetaminophen, and gabapentinoids and in some populations non-steroidal anti-inflammatories (NSAIDs), lidocaine infusions, and regional anesthesia. See Table 1 for non-opioid pharmacologic treatment options.
Table 1.
Non-Opioid Analgesics for the ICU
| Drug | Dose | Metabolism/Clearance | Considerations |
|---|---|---|---|
| Acetaminophen | <4 g/d | Hepatic | Dose adjust for cirrhosis: up to 2g/d |
| Gabapentinoids Gabapentin Pregabalin |
100–1200 mgTID 50–300 mg TID |
Renal |
Dose adjust for GFR; Commonly can cause somnolence, blurry vision |
| Ketamine Infusion | 2–5 mcg/kg/min | Hepatic | Avoid if history of PTSD/psychosis due to risk of hallucinations |
| Lidocaine Infusion* | 1–2 mg/min | Hepatic | Avoid if history of seizure disorder, on anti-arrhythmics |
| NSAIDs* | Varies | Renal | Caution with reduced GFR, peptic ulcers, age >65 |
| SNRIs* | Varies | Hepatic | Analgesic effect is faster onset than anti-depressant effect; helps with anxiety component |
Note: *Not recommended for routine use by SCCM.3
Abbreviations: TID, three times daily; GFR, glomerular filtration rate; PTSD, post-traumatic stress disorder; NSAIDs, non-steroidal anti-inflammatory drugs; SNRIs, serotonin/norepinephrine reuptake inhibitors.
Using a standardized pain assessment and management protocol is associated with a more efficient use of pharmacologic pain control, with lower doses of opioids and improved self-reported numeric pain scores, although this association is not always consistent across studies, with some showing increased doses in certain populations.21,53,54 In patients who were adequately assessed for pain, multi-modal adjuvant therapy use such as ketamine, paracetamol and nefopam increased, as did the use of dedicated treatment during procedures.8,55
Unlike many of the non-pharmacologic interventions, pharmacologic pain management has several adverse effects, the most concerning being ICU delirium.3,12,15,56–59
There is a complex interplay of pain, pharmacologic analgesia, and sedation when it comes to delirium, a needle that must be carefully threaded. Untreated pain has been associated with higher rates of delirium;60–64 however, the overuse of opioids and particularly benzodiazepines have been associated with increased delirium.65–67 This supports the analgosedation approach, in which the lowest possible dose to achieve target effect is recommended, and benzodiazepines in general should be avoided for sedation as they are strongly associated with delirium.3,10,22
Pain and Delirium
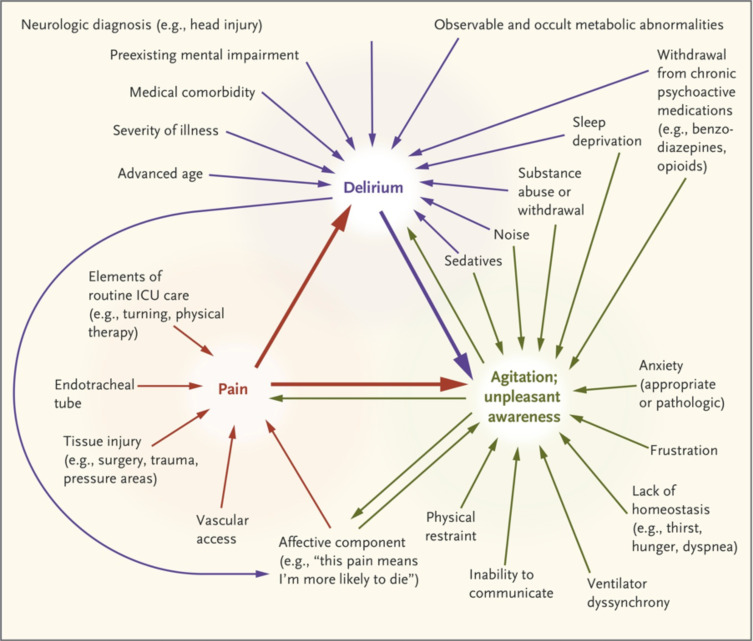
Pain has a complex role in the epidemiology of ICU delirium. Untreated pain can both perpetuate delirium, especially in surgical patients, and the pharmacologic treatment of pain can also perpetuate it,58 especially opioid therapy in older adults. Figure 3 shows the complex interaction between pain, delirium, and agitation.57
Figure 3.
Interaction between pain, delirium, and agitation. Notes: From The New England Journal of Medicine, Reade MC, Finfer S, Sedation and delirium in the intensive care unit, Volume No. 370(5), Page No. 444–454, Copyright © (2014) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.57
A common misconception is that sedated patients do not feel pain. In studies of patients and anecdotal recounts of ICU survivors of their ICU experience, this is untrue.68 These painful experiences tend to become distorted, and may become incorporated into severe and disturbing delusions of patients suffering from ICU delirium. This can be profoundly distressing to patients, even years after their critical illness, and is a contributing factor to Post-Intensive Care Syndrome (PICS),69 which will be discussed in more detail in a later section.
As mentioned previously, delirium is an important balancing risk factor when achieving adequate analgesia and sedation in the ICU. Delirium has effects on long-term cognition, even one to five years after hospital discharge.4,70–76 Many patients who experience ICU delirium develop Post-Traumatic Stress Disorder (PTSD) after hospitalization, with many patients reporting flashbacks to particularly painful experiences during their illness.77–82 Delirium is also a risk factor for mortality in ICU patients,83–85 making it critical to prevent and screen for delirium on a regular basis, and utilize pain management strategies that minimize delirium, by the avoidance of benzodiazepines and maintaining the lowest possible dose of pharmacologic agents by employing a multi-modal approach.
The utilization of an evidence-based and well-validated ICU Liberation Bundle1 the first portion of which we have referenced heavily in this article, which was previously titled the ABCDEF Bundle (Assess, Prevent and Manage Pain, Both Spontaneous Awakening Trial (SAT) and Spontaneous Breathing Trials (SBT), Choice of Analgesia or Sedation, Delirium: Assess, Prevent, Manage, Early Mobility and Exercise, Family Engagement86,87 and Empowerment)88 has shown to decrease ICU delirium, but did not improve pain scores.89 Given that the bundle seeks to reduce sedation and allow patients to communicate, this likely facilitated patients being able to self-report pain, which is more reliable than behavioral pain assessments and may explain why pain scores do not improve with the bundle alone.
Pain-Related Long-Term Outcomes in ICU Patients
Uncontrolled pain in critically ill patients is not just an ethical or humanistic concern, but also a medical one. Pain pathways are intended to relay signals of tissue damage to the brain, in order to avoid the painful stimulus (by physical withdrawal or avoidance) and further injury. Pain pathways are closely interconnected to the sympathetic nervous system. Many of our physiologic signs of pain are sympathetic in nature, such as tachycardia, hypertension, and diaphoresis. Uncontrolled pain can leave a critically ill patient in a state of persistent adrenergic activation, which can lead to additional stress to an already ailing cardiovascular system.90–92 Critically ill patients are typically in catabolism, and uncontrolled pain leads to even higher levels of metabolic energy expenditure,57,93 which may worsen an already precarious metabolic state. Uncontrolled pain can even have unwanted immunomodulatory effects in cancer.94,95
Post-Intensive Care Syndrome (PICS) is a constellation of symptoms related to physical, cognitive and psychological ailments that persist months to years after discharge from the ICU.69 Chronic pain is an aspect of PICS with between 33% and 77% of ICU survivors reporting persistent pain after discharge from the ICU that can last for years, with a third to three-quarters of patients still reporting pain at 1 year after ICU discharge.96–99
In a large prospective cohort study of almost 300 ICU survivors that sought more granular detail of chronic pain after the ICU, Hayhurst et al. found that at one year post-discharge, 74% of patients reported any pain, with 35% of patients rating this pain as moderate to severe.97 In a study by Baumbach et al., 33% of patients reported chronic pain at 6 months post-discharge with half (16%) reporting this pain as resulting directly from their ICU stay, without a pre-existing history of pain symptoms.96 In another smaller study, Devine et al. found that 66% a mixed medical/surgical ICU cohort reported a new pain disorder associated with their critical illness.100 There has been at least one study that this chronic pain may persist long after the one-year mark, with 57% of patients reporting pain at 6 to 11 years after discharge.101
Mental health disorders such as depression and anxiety are a large component of PICS, with one-third of ICU survivors suffering from depression, which is highly comorbid with pain conditions.80,101 This syndrome, including chronic pain, has a significant impact of quality of life and the ability of ICU survivors to return to work and other normal daily activities, even years after their illness. Chronic pain after critical illness affects patients’ ability to sleep, enjoy life, time with their families and/or return to work.96,97,100 At 5 years after ICU discharge, three-quarters of scores related to physical functioning on a quality of life assessment were below the population mean.102
Although there has yet to be an adequately powered or rigorously conducted study to test the hypothesis that untreated pain, or amount of opioid received in the ICU is directly associated with chronic pain conditions after discharge, there is enough literature to signal there exists a link, and further work is necessary in this space to better identify modifiable risk factors or interventions to prevent the development of chronic pain conditions in these patients.
Summary and Future Directions
Recognition of pain as a prevalent problem in the critically ill, including those who are sedated and non-communicative is essential. The approach to assessment and treatment of pain in the ICU should be well-protocolized and multi-modal, with a focus on adequate and frequent assessment of pain both at rest and during procedures, with specific guidelines for addressing pain scores that are out of an acceptable range. Incorporating non-pharmacologic methods is important and may allow for less of a need for pharmacologic interventions. These efforts may be time-consuming, so engagement of the patient’s family and loved ones is paramount for successful pain management in these patients. Although opioid therapy remains the mainstay of pharmacologic pain management in the ICU, there are several multi-modal adjuncts that should be used in addition to non-pharmacologic methods when possible in the appropriate patient populations, as this may allow the minimization of opioid therapy, which has several undesired side effects, one of which is ICU delirium, especially when benzodiazepine-based sedation is utilized.
A major area in need of further research is identifying modifiable risk factors or interventions that can help prevent the development of chronic pain syndromes in ICU survivors. Over a third of ICU survivors report functional or physical limitations even at one-year post-ICU discharge, and a third suffer from depression.80 Many patients report pain as a contributing factor to their physical limitations, which has a profound impact on these patients from a quality of life and economic standpoint, with one-third unable return to work at one year, and for those who return to work, a third experience job loss and up to two-thirds require an occupation change, with up to 84% reporting a worsening employment status.103 Rigorous prospective studies identifying pain-related interventions either in-hospital, such as locoregional therapies or strictly protocolized analgesia regimens, or shortly after discharge, such as early referral to pain management and/or physical therapy, that could prevent this tremendous economic and public health issue of chronic pain and debilitation of ICU survivors are desperately needed.
Disclosure
Dr Mina Nordness reports grants from NIH-NIA, during the conduct of the study. Dr Pratik Pandharipande reports grants from Pfizer, outside the submitted work. The author report no other conflicts of interest in this work.
References
- 1.Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam JJ, Jaber S. A prospective study of pain at rest: incidence and characteristics symptom in surgical and trauma versus intensive care patients. Anesthesiology. 2007;107(5):858–860. doi: 10.1097/01.anes.0000287211.98642.51 [DOI] [PubMed] [Google Scholar]
- 2.Damico V, Macchi G, Murano L, Forastieri Molinari A. Incidence of pain at rest and during nursing procedures in ICU patients: a longitudinal observational study. Ann Ig. 2020;32(4):407–418. doi: 10.7416/ai.2020.2364 [DOI] [PubMed] [Google Scholar]
- 3.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258–2263. doi: 10.1097/00003246-200112000-00004 [DOI] [PubMed] [Google Scholar]
- 6.Kanji S, MacPhee H, Singh A, et al. Validation of the critical care pain observation tool in critically ill patients with delirium: a Prospective Cohort Study. Crit Care Med. 2016;44(5):943–947. doi: 10.1097/CCM.0000000000001522 [DOI] [PubMed] [Google Scholar]
- 7.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15(4):420–427. doi: 10.4037/ajcc2006.15.4.420 [DOI] [PubMed] [Google Scholar]
- 8.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology. 2009;111(6):1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0 [DOI] [PubMed] [Google Scholar]
- 9.Frischenschlager O, Pucher I. Psychological management of pain. Disabil Rehabil. 2002;24(8):416–422. doi: 10.1080/09638280110108841 [DOI] [PubMed] [Google Scholar]
- 10.Posa PJ, Singh J, Stollings JL. ICU Liberation. Second ed. Mount Prospect, IL: Society of Critical Care Medicine; 2020. [Google Scholar]
- 11.Critical Care Statistics. Society of Critical Care Medicine (SCCM). Availbale from: https://www.sccm.org/Communications/Critical-Care-Statistics. Accessed January18, 2021.
- 12.Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56 [DOI] [PubMed] [Google Scholar]
- 13.Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106(4):687–695; quiz 891–682. doi: 10.1097/01.anes.0000264747.09017.da [DOI] [PubMed] [Google Scholar]
- 14.Puntillo KA, Max A, Timsit JF, et al. Determinants of procedural pain intensity in the intensive care unit. The Europain(R) study. Am J Respir Crit Care Med. 2014;189(1):39–47. doi: 10.1164/rccm.201306-1174OC [DOI] [PubMed] [Google Scholar]
- 15.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
- 16.Merskey HAFD, Bonica JJ, Carmon A, et al. Pain terms: a list with definitions and notes on usage. Recommended by the IASP subcommittee on taxonomy. Pain. 1979;6(3):249. [PubMed] [Google Scholar]
- 17.Puntillo KA, Miaskowski C, Kehrle K, Stannard D, Gleeson S, Nye P. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Crit Care Med. 1997;25(7):1159–1166. doi: 10.1097/00003246-199707000-00017 [DOI] [PubMed] [Google Scholar]
- 18.Puntillo KA, Max A, Timsit JF, et al. Pain distress: the negative emotion associated with procedures in ICU patients. Intensive Care Med. 2018;44(9):1493–1501. doi: 10.1007/s00134-018-5344-0 [DOI] [PubMed] [Google Scholar]
- 19.Yamashita A, Yamasaki M, Matsuyama H, Amaya F. Risk factors and prognosis of pain events during mechanical ventilation: a retrospective study. J Intensive Care. 2017;5:17. doi: 10.1186/s40560-017-0212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastrup M, von Dossow V, Seeling M, et al. Key performance indicators in intensive care medicine. A retrospective matched cohort study. J Int Med Res. 2009;37(5):1267–1284. [DOI] [PubMed] [Google Scholar]
- 21.Gelinas C, Arbour C, Michaud C, Vaillant F, Desjardins S. Implementation of the critical-care pain observation tool on pain assessment/management nursing practices in an intensive care unit with nonverbal critically ill adults: a before and after study. Int J Nurs Stud. 2011;48(12):1495–1504. doi: 10.1016/j.ijnurstu.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644 [DOI] [PubMed] [Google Scholar]
- 23.Chanques G, Viel E, Constantin JM, et al. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 2010;151(3):711–721. doi: 10.1016/j.pain.2010.08.039 [DOI] [PubMed] [Google Scholar]
- 24.Arbour C, Gélinas C. Are vital signs valid indicators for the assessment of pain in postoperative cardiac surgery ICU adults? Intensive Crit Care Nurs. 2010;26(2):83–90. doi: 10.1016/j.iccn.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 25.Gélinas C, Arbour C. Behavioral and physiologic indicators during a nociceptive procedure in conscious and unconscious mechanically ventilated adults: similar or different? J Crit Care. 2009;24(4):628.e627–617. doi: 10.1016/j.jcrc.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 26.Boitor M, Richard-Lalonde M, Bérubé M, Émilie G, Gélinas C. Vital signs fluctuations and their relationship with pain in the brain-injured adult critically ill - a repeated-measures descriptive-correlational study. Intensive Crit Care Nurs. 2019;55:102743. doi: 10.1016/j.iccn.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 27.Chen HJ, Chen YM. Pain assessment: validation of the physiologic indicators in the ventilated adult patient. Pain Manag Nurs. 2015;16(2):105–111. doi: 10.1016/j.pmn.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 28.Gélinas C, Joffe AM, Szumita PM, et al. A psychometric analysis update of behavioral pain assessment tools for noncommunicative, critically ill adults. AACN Adv Crit Care. 2019;30(4):365–387. doi: 10.4037/aacnacc2019952 [DOI] [PubMed] [Google Scholar]
- 29.Marmo L, Fowler S. Pain assessment tool in the critically ill post-open heart surgery patient population. Pain Manag Nurs. 2010;11(3):134–140. doi: 10.1016/j.pmn.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Severgnini P, Pelosi P, Contino E, Serafinelli E, Novario R, Chiaranda M. Accuracy of critical care pain observation tool and behavioral pain scale to assess pain in critically ill conscious and unconscious patients: prospective, observational study. J Intensive Care. 2016;4:68. doi: 10.1186/s40560-016-0192-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burry LD, Williamson DR, Perreault MM, et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Can J Anaesth. 2014;61(7):619–630. doi: 10.1007/s12630-014-0174-1 [DOI] [PubMed] [Google Scholar]
- 32.Martorella G, Boitor M, Michaud C, Gélinas C. Feasibility and acceptability of hand massage therapy for pain management of postoperative cardiac surgery patients in the intensive care unit. Heart Lung. 2014;43(5):437–444. doi: 10.1016/j.hrtlng.2014.06.047 [DOI] [PubMed] [Google Scholar]
- 33.Boitor M, Gélinas C, Richard-Lalonde M, Thombs BD. The effect of massage on acute postoperative pain in critically and acutely ill adults post-thoracic surgery: systematic review and meta-analysis of randomized controlled trials. Heart Lung. 2017;46(5):339–346. doi: 10.1016/j.hrtlng.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 34.Boitor M, Martorella G, Maheu C, Laizner AM, Gélinas C. Effects of massage in reducing the pain and anxiety of the cardiac surgery critically ill-a randomized controlled trial. Pain Med. 2018;19(12):2556–2569. doi: 10.1093/pm/pny055 [DOI] [PubMed] [Google Scholar]
- 35.Jagan S, Park T, Papathanassoglou E. Effects of massage on outcomes of adult intensive care unit patients: a systematic review. Nurs Crit Care. 2019;24(6):414–429. [DOI] [PubMed] [Google Scholar]
- 36.Aktaş YY, Karabulut N. The use of cold therapy, music therapy and lidocaine spray for reducing pain and anxiety following chest tube removal. Complement Ther Clin Pract. 2019;34:179–184. doi: 10.1016/j.ctcp.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 37.Ertuğ N, Ulker S. The effect of cold application on pain due to chest tube removal. J Clin Nurs. 2012;21(5–6):784–790. doi: 10.1111/j.1365-2702.2011.03955.x [DOI] [PubMed] [Google Scholar]
- 38.Gorji HM, Nesami BM, Ayyasi M, Ghafari R, Yazdani J. Comparison of ice packs application and relaxation therapy in pain reduction during chest tube removal following cardiac surgery. N Am J Med Sci. 2014;6(1):19–24. doi: 10.4103/1947-2714.125857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasanzadeh F, Kashouk NM, Amini S, et al. The effect of cold application and lavender oil inhalation in cardiac surgery patients undergoing chest tube removal. EXCLI J. 2016;15:64–74. doi: 10.17179/excli2015-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammadi N, Pooria A, Yarahmadi S, et al. Effects of cold application on chest tube removal pain in heart surgery patients. Tanaffos. 2018;17(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- 41.Chiasson AM, Linda Baldwin A, McLaughlin C, Cook P, Sethi G. The effect of live spontaneous harp music on patients in the intensive care unit. Evid Based Complement Alternat Med. 2013;2013:428731. doi: 10.1155/2013/428731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golino AJ, Leone R, Gollenberg A, et al. Impact of an active music therapy intervention on intensive care patients. Am J Crit Care. 2019;28(1):48–55. [DOI] [PubMed] [Google Scholar]
- 43.Jafari H, Emami Zeydi A, Khani S, Esmaeili R, Soleimani A. The effects of listening to preferred music on pain intensity after open heart surgery. Iran J Nurs Midwifery Res. 2012;17(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Meghani N, Tracy MF, Hadidi NN, Lindquist R. Part I: the effects of music for the symptom management of anxiety, pain, and insomnia in critically ill patients: an integrative review of current literature. Dimens Crit Care Nurs. 2017;36(4):234–243. doi: 10.1097/DCC.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 45.Saadatmand V, Rejeh N, Heravi-Karimooi M, Tadrisi SD, Vaismoradi M, Jordan S. Effects of natural sounds on pain: a randomized controlled trial with patients receiving mechanical ventilation support. Pain Manag Nurs. 2015;16(4):483–492. doi: 10.1016/j.pmn.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 46.Kyavar M, Karkhaneh S, Rohanifar R, et al. Effect of preferred music listening on pain reduction in mechanically ventilated patients after coronary artery bypass graft surgery. Res Cardiovasc Med. 2016;5(4):e33769. [Google Scholar]
- 47.Yaghoubinia F, Navidian A, Nasir-al-din Tabatabaei SM, Sheikh S. Effect of music on pain intensity among patients with loss of consciousness in an intensive care unit. Med Surg Nurs J. 2016;4(4):e68080. [Google Scholar]
- 48.Richard-Lalonde M, Gélinas C, Boitor M, et al. The effect of music on pain in the adult intensive care unit: a systematic review of randomized controlled trials. J Pain Symptom Manage. 2020;59(6):1304–1319.e1306. doi: 10.1016/j.jpainsymman.2019.12.359 [DOI] [PubMed] [Google Scholar]
- 49.Ziehm S, Rosendahl J, Barth J, Strauss BM, Mehnert A, Koranyi S. Psychological interventions for acute pain after open heart surgery. Cochrane Database Syst Rev. 2017;7:CD009984. doi: 10.1002/14651858.CD009984.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deisch P, Soukup SM, Adams P, Wild MC. Guided imagery: replication study using coronary artery bypass graft patients. Nurs Clin North Am. 2000;35(2):417–425. [PubMed] [Google Scholar]
- 51.Halpin LS, Speir AM, CapoBianco P, Barnett SD. Guided imagery in cardiac surgery. Outcomes Manag. 2002;6(3):132–137. [PubMed] [Google Scholar]
- 52.Friesner SA, Curry DM, Moddeman GR. Comparison of two pain-management strategies during chest tube removal: relaxation exercise with opioids and opioids alone. Heart Lung. 2006;35(4):269–276. doi: 10.1016/j.hrtlng.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 53.Radtke FM, Heymann A, Franck M, et al. How to implement monitoring tools for sedation, pain and delirium in the intensive care unit: an experimental cohort study. Intensive Care Med. 2012;38(12):1974–1981. doi: 10.1007/s00134-012-2658-1 [DOI] [PubMed] [Google Scholar]
- 54.Rose L, Haslam L, Dale C, Knechtel L, McGillion M. Behavioral pain assessment tool for critically ill adults unable to self-report pain. Am J Crit Care. 2013;22(3):246–255. doi: 10.4037/ajcc2013200 [DOI] [PubMed] [Google Scholar]
- 55.Williams TA, Martin S, Leslie G, et al. Duration of mechanical ventilation in an adult intensive care unit after introduction of sedation and pain scales. Am J Crit Care. 2008;17(4):349–356. doi: 10.4037/ajcc2008.17.4.349 [DOI] [PubMed] [Google Scholar]
- 56.Gélinas C, Klein K, Naidech A, Skrobik Y. Pain, sedation, and delirium management in the neurocritically ill: lessons learned from recent research. Semin Respir Crit Care Med. 2013;34(02):236–243. doi: 10.1055/s-0033-1342986 [DOI] [PubMed] [Google Scholar]
- 57.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. doi: 10.1056/NEJMra1208705 [DOI] [PubMed] [Google Scholar]
- 58.Ely EW, Barr J. Pain/agitation/delirium. Semin Respir Crit Care Med. 2013;34(2):151–152. doi: 10.1055/s-0033-1342974 [DOI] [PubMed] [Google Scholar]
- 59.Ely EW, Pisani MA. Monitoring and treatment of pain, anxiety, and delirium in the ICU. In: Albert RK, Slutsky A, Ranieri M, Takala J, Torres A, editors. Clinical Critical Care Medicine. Philedelphia: Mosby Elsevier; 2006:51–59. [Google Scholar]
- 60.Agnoletti V, Ansaloni L, Catena F, et al. Postoperative delirium after elective and emergency surgery: analysis and checking of risk factors. A study protocol. BMC Surg. 2005;5(1):12. doi: 10.1186/1471-2482-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chrispal A, Mathews KP, Surekha V. The clinical profile and association of delirium in geriatric patients with hip fractures in a tertiary care hospital in India. J Assoc Physicians India. 2010;58:15–19. [PubMed] [Google Scholar]
- 62.Maldonado JR, van der Starre PJ, Wysong A. Post-operative sedation and the incidence of ICU delirium in cardiac surgery patients. Anesthesiology. 2003;99:A465. [Google Scholar]
- 63.Mckoy JM. Effect of delirium on length of stay for post-operative surgical patients. J Am Geriatr Soc. 2004;52(4):S167–S167. [Google Scholar]
- 64.Santos FS, Velasco IT, Fraguas R Jr. Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16(2):175–193. doi: 10.1017/S1041610204000365 [DOI] [PubMed] [Google Scholar]
- 65.Pisani MA, Murphy TE, Araujo KLB, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavone KJ, Jablonski J, Cacchione PZ, Polomano RC, Compton P. Evaluating pain, opioids, and delirium in critically ill older adults. Clin Nurs Res. 2020;1054773820973123. doi: 10.1177/1054773820973123 [DOI] [PubMed] [Google Scholar]
- 68.Clukey L, Weyant RA, Roberts M, Henderson A. Discovery of unexpected pain in intubated and sedated patients. Am J Crit Care. 2014;23(3):216–220. doi: 10.4037/ajcc2014943 [DOI] [PubMed] [Google Scholar]
- 69.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 70.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869 [DOI] [PubMed] [Google Scholar]
- 72.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–191. doi: 10.1164/rccm.200903-0442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94 [DOI] [PubMed] [Google Scholar]
- 74.Jackson JC, Obremskey W, Bauer R, et al. Long-term cognitive, emotional, and functional outcomes in trauma intensive care unit survivors without intracranial hemorrhage. J Trauma. 2007;62:80–88. doi: 10.1097/TA.0b013e31802ce9bd [DOI] [PubMed] [Google Scholar]
- 75.Jutte JE, Erb CT, Jackson JC. Physical, cognitive, and psychological disability following critical illness: what is the risk? Semin Respir Crit Care Med. 2015;36(6):943–958. doi: 10.1055/s-0035-1566002 [DOI] [PubMed] [Google Scholar]
- 76.Karnatovskaia LV, Johnson MM, Benzo RP, Gajic O. The spectrum of psychocognitive morbidity in the critically ill: a review of the literature and call for improvement. J Crit Care. 2015;30(1):130–137. doi: 10.1016/j.jcrc.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 77.Jackson JC, Archer KR, Bauer R, et al. A prospective investigation of long-term cognitive impairment and psychological distress in moderately versus severely injured trauma intensive care unit survivors without intracranial hemorrhage. J Trauma. 2011;71(4):860–866. doi: 10.1097/TA.0b013e3182151961 [DOI] [PubMed] [Google Scholar]
- 78.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11(1):R27. doi: 10.1186/cc5707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson JC, Jutte JE, Hunter CH, et al. Posttraumatic stress disorder (PTSD) after critical illness: a conceptual review of distinct clinical issues and their implications. Rehabil Psychol. 2016;61(2):132–140. doi: 10.1037/rep0000085 [DOI] [PubMed] [Google Scholar]
- 80.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 82.Patel MB, Jackson JC, Morandi A, et al. Incidence and risk factors for intensive care unit-related post-traumatic stress disorder in veterans and civilians. Am J Respir Crit Care Med. 2016;193(12):1373–1381. doi: 10.1164/rccm.201506-1158OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van den Boogaard M, Peters SA, van der Hoeven JG, et al. The impact of delirium on the prediction of in-hospital mortality in intensive care patients. Crit Care. 2010;14(4):R146. doi: 10.1186/cc9214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759 [DOI] [PubMed] [Google Scholar]
- 85.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753 [DOI] [PubMed] [Google Scholar]
- 86.Gosselin É, Richard-Lalonde M. Role of family members in pain management in adult critical care. AACN Adv Crit Care. 2019;30(4):398–410. doi: 10.4037/aacnacc2019275 [DOI] [PubMed] [Google Scholar]
- 87.Richard-Lalonde M, Boitor M, Mohand-Saïd S, Gélinas C. Family members’ perceptions of pain behaviors and pain management of adult patients unable to self-report in the intensive care unit: a qualitative descriptive study. Can J Pain. 2018;2(1):315–323. doi: 10.1080/24740527.2018.1544458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243 [DOI] [PubMed] [Google Scholar]
- 89.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14. doi: 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janig W. Systemic and specific autonomic reactions in pain: efferent, afferent and endocrine components. Eur J Anaesthesiol. 1985;2(4):319–346. [PubMed] [Google Scholar]
- 91.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82(6):1474–1506. doi: 10.1097/00000542-199506000-00019 [DOI] [PubMed] [Google Scholar]
- 92.Burton AR, Fazalbhoy A, Macefield VG. Sympathetic responses to noxious stimulation of muscle and skin. Front Neurol. 2016;7:109. doi: 10.3389/fneur.2016.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swinamer DL, Phang PT, Jones RL, Grace M, King EG. Effect of routine administration of analgesia on energy expenditure in critically ill patients. Chest. 1988;93(1):4–10. doi: 10.1378/chest.93.1.4 [DOI] [PubMed] [Google Scholar]
- 94.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90(1–2):191–199. doi: 10.1016/S0304-3959(00)00403-6 [DOI] [PubMed] [Google Scholar]
- 95.Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167(1):174–179. doi: 10.1016/0002-9610(94)90070-1 [DOI] [PubMed] [Google Scholar]
- 96.Baumbach P, Gotz T, Gunther A, Weiss T, Meissner W. Prevalence and characteristics of chronic intensive care-related pain: the role of severe sepsis and septic shock. Crit Care Med. 2016;44(6):1129–1137. doi: 10.1097/CCM.0000000000001635 [DOI] [PubMed] [Google Scholar]
- 97.Hayhurst CJ, Jackson JC, Archer KR, Thompson JL, Chandrasekhar R, Hughes CG. Pain and its long-term interference of daily life after critical illness. Anesth Analg. 2018;127(3):690–697. doi: 10.1213/ANE.0000000000003358 [DOI] [PubMed] [Google Scholar]
- 98.Battle CE, Lovett S, Hutchings H. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 2013;17(3):R101. doi: 10.1186/cc12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langerud AK, Rustoen T, Brunborg C, Kongsgaard U, Stubhaug A. Prevalence, location, and characteristics of chronic pain in intensive care survivors. Pain Manag Nurs. 2018;19(4):366–376. doi: 10.1016/j.pmn.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 100.Devine H, Quasim T, McPeake J, Shaw M, McCallum L, Mactavish P. Chronic pain in intensive care unit survivors: incidence, characteristics and side-effects up to one-year post-discharge. J Rehabil Med. 2019;51(6):451–455. doi: 10.2340/16501977-2558 [DOI] [PubMed] [Google Scholar]
- 101.Timmers TK, Verhofstad MH, Moons KG, van Beeck EF, Leenen LP. Long-term quality of life after surgical intensive care admission. Arch Surg. 2011;146(4):412–418. doi: 10.1001/archsurg.2010.279 [DOI] [PubMed] [Google Scholar]
- 102.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14(1):R6. doi: 10.1186/cc8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamdar BB, Suri R, Suchyta MR, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17–27. doi: 10.1136/thoraxjnl-2019-213803 [DOI] [PMC free article] [PubMed] [Google Scholar]