Abstract
Objective
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors. Long non-coding RNA plays an important role in the development of HCC. This study analyzed the impact of MEG3 on malignant behavior of HCC and explored its possible molecular mechanism.
Methods
Expression of MEG3 in HCC tissues and cell lines was measured by qRT-PCR. Transfection efficiency of MEG3 was verified by qRT-PCR. Cell proliferation, transwell migration, invasion and cell cloning assays were used to detect the effect of MEG3 on the proliferation, migration and invasion ability of HCC cells. The bioinformatics analysis was applied to predict the binding between miR-544b and MEG3 as well as BTG2. Luciferase reporter assay was performed to verify their interaction. Finally, the m6A modification of MEG3 by METTL3 was identified through RIP experiments.
Results
MEG3 was lowly expressed in HCC tissues and cells. Overexpression of MEG3 inhibits the proliferation, migration and invasion of HCC cells. MiR-544b can be sponged by MEG3, and overexpression of miR-544b reverses the anti-cancer effect of MEG3. We further confirmed that BTG2 gene is the target gene of miR-544b. Epigenetic studies have shown that METTL3-mediated N6-methyladenosine modification led to MEG3 downregulation.
Conclusion
In HCC, MEG3 and BTG2 are lowly expressed while miR-544b is highly expressed. MEG3 regulates the expression of BTG2 through miR-544b, thus affecting the malignant behavior of HCC. METTL3 regulates the m6A modification of MEG3 and its expression. This study clarified the role of MEG3/miR-544b/BTG2 axis in HCC and also provided new targets for HCC research.
Keywords: lncRNA MEG3, miR-544b, HCC, N6-methyladenosine, BTG2
Introduction
Hepatocellular carcinoma (HCC) is a common highly malignant tumor, ranking third in mortality, high mortality, high metastatic and invasive tumor. The incidence of HCC is relatively insidious, and most of the patients with hepatocellular carcinoma are in the advanced stage at the time of diagnosis, which cannot be treated by surgery, and have poor sensitivity to chemotherapy drugs.1 In addition, the occurrence and development of HCC are also affected by epigenetics.2
Long noncoding RNAs (lncRNAs) are noncoding RNAs of more than 200 nucleotides. LncRNAs can modulate gene expression at transcriptional or posttranscriptional levels and influence physiological processes, including proliferation, differentiation and necrosis. Studies have shown the dysregulated expression of numerous lncRNAs indicating their critical roles in tumor progression.3 Matrilineal expression gene 3 (MEG3) is an imprinted gene located on human chromosome 14q32.3. MEG3 is widely expressed in normal tissues, in addition, its expression is decreased or absent in HCC, nasopharyngeal cancer and other tumors. Function studies have demonstrated the effect of MEG3 on the malignant behavior of various cancers.4 However, the effect and mechanism of MEG3 on HCC are still unclear.
MicroRNAs (miRs) are a type of non-coding RNA with a length of about 22 nucleotides. Several miR shave been found abnormally expressed in tumor cells and play an important role in the process of tumor cell proliferation, differentiation, apoptosis and metastasis. As one of the important miRNA members, miR-544 plays an important role in regulating the pathological changes of tumor cells. Besides, miR-544 has been reported to mediate the immune escape of liver cancer via targeting RUNX3.5 It indicated the critical role of miR-544 in the liver cancer progression.
B-cell translocation gene (BTG2) is an important member of the B-cell translocation growth factor family.6,7 In common with other members of the family, it inhibits cell proliferation. Previous studies have confirmed that B-cell translocated growth factor family proteins interact closely with many oncogenes and tumor suppressor gene protein molecules in cells,8,9 and these protein molecules may affect important biological functions of cells. In HCC development, BTG2 also plays a crucial role. It is down-regulated in HCC and mediates the suppressive function of circ_0014717 on tumorigenesis.10,11 However, the precise mechanism of BTG2 in HCC remains to be further elucidated.
In this study, we found that MEG3 affects the malignant behavior of hepatocellular carcinoma. MEG3 can directly regulate the expression of miR-544b. MEG3 silencing can promote tumor proliferation, invasion and metastasis by upregulation of miR-544b. Subsequently, we discovered that BTG2 is a target of miR-544b whereas miR-544b is involved in the regulation of BTG2 expression. Mechanistically, METTL3-mediated N6-methyladenosine (m6A) modification induced the downregulation of MEG3, which affects HCC progression through the miR-544b/BTG2 axis. Our study provides new insights for acknowledging the circRNA and miRNA interactions in tumors.
Methods
Clinical Specimen
The HCC tissues and corresponding para-cancer tissues were collected from patients undergoing primary hepatocellular carcinoma surgery in The First Affiliated Hospital of Guangzhou University of Chinese Medicine. All patients signed informed consent, and the study was approved by the ethics committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine and was conducted in accordance with the Declaration of Helsinki.
Cell Culture
Human HCC cells HepG2, Hep3B, MHCC-97H, Huh7 and human normal liver epithelial cells THLE-3 were purchased from American Type Culture Collection (ATCC, VA, USA). Fetal bovine serum, trypsin, and medium were purchased from Gibco (Gibco, USA). The cells were cultured in DMEM (containing 10% fetal bovine serum, 100 g/mL streptomycin and 100 u/mL penicillin) in an incubator at 37°C and 5% CO2. The solution was changed every 2 days, and when the cell growth and fusion degree reached more than 85%, 0.25% trypsin was used for digestion and passage of the cell.
Cell Transfection
HCC cells were inoculated into 6-well cell culture plates at a concentration of 2×105/mL per well. When the cell growth density reached more than 70%, plasmids were transfected using Invitrogen LipofectamineTM2000 (Life Technologies, USA) transfection kit according to the manufacturer’s instructions.
Cell Proliferation Assay
WST-1 (Beyotime, Shanghai, China) was used to determine the proliferation of HCC cells in each group. HepG2 and Huh7 cells in each group were collected 24 h after transfection. After washing with PBS and digestion with trypsin, the cells were resuspended with a complete medium. The cells were inoculated into 96-well plates at a concentration of 2×103 Wells per well, and 7 multiple Wells were set in each group. The cells were cultured in an incubator for 24 h, 48 h and 72 h. Each well was incubated with WST-1 solution for 2 h, and the absorbance (OD) value of cells in each well at the wavelength of 450 nm was determined.
Cell Clone Formation Experiment
Single-cell suspension was prepared by digestion with 0.25% trypsin and suspended in DMEM containing 10% fetal bovine serum. One milliliter cell suspension was placed in a 6 cm culture dish. The cells were cultured in an incubator for 2–3 weeks. Culture was stopped when visible clones appeared in the petri dish. The supernatant was discarded and washed with phosphate buffer solution (PBS) for 3 times. The cells were fixed with 4% paraformaldehyde (5 mL) and stained with GIMSA for 20 min. Further, the dye was slowly washed with running water and left to dry naturally in the air.
Transwell Assay
The HCC cells were collected 24 h after transfection. Serum-free medium resuspended cells. The cell concentration of each group was adjusted to 5×105 cells/mL. One hundred microliter drops were added to the upper transwell chamber. A complete medium containing 12% fetal bovine serum was added to the lower chamber. After 24 hours of culture, cells invaded to the lower surface were fixed with methanol followed by staining with 0.1%crystal violet at room temperature for 30 min. Finally, the number of invaded cells was counted under a microscope (Nikon, Japan). In the invasion detection, Matrigel (BD Biosciences, San Jose, CA) was used to pre-coat the upper of the membrane first. The other procedures were similar as above in the migration detection methods.
Dual-Luciferase Reporter Assay
MiR-544b Mimics and BTG2 or MEG3 3ʹ- untranslated region (3ʹUTR) wild-type (Wt) or mutant (Mut) were transfected into HepG2 and Huh7 cells simultaneously. Fragments of BTG2 or MEG3 3ʹUTR containing the predicted binding sites or mutated 3ʹUTR were cloned on the GV272 vector using the mRNA binding. After DNA sequencing was verified, miR-544b mimics or control plasmids were transfected into the cells using LipofectamineTM2000 according to the specification. After transfection for 48 h, cells were collected and analyzed using a dual-luciferase reporting system.
RNA Pull-Down
MEG3 and miR-544b sequences were etherized by Biotin RNA Labeling with Biotin Mix. After DNA digestion by RnasEI, they were purified by RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA structure buffer (10 mmol/L Tris pH=7, 0.1 mol/L KCl, 10 mmol/L MgCl2) was heated to 95°C with 1 g labeled RNA. After 2 min, RNA was moved to the ice for incubation for 3 min and then stood at room temperature for 30 min to form an appropriate secondary structure. HepG2 and Huh7 cells were taken and cell lysates (Sigma, USA) were added for 1h at 4°C. The lysate was centrifuged and the supernatant was collected and transferred to the RNase-free centrifuge tube. Add 50μL of Streptavidin agarose beads and proceed to incubate for 1h. The washing-up liquid was collected for RT-PCR detection of the target gene.
qRT-PCR
The total RNA in the cells after transfection was extracted using Trizol reagent according to the instructions. Thereafter, the total RNA was reverse-transcribed into cDNA by the reverse transcription kit (provided by Shanghai Sangon Biological Engineering Co., LTD.). The primers used are as follows: MEG3 F: 5ʹ-CGTTTGTAAGCCTGGGAT-3ʹ, R: 5ʹ-CTAAAGCGCACTCACCATGA-3ʹ. MiR-544F: 5ʹ-ATTCTGCATTTTTAGCAAGTT-3ʹ, R: 5ʹ-GCGAGCACAGAATTAATACGAC-3ʹ, U6 F: 5ʹ-GAAGGTGAAGGTCGGAGTC-3ʹ, R: 5ʹ-GAAGATGGTGATGGGATTT-3ʹ. BTG2 F: 5ʹ-TACCGCTGCATTCGCATCAAC3ʹ; R: 5ʹ-AGGGCCTAGCTGGAGACTGC-3ʹ. PCR reaction conditions were as follows: A: Pre-denaturation at 95°C for 10 min; B: Denaturation 95°C 15 s; Annealing at 60°C for 15 s, elongation at 72°C for 20 s, a total of 40 cycles. C: 72°C for 15 min. The reaction is terminated at 4°C. Three replicates were set for each sample, and 2−ΔΔCt was used for relative quantitative analysis of the data.
Metascape Enrichment Analysis
Metascape is a Cytoscape plug-in with fast data updates and wide coverage. It integrates several authoritative databases including GO, KEGG, UniProt and DrugBank, enabling it not only to complete pathway enrichment and biological process annotation but also to perform gene-related protein network analysis and relative drug analysis. Membership supports users to select data sets used in each annotation step, such as path enrichment, biological process enrichment, functional correlation and product analysis, and they can enter fields of interest in the search box. In this study, 296 target genes list of miR-544b were input, and the results including enrichment summary, gene list, gene annotation, enrichment analysis were obtained.
RIP Experiment
The cells in the culture dish were washed with cold PBS twice and centrifuged at 1500 rpm for 5 min at 4°C. The supernatant was discarded and the cells were collected. The cells were resuspended with the RIP lysate. Fifty microliters of the re-suspended magnetic bead suspension was absorbed into each Eppendorf tube, and 500 μL of RIP Wash Buffer was added to each tube to the vortex and oscillate. The magnetic beads were resuspended with 100 μL of RIP Wash Buffer, and about 5 ug corresponding antibodies were added to each sample. The RIP Immunoprecipitation Buffer is prepared and the Eppendorf tube in the previous step is put into the magnetic rack. As the supernatant was removed, 900 μL RIP Immunoprecipitation was added into each tube, and 100 μL supernatant was absorbed into the magnetic bead antibody complex in the previous step, resulting in the total volume of 1mL. Incubate at 4°C overnight. After the RNA was purified, the expression was detected by PCR.
Statistical Analysis
SPSS 20.0 (SPSS Inc., Chicago, IL, USA) statistical software was used for analysis, and measurement data were expressed as mean ± s. Comparison between the two groups was performed by T-test. Single-factor ANOVA analysis was used to compare multiple groups. Pearson correlation was used for correlation analysis. P<0.05 was considered statistically significant.
Results
MEG3 Expression is Down-Regulated in HCC
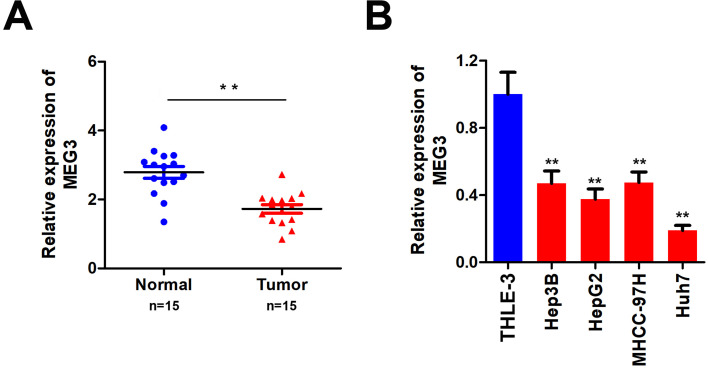
To study the role of MEG3 in HCC, we first analyzed the MEG3 expression in HCC tissues and cells using qPCR. The results showed that MEG3 was down-regulated in HCC compared with adjacent control tissues (Figure 1A). MEG3 was also down-regulated in HCC cells (Figure 1B). MEG3 has the lowest expression level in HepG2 and Huh7 cells, so these two types of cells are selected for subsequent experiments.
Figure 1.
Detection of MEG3 expression in hepatocellular carcinoma tissues and hepatocellular carcinoma cells. (A) Detection of MEG3 expression in tumor tissues. (B) Detection of MEG3 expression in normal liver cell lines and hepatocellular carcinoma cell lines. **P<0.01 vs QSG7701.
MEG3 Inhibits the Malignant Behavior of HCC Cells
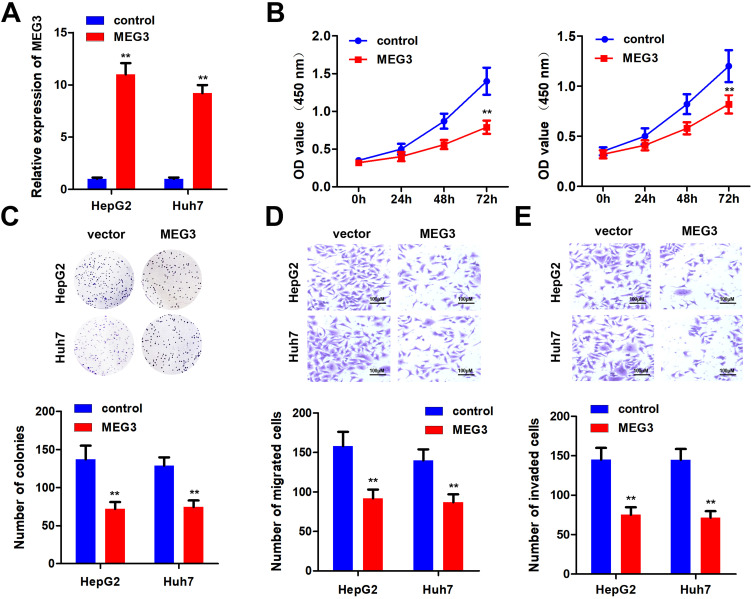
In order to further elucidate the effects of MEG3 on HCC cells, we studied the effects of MEG3 overexpression on proliferation, invasion and migration. First, we tested the efficiency of MEG3 overexpressing vector. The results show that MEG3 was effectively overexpressed in HepG2 and Huh7 cells (Figure 2A) after transfection. Furthermore, we detected the proliferation of hepatocellular carcinoma cells after MEG3 overexpression. Experimental results demonstrated that MEG3 might inhibit the proliferation of HepG2 and Huh7 cells (Figure 2B). Cell cloning and formation experiment results showed that MEG3 inhibited the cloning formation of HepG2 and Huh7 cells compared with the control group. Further transwell experiment results revealed that MEG3 overexpression could inhibit the migration and invasion of HepG2 and Huh7 cells compared with the control group (Figure 2C–E).
Figure 2.
Overexpression of MEG3 inhibits the malignant progression of hepatocellular carcinoma. (A) Detection of MEG3 overexpression efficiency. (B) After overexpression of MEG3, the proliferation ability of hepatocellular carcinoma cells HepG2 and Huh7 was detected. (C) After overexpressing MEG3, the cloning ability of hepatocellular carcinoma cells HepG2 and Huh7 was tested. (D) After overexpressing MEG3, the migration ability of hepatocellular carcinoma cells HepG2 and Huh7 was detected. (E) After overexpression of MEG3, the invasive ability of hepatocellular carcinoma cells HepG2 and Huh7 was detected. **P<0.01 vs control.
MEG3 Targets miR-544b in HCC Cell
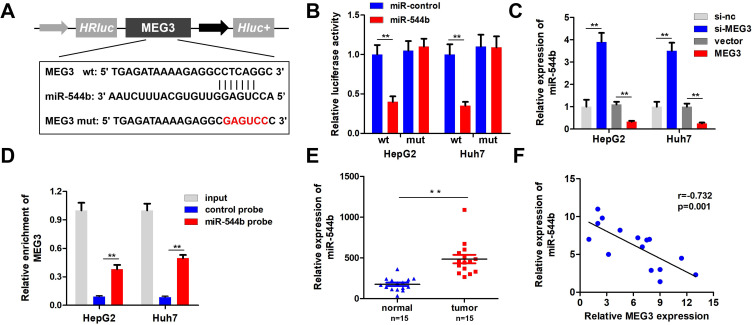
To unfold the mechanism of MEG3 inhibition into HCC, we analyzed the downstream miRNA associated with MEG3. The predicted results indicate that MEG3 can be bind to miR-544b (Figure 3A). Dual-luciferase reporter gene detection experiment markedly confirmed the binding between MEG3 and miR-544b (Figure 3B). Detection results of miR-544b expression also showed that overexpressed MEG3 could inhibit the expression of miR-544b, while knockdown of MEG3 could up-regulate the expression of miR-544b (Figure 3C). The RNA pull-down experiment represented that compared to the control group, miR-544b probe could effectively enrich MEG3 (Figure 3D). Further detection results showed that miR-544b was up-regulated in HCC tissues (Figure 3E). The correlation analysis between MEG3 and miR-544b shows that MEG3 had a negative co-expression correlation with miR-544b (r=−0.732) (Figure 3F). Therefore, the experimental results indicate that miR-544b is the target gene of MEG3, and miR-544b may play an important role in the malignant behavior of HCC.
Figure 3.
MiR-544b is the target gene of MEG3. (A) Schematic diagram of MEG3 binding site with miR-544b. (B) Double luciferase reporter gene detection experiment to verify MEG3 binding with miR-544b. (C) Detection of miR-544b expression level. (D) RNA pulled down confirmed MEG3 binding with miR-544b. (E) Detection of miR-544b expression in hepatocellular carcinoma tissues and adjacent tissues. (F) Correlation analysis between MEG3 and miR-544b in tumor tissues of hepatocellular carcinoma patients. **P<0.01.
BTG2 is the Target Gene of miR-544b
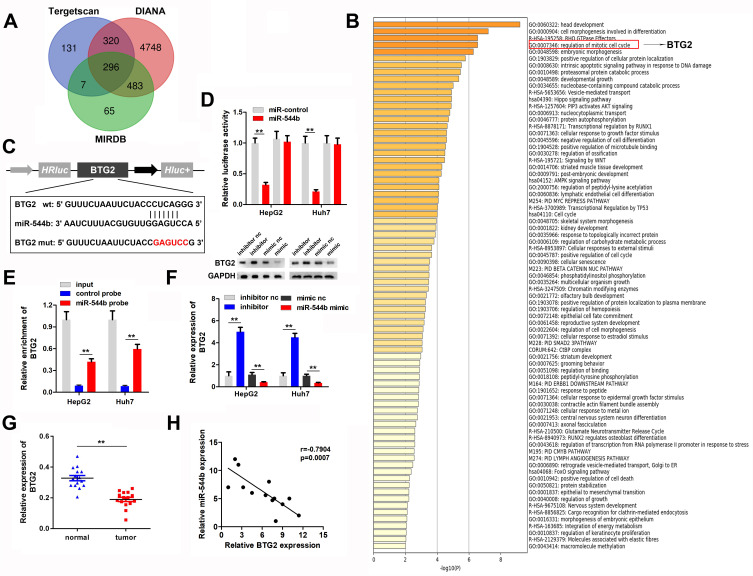
In order to evaluate the downstream regulatory target genes of miR-544b, we used bioinformatics tools to make predictions. The prediction results of the three database websites showed that 296 genes might be bound by miR-544b (Figure 4A). Further functional analysis of these target genes was performed using Metascape. The analysis results interpreted that the function of target genes was mainly related to transcriptional regulation, and BTG2 was further selected for in-depth analysis (Figure 4B). Figure 4C illustrates the binding site information of miR-544b and BTG2. The binding of miR-544b with BTG2 was confirmed by dual-luciferase reporter assay (Figure 4D). The RNA pull down experimental results confirmed that compared with the control group, miR-544b probe can significantly enrich BTG2 (Figure 4E). BTG2 expression analysis and detection results showed that overexpression of miR-544b could inhibit the expression of BTG2, while inhibition of miR-544b could up-regulate the expression of BTG2 (Figure 4F). Furthermore, BTG2 expression was down-regulated in HCC tissues (Figure 4G). Correlation analysis of MEG3 and BTG2 co-expression shows that MEG3 is negatively correlated with BTG2 co-expression (r=−0.7904) (Figure 4H).
Figure 4.
BTG2 is the target gene of miR-544b. (A) Prediction results of miR-544b target genes. (B) Enrichment analysis of miR-544b target gene. (C) Target site information of miR-544b and BTG2. (D) The binding of miR-544b to BTG2 was detected by reporter vector assay. (E) RNA pull down assay was used to detect the binding of miR-544b to BTG2. (F) Detection of BTG2 expression after different treatments. (G) Detection of BTG2 expression in tumors. (H) Correlation analysis between miR-544b and BTG2. **P<0.01.
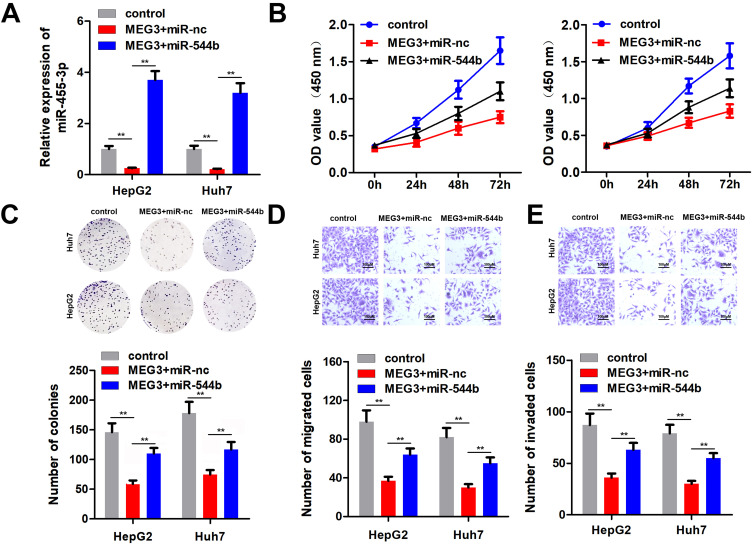
Overexpression of miR-544b Reversed the Anti-Cancer Effect of MEG3
To further confirm the interaction between miR-544b and MEG3, we performed rescue experiments. Detection results of miR-544b expression showed that overexpression of MEG3 could reduce the expression of miR-544b, while miR-544b Mimics could reverse the inhibition of MEG3 (Figure 5A). Cell proliferation experiment results manifested that compared with the control group, MEG3 could inhibit the proliferation of HepG2 and Huh, while overexpression of miR-544b could reverse the inhibition of MEG3 proliferation (Figure 5B). Moreover, the results of cell cloning and formation experiments showed that compared with the control group, MEG3 could inhibit the cloning formation of HepG2 and Huh, while overexpression of miR-544b could reverse the inhibition of MEG3 (Figure 5C). The results of cell migration and invasion experiments reveal that compared with the control group, MEG3 can inhibit the migration and invasion of HepG2 and Huh, while overexpression of miR-544b can reverse the inhibition of MEG3 (Figure 5D and E).
Figure 5.
Overexpression of miR-544b reverses the anti-cancer effect of MEG3. (A) Expression of miR-544b was detected. (B) Cell proliferation experiments after treatment in different groups. (C) Detection of clonal forming ability of cells after different treatments. (D) Cell migration ability test after different treatments. (E) Cell invasion ability tests after different treatments. **P<0.01.
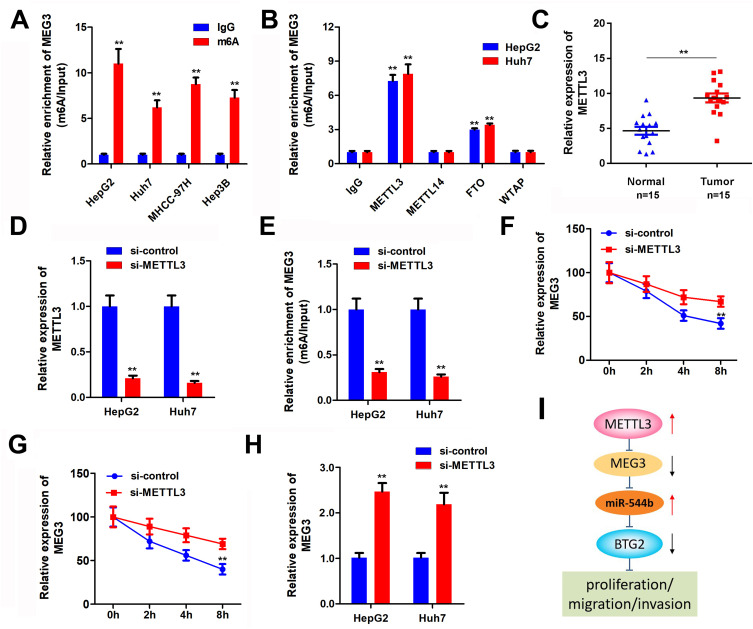
MEG3 is Regulated by m6A Modification of METTL3
Epigenetics modification plays an important role in HCC progression. To determine the regulatory mechanism of MEG3 expression, we analyzed whether MEG3 is modified with m6A. M6A antibody and control IgG antibody were used to carry out pull-down assay. In the compound pulled down, it was found that MEG3 expression in the m6A group was significantly higher than that in the IgG group (Figure 6A). Many enzymes can regulate m6A modification in cells. In order to explore the m6A enzyme that regulates MEG3 modification, we used antibodies of different m6A-related proteins to conduct a RIP experiment and detect the expression of MEG3 in the pulled products. The experimental results demonstrated that METTL3 and FTO can significantly enrich MEG3, indicating that METTL3 and FTO are involved in the m6A modification of MEG3 (Figure 6B). Further, we detected the expression level of METTL3 in HCC tissues, and the experimental results showed that compared with the control group, METTL3 was significantly up-regulated in hepatocellular carcinoma tissues (Figure 6C). Results of METTL3 interference efficiency test showed that siRNA of METTL3 could effectively reduce the expression of METTL3 (Figure 6D). Detection results of MEG3 expression indicated that after knockdown of METTL3, a reduction in MEG3 enriched by the m6A antibody was found, which further indicates that METTL3 regulates the m6A modification of MEG3 (Figure 6E). After cell METTL3 knockdown and actinomycin addition, the mRNA expression of MEG3 was detected at different time, proving that METTL3 silencing can reduce MEG3 RNA stability (Figure 6F and G). Furthermore, the MEG3 expression detection results noticeably proved that after METTL3 was suppressed, MEG3 expression was also down-regulated (Figure 6H). The mechanism flow gram is shown in Figure 6I.
Figure 6.
MEG3 is regulated by m6A modification of METTL3. (A) Protein immunoprecipitation assay to detect the m6A modification of MEG3. (B) RIP antibody test of different M6A-related proteins. (C) METTL3 expression in tumor tissues was detected. (D) METTL3 interference efficiency was detected. (E) Me-RIP assay was used to detect the m6A modification of MEG3 after METTL3 knockdown. (F and G) METTL3 interference can reduce the stability of MEG3 mRNA. (H) MEG3 expression was detected by qPCR after METTL3 interference. (I) The molecular mechanism flow gram. **P<0.01.
Discussion
It has been observed that lncRNAs play a role similar to tumor suppressor genes or oncogenes in the occurrence and development of tumors.12 LncRNAs also affect the proliferation, metastasis, invasion and other biological processes of HCC.13 For instance, Wang et al14 found that the OCT4-pg4 acts as a natural miRNA sponge to competitively bind miRNA-145, resulting in the reduced inhibitory effect of miRNA-145 on HCC development. Hepatocellular carcinoma high expression gene (HULC) is a significantly up-regulated LncRNA in HCC. It is located in 6p24.3 with two exons and one intron and plays an important role in the occurrence and development of HCC.15 HULC can form a regulatory network with CAMP-responsive element-binding protein (CREB) and miR-372 to participate in the occurrence and development of HCC.16
MEG3 is a matriline-imprinted gene, which is a potential cancer suppressor gene. It is uniformly expressed in various normal tissues, and its overexpression can inhibit tumor proliferation.17 For example, BTG2 mediates the effect of SRXN1 on the tumorigenesis and metastasis of HCC.18 It can also mediate the role of miR-6875-3p in HCC via regulating FAK/Akt signaling.19 Recently, a new MEG3 isoform was found to exert oncogenic function in vitro in HCC.20 This study examined the effects of MEG3 overexpression on proliferation, invasion and metastasis of HCC cells. The results showed that overexpression of MEG3 could significantly inhibit the proliferation, invasion and migration of HCC cells.
The regulatory mechanism of lncRNAs is complicated. To elucidate the mechanism of MEG3 in HCC progression, we focused on its effect as the miR sponge. It was found that MEG3 can directly bind with miR-544b. MiR-544b, as one of the important members of miRNA, plays an important role in regulating the pathological changes of tumor cells.21,22 However, the role of miR-544b in different tumor types is not the same.23 MiR-544b, as a tumor suppressor, inhibits the growth of triple-negative breast cancer by targeting BCL6 and STAT3.24 However, miR-544b, as an oncogene, promotes the occurrence and development of gastric cancer by targeting oncosuppressor gene IRX1 and activating EMT mediated by WNT signaling pathway.25 In this study, miR-544b was demonstrated to be up-regulated in HCC tissues. We firstly reported miR-544b as an oncogene in HCC development. It is well known that miRs can inhibit the expression of their targets, thus changing the behavior of cells. Here, BTG2 was found to be targeted by miR-544b. Members of this family, including BTG2, have the functions of inhibiting cell growth and promoting cell maturation and differentiation, but no clear conclusion has been formed.26,27 This study discovered that BTG2 expression was significantly down-regulated in hepatocellular carcinoma, reflecting that low BTG2 gene expression may be involved in the formation and progression of HCC.28
The methylated transferase catalyzes the production of m6A in the form of complexes in the nucleus. The complex consists of METTL3 and several other interacting proteins, including METTL14 and WTAP. At present, there are many new research achievements in m6A modification in non-coding RNA. Among them, lncRNA KCNK15-AS1 can inhibit the metastasis of pancreatic cancer through ALKBH5 demethylation.29 Modification of m6A was found on the A1114 bit of lncRNA TUG1,30 but the way it works is unclear. Meanwhile, it was also noted that m6A was distributed differently in mRNA and lncRNA, indicating that m6A modification of lncRNA may be different from mRNA, and its specific mechanism needs to be further studied.31 METTL3 has been indicated to be upregulated in HCC32. while METTL3 and METTL14 hold opposite regulatory roles in HCC.33 In this study, it was perceived that the MEG3 gene was modified by m6A in HCC cells. At the same time, it was found that METTL3 can directly interact with MEG3 indicating that METTL3 is involved in the m6A modification of MEG3.
However, there are a few limitations of this study. First, the effect of MEG3/miR-544b/BTG2 axis on hepatocellular carcinoma has not been studied at the animal level. Second, the miR-544b/BTG2 signaling mediates a part of the MEG3 function. More research should be carried out to elucidate the precise mechanism of MEG3. Third, the m6A modification of MEG3 only found METTL3, a methylated transferase. The specific effector enzymes such as YTHDC1 that changed MEG3 stability were not clarified. We will work on them in the future.
Conclusion
Our data indicate that MEG3 is downregulated in HCC tissues and cells. Overexpression of MEG3 inhibits malignant behaviors in HCC. In addition, MEG3 targets miR-544b, and overexpression of miR-544b may reverse MEG3’s anticancer effect by inhibiting BTG2. Meanwhile, MEG3 is regulated by the m6A modification of METTL3. In conclusion, MEG3/miR-544b/BTG2 pathway may be critical in regulating the development and progression of HCC and may be a therapeutic target for intervention of HCC.
Funding Statement
No funding was received to perform this study.
Disclosure
The authors reported no conflicts of interest for this work.
References
- 1.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular carcinoma surveillance among cirrhotic patients with commercial health insurance. J Clin Gastroenterol. 2016;50(3):258–265. doi: 10.1097/MCG.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 2.Han MS, Barrett T, Brehm MA, Davis RJ. Inflammation mediated by JNK in myeloid cells promotes the development of hepatitis and hepatocellular carcinoma. Cell Rep. 2016;15(1):19–26. doi: 10.1016/j.celrep.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendell JT. Targeting a long noncoding RNA in breast cancer. N Engl J Med. 2016;374(23):2287–2289. doi: 10.1056/NEJMcibr1603785 [DOI] [PubMed] [Google Scholar]
- 4.Ren S, Li G, Liu C, et al. Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep. 2016;36(4):1861–1867. doi: 10.3892/or.2016.4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan C, Xiang L, Pan Z, et al. MiR −544 promotes immune escape through downregulation of NCR1/NKp46 via targeting RUNX3 in liver cancer. Cancer Cell Int. 2018;18:52. doi: 10.1186/s12935-018-0542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirone F. The gene PC3TIS21/BTG2, prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol. 2001;187(2):155–165. doi: 10.1002/jcp.1062 [DOI] [PubMed] [Google Scholar]
- 7.Duriez C, Falette N, Audoynaud C, et al. The human BTG2/TIS21/PC3 gene: genomic structure, transcriptional regulation and evaluation as a candidate tumor suppressor gene. Gene. 2002;282(1–2):207–214. doi: 10.1016/S0378-1119(01)00825-3 [DOI] [PubMed] [Google Scholar]
- 8.Zhu G, Gilchrist R, Borley N, et al. Reduction of TSG101 protein has a negative impact on tumor cell growth. Int J Cancer. 2004;109(4):541–547. doi: 10.1002/ijc.20014 [DOI] [PubMed] [Google Scholar]
- 9.Hong JW, Ryu MS, Lim IK. Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death. J Biol Chem. 2005;280(22):21256–21263. doi: 10.1074/jbc.M500318200 [DOI] [PubMed] [Google Scholar]
- 10.Huang C-S, Zhai J-M, Zhu -X-X, et al. BTG2 is down-regulated and inhibits cancer stem cell-like features of side population cells in hepatocellular carcinoma. Dig Dis Sci. 2017;62(12):3501–3510. doi: 10.1007/s10620-017-4829-y [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Huang C, Huang Q, et al. Circular RNA circ_0014717 suppresses hepatocellular carcinoma tumorigenesis through regulating miR-668-3p/BTG2 axis. Front Oncol. 2021;10:592884. doi: 10.3389/fonc.2020.592884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859(1):169–176. doi: 10.1016/j.bbagrm.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Chen Z, An L, et al. Analysis of long non-coding RNA expression profiles in non-small cell lung cancer. Cell Physiol Biochem. 2016;38(6):2389–2400. doi: 10.1159/000445591 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Guo ZY, Zhang R, et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34(8):1773–1781. doi: 10.1093/carcin/bgt139 [DOI] [PubMed] [Google Scholar]
- 15.Matouk IJ, Abbasi I, Hochberg A, Galun E, Dweik H, Akkawi M. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol. 2009;21(6):688–692. doi: 10.1097/MEG.0b013e328306a3a2 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu X, Wu H, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38(16):5366–5383. doi: 10.1093/nar/gkq285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brock M, Schuoler C, Ulrich S, et al. The long noncoding RNA MEG3 is an emerging factor in hypoxia-induced pulmonary hypertension. J Heart Lung Transplant. 2016;35(4):S358. doi: 10.1016/j.healun.2016.01.1030 [DOI] [Google Scholar]
- 18.Lv X, Yu H, Zhang Q, et al. SRXN1 stimulates hepatocellular carcinoma tumorigenesis and metastasis through modulating ROS/p65/BTG2 signalling. J Cell Mol Med. 2020;24(18):10714–10729. doi: 10.1111/jcmm.15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y, Du J, Liu Z, et al. MiR-6875-3p promotes the proliferation, invasion and metastasis of hepatocellular carcinoma via BTG2/FAK/Akt pathway. J Exp Clin Cancer Res. 2019;38(1):7. doi: 10.1186/s13046-018-1020-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X, Feng D, Li M, et al. Transcriptomic analysis of mRNA-lncRNA-miRNA interactions in hepatocellular carcinoma. Sci Rep. 2019;9(1):16096. doi: 10.1038/s41598-019-52559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–333. doi: 10.1038/nrc3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z, Wang S, Zhu J, Yang Q, Dong H, Huang J. MicroRNA-544 down-regulates both Bcl6 and Stat3 to inhibit tumor growth of human triple negative breast cancer. Biol Chem. 2016;397(10):1087–1095. doi: 10.1515/hsz-2016-0104 [DOI] [PubMed] [Google Scholar]
- 24.Zhi Q, Guo X, Guo L, et al. Oncogenic miR-544 is an important molecular target in gastric cancer. Anti Cancer Agents Med Chem. 2013;13(2):270–275. [DOI] [PubMed] [Google Scholar]
- 25.Yanaka Y, Muramatsu T, Uetake H, Kozaki K-I, Inazawa J. miR-544a induces epithelial-mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36(11). doi: 10.1093/carcin/bgv106 [DOI] [PubMed] [Google Scholar]
- 26.Cho B-O, Jeong Y-W, Kim S-H, et al. Up-regulation of the BTG2 gene in TPA-or RA-treated HL-60 cell lines. Oncol Rep. 2008;19(3):633–637. [PubMed] [Google Scholar]
- 27.Terra R, Luo H, Qiao X, Wu J. Tissue-specific expression of B-cell translocation gene 2 (BTG2) and its function in T-cell immune responses in a transgenic mouse model. Int Immunol. 2008;20(3):317–326. doi: 10.1093/intimm/dxm152 [DOI] [PubMed] [Google Scholar]
- 28.Kwon Y-K, Jun J-M, Shin S-W, Cho J-W, Suh S-I. Curcumin decreases cell proliferation rates through BTG2-mediated cyclin D1 down-regulation in U937 cells. Int J Oncol. 2005;26(6):1597–1603. [PubMed] [Google Scholar]
- 29.He Y, Hu H, Wang Y, et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol Biochem. 2018;48(2):838–846. doi: 10.1159/000491915 [DOI] [PubMed] [Google Scholar]
- 30.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19(12):1848–1856. doi: 10.1261/rna.041178.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amort T, Rieder D, Wille A, et al. Distinct 5-methylcytosine profiles in poly (A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18(1):1. doi: 10.1186/s13059-016-1139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Sun G, Pan S, et al. The Cancer Genome Atlas (TCGA) based m6 a methylation-related genes predict prognosis in hepatocellular carcinoma. Bioengineered. 2020;11(1):759–768. doi: 10.1080/21655979.2020.1787764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Qin J, Gao T, et al. Analysis of METTL3 and METTL14 in hepatocellular carcinoma. Aging. 2020;12(21):21638–21659. doi: 10.18632/aging.103959 [DOI] [PMC free article] [PubMed] [Google Scholar]