X-ray free electron laser (XFEL) crystallography could revolutionize mechanistic studies of photosystem II (PSII) by providing three-dimensional structures of its reaction intermediates that cannot be obtained using traditional crystallographic techniques. However, care must be taken to ensure that the XFEL data are interpreted correctly. Here, we challenge the conclusion that a water molecule adds to the oxygen-evolving complex (OEC) of PSII when crystals of dark-adapted PSII are exposed to two flashes of light (1).
In each turn of its catalytic cycle, the OEC of PSII catalyzes a four-electron oxidation of two water molecules that produces a dioxygen (O2) molecule according to the Joliot–Kok model (2–5). A key mechanistic question is whether both of the oxygen atoms in the O2 product are already present in the dark-adapted OEC, or whether one of them is supplied by a water molecule (Ox) that adds to it after two flashes. Many investigators believe that the XFEL data support the latter (1, 6–8). However, it has been pointed out earlier that the alleged Ox (also known as O6 in refs. 6 and 8) is found completely outside the electron density feature assigned to it in σA-weighted 2Fo-Fc electron density maps computed using phases obtained from an atomic model that includes Ox (6, 9). Here, we examine the most recent XFEL data relevant to this issue (1), and again find no crystallographic evidence for Ox binding after two flashes.
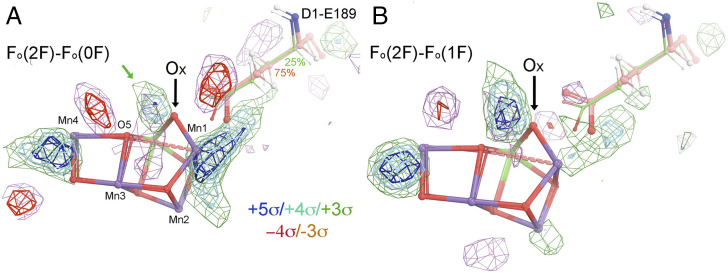
An atomic model that has two OEC conformations has been proposed for PSII crystals 200 ms after their exposure to two flashes of light (hereafter “2F structure”) (Protein Data Bank [PDB] ID 6w1v) (1): a major conformation (at 75% occupancy) that includes Ox, and a minor one lacking Ox (25% occupancy) (Fig. 1). Using the XFEL datasets reported by Ibrahim et al. (1), we calculated isomorphous difference Fourier maps between the 2F structure and the dark-adapted (0F) structure, and between the 2F structure and the one-flash (1F) structure (Fig. 1 A and B). As Fig. 1 shows, there are some significant positive and negative peaks near the OEC in both maps; but there is no positive difference feature in either map at the position assigned to Ox at any contouring level. All of these difference density features, however, can be fully explained using the OEC model for the 1F structure after it has been refined into the 2F data using standard methods (Fig. 2). Our observation also applies to the analysis of other 2F structures, including those at different time intervals (1, 6–8). Thus, there is concern over the interpretation of all the 2F XFEL experiments reported to date, since the 2F XFEL data can be explained without including the Ox-inserted OEC conformation in the structural model. Hence, it is important to note that all of the existing XFEL data do not necessarily and sufficiently support the hypothesis that a water molecule adds to the OEC during the 1F-to-2F transition.
Fig. 1.
The observed isomorphous difference Fourier maps. (A) Fo(6w1v/2F)-Fo(6w1o/0F) difference map contoured at +5σ (blue), +4σ (cyan), +3σ (green), −4σ (red), and −3σ (salmon) superimposed on the 6w1v/2F atomic model of ref. 1. (B) Fo(6w1v/2F)-Fo(6w1p/1F) difference map. The black arrows indicate the proposed position of Ox at which there is clearly no positive feature in either Fo-Fo map. The green arrow indicates the nearest positive peak to Ox, which is 1.1 Å away. It is also 1.6 Å away from O5. If one could model this peak as an O atom, this O atom and O5 would become a peroxide species.
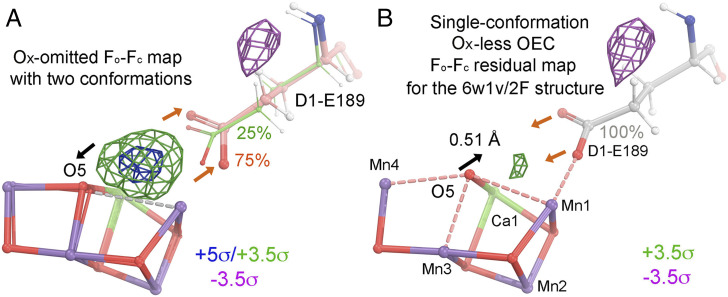
Fig. 2.
Two interpretations of the observed difference density features for the 6w1v/2F structure during the 1F-to-2F transition. (A) One interpretation using an Ox-omitted Fo-Fc difference Fourier map of an Ox-omit 6w1v/2F structure without any additional model refinement. The map is contoured +5σ (blue), +3.5σ (green), and −3.5σ (magenta) superimposed onto the atomic model. A major conformation (75% occupancy) is shown in salmon/blue/red, and a minor conformation (25%) is in green/red/blue. A large omit peak (greater than +5σ) between O5 and D1-E189 can be artificially created in this study when both O5 and D1-E189 (at 75% occupancy) are forced to move away from each other as indicated by the black and salmon-colored arrows regardless of the fact there is still no physical room to fit a new water molecule. (B) A simple straightforward crystallographic interpretation using an Fo(6w1v/2F)-Fc(single-conformation) residual map after standard model refinement starting with an OEC that lacks Ox, taken from the model before the 1F-to-2F transition, i.e., the single-conformation 1F model from ref. 1. The map is contoured at +3.5σ (green) and −3.5σ (magenta) superimposed onto the refined model. The black arrow indicates the displacement of O5 of the OEC toward the Fo-Fo positive peak. The salmon-colored arrows indicate that, after a displacement, the major conformation of D1-E189 is merged with the minor conformation that becomes a single conformation (silver/blue/red, at 100% occupancy). The largest Fo-Fc residual peak near the OEC is only +3.65σ, which we consider to be noise because this Fo-Fc map includes ∼1,200 peaks that are stronger than it. After refinement of the Ox-deleted model, the distance of O5 to Mn1, Mn3, Mn4, and Ca1 of the four nearest metal ions of the OEC in the first monomer (lowercase) is 2.63, 2.25, 2.54, and 2.60 Å, respectively, and it is 2.45, 2.30, 2.44, and 2.65 Å for the second crystallographically independent PSII monomer (uppercase).
Acknowledgments
V.S.B. acknowledges financial support from the US Department of Energy (DOE) Division of Chemical Sciences, Geosciences, and Biosciences; DOE Office of Basic Energy Sciences; and DOE Photosynthetic Systems. Computational work was funded by DOE Grant DESC0001423.
Footnotes
The authors declare no competing interest.
References
- 1.Ibrahim M., et al., Untangling the sequence of events during the S2 → S3 transition in photosystem II and implications for the water oxidation mechanism. Proc. Natl. Acad. Sci. U.S.A. 117, 12624–12635 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joliot P., Kok B., “Oxygen evolution in photosynthesis” in Bioenergetics of Photosynthesis (Academic Press, New York, 1975), pp. 388–413. [Google Scholar]
- 3.Vinyard D. J., Ananyev G. M., Dismukes G. C., Photosystem II: The reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 82, 577–606 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Vinyard D. J., Brudvig G. W., Progress toward a molecular mechanism of water oxidation in photosystem II. Annu. Rev. Phys. Chem. 68, 101–116 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Cox N., Pantazis D. A., Lubitz W., Current understanding of the mechanism of water oxidation in photosystem II and its relation to XFEL data. Annu. Rev. Biochem. 89, 795–820 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Suga M., et al., Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543, 131–135 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kern J., et al., Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563, 421–425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suga M., et al., An oxyl/oxo mechanism for oxygen-oxygen coupling in PSII revealed by an x-ray free-electron laser. Science 366, 334–338 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Askerka M., Brudvig G. W., Batista V. S., Crystallographic data support the carousel mechanism of water supply to the oxygen-evolving complex of photosystem II. ACS Energy Lett. 2, 2299–2306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]