Significance
Multiple sclerosis (MS) is the most common demyelinating disease in young adults. Advances in the understanding of the cellular mechanisms that contribute to MS may help to develop new therapies. Here, we elucidated the role of autophagy (a controlled intracellular pathway regulating the degradation of cellular components) and mitophagy (a specific form of autophagy that removes dysfunctional mitochondria) in MS. We found that human and experimental MS induces a mitochondrial deficit that leads to activation of autophagy and mitophagy. These phenomena play a causal role in MS because their inhibition with antipsychotic drugs prevents demyelination, induces remyelination, and reverts MS behavioral deficits. Our data suggest the repurposing of these Food and Drug Administration–approved drugs for the treatment of MS.
Keywords: multiple sclerosis, remyelination, autophagy, antipsychotic drugs, mitochondria
Abstract
Multiple sclerosis (MS) is a neuroinflammatory and neurodegenerative disease characterized by myelin damage followed by axonal and ultimately neuronal loss. The etiology and physiopathology of MS are still elusive, and no fully effective therapy is yet available. We investigated the role in MS of autophagy (physiologically, a controlled intracellular pathway regulating the degradation of cellular components) and of mitophagy (a specific form of autophagy that removes dysfunctional mitochondria). We found that the levels of autophagy and mitophagy markers are significantly increased in the biofluids of MS patients during the active phase of the disease, indicating activation of these processes. In keeping with this idea, in vitro and in vivo MS models (induced by proinflammatory cytokines, lysolecithin, and cuprizone) are associated with strongly impaired mitochondrial activity, inducing a lactic acid metabolism and prompting an increase in the autophagic flux and in mitophagy. Multiple structurally and mechanistically unrelated inhibitors of autophagy improved myelin production and normalized axonal myelination, and two such inhibitors, the widely used antipsychotic drugs haloperidol and clozapine, also significantly improved cuprizone-induced motor impairment. These data suggest that autophagy has a causal role in MS; its inhibition strongly attenuates behavioral signs in an experimental model of the disease. Therefore, haloperidol and clozapine may represent additional therapeutic tools against MS.
Multiple sclerosis (MS) is a neuroinflammatory disease, characterized by progressive neurological damage consisting in crumbling of the axonal myelin sheath in the central nervous system (CNS), followed by strong impairment in axonal conductance, axonal transection, and death of oligodendrocytes and neurons (1). MS onset is subacute or chronic, typically in a relapsing–remitting fashion, with phases of neuroinflammation corresponding to clinically evident disease followed by periods of oligodendrocyte proliferation, partial reconstitution of the myelin sheath, and corresponding clinical recovery. MS worsens over time, evolving into a progressive form, characterized by the disappearance of the relapsing–remitting phases and by permanent chronic lesions.
Inflammation plays a central and fundamental role in MS (2). The initial phase of MS is characterized by signs of an autoimmune response including disruption of the blood–brain barrier, invasion of the CNS by immune cells, and presence of antibodies against myelin. These are believed to play a causative role in the onset of the disease. Immunological aggression is sustained by a macrophage- or microglia-driven secretion of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) (3, 4) or interleukin-1 beta (IL-1-β) (5, 6), which have been shown to promote neuronal, oligodendrocyte, and vascular damage in models of MS.
Autophagy may also play a fundamental pathophysiological role in MS, but this has been only marginally explored thus far (1, 7). Autophagy represents a key intracellular function, which allows the cell to face periods of nutrient deprivation, meanwhile eliminating damaged and potentially harmful molecules, organelles, or invading microorganisms (8). These activities are grounded on the capacity of the cell to organize a double-membrane–lined autophagosome that, after engulfing the unwanted material, brings it to the lysosomal compartment, which in turn ensures degradation of proteins and recycling of nutrients.
Many neurodegenerative diseases are characterized by pathological accumulation of misfolded or damaged proteins, suggesting an important role of autophagy in their pathophysiology (9, 10). In Alzheimer’s disease, for example, impairment of the autophagic flux due to reduced vesicle clearance has been associated to accumulation of an aggregated form of hyperphosphorylated tau protein and to extracellular amyloid-β (A-β) plaques (11). Similarly, in Parkinson’s disease, dysfunctional lysosomes and accumulation of autophagosomes are associated with intracellular inclusions of α-synuclein and other polyubiquitinated proteins.
Several reports have demonstrated an increase in autophagic markers in human samples of MS patients (12). In particular, our and other groups have shown that autophagic and mitophagic markers are increased in serum and cerebrospinal fluid (CSF) of MS-affected individuals (13–15). However, the role of autophagy in MS remains still controversial. (16, 17).
In this study, we investigated in detail the involvement of autophagy in MS. We used in vitro, ex vivo, and in vivo models to test the effect of proinflammatory cytokines, lysolecithin (LPC), and cuprizone (CPZ) on myelin production, axonal myelination, and motor performance. In particular, we investigated the occurrence of autophagy in the different models and examined the effect of autophagy inhibitors and of two antipsychotic drugs, clozapine and haloperidol, on autophagy and behavior, revealing a significant improvement in motor function in MS animals.
The rationale for employing these antipsychotic drugs stems from neuroimaging and postmortem investigations on the brain of schizophrenia patients that revealed loss of white matter (18, 19) and down-regulation of genes involved in oligodendrocyte functioning and myelination (20, 21). Antipsychotic drugs, in particular haloperidol and clozapine, proved effective in improving cortical myelination as well as spatial working memory and locomotor activity in animal models (22–25). The molecular mechanism(s) underlying these effects are still obscure, but it has been recently suggested that these antipsychotic drugs may interfere with the autophagic process (26, 27). Our findings explore this mechanism of action of haloperidol and clozapine and disclose the opportunity of repurposing these drugs that are currently employed in other clinical settings for the treatment of MS.
Results
Patients with Active MS Have Increased Levels of Autophagy Markers.
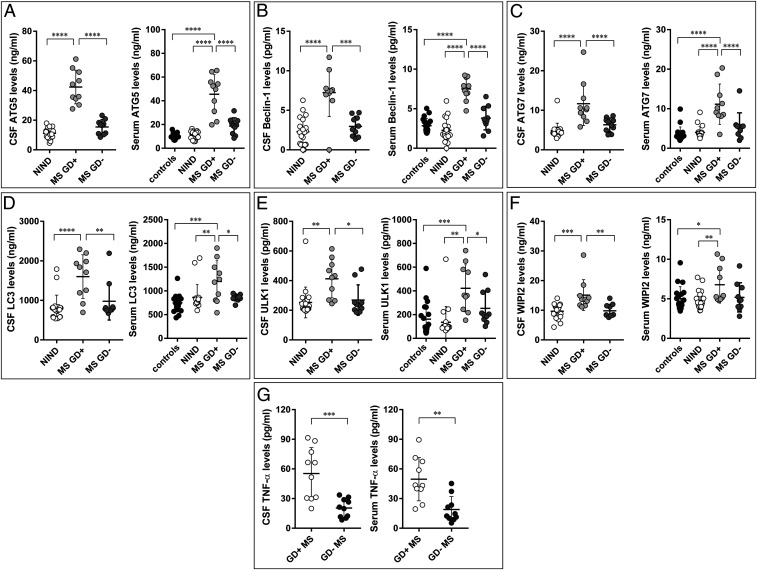
Recently, we detected the established marker of autophagy, autophagy-related 5 (ATG5), in the CSF of MS patients (13, 14). We found that this marker was increased in MS patients in the active phase of the disease (13, 14), identified on response to Gadolinium (Gd) (28). In the present study, by analyzing a new cohort of patients, we found increased levels not only of ATG5 but also of many other markers of autophagy (namely coiled-coil, moesin-like BCL2-interacting protein, Beclin-1; ATG7; microtubule-associated proteins 1A/1B light chain 3, LC3; unc-51–like autophagy-activating kinase 1, ULK1; and tryptophan-aspartic acid (WD) repeat domain phosphoinositide–interacting 2 WIPI2) in the CSF and serum of MS patients compared with patients with no inflammatory neurological disease, used as controls. In addition, we observed significantly increased levels of all these autophagic markers in the CSF and serum of Gd positive (Gd+) as compared with Gd negative (Gd−) patients (Fig. 1 A–F). Increased levels of all autophagic markers in the CSF of Gd+ patients were also detected by using the immunoblot technique (SI Appendix, Fig. S1). Similarly, levels of the proinflammatory cytokine TNF-α were significantly higher in Gd+ than in Gd− patients (Fig. 1G). Altogether, these data confirm and extend our previous observations highlighting increased levels of autophagy biomarkers during the acute phases of MS.
Fig. 1.
CSF and serum levels of autophagic elements. ATG5 (A), Beclin-1 (B), ATG7 (C), LC3 (D), ULK1 (E) WIPI2 (F), and TNF-α (G) levels in CSF and serum of patients with no inflammatory neurological diseases (NIND), healthy individuals (controls), and MS patients grouped according to MRI disease activity. MS Gd+: MRI-active MS; MS Gd-: MRI-inactive MS. Each point represents a single observation, including outliers. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Proinflammatory Cytokines Cause Autophagic Activation and Myelin Loss In Vitro.
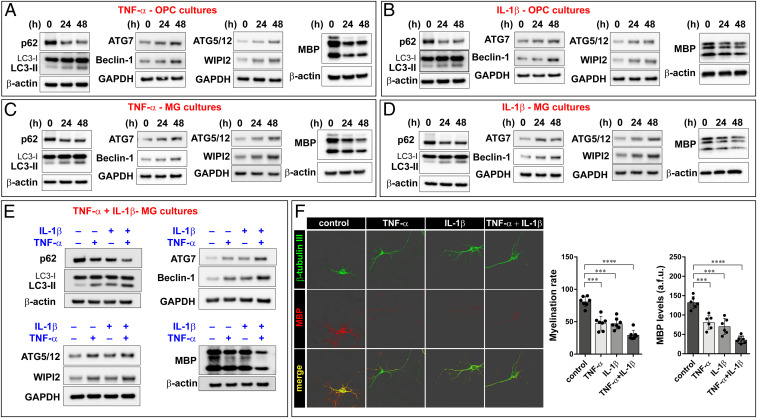
Based on these findings, we attempted to reproduce MS-like conditions in vitro to study the role of autophagy activation during the demyelination process. Undifferentiated primary oligodendrocyte precursor cells (OPC) cultures were treated with sublethal doses of the proinflammatory cytokines TNF-α and IL-1-β for 24 and 48 h. Treatment conditions were accurately chosen to avoid any interference with glial or neuronal differentiation (SI Appendix, Fig. S2A) as well as with cell viability (SI Appendix, Fig. S2B). The medium containing growth factors essential for OPC proliferation was replaced by the thyroid hormone triiodothyronine to stimulate cell differentiation. After 5 d, cells were collected for immunoblot analysis of myelin basic protein (MBP) and of the autophagy markers LC3, p62, Beclin-1, ATG7, ATG5/12, and WIPI2. We observed that the levels of both p62 and MBP roughly halved after 24-h exposure to proinflammatory cytokines, while the levels of the cleaved form of LC3 related to activation of the autophagic process (LC3-II), Beclin-1, ATG7, ATG5, and WIPI2 nearly doubled (Fig. 2 A and B). These data suggest a stimulation of the autophagic process.
Fig. 2.
TNF-α and IL-1-β boost autophagy and provoke demyelination in vitro MS models. Proinflammatory cytokines TNF-α (A) and IL-1-β (B) cause activation of autophagy in OPC cultures, as denoted by increases in LC3-II, ATG7, Beclin-1, ATG5/12, and WIPI2 and reduction in p62 protein levels. Cytokines exposure is also associated with decreased MBP levels. (C and D) Similar results were obtained in MG cell cultures. (E) The concurrent use of both TNF-α and IL-1-β determines increased activation of autophagy and higher demyelination than the single treatments in MG cultures. Immunoblots are representative of at least three independent experiments. Quantification of the Western blot performed is reported in SI Appendix, Table S1. Notably, the primary antibody against ATG5 also detect the ATG12-ATG5 conjugated form. (F) Fluorescence microscopy analysis of the axonal marker β-tubulin III and myelin marker MBP reveals that proinflammatory cytokines provoke a reduction in myelination capacity. Representative three-dimensional images are shown. All data presented are the mean ± SD of at least three separated experiments; two-way ANOVA with Dunnett’s multiple comparison test, ****P < 0.0001, ***P < 0.001.
In the CNS, oligodendrocyte metabolism and axon myelination are dependent on the relationship with other nervous cells (29). Hence, we decided to test the effect of proinflammatory cytokines on autophagic recruitment in a mixed glial cells (MG) culture, where oligodendrocytes were grown on a layer of astrocytes and neurons. The data we obtained were coherent with those observed in OPC cultures, proving that proinflammatory conditions cause both a reduction in MBP levels and activation of autophagy (Fig. 2 C and D) without affecting cell differentiation and viability (SI Appendix, Fig. S2 C and D) as well the protein levels of neuronal (β-tubulin III) and astrocyte (glial fibrillary acidic protein; GFAP) markers (SI Appendix, Fig. S2E). Further confirming the pivotal role of autophagy in MS, we also found that TNF-α and IL-1-β synergize not only in determining greater MBP loss but also increased activation of the autophagy process (Fig. 2E).
The autophagic flux is a multistep pathway (SI Appendix, Fig. S2F) consisting of induction and engagement of autophagosomal membranes, elongation and expansion into a closed autophagosome, fusion with a lysosome, and finally, degradation of the autophagic cargo (30, 31). Higher levels of autophagic markers may be due to either an increase in the autophagic flux or an inhibition of lysosomal activity. To understand whether proinflammatory cytokines would actually increase the autophagic flux, MG cultures previously treated with TNF-α or IL-1-β were exposed to bafilomycin A1 (BafA1), a potent inhibitor of the lysosomal V-ATPase. Under conditions of normal autophagic flux, BafA1 inhibits lysosomal degradation of the autophagic cargo and leads to accumulation of the newly formed markers, like LC3-II. The immunoblot reported in SI Appendix, Fig. S2G shows that BafA1 increased the levels of LC3-II under basal conditions and that cytokines and BafA1 had additive effects. This suggests that the increased levels of LC3-II in the presence of TNF-α or IL-1-β is due to a stimulation of autophagy flux and not to an inhibition of LC3-II degradation (i.e., of lysosomal activity).
We then asked if cytokine treatment can also cause an impairment in myelination. To pursue an answer to this question, we grew separately OPC and neuronal cultures. After 7 d, OPC cultures were first exposed to cytokines and next cocultured with neurons. Finally, the amount of myelin and the myelination rates were analyzed. We found that TNF-α and IL-1-β reduced MBP synthesis and impaired the ability of oligodendrocytes to sheath neuronal axons (Fig. 2F).
Recent studies demonstrated that many features of ferroptosis, the iron-dependent form of nonapoptotic cell death, are present in MS (32, 33). Autophagy and ferroptosis are highly connected [e.g., classical activators of ferroptosis increase the autophagic flux (34, 35)]. In addition, it has been found that a specific autophagy-related cellular mechanism, named ferritinophagy, regulates ferroptosis (36). During ferritinophagy, the iron storage protein ferritin, which is critical for the regulation of cellular iron levels, is sequestered into autophagosomes. The nuclear receptor coactivator 4 (NCOA4) has been identified as the receptor responsible for the selective autophagic degradation of ferritin (37). Ferritinophagy is involved in different cellular mechanisms, including the inflammatory process, as it has been discovered to trigger the extracellular release of the proinflammatory protein high-mobility group box 1 (HMGB1) (36, 38). Ferritinophagy has been also reported to play an active role in neurodegeneration, hepatic fibrosis, cancer (38–41), and most important in the frame of the present study, in MS (33). It has been demonstrated that, in the early stage of a demyelinating attack, the expression of NCOA4 and of the transferrin receptor (TFR) increase, whereas they decrease while the demyelination occurs (33), probably due to the execution of the autophagic process. In keeping with these studies, we found that the proinflammatory cytokines TNF-α and IL-1-β not only cause MBP loss and increase the autophagy flux but also activate the ferritinophagic flux, as demonstrated by reduced levels of NCOA4 and TFR (SI Appendix, Fig. S2H), and trigger the release of HMGB1 (SI Appendix, Fig. S2I).
Proinflammatory Conditions Cause Mitochondrial Stress Followed by Increased Glucose Catabolism and Mitophagy.
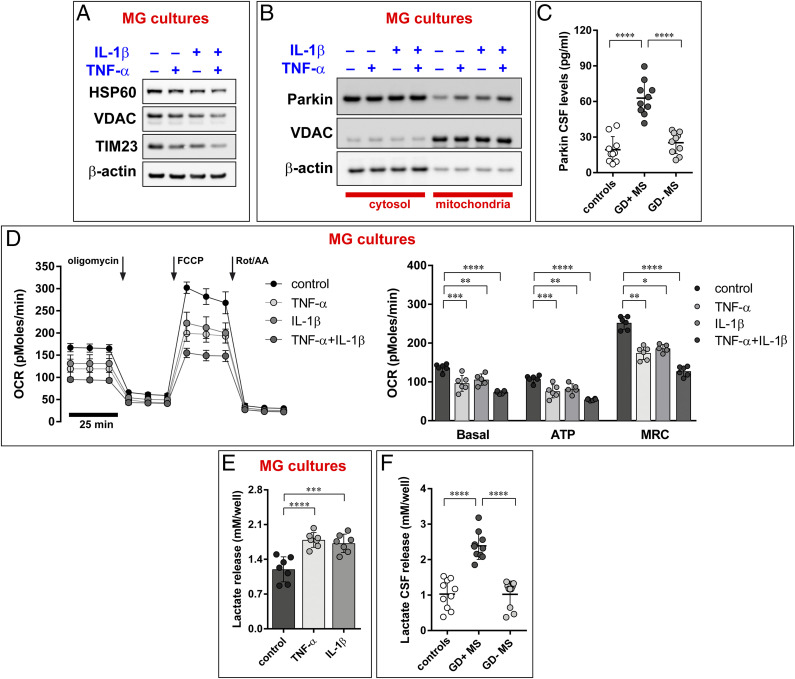
Several studies have demonstrated that mitochondrial impairment is a feature of MS (42, 43). In addition, alteration of the mitochondrial homeostasis and dynamics have been associated with autophagy programs (36). Therefore, having observed that proinflammatory cytokines can cause autophagy, we decided to focus on mitophagy, a particular form of autophagy. Mitophagy occurrence was detected by using live-cell imaging microscopy. Cell cultures were treated with TNF-α or IL-1-β, either alone or in combination for 48 h, loaded with mitochondrial (MitoTracker Green) and lysosomal (LysoTracker Red) markers, and imaged at confocal microscopy to analyze the colocalization of both markers (44). We found a significant increase in colocalization following cytokine exposure, suggesting an increased mitophagic process in MS-like conditions (SI Appendix, Fig. S3A). Accordingly, we found that the proinflammatory cytokines reduced the mitochondrial mass, as indicated by reduced levels of HSP60, VDAC, and TIM23, specific mitochondrial markers for matrix, outer, and inner mitochondrial membrane, respectively (Fig. 3A).
Fig. 3.
TNF-α and IL-1-β increase mitophagic events with consequent loss of mitochondrial functioning. (A) Proinflammatory cytokines prompt activation of mitophagic process, as indicated by a reduction in mitochondrial proteins. HSP60, VDAC, and TIM23 were used as markers of mitochondrial matrix, outer and inner membrane, respectively. (B) Parkin recruitment into mitochondria, analyzed after subcellular fractionation of MG cultures pretreated with TNF-α and IL-1-β. Immunoblots are representative of at least three independent experiments. Quantification of the Western blot performed is reported in SI Appendix, Table S1. (C) CSF Parkin levels in the CSF of control, MS patients in active phase, and MS patients in remission phase. The groups are different according to ANOVA (P < 0.0001): Gd+ MS > Gd− MS (Tukey’s test: P < 0.0001); Gd+ MS > Controls (Tukey’s test: P < 0.0001). (D) Mitochondrial respiration in MG cultures pretreated with TNF-α and IL-1-β was assessed by using the Seahorse Mito Stress test with oligomycin (ATP synthase inhibitor), FCCP (mitochondrial uncoupler), and a mix of rotenone and Rot/AA (specific inhibitors for the ETC complex I and III, respectively). The arrows indicate time of drug injection. The graphs depict the mean ± SD of at least n = 5 experiments; two-way ANOVA with Dunnett’s multiple comparison test, *P < 0.01. Calculated parameters shown are as follows: Basal rate (Basal) was calculated by the equation (OCR before addition of oligomycin − OCR after addition of Rot/AA). ATP production (ATP) was indicated as OCR before addition of oligomycin − OCR after addition of FCCP. Maximal rate was calculated as the highest OCR after addition of FCCP subtracted from the OCR after addition of Rot/AA. The groups are different according to two-way ANOVA with Dunnett’s multiple comparison test ****P < 0.0001, ***P < 0.001, and **P < 0.01. (E) Lactate release in media collected from MG cultures (the data are the mean ± SD of at least three independent experiments. Two-way ANOVA with Dunnett’s multiple comparison test, ****P < 0.0001, ***P < 0.001. (F) Lactate release in in the CSF of control, MS patients in active phase and MS patients in remission phase. The groups are different according to ANOVA (P < 0.0001): Gd+ MS > Gd− MS (Tukey’s test: P < 0.0001); Gd+ MS > Controls (Tukey’s test: P < 0.0001).
Such findings were further confirmed by analyzing the redistribution of Parkin from cytosol to mitochondria. The PTEN-induced putative kinase 1 (PINK1) and Parkin molecular axis represents a primary mechanism of mitophagy. Following mitophagy activation, Parkin is recruited from the cytosol into the outer mitochondrial membrane (OMM) by PINK1. Here, Parkin ubiquitinates multiple OMM proteins. Next, autophagic adaptors recognize these proteins and induce the removal of damaged mitochondria in autophagosomal vesicles (44). To test the involvement of this molecular axis in MS-like conditions, we performed a subcellular fractionation of cells exposed to TNF-α and IL-1-β. The immunoblot in Fig. 3B shows that, under resting conditions, the amount of Parkin localized in the mitochondrial compartment is very low but significantly increases after exposure to proinflammatory cytokines.
These data suggest that the PINK1-Parkin axis is involved in the regulation of the mitophagic process under MS-like conditions. To investigate whether an increase in mitophagy may be present in MS patients, levels of Parkin were measured in the CSF of MS-affected individuals. We found a significant increase in Parkin levels in MS patients in the active phase of the disease (Gd+) as compared with controls and with patients in the remission phase (Gd−) (Fig. 3C).
One hypothesis to explain these observations is that MS in active phase depresses mitochondrial activity, thereby triggering mitophagy. Hence, we tested if proinflammatory cytokines affect mitochondrial function. In particular, we focused on the oxygen consumption rate (OCR), a valuable indicator of mitochondrial respiratory capacity and energy production. MG cultures pretreated with TNF-α and IL-1-β were seeded into XF96 Seahorse plates, and the OCR was measured. This experimental approach can subdivide mitochondrial activity into different functional modules (basal, maximal, and ATP-linked respiration) by means of selective inhibitors for the different parts of the mitochondrial electron transport chain. All these modules were significantly lowered by proinflammatory cytokines (Fig. 3D). OCR measurements represent an essential parameter to evaluate metabolism in health and disease but may not be sufficient to demonstrate a corrupted mitochondrial function. Therefore, we also verified mitochondrial activity by using other parameters: mitochondrial membrane potential (ΔΨm), production of mitochondrial reactive oxygen species (mROS) and mitochondrial calcium (Ca2+) dynamics. These experiments confirmed that TNF-α and IL-1-β alter the functioning of the mitochondrial compartment, as denoted by an increase in mROS production (SI Appendix, Fig. S3B) and a reduction of both ΔΨm (SI Appendix, Fig. S3C) and mitochondrial Ca2+ uptake (SI Appendix, Fig. S3D). The cotreatment with TNF-α and IL-1-β further exacerbated mitochondrial impairment. These findings are consistent with previous reports demonstrating that proinflammatory conditions impair mitochondrial homeostasis and dynamics (45, 46) and demonstrate that, in our experimental settings, OCR measurements represent a reliable parameter to analyze mitochondrial metabolism and functioning.
Having observed an impairment of mitochondrial bioenergetics and activity as a consequence of treatments with proinflammatory cytokines, we measured the extracellular acidification rate in MG cultures to identify variations in the glycolytic contribution to metabolic equilibrium and to assess whether the loss of mitochondrial function could affect cellular metabolism. Pretreatment with TNF-α and IL-1-β enhanced the secretion of lactate in the MG culture medium, suggesting an increased rate of glycolysis (Fig. 3E). Interestingly, a significant increase in lactate production was also observed in CSF samples obtained from MS patients in active phase (Fig. 3F). Again, significance was found not only when comparing patients in the active phase of disease versus controls but also versus patients in the remission phase (Fig. 3F).
These metabolic data suggest that proinflammatory cytokines cause mitochondrial stress that is followed by increased glucose catabolism and compensatory increase of autophagy and mitophagy. Several studies demonstrate a direct relationship between metabolic status and autophagy, and specific regulatory molecular pathways have been identified (47). Autophagy is mostly regulated by the AMPK-mTORC-ULK1 pathway (48, 49), which also acts as a nutrient-sensing pathway. In response to low energy levels or stress conditions, AMPK (5′ adenosine monophosphate–activated protein kinase) becomes activated and prompts the autophagic process through direct or indirect modulation of mTORC (mammalian target of rapamycin complex) and ULK1 (Unc-51–like autophagy-activating kinase 1). Interestingly, all the components of this autophagy regulatory complex are important for mitochondria functioning and during MS progression (45, 50, 51). We found that MG cultures exposed to TNF-α or IL-1-β display increased phosphorylation of both AMPK and ULK1. More specifically, ULK1 was found to be hyperphosphorylated at SER317 and dephosphorylated at SER757, a target site of the inhibitory action of mTOR (52) (SI Appendix, Fig. S3E).
These results suggest that the metabolic imbalance observed in MS-like conditions is associated with an activation of AMPK and ULK1, which act as positive regulators of the autophagic machinery. This induced autophagy and mitophagy, however, would not affect oligodendrocyte viability or differentiation but impair the oligodendrocyte ability to synthetize myelin (as shown in Fig. 2 and SI Appendix, Fig. S2). We therefore speculated that autophagy inhibition could restore or improve myelin synthesis.
Inhibition of Autophagy Restores Myelin Production and Axonal Myelination.
Modulators of the autophagic process are currently in clinical trial for cancer treatment (53), but their possible use in the treatment of MS has not been yet investigated. Therefore, we decided to treat MG cultures previously exposed to the proinflammatory cytokine TNF-α with a series of antiautophagic compounds to test whether inhibition of autophagy may improve myelin production and axon myelination.
First, we modulated the activity of the AMPK-mTOR-ULK1 axis by using the so-called compound-C (CC), a specific AMPK inhibitor (54). We identified 2.5 µM as an optimal CC concentration that reduces the phosphorylated form of AMPK and the autophagic levels (SI Appendix, Fig. S4A) without affecting the normal autophagic flux (Fig. 4A). Next, we tested CC in MS-like conditions. We found that CC increased MBP levels to nearly normal values (Fig. 4B) and normalized the increased levels of phosphorylated AMPK caused by proinflammatory cytokines (SI Appendix, Fig. S4B). Similar experiments were also carried out after treatment with the early-stage inhibitor of autophagy 3-methyladenine (3-MA), which blocks autophagosome formation by controlling the activity of class 3 phosphatidylinositol 3-kinases (55). 3-MA reduced LC3-II levels (Fig. 4C) and restored MBP levels following TNF-α and IL-1-β treatment (Fig. 4D).
Fig. 4.
Modulation of autophagy increases myelin production in vitro. Immunoblots showing the ability of autophagy inhibitor compound CC (2.5 µM) to inhibit autophagy without affecting the normal autophagic flux (A) and to restore normal myelin levels following treatment with TNF-α or IL-1-β, either alone or in combination (B). Early-stage autophagy inhibitor 3-MA (2.5 µM) reduces autophagic activities, as suggested by a decrease in LC3-II levels (C) and reverts TNFα- or IL-1-β–mediated loss of myelin (D). Genetic interference on the essential autophagic gene ATG7 decreases the autophagosomal formation (E) and restores the myelin loss provoked by proinflammatory cytokines (F). The late-stage inhibitor CQ blocks autophagy by interfering with LC3-II degradation. At demonstration of this, the vacuolar H+-ATPase inhibitor BafA1 that inhibits the autophagosome–lysosome fusion does not increase LC3-II levels than the CQ (1 µM) treatment alone (G). Interestingly, clozapine (Cloz) and haloperidol (Halo) 1 µM exert the same effect as CQ (H and I). Clozapine and haloperidol (like the late-stage autophagy inhibitor CQ) also revert TNFα- or IL-1-β–mediated loss of myelin (J–L). All experiments are performed in MG cultures. Immunoblots are representative of at least three independent experiments. Quantification of the Western blot performed is reported in SI Appendix, Table S1.
To test whether autophagy was essential to modulate MBP levels, we performed genetic interference toward ATG7, a gene essential for autophagy. Silencing effectively lowered ATG7 levels (SI Appendix, Fig. S4C), reducing LC3-II levels (Fig. 4E) and preventing the diminution in MBP levels caused by TNF-α and IL-1-β treatment (Fig. 4F). These results support the notion that autophagy plays an active role in the loss of myelin and that autophagy inhibition could prevent demyelination.
Unfortunately, 3-MA, CC, and small interfering RNAs (siRNAs) cannot be used clinically. Furthermore, multiple studies suggest that these compounds may produce effects not restricted to autophagic mechanisms and may even paradoxically cause autophagic activation (56, 57). On the opposite, inhibitors of the late steps of the autophagic flux are already employed in the clinic. Among autophagic modulators, the most used is the antimalarial agent chloroquine (CQ) which, like its derivate hydroxychloroquine, counteracts the autophagic pathway at late stage by blocking the binding of autophagosomes to lysosomes (58). Interestingly, some antipsychotic drugs such as haloperidol and clozapine have been recently shown to exert potent late-stage autophagy inhibition (26, 27). However, other works suggest that haloperidol and clozapine may also act as autophagic inducers (59, 60). Therefore, we decided to test the efficacy of these drugs in our cellular settings. Fig. 4 G–I shows that these compounds, just like the classical inhibitor of autophagy CQ, effectively block the autophagic activity in our cells, as shown by the marked increase in LC3-II levels. Most importantly, clozapine and haloperidol, again like CQ, increase MBP levels (Fig. 4 J–L).
Clozapine and Haloperidol Enhance Myelination in an Ex Vivo MS Model by Blocking Autophagic Dynamics.
We then decided to restrict our investigation to clozapine and haloperidol. First of all, we evaluated the therapeutic potential of these antipsychotic compounds using organotypic slice cultures (OSC) from the mouse cerebellum and LPC as demyelinating agent. Rodent three-dimensional OSC have been widely used to investigate the complexity of the degenerative mechanisms which occur during MS, and the toxin LPC represents an established approach to investigate the demyelination events related to MS in OSC (61–64).
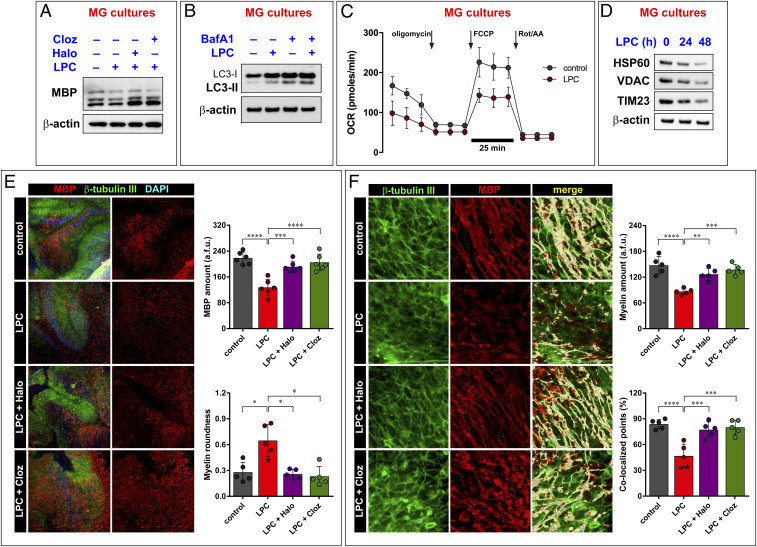
Initial Western blot (WB) analysis of LPC-treated MG cultures not only confirmed the fall on MBP levels (Fig. 5A) but also showed that LPC stimulates the autophagic flux, which is further increased by BafA1 (Fig. 5B). In vitro testing also revealed that LPC affected mitochondrial activity, reducing basal and maximal respiratory capacity (Fig. 5C and SI Appendix, Fig. S5) and eventually leading to a mitophagic reduction of the mitochondrial mass, as revealed by quantification of mitochondrial markers (Fig. 5D).
Fig. 5.
Haloperidol and Clozapine revert demyelination events in ex vivo MS model. The demyelinating agent LPC causes MBP loss in MG cultures (A). This feature is abolished by cotreating cells with the autophagy inhibitors haloperidol (Halo) and clozapine (Cloz). Similar to proinflammatory cytokines, LPC boosts autophagy (B), causes mitochondrial function impairment (C), and activates mitochondrial autophagy (D). Calculated values of the Seahorse assay are reported in SI Appendix, Fig. S5. (E) Ex vivo analysis performed in OSC confirms the ability of the antipsychotic agents Halo and Cloz to improve myelinization following LPC treatment. (F) Fluorescence shape (myelin roundness) and colocalization between MBP (red) and the neuronal marker β-tubulin III (green). Immunoblots are representative of at least three independent experiments. Quantification of the Western blot performed is reported in SI Appendix, Table S1. The graphs in C, E, and F represent the mean ± SD of five experiments; two-way ANOVA with Dunnett’s multiple comparison test, ****P < 0.0001, ***P < 0.001, **P < 0.01.
The immunostaining analysis of OSC for the myelin marker MBP revealed a dramatic demyelination in slices treated with LPC (Fig. 5E). Myelin appeared broken and punctate when compared with control OSC, with formation of MBP-positive debris. In contrast, a significant increase of MBP-positive cells was found in slices treated with clozapine or haloperidol after LPC-induced demyelination, and the filamentous morphological aspect of myelin was similar to that observed in control OSC (Fig. 5E). These results were further supported by shape analysis of the fluorescence signal. We extrapolated a fluorescence shape parameter, the circularity value, and found that MBP fluorescence changed from a maximum circularity value of 1 (representative of the maximum myelin fragmentation) to a minimal value of zero, which indicates the distribution of MBP fluorescence along the axon. Samples treated with LPC displayed the highest degree of circularity, while cotreatment with either clozapine or haloperidol rescued the circularity value to control levels (Fig. 5E).
We used confocal microscopy to verify if myelination occurred in alignment with axons. We examined the three-dimensional localization of the myelin and axonal markers (MBP and β-tubulin III, respectively) and evaluated the percentage of colocalized points shared by these two markers. LPC dramatically reduced the percentage of colocalized signal. In OSC treated either with clozapine or haloperidol, the degree of alignment of the myelin and the axonal markers was similar to that of control slices, indicating the presence of a good myelin sheath (Fig. 5F).
Treatment with Antipsychotic Drugs Completely Reverses CPZ-Induced MBP Loss In Vivo.
Next, we used the CPZ mouse model of MS (65, 66) to study the effect of the two antipsychotic drugs in vivo. The CPZ model mimics different pathological courses of MS and represents a useful approach to test new pharmacological treatments protecting from demyelination and/or stimulating remyelination (66–69).
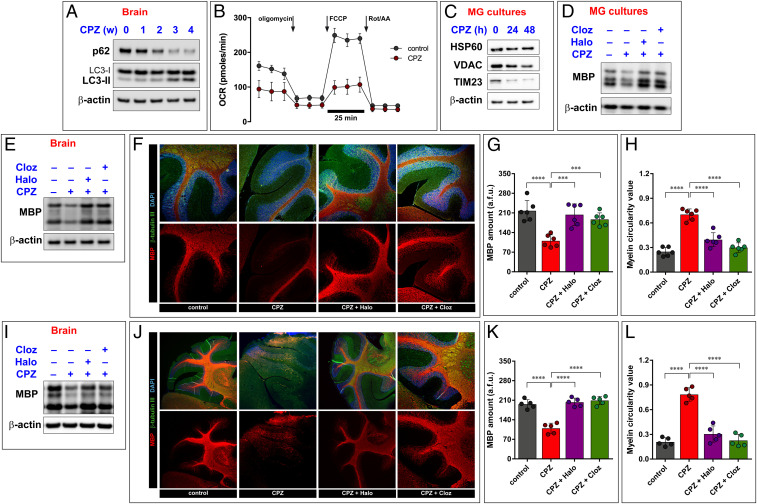
WB analysis of brain homogenates prepared from mice treated for 4 wk with CPZ suggests stimulation of autophagy, as shown by a progressive reduction, up to a total disappearance of the p62 protein and by a progressive increase of LC3-II (Fig. 6A). Identical findings were obtained in MG cultures (SI Appendix, Fig. S6A) [i.e., CPZ and proinflammatory cytokines produced identical effects (compare with Fig. 2 C and D)]. Again, like with proinflammatory cytokines (SI Appendix, Fig. S2F), the cotreatment with BafA1 further increased LC3-II in CPZ-treated MG cultures (SI Appendix, Fig. S6A), suggesting that its proautophagic effect is due to increase of autophagic flux and not to unspecific autophagy inhibition. The fact that CPZ could increase the autophagic flux was also confirmed in mice brains, as we found incremented LC3-II and a parallel reduction of p62 after CPZ treatment (SI Appendix, Fig. S6B). Increased autophagy occurs together with an increase in mitophagy, as indicated by impairment of mitochondrial functions (Fig. 6B and SI Appendix, Fig. S6C) and by reduced mitochondrial content (Fig. 6C). Finally, as expected based on this profile, CPZ caused a reduction of MBP levels in both MG cultures (Fig. 6D) and brain homogenates (Fig. 6E).
Fig. 6.
Antipsychotic drugs improve myelination in a mouse model of demyelination. CPZ administration causes activation of autophagy in brains of mice treated for 4 wk, as shown by a progressive increase in the lipidated form of LC3 (LC3-II) and a parallel decrease of p62. This event also excludes that CPZ may interfere with the correct execution of the autophagic flux (A). CPZ also provokes loss of mitochondrial functioning and activation of mitophagy, as denoted by Seahorse assay (B) and immunoblot for mitochondrial proteins (C). Calculated values for Seahorse assay are shown in SI Appendix, Fig. S6C. As a result of these effects, CPZ causes loss of MBP both in in MG cultures (D) and in brain homogenates (E). Before starting CPZ administration, mice were treated with haloperidol (Halo) and clozapine (Cloz). These drugs demonstrated to be efficacious in preventing the CPZ-dependent loss of myelin, as shown by immunoblot (E) and immunohistochemistry (F) performed in cerebellar slices. The graphs indicate the myelin levels (G) and linearity (H) detected in the cerebellar slices obtained from CPZ-treated mice. Antipsychotic compounds not only prevent the demyelination induced by CPZ but also improve the remyelination process. In this case, haloperidol and clozapine were administered after the 5-wk CPZ period. Remyelination events have been assessed by immunoblot of brain homogenates (I) and by immunohistochemistry of cerebellar slices (J). Relative quantifications of myelin amount (K) and linearity (L) are shown. All Immunoblots are representative of at least three independent experiments, and their quantification is reported in SI Appendix, Table S1. Two-way ANOVA with Dunnett’s multiple comparison test, ****P < 0.0001, ***P < 0.001.
MBP loss was further confirmed by immunohistochemistry in sagittal cerebellar sections, which displayed a dramatic loss of MBP from the white matter as a consequence of CPZ treatment (Fig. 6F). A detailed analysis of the morphological features of the fluorescent MBP signal demonstrated that it was not only strongly reduced but also fragmented along the axon (Fig. 6 G and H). A group of mice was pretreated with haloperidol and clozapine for 1 wk before starting CPZ administration, and the treatment was then continued for the entire duration of the experiment. Immunoblot (Fig. 6E) and immunohistochemistry (Fig. 6F) data indicate that both drugs effectively prevented CPZ-induced demyelination. In addition, a second group of mice was treated with haloperidol or clozapine after 5-wk administration of CPZ (i.e., when myelin loss was already in place). The two antipsychotics reversed CPZ-induced MBP loss (Fig. 6 I–K) and myelin fragmentation (Fig. 6 J and L). All these effects appear to be due to interference with the autophagic flux, as indicated by a concomitant increase of both LC3-II and p62 levels (SI Appendix, Fig. S6B).
We also asked if clozapine and haloperidol may impact on the inflammatory microenvironment that occur during demyelination and found that they caused significant decreases in CPZ-stimulated TNF-α and IL-1-β production (SI Appendix, Fig. S6 D and E). In addition, we found that NCOA4 and TFR levels increased during the first week of CPZ treatment (SI Appendix, Fig. S6F), indicating activation of ferritinophagy. In the subsequent weeks, when the autophagic degradation occurs (as demonstrated by decrease in p62 levels in Fig. 6A), NCOA4 and TFR levels also decreased (SI Appendix, Fig. S6F). Recent findings demonstrate that ferritinophagy may be an important contributor to inflammation, in particular by triggering HMGB1 release, which favors inflammatory and immune response. Indeed, we found increased HMGB1 levels in the brains of CPZ-treated mice (SI Appendix, Fig. S6G) that were prevented by clozapine and haloperidol administration (SI Appendix, Fig. S6G). Coherent with these findings, we found increased levels of HMGB1 in the CSF of MS patients during the active phase of the disease (SI Appendix, Fig. S6H).
Taken together, these data demonstrate that autophagy and its selective forms (mitophagy and ferritinophagy) are activated during MS, exacerbating inflammation, causing mitochondrial impairment and alteration of the normal cellular metabolism, and ultimately leading to cellular damage and demyelination. Blocking autophagic dynamics may therefore represent an approach to counteract these processes (SI Appendix, Fig. S7).
Antipsychotic Drugs Improve Motor Performance in the CPZ Model.
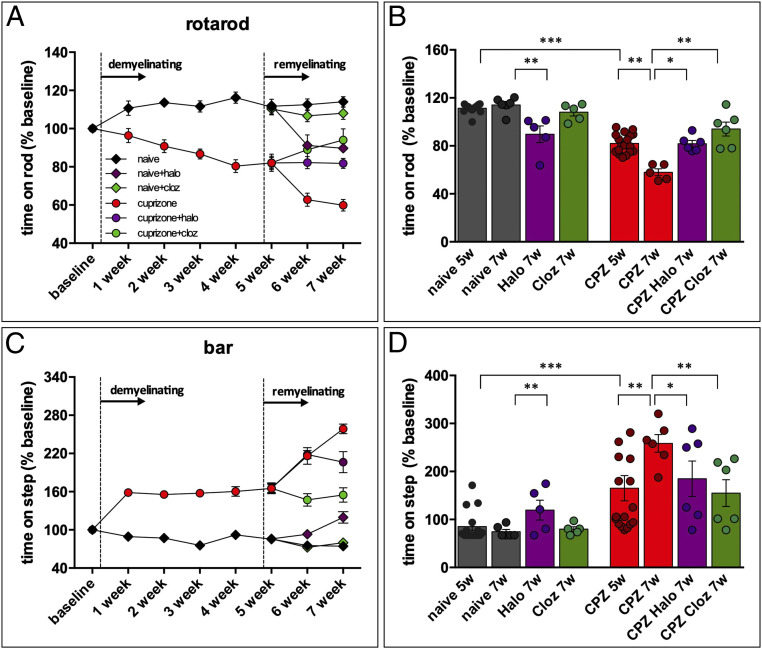
Repeated CPZ administrations cause a progressive motor impairment in the mouse, as assessed by two behavioral tests. The rotarod test, used to assess neuromuscular coordination and balance, measures the animal’s ability to maintain itself on a rod that turns at accelerating speed (70–72). Deficits in the rotarod test are particularly clear in animals with altered cerebellar or spinal cord function and are indicated by loss of equilibrium and fall from the rod. Catalepsy, defined as muscular rigidity and failure to correct an externally imposed posture for a prolonged period of time, was assessed using the bar test. Intensity of catalepsy was measured as the length of time (behavioral immobility) during which mice maintained this abnormal imposed posture. The same tests were used to evaluate the effect of antipsychotic during the 2 wk following CPZ withdrawal.
During the first 5 wk of experiment, control animals improved their performance in the rotarod and in the bar test. On the contrary, CPZ-treated animals became more likely to fall during the rotarod test (Fig. 7A) and to maintain the imposed posture for a prolonged period in the bar test (Fig. 7C). During the 2 wk that followed CPZ withdrawal, CPZ-treated mice continued to experience a progressive impairment of their motor performance, whereas animals which received clozapine or haloperidol significantly improved it. Notably, clozapine produced better results than haloperidol (Fig. 7 A–D). This latter observation may relate to the fact that haloperidol is a typical antipsychotic drug with parkinsonian extrapyramidal effects, whereas clozapine is an atypical antipsychotic, less likely to produce these effects. To test this hypothesis, we administered clozapine or haloperidol to control animals. As expected, clozapine-treated control animals displayed no significant difference with untreated animals in both the rotarod and the bar test, while haloperidol significantly worsened both (Fig. 7 A–D).
Fig. 7.
Haloperidol (Halo) and clozapine (Cloz) reverse motor deficit in the CPZ mouse model. Following a 5-wk CPZ treatment, Haloperidol (Halo) or Clozapine (Cloz) (0.5 mg/kg) were administered intraperitoneal twice per week for 2 wk. Motor activity was evaluated in the rotarod (A and B) and bar test (C and D). The dark gray diamonds and bars represent control animals; the red circles and bars represent CPZ-treated animals; the purple diamonds and bars are naïve animals treated with Halo; the purple circles and bars are CPZ animals treated with Halo; the green diamonds and bars are naïve animals treated with Cloz; the green circles and bars are CPZ animals treated with Cloz. The data are means ± SEM of eight determinations per group. *P < 0.05, **P < 0.01, two-way ANOVA for repeated measures followed by the Bonferroni test.
Discussion
Main Findings.
Three main findings emerge from the present work. First, autophagy and its selective forms (mitophagy and ferritinophagy) occur in MS patients and in experimental models of MS; second, these phenomena play a causal role in MS because their inhibition prevents myelin loss; third, two clinically used drugs can inhibit autophagy, prevent demylinization, induce remyelination, and revert MS behavioral deficits.
Autophagy Occurs in MS.
In two recent studies, we found increased levels of autophagy and mitophagy markers both in the serum and in the CSF of MS patients (13, 14). Here, we demonstrate that these and other markers increase during active periods of disease and return near baseline levels in remitting periods. While this suggests that autophagy and mitophagy are activated during recurrent MS, their contribution to the pathophysiology of the disease remains controversial. On the one hand, autophagy and mitophagy are known to advantage cell survival. Autophagy can remove extracellular materials and intracellular dysfunctional or damaged molecules, not only clearing useless and potentially dangerous material but also ensuring availability of metabolic substrates under shortage conditions. In addition, autophagy can limit the inflammatory response by digesting inflammasome complexes and related activators, thereby limiting the autoinflammatory priming that contributes to MS. On the other hand, dysregulated autophagy and mitophagy may favor myelin damage (13, 73, 74). Moreover, autophagy supports the activity of cells that contribute to autoimmune disorders (75, 76), and this may also exert an unfavorable effect on MS.
We attempted to clarify the involvement of autophagy and its selective forms (mitophagy and ferritinophagy) in MS by using several in vitro and in vivo models of the disease: cell cultures, where MS-like conditions are induced using the proinflammatory cytokines TNF-α and IL-1-β; organotypic cerebellar slices treated with the demyelinating agent LPC; and mice treated with the copper chelator CPZ, an established in vivo model that replicates MS type III and IV and is widely used to test new pharmacological treatments protecting from demyelination and stimulating remyelination (67, 69, 77). All these experimental conditions cause demyelization, as documented by a reduction in several parameters (i.e., the amount of MBP, its colocalization to neurites, and the linearization of the MBP fluorescence signal in slices of mouse cerebellum), which indicates a reduction of myelin wrapping around the axons. We found that activation of the autophagic flux is a common feature of all these treatments. Importantly, this occurs in the absence of cell damage or interference with cell differentiation.
Cytokines (1, 45), LPC (78–80), and CPZ (81) can also induce an impairment of mitochondrial function, with a reduction of basal and maximal respiratory capacity. This mitochondrial defect shifts the metabolic balance toward an increase in glycolysis and cytoplasmic ATP production, with a consequent accumulation and excretion of lactic acid. However, the overall metabolic demand exceeds the ATP supply ensured by glycolysis, and this energy shortage is sensed by the regulatory kinases mTOR and AMPK. In particular, mTOR is inhibited under starvation conditions, whereas AMPK is activated by impairment of mitochondrial activity [i.e., low ATP synthesis, reduced O2 consumption, increased production of reactive oxygen species, or Ca2+ mobilization (78)].
Coherently with these premises, we found a change in the pattern of phosphorylation of the ULK1 protein, a substrate of both mTOR and AMPK and an important protein in autophagy and mitophagy. ULK1 becomes hyperphosphorylated in the SER317 excitatory position, most likely by AMPK (52, 82), and dephosphorylated at the SER757 position, where the phosphorylation is operated by mTOR. This altered pattern of ULK1 phosphorylation is expected to facilitate autophagy and mitophagy (30, 82), and in fact, we documented both an increase in mitochondrial levels of Parkin, which can trigger mitophagy, and the delivery of mitochondria to lysosomal degradation.
Taken together, these data suggest that different stimuli that cause MS-like demyelination affect mitochondrial function and reduce mitochondrial mass, leading to increased autophagy and mitophagy. At first sight, the increase in autophagy may be viewed as an attempt to provide new metabolic intermediates for compensating the energetic deficit, and mitophagy may aim to reshape the mitochondrial population, eliminating damaged mitochondria (83). However, activation of mitophagy should be balanced by increased mitochondria replication (84), and this does not seem to occur under our experimental conditions. Thus, excessive mitophagy can further unbalance cell metabolism and reinforce the need for autophagy, fueling a vicious circle. Hence, increased autophagy and mitophagy may cause demyelination. In turn, demyelination generates a very demanding energy need in neurons because demyelinated axons become more dependent on mitochondrial ATP production to maintain an increased Na+/K+ pump activity and suffer of an up-regulated Na+/Ca2+ exchange, which requires an efficient mitochondrial Ca2+ buffering (85). As a consequence, mitochondrial electron chain (ETC) alterations may occur also in neurons (86), leading, in time, to axonal degeneration and neuron loss. In sum, our data suggest that demyelination may depend on mitochondrial impairment due to autophagy/mitophagy in the absence of a compensatory mitochondrial biogenesis.
Autophagy Plays a Causal Role in Myelin Loss.
To explore the possibility that autophagy plays a causal role in MS, we employed multiple, structurally and mechanistically unrelated inhibitors: CC, an AMPK inhibitor; siATG7, a short interfering RNA against ATG7, a gene essential for autophagy; 3MA, an inhibitor of autophagosome formation; CQ, a blocker of the binding of autophagosomes to lysosomes; and the antipsychotic drugs haloperidol and clozapine, which have been recently shown to be potent late-stage autophagy inhibitors (27). In both in vitro and ex vivo experiments, these inhibitors reduced not only autophagy but also MBP degradation and axonal demyelination. We also observed remyelination in in vivo experiments. These findings suggest that activation of autophagy may play a causal role in myelin loss, and it can be speculated that in a wider time frame, this reduced demyelination (and remyelination) may also slow down axonal degeneration and neuron loss.
As mentioned above, it can be speculated that autophagy inhibition may act on immune cells, increasing the production of inflammatory cytokines and thereby contributing to the autoinflammatory phase of the illness. However, our data do not support this possibility because both haloperidol and clozapine reduced the inflammatory environment in the in vivo MS model. In particular, antipsychotics appear to counteract the formation of the inflammatory microenvironment, as they not only reduced the levels of classical proinflammatory cytokines (e.g., TNF-α and IL-1-β) but also the release of HMGB1, which is known to cause exacerbation of inflammation in different pathologic contexts (49), including neurodegeneration (87) and MS (23, 88). While it has been previously suggested that autophagic inhibitors (such as CQ) reduce the inflammatory microenvironment by interfering with the release of HMGB1 (49), the finding that haloperidol and clozapine can also achieve this effect is interesting. In addition, the present study supports the possibility that autophagy is involved in the regulation of HMGB1 release. First, increased HMGB1 levels were detected in the CSF of MS patients in the active phase of the disease, when multiple markers of the autophagy process are found in both CSF and serum. Second, we found that our MS models not only were associated with increased release of HMGB1 but were also characterized by activation and execution of the ferritinophagic process, and ferritinophagy can prompt the release of HMGB1 (37).
Translational Potential.
If inhibition of autophagy can improve myelinization, is it also sufficient to ameliorate MS symptomatology? To pursue an answer to this key question, we tested the effects of the autophagy inhibitors haloperidol and clozapine in the CPZ model. CPZ causes a dramatic demyelination in the cerebellar white matter, which brings about a significant impairment of motor function, as documented in the rotarod and the bar tests. As expected, both haloperidol and clozapine improved motor performance in CPZ-treated animals. Clozapine provided better results than haloperidol in these tests, probably due to the strong antidopaminergic parkinsonian effect of haloperidol (89). These data support the notion that these drugs may represent a therapeutic option for MS.
Unfortunately, the mechanism of this interesting and promising action of antipsychotics remains obscure. It has been suggested that they may exert their beneficial effects by acting on D3 receptors expressed in oligodendrocytes (90). In fact, oligodendrocyte differentiation is impaired after administration of the D3 agonist quinpirole, and this effect is blocked by the antagonist haloperidol (90). However, the most provocative findings suggesting that antipsychotics may be useful against MS came from investigations in the schizophrenia field. Swollen oligodendrocytes were observed in postmortem brains of patients affected by schizophrenia in 1938 (91). Despite the fact that imaging techniques permitted to highlight impairments in oligodendroglial function and abnormalities (including demyelination) in the white matter of people with schizophrenia, the demonstration that antipsychotic compounds may improve oligodendrocyte development and brain function came only at the beginning of the 21st century (18, 19). Hypotheses have been made to interpret these effects. Many such antipsychotic drugs (chlorpromazine, clozapine, ziprasidone, risperidone, haloperidol, olanzapine, and ziprasidone) increase the expression of genes involved in cholesterol and fatty acid biosynthesis (92, 93), and cholesterol-synthesis gene pathways have been reported to be strongly up-regulated in oligodendrocytes during remyelination (69). Improvements in the differentiation of oligodendrocyte were also achieved by using quetiapine, in a ERK1/2 pathway–dependent manner (25), a molecular pathway that may be activated by antipsychotic drugs. Finally, the present study supports the notion that autophagy and its selective forms are a possible target of action of clozapine and haloperidol (SI Appendix, Fig. S7)
In conclusion, our findings suggest to repurpose haloperidol and clozapine for the treatment of MS, at least in patients with MS variants that are more closely modeled by CPZ, like type III and IV. These antipsychotic drugs may accelerate recovery from a demyelinating attack and prevent relapses. Unfortunately, it is difficult (if not impossible) to predict when a relapse may occur, and in addition, the active phase of the disease may begin before a clinically evident relapse. Therefore, the most plausible use of these drugs may be in the late phases of MS, when remyelination after a relapse becomes incomplete and the disease takes a progressive course. Further studies will be needed to validate this interesting possibility.
Materials and Methods
MG and OPC Cultures Generation.
For detailed materials and methods, please refer to SI Appendix, Supplementary Material.
MG and OPC Cultures Generation.
MG cultures were obtained from brain cortexes that were mechanically and enzymatically dissociated, then cultured in T75 flasks. The OPC population was isolated by shaking the T75 flasks on an orbital shaker. Detailed information is reported in SI Appendix, Supplementary Material.
Patients.
The retrospective study of relapsing–remitting MS patients was approved by the Committee for Medical Ethics in Research of Ferrara and written informed consent was obtained from all participants. CSF and serum levels of biomarkers were determined using commercially available ELISA kits. Detailed information is reported in SI Appendix, Supplementary Material.
Animal Studies.
Experiments involving animals were conducted in accordance with European Community (EU Directive 2010/63/EU), national and local laws and policies. The Institutional Animal Care and Use Committee of the University of Ferrara approved this research that was then authorized by the Italian Ministry for Health (DGSAF 0023994-P-16/09/2019). Detailed information for in vivo experiments is reported in SI Appendix, Supplementary Material.
Statistical Analysis.
The comparison between three groups by means of the one-way ANOVA test, while the comparison between two groups by the use of the unpaired t test. Two-tailed P values < 0.01 were considered statistically significant. Detailed information is reported in SI Appendix, Supplementary Material.
Supplementary Material
Acknowledgments
P.P. is grateful to Camilla degli Scrovegni for continuous support. The Signal Transduction Laboratory is supported by the Italian Association for Cancer Research (AIRC; Grant IG-23670 to P.P. and Grant IG-19803 to C.G.), Associazione Ricerca Oncologica Sperimentale Estense (A-ROSE), Telethon (Grant GGP11139B to P.P.), the Ministry of Education, University and Research–Progetti di Rilevante Interesse Nazionale (Grant PRIN2017E5L5P3 to P.P. and Grant PRIN20177E9EPY to C.G.), the Italian Ministry of Health (Grant GR-2013-02356747 to C.G.), the European Research Council (Grant InflaPML 853057 to C.G.), and by local funds from the University of Ferrara to P.P. and C.G. M.R.W. was supported by the National Science Centre, Poland (Grant UMO-2018/29/B/NZ1/00589). S.P. was supported by Fondazione Umberto Veronesi. Preliminary results were also obtained thanks to the support of the Italian Multiple Sclerosis Foundation to S.P. (code 2014/B3).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020078118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Patergnani S., et al., Mitochondria in multiple sclerosis: Molecular mechanisms of pathogenesis. Int. Rev. Cell Mol. Biol. 328, 49–103 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Thompson A. J., Baranzini S. E., Geurts J., Hemmer B., Ciccarelli O., Multiple sclerosis. Lancet 391, 1622–1636 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Sharief M. K., Hentges R., Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 325, 467–472 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Palle P., Monaghan K. L., Milne S. M., Wan E. C. K., Cytokine signaling in multiple sclerosis and its therapeutic applications. Med. Sci. (Basel) 5, E0023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seppi D., et al., Cerebrospinal fluid IL-1β correlates with cortical pathology load in multiple sclerosis at clinical onset. J. Neuroimmunol. 270, 56–60 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Hauser S. L., Doolittle T. H., Lincoln R., Brown R. H., Dinarello C. A., Cytokine accumulations in CSF of multiple sclerosis patients: Frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology 40, 1735–1739 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Liang P., Le W., Role of autophagy in the pathogenesis of multiple sclerosis. Neurosci. Bull. 31, 435–444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshii S. R., Mizushima N., Autophagy machinery in the context of mammalian mitophagy. Biochim. Biophys. Acta 1853, 2797–2801 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Dikic I., Elazar Z., Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Menzies F. M., et al., Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Yoon S. Y., Kim D. H., Alzheimer’s disease genes and autophagy. Brain Res. 1649, 201–209 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Giorgi C., et al., Relevance of autophagy and mitophagy dynamics and markers in neurodegenerative diseases. Biomedicines 9, 149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patergnani S., et al., Autophagy and mitophagy elements are increased in body fluids of multiple sclerosis-affected individuals. J. Neurol. Neurosurg. Psychiatry 89, 439–441 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Castellazzi M., et al., Correlation between auto/mitophagic processes and magnetic resonance imaging activity in multiple sclerosis patients. J. Neuroinflammation 16, 131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alirezaei M., et al., Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy 5, 152–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z., et al., NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misrielal C., Mauthe M., Reggiori F., Eggen B. J. L., Autophagy in multiple sclerosis: Two sides of the same coin. Front. Cell. Neurosci. 14, 603710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubicki M., et al., DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage 26, 1109–1118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlösser R. G., et al., White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophr. Res. 89, 1–11 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Hakak Y., et al., Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 98, 4746–4751 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsel P., Davis K. L., Haroutunian V., Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: A gene ontology study. Schizophr. Res. 79, 157–173 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Wang H., et al., Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr. Res. 119, 164–174 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Green L. K., et al., Enhanced disease reduction using clozapine, an atypical antipsychotic agent, and glatiramer acetate combination therapy in experimental autoimmune encephalomyelitis. Mult. Scler. J. Exp. Transl. Clin. 3, 2055217317698724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Sullivan D., et al., Treatment with the antipsychotic agent, risperidone, reduces disease severity in experimental autoimmune encephalomyelitis. PLoS One 9, e104430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L., et al., Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry 13, 697–708 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Rimessi A., et al., Protein kinase C beta: A new target therapy to prevent the long-term atypical antipsychotic-induced weight gain. Neuropsychopharmacology 42, 1491–1501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J., et al., Haloperidol and clozapine block formation of autophagolysosomes in rat primary neurons. Neuroscience 209, 64–73 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Cramer S. P., Simonsen H., Frederiksen J. L., Rostrup E., Larsson H. B., Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 4, 182–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bercury K. K., Macklin W. B., Dynamics and mechanisms of CNS myelination. Dev. Cell 32, 447–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshii S. R., Mizushima N., Monitoring and measuring autophagy. Int. J. Mol. Sci. 18, E1865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klionsky D. J., et al., Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 12, 1–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu C. L., et al., Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurochem. 148, 426–439 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jhelum P., et al., Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J. Neurosci. 40, 9327–9341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J., et al., PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 117, 14376–14385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao M., et al., Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., et al., Autophagy-dependent ferroptosis: Machinery and regulation. Cell Chem. Biol. 27, 420–435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancias J. D., Wang X., Gygi S. P., Harper J. W., Kimmelman A. C., Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Q., Liu J., Kang R., Zhou B., Tang D., The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 510, 278–283 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z., et al., Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14, 2083–2103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quiles Del Rey M., Mancias J. D., NCOA4-mediated ferritinophagy: A potential link to neurodegeneration. Front. Neurosci. 13, 238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui S., et al., Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 10, 331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bargiela D., Chinnery P. F., Mitochondria in neuroinflammation–Multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci. Lett. 710, 132932 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Klotz L., et al., Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med. 11, eaao5563 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Patergnani S., Pinton P., Mitophagy and mitochondrial balance. Methods Mol. Biol. 1241, 181–194 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Bonora M., et al., Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 21, 1198–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziabreva I., et al., Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia 58, 1827–1837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galluzzi L., Pietrocola F., Levine B., Kroemer G., Metabolic control of autophagy. Cell 159, 1263–1276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Missiroli S., et al., PML at mitochondria-associated membranes is critical for the repression of autophagy and cancer development. Cell Rep. 16, 2415–2427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue J., et al., Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc. Natl. Acad. Sci. U.S.A. 117, 25543–25552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs H. H., Bercury K. K., Popescu D. C., Narayanan S. P., Macklin W. B., A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro 6, 1759091414551955 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller C. W., Lünemann J. D., Autophagy and autophagy-related proteins in CNS autoimmunity. Front. Immunol. 8, 165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J., Kundu M., Viollet B., Guan K. L., AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy J. M. M., Towers C. G., Thorburn A., Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dasgupta B., Seibel W., Compound C/dorsomorphin: Its use and misuse as an AMPK inhibitor. Methods Mol. Biol. 1732, 195–202 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Petiot A., Ogier-Denis E., Blommaart E. F., Meijer A. J., Codogno P., Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Wu Y. T., et al., Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 285, 10850–10861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vucicevic L., et al., Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy 7, 40–50 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Mauthe M., et al., Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14, 1435–1455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S. H., et al., The antipsychotic agent clozapine induces autophagy via the AMPK-ULK1-Beclin1 signaling pathway in the rat frontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 96–104 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Yin Y. C., et al., Clozapine induces autophagic cell death in non-small cell lung cancer cells. Cell. physiol. Biochem. 35, 945–956 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Rutkowska A., Sailer A. W., Dev K. K., EBI2 receptor regulates myelin development and inhibits LPC-induced demyelination. J. Neuroinflammation 14, 250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eleuteri C., et al., A staged screening of registered drugs highlights remyelinating drug candidates for clinical trials. Sci. Rep. 7, 45780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miron V. E., et al., Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am. J. Pathol. 176, 2682–2694 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Sullivan C., Schubart A., Mir A. K., Dev K. K., The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflammation 13, 31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denic A., et al., The relevance of animal models in multiple sclerosis research. Pathophysiology 18, 21–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Praet J., Guglielmetti C., Berneman Z., Van der Linden A., Ponsaerts P., Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci. Biobehav. Rev. 47, 485–505 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Choi I. Y., et al., A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 15, 2136–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz V., et al., Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia 65, 1350–1360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voskuhl R. R., et al., Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 116, 10130–10139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zausinger S., Hungerhuber E., Baethmann A., Reulen H., Schmid-Elsaesser R., Neurological impairment in rats after transient middle cerebral artery occlusion: A comparative study under various treatment paradigms. Brain Res. 863, 94–105 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Lüesse H. G., et al., Evaluation of R6/2 HD transgenic mice for therapeutic studies in Huntington’s disease: Behavioral testing and impact of diabetes mellitus. Behav. Brain Res. 126, 185–195 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Karl T., Pabst R., von Horsten S., Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 55, 69–83 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Keller C. W., et al., ATG-dependent phagocytosis in dendritic cells drives myelin-specific CD4+ T cell pathogenicity during CNS inflammation. Proc. Natl. Acad. Sci. U.S.A. 114, E11228–E11237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albert M., et al., Synaptic pathology in the cerebellar dentate nucleus in chronic multiple sclerosis. Brain Pathol. 27, 737–747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei J., et al., Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 17, 277–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhattacharya A., Parillon X., Zeng S., Han S., Eissa N. T., Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J. Biol. Chem. 289, 26525–26532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lucchinetti C., et al., Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Jiang S., et al., Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J. Biol. Chem. 288, 26013–26026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., et al., Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler. Thromb. Vasc. Biol. 36, 1090–1100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hollie N. I., et al., Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 1841, 888–895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandur E., et al., Relationship of iron metabolism and short-term cuprizone treatment of C57BL/6 mice. Int. J. Mol. Sci. 20, E2257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egan D. F., et al., Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoitzing H., Johnston I. G., Jones N. S., What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research. BioEssays 37, 687–700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uittenbogaard M., Chiaramello A., Mitochondrial biogenesis: A therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 20, 5574–5593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kornek B., et al., Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 124, 1114–1124 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Aboul-Enein F., et al., Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J. Neuropathol. Exp. Neurol. 62, 25–33 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Walker L. E., et al., A comparison of HMGB1 concentrations between cerebrospinal fluid and blood in patients with neurological disease. Biomarkers 22, 635–642 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Andersson A., et al., Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leukoc. Biol. 84, 1248–1255 (2008). [DOI] [PubMed] [Google Scholar]
- 89.Madras B. K., History of the discovery of the antipsychotic dopamine D2 receptor: A basis for the dopamine hypothesis of schizophrenia. J. Hist. Neurosci. 22, 62–78 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Bongarzone E. R., Howard S. G., Schonmann V., Campagnoni A. T., Identification of the dopamine D3 receptor in oligodendrocyte precursors: Potential role in regulating differentiation and myelin formation. J. Neurosci. 18, 5344–5353 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elvidge A. R., Reed G. E., Biopsy studies of cerebral pathologic changes in schizophrenia and manic-depressive psychosis. Arch. Neurol. Psychiatry 40, 227–268 (1938). [Google Scholar]
- 92.Fernø J., et al., Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: A novel mechanism of action? Pharmacogenomics J. 5, 298–304 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Fernø J., Skrede S., Vik-Mo A. O., Håvik B., Steen V. M., Drug-induced activation of SREBP-controlled lipogenic gene expression in CNS-related cell lines: Marked differences between various antipsychotic drugs. BMC Neurosci. 7, 69 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.