Abstract
Exposure to adversity can accelerate biological aging. However, existing biomarkers of early aging are either costly and difficult to collect, like epigenetic signatures, or cannot be detected until late childhood, like pubertal onset. We evaluated the hypothesis that early adversity is associated with earlier molar eruption, an easily assessed measure that has been used to track the length of childhood across primates. In a preregistered analysis (n = 117, ages 4 to 7 y), we demonstrate that lower family income and exposure to adverse childhood experiences (ACEs) are significantly associated with earlier eruption of the first permanent molars, as rated in T2-weighted magnetic resonance images (MRI). We replicate relationships between income and molar eruption in a population-representative dataset (National Health and Nutrition Examination Survey; n = 1,973). These findings suggest that the impact of stress on the pace of biological development is evident in early childhood, and detectable in the timing of molar eruption.
Keywords: income, adversity, molar eruption, development
Exposure to early life stress, including poverty and adverse childhood experiences (ACEs), undermines physical and mental health (1). Recently, accelerated biological aging has gained attention as a potential mechanism linking experiences of poverty and maltreatment with increased risk for poor health (2). Childhood adversity has been linked to earlier puberty, faster epigenetic aging, and earlier brain maturation (3). Some life history research (4) suggests that individuals who grow up in secure, enriched environments prioritize a slower pace of development, in order to maximize parental investment and extend periods of plasticity that facilitate learning. In contrast, individuals who grow up in environments characterized by psychosocial adversity may prioritize a faster pace of development, leading to earlier attainment of adult-like abilities and increased reproductive fitness, but at a steep cost to later physical and mental health.
Here, we investigate a domain of biological maturation that has not previously been considered in the childhood adversity literature: molar eruption. Tooth eruption patterns have been used to estimate age in fields including forensic science, anthropology, and archeology (5), and have also provided insights into the evolution of human development. Across broad samples of primates, age at molar eruption has been found to correlate with age at weaning, age of sexual maturity, and brain weight (refs. 6, 7, but see ref. 8). Furthermore, the evolution of an extended childhood in humans is evident in the later age of first molar emergence and later age for completion of brain growth in humans, as compared with nonhuman primates and early human ancestors (9). Psychosocial stressors can alter tooth development, resulting in caries and dental enamel defects (10). However, no studies to date have investigated whether adversity impacts the timing of molar eruption. Molar eruption is assessed routinely, unambiguously, and at an early age, as the first permanent molars typically emerge between ages 6 and 7 y. If early adversity drives early molar development, molar eruption timing could serve as an easily assessed biomarker to identify individuals at risk for accelerated development, and earlier morbidity and mortality.
Results
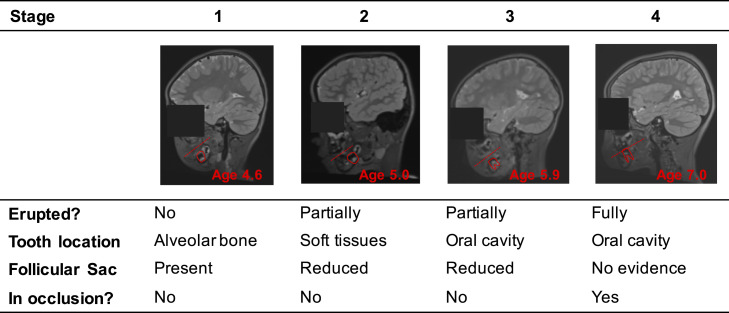
We conducted a preregistered analysis (https://osf.io/f6snd) to investigate how experiences of early adversity relate to the timing of emergence of the first permanent molars. We focused on two measures: family income, as low income is associated with high risk for chronic stress, and ACEs, as they have been consistently linked with poor health outcomes (1). We measured molar eruption by rating T2-weighted MRI scans, which are sensitive to tissues in the dental follicle (Fig. 1). Our analyses included 117 4- to 7-y-old children (64 female/53 male; 48 Black/36 white/5 Asian/14 Hispanic/14 multiracial or other).
Fig. 1.
Molar eruption rating criteria in MRI. The dashed lines represent the planes of occlusion (i.e., the planes at which the top and bottom teeth meet).
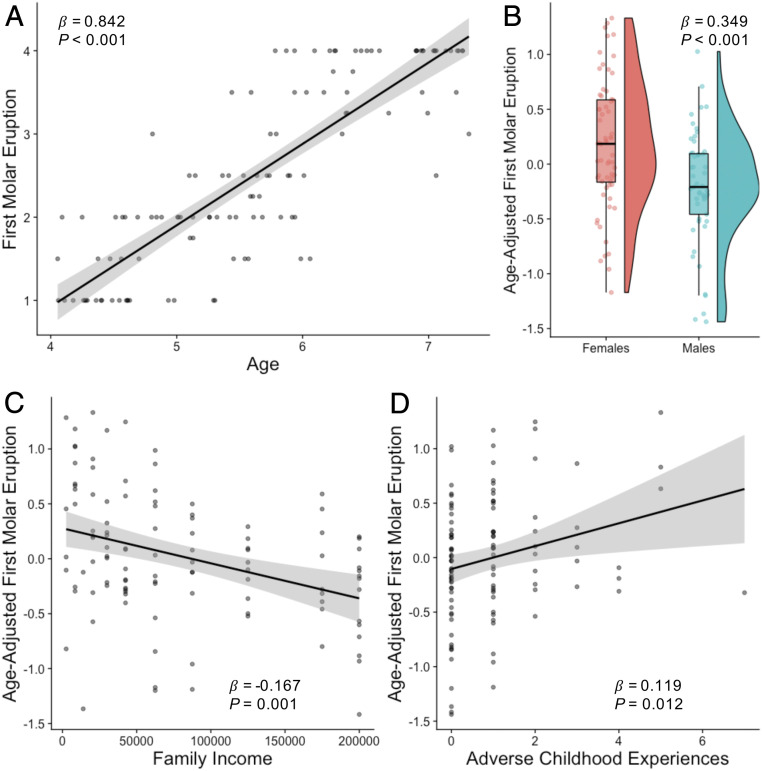
Effects of age, gender, and race/ethnicity on molar eruption were consistent with previous reports (11). Molar eruption was positively associated with age (β = 0.842, 95% CI [0.743, 0.942], P < 0.001; Fig. 2A). Girls had earlier molar eruption than boys (β = 0.349, 95% CI [0.159, 0.539], P < 0.001 controlling for age; Fig. 2B). Compared with Black children, white (β = −0.307, 95% CI [−0.524, −0.090], P = 0.006), Asian (β = −0.548, 95% CI [−1.011, −0.086], P = 0.021), and multiracial (β = −0.340, 95% CI [−0.639, −0.041] P = 0.026) children had later molar eruption. Body mass index (BMI) was not associated with molar eruption (β = 0.050, 95% CI [−0.063, 0.163], P = 0.381). As preregistered, age and gender were included as covariates in all subsequent models of molar eruption, and models were run with and without race/ethnicity and BMI.
Fig. 2.
Associations between molar eruption and (A) age, (B) gender, (C) income, and (D) ACEs.
Lower family income was significantly associated with earlier molar eruption (β = −0.167, 95% CI [−0.261, −0.073], P = 0.001; Fig. 2C). This association remained significant after controlling for BMI (β = −0.199, 95% CI [−0.301, −0.098], P < 0.001), racial/ethnic group (β = −0.144, 95% CI [−0.261, −0.028], P = 0.015), or both BMI and racial/ethnic group (β = −0.184, 95% CI [−0.306, −0.061], P = 0.004). Greater exposure to ACEs was also significantly associated with earlier molar eruption (β = 0.119, 95% CI [0.027, 0.212], P = 0.012; Fig. 2D). This association remained significant when controlling for BMI (β = 0.126, 95% CI [−0.024, 0.228], P = 0.016) but was at trend level when including racial/ethnic group (β = 0.086, 95% CI [−0.005, 0.178], P = 0.065). When family income and ACEs were included in the same model, only family income was significantly associated with molar eruption (income: β = −0.150, 95% CI [−0.246, −0.053], P = 0.003; ACEs: β = 0.065, 95% CI [−0.030, 0.161], P = 0.178).
We conducted a parallel set of analyses using data from the National Health and Nutrition Examination Survey (NHANES; 2011–2016). Analyses were restricted to subjects between ages 4.8 and 7.8 y with available oral health data, resulting in a sample of 1,973 participants. Age was related to number of first molars (β = 0.736, 95% CI [0.712, 0.76], P < 0.001), and girls had more first molars than boys (β = 0.113, 95% CI [0.03, 0.196], P = 0.01, controlling for age). Black (β = 0.137, 95% CI [0.043, 0.23], P = 0.006), Hispanic (β = 0.144, 95% CI [0.072, 0.217], P < 0.001), and multiracial (β = 0.156, 95% CI [0.036, 0.277], P = 0.015) children had more first molars than white children. Lower family income was significantly associated with having more first molars erupted (β = −0.060, 95% CI [−0.101, −0.019], P = 0.006, controlling for age and gender). Family income was significantly associated with number of first molars after controlling for BMI (β = −0.047, 95% CI [−0.087, −0.006], P = 0.029), but not racial/ethnic category (β = −0.042, 95% CI [−0.087, 0.003], P = 0.076).
Finally, we tested whether relationships between income and molar eruption extended to second molars, which erupt, on average, at age 12 y. Among 2,993 children aged 9.1 to 14.3 y, family income was significantly associated with number of erupted second molars (β = −0.046, 95% CI [−0.074, −0.018], P = 0.002, controlling for age and gender). Family income was no longer significantly associated with number of second molars after controlling for BMI (β = −0.026, 95% CI [−0.055, 0.002], P = 0.079) or racial/ethnic category (β = −0.025, 95% CI [−0.057, 0.006], P = 0.126). Consistent with prior work (12), molar eruption was associated with pubertal timing: 12- to 14-y-old girls who had not yet started their menstrual period had significantly fewer second molars than girls who had already started their menstrual period (β = −0.359, 95% CI [−0.690, −0.028], P = 0.039, controlling for age), and this association held when controlling for income (β = −0.393, 95% CI [−0.741, −0.046], P = 0.032) but not when controlling for BMI or race.
Discussion
Combining evidence from detailed characterization of molar eruption in MRI with nationally representative epidemiological research, we demonstrate that children from lower-income backgrounds grow up faster: Their permanent molars emerge before those of their more advantaged peers. Across both analyses, Black children show earlier molar eruption than white children, which is consistent with pervasive racial disparities in income and other contextual stressors that have roots in structural racism (13). We also present preliminary evidence that earlier second molar eruption is associated with earlier pubertal timing. Our findings contribute to a growing literature indicating that exposure to psychosocial stress may contribute to accelerated biological maturation.
The mechanisms underlying accelerated molar eruption remain unknown. The timing of tooth emergence is under partial genetic control, but is also regulated by hormones that are sensitive to stress, including osteocalcin, thyroid hormones, sex hormones, and cortisol (2, 14–18). Work in preclinical models is needed to illuminate mechanisms linking stress to accelerated dental development.
This study has several limitations. First, we investigated family income as a contextual condition that affects stress exposure in childhood; we did not measure stress hormones or child-reported stress. Second, both samples were cross-sectional, so we cannot test for stress-induced changes in developmental trajectories. Third, the measurement of molar eruption in NHANES (present/absent) is coarse compared to techniques like MRI and X-ray, which capture variability before molars emerge into the oral cavity. Furthermore, the NHANES measure of family income fails to capture differences in cost of living across the United States, and the dataset does not include other measures of psychosocial stress. These limitations may contribute to the weaker effect of income on molar eruption in NHANES as compared to the MRI analysis. Finally, our work was done only in the United States, and may not generalize to other contexts (19).
Despite these limitations, our work provides insight into the timing of molar eruption among children from lower-income families. Molar eruption can be characterized both in existing MRI datasets and in radiographs obtained through routine dental care. Longitudinal research is necessary to evaluate downstream correlates of early molar eruption, including early puberty, early brain development, and mental and physical health. If molar eruption timing can identify children at risk for accelerated aging early in childhood, it may serve as a useful screening tool to direct early intervention resources to the children who need them most.
Methods
Detailed materials and methods are provided in the SI Appendix.
MRI Sample.
This study was approved by the Institutional Review Board at the University of Pennsylvania. All parents provided written informed consent. Molar eruption was rated from raw T2-weighted MRI scans (Fig. 1). Molar eruption associations with age, gender, race/ethnicity, BMI percentile, family income, and ACEs were examined using linear models in R.
Replication in NHANES.
We combined three cycles of NHANES (2011−2012, 2013−2014, and 2015–2016). The number of erupted molars was summed from the Coronal Caries: Tooth Count variables. Molar eruption associations with age, gender, race/ethnicity, BMI percentile, and family income were examined using linear models with appropriate sample weights applied using the “survey” package in R (20).
Supplementary Material
Acknowledgments
We thank the families who participated in this research. This study was supported by the Jacobs Foundation Early Career Award (A.P.M.); the National Institute on Drug Abuse (Award 1R34DA050297-01 to A.P.M.); and NSF Graduate Research Fellowships to C.L.M., U.A.T., and A.L.B. We thank Megan Gunnar, Ph.D., and Sara Jaffee, Ph.D., for helpful comments.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2105304118/-/DCSupplemental.
Data Availability
Deidentified data and code for the MRI sample are deposited on OSF (https://osf.io/j4twn/) (21). NHANES data are publicly available through the National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2011, https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013, and https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2015) (22).
References
- 1.Felitti V. J., et al., Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 14, 245–258 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Belsky J., Early-life adversity accelerates child and adolescent development. Curr. Dir. Psychol. Sci. 28, 241–246 (2019). [Google Scholar]
- 3.Colich N. L., Rosen M. L., Williams E. S., McLaughlin K. A., Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol. Bull. 149, 721–764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Giudice M., Gangestad S. W., Kaplan H. S., “Life history theory and evolutionary psychology” in The Handbook of Evolutionary Psychology: Foundations Buss D. M., Ed. (John Wiley, ed. 2, 2016), vol. 1, pp. 88–114. [Google Scholar]
- 5.Manjunatha B. S., Soni N. K., Estimation of age from development and eruption of teeth. J. Forensic Dent. Sci. 6, 73–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith B. H., Dental development as a measure of life history in primates. Evolution 43, 683–688 (1989). [DOI] [PubMed] [Google Scholar]
- 7.Smith B. H., Crummett T. L., Brandt K. L., Ages of eruption of primate teeth: A compendium for aging individuals and comparing life histories. Am. J. Phys. Anthropol. 37, 177–231 (1994). [Google Scholar]
- 8.Smith T. M., Teeth and human life-history evolution. Annu. Rev. Anthropol. 42, 191–208 (2013). [Google Scholar]
- 9.Bernstein R., Hormones and the evolution of childhood in human and nonhuman primates. Horm. Res. Paediatr. 88, 15–21 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Davis K. A., et al., Teeth as potential new tools to measure early-life adversity and subsequent mental health risk: An interdisciplinary review and conceptual model. Biol. Psychiatry 87, 502–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pahel B. T., Vann W. F. Jr, Divaris K., Rozier R. G., A contemporary examination of first and second permanent molar emergence. J. Dent. Res. 96, 1115–1121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hägg U., Taranger J., Dental development assessed by tooth counts and its correlation to somatic development during puberty. Eur. J. Orthod. 6, 55–64 (1984). [DOI] [PubMed] [Google Scholar]
- 13.Geronimus A. T., Hicken M., Keene D., Bound J., “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96, 826–833 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almonaitiene R., Balciuniene I., Tutkuviene J., Factors influencing permanent teeth eruption. Stomatologija 12, 67–72 (2010). [PubMed] [Google Scholar]
- 15.Pirinen S., Endocrine regulation of craniofacial growth. Acta Odontol. Scand. 53, 179–185 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Danese A., McEwen B. S., Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Machado T. D., et al., Early life trauma is associated with decreased peripheral levels of thyroid-hormone T3 in adolescents. Int. J. Dev. Neurosci. 47, 304–308 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Patterson-Buckendahl P., Osteocalcin is a stress-responsive neuropeptide. Endocr. Regul. 45, 99–110 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Kutesa A. M., et al., Socioeconomic and nutritional factors associated with age of eruption of third molar tooth among Ugandan adolescents. J. Forensic Dent. Sci. 11, 22–27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumley T., Analysis of complex survey samples. J. Stat. Soft. 9, 48612 (2020). [Google Scholar]
- 21.McDermott C., Accelerated maturation. Open Science Framework (OSF). https://osf.io/j4twn. Deposited 24 May 2021.
- 22.Centers for Disease Control and Prevention (CDC) , National health and nutrition examination survey data. National Center for Health Statistics (NCHS). https://www.cdc.gov/nchs/nhanes/. Accessed 8 June 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data and code for the MRI sample are deposited on OSF (https://osf.io/j4twn/) (21). NHANES data are publicly available through the National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2011, https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013, and https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2015) (22).