Significance
Plants have evolved to produce an abundance of neuroactive small molecules. Many major classes of these compounds are derived from the amino acid lysine, including the Lycopodium alkaloids, which are produced by club moss species that are traditional herbal medicines in several cultures. In this work, we describe a likely metabolic regulon, or transcriptionally coregulated group of metabolic genes, for the Lycopodium alkaloids in the club moss Phlegmariurus tetrastichus, which we used to identify six enzymes within the biosynthesis of huperzine A (HupA), an acetylcholinesterase inhibitor with clinical interest as a treatment for neurological disease. Our results demonstrate precise transcriptional coregulation of biosynthetic steps within Lycopodium alkaloid metabolism and further uncover diverse enzymatic transformations for lysine-derived alkaloid biosynthesis in plants.
Keywords: biosynthesis, alkaloid, dioxygenase, huperzine A, Lycopodium
Abstract
Plants synthesize many diverse small molecules that affect function of the mammalian central nervous system, making them crucial sources of therapeutics for neurological disorders. A notable portion of neuroactive phytochemicals are lysine-derived alkaloids, but the mechanisms by which plants produce these compounds have remained largely unexplored. To better understand how plants synthesize these metabolites, we focused on biosynthesis of the Lycopodium alkaloids that are produced by club mosses, a clade of plants used traditionally as herbal medicines. Hundreds of Lycopodium alkaloids have been described, including huperzine A (HupA), an acetylcholine esterase inhibitor that has generated interest as a treatment for the symptoms of Alzheimer’s disease. Through combined metabolomic profiling and transcriptomics, we have identified a developmentally controlled set of biosynthetic genes, or potential regulon, for the Lycopodium alkaloids. The discovery of this putative regulon facilitated the biosynthetic reconstitution and functional characterization of six enzymes that act in the initiation and conclusion of HupA biosynthesis. This includes a type III polyketide synthase that catalyzes a crucial imine-polyketide condensation, as well as three Fe(II)/2-oxoglutarate–dependent dioxygenase (2OGD) enzymes that catalyze transformations (pyridone ring-forming desaturation, piperidine ring cleavage, and redox-neutral isomerization) within downstream HupA biosynthesis. Our results expand the diversity of known chemical transformations catalyzed by 2OGDs and provide mechanistic insight into the function of noncanonical type III PKS enzymes that generate plant alkaloid scaffolds. These data offer insight into the chemical logic of Lys-derived alkaloid biosynthesis and demonstrate the tightly coordinated coexpression of secondary metabolic genes for the biosynthesis of medicinal alkaloids.
The production of small-molecule natural products allows plants to modulate interactions with both their biotic and abiotic environment (1). Many of these natural products have toxic bioactivities that deter herbivory of the plant (2), and a striking number do this by targeting neural signaling or perception in animals (3). Humans have long recognized the neurological effects associated with certain plants and have co-opted many plant species and their associated molecules to potently alter the central nervous system (CNS). This includes the use of plants for both recreational purposes (e.g., tobacco, coffee, kava, and marijuana) as well as for the clinical treatment of neurological diseases (e.g., galantamine as a treatment for Alzheimer’s dementia) (4).
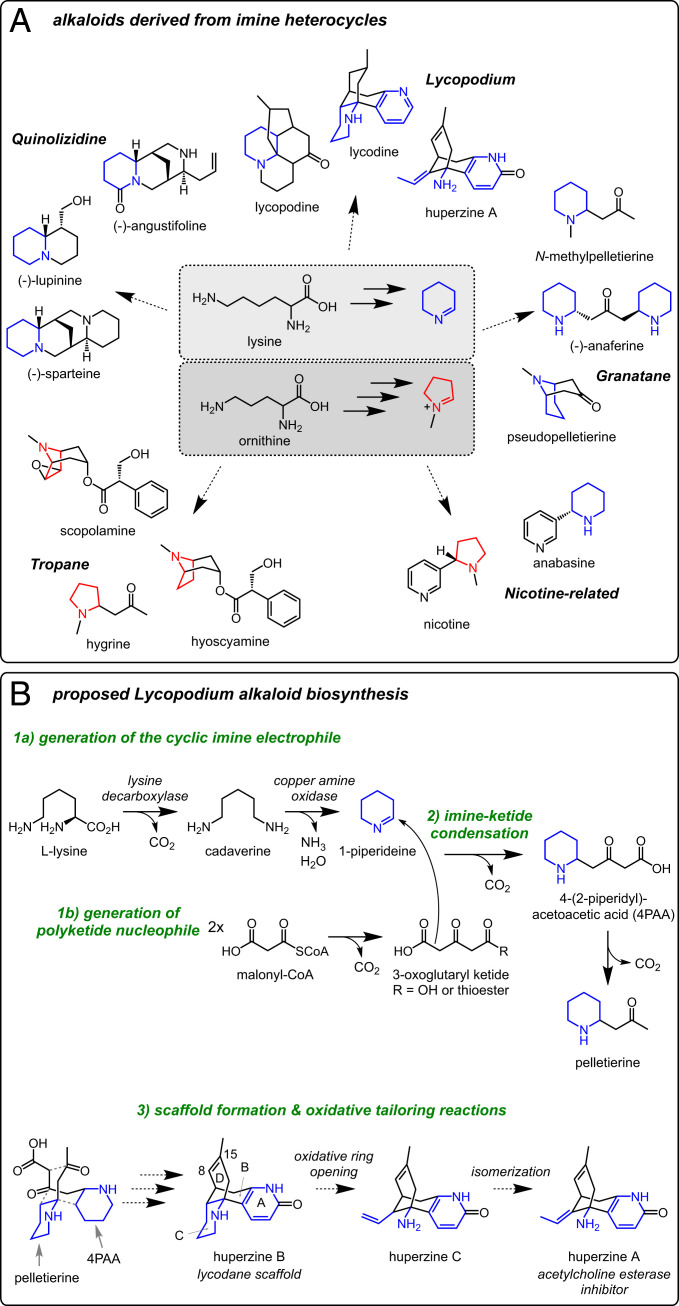
Many CNS-altering natural products are alkaloids, which contain basic nitrogen atoms predominantly derived from amino acid precursors (4, 5). These neuroactive alkaloids seem to mimic amino acid–derived mammalian neurotransmitters and function by interacting with and modulating specific neural signaling proteins (6). Though the structures of alkaloids from across the plant kingdom have notable diversity, many phylogenetically unrelated alkaloid classes are thought to share similar biosynthetic origins (7). For example, aliphatic plant alkaloids are often initially synthesized through the decarboxylation of the amino acids lysine (Lys) or ornithine (Orn) to produce polyamine precursors that are subsequently oxidized to form heterocyclic imines. These imine heterocycles can then be used as building blocks for the construction of diverse, often polycyclic carbon scaffolds. Notably, the inherent reactivity of these imines poises them for condensation with polyketides, phenylpropanoids, and even other heterocycles (7–9). These condensations not only serve as potential committed steps in the biosynthesis of secondary metabolites (8) but also connect distinct branches of plant primary metabolism to generate a wealth of chemically diverse carbon scaffolds (Fig. 1A). Subsequent enzymatic tailoring of these carbon scaffolds (oxidations, rearrangements, acylations, etc) provides an additional mechanism for diversifying these carbon scaffolds even further, thereby constructing the many bioactive small molecules found in plants. Accordingly, natural product biosynthesis requires the use of many diverse chemical transformations and an accompanying set of specialized enzymes, many of which remain unexplored in plants.
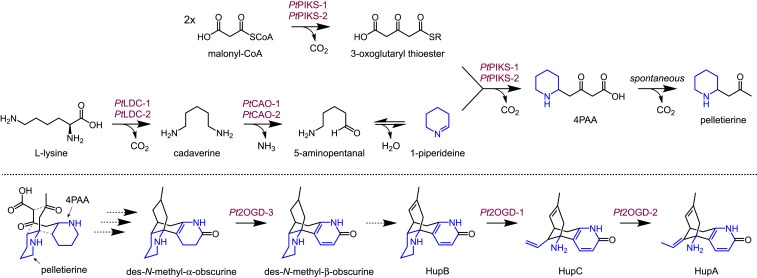
Fig. 1.
Chemical logic of aliphatic alkaloids derived from heterocyclic imines. (A) Representative alkaloids that incorporate lysine- or ornithine-derived imine heterocycles. (B) Proposed chemical transformations for Lycopodium alkaloid biosynthesis, with a focus on huperzine A.
Our collective understanding of plant alkaloid biosynthesis has largely been guided by the study of relatively few classes derived from aromatic amino acids [e.g., benzylisoquinoline, colchicine (10), and monoterpene indole alkaloids (11)]. While pathways from Orn to nicotine and tropane alkaloids have been well studied (12, 13), a relative paucity of information is available for the biosynthesis of alkaloids derived from aliphatic amines, including many Lys-derived alkaloids (14) (Fig. 1A). Accordingly, our ability to biosynthetically reconstitute the production of these molecules, either for manufacture or to study their biological role, is limited by a lack of biosynthetic genes. To expand our understanding of the chemical logic that is used for the biosynthesis of Lys-derived alkaloids, we focused on the presumably conserved biosynthesis of Lycopodium alkaloids, an expansive class (∼300 known structures) produced by club moss species of the Lycopodiaceae, an early diverging clade of vascular plants (15). Club moss species have served as traditional medicinal herbs in many different cultures, particularly for their psychoactive effects, which are now recognized to be largely due to the Lycopodium alkaloids within the plants (16, 17). For example, Huperzia serrata has been used for over a millennium in traditional Chinese medicine to treat a myriad of ailments, including contusions, schizophrenia, and myasthenia gravis (16). This species, as well as several others from the Huperzia and Phlegmariurus genera, produce huperzine A (HupA) (17), a Lycopodium alkaloid that has been explored as a treatment for neurological diseases due to its ability to reversibly inhibit acetylcholine esterase (AChE) (18), which presumably causes some of the bioactivity attributed to this medicinal plant (19).
The Lycopodium alkaloids have intrigued chemists for over a century (20, 21), due in large part to their complex, polycyclic carbon scaffolds, as well as their potential pharmaceutical use. Critically, extensive isotope labeling studies with predicted precursors have defined a plausible metabolic route for these alkaloids (15) (Fig. 1B). This past work has determined that Lys is processed into a heterocyclic imine (22–24), which is subsequently coupled to a polyketide substrate (25–28), thereby generating the initial building blocks for Lycopodium alkaloid formation. Previous efforts have uncovered putative enzymes for these initial stages (29–32), but our overall understanding of the Lycopodium alkaloid biosynthetic machinery was still largely incomplete. Here, we use a combination of long-read and short-read RNA sequencing (RNA-seq) to develop a robust transcriptomic data set for Phlegmariurus tetrastichus, a previously unsequenced club moss that produces HupA at high levels (33). Through paired transcriptomic and metabolomic analyses, we identified a highly coexpressed set of biosynthetic enzymes that appear to constitute a Lycopodium alkaloid biosynthetic regulon, and we further determined biochemical functions for six of these enzymes via heterologous biosynthetic reconstitution. Our results establish both early and terminal reactions in the biosynthesis of HupA and provide insight into the regulation of neuroactive alkaloid biosynthesis.
Results
Metabolomics and Transcriptomics of P. tetrastichus.
To guide our study of Lycopodium alkaloid biosynthesis, we focused on HupA due to its known activity as an AChE inhibitor and potential as a pharmaceutical (19). HupA is produced by species within the Huperzia and Phlegmariurus genera, but the level of HupA accumulation varies drastically within these species (34). We hypothesized that increased HupA in a given species will likely be associated with up-regulated biosynthetic gene expression. Therefore, we measured HupA content in several species from the Phlegmariurus genus, which accumulate HupA to high levels (33), and found that P. tetrastichus (Fig. 2A) accumulated HupA to the highest level (SI Appendix, Fig. S1). Thus, we focused our discovery efforts in this species. Though HupA accumulates to a reasonably high level in all tissues within the plant, we had previously established through D2O labeling studies that de novo HupA biosynthesis is specific to new growth in the shoots of P. tetrastichus (35). We confirmed through D2O labeling studies that active biosynthesis of HupA is only found within the new growth of the shoot (although other primary metabolites are actively made throughout the shoot) (35). These experiments also revealed that HupA labeling was more extensive in the leaves of this region compared to the stem, suggesting an enrichment of biosynthetic gene expression here (Fig. 2B). This analysis was critical, as it indicated that differential RNA-seq analysis should reveal enrichment of Lycopodium alkaloid biosynthetic genes in the active tissues. Having confidence in a site of active HupA biosynthesis, we then performed RNA-seq on multiple tissues from this species, including both biosynthetic and nonbiosynthetic tissues.
Fig. 2.
Metabolomics and transcriptomics of a HupA-producing plant. (A) Tissues from P. tetrastichus were dissected for paired metabolite analysis and RNA-seq. (B) Deuterium labeling experiments using 20% D2O (right half of bar graph) compared to a nondeuterated control (100% H2O, left half of bar graph) determine active, de novo biosynthesis of HupA in the shoots of P. tetrastichus. Location of tissue types (e.g., “1-leaf”) are defined in panel “a”. Statistical comparisons between tissues were made using a one-way ANOVA and Tukey’s multiple comparisons test. Different letters indicate P < 0.05 between tested data sets. (C) RNA-seq of multiple tissue types and Pearson’s correlation using PtLDC-1 as the query gene identifies a set of strongly coexpressing biosynthetic gene candidates out of 46,049 unique transcripts. Genes found to have a role in Lycopodium alkaloid biosynthesis are shown in bold. Expression values within the heat map represent log2-transformed fold changes relative to the median expression value for a given transcript. Initial expression values were calculated as Trimmed mean of M (TMM)-normalized counts per million (CPM).
Transcriptomic-guided, differential expression analyses have proven to be very effective for the discovery of genes within plant biosynthetic pathways (10, 36). However, the ability to perform robust gene expression analysis from plants lacking a sequenced genome relies upon the availability of a protein-coding transcriptome, which has traditionally been accessed via de novo assembly of short reads from Illumina sequencing. While this method can be used successfully, it often results in fragmented or misassembled contigs that confound transcriptomic analyses (37). To avoid the technical limitations of de novo transcriptome assembly, we utilized PacBio Single Molecule Real-time (SMRT) sequencing to obtain full-length complementary DNA (cDNA) reads for RNA isolated from P. tetrastichus tissue, thus generating a reference transcriptome (46,049 transcripts after processing; SI Appendix). We then utilized Illumina sequencing with libraries from multiple plant tissues (both biosynthetic and nonbiosynthetic) to quantify relative expression within this transcriptome (raw sequencing data has been deposited in the Sequence Read Archive with the BioProject ID PRJNA731132) (38).
Generation of Piperidyl-Ketide Intermediates.
The generation of heterocyclic imines in plants, such as the proposed 1-piperideine precursor to Lycopodium alkaloids, involves the decarboxylation of Lys/Orn amino acids, followed by oxidation of the resulting polyamine, both of which appear to be common metabolic processes in plants (39). The generation of polyamines is ubiquitous in plants and known to be catalyzed through the action of PLP-dependent decarboxylase enzymes that act on Orn or Lys; however, lysine decarboxylase (LDC) enzymes seem to have specifically evolved for secondary metabolism in certain lineages (29, 40). The oxidation of these polyamines is commonly catalyzed by copper amine oxidase (CAO) enzymes, and this activity is critical for numerous aspects of plant development and stress responses (41). Labeling studies have indicated that Lycopodium alkaloid biosynthesis is initiated via decarboxylation of L-Lys to form cadaverine (22, 23), suggesting the involvement of an LDC enzyme from the group IV pyridoxal-dependent decarboxylase protein family (40, 42) (Fig. 1B). Subsequent oxidation of cadaverine, an activity known to be catalyzed by CAO enzymes (41), would then ultimately yield 1-piperideine, the cyclic imine precursor to the Lycopodium alkaloids (24).
Within the Lycopodiaceae, enzymes with LDC and CAO activity have been biochemically characterized (29–31, 43). Of the characterized LDCs (LcLDC from Lycopodium clavatum and HsLDC-X1 from H. serrata), only LcLDC showed a notable substrate preference for lysine over ornithine (29, 30). Though an ortholog of LcLDC has been identified in H. serrata (81% amino acid identity to LcLDC), this seems to be distinct from HsLDC-X1 (59% amino acid identity) (30, 43). Furthermore, the previously assessed CAO (HsCAO from H. serrata) did not appear to have specificity for cadaverine, suggesting it may not be dedicated to Lycopodium alkaloid metabolism (31). In an effort to better understand the genes that are involved in the early steps of this pathway, we were first interested in further investigating LDC and CAO genes from P. tetrastichus.
As an initial assessment of our transcriptome, we searched for LDC genes that showed high and specific expression within new growth tissues. This analysis revealed two LDC homologs (PtLDC-1 and PtLDC-2; 84% amino acid identity) that met these criteria (Fig. 2C and SI Appendix, Fig. S2), and these appeared to be orthologs of LcLDC (∼75% amino acid identity for each) (29). Encouraged by the tissue-specific expression of these LDC genes, we performed Pearson correlation analyses of our transcript expression data using PtLDC-1 as the query gene. This analysis demonstrated that two related CAO genes (PtCAO-1 and PtCAO-2; 88% amino acid identity) were within the top 25 highest-correlating transcripts (out of over 46,000 transcripts, Fig. 2C and SI Appendix, Fig. S2). These appeared to be unique CAO genes, as both PtCAO-1 and PtCAO-2 shared less than 30% amino acid identity with HsCAO. An ortholog of HsCAO could be found in our transcriptome (83% amino acid identity), but this gene had relatively consistent expression across all tissue types (SI Appendix, Fig. S2). Accordingly, the HsCAO ortholog did not share strong coexpression with the identified LDC genes, suggesting that this CAO may not be specific to Lycopodium alkaloid biosynthesis. Conversely, the strong coexpression of PtCAO homologs with PtLDC genes supports their involvement within the same metabolic pathway, presumably toward the biosynthesis of Lycopodium alkaloids.
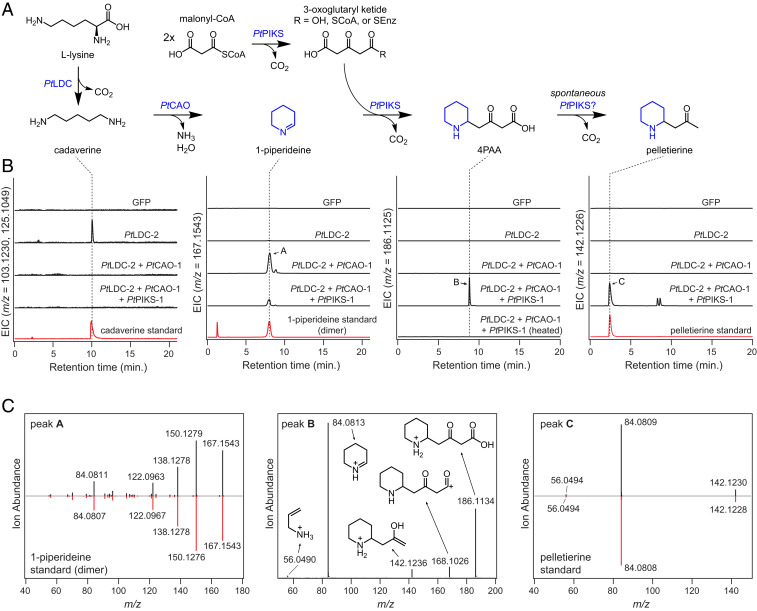
To confirm the functions of the PtLDC and PtCAO genes, they were cloned from cDNA and expressed individually and in tandem within Nicotiana benthamiana leaves via Agrobacterium-mediated transient expression. Leaf extracts were then analyzed via liquid chromatography-mass spectrometry (LC-MS) to assess changes in metabolite accumulation. In agreement with previous characterization of LcLDC (29), both PtLDC homologs yielded elevated levels of cadaverine when expressed in N. benthamiana (Fig. 3). Coexpression of either PtCAO homolog with PtLDC homologs led to consumption of the generated cadaverine and production of 1-piperideine dimer and trimer (Fig. 3 and SI Appendix, Figs. S3 and S4). It has previously been noted that 1-piperideine will spontaneously self-react to form these structures (44–46) (SI Appendix, Fig. S3), and thus we propose that PtCAO-1 and PtCAO-2 act as cadaverine oxidases to produce 5-aminopentanal, which can spontaneously cyclize to form 1-piperideine. While PtLDC-1 and PtLDC-2 appeared to be functional orthologs of LcLDC (SI Appendix, Fig. S5), we did not detect consumption of cadaverine or production of 1-piperideine when the previously characterized CAO gene (HsCAO) (31) was coexpressed within the transient coexpression system (SI Appendix, Fig. S5). The lack of activity from HsCAO in our system, together with the observed activity and expression pattern of PtCAO-1 and PtCAO-2, suggests that the enzymes we identified are likely the specialized CAO enzymes used within Lycopodium alkaloid biosynthesis. The PtLDC and PtCAO variants described here were functionally redundant within our transient expression system (SI Appendix, Fig. S4), and we utilized PtLDC-2 and PtCAO-1 for 1-piperideine production in all subsequent experiments.
Fig. 3.
A reconstituted pathway for pelletierine biosynthesis. (A) Proposed biosynthetic pathway for 4PAA and pelletierine biosynthesis via a combination of three genes (PtLDC-2, PtCAO-1, and PtPIKS-1) expressed transiently in N. benthamiana. The PtLDC, PtCAO, and PtPIKS homologs identified in this study are functionally analogous, as shown in SI Appendix, Fig. S4, and thus, the results of this figure are representative for each homolog. (B) LC–MS chromatograms demonstrating the heterologous production of the pathway intermediates cadaverine ([M + H]+ = m/z 103.1230, [M + Na]+ = m/z 125.1049), 1-piperideine (observed as dimer, [M + H]+ = m/z 167.1543), 4PAA ([M + H]+ = m/z 186.1125), and pelletierine ([M + H]+ = m/z 142.1226) with comparison to authentic standards, where applicable. EIC = extracted ion chromatogram. (C) MS/MS fragmentation of three products produced within this biosynthetic reconstitution: 1-piperideine dimer (m/z 167.1543, 20V), 4PAA (m/z 186.1125, 20V), and pelletierine (m/z 142.1226, 20V). The MS/MS spectra for putative 1-piperideine and pelletierine are compared to those of authentic standards, while proposed ion fragment structures are shown for putative 4PAA.
Biosynthesis is next predicted to proceed through the addition of a malonyl-CoA–derived polyketide chain to 1-piperideine to produce 4-(2-piperidyl)acetoacetic acid (4PAA), which could decarboxylate to produce pelletierine (24–28) (Fig. 1B). The production of a polyketide substrate suggests the involvement of a polyketide synthase (PKS) enzyme in catalyzing this imine-ketide condensation. Indeed, recent work on tropane alkaloid biosynthesis has demonstrated that a novel type III PKS (pyrrolidine ketide synthase; PYKS) catalyzes an analogous condensation of a five-membered cyclic imine derived from Orn with two units of malonyl-CoA (47). This led us to hypothesize that a type III PKS enzyme could be acting in the biosynthesis of Lycopodium alkaloids as a putative piperidyl-ketide synthase (PIKS). Further analysis of our transcriptomic data revealed two PKS candidate genes (PtPIKS-1 and PtPIKS-2; 82% amino acid identity) that were among the top coexpressing genes to PtLDC-1 (Fig. 2C). Transient coexpression in N. benthamiana leaves of either PtPIKS-1 or PtPIKS-2 with PtLDC-2 and PtCAO-1 led to the consumption of 1-piperidiene metabolites and production of several new compounds. One of these compounds possessed an exact mass that corresponded to the putative Lycopodium alkaloid precursor pelletierine ([M + H]+= m/z 142.1226) (24, 25), and this was verified via comparison to a synthesized standard (Fig. 3). Additionally, we observed production of a compound with the same exact mass as 4PAA ([M + H]+= m/z 186.1125), for which structural assignment was supported by tandem mass spectrometry (MS/MS) fragmentation (Fig. 3). We considered that spontaneous decarboxylation of 4PAA, a β-keto acid, would lead to the production of pelletierine; indeed, heating of N. benthamiana leaf extracts led to a depletion of this metabolite and a corresponding increase in pelletierine (Fig. 3 and SI Appendix, Fig. S6). We also observed two peaks with mass signatures and MS/MS fragmentation patterns that match those expected for anaferine diastereomers ([M + H]+= m/z 225.1961; SI Appendix, Fig. S7). Similar to what has been observed in tropane alkaloid biosynthesis, the formation of anaferine would result from decarboxylative condensation of 4PAA with 1-piperideine (47) (SI Appendix, Fig. S7). Collectively, our results indicate that PtPIKS-1 and PtPIKS-2 are likely the key enzymes for forming 4PAA and pelletierine in Lycopodium alkaloid biosynthesis. This is in agreement with recent work that identified PIKS orthologs from two other Lycopodiaceae species with the same biosynthetic capacity (HsPKS4 from H. serrata, an ortholog of PtPIKS-2, 93% amino acid identity; PcPKS1 from Phlegmariurus cryptomerianus, an ortholog of PtPIKS-1, 97% amino acid identity) (32).
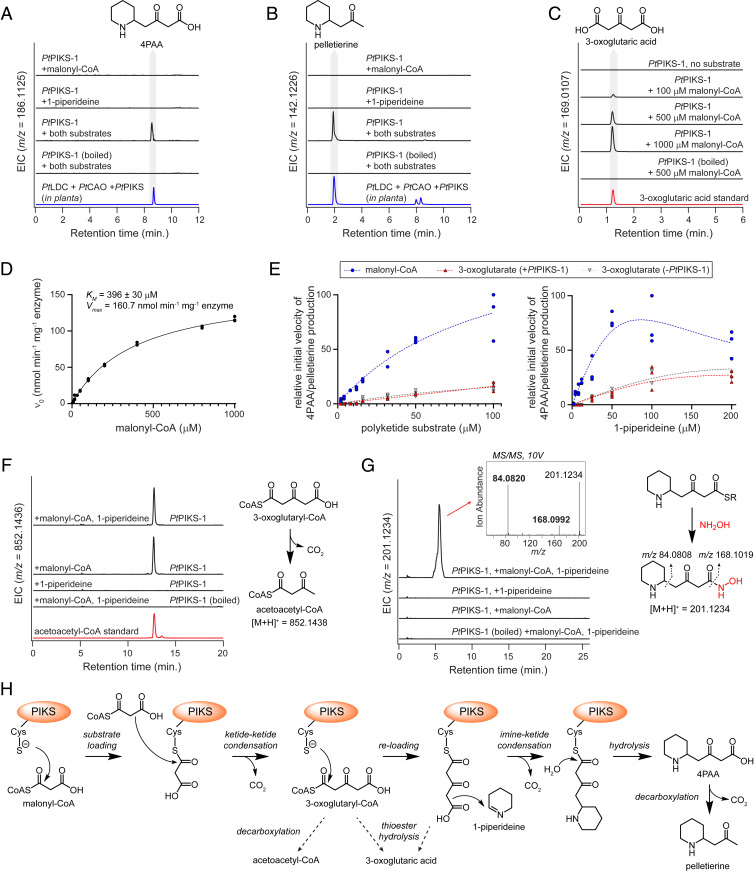
The PIKS enzymes identified here belong to the chalcone/stilbene synthase protein superfamily. The archetypical enzyme within this family, chalcone synthase (CHS), catalyzes the condensation of a phenylpropanoid CoA starter unit with three malonyl-CoA units to yield a key flavonoid precursor (48). Though variations on this mechanism exist throughout this protein family (SI Appendix, Fig. S8), the condensation of an imine heterocycle with a malonyl-CoA–derived ketide chain represents a relatively unique variation in the biochemical repertoire for this enzyme family, as PIKS and PYKS orthologs from Lycopodium and tropane alkaloid biosynthesis, respectively, are the only PKS enzymes yet found to have a role in such condensations. To better understand the mechanism of this imine-ketide condensation, purified protein was obtained for both PtPIKS homologs for in vitro analysis. Consistent with our results in N. benthamiana, both PtPIKS-1 and PtPIKS-2 produced 4PAA and pelletierine within in vitro assays when 1-piperideine and malonyl-CoA were added as substrates (Fig. 4 A and B and SI Appendix, Fig. S9), in addition to several other unknown compounds that we predict to be side reactions of product with 1-piperideine substrate (SI Appendix, Fig. S10). While no obvious products were observed when 1-piperideine was incubated alone as a substrate, addition of only malonyl-CoA to PtPIKS-1 led to accumulation of 3-oxoglutaric acid (Fig. 4 C and D), much like what was observed in analogous experiments with PYKS from Anisodus acutangulus (AaPYKS) (49), as well as the other described PIKS orthologs (32). The accumulation of 3-oxoglutaric acid suggests that PIKS is catalyzing the condensation of two malonyl-CoA units into a thioester-linked 3-oxoglutaryl moiety, and that this polyketide is either enzymatically or nonenzymatically hydrolyzed from the thioester linkage.
Fig. 4.
In vitro analysis of the PtPIKS-catalyzed imine-ketide condensation. (A) In vitro reactions with PtPIKS-1 produce 4PAA ([M + H]+ = m/z 186.1125) and (B) pelletierine ([M + H]+ = m/z 142.1226) when malonyl-CoA and 1-piperideine are used as substrates. (C) Addition of malonyl-CoA alone as a substrate leads to accumulation of 3-oxoglutaric acid ([M + Na]+ = m/z 169.0107). (D) Michaelis–Menten kinetics for the PtPIKS-catalyzed formation of 3-oxoglutaric acid. n = 3 replicates for each substrate concentration. (E) Comparison of the initial velocity for the imine-ketide condensation when either malonyl-CoA or 3-oxoglutaric acid was provided as the polyketide cosubstrate with 1-piperideine. The sum total of 4PAA and pelletierine ion abundance was used as the metric for product accumulation. Dotted lines only represent a visual summary of the data. Reference SI Appendix, Fig. S10 for additional details. (F) Detection of acetoacetyl-CoA ([M + H]+ = m/z 852.1436) when malonyl-CoA is included as a substrate. (G) Treatment of PtPIKS-1 reactions with hydroxylamine (NH2OH) allows for the capture of a potential 4PAA thioester intermediate. The structure of the putative hydroxamic acid derivative ([M + H]+ = m/z 201.1234) is supported by MS/MS fragmentation. (H) Proposed biosynthetic mechanism for the PtPIKS-catalyzed formation of 4PAA and pelletierine.
It has been proposed that the imine-ketide condensations observed in Lycopodium and tropane alkaloid biosynthesis occur via the nonenzymatic decarboxylative condensation of 3-oxoglutaric acid with the cyclic imine cosubstrate (32, 49). Even though this nonenzymatic condensation is possible, we wanted to further assess the relevance of 3-oxoglutaric acid as a biosynthetic intermediate. To do this, we compared the reaction rate for 4PAA/pelletierine production when either 3-oxoglutaric acid or malonyl-CoA was paired with 1-piperideine within in vitro reactions containing PtPIKS-1. Although spontaneous condensation of 1-piperideine and 3-oxoglutaric acid was observed at low levels, the rate of product formation was dramatically greater when malonyl-CoA was used as a cosubstrate with 1-piperideine (Fig. 4E and SI Appendix, Fig. S10). Moreover, the presence of PtPIKS-1 did not appear to affect the rate of condensation for 3-oxoglutaric and 1-piperideine at the measured substrate concentrations (2 to 200 µM; Fig. 4E), while condensation between malonyl-CoA and 1-piperideine was clearly enzyme dependent (Fig. 4 A and B). Thus, while the spontaneous condensation between 1-piperideine and 3-oxoglutaric acid can occur at low levels, our data support a model in which the PIKS-catalyzed condensation occurs between 1-piperideine and a polyketide thioester derived from malonyl-CoA. In support of this, we could detect accumulated acetoacetyl-CoA when malonyl-CoA was included as a substrate for PtPIKS-1 in vitro (Fig. 4F). Though indirect, the accumulation of acetoacetyl-CoA suggests production of a 3-oxoglutaryl-CoA metabolite that rapidly undergoes decarboxylation. We were unable to detect 3-oxoglutaryl-CoA directly and speculate that this is due to the transient, unstable nature of this intermediate. Interestingly, acetoacetyl-CoA undergoes enzyme-independent condensation with 1-piperideine to yield pelletierine (SI Appendix, Fig. S11). However, this condensation does not yield 4PAA, and thus its relevance within Lycopodium alkaloid biosynthesis is yet unclear.
No metabolites pertaining to a 4PAA-CoA thioester could be detected via LC-MS analysis. Thus, we considered the possibility that the imine-ketide condensation occurs following reloading of the 3-oxoglutaryl moiety onto the catalytic cysteine of PtPIKS. To investigate the presence of any acyl intermediates bound to the catalytic cysteine of PtPIKS-1, we treated in vitro enzyme reactions with hydroxylamine, which has been utilized to capture thioester intermediates as the corresponding hydroxamic acid derivative (50). When both malonyl-CoA and 1-piperideine were supplied as substrate, we noted the hydroxylamine-dependent appearance of a mass corresponding to the hydroxamic acid of 4PAA ([M + H]+ = m/z 201.1234, Fig. 4G and SI Appendix, Fig. S12). This observation, along with the inability to detect a 4PAA-CoA metabolite, suggests the presence of a transient enzyme-bound 4PAA thioester.
Collectively, these data support a tentative mechanism (Fig. 4H) for this imine-ketide condensation: 1) loading of malonyl-CoA onto the catalytic cysteine of PIKS; 2) Claisen condensation of a second malonyl-CoA nucleophile with the enzyme-bound malonyl group, thus forming 3-oxoglutaryl-CoA; 3) reloading of the 3-oxoglutaryl moiety onto the enzyme; 4) decarboxylative condensation of the enzyme-bound polyketide with 1-piperideine, producing an enzyme-bound 4PAA; and 5) thioester hydrolysis to yield 4PAA, which can spontaneously decarboxylate under mild conditions to form pelletierine. While additional experimentation will be necessary to uncover the precise order of events and mechanistic details of this reaction, our data demonstrate the critical role for PIKS in catalyzing the imine-ketide condensation in Lycopodium alkaloid biosynthesis.
Late-Stage Oxidative Processing of HupB.
Although labeling studies have not confirmed any downstream intermediates in HupA biosynthesis, isolation of alkaloids from various club moss species has led to a biosynthetic hypothesis for the incorporation of two 8-carbon, piperidine-containing subunits into the Lycopodium alkaloids (22, 24) (Fig. 1B). While the decarboxylative condensation of 4PAA and pelletierine would satisfy this, it was not immediately apparent what class of enzyme could utilize these substrates for constructing the Lycopodium alkaloid scaffold, nor were we certain that these compounds are utilized directly as substrates without further modification. Thus, as an alternative approach to stepwise discovery from the beginning of the pathway, we reasoned that downstream Lycopodium alkaloid biosynthesis could be assessed independently by screening enzyme candidates against isolated Lycopodium alkaloid substrates, which can be coinfiltrated with transiently expressed biosynthetic genes (10, 36).
The identification of three highly coexpressed genes for the biosynthesis of 4PAA/pelletierine suggested that our combined approach of metabolomics and transcriptomics had uncovered a Lycopodium alkaloid biosynthetic regulon, that is, a transcriptionally coregulated group of biosynthetic genes (51). Indeed, along with lysine biosynthetic genes (diaminopimelate epimerase, dihydropicolinate reductase, and diaminopimelate decarboxylase), which would be required to maintain high levels of this amino acid precursor, many putative oxidoreductase enzymes, presumed to be involved in downstream scaffold tailoring reactions, were among the top coexpressing candidates with PtLDC, PtCAO, and PtPIKS genes (Fig. 2C). The most notably enriched class of enzymes were Fe(II)/2-oxoglutarate–dependent dioxygenases (2OGDs), which represented nearly one-third of the top 23 coexpressing transcripts to PtLDC (Fig. 2C). Several distinct oxidations and scaffold tailoring reactions would be required to eventually form the characteristic structure of HupA from a proposed lycodane carbon scaffold (Fig. 1B), and thus we considered these highly coexpressing 2OGDs to be strong candidates within downstream Lycopodium alkaloid metabolism.
Multiple Lycopodium alkaloids that naturally cooccur with HupA, including huperzine B (HupB) (19) and huperzine C (HupC) (52), appear to be logical pathway intermediates for its production and thus may be potential precursors (15) (Fig. 1B). Although neither of these molecules had previously been validated as HupA precursors, both HupB and HupC could be detected in the new growth of P. tetrastichus (SI Appendix, Fig. S13), which supports their viability as potential intermediates in planta. The proposed transformation of HupB would require the oxidative cleavage of the C-ring piperidine moiety (Fig. 1B; reference SI Appendix, Fig. S14 for full carbon numbering and ring annotations), which necessitates the breaking of both carbon–carbon (C–C) and carbon–nitrogen (C–N) bonds. As such, we first considered the aforementioned 2OGDs, as well as cytochromes P450 (CYPs) and flavin-containing monooxygenases (FMOs), as these enzyme families have been shown to catalyze cleavage of C–C and C–N bonds and are capable of performing a wide diversity of oxidative chemical transformations (53–57). Candidate genes from these families were selected based upon having high coexpression with PtLDC, PtCAO, and PtPIKS genes (SI Appendix, Fig. S15), and these enzymes were then tested heterologously in N. benthamiana in combined batches with coinfiltration of HupB substrate. Importantly, this strategy enabled high throughput of enzyme testing, as multiple gene constructs can readily be coexpressed in N. benthamiana simultaneously (10, 36, 58).
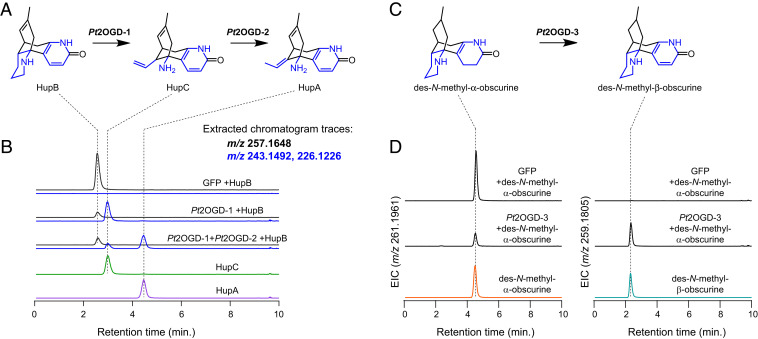
In an initial experiment, we observed consumption of coinfiltrated HupB by a panel of eight transiently coexpressed 2OGDs. This consumption of HupB was concomitant with production of both HupC and HupA ([M + H]+ = m/z 243.1492, [M − NH2]+ = m/z 226.1226 for each compound), which were each verified by comparison to authentic standards and published MS/MS data (59) (SI Appendix, Fig. S16). This suggested that one 2OGD, or a combination of those tested, was capable of performing the ring cleavage of HupB, as well as a double bond isomerization to interconvert HupC and HupA. Subsequent testing identified one 2OGD enzyme (Pt2OGD-1) that consumed HupB and produced HupC, while a second 2OGD (Pt2OGD-2) consumed HupC to yield HupA (Fig. 5 and SI Appendix, Fig. S17). These functionalities were further confirmed via in vitro assays with purified Pt2OGD-1 and Pt2OGD-2 (SI Appendix, Fig. S18), which also demonstrated the canonical dependence of 2-oxoglutarate, iron, and ascorbate for these enzymes. Thus, a pair of 2OGD enzymes is responsible for converting HupB into HupA in Lycopodium alkaloid biosynthesis via stepwise oxidative ring cleavage and, remarkably, a redox-neutral double bond isomerization. Furthermore, though HupB and HupC are consistently found within HupA-producing plants (17, 19), their status within the context of HupA biosynthesis had not been shown, and thus our results demonstrate their intermediacy within this pathway.
Fig. 5.
Three 2OGD enzymes catalyze crucial transformations in HupA biosynthesis. (A) Proposed biosynthesis of HupA from HupB, with HupC as a logical intermediate. (B) LC–MS chromatograms demonstrate the consumption of HupB ([M + H]+ = m/z 257.1648) by Pt2OGD-1, which produces HupC ([M + H]+ = m/z 243.1492, [M − NH2]+ = m/z 226.1226). HupC is then consumed by Pt2OGD-2 to produce HupA ([M + H]+ = m/z 243.1492, [M − NH2]+ = m/z 226.1226). (C) Proposed conversion of des-N-methyl-α-obscurine (DNMAO) to des-N-methyl-β-obscurine (DNMBO). (D) Conversion of DNMAO ([M + H]+ = m/z 261.1961) to DNMBO ([M + H]+ = m/z 259.1805) catalyzed by Pt2OGD-3, as shown here by LC–MS chromatograms. See SI Appendix for the corresponding MS/MS data of each compound.
Encouraged by these results, we next assessed our ability to define other downstream biosynthetic transformations. In particular, two Lycopodium alkaloids, des-N-methyl-α-obscurine (DNMAO) and des-N-methyl-β-obscurine (DNMBO), share the same carbon scaffold as HupB and appear to be logical precursors (Fig. 5). Specifically, initial desaturation of ring A on DNMAO would form the aromatic pyridone ring of DNMBO, which in turn could then be further desaturated on ring D to produce HupB. Although access to authentic compounds can often be an obstacle in the study of plant specialized metabolism, we were able to obtain ample quantities of DNMAO and DNMBO, which were purified from large-scale extractions of HupA-producing plants (60). In a similar screening method to that described above, we found that another distinct 2OGD enzyme (Pt2OGD-3) acts to convert DNMAO into DNMBO (Fig. 5 and SI Appendix, Fig. S17), thus forming the pyridone ring ultimately found in HupA. Interestingly, we also found that Pt2OGD-1 and Pt2OGD-2 can act sequentially on DNMBO to produce the presumed 8,15-dihydro congeners of HupC and HupA (SI Appendix, Fig. S19). Pt2OGD-1 did not act on DNMAO, suggesting that presence of the pyridone ring found in DNMBO and HupB is critical for Pt2OGD-1 substrate binding and/or catalytic activity. While we were unable to identify an enzyme that desaturates DNMBO or either of the dihydro congeners of HupC and HupA, it is possible that the 8,15-double bond in HupB and other downstream alkaloids is a relic of scaffold formation (SI Appendix, Fig. S20). Enzymatic saturation of this double bond following scaffold generation could then produce DNMAO and/or DNMBO. Though enzyme-catalyzed saturations of unactivated double bonds are possible (61), these reactions are rare. Thus, it may be more plausible that early reduction of intermediates prior to tetracyclic scaffold formation results in a saturated 8,15-bond (SI Appendix, Fig. S20) and that we have simply not yet cloned and tested the correct desaturase gene or provided the appropriate substrate(s).
All three of the 2OGDs identified in this work were among the top 25 correlating genes to PtLDC-1 without any additional filters being applied to the transcriptomic data (Fig. 2C). Importantly, these results provide further support that our coexpression analysis has identified a Lycopodium alkaloid biosynthetic regulon and that the identified genes are likely acting within the same metabolic pathway. Although we do not know the nature of the transcriptional control for these coexpressed genes, their striking coexpression and involvement in a specific metabolic pathway would suggest that they are likely under similar transcriptional regulation (51). Overall, through our combined use of metabolomic and transcriptomic analyses, we have identified a tightly coexpressed module of Lycopodium alkaloid metabolic genes, thereby enabling our discovery of several biosynthetic enzymes within the biosynthesis of HupA (Fig. 6).
Fig. 6.
Summary of characterized HupA biosynthetic reactions. The gray dotted lines between 4PAA and pelletierine in the bottom scheme represent presumed bonds to be formed during scaffold generation. For a detailed biosynthetic proposal, reference SI Appendix, Fig. S20.
Discussion
The generation of many Lys-derived alkaloids requires both the generation of cyclic imine and polyketide precursors, as well as their subsequent condensation. Through our study of the Lycopodium alkaloids, we have identified a transcriptionally coregulated three-enzyme system that recapitulates the required activity needed to produce 4PAA and pelletierine, two presumed precursors to this class of alkaloids. Along with orthologs to the previously characterized LDC from L. clavatum (PtLDC-1 and PtLDC-2) (29), we have uncovered distinct, previously uncharacterized CAO homologs (PtCAO-1 and PtCAO-2) that appear to be specific for Lycopodium alkaloid biosynthesis, as well as two type III PKS (PtPIKS-1 and PtPIKS-2) that generate the polyketide substrate and catalyze the crucial imine-ketide condensation. The biosynthesis of 4PAA and pelletierine represents a key, defining step in the production of Lycopodium alkaloids. This reaction not only bridges two different aspects of plant metabolism (amino acid and polyketide) but also represents a committed biosynthetic step that distinguishes the secondary metabolism of Lycopodium alkaloids from the primary metabolites from which they derive, as generation and oxidation of polyamines is common throughout the plant kingdom (39).
The next major step in Lycopodium alkaloid biosynthesis is the generation of a 16-carbon scaffold. The biochemistry of this transformation remains somewhat unclear in terms of both substrates and the relevant enzymes that may be involved. While labeling studies have confirmed that pelletierine (8 carbons) is incorporated into one “half” of the 16-carbon scaffold (24, 25), the precursor for the other “half” has not been determined. However, isotope labeling has strongly suggested that the other “half” is likely to be derived from 4PAA or a very similar compound (24) (Fig. 1B and SI Appendix, Fig. S20). Additionally, though labeling studies establish pelletierine as a precursor to the Lycopodium alkaloids, it has not been determined whether this substrate needs to be further processed prior to scaffold formation. Thus, additional work will be necessary to understand the biosynthetic transformations that yield the 16-carbon scaffold precursor to these alkaloids. Similarly, additional pathway discovery will be required to connect formation of the lycodane-type scaffold to the downstream oxidation and tailoring steps identified here. This includes finding enzymes for the formation of the A-ring lactam, as well as identification of an 8,15-desaturase enzyme to produce the D-ring double bond. However, it is unclear whether this bond is reduced or oxidized following scaffold formation. Thus, it is possible that either 1) the 8,15-double bond is a vestige of scaffold formation and that DNMAO and DNMBO are products of double bond hydrogenation by a reductase enzyme, or 2) the 8,15-bond is saturated after scaffold formation as a result of earlier reduction of scaffold precursors, and thus a desaturase enzyme is required to form the 8,15-double bond (SI Appendix, Fig. S20).
Overall, the initial steps in Lycopodium alkaloid biosynthesis are strikingly similar to those recently reported within the production of tropane alkaloids such as hyoscyamine and scopolamine (47, 49). Of particular note is the similarity in enzymatic function between PIKS (Lycopodium alkaloid biosynthesis) and PYKS (tropane alkaloid biosynthesis), as both enzymes act to promote the condensation of a polyketide substrate with a cyclic imine. To accomplish this, each enzyme appears to initially catalyze the condensation of two malonyl-CoA units, which would result in the formation of 3-oxoglutaryl-CoA. In agreement with this, 3-oxoglutaric acid, a presumed hydrolysis product of the corresponding thioester, can be detected as a byproduct of both PIKS and PYKS. It has been postulated for both Lycopodium and tropane alkaloid biosynthesis that product formation occurs via nonenzymatic decarboxylative condensation of 3-oxoglutaric acid with the cyclic imine (32, 49). However, we have shown that PIKS not only generates the requisite polyketide substrate but also catalyzes the condensation of this ketide with 1-piperideine, an important distinction for this crucial step in Lycopodium alkaloid biosynthesis. Specifically, our data show that the rate of 4PAA/pelletierine biosynthesis is considerably greater when malonyl-CoA is used as a substrate in place of 3-oxoglutaric acid, especially at low (micromolar) concentrations (Fig. 4E and SI Appendix, Fig. S10). Moreover, the use of malonyl-CoA in this condensation reaction is clearly enzyme dependent (Fig. 4 A–C). This suggests that a thioester-linked 3-oxoglutaryl moiety, and not the free diacid, is the major nucleophile that condenses with 1-piperideine, which is supported by our indirect detection of relevant thioester intermediates (Fig. 4 F and G). Interestingly, structural studies with PYKS have identified an enzyme-bound 3-oxoglutaryl thioester (49), which suggests reloading of the 3-oxoglutaryl group from coenzyme A onto the enzyme after one round of malonyl-CoA condensation. Though PIKS and PYKS likely evolved independently (32), the similarity of reactions suggests that an enzyme-linked 3-oxoglutaryl thioester may be relevant within Lycopodium alkaloid biosynthesis as well (Fig. 4H). Indeed, all identified PIKS orthologs (PtPIKS-1, PtPIKS-2, HsPKS4, and PcPKS1) possess an analogous active-site arginine residue to that found in AaPYKS [R134 in AaPYKS (49)], which appears to be critical for formation and stabilization of the enzyme-bound 3-oxoglutaryl thioester (32). Structural modeling of HsPKS4 and PcPKS1 using the AaPYKS crystal structure suggests that this arginine is similarly poised within the PIKS active site, which may indicate a similar mechanism for PIKS and PYKS enzymes within imine-ketide condensations (32). While future efforts will be needed to refine the mechanistic details of these PKS-catalyzed condensations, we have shown that the imine-ketide condensation in Lycopodium alkaloid biosynthesis is enzyme catalyzed, and we further speculate that the same is true within biosynthesis of the tropane alkaloids.
More broadly, the discovery of a type III PKS enzyme within Lycopodium alkaloid metabolism expands and reinforces the role of these enzymes within alkaloid biosynthesis. This condensation of polyketides with imines represents a notable chemical theme that plants utilize for producing bioactive alkaloids, as functionally analogous type III PKS enzymes are likely involved within other phylogenetically distant alkaloid classes such as the granatane and tropane-related coca alkaloids (7, 8, 47, 62). Just as PIKS and PYKS seem to have arisen independently (32), we expect for imine-ketide condensation activity to have distinctly evolved in type III PKS enzymes for the biosynthesis of alkaloid classes from other plant lineages as well.
The use of transcriptomics and coexpression analysis within plant pathway discovery is decidedly enhanced if a known pathway gene can be used as “bait” to identify coexpressing candidate genes. Critically, the identification of a set of genes acting within the initial steps of Lycopodium alkaloid metabolism greatly facilitated our prioritization of oxidative enzyme candidates and allowed us to readily define reactions at the end of HupA biosynthesis. Collectively, these results revealed that 2OGD enzymes play a prominent role within the downstream biosynthesis of HupA. Although the archetypical role of 2OGD enzymes is to catalyze hydroxylations (54), here we have determined three relatively distinct reactions catalyzed by these enzymes: desaturation to yield an aromatic pyridone, oxidative ring cleavage, and redox-neutral double bond isomerization. It is intriguing that 2OGDs, rather than CYPs, which are common oxidative enzymes within plant specialized metabolisms (63), play such a prominent role in this pathway. It has been speculated that because of their nature as soluble enzymes, 2OGDs tend to act more on polar intermediates (e.g., lysine/polyamine-derived alkaloids), while CYPs, which are anchored to the endoplasmic reticulum, may be more crucial for oxidations of nonpolar metabolites (53).
The chemical transformations catalyzed by these 2OGDs expand the known biosynthetic repertoire for this class of enzyme. Perhaps the most straightforward of these reactions is the desaturation catalyzed by Pt2OGD-3, which results in formation of the aromatic pyridone ring. Though 2OGD-catalyzed desaturations are not uncommon, they are typically assisted by a heteroatom adjacent to the site of desaturation (64). However, given the position of the double bond installed by Pt2OGD-3, we predict that desaturation may instead be promoted by resonance stabilization from the adjacent double bond and nitrogen atom of the A ring (SI Appendix, Fig. S21).
Even more noteworthy are the reactions catalyzed by Pt2OGD-1 (piperidine ring cleavage) and Pt2OGD-2 (double bond isomerization). The ring cleavage catalyzed by Pt2OGD-1 involves breaking of both a C–N and C–C bond by a single enzyme, ultimately resulting in loss of a single carbon. We speculate that enzyme-catalyzed oxidative cleavage of the C–N bond could open the C ring and yield a carboxylic acid, which could then be oxidatively decarboxylated to yield the terminal olefin of HupC (65) (SI Appendix, Fig. S21). To our knowledge, Pt2OGD-1 is the only described 2OGD that catalyzes both piperidine ring cleavage and carbon loss, though a chemically analogous reaction is poised to occur within quinolizidine alkaloid biosynthesis, another class of lysine-derived alkaloids. Specifically, the quinolizidine alkaloid (+)−lupanine, bearing structural similarity to HupB, appears to be a logical substrate for oxidative piperidine ring cleavage to produce (−)−angustifoline, which contains a terminal double bond analogous to that found in HupC (66) (SI Appendix, Fig. S22). Our identification of a 2OGD enzyme that catalyzes this reaction sets a precedent in the biosynthesis of aliphatic alkaloids and may assist in the future identification of other enzymes involved in the biosynthesis of neuroactive metabolites found in plants.
The most unexpected activity identified within this group of 2OGDs is the double bond isomerization catalyzed by Pt2OGD-2. The catalytic cycle of 2OGD enzymes typically results in the net oxidation of a substrate, often via hydrogen abstraction and oxygen rebound. Though 2OGD enzymes have been found to exhibit redox-neutral epimerase activity (54), to our knowledge, Pt2OGD-2 appears to be the first double bond isomerase within this protein family. Mechanistic details still need to be uncovered for this enzyme, but we speculate that the mechanism may be similar to that of CarC, a 2OGD that catalyzes an epimerization within the biosynthesis of carbapenem antibiotics (67) (SI Appendix, Fig. S21). Regardless of mechanism, we predict that 2OGD isomerase functionality, while perhaps uncommon, will likely be found to contribute to other plant biosynthetic pathways.
Collectively, our discovery of highly coexpressing transcripts for genes involved in both the early and late stages of HupA biosynthesis strongly supports the identification of a biosynthetic regulon for Lycopodium alkaloid metabolism in P. tetrastichus. Our deuterium labeling experiments (35) (Fig. 2B) have determined that at least within the shoots of P. tetrastichus, Lycopodium alkaloid biosynthesis is only occurring to a notable level within the new growth, with particularly high activity within the new leaves. For the purpose of biosynthetic pathway discovery, this metabolic activity distinction is important, as HupA accumulates to high levels (milligrams per gram dry weight) in all tissues from P. tetrastichus, which otherwise would have suggested that biosynthetic genes may be expressed to a high level throughout the plant. Thus, the deuterium incorporation study gave us confidence in determining a set of conditions/tissues in which we could anticipate differential expression of candidate biosynthetic genes. Accordingly, we observed that the biosynthetic genes identified here all show enriched expression within the new growth tissues and that all shared a highly similar expression profile. Importantly, the coexpression of these genes was strong enough that simple correlative analysis with a likely pathway gene (PtLDC-1) allowed for the facile identification of genes from both the beginning and end of the HupA pathway. While the nature of the scaffold-generating enzyme(s) necessary for processing 4PAA and pelletierine remain unknown, we anticipate that further assessment of the genes within the putative metabolic regulon identified here should guide future discoveries of novel enzymes.
The specific biosynthetic activity in new growth (35), accompanied by our transcriptomic data, suggests developmental control of Lycopodium alkaloid biosynthesis. Tissue- and cell-specific production of plant alkaloids is well-documented (10, 68, 69), and the production of bioactive alkaloids within new growth supports a role for these molecules in defending these susceptible new tissues from herbivores. The slow growth and difficulty in cultivating species from the Lycopodiaceae would make it challenging to determine an in vivo role for the Lycopodium alkaloids in native plants. However, continued biosynthetic discovery for these alkaloids will not only allow their reconstitution for large-scale metabolite production but will also enable synthetic biology approaches to assess the potential defensive functions of these molecules in nature. Overall, our insight into the transcriptional and biochemical basis for HupA biosynthesis provides a foundational understanding for future investigations into the Lycopodium class of alkaloids, as well as many other neuroactive, aliphatic alkaloids produced by plants.
Materials and Methods
Chemical Reagents.
All standard biological and chemical reagents were purchased from commercial vendors unless noted otherwise. Authentic standards of 1-piperideine and pelletierine were synthesized from commercially available chemicals. The following Lycopodium alkaloids were acquired from commercial sources: (-)-huperzine A (ApexBio Technology LLC), huperzine B (MilliporeSigma), and huperzine C (two sources: Shanghai Tauto Biotech Co., Ltd and Toronto Research Chemicals Inc.). The alkaloids des-N-methyl-α-obscurine and des-N-methyl-β-obscurine were previously isolated from Lycopodium platyrhizoma (60). Data confirming the structures of these molecules is provided in SI Appendix.
Plant Materials and Growth.
The following species from the Phlegmariurus genus (previously classified as Huperzia) (70) were obtained from Charles Alford Plants (https://rareferns.com): P. brassii, P. carinata, P. goebelli, P. salvinoides, P. squarrosus, and P. tetrastichus. All plants were grown at ambient room temperature and lighting and were watered occasionally by spraying their roots with deionized (DI) water. Once per week, plants were supplied with a nutrient solution that was applied directly to the roots (Ionic Grow for Soil 3-1-5, Hydrodynamics International).
The N. benthamiana plants used for heterologous gene expression were grown in PRO MIX HP Mycorrhizae soil (Premier Tech Horticulture) at ambient laboratory temperature under growth lights with a 16/8-h light/dark cycle. Plants were selected for Agrobacterium-mediated transformation at 4 to 5 wk postgermination.
Measuring HupA Concentration in Phlegmariurus Species.
As an initial screen of HupA abundance in various Phlegmariurus specimen, shoot tips were excised from each species (three replicates for each plant) and immediately snap frozen in liquid nitrogen. Samples were lyophilized to dryness and weighed to calculate the dry mass. Samples were then processed for analysis via LC–MS (Metabolite Extraction and LC–MS Analysis of Metabolites). The presence of HupA in these plants was confirmed via LC–MS retention time and MS/MS comparison to an authentic commercial standard (SI Appendix, Fig. S1).
D2O Labeling to Identify Active Metabolism.
D2O labeling studies were performed much as previously described (35). Additional details are available in SI Appendix.
RNA-Seq and Analysis.
Frozen plant tissues were homogenized to a fine powder under liquid nitrogen via mortar/pestle, which were pretreated with RNaseZap RNase Decontamination Solution (Thermo Fisher Scientific). Total RNA was isolated from the homogenized P. tetrastichus tissues using the Spectrum Plant Total RNA kit (MilliporeSigma) or the RNeasy Plant Mini kit (Qiagen), as per the manufacturer’s instructions. RNA intended for SMRT sequencing (Pacific Biosciences, PacBio) was isolated from four different tissues: 1) new growth, leaves; 2) new growth, stem; 3) mature shoot (combined leaves and stem); and 4) roots. Each sample contained a pooled combination of four distinct samples of the same tissue type (e.g., new growth leaves were isolated from four different shoots and combined into one pooled sample). Following tissue homogenization, a portion of each sample was saved for future metabolite extraction and LC–MS analysis such that metabolites in a sequenced tissue could be directly compared to RNA-seq results. A 100-mg homogenized tissue sample was utilized for RNA isolation for each pooled sample. For the RNA intended for Illumina sequencing, the same general strategy of pooled samples was utilized. However, for Illumina library preparation, we isolated RNA from six different types of tissue samples: 1) new growth, leaves; 2) new growth, stem; 3) subnew growth, leaves; 4) subnew growth, stem; 5) mature shoot (leaves and stem together); and 6) roots. For each tissue type, a total of four separate pooled samples were utilized to provide biological replicates. Samples were homogenized in the same method as above, and a portion of each sample was saved for future LC–MS analysis. RNA for every sample was quantified via NanoDrop and with the Qubit HS (high sensitivity) RNA Assay kit (Thermo Fisher Scientific), and quality was assured with analysis using an RNA 6000 Nano chip on a 2100 Bioanalyzer (Agilent).
Preparation of the PacBio SMRT sequencing library was performed much as described in the Iso-Seq Template Preparation for Sequel Systems manual (with no size selection). Library preparation for Illumina HiSeq 4000 sequencing was performed using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs, or NEB), as per the manufacturer instructions. Reference SI Appendix for specific details of library preparations, sequencing, and data processing.
Cloning of Candidate Biosynthetic Genes.
All biosynthetic genes tested here were cloned from unprocessed cDNA generated for PacBio SMRT sequencing. Additional cloning details are available in SI Appendix.
Agrobacterium-Mediated Transient Expression.
Transient expression of biosynthetic gene candidates was performed as previously described (10). Reference SI Appendix for additional details.
Metabolite Extraction.
Following lyophilizing and weighing to calculate dry mass, plant samples in 2 mL Safe-Lock tubes (Eppendorf) were homogenized to a fine powder on a ball mill (Retsch MM 400) using 5-mm stainless steel beads with shaking at 25 Hz for 2 min. For metabolite extraction from this tissue, a solution of 80:20 methanol (MeOH):water was utilized, with 10 to 20 µL solution used per milligram of dry weight for N. benthamiana leaves and 300 µL per milligram dry weight used for Phlegmariurus tissues. After addition of the solution, samples were mixed by vortexing and allowed to incubate at room temperature for 30 min. Samples were then centrifuged at 10,000 × g for 5 min to pellet plant debris. For the detection of all identified pathway compounds up to and including 4PAA and pelletierine, hydrophilic interaction liquid chromatography (HILIC) was utilized. As such, extracts from these experiments were diluted 10-fold in acetonitrile (ACN) to better match the starting HILIC buffer conditions and were then filtered through 0.45 μm PTFE filters and transferred into appropriate LC–MS vials for subsequent analysis. In experiments analyzing downstream pathway metabolites (DNMAO, DNMBO, HupB, HupC, and HupA), C18 chromatography was used, and sample extracts were diluted 1:5 in water with 0.1% formic acid prior to being filtered. It was eventually determined that some products (e.g., 4PAA and 3-oxoglutaric acid) were temperature sensitive and decomposed even at room temperature; as such, we opted to use ice-cold solutions for all extractions, and samples were kept on ice or at 4 °C for the entirety of the extraction process. Additionally, all samples were stored at −80 °C prior to LC–MS analysis. For the robust detection of 4PAA, it was critical to immediately analyze samples via LC–MS following removal from −80 °C.
To assess the presumed decomposition of 4PAA into pelletierine, newly prepared N. benthamiana methanolic plant extracts were diluted 10-fold into ice-cold ACN that contained caffeine (100 µM) as an internal standard for quantification. Samples were then filtered and divided into two aliquots in capped LC–MS vials; one of these was immediately stored at −80 °C, while the other was left at room temperature (“heated”) for several hours. The heated samples were then stored at –80 °C until LC–MS analysis.
In Vitro Reactions with Lycopodium Alkaloid Biosynthetic Enzymes.
Details regarding the production, purification, and in vitro reactions with PtPIKS-1, PtPIKS-2, Pt2OGD-1, and Pt2OGD-2 can be found in SI Appendix.
LC–MS Analysis of Metabolites.
Metabolites assessed in this study were analyzed via LC–MS by using an Agilent 1260 high-performance liquid chromatography (HPLC) instrument paired with a coupled Agilent 6520 Accurate-Mass quadrupole time-of-flight (Q-TOF) electrospray ionization (ESI) mass spectrometer in positive ion mode. For detection of relatively nonpolar metabolites (DNMAO, DNMBO, HupB, HupC, and HupA), samples were analyzed using a Gemini NX-C18 column (Phenomenex, 5 μm, 2 × 100 mm) using water with 0.1% formic acid and ACN with 0.1% formic acid as mobile phases. Detection of polar intermediates (e.g., L-lysine, cadaverine, 1-piperideine, and pelletierine) was performed via HILIC analysis with an XBridge BEH Amide column (Waters, 5 µm, 2.1 × 100 mm) using water and 95:5 ACN:water, each with 0.125% formic acid and 10 mM ammonium formate, as mobile phases. For the analysis of CoA thioesters, a Poroshell 120 HILIC-Z column (Agilent, 2.7 µm, 2.1 × 150 mm) was utilized with high pH buffers (water with 10 mM ammonium acetate and 5 µM InfinityLab Deactivator Additive, pH 9; 85:15 ACN:water with 10 mM ammonium acetate and 5 µM InfinityLab Deactivator Additive, pH 9), and MS data were collected in both positive and negative ion mode. Additional information on LC–MS conditions can be found in SI Appendix.
Metabolomic Analyses.
In general, LC–MS data were analyzed using Agilent MassHunter software. To quantify substrate and product abundance, extracted ion chromatograms were generated for the monoisotopic mass of a given compound (with a 20 to 50 ppm mass tolerance), and the area under the peak was calculated using the Agile2 method with default parameters. Where applicable, peak areas were converted to concentrations by utilizing a standard curve generated from the LC–MS analysis of standards with known concentrations. Additional information regarding metabolomic analysis can be found in SI Appendix.
Synthesis of 1-Piperideine and Pelletierine.
1-piperideine and pelletierine were synthesized by following a previously described protocol (71). Specific details regarding these syntheses are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. G. Lomonossoff (John Innes Centre) for providing the pEAQ-HT vector and Dr. K. Smith (Stanford University) for assistance with chemical synthesis and NMR analysis. We acknowledge the Stanford Center for Genomics and Personalized Medicine for RNA-seq services and the Stanford Genetics Bioinformatics Service Center for the use of computational resources supported by NIH S10 Instrumentation Grant S10OD023452. The research in this manuscript was supported by an NIH U01 GM110699, an R01 GM121527, and an HHMI-Simons Faculty Scholar award (to E.S.S.). R.S.N. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2102949118/-/DCSupplemental.
Data Availability
RNA-seq data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under the BioProject ID PRJNA731132 (38). Cloned sequences have been deposited in GenBank (accession nos. MZ190751–MZ190759). All other raw/source data are available upon request.
References
- 1.Dixon R. A., Natural products and plant disease resistance. Nature 411, 843–847 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Gershenzon J., Dudareva N., The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Pluskal T., Weng J. K., Natural product modulators of human sensations and mood: Molecular mechanisms and therapeutic potential. Chem. Soc. Rev. 47, 1592–1637 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Bharate S. S., Mignani S., Vishwakarma R. A., Why are the majority of active compounds in the CNS domain natural products? A critical analysis. J. Med. Chem. 61, 10345–10374 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Yang L., Stöckigt J., Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 27, 1469–1479 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Wink M., Interference of alkaloids with neuroreceptors and ion channels. Stud. Nat. Prod. Chem. 21, 3–122 (2000). [Google Scholar]
- 7.Kim N., Estrada O., Chavez B., Stewart C., D’Auria J. C., Tropane and granatane alkaloid biosynthesis: A systematic analysis. Molecules 21, 1–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichman B. R., The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 38, 103–129 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Sigrist R., da Costa B. Z., Marsaioli A. J., de Oliveira L. G., Nature-inspired enzymatic cascades to build valuable compounds. Biotechnol. Adv. 33, 394–411 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Nett R. S., Lau W., Sattely E. S., Discovery and engineering of colchicine alkaloid biosynthesis. Nature 584, 148–153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caputi L., et al., Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science 360, 1235–1239 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Dewey R. E., Xie J., Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94, 10–27 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan P., Smolke C. D., Biosynthesis of medicinal tropane alkaloids in yeast. Nature 585, 614–619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunsupa S., Yamazaki M., Saito K., Lysine-derived alkaloids: Overview and update on biosynthesis and medicinal applications with emphasis on quinolizidine alkaloids. Mini Rev. Med. Chem. 17, 1002–1012 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Ma X., Gang D. R., The Lycopodium alkaloids. Nat. Prod. Rep. 21, 752–772 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Tan C., Zhu D., Gang D. R., Xiao P., Huperzine A from Huperzia species–An ethnopharmacolgical review. J. Ethnopharmacol. 113, 15–34 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Xu M., Heidmarsson S., de Boer H. J., Kool A., Olafsdottir E. S., Ethnopharmacology of the club moss subfamily Huperzioideae (Lycopodiaceae, Lycopodiophyta): A phylogenetic and chemosystematic perspective. J. Ethnopharmacol. 245, 112130 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Raves M. L., et al., Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat. Struct. Biol. 4, 57–63 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Liu J.-S., et al., The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 64, 837–839 (1986). [Google Scholar]
- 20.Bödeker K., Lycopodin, das erste Alkaloïd der Gefässkryptogamen. Justus Liebigs Ann. Chem. 208, 363–367 (1881). [Google Scholar]
- 21.Siengalewicz P., Mulzer J., Rinner U., “Lycopodium alkaloids–Synthetic highlights and recent developments” in Alkaloids: Chemistry and Biology, Knolker H.-J., Ed. (Elsevier Inc., 2013), pp. 1–151. [DOI] [PubMed] [Google Scholar]
- 22.Gupta R. N., Castillo M., MacLean D. B., Spenser I. D., Wrobel J. T., Biosynthesis of lycopodine. J. Am. Chem. Soc. 90, 1360–1361 (1968). [DOI] [PubMed] [Google Scholar]
- 23.Castillo M., Gupta R. N., MacLean D. B., Spenser I. D., Biosynthesis of lycopodine from lysine and acetate. The pelletierine hypothesis. Can. J. Chem. 48, 1893–1903 (1970). [Google Scholar]
- 24.Castillo M., Gupta R. N., Ho Y. K., MacLean D. B., Spenser I. D., Biosynthesis of lycopodine. Incorporation of Δ1-piperideine and of pelletierine. Can. J. Bot. 48, 2911–2918 (1970). [Google Scholar]
- 25.Braekman J.-C., Gupta R. N., MacLean D. B., Spenser I. D., Biosynthesis of lycopodine. Pelletierine as an obligatory intermediate. Can. J. Chem. 50, 2591–2602 (1972). [Google Scholar]
- 26.Marshall W. D., Spenser I. D., Nguyen T. T., MacLean D. B., Biosynthesis of lycopodine. The question of the intermediacy of piperidine-2-acetic acid. Can. J. Chem. 53, 41–50 (1975). [Google Scholar]
- 27.Hemscheidt T., Spenser I. D., Biosynthesis of lycopodine: Incorporation of acetate via an intermediate with C2v symmetry. J. Am. Chem. Soc. 115, 3020–3021 (1993). [Google Scholar]
- 28.Hemscheidt T., Spenser I. D., A classical paradigm of alkaloid biogenesis revisited: Acetonedicarboxylic acid as a biosynthetic precursor of lycopodine. J. Am. Chem. Soc. 118, 1799–1800 (1996). [Google Scholar]
- 29.Bunsupa S., et al., Molecular evolution and functional characterization of a bifunctional decarboxylase involved in Lycopodium alkaloid biosynthesis. Plant Physiol. 171, 2432–2444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B., Lei L., Zhu X., Zhou Y., Xiao Y., Identification and characterization of L-lysine decarboxylase from Huperzia serrata and its role in the metabolic pathway of lycopodium alkaloid. Phytochemistry 136, 23–30 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Sun J., Morita H., Chen G., Noguchi H., Abe I., Molecular cloning and characterization of copper amine oxidase from Huperzia serrata. Bioorg. Med. Chem. Lett. 22, 5784–5790 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Wang J., et al., Deciphering the biosynthetic mechanism of pelletierine in Lycopodium alkaloid biosynthesis. Org. Lett. 22, 8725–8729 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Ma X., Tan C., Zhu D., Gang D. R., Is there a better source of huperzine A than Huperzia serrata? Huperzine A content of Huperziaceae species in China. J. Agric. Food Chem. 53, 1393–1398 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Ishiuchi K., Park J. J., Long R. M., Gang D. R., Production of huperzine A and other Lycopodium alkaloids in Huperzia species grown under controlled conditions and in vitro. Phytochemistry 91, 208–219 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Nett R. S., et al., D2O labeling to measure active biosynthesis of natural products in medicinal plants. AIChE J. 64, 4319–4330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau W., Sattely E. S., Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349, 1224–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conesa A., et al., A survey of best practices for RNA-seq data analysis. Genome Biol. 17, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nett R. S., Sattely E. S., Transcriptomic profiling of Phlegmariurus tetrastichus to study Lycopodium alkaloid biosynthesis. Sequence Read Archive. http://www.ncbi.nlm.nih.gov/bioproject/731132. Deposited 19 May 2021.
- 39.Wang W., Paschalidis K., Feng J. C., Song J., Liu J. H., Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 10, 561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunsupa S., et al., Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in Leguminosae. Plant Cell 24, 1202–1216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cona A., Rea G., Angelini R., Federico R., Tavladoraki P., Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11, 80–88 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Sandmeier E., Hale T. I., Christen P., Multiple evolutionary origin of pyridoxal-5′-phosphate-dependent amino acid decarboxylases. Eur. J. Biochem. 221, 997–1002 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Yang M., et al., Global transcriptome analysis of Huperzia serrata and identification of critical genes involved in the biosynthesis of huperzine A. BMC Genomics 18, 245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monaco M. R., Renzi P., Scarpino Schietroma D. M., Bella M., Biomimetic organocatalytic asymmetric synthesis of 2-substituted piperidine-type alkaloids and their analogues. Org. Lett. 13, 4546–4549 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Leeper F. J., Grue-Sørensen G., Spenser I. D., Biosynthesis of the quinolizidine alkaloids. Incorporation of Δ1-piperideine into matrine. Can. J. Chem. 59, 106–115 (1981). [Google Scholar]
- 46.Wanner M. J., Koomen G. J., Oxidative deamination of tetrahydroanabasine with o-quinones: An easy entry to lupinine, sparteine, and anabasine. J. Org. Chem. 61, 5581–5586 (1996). [Google Scholar]
- 47.Bedewitz M. A., Jones A. D., D’Auria J. C., Barry C. S., Tropinone synthesis via an atypical polyketide synthase and P450-mediated cyclization. Nat. Commun. 9, 5281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe I., Morita H., Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 27, 809–838 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Huang J.-P., et al., Tropane alkaloids biosynthesis involves an unusual type III polyketide synthase and non-enzymatic condensation. Nat. Commun. 10, 4036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belecki K., Townsend C. A., Biochemical determination of enzyme-bound metabolites: Preferential accumulation of a programmed octaketide on the enediyne polyketide synthase CalE8. J. Am. Chem. Soc. 135, 14339–14348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mentzen W. I., Wurtele E. S., Regulon organization of Arabidopsis. BMC Plant Biol. 8, 99 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J.-S., Huang M.-F., The alkaloids huperzines C and D and huperzinine from Lycopodiastrum casuarinoides. Phytochemistry 37, 1759–1761 (1994). [Google Scholar]
- 53.Kawai Y., Ono E., Mizutani M., Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 78, 328–343 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Martinez S., Hausinger R. P., Catalytic mechanisms of Fe(II)- and 2-oxoglutarate-dependent oxygenases. J. Biol. Chem. 290, 20702–20711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guengerich F. P., Yoshimoto F. K., Formation and cleavage of C-C bonds by enzymatic oxidation-reduction reactions. Chem. Rev. 118, 6573–6655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagel J. M., Facchini P. J., Biochemistry and occurrence of O-demethylation in plant metabolism. Front. Physiol. 1, 14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huijbers M. M. E., Montersino S., Westphal A. H., Tischler D., van Berkel W. J. H., Flavin dependent monooxygenases. Arch. Biochem. Biophys. 544, 2–17 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Christ B., et al., Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat. Commun. 10, 3206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Wang D., Luo H., Yang M., Simultaneous determination of three alkaloids in Huperzia serrata by UPLC-PDA and UPLC-Q/TOF-MS. Anal. Methods 7, 1770–1776 (2015). [Google Scholar]
- 60.Yeap J. S. Y., et al., Lycopodium alkaloids: Lycoplatyrine A, an unusual lycodine-piperidine adduct from Lycopodium platyrhizoma and the absolute configurations of lycoplanine D and lycogladine H. J. Nat. Prod. 82, 324–329 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Keller Y., Bouvier F., d’Harlingue A., Camara B., Metabolic compartmentation of plastid prenyllipid biosynthesis–Evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur. J. Biochem. 251, 413–417 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Abe I., Biosynthesis of medicinally important plant metabolites by unusual type III polyketide synthases. J. Nat. Med. 74, 639–646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamberger B., Bak S., Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120426 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunham N. P., et al., Two distinct mechanisms for C-C desaturation by iron(II)- and 2-(oxo)glutarate-dependent oxygenases: Importance of α-heteroatom assistance. J. Am. Chem. Soc. 140, 7116–7126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu C. P., et al., Elucidating the reaction pathway of decarboxylation-assisted olefination catalyzed by a mononuclear non-heme iron enzyme. J. Am. Chem. Soc. 140, 15190–15193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang T., et al., Transcript profiling of a bitter variety of narrow-leafed lupin to discover alkaloid biosynthetic genes. J. Exp. Bot. 68, 5527–5537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang W. C., et al., Mechanism of the C5 stereoinversion reaction in the biosynthesis of carbapenem antibiotics. Science 343, 1140–1144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Courdavault V., et al., A look inside an alkaloid multisite plant: The Catharanthus logistics. Curr. Opin. Plant Biol. 19, 43–50 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Singh A., Menéndez-Perdomo I. M., Facchini P. J., Benzylisoquinoline alkaloid biosynthesis in opium poppy: An update. Phytochem. Rev. 18, 1457–1482 (2019). [Google Scholar]
- 70.Field A. R., Testo W., Bostock P. D., Holtum J. A. M., Waycott M., Molecular phylogenetics and the morphology of the Lycopodiaceae subfamily Huperzioideae supports three genera: Huperzia, Phlegmariurus and Phylloglossum. Mol. Phylogenet. Evol. 94, 635–657 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Quick J., Oterson R., A convenient synthesis of pelletierine (2-piperidylpropanone). Synth. 1976, 745–746 (1976). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under the BioProject ID PRJNA731132 (38). Cloned sequences have been deposited in GenBank (accession nos. MZ190751–MZ190759). All other raw/source data are available upon request.