Significance
Pathogenic autoantibodies are a feature of systemic lupus erythematosus (SLE), of which 85% of patients are women. Multiple X chromosomes increase the risk for SLE, suggesting an important role for X-linked gene expression for the female sex bias. X-chromosome inactivation (XCI) regulates X-linked gene expression on the inactive X. This study examines XCI maintenance across multiple human B cell subsets from healthy individuals and SLE patients. Importantly, we found that both pediatric and adult SLE patient B cells have significant reductions with epigenetic modifications on the inactive X and aberrant X-linked gene expression. Our findings will be instrumental for future investigations of disease mechanisms underlying the female bias of SLE and abnormal autoantibody production in B cells.
Keywords: X-chromosome inactivation, XIST RNA, human B cells, lupus, sex differences
Abstract
Systemic lupus erythematous (SLE) is a female-predominant disease characterized by autoimmune B cells and pathogenic autoantibody production. Individuals with two or more X chromosomes are at increased risk for SLE, suggesting that X-linked genes contribute to the observed sex bias of this disease. To normalize X-linked gene expression between sexes, one X in female cells is randomly selected for transcriptional silencing through X-chromosome inactivation (XCI), resulting in allele-specific enrichment of epigenetic modifications, including histone methylation and the long noncoding RNA XIST/Xist on the inactive X (Xi). As we have previously shown that epigenetic regulation of the Xi in female lymphocytes from mice is unexpectedly dynamic, we used RNA fluorescence in situ hybridization and immunofluorescence to profile epigenetic features of the Xi at the single-cell level in human B cell subsets from pediatric and adult SLE patients and healthy controls. Our data reveal that abnormal XCI maintenance in B cells is a feature of SLE. Using single-cell and bulk-cell RNA sequencing datasets, we found that X-linked immunity genes escape XCI in specific healthy human B cell subsets and that human SLE B cells exhibit aberrant expression of X-linked genes and XIST RNA interactome genes. Our data reveal that mislocalized XIST RNA, coupled with a dramatic reduction in heterochromatic modifications at the Xi in SLE, predispose for aberrant X-linked gene expression from the Xi, thus defining a genetic and epigenetic pathway that affects X-linked gene expression in human SLE B cells and likely contributes to the female bias in SLE.
Systemic lupus erythematosus (SLE) is an incurable autoimmune disease with multiorgan system manifestations. B cells contribute to various aspects of SLE by secreting pathogenic autoantibodies, presenting autoantigens to T cells, and producing inflammatory cytokines. In addition, the representation of B cell subsets changes in SLE, which can accelerate the production of autoantibodies. In particular, CD27− memory B cells (1), CD19hiCXCR3hi B cells (2), CD24−-activated naïve B cells (3), and age-associated CD11c+ B cells that express T-bet (4, 5) are increased in autoimmunity. Thus, a further understanding of the mechanisms involved in autoimmune B cell dysregulation is critical for future efforts to control the development and progression of SLE.
Like many autoimmune diseases, SLE exhibits a strong female bias, with 85% of patients being women. The underlying mechanisms responsible for this sex difference are not well understood, yet it is clear that the genetics of the X chromosome impact disease susceptibility (6). Indeed, individuals with two or more X chromosomes are at increased risk for SLE (7), suggesting that X-linked genes have a significant role in disease. Immunity-related genes are enriched on the X chromosome (8, 9), and some of these genes are routinely overexpressed in SLE patient B cells (10–13). In addition, mouse models with X-linked gene duplication [such as the BXSB-Yaa mouse model (14, 15)] or transgenic overexpression of either of the X-linked genes Tlr7 (16, 17) or Btk (18, 19) exhibit disease resembling human SLE, with production of double-stranded DNA autoantibodies. Thus, abnormal dosage or expression of particular X-linked genes is associated with SLE disease in mice and humans.
Female mammalian cells with two X chromosomes regulate X-linked gene expression using X-chromosome inactivation (XCI), in which one X is randomly selected for transcriptional silencing to equalize gene expression between the sexes (20, 21). Numerous epigenetic modifications, including histone methylation (22, 23), DNA methylation (24, 25), and the long noncoding RNA XIST/Xist (26–28) are enriched allele specifically on the inactive X (Xi) and maintain transcriptional repression of most of the X chromosome. However, some X-linked genes escape XCI, and human cells exhibit higher levels of XCI escape (15 to 25% of the X chromosome) compared to mice (3% escape) (29, 30). While most somatic cells maintain XCI with static enrichment of Xist RNA and heterochromatin marks on the Xi, we found that lymphocytes exhibit a unique dynamic localization of these modifications to the Xi following stimulation (31–33). These observations are likely to be significant to pathogenesis, as we recently showed that T cells from SLE patients have dispersed XIST RNA transcripts and aberrant overexpression of many X-linked gene transcripts compared to T cells from healthy controls (32).
In this study, we determined the epigenetic profile of the Xi in human B cell subsets at the single-cell level and found that typical heterochromatic modifications are missing from the Xi, suggestive of high levels of XCI escape across B cells. Remarkably, we found mislocalized XIST RNA and reductions with the heterochromatin mark H2AK119Ub at the Xi in activated B cells from pediatric and adult SLE patients and, accordingly, discovered aberrant gene expression profiles of X-linked genes in activated SLE B cells. Our study demonstrates that the unique chromatin features of the Xi in human B cells facilitates XCI escape, and we propose that impaired XCI maintenance in SLE results in aberrant gene expression of X-linked genes that may further contribute to autoimmunity.
Results
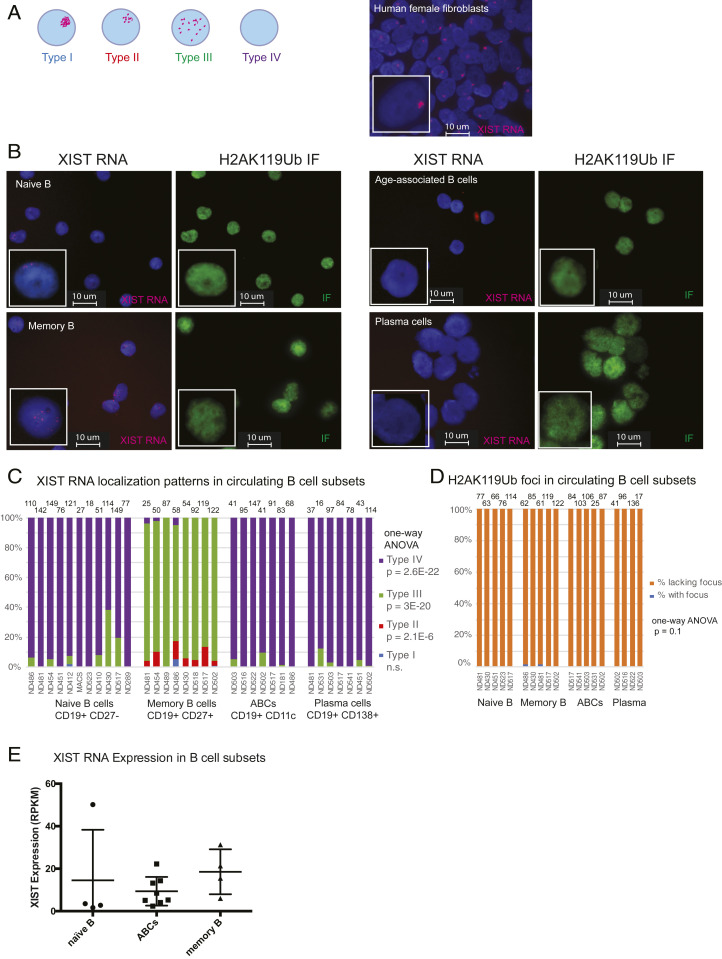
Circulating Human B Cell Subsets Lack Robust XIST RNA Localization at the Xi.
We previously reported that naïve B cells from female humans lack detectible XIST RNA signals at the Xi and that naïve B cells from female mice are missing both Xist RNA and enrichment of the heterochromatin modification H3K27me3 at the Xi. XIST RNA localization patterns in lymphocytes can be classified into four groups (31, 32): Type I cells have robust XIST RNA localized on the Xi; Type II cells have diffuse XIST RNA signals within a nuclear territory encompassing the X chromosome; Type III cells have XIST RNA pinpoints across the nucleus; and Type IV cells lack XIST RNA signals (Fig. 1A). To determine whether XIST RNA and the heterochromatin modification H2AK119-ubiquitin (H2AK119Ub) were missing from the Xi in human B cell subsets, we isolated circulating naïve B (CD19+CD10−CD21+IgD+), memory B (CD19+ CD27+), plasma (CD19+ CD138+), and age-associated B cells (ABCs; CD19+CD11c+) from healthy human donors for sequential XIST RNA fluorescence in situ hybridization (FISH) and immunofluorescence (IF). Remarkably, we found that both XIST RNA transcripts and H2AK119Ub foci were missing from the Xi in naïve B cells, plasma cells, and ABCs (Fig. 1B). Memory B cells had dispersed XIST RNA signals across the nucleus yet also lacked H2AK119Ub foci (Fig. 1B). We quantified the XIST RNA localization patterns for human B cell subsets and found that naïve B, ABCs, and plasma B cells are predominantly Type IV, and memory B cells were mostly Type III with some Type II patterns (one-way ANOVA for Type II, Type III, Type IV, P < 0.05; Fig. 1C). All four B cell subsets examined lacked detectable H2AK119Ub foci (Fig. 1D), including memory B cells that displayed Type III XIST RNA pinpoints across the nucleus. As a previously published RNA sequencing (RNA-seq) dataset (34) revealed that XIST is continuously expressed in naïve, memory, and ABC cells (Fig. 1E), our findings indicate that XIST RNA localization and transcription of the XIST gene are genetically uncoupled in human B cell subsets.
Fig. 1.
XIST RNA signals are missing from the Xi in human B cell populations. (A) Cartoon representing each type of XIST RNA localization pattern observed in human B cell subsets (Left). Representative XIST RNA FISH using primary human female fibroblasts (IMR-90). (B) Sequential XIST RNA FISH (red) then IF detection (green) for H2AK119-ubiquitin (Ub) for naïve B cells, memory B cells, ABCs, and plasma cells from healthy PBMCs. (C) Quantification of XIST RNA localization patterns from each B cell subset. Each column represents an individual donor. Number of nuclei counted is shown above each sample. Statistical significance for each XIST RNA localization pattern was determined using one-way ANOVA. (D) Quantification of H2AK119Ub foci in human B cell subsets. Each column represents an individual donor. (E) XIST RNA reads for naïve B, ABCs, and memory B cells from a previously published RNA-seq dataset (34).
XIST RNA and Heterochromatin Modifications H2AK119Ub and H3K27me3 Are Localized at the Xi after In Vitro Activation of Mature Naïve Human B Cells.
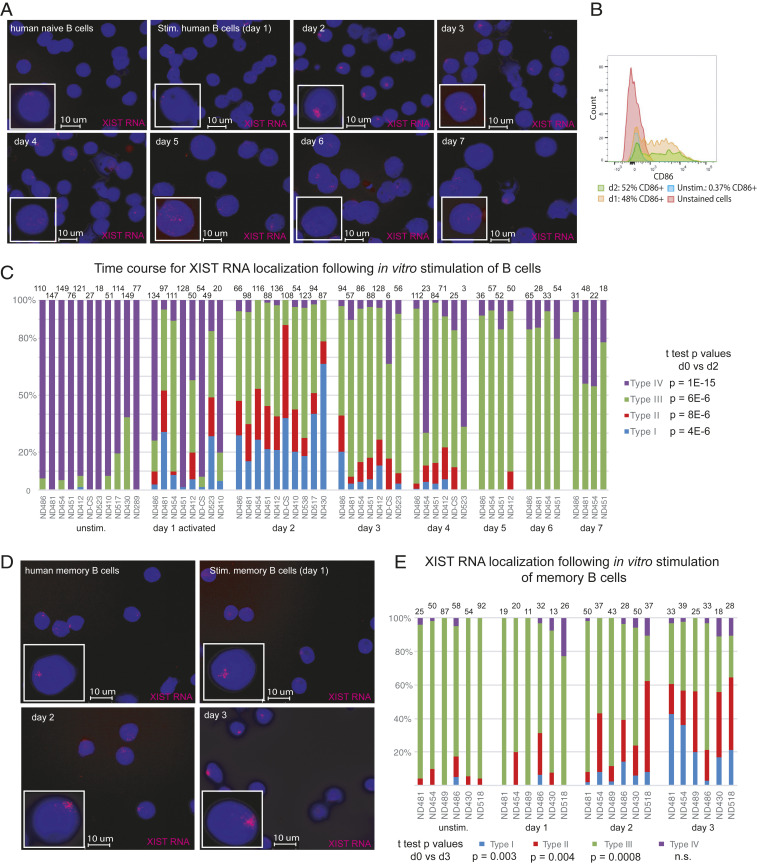
While naïve B cells from female mice lack Xist RNA and heterochromatin mark enrichment on the Xi, we have shown that these epigenetic modifications return to the Xi at 24 to 30 h poststimulation (35). Here, we determined if the dynamic localization of XIST RNA is similarly observed in healthy naïve and memory B cells stimulated in vitro using CpG for 3 to 7 d. Using RNA FISH, we find that XIST RNA transcripts were first detected in naïve B cells at 1 to 2 d poststimulation, and signals decreased by 3 to 4 d poststimulation (Fig. 2A). The efficiency of in vitro stimulation was assessed by CD86+ staining, with ∼50 to 60% of B cells being positive for this marker in each XIST RNA experiment (Fig. 2B). We quantified the XIST RNA localization patterns during human B cell activation and found that day 2 stimulated B cells had the highest levels of Type I and Type II XIST RNA localization patterns (Fig. 2C), with such patterns appearing at day 1 poststimulation. Type III XIST RNA localization patterns were predominant at days 3 to 7 post in vitro activation (Fig. 2C). XIST RNA transcript levels were relatively similar between naïve and in vitro stimulated B cells (SI Appendix, Fig. S1), as previously observed in mouse B cells (35) and reflecting uncoupled XIST transcription and localization to the Xi. Similar analysis of circulating memory B cells revealed that Type I and II XIST RNA patterns predominated after 3 d of culture poststimulation for memory B cells (Fig. 2 D and E). In sum, in vitro activation using CpG stimulates the return of XIST RNA transcripts to the Xi in both naïve and memory B cells.
Fig. 2.
Timing for XIST RNA localization to the Xi during human B cell stimulation for naïve and memory B cells. (A) Time course analysis for XIST RNA FISH to monitor XIST RNA localization changes after B cell stimulation using CpG, over 7 d. (B) CD86+ staining of in vitro activated B cells (day 1 and day 2) to measure efficiency of in vitro stimulation. (C) Quantification of XIST RNA localization patterns for in vitro stimulated B cells over 7 d in culture. The number of nuclei counted is shown above each column. Statistical significance was determined using unpaired t test comparing day 0 to day 2. (D) XIST RNA FISH for in vitro stimulated memory B cells using CpG, over 3 d. (E) Quantification of XIST RNA localization patterns for in vitro activated memory B cells over 3 d. Statistical significance determined using an unpaired t test comparing day 0 to day 3.
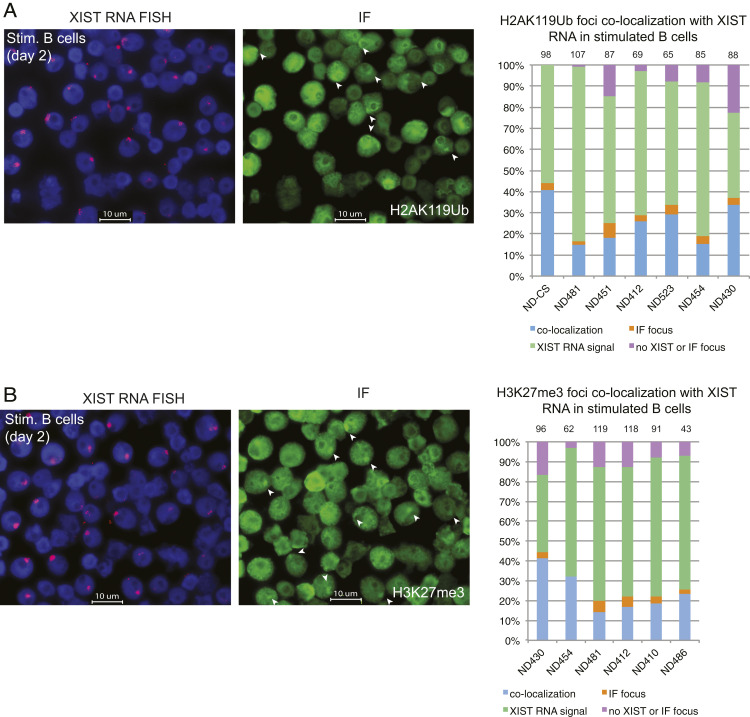
We next asked whether XIST RNA recruitment to the Xi coincided with the enrichment of heterochromatic foci typical of the Xi in somatic cells (22). Circulating naïve B cells were activated with CpG for 2 d and then used for sequential XIST RNA FISH followed by IF using antibodies for H3K27me3 and H2AK119Ub. We quantified the number of nuclei that exhibited colocalization of XIST RNA with a heterochromatic focus (Fig. 3). The majority of activated B cells (40 to 80%) contained a focus that colocalized with Type I XIST RNA patterns and either H2AK119Ub (Fig. 3A) or H3K27me3 (Fig. 3B). There were very few nuclei with XIST Type IV patterns (purple bars) and a focus with either H2AK119Ub or H3K27me3 (2 to 4%), suggesting that XIST RNA localization to the Xi may be necessary for enrichment of these repressive modifications. Return of epigenetic marks to the Xi during human B cell activation occurs in one phase in which both XIST RNA and heterochromatin modifications appear concurrently at the Xi beginning at day 1 poststimulation using CpG, with peak enrichment occurring at day 2.
Fig. 3.
Colocalization of XIST RNA and heterochromatin marks H2AK119Ub and H3K27me for in vitro activated human B cells. Sequential XIST RNA FISH (red) followed by immunofluorescence detection (green) for (A) H2AK119Ub and (B) H3K27me3. Representative images (showing the same field) are shown. Quantification of colocalization patterns for XIST RNA and each heterochromatin mark at the Xi. Colocalization of XIST RNA (Types I and II) and IF focus (blue bars), XIST RNA signals alone (Type III; green), nuclei without either signal (purple), or IF focus (orange). Number of nuclei counted is above column.
Next, we asked whether the histone variant macroH2A, which is also typically enriched on the Xi in fibroblasts, was localized to the Xi of in vitro activated healthy human B cells. We observed very few macroH2A1 foci in these cells, with ∼10% colocalization of XIST RNA with a focus of macroH2A1 (SI Appendix, Fig. S2 A and B). Use of qRT-PCR revealed expression of both macroH2A1 transcript variants, but these levels still remained below those observed in human female fibroblasts (SI Appendix, Fig. S2C). We also investigated whether the active chromatin modification H3K4me3 was depleted within the territory of the Xi in activated B cells, as typically observed in female fibroblasts (36, 37). Using sequential XIST RNA FISH followed by IF, we observed the characteristic H3K4me3 “holes,” reflecting active transcription, which overlapped with XIST RNA Type I and II signals in 70 to 85% of the nuclei (SI Appendix, Fig. S3). In sum, the chromatin of the Xi in CpG-activated human B cells is enriched for some, but not all, silent and active chromatin modifications, underscoring important differences with other somatic cells.
Single-Cell Transcriptional Profiling of Human B Cell Subsets Reveals Cell Type–Specific Biallelic Expression of X-Linked Genes.
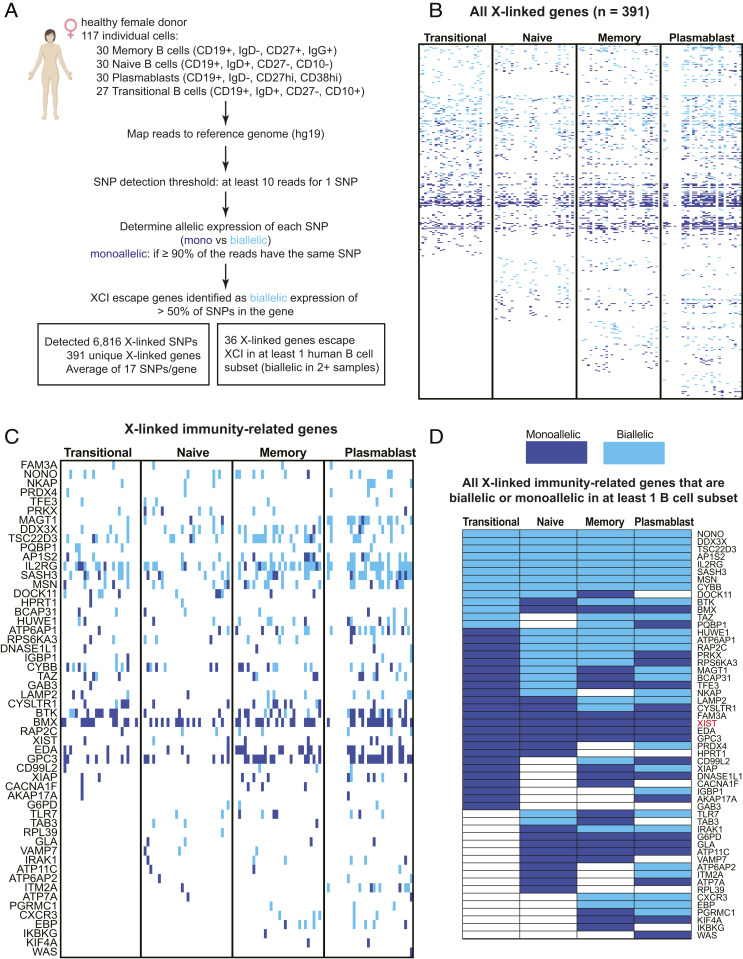
The absence of XIST RNA and heterochromatic modifications H2AK119Ub, H3K27me3, and macroH2A from the Xi in circulating B cell subsets suggests that there may be either increased overall transcription from this chromosome or that the number of genes that escape from XCI would be increased. To investigate this possibility, we queried single-cell RNA-seq (scRNA-seq) data from a recent study (38) examining 117 human B cells isolated from a healthy female donor. These cells were sorted by surface markers and consist of 30 memory B cells, 30 naïve B cells, 29 plasmablasts, and 27 transitional B cells. We determined the X-linked single nucleotide polymorphism (SNP) expression for genes in each cell using an SNP detection threshold of at least 10 reads/SNP (Fig. 4A). Each X-linked gene containing an SNP was called “monoallelic” if greater than 90% of the reads had the same SNP, otherwise the gene was called “biallelic.” We detected 6,816 individual X-linked SNPs and 391 unique X-linked genes that were expressed across the B cell subsets (Fig. 4A and Datasets S1 and S2). We observed cell type–specific XCI escape with higher levels of biallelic expression in memory B cells (98 biallelic genes) and plasmablasts (122 biallelic genes) compared to transitional B and naïve B cells (light blue, Fig. 4B and Dataset S3). We detected a total of 190 X-linked genes that escape XCI in at least one B cell subset and 77% of these genes were XCI escape genes, as they had not been reported previously (Dataset S3). We also observed expression of 53 X-linked immunity-related genes across the B cell subsets and found that 38 of these genes (72%) were biallelically expressed (light blue, Fig. 4C and Dataset S4). We summarized the expression status for the X-linked immunity-related genes across the four human B cell subsets in Fig. 4D. Biallelic expression (light blue) of DDX3X in all four B cell subsets was expected, as DDX3X ubiquitously escapes XCI in multiple tissues (30). While NONO, AP1S2, TSC22D3, AP1S2, IL2RG, SASH3, MSN, and CYBB also escaped XCI across all four B cell subsets, the significance of this biallelic expression is unknown as overexpression of these genes has not been reported in autoimmune diseases. As expected, XIST was exclusively monoallelic (dark blue) across all B cells (Fig. 4D), given its selective expression from the Xi. We also detected variable XCI escape of TLR7 in naïve B cells and plasmablasts, which has been observed previously by our group and others (31, 39). Interestingly, we also observed variable XCI escape for BTK, which is notable because dosage imbalances of this gene are associated with lupus-like phenotypes (18, 19). As a control, we repeated the analysis for chromosome 11 and detected 18,025 individual SNPs and 670 unique genes that were expressed across the B cell subsets (Dataset S5). We observed more biallelic expression for chromosome 11 compared to the X and monoallelic expression of known imprinted genes KCNQ1OT1 and KCNQ1 (Datasets S5 and S4). In sum, human B cells exhibit cell type–specific XCI escape of important immune regulatory genes that could potentially contribute to sex-dependent differences in cellular function, thereby impacting autoimmune disease.
Fig. 4.
Biallelic expression of X-linked genes in human B cell populations. (A) Schematic of the bioinformatics analysis pipeline to identify XCI escape genes in circulating memory B cells, naïve B cells, transitional B cells, and plasmablasts. (B) Heatmap for all X-linked genes with detectable expression across the four human B cell subsets. Each individual cell is a column. Light blue indicates biallelic expression (minor allele frequency ≥ 0.1 for > 50% of SNPs per gene), dark blue indicates monoallelic expression, and white is undetectable expression. Gene lists for individual SNPs in each cell (across B cell populations) in Dataset S1; complete list of all expressed X-linked genes in Dataset S2. Dataset S3 contains gene lists for each B cell subset. (C) Expression of X-linked immunity-related genes across all four B cell populations. Individual cells for a particular B cell subset shown in columns; monoallelic expression (dark blue); biallelic expression (light blue). (D) Allelic expression summary for the X-linked immunity-related genes, either monoallelic (dark blue) or biallelic (light blue) across human B cell subsets. A gene was called “biallelic” for a particular B cell subset if two or more cells within that group were biallelic. A gene was considered monoallelic for a B cell subset if two or more cells within that subset were monoallelic. Dataset S4 contains a complete list of X-linked immunity-related genes that were biallelically expressed in each B cell subset, along with allelic expression information.
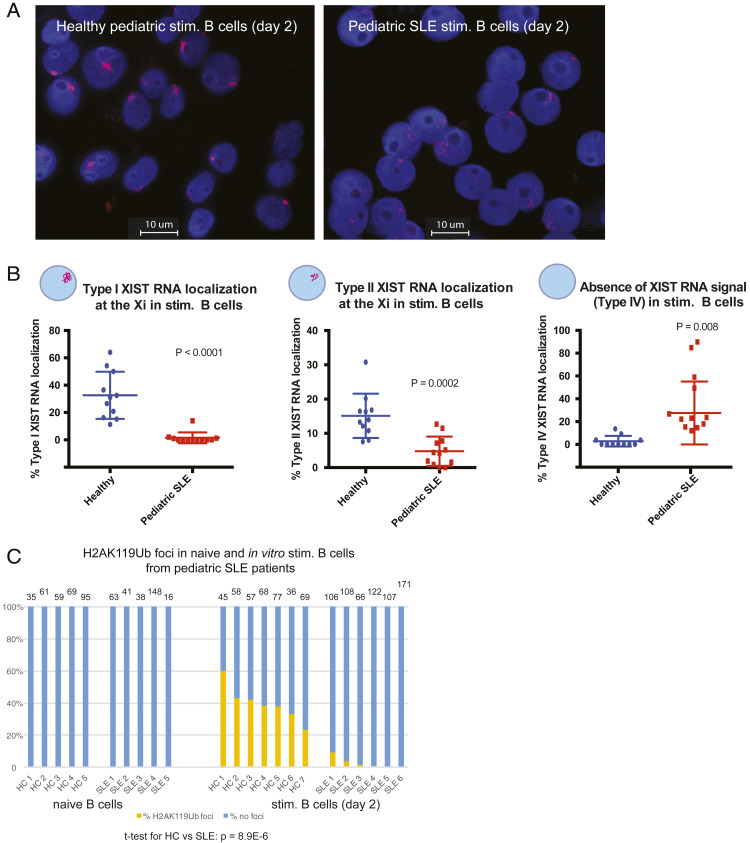
Pediatric SLE Patient B Cells Have Missing or Mislocalized XIST RNA Transcripts from the Xi and Lack H2AK119Ub Foci.
Cell lines derived from SLE patient B cells exhibited differences in XIST RNA localization patterns compared to cell lines from age-matched, healthy individuals (31). Here, we investigated whether primary naïve B cells from SLE patients would also have mislocalized XIST RNA patterns. Naïve B cells isolated from pediatric SLE patients in disease remission (SLE disease activity index [SLEDAI] score 0 to 1) were stimulated in vitro using CpG for 2 d and then used for XIST RNA FISH analyses. We quantified the percentage of each type of XIST RNA localization pattern for both circulating naïve B cells and activated B cells that had been stimulated in vitro (SI Appendix, Fig. S5). There were no significant differences between XIST RNA localization patterns for circulating naïve B cells from pediatric SLE patients and age-matched, healthy controls (SI Appendix, Fig. S5A). In contrast, we found that there were very few examples of Type I and significantly reduced levels of Type II XIST RNA patterns for pediatric SLE in vitro stimulated B cells compared to healthy controls (Fig. 5 A and B and SI Appendix, Fig. S5B; P < 0.0001 and P = 0.0002). In vitro activated SLE patient B cells also had significantly higher levels of Type IV XIST RNA patterns, where nuclei lack detectable XIST signals (Fig. 5B and SI Appendix, Fig. S5B; P = 0.008). Aberrant XIST RNA localization patterns in activated pediatric SLE B cells were not a result of impaired or ineffective stimulation using CpG (a TLR9 agonist), as in vitro stimulated SLE B cells had similar levels of the activation marker CD86 (SI Appendix, Fig. S5C). XIST RNA localization patterns were also disrupted in SLE patient memory B cells compared to healthy controls, with predominantly Type III and Type IV patterns (SI Appendix, Fig. S5D; P = 0.004). To further assess if enrichment of the heterochromatic modification H2AK119Ub was also affected, we performed IF for H2AK119Ub on circulating and in vitro activated B cells from pediatric SLE patients and healthy controls. We observed a significant reduction in H2K119Ub foci in the activated B cells from pediatric SLE patient samples relative to healthy controls (Fig. 5C; P = 8.9E-6). Circulating naïve B cells from both SLE patients and healthy controls lacked detectible H2AK119Ub foci, as expected (Fig. 1). In sum, mislocalization of XIST RNA and near absence of H2AK119Ub foci on the Xi is a feature of activated pediatric SLE patient B cells in disease remission.
Fig. 5.
Peripheral B cells from pediatric SLE patients have mislocalized XIST RNA patterns and lack H2AK119Ub foci at the Xi. (A) Representative XIST RNA FISH images from in vitro activated B cells (cultured 2 d) from one pediatric SLE patient (Right) and a healthy, age-matched control (Left). (B) Quantification of Type I (Left), Type II (Center), and Type IV (Right) XIST RNA localization patterns for in vitro activated B cells from pediatric SLE patients (red) and healthy controls (blue). Each data point represents an individual donor. Error bars denote mean ± SD, and statistical significance was determined using two-tailed unpaired t test. (C) Quantification of H2AK119Ub foci for in vitro activated B cells from pediatric SLE patients and healthy control samples. The number of nuclei counted is above each sample; statistical significance comparing SLE to healthy controls was determined using two-tailed unpaired t test. Note that some PBMC samples yielded too few B cells for a naïve B sample timepoint, and all purified cells were activated using CpG.
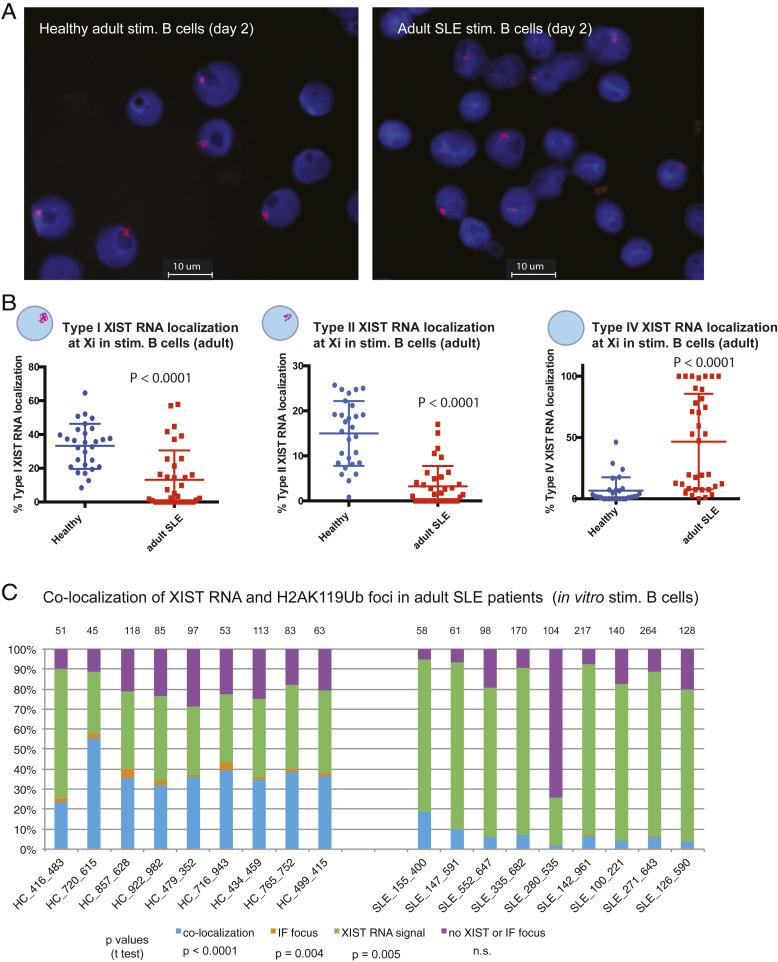
B Cells from Adult SLE Patients Have Aberrant XIST RNA Localization Patterns and Significant Reductions in H2AK119Ub Enrichment on Xi, Irrespective of Disease Activity.
We initially confirmed that the CD27-isolated cells from SLE patients had a relatively similar representation of all B cell populations relative to healthy controls (SI Appendix, Fig. S6). Similar to in vitro activated pediatric SLE patient B cells, in vitro activated B cells from adult SLE patients had significantly fewer Type I (P < 0.0001) and Type II (P < 0.0001) XIST RNA patterns and significantly more Type IV patterns (P < 0.0001), signifying abnormal XIST RNA localization patterns in B cells from adult SLE patients (Fig. 6 A and B and SI Appendix, Fig. S7A). In contrast to the pediatric SLE population, about half of the adult SLE patients had similar levels of Type I and Type IV XIST RNA patterns as healthy controls (Fig. 6B). Colocalization of XIST RNA with H2AK119Ub foci were significantly reduced in adult SLE patient B cells compared to healthy controls (blue bars, P < 0.0001; Fig. 6C), and cells containing an H2AK119Ub focus independent of XIST RNA signals (Types I and II) were absent in SLE samples (orange bars). Activation of naïve B cells following stimulation with CpG (as determined by CD86+ levels) was not significantly affected across adult SLE samples (SI Appendix, Fig. S7B). In sum, enrichment of both XIST RNA and the heterochromatic modification H2AK119Ub at the Xi are significantly reduced for in vitro activated adult SLE B cells.
Fig. 6.
Peripheral B cells from adult SLE patients have mislocalized XIST RNA patterns and reduced H2AK119Ub foci at the Xi. (A) Representative XIST RNA FISH images from in vitro activated B cells (cultured 2 d) from one adult SLE patient (Right) and a healthy, age-matched control (Left). (B) Quantification of Type I (Left), Type II (Center), and Type IV (Right) XIST RNA localization patterns for in vitro activated B cells from adult SLE patients (red) and healthy controls (blue). Error bars denote mean ± SD, and statistical significance was determined using two-tailed unpaired t test. (C) Quantification of colocalization patterns for XIST RNA and H2AK119Ub at the Xi for in vitro activated B cells from adult SLE patients and healthy control samples. Colocalization of XIST RNA (Types I and II) and IF focus (blue bars), XIST RNA signals alone (Type III; green), nuclei without either signal (purple), or IF focus (orange). The number of nuclei counted is above each sample; statistical significance comparing SLE to healthy controls was determined using two-tailed unpaired t test.
We next asked whether the distribution of XIST RNA patterns correlated with SLE disease activity (SLEDAI values), patient medications, age, or disease duration. For these analyses, we removed patients with known comorbidities, including thyroid illnesses (such as Graves’ disease) as they exhibit greater Type III and fewer Type IV XIST RNA patterns (SI Appendix, Fig. S8, Left). Analysis of each XIST RNA localization pattern and the six medications typically used to treat SLE symptoms demonstrated that only hydroxychloroquine showed significant correlation with Type I XIST RNA localization patterns, comparable to healthy controls (SI Appendix, Fig. S8 A and E). We then performed multiple linear regressions between continuous patient metrics (age at sample draw, SLEDAI score, antinuclear antibody titer, and disease duration) and the percentage of Type I to IV XIST RNA localization patterns (SI Appendix, Fig. S9). Of all supplied metrics, SLE patient age was the only one that correlated positively with the Type IV XIST RNA patterns (P value: 0.030, R2: 0.214; SI Appendix, Fig. S9B). Healthy control samples did not exhibit a similar correlation (SI Appendix, Fig. S9B). Taken together, activated B cells from adult SLE patients exhibit aberrant XIST RNA localization and reduced H2AK119Ub enrichment on the Xi, irrespective of disease activity but correlated with patient age, suggestive of impairments with gene expression on this chromosome.
Activated B Cells from SLE Patients Exhibit Abnormal Expression of X-Linked Genes and XIST RNA Interactome Genes.
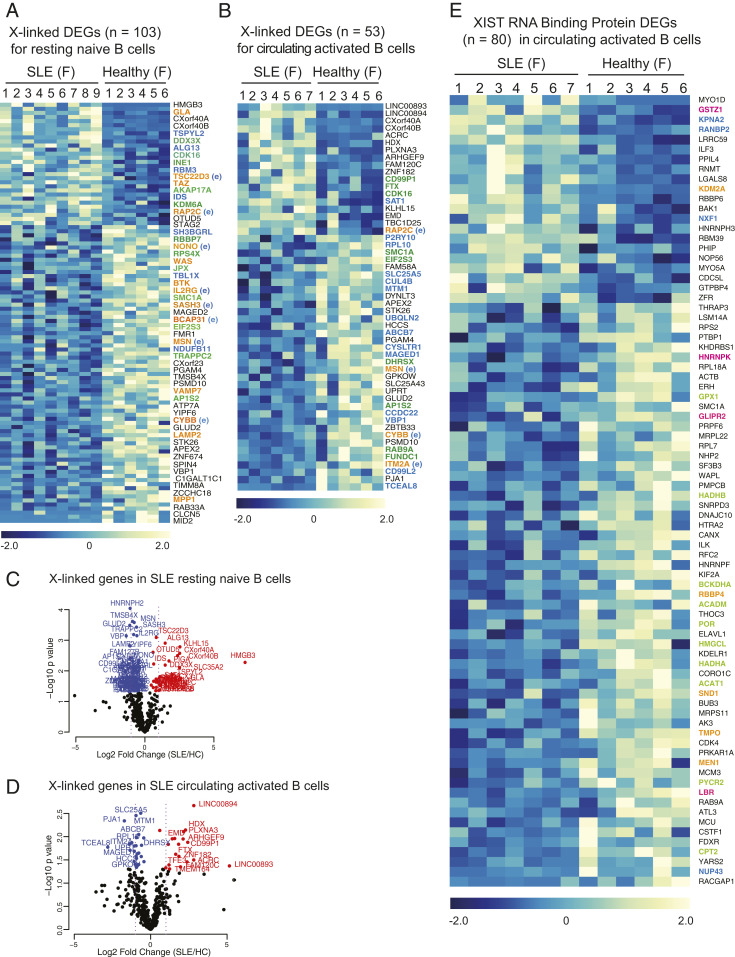
Because SLE activated B cells from both pediatric and adult SLE patients exhibited abnormal XIST RNA localization and reduced/missing H2AK119Ub enrichment at the Xi, we asked whether these changes were also associated with abnormal X-linked gene expression. To answer this, we utilized a previously published RNA-seq dataset (GSE118254) that profiled resting naïve (CD19+, IgD+, CD27−, MTG−, CD24+, CD38+) and circulating activated B cells (CD19+, IgD+, CD27−, MTG+, CD24−, CD38−) in female SLE patients and healthy controls (40). In this dataset, we found that 103 X-linked genes were differentially expressed in resting naïve B cells from SLE patients (Fig. 7A), and 53 X-linked genes were differentially expressed in circulating activated B cells from SLE patients (Fig. 7B). We found that X-linked genes were up- and down-regulated in SLE patient B cell samples, with more down-regulated genes for SLE samples (Fig. 7 A and B; complete list of differentially expressed X-linked genes for naïve cells in Dataset S6). Among the differentially expressed X-linked genes, some have known immune functions (genes in orange), and these genes exhibited XCI escape in at least one B cell subset (Fig. 4D and Dataset S3). Comparison of the X-linked genes altered in circulating naïve and activated SLE B cells with our results from Dataset S3 and published lists of XCI escape genes from various cell types (30) indicate that a large number of the genes in Fig. 7 A and B (all the genes in color) likely escape XCI in either naïve or in vivo activated B cells. Notably, some X-linked genes were aberrantly overexpressed (red genes) in SLE patient naïve and activated B cell samples, yet down-regulated genes were not as significantly affected (blue genes; Fig. 7 C and D). Examination of differentially expressed genes (DEGs) for chromosome 8 revealed more down-regulated genes in SLE patients compared to healthy controls, with fewer significantly up-regulated genes (SI Appendix, Fig. S10). Unexpectedly, we found that the majority of these putative XCI escape genes are down-regulated in SLE naïve and activated B cells (Fig. 7 A and B), suggesting impairments with the regulation of XCI escape on the Xi in SLE. In sum, we identified a set of X-linked genes whose expression is altered in SLE patient activated B cells, and the majority of these genes should be subject to XCI silencing, reflecting aberrant gene regulation on both Xs.
Fig. 7.
X-linked gene expression and XIST RNA interactome genes are altered in SLE patient activated B cells. (A) X-linked DEGs (103 genes) in resting naïve B cells in circulation from SLE patients (nine female samples) and adult, healthy controls (six female samples). XCI escape genes are in color: orange are immunity-related genes that may escape in naïve B cells; green are known XCI escape genes in other somatic cells; and blue are putative XCI escape based on Dataset S3. The complete gene list is shown in Dataset S5. The color gradient represents row Z-scores for each gene. (B) X-linked DEGs (53 genes) in activated B cells in circulation from SLE patients (seven female samples) and adult, healthy controls (six female samples). The color gradient represents row Z-scores for each gene. Gene symbols in color denote XCI escape: orange are immunity-related genes that may escape in activated B cells; green are known XCI escape genes in other somatic cells; and blue are putative XCI escape based on Dataset S3. Genes in orange exhibited XCI escape in at least one B cell subset in Dataset S3 (E). (C and D) Volcano plot showing X-linked DEGs in circulating naïve B cells (C) and flow-sorted in vivo activated B cells in circulation (D). Genes significantly up-regulated in SLE patients are in red; genes significantly down-regulated in SLE are in blue (P < 0.05). (E) XIST RNA binding protein genes that are differentially expressed in activated B cells from SLE patients and healthy controls. Nuclear matrix/nuclear envelop genes in blue; cell metabolism/cell growth genes in green; chromatin regulators in orange; and XIST RNA binding protein genes whose expression was also altered in SLE patient T cells in pink.
One possible mechanism for aberrant XIST RNA localization in SLE patient activated B cells could result from impairments with nuclear proteins that bind XIST RNA. The XIST RNA interactome consists of ∼275 proteins (41–44), and we have previously reported that two XIST RNA binding proteins, YY1 and hnRNP-U, are required for localization of XIST RNA and heterochromatin marks to the Xi in lymphocytes (31, 35). Notably, SLE patient T cells have altered expression of XIST RNA interactome genes (32). Thus, we asked whether the expression of genes encoding XIST RNA binding proteins was also abnormal in the activated B cells from SLE patients. We found that 80 XIST RNA binding protein genes were differentially expressed in activated B cells, and the majority of these genes (59/80; 74%) were down-regulated (Fig. 7E and Dataset S7). These genes function in cell metabolism/cell growth (genes in green), nuclear matrix/nuclear envelope/transport (blue, but also includes lamin B receptor [LBR] and hnRNPK), and chromatin regulation (orange) (Fig. 7E). Down-regulated genes LBR, hnRNP K, and GLIPR2 (pink) also exhibited altered gene expression among SLE T cells relative to healthy controls (32). Taken together, the XIST RNA interactome is dysregulated in activated B cells from SLE patients and may be responsible for mislocalization of XIST RNA and heterochromatin modifications from the Xi, resulting in aberrant XCI maintenance.
Discussion
B cells contribute to the pathogenesis of SLE, an autoimmune disease that predominantly affects women. Here, we sought to investigate the genetic basis for the female bias of SLE, focusing first on how XCI is maintained across distinct B cell subsets in healthy individuals and then determining if XCI maintenance and X-linked gene expression are affected in SLE. We discovered a diverse enrichment of epigenetic modifications at the Xi across activated human B cell subsets and found B cell-specific patterns of XCI escape in healthy adults, including the escape of important immune-related genes. Our profiling of pediatric and adult SLE patient B cells revealed significant impairments with XIST RNA and H2AK119Ub enrichment on the Xi irrespective of disease activity and aberrant expression of X-linked genes. Together, our results suggest that facultative chromatin of the Xi is relaxed in healthy B cells, thereby altering XCI maintenance and permitting gene-specific escape from XCI. SLE disease further impacts the heterochromatic composition of this chromosome, resulting in abnormal gene expression changes across the X. Our epigenetic profiling of the Xi in healthy and SLE B cells provides a foundation for future studies investigating the molecular mechanisms of XIST RNA and heterochromatin mark localization and spreading across the Xi and how these mechanisms become altered in SLE.
All four human B cell subsets assessed—naïve B, classical memory B, plasma cells, and ABCs—are missing XIST RNA and the heterochromatic modification H2AK119Ub on the Xi (Fig. 1). Unexpectedly, our analyses show that unlike naïve B cells, ABCs, and plasma cells, memory B cells have XIST RNA transcripts dispersed across the nucleus yet still lack H2AK119Ub foci on the Xi (Fig. 1 C and D). At present, the significance of dispersed XIST RNA transcripts in memory B cells is unknown. However, it is likely to impact gene expression on the Xi as ex vivo YY1 deletion generates similar Type III dispersed patterns with altered expression of ∼70 X-linked genes (35). The XIST gene is expressed across all resting human B cell subsets (Fig. 1E), thus transcriptional changes do not account for the absence of XIST RNA transcripts on the Xi in circulating human B cells. XIST RNA and the heterochromatin modifications H2AK119Ub, H3K27me3, and low levels of macroH2A returned to the Xi when using CpG to activate human naïve B cells (Figs. 2 and 3 and SI Appendix, Fig. S2). As for mouse naïve B cells, XIST RNA and heterochromatin marks return to the Xi in human naïve B cells before the first cell division (35), with peak enrichment at day 2 poststimulation and prior to cell division (45). We propose the Xi chromatin in circulating human B cell subsets is more relaxed compared to somatic cells, in which XIST RNA and heterochromatin marks are localized to the Xi, and this may allow additional X-linked genes to escape transcriptional silencing. However, as our cytogenetic RNA FISH and IF analyses lack resolution at the gene level, it will be important to determine allele-specific enrichment of silent and active histone modifications at genes exhibiting cell-specific XCI escape and silencing across B cell subsets and to further assess if such modifications are altered upon activation.
scRNA-seq profiling of four human B cell subsets from a healthy female individual reveals cell type–specific XCI escape in naïve B cells, memory B cells, plasmablasts, and transitional B cells. The percentage of biallelically expressed X-linked genes increase with B cell differentiation (Dataset S3), which may reflect a requirement for higher dosage of X-linked genes for proper function of memory B cells and plasmablasts. For example, LAMP2, a lysosomal protein important for autophagy and intracellular antigen presentation, is monoallelically expressed in transitional and naïve B cells yet biallelically expressed in memory B and plasmablasts (Fig. 4D). Such results raise the intriguing possibility that biallelic expression of LAMP2 may increase autophagy and antigen presentation, thereby contributing to enhanced immune responses observed in females (46). The X-linked gene IRAK1, responsible for IL1-induced up-regulation of NF-kappa B, is also biallelically expressed in memory B cells and plasmablasts (Fig. 4D). Female neonates have higher levels of IRAK1 mRNA and protein in cord blood and mononucleated cells compared to males (47), potentially contributing to reduced infection rates and female-specific immune advantages in infants. However, these scRNA-seq analyses are limited by the fact that the sample was taken from one individual and does not contain in vivo stimulated B cells, which may have distinct XCI escape profiles. As XCI escape exhibits individual variability when comparing across human samples (48), it will be important to repeat the allelic expression profiling for human B cell subsets, especially activated B cells, using healthy female individuals of different ages to determine which X-linked genes consistently escape transcriptional silencing, and whether XCI escape increases with age.
Our investigations revealed abnormal XCI maintenance, as evidenced by reduced XIST RNA and H2AK119Ub enrichment at the Xi, in both pediatric and adult SLE patient B cells, irrespective of disease activity. Perturbed XIST RNA and heterochromatin mark localization to the Xi in lymphocytes is a feature of both human SLE and the analogous lupus-like disease in the female-biased spontaneous mouse model NZB/W F1 (32, 49). SLE patient B cells exhibit altered expression of about 50 to 100 X-linked genes (Fig. 7 A and B), and the majority of these genes were down-regulated in SLE patients compared to healthy controls. It is surprising that more than half of the down-regulated X-linked genes in circulating activated B cell SLE samples are putative XCI escape genes based on our studies (Fig. 4) and previous studies in human fibroblasts. Yet this number is much smaller in resting naïve B cells (25 down-regulated X-linked genes are putative XCI escape genes out of 71 down-regulated genes), which would be expected as quiescent cells are less transcriptionally active. It is theoretically possible that impairments with nuclear organization of the Xi, as a result of SLE disease, could shift XCI escape genes into heterochromatic territory resulting in reduced expression but not complete silencing (Dataset S6). The implications of reduced expression of these X-linked genes for B cell function is unknown at this time. It is possible that aberrant XIST RNA localization and reduced heterochromatic enrichment reflects alterations in the nuclear organization of the Xi in SLE patient B cells. The Xi, unlike the active X and autosomes, is organized into two “megadomains” separated by a boundary region near the microsatellite repeat Dxz4 (50, 51). During XCI initiation, Xist RNA plays an important structural role for configuring the Xi territory, and Xist deletion impairs megadomain formation (52). XIST RNA mislocalization in SLE patient B cells may reflect impairments to the Xi nuclear territory, possibly resulting in abnormal gene silencing of some X-linked genes.
While altered X-linked gene expression can clearly impact SLE progression, our studies cannot determine if abnormal XCI maintenance causes SLE disease or is instead a consequence of the disease. To date, there is no evidence that changes to the extracellular environment can influence XCI maintenance in somatic cells. However, the nuclear pore complex NUP43 and LBR, which are Xist RNA binding proteins, were down-regulated in SLE patient B cells (Fig. 7C). This may contribute to aberrant organization of the Xi nuclear territory in SLE patient B cells, as LBR protein directly binds Xist RNA, and this interaction is necessary for tethering the Xi to the nuclear lamina and gene silencing (53). While it is currently unclear if the inflammatory environment of SLE affects nuclear architecture, it is tempting to speculate that inflammatory cytokines or type I interferons, which are highly elevated in SLE patients experiencing disease flares, may perturb XCI maintenance in B cells, resulting in altered X-linked gene expression. Future studies to determine whether extrinsic factors can influence XCI maintenance in lymphocytes will certainly reveal exciting new insights into genetic and epigenetic factors responsible for sex-biased autoimmune disease.
Methods
Source of Human B Cell Samples from Healthy Donors and SLE Patients.
Fresh and frozen peripheral blood mononuclear cells (PBMCs) from adult healthy female donors were obtained from the Penn Pathology BioResource core facility at the Perelman School of Medicine, University of Pennsylvania. For comparative study of pediatric SLE patients (SLEDAI score = 0) and age-matched, healthy controls, we recruited patients from the Children’s Hospital of Philadelphia (CHOP). We also obtained frozen PBMC samples from adult SLE patients (SLEDAI score: 0 to 20; age 18 to 63 y) and age-matched, healthy controls from the Benaroya Research Institute, Seattle, Washington. The acquisition of blood samples from pediatric SLE patients and healthy controls from CHOP, including written informed consent forms, was approved by the Institutional Review Board (IRB) at CHOP. The acquisition of blood from adult SLE patients and healthy controls from the Benaroya Institute, including written informed consent forms, was approved by the IRB at the Benaroya Institute. All samples were deidentified prior to use in this study.
Human B Cell Culture and Activation.
B cells were cultured in X-VIVO15 media (04-744Q, Lonza) with penicillin streptomycin (100 units/mL) and activated using 3 µM CpG (ODN 7909) (tlrl-2006–1, InvivoGen). Cells were cultured in 200 µL medium for 1 to 8 d using round bottom 96-well plates. B cell stimulation was determined by staining for the activation marker CD86, which was quantified using flow cytometry.
Sequential XIST RNA FISH and IF.
Sequential XIST RNA FISH and IF was performed using established protocols (31, 32). For human XIST RNA FISH, we used two Cy3-labeled oligonucleotide probes, which target repetitive regions within XIST exons 1, 3, and 4 (31).
Supplementary Material
Acknowledgments
We thank C. Berry for statistical consultation for correlation analyses, N. Jiwrajka and L. King for assistance with editing of the manuscript, and all members of the M.C.A. laboratory for helpful discussions. This research was supported by a University Research Foundation grant, American Chemical Society grant, McCabe Foundation grant, NIH R21 AI124084, National Institute of Child Health and Disease 5K12 HD085848-03, NIH R01 AI 134834, Department of Defense Grant LR170055: W81XWH-18-1-06 to M.C.A., and NIH 1F32AI154797 to S.P.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024624118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Wei C., et al., A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178, 6624–6633 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Nicholas M. W., et al., A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin. Immunol. 126, 189–201 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipton C. M., et al., Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubtsov A. V., Rubtsova K., Kappler J. W., Marrack P., TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol. Res. 55, 210–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao Y., O’Neill P., Naradikian M. S., Scholz J. L., Cancro M. P., A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118, 1294–1304 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libert C., Dejager L., Pinheiro I., The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 10, 594–604 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Liu K., et al., X chromosome dose and sex bias in autoimmune diseases: Increased 47,XXX in systemic lupus erythematosus and Sjogren’s syndrome. Arthritis Rheumatol. 68, 1290–1300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross M. T., et al., The DNA sequence of the human X chromosome. Nature 434, 325–337 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi I., Lleo A., Gershwin M. E., Invernizzi P., The X chromosome and immune associated genes. J. Autoimmun. 38, J187–J192 (2012). [DOI] [PubMed] [Google Scholar]
- 10.García-Ortiz H., et al., Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann. Rheum. Dis. 69, 1861–1865 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Desai-Mehta A., Lu L., Ramsey-Goldman R., Datta S. K., Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Invest. 97, 2063–2073 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadasz Z., et al., The expansion of CD25 high IL-10 high FoxP3 high B regulatory cells is in association with SLE disease activity. J. Immunol. Res. 2015, 254245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong W., et al., Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin. Rheumatol. 37, 43–49 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun P., et al., Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Subramanian S., et al., A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. U.S.A. 103, 9970–9975 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairhurst A. M., et al., Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 38, 1971–1978 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang S. H., et al., B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J. Immunol. 189, 5786–5796 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kil L. P., et al., Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Corneth O. B., et al., Enhanced expression of Bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting T cell homeostasis. J. Immunol. 197, 58–67 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Lyon M. F., Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190, 372–373 (1961). [DOI] [PubMed] [Google Scholar]
- 21.Payer B., Lee J. T., X chromosome dosage compensation: How mammals keep the balance. Annu. Rev. Genet. 42, 733–772 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Plath K., et al., Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Silva J., et al., Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 4, 481–495 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Cotton A. M., et al., Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum. Mol. Genet. 24, 1528–1539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp A. J., et al., DNA methylation profiles of human active and inactive X chromosomes. Genome Res. 21, 1592–1600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brockdorff N., et al., Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351, 329–331 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Brown C. J., et al., A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349, 38–44 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Penny G. D., Kay G. F., Sheardown S. A., Rastan S., Brockdorff N., Requirement for Xist in X chromosome inactivation. Nature 379, 131–137 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Carrel L., Willard H. F., X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Balaton B. P., Cotton A. M., Brown C. J., Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol. Sex Differ. 6, 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., et al., Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl. Acad. Sci. U.S.A. 113, E2029–E2038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syrett C. M., et al., Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4, 126751 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syrett C. M., et al., Diversity of epigenetic features of the inactive X-chromosome in NK cells, dendritic cells, and macrophages. Front. Immunol. 9, 3087 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S.et al.; Autoimmunity Molecular Medicine Team , IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syrett C. M., et al., Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLoS Genet. 13, e1007050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridings-Figueroa R., et al., The nuclear matrix protein CIZ1 facilitates localization of Xist RNA to the inactive X-chromosome territory. Genes Dev. 31, 876–888 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lessing D., Anguera M. C., Lee J. T., X chromosome inactivation and epigenetic responses to cellular reprogramming. Annu. Rev. Genomics Hum. Genet. 14, 85–110 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Rizzetto S., et al., B-cell receptor reconstruction from single-cell RNA-seq with VDJPuzzle. Bioinformatics 34, 2846–2847 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Souyris M., et al., TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Scharer C. D., et al., Epigenetic programming underpins B cell dysfunction in human SLE. Nat. Immunol. 20, 1071–1082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu C., et al., Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh C. A., et al., The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minajigi A., et al., Chromosomes. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science 349, aab2276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monfort A., et al., Identification of spen as a crucial factor for xist function through forward genetic screening in haploid embryonic stem cells. Cell Rep. 12, 554–561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangye S. G., Avery D. T., Deenick E. K., Hodgkin P. D., Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 170, 686–694 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Klein S. L., Flanagan K. L., Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 47.O’Driscoll D. N., et al., Expression of X-linked Toll-like receptor 4 signaling genes in female vs. male neonates. Pediatr. Res. 81, 831–837 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Tukiainen T., et al., Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syrett C. M., Sierra I., Beethem Z. T., Dubin A. H., Anguera M. C., Loss of epigenetic modifications on the inactive X chromosome and sex-biased gene expression profiles in B cells from NZB/W F1 mice with lupus-like disease. J. Autoimmun. 107, 102357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng X., et al., Bipartite structure of the inactive mouse X chromosome. Genome Biol. 16, 152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giorgetti L., et al., Structural organization of the inactive X chromosome in the mouse. Nature 535, 575–579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Splinter E., et al., The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA. Genes Dev. 25, 1371–1383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C. K., et al., Xist recruits the X chromosome to the nuclear lamina to enable chromosome-wide silencing. Science 354, 468–472 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.