Abstract
The objective of this study was to describe the pharmacokinetics (PK) of intravenous phenobarbital in neonates and infants on extracorporeal membrane oxygenation (ECMO) and to provide dosing recommendations in this population. We performed a retrospective single-center PK study of phenobarbital in neonates and infants on ECMO between January 1, 2014, and December 31, 2018. We developed a population PK model using nonlinear mixed-effects modeling, performed simulations using the final PK parameters, and determined optimal dosing based on attainment of peak and trough concentrations between 20 and 40 mg/L. We included 35 subjects with a median (range) age and weight of 14 days (1–154 days) and 3.4 kg (1.6–8.1 kg), respectively. A total of 194 samples were included in the analysis. Five children (14%) contributing 30 samples (16%) were supported by continuous venovenous hemodiafiltration (CVVHDF). A 1-compartment model best described the data. Typical clearance and volume of distribution for a 3.4–kg infant were 0.038 L/h and 3.83 L, respectively. Clearance increased with age and CVVHDF. Although on ECMO, phenobarbital clearance in children on CVVHDF was 6-fold higher than clearance in children without CVVHDF. In typical subjects, a loading dose of 30 mg/kg/dose followed by maintenance doses of 6–7 mg/kg/day administered as divided doses every 12 hours reached goal concentrations. Age did not impact dosing recommendations. However, higher doses were needed in children on CVVHDF. We strongly recommend therapeutic drug monitoring in children on renal replacement therapy (excluding slow continuous ultrafiltration) while on ECMO.
Keywords: seizures, pharmacotherapy, pharmacology, children, pediatrics
Phenobarbital is one of the oldest antiseizure medications currently in routine clinical use.1 It is a long-acting barbiturate with a half-life of 70–140 hours in adults and 100–200 hours in neonates.2,3 Phenobarbital has low-intermediate protein binding and is primarily metabolized by hepatic cytochromes CYP2C9, CYP2C19, and CYP2E1, with approximately 25% excreted unchanged by the kidneys.2 It has demonstrated efficacy4–7 and is the most commonly administered first-line therapy antiseizure medication for neonatal seizures.8,9 Although the efficacy of phenobarbital has been described in older infants and children, adverse effects including excessive sedation, respiratory depression, and hypotension limit its use in these cohorts.10–12
Electrographic seizures are common in neonates and children undergoing extracorporeal membrane oxygenation (ECMO) and are associated with poor short- and long-term outcomes.13,14 There are limited data to guide optimal dosing of antiseizure medications in pediatric patients undergoing ECMO, including a paucity of data on the pharmacokinetics (PK) of phenobarbital.15 Organ dysfunction, inflammation, capillary leak, fluid shifts, hypoalbuminemia, altered protein binding, and drug sequestration by the circuit are among the factors responsible for altered drug disposition in critically ill children on ECMO.16 Therefore, dosing adjustment may be needed. There are data that suggest children on ECMO require higher phenobarbital doses and reach lower serum concentrations compared with children not on ECMO.17 However, the optimal dosing strategy in this population remains unclear. Improved dosing guidance could increase treatment efficacy and potentially improve outcomes.18,19 We aimed to characterize phenobarbital PK in neonates and infants on ECMO and to provide dosing guidance using a simulation-based analysis.
Methods
Patient Population and Data Collection
This study was approved by Children’s Hospital of Philadelphia’s Institutional Review Board. This was a single-center retrospective PK study of consecutive children (age < 18 years) on ECMO who received intravenous phenobarbital for seizure management between January 1, 2014, and December 31, 2018, at Children’s Hospital of Philadelphia. Children were included if they received ≥1 dose of intravenous phenobarbital with ≥1 phenobarbital plasma concentration drawn for therapeutic drug monitoring (TDM) per standard care while on ECMO. Children were identified by querying the individual electronic medical records of all children in the institutional ECMO database. Data collected included: (1) patient demographics (gestational age, postnatal age, weight, height, presence of an extracardiac shunt); (2) ECMO-related data (ECMO type [venoarterial or venovenous], ECMO flow during PK sampling, date/time of ECMO cannulation and decannulation, date/time of circuit components changes); (3) phenobarbital data (date/time and amount of phenobarbital doses received, phenobarbital plasma concentrations); (4) laboratory values (blood urea nitrogen, serum creatinine, albumin, and alanine aminotransferase); and (5) concomitant medications known to alter phenobarbital metabolism (pantoprazole, midazolam, and [fos]phenytoin).20 When applicable, the presence of renal replacement therapy (RRT, including continuous venovenous hemofiltration [CVVH], continuous venovenous hemodiafiltration [CVVHDF], peritoneal dialysis, and slow continuous ultrafiltration [SCUF]) was collected. Data were collected through the duration of ECMO course or phenobarbital treatment, whichever ended first.
Drug Dosing and PK Sampling
At our institution, phenobarbital is usually administered as a loading dose of 10–20 mg/kg, with additional boluses as needed to terminate seizures. The loading dose is often divided into smaller aliquots of 5 mg/kg in children with hemodynamic instability to limit the potential hypotensive effect associated with phenobarbital administration.21 This is typically followed by maintenance doses of 5 mg/kg/day administered as divided doses every 12 hours. Phenobarbital is infused at a rate of 1 mg/kg/min, and TDM is routinely done per standard care. Typical goal concentrations are between 20 and 40 mg/L for both peak and trough concentrations.20,22 For this study, loading doses were defined as any single boluses given with the intent of quickly rising concentrations and reaching a steady state, whereas maintenance doses were defined as any scheduled doses aiming to maintain that steady state. Peak and trough concentrations were defined as concentrations obtained 1–2 hours following and before a scheduled dose, respectively.
Population PK Analysis
Population PK analysis was performed using the software NONMEM (version 7.3.4) through the interface provided by PDx-POP (version 5.2.2; ICON plc, Leopardstown, Dublin, Ireland). Output was summarized using R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org)andStata(version14.2;StataCorp,College Station, Texas). All models were run with the first-order conditional estimation with interaction method. One- and 2-compartment models were evaluated. Interindividual variability was assessed using an exponential model. Residual variability was evaluated using an additive, a proportional, and a combined (additive and proportional) model. The potential effect of covariates was assessed if a relationship was physiologically plausible. Weight was included in the base model using an allometrically scaled relationship normalized to our population median weight. Both estimated and fixed (0.75 for clearance and 1 for volume of distribution) allometric exponents were evaluated. For comparison with previously published PK data, our final model was also run using an allometrically scaled weight normalized to a standard adult weight of 70 kg. Based on available literature,20,23–27 age was considered an essential covariate because of the ontogeny of metabolic and elimination pathways, and was placed as a covariate on clearance before inclusion of other covariates. Postnatal age and postmenstrual age were both evaluated as (1) continuous variables using exponential relationships and (2) parameters of sigmoidal maturation equations (Hill equations) with evaluation of both estimated and fixed parameters based on published values.20,24,28 Following the inclusion of weight and age, other covariates were evaluated using exponential models for continuous and categorical variables. Evaluated covariates included: the presence of RRT (as a dichotomous variable), ECMO day, age of the ECMO circuit, ECMO flow, presence of an extracardiac shunt, blood product transfusion during PK sampling (as a dichotomous variable), and concomitant medications capable of altering phenobarbital metabolism (phenytoin, fosphenytoin, midazolam, and pantoprazole). The effects of CVVH, CVVHDF, and peritoneal dialysis were evaluated both independently and together as a single covariate (renal replacement therapy [RRT]), whereas the impact of slow continuous ultrafiltration was independently evaluated. As phenobarbital induces its own clearance through hepatic microsome activation when administered for approximately 1 week, the number of days on therapy was also evaluated on clearance if phenobarbital was administered for ≥7 days.29 Covariates were added using a stepwise forward additive approach with a P = .05 (ΔOFV, 3.84). Covariates were then removed from the full model using a stepwise backward elimination approach with a P = .005 (ΔOFV, 7.88).
The goodness-of-fit for each run was assessed by examining the following criteria: visual inspection of diagnostic scatterplots, the precision of the parameter estimates, successful minimization, relative changes in Akaike information criteria and objective function value (OFV), and differences in interindividual and residual variabilities. The robustness of our final model was evaluated using goodness-of-fit plots and the precision of the parameter estimates. Bootstrap simulations (1000 replicates) and log-likelihood profiling were also performed to evaluate the precision of the estimated PK parameters and establish 95% confidence intervals.
Simulations
Simulations were performed using the final phenobarbital PK model. Virtual children with baseline characteristics similar to our population were created. Loading doses ranging from 10 to 60 mg/kg/dose given at a rate of 1mg/kg/min were simulated, and an optimal loading dose was determined based on attainment of goal concentrations. Maintenance doses between 3 and 8 mg/kg/day administered as divided doses every 12 hours were then simulated using our optimal loading dose. Our goal concentrations were 20–40 mg/L for both initial (peak concentration 2 hours following a loading dose) and sustained (trough concentration after 7 days of therapy) efficacy. Given the long half-life of phenobarbital, trough concentrations at 20 days were also simulated in a typical child to approximate a steady state. Simulations included 1000 replicates and were summarized by mean value with standard deviations.
Results
Study Population
A total of 35 neonates and infants were included (Table 1). The median (range) postnatal age, postmenstrual age, and weight were 14 days (1–154 days), 40.4 weeks (35.7–55.9 weeks), and 3.4 kg (1.6–8.1kg), respectively. Neonates comprised the majority of subjects (27 [77%]), whereas 8 subjects (23%) were infants (31 days to <2 years). Nineteen children (54%) were placed on ECMO following cardiac surgery. Five children (14%) received CVVHDF, and 8 children (23%) received slow continuous ultrafiltration. During PK sampling, 4 children (11%) received concomitant fosphenytoin therapy, 2 children (6%) received concomitant phenytoin therapy, 20 children (57%) received concomitant midazolam therapy, and 7 children (20%) received concomitant pantoprazole therapy.
Table 1.
Demographics and clinical characteristics (N=35)
| Variable | N (%) or Median (range) |
|---|---|
| Male | 21 (60%) |
| Postnatal age (days) | 14 (1, 154) |
| Postmenstrual age (weeks) | 40.4 (35.7, 55.9) |
| Weight (kg) | 3.4 (1.6, 8.1) |
| Height (cm) | 50.4 (31.5, 74.8) |
| Hospitalization unit | |
| Neonatal ICU | 13 (37.1%) |
| Cardiac ICU | 22 (62.9%) |
| Presence of an extracardiac shunt | 17 (48.6%) |
| Main indication for ECMO | |
| Postoperative from cardiac surgery | 19 (54.3%) |
| Cardiac failure unrelated to cardiac surgery | 3 (8.6%) |
| Respiratory failure ± pulmonary hypertension | 13 (37.1%) |
| ECMO mode | |
| Veno-arterial | 35 (100%) |
| ECMO duration (days) | 8.1 (1.5, 39.8) |
| RRT | |
| CVVHDF | 5 (14.3%) |
| SCUF | 8 (22.9%) |
| SCR value (mg/dL) (normal laboratory values: 0.1–0.4 mg/dL) | 0.5 (0.2, 1.4) |
| BUN value (mg/dL) (normal laboratory values: 2–19 mg/dL) | 23 (3, 43) |
| ALT1 value (U/L) (normal laboratory values: 7–50 U/L) | 35 (20, 400) |
| Albumin1 value (g/dL) (normal laboratory values: 2.8–4.0 g/dL) | 2.6 (1.7, 4) |
| Co-medication | |
| Fosphenytoin | 4 (11.4%) |
| Phenytoin | 2 (5.7%) |
| Midazolam | 20 (57.1%) |
| Pantoprazole | 7 (20%) |
| Survive to discharge | 24 (68.6%) |
ALT and albumin values available for 34 children
ALT: alanine aminotransferase, BUN: blood urea nitrogen, CVVHDF: continuous venovenous hemodiafiltration, ECMO: extracorporeal membrane oxygenation, ICU: intensive care unit, RRT: renal replacement therapy, SCR: serum creatinine, SCUF: slow continuous ultrafiltration
Phenobarbital Dosing and PK Specimens
Over the first 72 hours of phenobarbital treatment, children received a median of 3 loading doses (1–14 doses) of 10.1 mg/kg/dose (2.4–29.4 mg/kg/dose), resulting in a median total loading amount in this initial period of 49.8 mg/kg (10.1–165.3 mg/kg). Additional loading doses were administered despite reaching goal concentrations (>20 mg/L) in 24 children (69%) and reaching above-goal concentrations (>40 mg/L) in 8 children (23%). Loading doses were followed by maintenance doses of 2.5 mg/kg/dose (1.2–10.7 mg/kg/dose) given every 12 hours (8–12 hours).
A total of 194 concentrations contributed to the analysis (Table S1). Of those, 106 (55%) were peak concentrations, 41 (21%) were trough concentrations, and 47 (24%) were drawn at other times during the dosing interval. Samples collected during RRT represented 16% of all concentraitons (30 of 194), and 21% (40 of 194) were also included in a previous study conducted by our group.27 The mean ± SD peak and trough concentrations were 34.5 ± 16.1 and 29.1 ± 15.3 mg/L, respectively. None of the samples were below the quantification limit.
Population PK Model
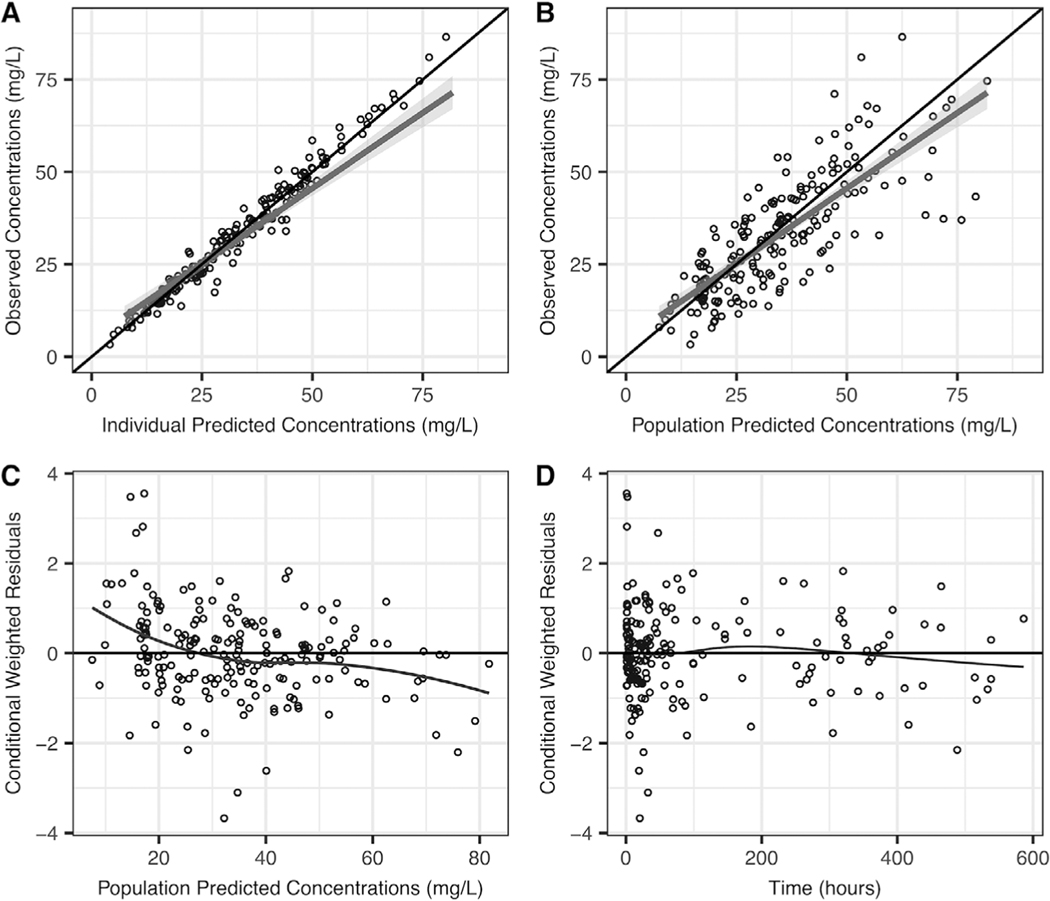
A 1-compartment model best described our data. Fixed allometric exponents (0.75 for clearance and 1 for volume of distribution [V]) characterized the relationship between weight and the PK parameters adequately. The impact of age was best characterized using a sigmoidal equation including postnatal age, where Hill was fixed to 1 and TM50 (the age when clearance reaches 50% of adult clearance) was estimated at 5.91 days. The progression of model development is shown in Table S2. After accounting for weight and age, RRT on clearance was the only significant covariate. Interindividual variability was 39% for clearance and 26% for V. A proportional model characterized residual variability well, and it was estimated at 11%. Diagnostic plots, bootstraps, and log-likelihood profiles confirmed the robustness of our model (Table 2 and Figures 1 and S1).
Table 2.
Final model and bootstrap analysis1
| Final Model | Bootstrap Analysis2 | ||||||
|---|---|---|---|---|---|---|---|
| Point Estimate | RSE (%) | 95% CI | CV (%) | 2.5th percentile | Median | 97.5th percentile | |
| CL (L/h) for a 3.4-kg neonate | 0.038 | 11.9 | 0.03, 0.05 | 0.03 | 0.037 | 0.05 | |
| V (L/3.4 kg) | 3.83 | 4.73 | 3.48, 4.18 | 3.50 | 3.83 | 4.21 | |
| TM50 on CL (days) | 5.91 | 31.5 | 2.26, 9.56 | 1.84 | 5.96 | 12.30 | |
| RRT3 on CL | 6.23 | 4.35 | 5.70, 6.76 | 4.71 | 7.97 | 17.60 | |
| Interindividual variability on CL | 0.15 | 34.8 | 0.05, 0.25 | 38.9 | 0.04 | 0.14 | 0.24 |
| Interindividual variability on V | 0.07 | 24.3 | 0.04, 0.10 | 26.2 | 0.03 | 0.07 | 0.10 |
| Residual variability4 | 0.01 | 26.8 | 0.006, 0.02 | 10.9 | 0.005 | 0.01 | 0.02 |
Parameter estimates are for a 3.4–kg neonate
Bootstrap successful in 100% of runs
Excluding slow continuous ultrafiltration
Proportional error
CL (L/h)=0.038*(WT/3.4)0.75*(AGE/(5.91+AGE)) *6.23 (if on RRT)
V (L)=3.83*(WT/3.4)
ALB: albumin, CL: clearance, CV: coefficient of variation, RRT: renal replacement therapy, RSE: relative standard error, TM50: age at which clearance reaches 50% of adult values, V: volume of distribution
Figure 1.
Final phenobarbital diagnostic plots: observed versus individual-predicted concentrations (A) or population-predicted concentrations (B), and conditional weighted residuals versus population-predicted concentrations (C), or time (D).
Our population estimates for a 3.4-kg infant were 0.038 L/h and 3.83 L for clearance and V, respectively. When normalized to a 70-kg weight, this correlates to a clearance of 0.37 L/h, and a volume of 78.9 L. Clearance in children on RRT was 6.2-fold higher than clearance in children without RRT. Clearance increased with age and was 2.6-fold higher in a 4-monthold infant (5 kg) compared with a 7-day-old neonate (3 kg).
Simulations
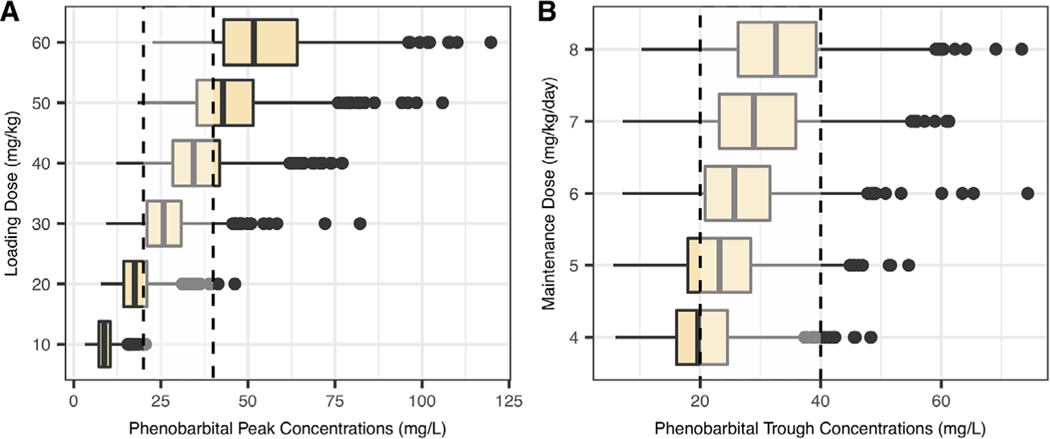
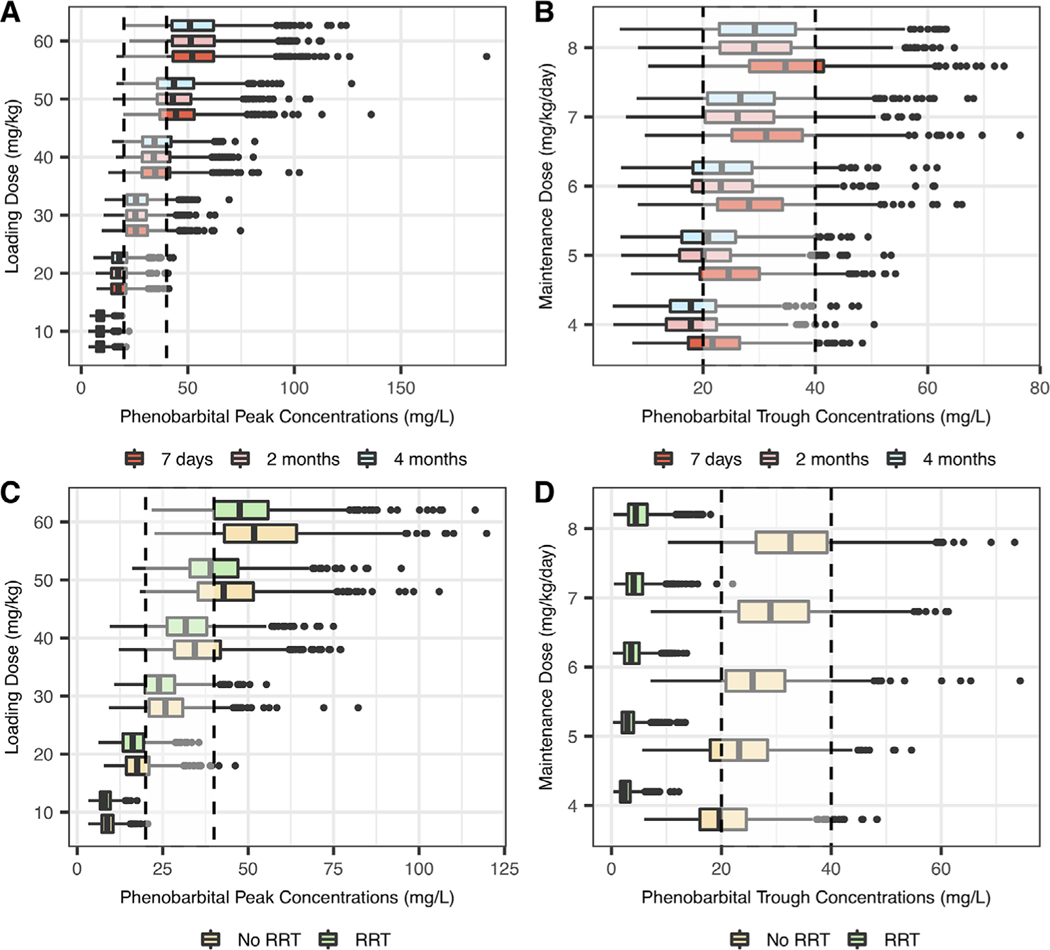
A typical child with baseline characteristics corresponding to our population median values was created. In addition, 4 virtual children with different characteristics (RRT and postnatal age from 7 to 120 days with corresponding weight from 3 to 5 kg) were created to illustrate the effects of covariates on phenobarbital concentrations. A loading dose of 30 mg/kg reached our goal peak concentrations in a typical child on ECMO, with an attainment rate of 75% (Table 3 and Figure 2). A loading dose of 40 mg/kg showed a higher attainment rate in children on RRT compared with a loading dose of 30 mg/kg (76% vs 72%). Age did not have a significant impact on the optimal loading dose (Figure 3). Maintenance doses of 6 and 7 mg/kg/day administered as divided doses every 12 hours showed equal attainment rates of goal trough concentrations at 7 days (73% and 73%, respectively). Following the first 10 days of treatment, phenobarbital concentrations remained relatively stable in a typical child, as shown in Figure S2. Standard maintenance dosing regimens did not reach goal trough concentrations on RRT (Figure 3). Therefore, additional maintenance dosing regimens were evaluated, ranging from 10 to 52.5 mg/kg/day administered as divided doses every 6–12 hours. The optimal maintenance dosing regimen in children on RRT was 40 mg/kg/day administered as divided doses every 6 hours, reaching goal trough concentrations at 7 days in 57% of subjects (mean concentration of 28.2 ± 12.7 mg/L).
Table 3.
Attainment of goal concentrations from simulations
| Dose | Target attainment (%) | Concentrations1 (mg/L) |
|---|---|---|
| Goal: Peak concentration between 20–40 mg/L 2 hours post-dose | ||
| 30 mg/kg | 74.8% | 26.7 ± 7.9 |
| 40 mg/kg | 68.9% | 35.7 ± 10.0 |
| Goal: Trough concentration between 20–40 mg/L at 7 days | ||
| 5 mg/kg/day | 61.0% | 23.6 ± 7.6 |
| 6 mg/kg/day | 72.6% | 26.4 ± 8.3 |
| 7 mg/kg/day | 72.7% | 29.9 ± 9.1 |
| Goal: Trough concentration between 20–40 mg/L at 20 days | ||
| 5 mg/kg/day | 49.6 % | 22.7 ± 9.4 |
| 6 mg/kg/day | 59.0 % | 26.4 ± 10.7 |
| 7 mg/kg/day | 59.5 % | 31.2 ± 12.8 |
| On renal replacement therapy | ||
| Goal: Peak concentration between 20–40 mg/L 2 hours post-dose | ||
| 30 mg/kg | 72.0% | 24.6 ± 6.5 |
| 40 mg/kg | 76.1% | 32.7 ± 9.0 |
| Goal: Trough concentration between 20–40 mg/L at 7 days | ||
| 45 mg/kg/day given as divided doses q12h | 50.4% | 27.6 ± 14.4 |
| 45 mg/kg/day given as divided doses q8h | 52.3% | 31.1 ± 15.4 |
| 40 mg/kg/day given as divided doses q6h | 57.1% | 28.2 ± 12.7 |
Mean value ± SD
Figure 2.
Simulated phenobarbital peak (A) and trough concentrations at 7 days (B). Simulations of trough concentrations included a loading dose of 30 mg/kg. The gray box represents goal concentrations between 20 and 40 mg/dL.
Figure 3.
Simulated peak phenobarbital concentrations according to age (A) or the presence of RRT (C), and trough concentrations at 7 days according to age (B) or the presence of RRT (D). Estimated weights were: 3 kg for a 7-day-old neonate, 4 kg for a 2-month-old infant, and 5 kg for a 4-month-old infant. Maintenance dose simulations included a loading dose of 30 mg/kg in children without RRT and 40 mg/kg in children with RRT. The dotted lines represent goal concentrations between 20 and 40 mg/dL. RRT, renal replacement therapy.
Discussion
Although phenobarbital PK was previously described using noncompartmental analysis in neonates and infants supported by ECMO,30 to our knowledge this is the first study using population PK analysis to characterize phenobarbital disposition in the same population. A 1-compartment model resulted in the best fit for our data, as previously reported in children.20,23–27,31–33 The typical clearance and V values for a 3.4-kg in this study were 0.038 L/h and 3.83 L, respectively. Clearance increased with age and RRT. In typical children, a loading dose of 30 mg/kg/dose followed by maintenance doses of 6–7 mg/kg/day administered as divided doses every 12 hours reached goal concentrations. Age did not impact dosing recommendations. However, a loading dose of 40 mg/kg/dose followed by much higher maintenance doses (40 mg/kg/day administered as divided doses every 6 hours) were needed in children on RRT.
Pokorna et al previously used noncompartmental analysis to describe phenobarbital PK in 16 children undergoing ECMO.30 When adjusting their results on a 70-kg weight for comparison, our population estimate for clearance was lower (0.37 vs 0.55 L/h/70 kg), whereas our population estimate for V was higher (78.9 vs 34.3 L/70 kg). As both clearance values are consistent with previously described values in critically ill children (0.14–0.67 L/h/70 kg),24,27,32 the difference potentially represents the expected variability in clearance in this population. Our population estimate for V (78.9 L/70 kg) was higher than reported values in critically ill children without ECMO (44.667.2 L/70 kg),24,27,32 but it was consistent with a published study conducted by our group in a neonatal cohort following cardiac surgery. In that previous study, which included 12 neonates undergoing ECMO, ECMO was as significant co variate on V, and its presence resulted in a V of 80.7 L/70 kg.27 However, the comparison between both studies is limited by 10 infants (29%) overlapping both studies (Table S1). The increased V associated with ECMO in children may be caused by multiple factors, including a large amount of blood products used for circuit priming and hemostasis, the higher likelihood for hemodynamic instability requiring fluid resuscitation and the presence of an inflammatory reaction associated with capillary leak syndrome and fluid extravasation. A potential explanation for our higher V compared with that of Pokorna et al may be the inclusion of children with higher illness acuity and systemic inflammation requiring more aggressive fluid resuscitation, as the presence of RRT in some of our subjects may suggest. In a previously published in vitro study using older ECMO circuits, up to 17% of the dose was sequestrated by the circuit, thereby increasing V.34 However, drug sequestration does not appear to be significant with contemporary circuits.35
The presence of slow continuous ultrafiltration, analyzed separately from other RRT modalities, did not significantly impact phenobarbital clearance, as expected, because it is not an efficient solute removal modality. However, the presence of RRT, which exclusively comprised CVVHDF in our cohort, was the most significant covariate in our model (OFV, −335, when added in the univariable analysis step; Table S2). Phenobarbital’s low molecular weight (254.22 g/mol)36 and elevated free fraction because of (1) low-intermediate protein binding (28%−36% in neonates)37 and (2) our low albumin values (median, 2.6 g/dL) may explain its effective removal by continuous RRT.38 According to our model, clearance was 6-fold higher in children on RRT compared with typical children without RRT. Although our small number of subjects (only 5 children on RRT, all of them supported by CVVHDF) may limit the accuracy and external validity of this value, the impact of RRT on clearance was precisely estimated (Table 2). Our findings are also consistent with a previously reported 6-fold higher extracorporeal clearance compared with baseline in a 14-year-old adolescent on CVVHDF.39 Our simulations indicated that standard maintenance doses do not reach goal trough concentrations in children on CVVHDF, and higher maintenance doses of 40 mg/kg/day administered as divided doses every 6 hours were required. This is consistent with previous case reports indicating the need to administer significantly higher doses in CVVHD (3.6-fold increase in a neonate)40 and CVVHDF (8.3fold increase [assuming a bioavailability of 89%20] in a 14-year-old adolescent).39 However, caution should be applied when interpreting this suggested dosing regimen, as it is based on very few data and it may not be valid in a different cohort. Indeed, although this study provides an estimation of the required maintenance doses in neonates and infants on CVVHDF, more data are needed before providing clear dosing recommendations. Meanwhile, we recommend TDM in children on RRT to ensure adequate PHB exposure. Alternative antiseizure medications with established PK profiles during RRT may also be considered in children on RRT.
Phenobarbital clearance was repeatedly found to increase with either postnatal or postmenstrual age,20,23–27 and we decided to include a parameter characterizing age and maturation in our model before the inclusion of other covariates. The best fit for our data was obtained using a sigmoidal equation that included postnatal age, a fixed Hill value to 1 and an estimated TM50 to 5.91 days. Our TM50 value is lower than previously published values (41 weeks postmenstrual age20 and 22.1 days postnatal age24) but is estimated with poor precision. We believe TM50 may be difficult to precisely estimate in our population because, in addition to the maturation of metabolic and elimination pathways, other factors may explain the progressive increase in clearance with time including (1) clinical improvement and resolution of organ dysfunction, and (2) autoinduction of clearance by phenobarbital. Although the respective effect of those covariates could not be independently characterized because of their collinearity, the covariate “age” may be a surrogate for these effects, and they may in turn influence the TM50 parameter estimate. Moreover, our cohort’s age range is relatively young, with 77% of our population < 1 month old, and we may not be able to characterize the whole maturation period well.
Our simulations showed that loading doses of 30 mg/kg/dose and maintenance doses of 6–7 mg/kg/ day administered as divided doses every 12 hours reached goal concentrations in a typical 3.4-kg infant undergoing ECMO. This is in the higher range of previously recommended dosing regimens in children with and without ECMO with suggested loading doses varying between 15 and 40 mg/kg.20,27,30,41 Of note, we used goal peak and trough concentrations of 20–40 mg/dL to guide our simulations. Higher doses and consequently higher concentrations were shown effective for refractory seizures in children.42 Similarly, in the current study, 69% and 23% of children received additional loading doses despite reaching concentrations >20 and >40 mg/dL, respectively. Higher loading and maintenance doses may therefore be needed depending on the clinical situation and goals of therapy.
Our study has limitations. First, its retrospective nature implies some imprecision in the data, as it relies on variable chart documentation. Second, it mostly includes neonates, and the whole pediatric age spectrum is not represented. Therefore, results should be interpreted with caution in infants and may not apply to older children. However, phenobarbital is mostly used in very young children, and they represent the main population of interest. Although generalizability to an older population may be difficult, the validity of the model in this population is robust. Third, because of the small number of patients undergoing RRT, its effect on clearance may have been mischaracterized despite being precisely estimated. Moreover, as CVVHDF was the only RRT modality used in our population, the effect may be different in children supported by other RRT modalities. CVVH, intermittent hemodialysis, and peritoneal dialysis are also frequently used in children on ECMO.43,44 Their effectiveness in treating phenobarbital intoxication suggests that those modalities are associated with an increase in phenobarbital clearance, although the extent of this effect remain to be characterized.45–48 Therefore, we suggest routine TDM use in children on RRT (excluding slow continuous ultrafiltration). The optimal dosing regimen in children on CVVHDF while supported by ECMO in the current study (40 mg/kg/day divided every 6 hours) may need to be adjusted based on further studies and should be interpreted with caution. Our study also has considerable strengths. It is the largest phenobarbital PK study in children on ECMO and provides useful dosing recommendations. It also is the first phenobarbital PK study to include children on RRT and attempt to characterize its relationship with CL. Our observed increased CL in children on CVVHDF while on ECMO is clinically highly relevant and warrants further studies.
Conclusions
Loading intravenous doses of 30 mg/kg reached goal peak concentrations in all children on ECMO. Maintenance intravenous doses of 6–7 mg/kg/day given every 12 hours achieved goal trough concentrations in children on ECMO not supported by RRT. Standard maintenance dosing regimens were insufficient in children on CVVHDF, and alternative antiseizure medications may be considered in children on CVVHDF while on ECMO. We strongly recommend TDM in children on RRT (excluding slow continuous ultrafiltration) while on ECMO. The impact of RRT on phenobarbital disposition needs to be better characterized in future studies.
Supplementary Material
Acknowledgments
We thank James T. Connelly, manager of the ECMO center at Children’s Hospital of Philadelphia, for support of this study. NSA is supported by NIH grant funding (NS-096058) and receives funding from the Patient-Centered Outcomes Research Institute and the Epilepsy Foundation.
Funding
The authors received no financial support for this research.
The data supporting the findings of this study may be shared on request by the corresponding author (C.T.).
Footnotes
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Supplemental Information
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of web-based version of this article.
References
- 1.Yasiry Z, Shorvon SD. How phenobarbital revolutionized epilepsy therapy: the story of phenobarbital therapy in epilepsy in the last 100 years. Epilepsia. 2012;53(suppl 8): 26–39. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici GM. Clinical pharmacology of phenobarbital in neonates: effects, metabolism and pharmacokinetics. Curr Pediatr Rev. 2016;12(1):48–54. [DOI] [PubMed] [Google Scholar]
- 3.Patsalos PN. Phenobarbital. In: Patsalos PN, ed. Antiepileptic Drug Interactions: A Clinical Guide. Cham, Germany: Springer International Publishing; 2016:81–85. [Google Scholar]
- 4.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–489. [DOI] [PubMed] [Google Scholar]
- 5.Boylan GB, Rennie JM, Chorley G, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004;62(3):486–488. [DOI] [PubMed] [Google Scholar]
- 6.Pathak G, Upadhyay A, Pathak U, Chawla D, Goel SP. Phenobarbitone versus phenytoin for treatment of neonatal seizures: an open-label randomized controlled trial. Indian Pediatr. 2013;50(8):753–757. [DOI] [PubMed] [Google Scholar]
- 7.Glass HC, Soul JS, Chu CJ, et al. Response to antiseizure medications in neonates with acute symptomatic seizures. Epilepsia. 2019;60(3):e20–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellström-Westas L, Boylan G, Ågren J. Systematic review of neonatal seizure management strategies provides guidance on anti-epileptic treatment. Acta Paediatr. 2015;104(2):123–129. [DOI] [PubMed] [Google Scholar]
- 9.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37(2):85–90. [DOI] [PubMed] [Google Scholar]
- 10.Malamiri RA, Ghaempanah M, Khosroshahi N, Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16(5):536–541. [DOI] [PubMed] [Google Scholar]
- 11.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38(2):202–207. [DOI] [PubMed] [Google Scholar]
- 12.Phenobarbital. Wolters Kluwer Clinical Drug Information, Inc. http://online.lexi.com. Published 2019. Accessed September 3, 2019.
- 13.Okochi S, Shakoor A, Barton S, et al. Prevalence of seizures in pediatric extracorporeal membrane oxygenation patients as measured by continuous electroencephalography. Pediat Crit Care Med. 2018;19(12):1162–1167. [DOI] [PubMed] [Google Scholar]
- 14.Polito A, Barrett CS, Wypij D, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. 2013;39(9):1594–1601. [DOI] [PubMed] [Google Scholar]
- 15.Thibault C, Collier H, Naim MY, Heichel J, Schwartz E, Zuppa AF. Patterns of medication exposure in children on extracorporeal membrane oxygenation: a step in prioritizing future pharmacologic studies. Crit Care Explor. 2019;1(9): e0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raffaeli G, Pokorna P, Allegaert K, et al. Drug disposition and pharmacotherapy in neonatal ECMO: from fragmented data to integrated knowledge. Front Pediatr. 2019;7:360. https://www.frontiersin.org/articles/10.3389/fped.2019.00360/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillman NO, Messinger MM, Dinh KN, et al. Evaluation of the effects of extracorporeal membrane oxygenation on antiepileptic drug serum concentrations in pediatric patients. J Pediatr Pharmacol Ther. 2017;22(5):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RP, Banwell B, Berg RA, et al. Impact of an ICUEEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia. 2016;57(5):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris ML, Malloy KM, Lawson SN, Rose RS, Buss WF, Mietzsch U. Standardized treatment of neonatal status epilepticus improves outcome. J Child Neurol. 2016;31(14): 1546–1554. [DOI] [PubMed] [Google Scholar]
- 20.Moffett BS, Weingarten MM, Galati M, et al. Phenobarbital population pharmacokinetics across the pediatric age spectrum. Epilepsia. 2018;59(7):1327–1333. [DOI] [PubMed] [Google Scholar]
- 21.Thibault C, Naim MY, Abend NS, et al. A retrospective comparison of phenobarbital and levetiracetam for the treatment of seizures following cardiac surgery in neonates. Epilepsia. 2020;61(4):627–635. [DOI] [PubMed] [Google Scholar]
- 22.Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–1276. [DOI] [PubMed] [Google Scholar]
- 23.Yukawa M, Yukawa E, Suematsu F, et al. Population pharmacokinetics of phenobarbital by mixed effect modelling using routine clinical pharmacokinetic data in Japanese neonates and infants: an update. J Clin Pharm Ther. 2011;36(6): 704–710. [DOI] [PubMed] [Google Scholar]
- 24.Shellhaas RA, Ng CM, Dillon CH, Barks JD, Bhatt-Mehta V. Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatr Crit Care Med. 2013;14(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voller S, Flint RB, Stolk LM, et al. Model-based clinical dose optimization for phenobarbital in neonates: an illustration of the importance of data sharing and external validation. Eur J Pharm Sci. 2017;109s:S90–S97. https://www.sciencedirect.com/science/article/pii/S0928098717302543 [DOI] [PubMed] [Google Scholar]
- 26.Yukawa E, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of phenobarbital by mixed effect modelling using routine clinical pharmacokinetic data in Japanese neonates and infants. J Clin Pharm Ther. 2005;30(2):159–163. [DOI] [PubMed] [Google Scholar]
- 27.Thibault C, Massey SL, Naim MY, Abend NS, Zuppa AF. Population pharmacokinetics of IV phenobarbital in neonates after congenital heart surgery. Pediatr Crit Care Med. 2020;21(8):e557–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–2952. [DOI] [PubMed] [Google Scholar]
- 29.Dossing M, Pilsgaard H, Rasmussen B, Poulsen HE. Time course of phenobarbital and cimetidine mediated changes in hepatic drug metabolism. Eur J Clin Pharmacol.1983;25(2):215222. [DOI] [PubMed] [Google Scholar]
- 30.Pokorna P, Sima M, Vobruba V, Tibboel D, Slanar O. Phenobarbital pharmacokinetics in neonates and infants during extracorporeal membrane oxygenation. Perfusion. 2018;33(1_suppl):80–86. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Chung JY, Lee YM, et al. Effects of cytochromeP450 (CYP)2C19 polymorphisms on pharmacokinetics of phenobarbital in neonates and infants with seizures. Arch Dis Child. 2012;97(6):569–572. [DOI] [PubMed] [Google Scholar]
- 32.Marsot A, Brevaut-Malaty V, Vialet R, Boulamery A, Bruguerolle B, Simon N. Pharmacokinetics and absolute bioavailability of phenobarbital in neonates and young infants, a population pharmacokinetic modelling approach. Fundam Clin Pharmacol. 2014;28(4):465–471. [DOI] [PubMed] [Google Scholar]
- 33.van den Broek MP, Groenendaal F, Toet MC, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermo pharmacological approach. Clin Pharmacokinet. 2012;51(10):671–679. [DOI] [PubMed] [Google Scholar]
- 34.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15(4):263–266. [DOI] [PubMed] [Google Scholar]
- 35.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33(6):1018–1024. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Biotechnology Information. PubChem Database. Phenobarbital sodium, CID = 23674889, https://pubchem.ncbi.nlm.nih.gov/compound/Phenobarbitalsodium. Accessed February 27, 2020.
- 37.Morselli PL. Clincial pharmacokinetics in neonates. Clin Pharmacokinet. 1976;1(2):81–98. [DOI] [PubMed] [Google Scholar]
- 38.Pea F. Chapter 147 - Principles of pharmacodynamics and pharmacokinetics of drugs used in extracorporeal therapies. In: Ronco C, Bellomo R, Kellum JA, Ricci Z, eds. Critical Care Nephrology. 3rd ed. Philadelphia, PA: Saunders Elsevier; 2019:892–896.e891. [Google Scholar]
- 39.Rosenborg S, Saraste L, Wide K. High phenobarbital clearance during continuous renal replacement therapy: a case report and pharmacokinetic analysis. Medicine. 2014;93(7):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasko DA, Annich GM. Phenobarbital (PB) Pharmacokinetics of an Infant on ECMO and CVVHD: A case report. 2004; Abstract presented at the PCRRT conference, Orlando FL, 2004. http://www.pcrrt.com/pcrrt-abstracts-2004.html. Accessed July 29, 2020. [Google Scholar]
- 41.Schloss B, Hayes D Jr, Tobias JD. Phenobarbital use in an infant requiring extracorporeal membrane life support. J Anaesthesiol Clin Pharmacol. 2013;29(1):92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crawford TO, Mitchell WG, Fishman LS, Snodgrass SR. Very high-dose phenobarbital for refractory status epilepticus in children. Neurology. 1988;38(7):1035–1040. [DOI] [PubMed] [Google Scholar]
- 43.Beltramo F, DiCarlo J, Gruber JB, Taylor T, Totapally BR. Renal replacement therapy modalities in critically ill children. Pediatr Crit Care Med. 2019;20(1):e1–e9. [DOI] [PubMed] [Google Scholar]
- 44.Sasser WC, Robert SM, Askenazi DJ, O’Meara LC, Borasino S, Alten JA. Peritoneal dialysis: an alternative modality of fluid removal in neonates requiring extracorporeal membrane oxygenation after cardiac surgery. J Extra Corpor Technol. 2014;46(2):157–161. [PMC free article] [PubMed] [Google Scholar]
- 45.Le Page AK, Stewart AE, Roehr CC, Johnstone LM, Graudins A. The use of peritoneal dialysis in phenobarbitone toxicity in a critically unwell neonate. Neonatology. 2018;113(2): 117–121. [DOI] [PubMed] [Google Scholar]
- 46.Porto I, John EG, Heilliczer J. Removal of phenobarbital during continuous cycling peritoneal dialysis in a child. Pharmacotherapy. 1997;17(4):832–835. [PubMed] [Google Scholar]
- 47.Jacobs F, Brivet FG. Conventional haemodialysis significantly lowers toxic levels of phenobarbital. Nephrol Dial Transplant. 2004;19(6):1663–1664. [DOI] [PubMed] [Google Scholar]
- 48.Söylemezoglu O, Bakkaloglu A, Yigit S, Saatçi U. Haemodial-ysis treatment in phenobarbital intoxication in infancy. Int Urol Nephrol. 1993;25(1):111–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.