Abstract
Preeclampsia is a hypertensive disorder of pregnancy effecting ~5–8% of pregnancies in the United States, and ~8 million pregnancies worldwide. Preeclampsia is clinically diagnosed after the 20th week of gestation and is characterized by new onset hypertension accompanied by proteinuria and/or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. This broad definition emphasizes the heterogeneity of the clinical presentation of preeclampsia, but also underscores the role of the microvascular beds, specifically the renal, cerebral, and hepatic circulations, in the pathophysiology of the disease. While the diagnostic criteria for preeclampsia relies on the development of de novo hypertension and accompanying clinical symptoms after 20-week gestation, it is likely that subclinical dysfunction of the maternal microvascular beds occurs in parallel and may even precede the development of overt cardiovascular symptoms in these women. However, little is known about the physiology of the non-reproductive maternal microvascular beds during preeclampsia, and the mechanism(s) mediating microvascular dysfunction during preeclamptic pregnancy are largely unexplored in humans despite their integral role in the pathophysiology of the disease. Therefore, the purpose of this review is to provide a summary of the existing literature on maternal microvascular dysfunction during preeclamptic pregnancy by reviewing the functional evidence in humans, highlighting potential mechanisms, and providing recommendations for future work in this area.
Introduction
Preeclampsia is a hypertensive disorder of pregnancy effecting ~5–8% of pregnancies in the United States, and ~8 million pregnancies worldwide [1]. Preeclampsia is diagnosed after the 20th week of gestation and is characterized by new onset hypertension (systolic and/or diastolic pressures ≥140/90 mmHg, respectively), accompanied by proteinuria (0.3 g per 24 h) or, in the absence of proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or new-onset headache unresponsive to medication and not accounted for by alternative diagnoses or visual symptoms [2]. This broad definition emphasizes the heterogeneity of the clinical presentation of preeclampsia, but also underscores the role of the microvascular beds, specifically the renal, hepatic, and cerebral beds, in the pathophysiology of the disease. While the diagnostic criteria for preeclampsia relies on the development of de novo hypertension after 20-week gestation, the etiology of preeclampsia begins much earlier in pregnancy with insufficient uterine artery remodeling and subsequent placental ischemia. This ischemia triggers the release of anti-angiogenic factors which enter the maternal circulation and mediate the multifaceted maternal syndrome through their effects on systemic endothelial and vascular smooth muscle cell function in non-reproductive vascular beds [3,4]. A discussion of the mechanisms contributing to placental vascular dysfunction in preeclampsia is outside the scope of the present review, and the putative molecular mechanisms mediating this inadequate vascularization and its role in the maternal and fetal sequelae of preeclampsia are eloquently reviewed elsewhere by Staff et al. [5,6]. However, given that this inadequate vascularization occurs early in pregnancy, it is likely that subclinical dysfunction of the maternal microvascular beds occurs in parallel and may even precede the development of overt (i.e. reaching a clinical threshold for) cardiovascular dysfunction in these women. It is clear that large artery endothelial function is attenuated during preeclamptic pregnancy [7–9], even before the clinical diagnosis of the disease [10,11]. However, considerably less is known about the non-reproductive maternal microvascular beds, and the mechanism(s) mediating this dysfunction are largely unexplored in humans despite their integral role in the pathophysiology of the disease. Therefore, the purpose of this review is to provide a summary of the existing literature on maternal microvascular dysfunction during preeclamptic pregnancy by reviewing the functional evidence in humans, highlighting potential mechanisms, and providing recommendations for future work in this area.
Renal microcirculatory dysfunction
In healthy pregnancy, the kidneys play a central role in the development and maintenance of the cardiovascular milieu that supports the mother and fetus throughout gestation and undergo several anatomic and functional physiological changes to sustain this role [12]. Vasodilation of the renal circulation results in a 50% increase in renal plasma flow and glomerular filtration rate (GFR) compared with pre-pregnancy values. These changes are mediated by vasodilation of the kidneys that occurs as early as 5-week gestation and precedes full placentation and the development of the uteroplacental circulation [13,14]. Attributable to this increase in GFR, there is an increase in total urinary protein and albumin excretion above non-pregnant values, especially notable after 20 weeks and later into gestation. However, this protein content consists of low-molecular weight proteins, mainly uromodulin, with a small amount of albumin and other circulating proteins.
While the development of new onset proteinuria is no longer an absolute clinical requirement for the diagnosis of preeclampsia, only ~10% of preeclamptic women will present with gestational hypertension and accompanying signs of end-organ dysfunction in the absence of proteinuria [15]. Therefore, the most common presentation of preeclampsia includes proteinuria, defined as ≥0.3 g per 24-h period, or protein/creatinine ratio ≥ 0.3, or a dipstick reading of 2+ when other diagnostic criteria are not available [2]; values which are twice the normal limit in non-pregnant women. The pathogenesis of proteinuria in preeclampsia is driven primarily by glomerular changes, specifically the development of glomerular endotheliosis, and significant damage to the podocytes [16]. Glomerular endotheliosis, a specific variant of thrombotic microangiopathy, is characterized by swollen, vacuolated glomerular endothelial cells with loss of endothelial fenestrae and narrowing or occlusion of the capillary lumens [16–18]. In preeclampsia, renal biopsy studies have revealed that this endotheliosis occurs on a continuum and is present, to some degree, in normal pregnancy [18]. However, the exaggerated form observed in preeclampsia leads to reductions in glomerular filtration through reductions in capillary perfusion secondary to narrowing and in some cases complete occlusion of the capillary lumen. While the mechanisms mediating this glomerular endotheliosis are unclear in preeclampsia, it is likely that the imbalance in pro-angiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) and anti-angiogenic proteins soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin leads to endothelial and podocyte damage. Both sFlt-1 and soluble endoglin bind circulating VEGF and PlGF and prevent their interaction with endothelial cell-surface receptors, and experimental models of VEGF knockout or inhibition with sFlt-1 and endoglin show renal endothelial dysfunction [19–22]. Specifically, animal models of preeclampsia demonstrate that increasing circulating sFlt-1 concentrations during pregnancy results in increased blood pressure accompanied by proteinuria and glomerular endotheliosis [20,21,23], while anti-VEGF therapies or podocyte-specific VEGF knockout in nonpregnant rodents causes glomerular endotheliosis and proteinuria [19,21]. Indeed, the renal pathophysiology of preeclampsia is similar to that observed in anti-VEGF ablation therapy in humans [24], and similar proteinuria develops in cancer patients receiving anti-VEGF treatments [25–27]. Together, these data suggest that the anti-angiogenic actions of elevated circulating sFlt-1 and endoglin during preeclamptic pregnancy likely mediate this endotheliosis, but direct investigation of these mechanisms in pregnant women is lacking. Future studies exploring the role of this anti-angiogenic imbalance and its relation to the severity of renal insufficiency during preeclampsia may begin to shed light on these mechanisms in women.
From a functional standpoint, renal Doppler ultrasonography offers a non-invasive tool for evaluating renal arterial and venous blood flow, providing information about renal microcirculatory flow with little to no risk to the patient. Renal resistive index (RRI) and pulsatility index (PI) are measures of renal vascular impedance and are mainly affected by renal wedge capillary pressure, though they are also influenced by systemic vascular dynamics including pulse pressure, arterial resistance, and vascular compliance [28]. RRI has been suggested to have predictive value in a variety of vascular and renal complications, as high RRI values have been associated with increased risk of cardiovascular events in chronic kidney disease patients and patients with essential hypertension or coronary artery disease [29–32]. However, whether elevated RRI is present in preeclampsia is unresolved. In a study examining 24 women with preeclampsia compared with 24 pregnant controls matched for gestational age, Bahser et al. demonstrated that RRI and PI are elevated in preeclamptic pregnancy [33]. Similarly, Gyselaers et al. examined RRI and PI in control pregnant women, women with gestational hypertension, and those with late (onset > 34 weeks gestation)- or early (onset < 34 weeks gestation)-onset preeclampsia and found that both RRI and PI increased on a continuum from normal pregnancy (reference value) to late-onset (elevated compared with normal pregnancy) to early-onset preeclampsia (highest RRI and PI) [34]. In contrast, findings by Bateman et al. suggest that RRI may not be elevated in preeclampsia compared with matched controls [35]. A recent meta-analysis of renal Doppler ultrasonography findings in preeclampsia concluded that RRI and PI are not altered in preeclampsia, however the variability of findings across studies and the relative dearth of prospective clinical trials utilizing this technique highlight the need for further work in this area [36]. Interestingly, while the meta-analysis by Bellos and Pergialiotis found no consistent effect of preeclampsia on renal resistance or impedance measures, they did conclude that there was an effect on renal interlobar vein impedance index (RIVI) and shorter pulse transit time in the renal vascular bed, indicating involvement of the renal venous compartment in the pathological dysfunction associated with the disease [36]. RIVI is the venous counterpart to RRI, reflects the compliance of the renal parenchyma, and has been reported to be useful in clinical evaluation of obstructive uropathies [37–39]. Maternal RIVI is higher in all preeclampsia (early and late-onset combined) compared with uncomplicated pregnancy, and highest in early-onset preeclampsia [35,40,41]. Interestingly, RIVI is elevated only in women with preeclampsia, not in gestational hypertension [42], and correlates with proteinuria, but only in late-onset preeclampsia despite both proteinuria and RIVI being higher in early-onset disease [40,42,43].
Collectively, the available data from studies in humans describe the renal injury of preeclampsia as characterized by significant damage and/or degradation of the vascular networks within the glomeruli, and vasospasm of the renal microvasculature. This leads to the relative decreases in renal plasma flow and GFR compared with normal pregnancy (Figure 1). While the mechanistic determinants of these changes remain relatively unexplored in preeclampsia because of the challenges of performing in vivo studies in pregnant women, existing evidence from cell and animal models suggests that the angiogenic imbalance characteristic of preeclampsia likely mediates in part the microvascular dysfunction and damage of the renal circulation. In addition to the role(s) of sFlt-1 and soluble endoglin, it is also highly likely that alterations in the angiotensin and endothelin [23] systems observed in preeclampsia mediate the renal microvascular vasospasm. However, mechanistic investigations of these pathways in pregnant women are similarly limited by the teratogenic effects of the available mechanism-specific pharmacological inhibitors (e.g. inhibitors of the renin–angiotensin–aldosterone system and endothelin-1 (ET-1) antagonists). With the upcoming promise of maternally sequestered pharmacological treatment strategies, which stabilize protein-tagged therapeutics in the maternal circulation and prevent their placental transfer [44–46], future work in this area will be essential for describing the mechanisms that mediate this dysfunction and identifying potential therapeutic options for the treatment or prevention of renal microvascular dysfunction in preeclampsia.
Figure 1. The renal injury of preeclampsia is likely mediated by circulating anti-angiogenic factors sFlt-1 and soluble endoglin, inflammatory cytokines such as IL-6 and TNFα, reactive oxygen species, elevated circulating ET-1, and agonistic antibodies to the angiotensin II type 1 receptor (AT1-AA).
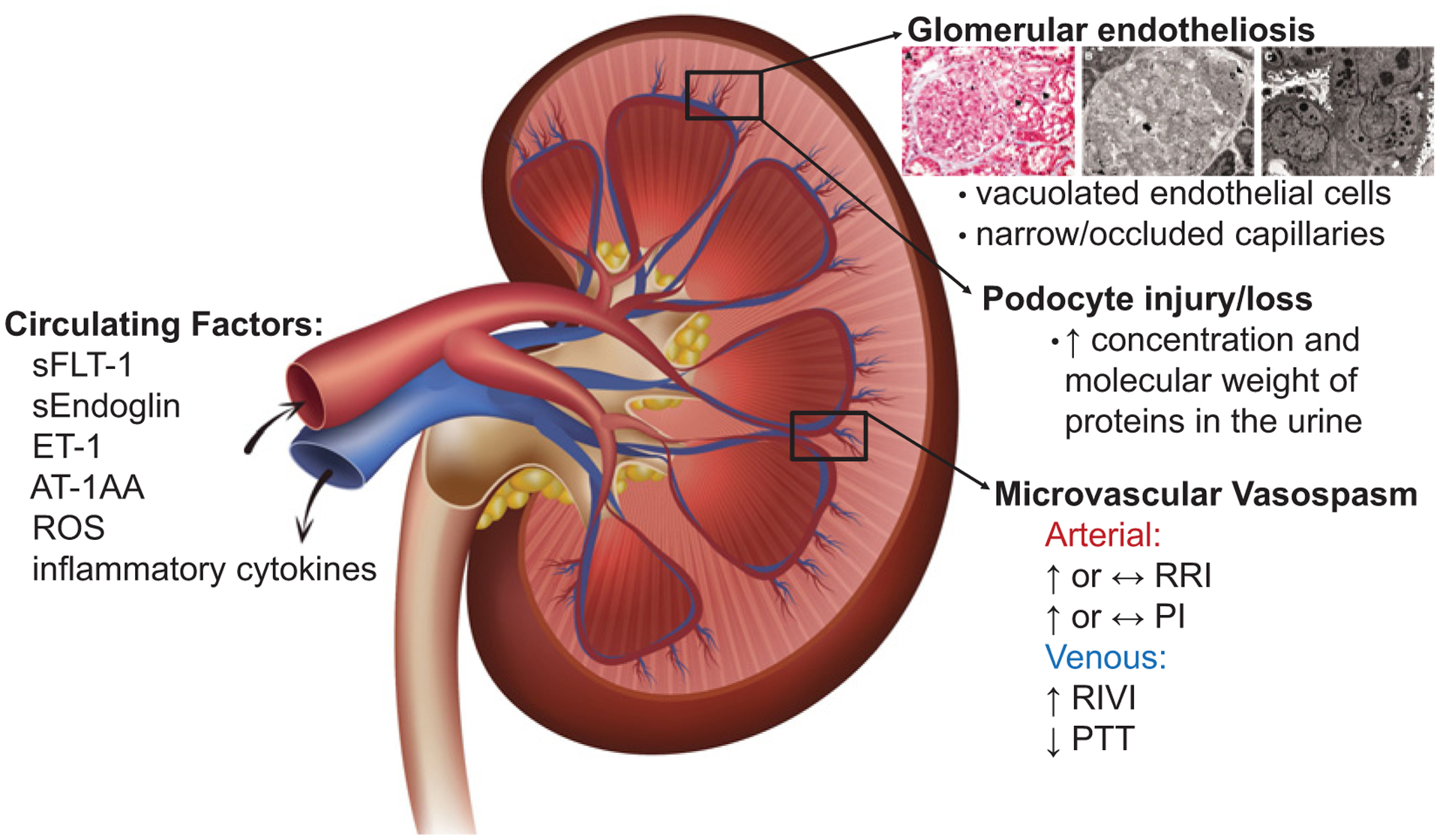
This injury is characterized by significant damage and degradation of the vascular networks within the glomeruli leading to glomerular endotheliosis, podocyte injury or loss, and vasospasm of the renal microvasculature [increased RRI, PI, and RIVI, and shorter pulse transit time (PTT)], leading to the relative decreases in renal plasma flow and GFR and increased concentration and molecular weight of proteins in the urine compared with physiological pregnancy. Image of glomerular endotheliosis adapted from Stillman and Karumanchi [16] with permission.
Hepatic microcirculatory dysfunction
Hepatic blood flow and liver size remain unchanged during healthy pregnancy despite increases in cardiac output. Liver diseases of pregnancy affect ~3–5% of pregnancies and although these diseases arise from many causes, preeclampsia with accompanying liver dysfunction is the most common cause of abnormal liver function, followed by Hemolysis, Elevated Liver transaminases, Low Platelet count (HELLP) syndrome [47]. Preeclampsia and HELLP syndrome are associated with hepatic dysfunction, and in some cases hepatic failure, in pregnancy. Approximately 20–30% of patients with preeclampsia have abnormal liver function tests. Liver dysfunction in preeclampsia is secondary to vasospasm of the hepatic vascular bed leading to increased hepatic microcirculatory resistance, sinusoidal obstruction and ischemia, and subsequently elevated circulating liver enzymes [48,49]. Approximately 12% of preeclamptic patients develop HELLP, but notably, these elevated transaminases can be observed in preeclamptic patients even in the absence of HELLP. It is assumed that the circulating anti-angiogenic, inflammatory, and vasoconstrictor factors in preeclampsia mediate endothelial injury, vasospasm, and fibrin deposition in the hepatic microcirculation, which can lead to large hematomas, capsular tears, and intraperitoneal hemorrhage [50–52]. While these mechanisms are essentially unexplored in preeclampsia, studies of portal hypertension demonstrate that the intrahepatic microcirculation is highly sensitive to circulating vasoactive mediators, which modulate intrahepatic endothelial function and vascular resistance. For example, liver sinusoidal endothelial cells (LSECs) are highly dependent on VEGF for the maintenance of their phenotype and anti-VEGF treatment leads to LSEC dysfunction and subsequent reductions in NO production [53–55]. In preeclampsia, therefore, it is likely that reductions in circulating VEGF contribute to LSEC dysfunction and subsequent attenuations in NO bioavailability within the hepatic microcirculation. The hepatic microcirculation is sensitive to the vasoconstrictor activity of ET-1 [56–58] and angiotensin II [59], both of which are implicated in the exaggerated constrictor tone of the maternal microvasculature in preeclampsia. Similarly, increases in oxidative stress and inflammation have been demonstrated to mediate LSEC dysfunction and increase intrahepatic resistance in portal hypertension [60–62] suggesting that these mechanisms may similarly contribute to hepatic resistance in preeclampsia.
Taken together, although hepatic microcirculatory dysfunction plays a role in the maternal syndrome of preeclampsia and HELLP, the mechanisms and mediators of this dysfunction remain largely unstudied in this condition (Figure 2). Future work informed by the mechanistic overlap between circulating anti-angiogenic factors in preeclampsia and known mediators of portal hypertension is warranted to understand the pathophysiology of hepatic microcirculatory dysfunction and identify mechanistic targets for the treatment or prevention of this complication in preeclampsia and HELLP syndrome.
Figure 2. Hepatic microcirculatory dysfunction in preeclampsia is initiated by vasoactive factors [ET-1, receptor agonistic antibodies to the angiotensin II type 1 receptor (AT1-AA), reactive oxygen species, reduced circulating VEGF] and inflammatory cytokines which likely mediates LSEC dysfunction characterized by reduced nitric oxide production, vasospasm, and fibrin deposition in the hepatic microcirculation.
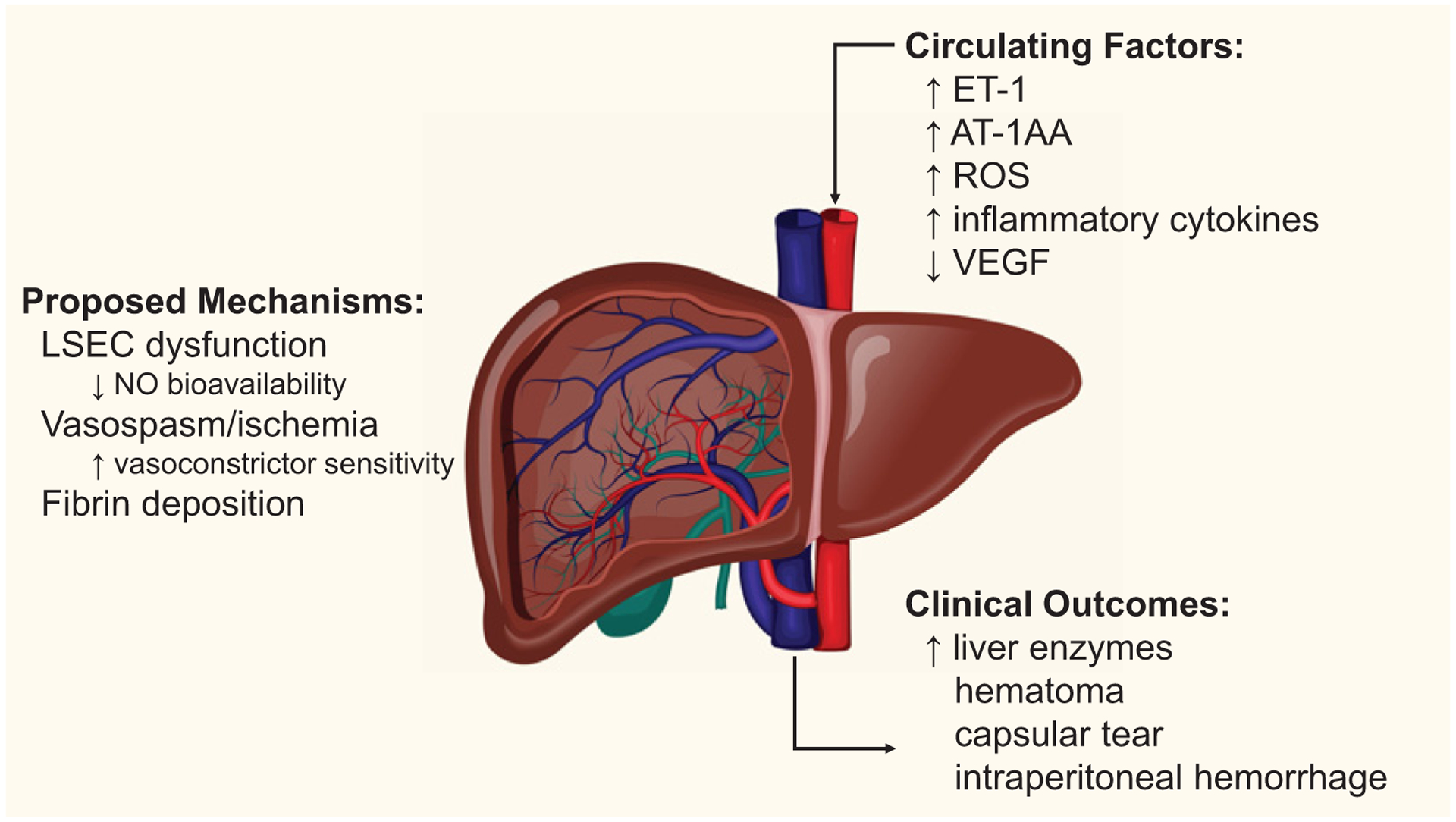
These mechanisms are thought to then cause the clinical outcomes and indicators of liver dysfunction observed in 20–30% of women who develop preeclampsia.
Cerebral microcirculatory dysfunction
The cerebral circulation is tasked with precisely coupling cerebral blood flow to neuronal activity and dampening pulsatile pressure transmission to the microvessels of the brain through efficient autoregulatory mechanisms [63]. Cerebral autoregulation describes the intrinsic myogenic mechanism that modulates cerebrovascular resistance to maintain cerebral blood flow across a wide range of arterial pressures. The cerebral arteries, including middle, anterior, and posterior cerebral arteries, arise from the circle of Willis and further branch into pial arteries that travel along the surface of the brain. The cerebral microcirculation comprises arterioles that penetrate the brain parenchyma and distal capillary beds enveloped by neuroglia to form the neurovascular unit [64]. The cerebral and pial arteries account for approximately two-thirds of cerebral vascular resistance, while the remaining one-third is attributed the microcirculation [65]. The cerebral capillary endothelium is the interface between the systemic circulation and nervous tissue and uniquely features tight cellular junctions that form the blood–brain barrier (BBB) [66]. Cerebral microvascular damage and dysfunction promotes disruption of the BBB and compromises local blood supply, resulting in focal ischemia, to which watershed regions of the cerebral white matter are particularly vulnerable [67].
In healthy pregnancy, cerebral blood flow velocity is reduced across gestation [68–72]. This alteration in cerebral blood flow velocity likely reflects reduced cerebral artery resistance related to circulating vasoactive hormones elevated in pregnancy [70,71] or local endothelium-derived mechanisms [69,71]. Sympathoexcitatory stimuli (i.e., isometric handgrip exercise) do not elicit a change in cerebral blood flow velocity at early-, mid-, or late-gestation, despite a robust increase in arterial pressure [73]. This indicates that cerebrovascular autoregulation is preserved throughout the progression of healthy pregnancy. Moreover, cerebrovascular reactivity to hypercapnia [74,75] and to a visual stimulus (i.e., neurovascular coupling) is unchanged in healthy pregnancy compared with non-pregnant women [74]. Taken together, these studies indicate that cerebrovascular functions are unaffected or represent a beneficial adaptation to systemic cardiovascular changes that occur during healthy pregnancy.
Cerebral artery dynamics in preeclampsia
Cerebrovascular function in pregnancy and preeclampsia has primarily been evaluated via Transcranial Doppler ultrasonography measures of middle cerebral artery (MCA) hemodynamics. In this regard, vascular resistance and cerebral perfusion pressure parameters are derived from MCA blood flow velocity and mean arterial pressure. This technique is not without limitations [76], but is supported by invasive epidural pressure transducer measurements in pregnant women [77]. Women with chronic hypertension in pregnancy demonstrate similar MCA blood flow hemodynamics compared with normotensive pregnancy [78] which indicates that hypertension alone does not account for altered cerebral artery function in preeclampsia. At 19–28 weeks of gestation, normotensive women who later present with either mild or severe clinical signs of preeclampsia exhibit lower MCA resistance index compared with controls, whereas cerebral perfusion pressure does not differ [79]. This finding indicates that altered cerebral hemodynamics antedate overt preeclampsia. Similarly, MCA resistance index is lower in women with preeclampsia in the third trimester despite greater cerebral perfusion pressure compared with healthy pregnancy controls [80–85]. This pattern is observed in women with preeclampsia even after initiating anti-hypertensive medication [86]. Furthermore, women with preeclampsia with cerebral symptoms (e.g. visual disturbances, headache, mental status changes etc.) exhibit greater cerebral perfusion pressure compared with those without cerebral symptoms [82] and a paradoxical inverse association between mean arterial pressure and MCA resistance [87]. Taken together, these findings suggest that the maternal brain is exposed to deleterious hyperperfusion during pregnancy complicated by preeclampsia which may contribute in part to cerebral symptoms.
The mechanisms underlying cerebral hyperperfusion in preeclampsia remain unclear. Low MCA resistance in the presence of elevated perfusion pressure may indicate that (1) cerebral autoregulation is compromised in preeclampsia or (2) arterial pressure exceeds the upper autoregulatory range (i.e., autoregulatory breakthrough) and cerebral blood flow is directly influenced by arterial pressure. The limits of cerebrovascular autoregulation shift to higher arterial pressures in chronic hypertension [88], but the impact of pregnancy and preeclampsia on the autoregulatory range is not known. Consequently, it is impossible to interpret conflicting findings of correlations between mean arterial pressure and MCA flow velocity at rest [86,89] or a lack thereof [90] as evidence of autoregulatory breakthrough or intact autoregulation in preeclampsia, respectively. Among women with preeclampsia, sublingual administration of the nitric oxide (NO) donor isosorbide dinitrate notably reduced MCA blood flow velocity and blood pressure and the degree of change strongly correlated [91]. The reduction in MCA resistance index could reflect an intact autoregulatory response to a fall in arterial pressure, but this result cannot be distinguished from the concomitant effect of the drug on peripheral resistance directly. Dynamic cerebral autoregulation is quantified using continuous MCA flow velocity and arterial pressure without provocation and captures more rapid shifts in cerebral blood flow in response to changes in arterial pressure [92]. Notably, women with preeclampsia exhibit a lower dynamic cerebral autoregulation index compared with healthy pregnancy controls unrelated to differences in arterial blood pressure [84,93]. Moreover, dynamic cerebral autoregulation is lower among women who develop superimposed preeclampsia compared with chronic hypertension in pregnancy [93]. Thus, these findings indicate that elevated blood pressure alone does not explain dysfunctional autoregulation in women with preeclampsia. However, Williams et al. report contradictory findings of efficient dynamic cerebral autoregulation indices among women with preeclampsia compared with controls; this discrepancy is potentially attributable to an alternate analytical approach, measurement time shorter than the recommended duration (i.e., 5 min [94]) and smaller sample size (n=10) [95]. Collectively, transcranial Doppler-assessed MCA hemodynamics are consistent with cerebral hyperperfusion and measures of dynamic cerebral autoregulation provide evidence for compromised cerebral autoregulation in preeclampsia.
Cerebrovascular reactivity describes the degree to which cerebral arterioles respond to vasoactive stimuli [96]. To date, several studies have demonstrated altered cerebrovascular reactivity to hypercapnia in women with preeclampsia compared with controls during transcranial Doppler measurements [80,81,97], and lower cerebrovascular reactivity among women with preeclampsia with severe compared with mild features [81]. Hypercapnia is a potent vasodilatory stimulus at the level of the cerebral microcirculation, eliciting ‘downstream’ increases in MCA flow velocity. Indeed, MCA resistance is reduced in normotensive pregnant women following controlled CO2 inhalation, whereas this effect is absent from women with preeclampsia [80,81]. Interestingly, cerebrovascular reactivity does not differ between normotensive pregnant women and women with chronic hypertension in late pregnancy [78]. In this regard, blood pressure alone may not explain blunted cerebrovascular reactivity in women with preeclampsia compared with healthy pregnancy. In contrast with experimentally controlled CO2 stimulus, breath-hold protocols to induce hypercapnia have yielded mixed results, providing evidence for reduced cerebrovascular reactivity among women with preeclampsia [97] or showing no difference between groups [98]. It is important to note that the former study demonstrated comparable end-tidal CO2 between groups during the breath-hold protocol, [97] while the null study did not assess CO2.
Overall, these findings indicate that cerebral microvascular response to vasodilatory stimuli is attenuated in preeclampsia, particularly as evidenced under controlled CO2 inhalation conditions. While mechanisms that underpin cerebrovascular reactivity to CO2 are not completely understood, this phenomenon is likely driven in part by cerebrovascular endothelium-derived NO-mediated reductions in vascular smooth muscle tone [96]. Indeed, macrovascular endothelial dysfunction is associated with reduced cerebrovascular reactivity in middle-aged and older adults [99]. Endothelial dysfunction is central to the pathophysiology and maternal syndrome in preeclampsia [100]; however, this putative mechanism of altered cerebrovascular reactivity has not been measured in preeclampsia.
Cerebral microvascular dynamics in preeclampsia
Abnormalities in cerebral tissue structure detected by Magnetic Resonance Imaging (MRI) are indicative of cerebral small vessel disease and cerebral microvascular dysfunction [101]. Although MRI is not associated with adverse maternal–fetal outcomes and considered a safe imaging modality in pregnant women [102], clinical recommendations limit use of MRI during pregnancy by clinical indication only [103]. In this regard, assessment of cerebral microvascular function by MRI during preeclampsia is primarily representative of cases with severe features that include cerebral symptoms. Posterior reversible encephalopathy syndrome is defined as vasogenic cerebral edema with neurologic symptoms and is typical of eclampsia [104] but is less characterized in preeclampsia. Case studies demonstrate diffuse cerebral vasospasm in preeclampsia with severe features using MRI angiography [105,106]. Among women with preeclampsia with severe features including headache, most had MRI abnormalities characteristic of diffuse ischemic damage and micro-hemorrhage [107] This finding is extended by Schwatrz et al., who observed vasogenic cerebral edema and multifocal lesions indicative of BBB dysfunction in 20 out of 28 women with preeclampsia, regardless of cerebral symptoms [108]. Notably, cerebral edema was related to serum concentrations of lactate dehydrogenase (LDH) but not blood pressure level [108]. In the brain, LDH is expressed by both astrocytes, glial cells that mediate neurovascular coupling, and neurons and greater levels of LDH in the cerebral spinal fluid and serum are thought to be indicative of cerebral hypoxic injury [109,110]. Indeed, posterior reversible encephalopathy syndrome is thought to be precipitated by systemic endothelial dysfunction that further mediates altered cerebral vasoregulation and disrupts the BBB, leading to local hypoperfusion [111]. In support of this, human cerebrovascular endothelial cells exhibit greater permeability in vitro when exposed to plasma from women with preeclampsia compared with healthy pregnancy [112]. Moreover, BBB permeability increased significantly in cerebral arteries from non-pregnant rats when exposed to plasma from women with severe, purportedly preterm preeclampsia compared with healthy pregnant women [113]. The degree to which BBB disruption is altered by plasma from women with early-onset compared with late-onset preeclampsia phenotypes is contradictory, which may be related in part to differences in exposed tissues [112,114]. Circulating biomarkers of neuroaxonal damage, hypothesized to be a consequence of BBB injury, are elevated in mid- and late-pregnancy in women with preeclampsia compared with healthy pregnancy [115,116], indicating that the BBB injury precedes the onset of overt preeclampsia signs. Although the underlying mechanisms are yet to be elucidated, consistent MRI findings of focal cerebral ischemia and excess BBB permeability in vitro in women with preeclampsia are suggestive of cerebral microvascular endothelial dysfunction that likely contribute to severe cerebral symptoms.
The evidence of cerebral microcirculatory dysfunction in preeclampsia is amassed from non-invasive transcranial Doppler of the MCA, MRI indices of cerebral regional ischemia and BBB disruption, circulating markers of neuronal injury and in vitro models of the BBB permeability (Figure 3). Taken together, these data depict compromised autoregulatory capacity of the cerebral arteries, exposing the ‘upstream’ cerebral microcirculation to injurious hyperperfusion, which likely precipitates BBB damage and local ischemia eventually resulting in neuronal degradation. Although a complete discussion of preclinical models are outside of the scope of the current review, animal models of varied methodology replicate the cerebral microvascular sequelae of preeclampsia and support a casual relation. Indeed, preeclampsia-like syndromes modeled by both mechanically induced placental ischemia [117] and infusion of anti-angiogenic factors [118] elicit increased BBB permeability and impaired cerebral autoregulation, which may be mediated in part by enhanced angiotensin-II type 1 receptor sensitivity or expression [119,120]. However, the cellular pathways underlying cerebrovascular dysregulation in preeclampsia remain unclear. The reader is referred to the following review for a comprehensive description of the preclinical model insights into the impact of pregnancy and preeclampsia on cerebral hemodynamics [121]. While evidence from animal models supports the idea that preeclampsia is causally related to cerebrovascular dysfunction and further implicates placental factors, assessment of cerebral microcirculatory dysfunction in humans is primarily reliant on neuroimaging techniques such as MRI or positron emission tomography. Restricted use of these modalities during pregnancy poses significant challenges to elucidating possible cerebral microcirculatory adaptations to pregnancy or mechanisms that underpin cerebral microvascular damage in women with preeclampsia. Future studies that explore novel application of cerebral oximetry measures (i.e., near-infrared spectrometry) [122] or combine complementary non-invasive measures of cerebrovascular function (i.e., ultrasonography and circulating factors) are needed to provide further insight into cerebral microcirculatory function in human pregnancy and preeclampsia.
Figure 3. Schematic representation of aggregate evidence of cerebral microcirculatory dysfunction during preeclamptic pregnancy in women assessed by transcranial doppler (TCD), circulating biomarkers, MRI, and in vitro models of the BBB.
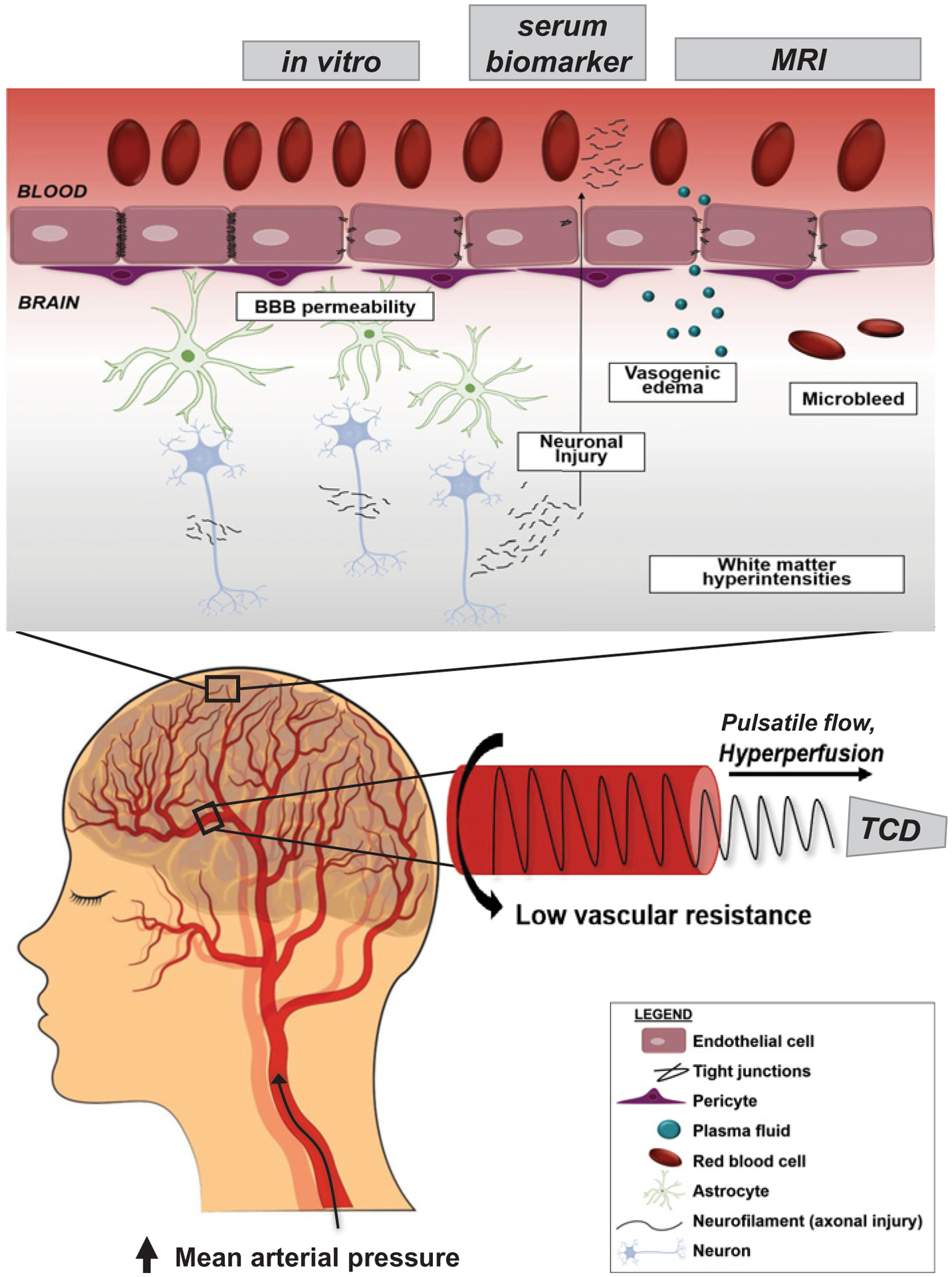
Blood flow velocity in the MCA measured via TCD is consistent with paradoxically reduced cerebral artery resistance in women with preeclampsia despite elevated central blood pressure and pulse pressure. This pulsatile pressure may be propagated into the low resistance cerebral microcirculation and represents a putative mechanism of cerebral endothelial damage and tight junction disruption. This microvascular damage may contribute in part to higher BBB permeability which ultimately promotes vasogenic edema, microbleeds, cerebral white matter ischemia, and axonal injury.
Microvascular endothelial glycocalyx dysfunction as a convergent pathway in preeclampsia
Vascular endothelial dysfunction of large conduit arteries, expressed by reduced brachial artery flow-mediated dilation, occurs early in pregnancy prior to the onset of and during clinical signs of preeclampsia [10]. Similarly, reduced endothelium-dependent vasodilation of microvascular beds is also present in women with preeclampsia [123–126], as a result of either reduced NO bioavailability and/or enhanced constrictor responsiveness to angiotensin II [123,124,127]. Moreover, dysfunction of the endothelial glycocalyx, sometimes called the endothelial surface layer, of the microvascular circulation has emerged as a possible convergent pathway in all tissues/organs in preeclampsia in humans during or preceding preeclampsia.
The glycocalyx is a dynamic, gel-like lining of the luminal side of the endothelium that acts as a protective barrier between the blood and the microvascular wall and regulates endothelial homeostasis [128,129] that becomes damaged in conditions such as aging, autoimmune diseases, and diabetes [130–133]. Given that 95% of the endothelium is located within microvascular capillaries, the glycocalyx plays a critical role in regulating a variety of microvascular functions. [129] A healthy endothelial glycocalyx prevents the adhesion of white blood cells and inflammatory proteins [128], mediates mechanotransduction of hemodynamic shear stress forces from laminar blood flow [134,135], promotes homogeneous microvascular blood flow [128,136] and resistance [137,138], and regulates endothelial permeability [139]. The glycocalyx is composed of a network of proteoglycans with negatively charged long unbranched glycosaminoglycan side-chains (GAGs), and glycoproteins with short-branched carbohydrate side chains, bound to the endothelial membrane [128,129]. Proteoglycans, considered the ‘backbone’ of the glycocalyx, consist of multiple core proteins including syndecans 1–4 and glypicans, with the former binding the plasma membrane via a transmembrane domain and the latter bound via a glycosylphosphatidylinositol anchor [140]. The proteoglycans covalently bind multiple types of GAG chain molecules including heparin sulfate and chondroitin sulfate, and noncovalently bind hyaluronan (i.e., hyaluronic acid, HA) [128,138,140] (see Figure 4A). Heparin sulfate and chondroitin sulfate make up the most common proteoglycan GAGs in the glycocalyx with heparin sulfate and hyaluronan playing critical mechanosensing roles in shear stress-mediated endothelial NO production and cytoskeleton reorganization, while hyaluronan is also critically involved in maintaining glycocalyx integrity, mechanotransduction and vascular permeability [128]. The GAGs are covered by a dynamic layer of plasma proteins, including extracellular superoxide dismutase, albumin, and antithrombin III, that loosely bind with proteoglycans and GAGs promoting protective functions (e.g., antioxidant, anticoagulant) of the endothelium and create a cross-linked mesh providing further stability to the glycocalyx layer [128,140]. Importantly, when the glycocalyx is damaged in certain pathological conditions, not only is the glycocalyx thickness and vasoprotective function compromised, but some GAGs such as heparin sulfate and hyaluronan, and proteoglycans syndecan-1, are shed into the circulation resulting in elevated soluble concentrations in the blood [128,140]. However, there are very little data on the status of the microvascular glycocalyx and shed circulating proteoglycans and GAGs during pregnancies complicated by preeclampsia in humans.
Figure 4. Schematic representation of the endothelial glycocalyx.
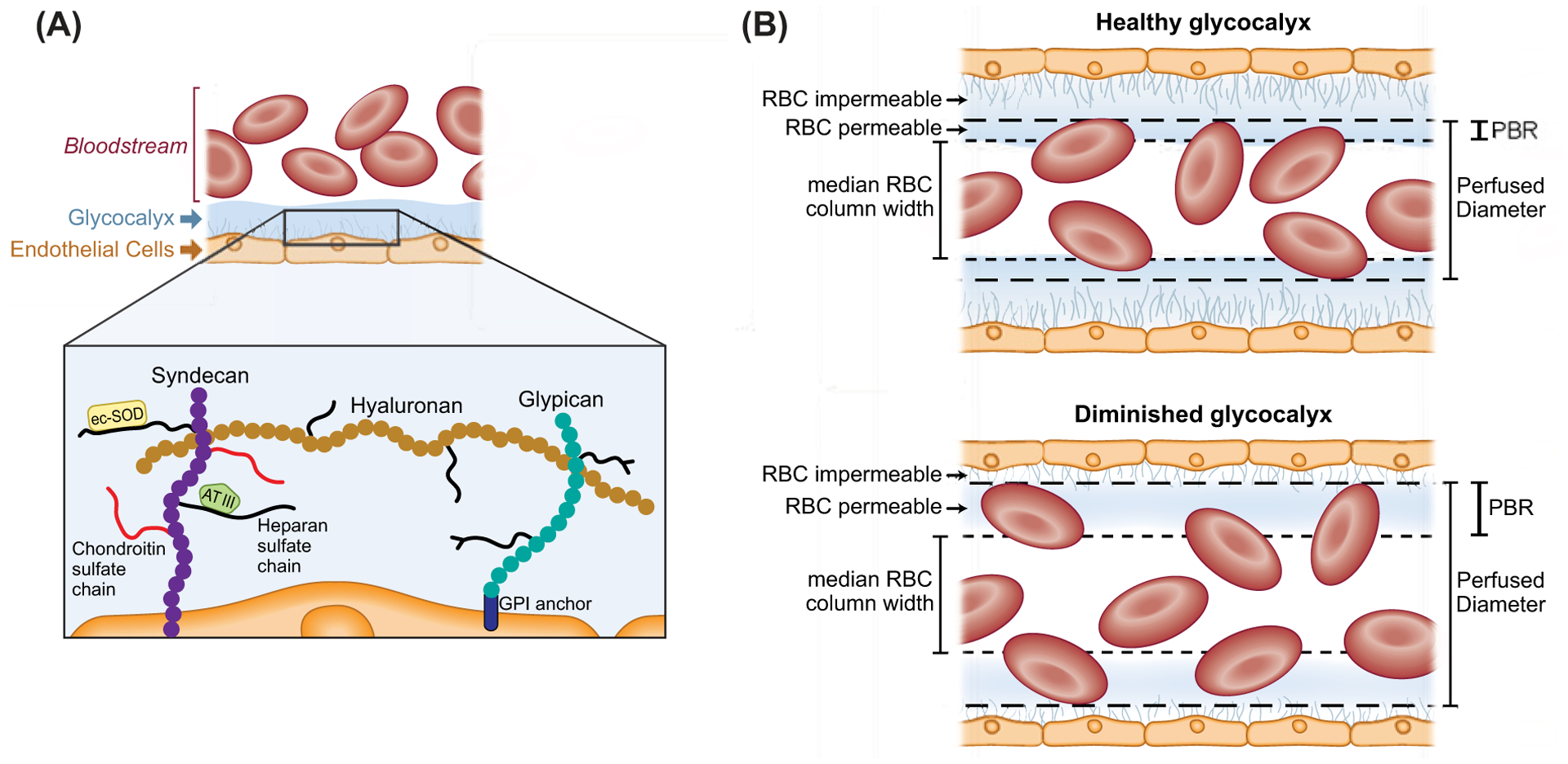
(A) Left side illustrating microvascular endothelial glycocalyx components including the network of core proteoglycans syndecan-1, with covalently bound long unbranched glycosaminoglycan side-chains (GAGs) heparin sulfate and chondroitin sulfate, noncovalently bound hyaluronan, and glypican with short-branched carbohydrate side chains. Also depicted are the dynamic layer of plasma proteins, including extracellular superoxide dismutase (ecSOD) and antithrombin III (AT III) that loosely bind with proteoglycans and glycosaminoglycans promoting protective functions of the endothelium and create a cross-linked mesh providing further stability to the glycocalyx layer. Syndecans bind the plasma membrane via a transmembrane domain and glypicans bind via a glycosylphosphatidylinositol (GPI) anchor. (B) Right side, illustrating a healthy endothelial glycocalyx which is relatively impermeable to red blood cells (RBCs) and other circulating cells in the microcirculation (right, upper panel), whereas a diminished glycocalyx allows for deeper penetration of RBCs into the glycocalyx layer (right, lower panel). This greater penetration of RBCs into the glycocalyx can be quantified in vivo as an increase in the perfused boundary region (PBR) in sublingual microvessels between 5 and 25 μm in diameter in humans. A larger PBR reflects a diminished or thinner glycocalyx layer compared with a smaller PBR and thicker glycocalyx.
The thickness of the healthy glycocalyx is in dynamic equilibrium with enzymatic and shear-induced shedding of these membrane-bound proteogylcans and GAGs being continuously replaced with biosynthesis of new molecules [128]. Given that the glycocalyx is critical for numerous vasoprotective mechanisms of the microvascular endothelium, a degraded or diminished glycocalyx is hypothesized to contribute to the pathophysiology of numerous clinical disorders associated with elevated cardiovascular disease risk in humans [138,141]. However, until recently it was challenging to quantify glycocalyx thickness or degradation in vivo in humans. In this regard, technical advances using video microscopy sidestream dark-field imaging of the sublingual microcirculation coupled with automated commercially available analysis software allows real-time estimation of glycocalyx thickness and microvascular perfusion in humans [138,141]. This technique uses a non-invasive hand-held camera to capture real-time flow of red blood cells in sublingual microvessels by green light-emitting diodes that is absorbed by hemoglobin in red blood cells allowing them to be viewed with video microscopy [130,141,142]. A healthy endothelial glycocalyx is relatively impermeable to red blood cells and other circulating cells in the microcirculation, whereas a degraded or thinner glycocalyx allows for deeper penetration of red blood cells into the glycocalyx layer. This greater penetration of flowing red blood cells into the glycocalyx can be quantified in vivo as an increase in the perfused boundary region (PBR) in sublingual microvessels between 5 and 25 μm in diameter in humans. Thus, a larger PBR reflects a diminished or thinner glycocalyx layer compared with a smaller PBR or thicker glycocalyx [130,141,142] (see Figure 4B). Thus, glycocalyx thickness is reduced (i.e., larger PBR) in healthy aged adults [130], and adults with diabetes [132], end-stage kidney disease [133] and autoimmune disease [131]. In addition, the red blood cell fraction, or the percentage of red blood cells in a given perfused microvessel segment, can be used as an estimate of microvascular perfusion [138,141,142]. However, whether microvascular endothelial glycocalyx thickness and red blood cell perfusion is diminished in preeclampsia has been completely unknown until recently.
Weissgerber et al. (2019) recently found that sublingual PBR was significantly higher, indicating thinner glycocalyx/greater degradation, among third trimester women with early-onset preeclampsia compared with late-onset preeclampsia or healthy pregnancy [143]. In addition, women with early-onset preeclampsia also demonstrated lower red blood cell fraction compared with late-onset preeclampsia or normal pregnancy, suggesting thinner glycocalyx and reduced microvascular perfusion. Of note was that microvascular PBR and red blood cell fraction among pregnant women with late-onset preeclampsia were not different than women with a healthy pregnancy or with gestational diabetes, indicating that early-onset but not late-onset preeclampsia is associated with a dysfunctional microvascular glycocalyx phenotype at the time of clinical presentation of preeclampsia. However, it remains unknown if this glycocalyx phenotype is present before clinical signs of preeclampsia manifested or if these alterations to the glycocalyx were a consequence of the disorder. Additionally, women with early-onset preeclampsia demonstrated elevated circulating concentrations of soluble heparin sulfate and HA, but not syndecan-1, compared with women with late-onset preeclampsia, healthy pregnancy, or gestational diabetes. Thus, these data support the idea of glycocalyx shedding of heparin sulfate and HA at the time of preeclampsia but only in the women with early onset of the disorder.
The observation of a lack of change in soluble syndecan-1 in the circulation of women with preeclampsia was different than several previous studies that demonstrated paradoxically lower soluble syndecan-1 concentrations before the onset and at the time of clinical preeclampsia [144–147]. Because syndecan-1 is also expressed in placental syncytiotrophoblast microvilli and maternal circulating syndecan-1 rises across normal gestation and decreases withing 1–2 days postpartum, this suggests that the placenta is a major source of maternal syndecan-1 in the blood during pregnancy. Moreover, placental syndecan-1 protein and mRNA expression and circulating maternal syndecan-1 concentrations are lower in delivered placenta of women with preeclampsia compared with healthy pregnancy, suggesting down-regulation of placental syndecan-1 or disruption of placental glycocalyx is associated with preeclampsia [144]. However, whether early decreased glycocalyx placental expression, and subsequently reduced soluble syndecan-1, is present before clinical signs of preeclampsia and involved in the pathogenesis of preeclampsia requires further study.
In summary, women with early gestation onset preeclampsia appear to have microvascular endothelial glycocalyx thinning and reduced microvascular perfusion at the time of signs/symptoms of preeclampsia compared with late-onset preeclampsia and healthy pregnancy. Consistent with these structural and functional changes in the microvascular glycocalyx, circulating concentrations of several proteoglycan GAGs are also elevated in the blood suggesting accelerated shedding in early-onset preeclampsia. In contrast, syndecan-1 a core proteoglycan is paradoxically lower in preeclampsia possibly from reduced expression in placental syncytiotrophoblast microvilli, a major source of syndecan-1, but these findings need to be verified in additional human studies. Nevertheless, we propose that disruption of the microvascular endothelial glycocalyx may be a convergent pathway in all maternal tissues/organs present before the clinical onset of preeclampsia that contributes to the pathogenesis of the disorder and therefore could be a common therapeutic target to prevent the onset or attenuate the clinical sequelae of the disorder.
Summary and clinical perspectives
Preeclampsia is a multisystem disorder that is characterized by its maternal cardiovascular sequelae. Among the most common symptoms and complications are those associated with maternal microvascular dysfunction, particularly of the renal, hepatic, and cerebral circulation as well as systemic and placental microvascular glycocalyx dysfunction (Figure 5). While the functional and histological evidence of this dysfunction is evident in preeclampsia, few, if any, in vivo mechanistic studies have been performed in pregnancy. A stark example of this is the complete lack of data on coronary microvascular function in these patients, despite the evidence that cardiac remodeling follows a pathological phenotype in preeclampsia, and postpartum data which demonstrate that coronary flow reserve is reduced in the year(s) following preeclamptic pregnancy [148–150]. There is a clinical need to translate the mechanistic animal and cell culture data to humans in order to fully describe the pathophysiology of the maternal vascular dysfunction and validate the mechanistic targets for treatment in human disease. However, challenges in the use of pharmacology and invasive measurement techniques during pregnancy are major limiters to these types of in vivo investigations. Recent advances in maternally sequestered pharmacologic strategies hold promise for the treatment and investigation of these mechanisms in pregnancy [44–46], but these drug delivery systems are not yet tested or approved for human use. Current and emerging treatment strategies for use during human pregnancy (e.g. aspirin and statin therapy) likely do target the putative endothelial mechanisms mediating the maternal microvascular dysfunction during preeclampsia, but the mechanisms underlying the efficacy of these treatments are still largely hypothetical and remain unexplored in in vivo human trials.
Figure 5. Preeclampsia is a multisystem disorder that is clinically characterized by its effects on the maternal microvasculature.
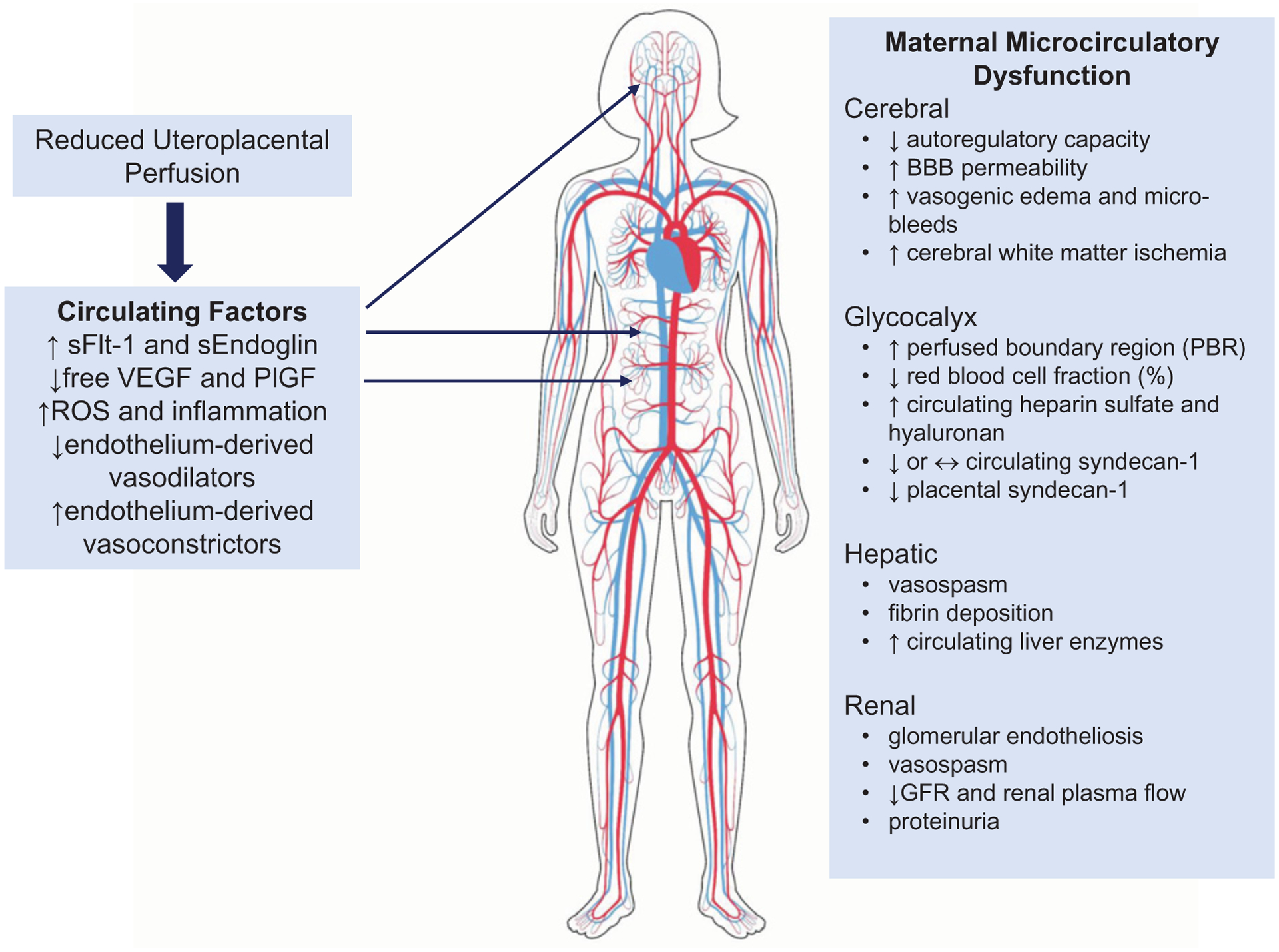
Reduced uteroplacental perfusion triggers the placental release of vasoactive factors that directly interact with the maternal endothelium and likely initiate the multisystem vascular dysfunction observed in the non-reproductive vascular beds of the mother. Abbreviation: ROS, reactive oxygen species.
While it is clear that maternal microvascular dysfunction is present and contributes to the clinical sequelae of preeclampsia during pregnancy, it is unclear whether this dysfunction is present as a risk factor prior to pregnancy or develops as a feature of the disease. To date, no studies have prospectively examined microvascular function prior to pregnancy and then subsequently assessed pregnancy outcomes. This lack of pre-pregnancy data introduces the question of whether women who go on to develop preeclampsia during pregnancy were already predisposed to vascular complications and the substantial cardiovascular strain of the pregnancy simply unmasks this underlying risk. This hypothesis is supported by the fact that preexisting cardiovascular and/or metabolic diseases (e.g. hypertension, type 2 diabetes, elevated blood cholesterol, overweight/obesity etc), which are known to decrease microvascular function, increase the risk of preeclampsia. However, women with no preexisting conditions still make up the majority of preeclampsia patients, underscoring the fact that these risk factors are not obligatory predictors of preeclampsia. The majority of investigations that have sought to examine vascular function prior to preeclampsia have done so in early pregnancy (≤20-week gestation), prior to the development of clinical symptoms. Overwhelmingly, these studies demonstrate a decrease in conduit artery endothelial function in women who go on to develop preeclampsia [10,151–154]. However, it is important to note that although these women were not diagnosed with preeclampsia at the time of the vascular measurements, they were already pregnant and the initiating factors in preeclampsia—i.e. improper placentation and subsequent placental release of anti-angiogenic, oxidative, and inflammatory mediators—arise very early in pregnancy. As such, it is likely that by the time these measurements were made the maternal endothelium had been exposed to the circulating mediators of microvascular dysfunction in preeclampsia for many weeks. To date, only one study has examined the relation between pre-pregnancy measures of vascular function and adverse pregnancy outcomes. Using retrospective data from 359 women in the Cardiovascular Risk in Young Finns Study that were linked with the national birth registry, Harville et al. found no relation between brachial artery flow-mediated dilation, carotid intima-media thickness, Young’s elastic modulus, or distensibility and subsequent hypertensive disorders of pregnancy [155]. While these data suggest that the vascular dysfunction observed during preeclamptic pregnancy is not necessarily reflective of or influenced by pre-pregnancy vascular function, only prospective studies examining vascular function before pregnancy can fully elucidate the role(s) of preceding vascular dysfunction in the risk for developing preeclampsia and the accompanying cardiovascular sequalae. Furthermore, no studies have yet examined microvascular function or the mechanisms mediating this function prior to pregnancy and this area remains wide open for investigation.
Despite the remission of clinical symptoms of preeclampsia postpartum, women who develop preeclampsia during pregnancy are at a significantly greater risk for the development of hypertension and CVD events [156]. These women develop primary hypertension at a younger age (~30–40 years of age vs. ~50–60 years in women who have a normal pregnancy) and with greater frequency than women who have healthy pregnancies [157–160] and they are significantly more likely to die of stroke, myocardial infarction, and end-stage renal disease [161–163]. Increasing evidence collected in the years following a preeclamptic pregnancy suggests that the dysfunction observed during pregnancy remains aberrant postpartum [164–166] and that similar mechanisms, namely changes in the vasoconstrictor sensitivity of the renin-angiotensin [164,166–168] and ET-1 systems [165], mediate this persistent vascular dysfunction. These persistent alterations likely contribute to the increased risk of hypertension, chronic kidney disease, myocardial infarction and heart failure, stroke, and cognitive impairment in these patients [169]. Developing a deeper understanding of the maternal microvascular dysfunction that presents during a preeclamptic pregnancy presents a unique opportunity to not only more fully understand the etiology of the dysfunction that persists postpartum, but also to appropriately intervene to halt or slow the accelerated CVD progression with advancing age in these women.
Building on the functional and mechanistic data reviewed, Table 1 presents a series of future research questions for the continued examination of maternal microvascular dysfunction in preeclampsia. Collectively, understanding the mechanisms of maternal microvascular dysfunction that underlies the clinical symptoms of preeclampsia is essential for the recognition and management of the maternal syndrome, and lends insight into the accelerated CVD risk postpartum. Future in vivo human studies aimed at identifying aberrant microvascular mechanisms during preeclampsia will be essential for the identification/confirmation of interventional targets and treatment strategies that may prevent or reverse the endotheliosis that characterizes the maternal syndrome.
Table 1.
Future research questions
| Renal microcirculatory dysfunction |
|
| Hepatic microcirculatory dysfunction |
|
| Cerebral microcirculatory dysfunction |
|
| Microvascular endothelial glycocalyx dysfunction |
|
Funding
This work was supported by the NIH [grant number R00HL138133 (to A.E.S.)]; the University of Iowa Graduate College Diversity Fellowship (to V.R.N.); the NIH [grant number AG063790 (to G.L.P.)]; and the American Heart Association [grant numbers 18SCG34350001, 15SFRN23760002, 19TPA34910016 (to G.L.P.)].
Abbreviations
- BBB
blood–brain barrier
- ET-1
endothelin-1
- GAG
glycosaminoglycan
- GFR
glomerular filtration rate
- HA
hyaluronic acid
- HELLP
Hemolysis, Elevated Liver transaminases, Low Platelet count
- LDH
lactate dehydrogenase
- LSEC
liver sinusoidal endothelial cell
- MCA
middle cerebral artery
- MRI
magnetic resonance imaging
- NO
nitric oxide
- PBR
perfused boundary region
- PI
pulsatility index
- PlGF
placental growth factor
- RIVI
renal interlobar vein impedance index
- ROS
reactive oxygen species
- RRI
renal resistive index
- sFlt-1
soluble fms-like tyrosine kinase-1
- VEGF
vascular endothelial growth factor
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Uzan J, Carbonnel M, Piconne O, Asmar R and Ayoubi JM (2011) Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc. Health Risk Manag 7, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of O, Gynecologists’ Committee on Practice B-O (2020) Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol 135, e237–e260, 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 3.Qu H and Khalil RA (2020) Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol 319, H661–H681, 10.1152/ajpheart.00202.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu W, Gao W, Rong D, Wu Z and Khalil RA (2018) Molecular determinants of microvascular dysfunction in hypertensive pregnancy and preeclampsia. Microcirculation e12508, Online ahead of print, 10.1111/micc.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staff AC, Fjeldstad HE, Fosheim IK, Moe K, Turowski G, Johnsen GM et al. (2020) Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am. J. Obstet. Gynecol S0002–9378(20)31116–9, 10.1016/j.ajog.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 6.Staff AC (2019) The two-stage placental model of preeclampsia: an update. J. Reprod. Immunol 134–135, 1–10, 10.1016/j.jri.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 7.Guimaraes MF, Brandao AH, Rezende CA, Cabral AC, Brum AP, Leite HV et al. (2014) Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch. Gynecol. Obstet 290, 441–447, 10.1007/s00404-014-3220-x [DOI] [PubMed] [Google Scholar]
- 8.Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K et al. (2014) Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens. Res 37, 145–150, 10.1038/hr.2013.131 [DOI] [PubMed] [Google Scholar]
- 9.Oliveira OP, Araujo Junior E, Lima JW, Salustiano EM, Ruano R, Martins WP et al. (2015) Flow-mediated dilation of brachial artery and endothelial dysfunction in pregnant women with preeclampsia: a case control study. Minerva Ginecol 67, 307–313 [PubMed] [Google Scholar]
- 10.Weissgerber TL, Milic NM, Milin-Lazovic JS and Garovic VD (2016) Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension 67, 415–423, 10.1161/HYPERTENSIONAHA.115.06554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takase B, Goto T, Hamabe A, Uehata A, Kuroda K, Satomura K et al. (2003) Flow-mediated dilation in brachial artery in the second half of pregnancy and prediction of pre-eclampsia. J. Hum. Hypertens 17, 697–704, 10.1038/sj.jhh.1001599 [DOI] [PubMed] [Google Scholar]
- 12.Yip W, Sabanayagam C, Ong PG, Patel UD, Chow KY, Tai ES et al. (2016) Joint effect of early microvascular damage in the eye & kidney on risk of cardiovascular events. Sci. Rep 6, 27442, 10.1038/srep27442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D et al. (1998) Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54, 2056–2063, 10.1046/j.1523-1755.1998.00217.x [DOI] [PubMed] [Google Scholar]
- 14.Varga I, Rigo J Jr, Somos P, Joo JG and Nagy B (2000) Analysis of maternal circulation and renal function in physiologic pregnancies; parallel examinations of the changes in the cardiac output and the glomerular filtration rate. J. Matern. Fetal Med 9, 97–104 [DOI] [PubMed] [Google Scholar]
- 15.Payne B, Magee LA, Cote AM, Hutcheon JA, Li J, Kyle PM et al. (2011) PIERS proteinuria: relationship with adverse maternal and perinatal outcome. J. Obstet. Gynaecol. Can 33, 588–597, 10.1016/S1701-2163(16)34907-6 [DOI] [PubMed] [Google Scholar]
- 16.Stillman IE and Karumanchi SA (2007) The glomerular injury of preeclampsia. J. Am. Soc. Nephrol 18, 2281–2284, 10.1681/ASN.2007020255 [DOI] [PubMed] [Google Scholar]
- 17.Han L, Yang Z, Li K, Zou J, Li H, Han J et al. (2014) Antepartum or immediate postpartum renal biopsies in preeclampsia/eclampsia of pregnancy: new morphologic and clinical findings. Int. J. Clin. Exp. Pathol 7, 5129–5143 [PMC free article] [PubMed] [Google Scholar]
- 18.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S et al. (2003) Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG 110, 831–836, 10.1111/j.1471-0528.2003.02162.x [DOI] [PubMed] [Google Scholar]
- 19.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N et al. (2003) Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest 111, 707–716, 10.1172/JCI17423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S et al. (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest 111, 649–658, 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A et al. (2003) Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem 278, 12605–12608, 10.1074/jbc.C300012200 [DOI] [PubMed] [Google Scholar]
- 22.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM et al. (2006) Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med 12, 642–649, 10.1038/nm1429 [DOI] [PubMed] [Google Scholar]
- 23.Murphy SR, LaMarca BB, Cockrell K and Granger JP (2010) Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55, 394–398, 10.1161/HYPERTENSIONAHA.109.141473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Deile J and Schiffer M (2011) Renal involvement in preeclampsia: similarities to VEGF ablation therapy. J. Pregnancy 2011, 176973, 10.1155/2011/176973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncone D, Satoskar A, Nadasdy T, Monk JP and Rovin BH (2007) Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat. Clin. Pract. Nephrol 3, 287–293, 10.1038/ncpneph0476 [DOI] [PubMed] [Google Scholar]
- 26.Izzedine H, Brocheriou I, Deray G and Rixe O (2007) Thrombotic microangiopathy and anti-VEGF agents. Nephrol. Dialysis Transplant 22, 1481–1482, 10.1093/ndt/gfl565 [DOI] [PubMed] [Google Scholar]
- 27.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL et al. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med 349, 427–434, 10.1056/NEJMoa021491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Nicolo P and Granata A (2017) Renal Resistive Index: not only kidney. Clin. Exp. Nephrol 21, 359–366, 10.1007/s10157-016-1323-3 [DOI] [PubMed] [Google Scholar]
- 29.Komuro K, Yokoyama N, Shibuya M, Soutome K, Hirose M, Yonezawa K et al. (2016) Associations between increased renal resistive index and cardiovascular events. J. Med. Ultrason 43, 263–270, 10.1007/s10396-015-0680-y [DOI] [PubMed] [Google Scholar]
- 30.Doi Y, Iwashima Y, Yoshihara F, Kamide K, Hayashi S, Kubota Y et al. (2012) Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension 60, 770–777, 10.1161/HYPERTENSIONAHA.112.196717 [DOI] [PubMed] [Google Scholar]
- 31.Andrikou I, Tsioufis C, Konstantinidis D, Kasiakogias A, Dimitriadis K, Leontsinis I et al. (2018) Renal resistive index in hypertensive patients. J. Clin. Hypertens 20, 1739–1744, 10.1111/jch.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wybraniec MT, Bozentowicz-Wikarek M, Olszanecka-Glinianowicz M, Chudek J and Mizia-Stec K (2020) Renal resistive index and long-term outcome in patients with coronary artery disease. BMC Cardiovasc. Disord 20, 322, 10.1186/s12872-020-01607-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahser N, Godehardt E, Hess AP and Blume C (2014) Examination of intrarenal resistance indices indicate the involvement of renal pathology as a significant diagnostic classifier of preeclampsia. Am. J. Hypertens 27, 742–749, 10.1093/ajh/hpt233 [DOI] [PubMed] [Google Scholar]
- 34.Gyselaers W, Tomsin K, Staelens A, Mesens T, Oben J and Molenberghs G (2014) Maternal venous hemodynamics in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 14, 212, 10.1186/1471-2393-14-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bateman GA, Giles W and England SL (2004) Renal venous Doppler sonography in preeclampsia. J. Ultrasound Med 23, 1607–1611, 10.7863/jum.2004.23.12.1607 [DOI] [PubMed] [Google Scholar]
- 36.Bellos I and Pergialiotis V (2020) Doppler parameters of renal hemodynamics in women with preeclampsia: a systematic review and meta-analysis. J. Clin. Hypertens 22, 1134–1144, 10.1111/jch.13940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bateman GA and Cuganesan R (2002) Renal vein Doppler sonography of obstructive uropathy. Am. J. Roentgenol 178, 921–925, 10.2214/ajr.178.4.1780921 [DOI] [PubMed] [Google Scholar]
- 38.Vadana BM, Pasumarthy A, Penumalli N and Bellapa NC (2015) Renal venous doppler study in obstructive uropathy. J. Clin. Diagn. Res 9, TC13–TC15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oktar SO, Yucel C, Ozdemir H and Karaosmanoglu D (2004) Doppler sonography of renal obstruction: value of venous impedance index measurements. J. Ultrasound Med 23, 929–936, 10.7863/jum.2004.23.7.929 [DOI] [PubMed] [Google Scholar]
- 40.Gyselaers W, Mesens T, Tomsin K, Molenberghs G and Peeters L (2010) Maternal renal interlobar vein impedance index is higher in early- than in late-onset pre-eclampsia. Ultrasound Obstet. Gynecol 36, 69–75, 10.1002/uog.7591 [DOI] [PubMed] [Google Scholar]
- 41.Gyselaers W, Molenberghs G, Van Mieghem W and Ombelet W (2009) Doppler measurement of renal interlobar vein impedance index in uncomplicated and preeclamptic pregnancies. Hypertens. Pregnancy 28, 23–33, 10.1080/10641950802233056 [DOI] [PubMed] [Google Scholar]
- 42.Gyselaers W, Staelens A, Mesens T, Tomsin K, Oben J, Vonck S et al. (2015) Maternal venous Doppler characteristics are abnormal in pre-eclampsia but not in gestational hypertension. Ultrasound Obstet. Gynecol 45, 421–426, 10.1002/uog.13427 [DOI] [PubMed] [Google Scholar]
- 43.Mesens T, Tomsin K, Staelens AS, Oben J, Molenberghs G and Gyselaers W (2014) Is there a correlation between maternal venous hemodynamic dysfunction and proteinuria of preeclampsia? Eur. J. Obstet. Gynecol. Reprod. Biol 181, 246–250, 10.1016/j.ejogrb.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 44.Logue OC, Mahdi F, Chapman H, George EM and Bidwell GL III (2017) A maternally sequestered, biopolymer-stabilized vascular endothelial growth factor (VEGF) chimera for treatment of preeclampsia. J. Am. Heart Assoc 6, e007216, 10.1161/JAHA.117.007216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George EM, Liu H, Robinson GG and Bidwell GL (2014) A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. J. Drug Target 22, 935–947, 10.3109/1061186X.2014.950666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy AC, Howell JA, Chapman H, Taylor E, Mahdi F, George EM et al. (2020) Biopolymer-delivered, maternally sequestered NF-kappaB (nuclear factor-kappaB) inhibitory peptide for treatment of preeclampsia. Hypertension 75, 193–201, 10.1161/HYPERTENSIONAHA.119.13368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen AM, Kim WR, Larson JJ, Rosedahl JK, Yawn BP, McKeon K et al. (2016) The epidemiology of liver diseases unique to pregnancy in a US Community: a population-based study. Clin. Gastroenterol. Hepatol 14, 287e1–2–294e1–2, 10.1016/j.cgh.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman TM and Wendon J (2002) Severe hepatic dysfunction in pregnancy. QJM 95, 343–357, 10.1093/qjmed/95.6.343 [DOI] [PubMed] [Google Scholar]
- 49.Steingrub JS (2004) Pregnancy-associated severe liver dysfunction. Crit. Care Clin 20, 763–776, xi, 10.1016/j.ccc.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 50.Westbrook RH, Dusheiko G and Williamson C (2016) Pregnancy and liver disease. J. Hepatol 64, 933–945, 10.1016/j.jhep.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 51.Shekhar S and Diddi G (2015) Liver disease in pregnancy. Taiwan J. Obstet. Gynecol 54, 475–482, 10.1016/j.tjog.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 52.Mikolasevic I, Filipec-Kanizaj T, Jakopcic I, Majurec I, Brncic-Fischer A, Sobocan N et al. (2018) Liver disease during pregnancy: a challenging clinical issue. Med. Sci. Monit 24, 4080–4090, 10.12659/MSM.907723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeLeve LD, Wang X, Hu L, McCuskey MK and McCuskey RS (2004) Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am. J. Physiol. Gastrointest. Liver Physiol 287, G757–G763, 10.1152/ajpgi.00017.2004 [DOI] [PubMed] [Google Scholar]
- 54.Bosch J (2007) Vascular deterioration in cirrhosis: the big picture. J. Clin. Gastroenterol 41, S247–S253, 10.1097/MCG.0b013e3181572357 [DOI] [PubMed] [Google Scholar]
- 55.Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D et al. (2017) Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol 66, 212–227, 10.1016/j.jhep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 56.Rockey DC and Weisiger RA (1996) Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology 24, 233–240, 10.1002/hep.510240137 [DOI] [PubMed] [Google Scholar]
- 57.Bauer M, Bauer I, Sonin NV, Kresge N, Baveja R, Yokoyama Y et al. (2000) Functional significance of endothelin B receptors in mediating sinusoidal and extrasinusoidal effects of endothelins in the intact rat liver. Hepatology 31, 937–947, 10.1053/he.2000.5922 [DOI] [PubMed] [Google Scholar]
- 58.Kaneda K, Ekataksin W, Sogawa M, Matsumura A, Cho A and Kawada N (1998) Endothelin-1-induced vasoconstriction causes a significant increase in portal pressure of rat liver: localized constrictive effect on the distal segment of preterminal portal venules as revealed by light and electron microscopy and serial reconstruction. Hepatology 27, 735–747, 10.1002/hep.510270315 [DOI] [PubMed] [Google Scholar]
- 59.Hennenberg M, Trebicka J, Kohistani AZ, Heller J and Sauerbruch T (2009) Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur. J. Clin. Invest 39, 906–913, 10.1111/j.1365-2362.2009.02181.x [DOI] [PubMed] [Google Scholar]
- 60.Karaa A, Kamoun WS, Xu H, Zhang J and Clemens MG (2006) Differential effects of oxidative stress on hepatic endothelial and Kupffer cell eicosanoid release in response to endothelin-1. Microcirculation 13, 457–466, 10.1080/10739680600776278 [DOI] [PubMed] [Google Scholar]
- 61.Lavina B, Gracia-Sancho J, Rodriguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J et al. (2009) Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut 58, 118–125, 10.1136/gut.2008.149880 [DOI] [PubMed] [Google Scholar]
- 62.Iwakiri Y (2012) Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int 32, 199–213, 10.1111/j.1478-3231.2011.02579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willie CK, Tzeng YC, Fisher JA and Ainslie PN (2014) Integrative regulation of human brain blood flow. J. Physiol 592, 841–859, 10.1113/jphysiol.2013.268953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA and Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468, 232–243, 10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faraci FM, Baumbach GL and Heistad DD (1989) Myogenic mechanisms in the cerebral circulation. J. Hypertens. Suppl 7, S61–S64, discussion S5 [PubMed] [Google Scholar]
- 66.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR and Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol. Dis 37, 13–25, 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 67.Pantoni L, Garcia JH and Gutierrez JA (1996) Cerebral white matter is highly vulnerable to ischemia. Stroke 27, 1641–1646, discussion 7, 10.1161/01.STR.27.9.1641 [DOI] [PubMed] [Google Scholar]
- 68.Belfort MA, Tooke-Miller C, Allen JC Jr, Saade GR, Dildy GA et al. (2001) Changes in flow velocity, resistance indices, and cerebral perfusion pressure in the maternal middle cerebral artery distribution during normal pregnancy. Acta Obstet. Gynecol. Scand 80, 104. [PubMed] [Google Scholar]
- 69.Zeeman GG, Hatab M and Twickler DM (2003) Maternal cerebral blood flow changes in pregnancy. Am. J. Obstet. Gynecol 189, 968–972, 10.1067/S0002-9378(03)00820-2 [DOI] [PubMed] [Google Scholar]
- 70.Serra-Serra V, Kyle PM, Chandran R and Redman CW (1997) Maternal middle cerebral artery velocimetry in normal pregnancy and postpartum. BJOG 104, 904–909, 10.1111/j.1471-0528.1997.tb14349.x [DOI] [PubMed] [Google Scholar]
- 71.Williams K and Wilson S (1994) Maternal middle cerebral artery blood flow velocity variation with gestational age. Obstet. Gynecol 84, 445–448 [PubMed] [Google Scholar]
- 72.Brackley KJ, Ramsay MM, Pipkin FB and Rubin PC (1998) A longitudinal study of maternal bloodflow in normal pregnancy and the puerperium: analysis of Doppler waveforms using Laplace transform techniques. BJOG 105, 68–77, 10.1111/j.1471-0528.1998.tb09353.x [DOI] [PubMed] [Google Scholar]
- 73.Bergersen TK, Hartgill TW and Pirhonen J (2006) Cerebrovascular response to normal pregnancy: a longitudinal study. Am. J. Physiol. Heart Circ. Physiol 290, H1856–H1861, 10.1152/ajpheart.00919.2005 [DOI] [PubMed] [Google Scholar]
- 74.Matenchuk BA, James M, Skow RJ, Wakefield P, MacKay C, Steinback CD et al. (2019) Longitudinal study of cerebral blood flow regulation during exercise in pregnancy. J. Cereb. Blood Flow Metab 40, 2278–2288, 10.1177/0271678X19889089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherman R, Bowie R, Henfrey M, Mahajan R and Bogod D (2002) Cerebral haemodynamics in pregnancy and pre-eclampsia as assessed by transcranial Doppler ultrasonography. Br. J. Anaesth 89, 687–692, 10.1093/bja/89.5.687 [DOI] [PubMed] [Google Scholar]
- 76.de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA et al. (2012) Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit. Care 17, 58–66, 10.1007/s12028-012-9672-6 [DOI] [PubMed] [Google Scholar]
- 77.Belfort MA, Tooke-Miller C, Varner M, Saade G, Grunewald C, Nisell H et al. (2000) Evaluation of a noninvasive transcranial Doppler and blood pressure-based method for the assessment of cerebral perfusion pressure in pregnant women. Hypertens. Pregnancy 19, 331–340, 10.1081/PRG-100101995 [DOI] [PubMed] [Google Scholar]
- 78.Riskin-Mashiah S and Belfort MA (2004) Cerebrovascular hemodynamics in pregnant women with mild chronic hypertension. Obstet. Gynecol 103, 294–298, 10.1097/01.AOG.0000110250.48579.21 [DOI] [PubMed] [Google Scholar]
- 79.Riskin-Mashiah S, Belfort MA, Saade GR and Herd JA (2002) Transcranial Doppler measurement of cerebral velocity indices as a predictor of preeclampsia. Am. J. Obstet. Gynecol 187, 1667–1672, 10.1067/mob.2002.127594 [DOI] [PubMed] [Google Scholar]
- 80.Riskin-Mashiah S, Belfort MA, Saade GR and Herd JA (2001) Cerebrovascular reactivity in normal pregnancy and preeclampsia. Obstet. Gynecol 98, 827–832, 10.1097/00006250-200111000-00020 [DOI] [PubMed] [Google Scholar]
- 81.Sariri E, Vahdat M, Behbahani AS, Rohani M and Kashanian M (2013) Cerebro vascular reactivity (CVR) of middle cerebral artery in response to CO25% inhalation in preeclamptic women. J. Mater. Fetal Neonatal Med 26, 1020–1023, 10.3109/14767058.2013.765844 [DOI] [PubMed] [Google Scholar]
- 82.Lee Y-J, Lee S, Jo HN, Kim JM, Kwon BS, Joo JK et al. (2019) Alterations in transcranial Doppler indices of pregnant women with complicated preeclampsia. Pregnancy Hypertens 15, 189–194, 10.1016/j.preghy.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 83.Ohno Y, Kawai M, Wakahara Y, Kitagawa T, Kakihara M and Arii Y (1997) Transcranial assessment of maternal cerebral blood flow velocity in patients with pre-eclampsia. Acta Obstet. Gynecol. Scand 76, 928–932, 10.3109/00016349709034904 [DOI] [PubMed] [Google Scholar]
- 84.van Veen TR, Panerai RB, Haeri S, Griffioen AC, Zeeman GG and Belfort MA (2013) Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet. Gynecol 122, 1064–1069, 10.1097/AOG.0b013e3182a93fb5 [DOI] [PubMed] [Google Scholar]
- 85.Belfort MA, Varner MW, Dizon-Townson DS, Grunewald C and Nisell H (2002) Cerebral perfusion pressure, and not cerebral blood flow, may be the critical determinant of intracranial injury in preeclampsia: a new hypothesis. Am. J. Obstet. Gynecol 187, 626–634, 10.1067/mob.2002.125241 [DOI] [PubMed] [Google Scholar]
- 86.Sonneveld MJ, Brussé IA, Duvekot JJ, Steegers EA, Grune F and Visser GH (2014) Cerebral perfusion pressure in women with preeclampsia is elevated even after treatment of elevated blood pressure. Acta Obstet. Gynecol. Scand 93, 508–511, 10.1111/aogs.12358 [DOI] [PubMed] [Google Scholar]
- 87.Belfort MA, Grunewald C, Saade GR, Varner M and Nisell H (1999) Preeclampsia may cause both overperfusion and underperfusion of the brain, A cerebral perfusion based model. Acta Obstet. Gynecol. Scand 78, 586–591 [PubMed] [Google Scholar]
- 88.Pavy-Le Traon A, Costes-Salon M-C, Galinier M, Fourcade J and Larrue V (2002) Dynamics of cerebral blood flow autoregulation in hypertensive patients. J. Neurol. Sci 195, 139–144, 10.1016/S0022-510X(02)00010-2 [DOI] [PubMed] [Google Scholar]
- 89.Zunker P, Ley-Pozo J, Louwen F, Schuierer G, Holzgreve W and Ringelstein E (1995) Cerebral hemodynamics in pre-eclampsia/eclampsia syndrome. Ultrasound Obstet. Gynecol 6, 411–415, 10.1046/j.1469-0705.1995.06060411.x [DOI] [PubMed] [Google Scholar]
- 90.Belfort MA, Saade GR, Grunewald C, Dildy GA, Varner MA and Nisell H (1999) Effects of blood pressure on orbital and middle cerebral artery resistances in healthy pregnant women and women with preeclampsia. Am. J. Obstet. Gynecol 180, 601–607, 10.1016/S0002-9378(99)70261-9 [DOI] [PubMed] [Google Scholar]
- 91.Nevo O, Thaler I, Shik V, Vortman T and Soustiel JF (2003) The effect of isosorbide dinitrate, a donor of nitric oxide, on maternal cerebral blood flow in gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol 188, 1360–1365, 10.1067/mob.2003.300 [DOI] [PubMed] [Google Scholar]
- 92.Tiecks FP, Lam AM, Aaslid R and Newell DW (1995) Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26, 1014–1019, 10.1161/01.STR.26.6.1014 [DOI] [PubMed] [Google Scholar]
- 93.Van Veen TR, Panerai RB, Haeri S, Singh J, Adusumalli JA, Zeeman GG et al. (2015) Cerebral autoregulation in different hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol 212, 513, e1–e7, 10.1016/j.ajog.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 94.Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB and Network ICAR (2016) Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow Metab 36, 665–680, 10.1177/0271678X15626425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams KP, Galerneau F and Small M (2015) Transfer function analysis of dynamic cerebral autoregulation in preeclampsia. Pregnancy Hypertens 5, 322–324, 10.1016/j.preghy.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 96.Ainslie PN and Duffin J (2009) Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol 296, R1473–R1495, 10.1152/ajpregu.91008.2008 [DOI] [PubMed] [Google Scholar]
- 97.van Veen TR, Panerai RB, Haeri S, Zeeman GG and Belfort MA (2015) Effect of breath holding on cerebrovascular hemodynamics in normal pregnancy and preeclampsia. J. Appl. Physiol 118, 858–862, 10.1152/japplphysiol.00562.2014 [DOI] [PubMed] [Google Scholar]
- 98.Zatik J, Aranyosi J, Settakis G, Pall D, Toth Z, Limburg M et al. (2002) Breath holding test in preeclampsia: lack of evidence for altered cerebral vascular reactivity. Int. J. Obstet. Anesth 11, 160–163, 10.1054/ijoa.2002.0950 [DOI] [PubMed] [Google Scholar]
- 99.Lavi S, Gaitini D, Milloul V and Jacob G (2006) Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, H1856–H1861, 10.1152/ajpheart.00014.2006 [DOI] [PubMed] [Google Scholar]
- 100.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M and Kooner JS (2001) Association of maternal endothelial dysfunction with preeclampsia. JAMA 285, 1607–1612, 10.1001/jama.285.12.1607 [DOI] [PubMed] [Google Scholar]
- 101.Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9, 689–701, 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 102.Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ and Park AL (2016) Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316, 952–961, 10.1001/jama.2016.12126 [DOI] [PubMed] [Google Scholar]
- 103.Practice ACoO. ACOG Committee Opinion. Number 299, September 2004 (replaces no. 158, September 1995) (2004) Guidelines for diagnostic imaging during pregnancy. Obstet. Gynecol 104, 647–651, 10.1097/00006250-200409000-00053 [DOI] [PubMed] [Google Scholar]
- 104.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M et al. (2013) Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am. J. Obstet. Gynecol 208, 468, e1–e6, 10.1016/j.ajog.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 105.Ito T, Sakai T, Inagawa S, Utsu M and Bun T (1995) MR angiography of cerebral vasospasm in preeclampsia. Am. J. Neuroradiol 16, 1344–1346 [PMC free article] [PubMed] [Google Scholar]
- 106.Tsukimori K, Ochi H, Yumoto Y, Iwasaki S, Hojo S, Noguchi T et al. (2008) Reversible posterior encephalopathy syndrome followed by MR angiography-documented cerebral vasospasm in preeclampsia-eclampsia: report of 2 cases. Cerebrovasc. Dis 25, 377, 10.1159/000120689 [DOI] [PubMed] [Google Scholar]
- 107.Chao A-S, Chen Y-L, Chang Y-L, Chao A, Su S-Y and Wang T-H (2020) Severe pre-eclamptic women with headache: is posterior reversible encephalopathy syndrome an associated concurrent finding? BMC Pregnancy Childbirth 20, 1–8, 10.1186/s12884-020-03017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM et al. (2000) Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 217, 371–376, 10.1148/radiology.217.2.r00nv44371 [DOI] [PubMed] [Google Scholar]
- 109.Park JS, You Y, Ahn HJ, Min JH, Jeong W, Yoo I et al. (2020) Cerebrospinal fluid lactate dehydrogenase as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J. Crit. Care 57, 49–54, 10.1016/j.jcrc.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 110.Kärkelä J, Pasanen M, Kaukinen S, Mörsky P and Harmoinen A (1992) Evaluation of hypoxic brain injury with spinal fluid enzymes, lactate, and pyruvate. Crit. Care Med 20, 378–386, 10.1097/00003246-199203000-00015 [DOI] [PubMed] [Google Scholar]
- 111.Bartynski W (2008) Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am. J. Neuroradiol 29, 1043–1049, 10.3174/ajnr.A0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergman L, Acurio J, Leon J, Gatu E, Friis T, Nelander M et al. (2020) Preeclampsia and increased permeability over the blood brain barrier-a role of vascular endothelial growth receptor 2. Am. J. Hypertens 34, 73–81, 10.1093/ajh/hpaa142 [DOI] [PubMed] [Google Scholar]
- 113.Amburgey OA, Chapman AC, May V, Bernstein IM and Cipolla MJ (2010) Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension 56, 1003–1008, 10.1161/HYPERTENSIONAHA.110.158931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schreurs MP, Hubel CA, Bernstein IM, Jeyabalan A and Cipolla MJ (2013) Increased oxidized low-density lipoprotein causes blood-brain barrier disruption in early-onset preeclampsia through LOX-1. FASEB J 27, 1254–1263, 10.1096/fj.12-222216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evers KS, Atkinson A, Barro C, Fisch U, Pfister M, Huhn EA et al. (2018) Neurofilament as neuronal injury blood marker in preeclampsia. Hypertension 71, 1178–1184, 10.1161/HYPERTENSIONAHA.117.10314 [DOI] [PubMed] [Google Scholar]
- 116.Andersson M, Oras J, Thörn SE, Karlsson O, Kälebo P, Zetterberg H et al. (2021) Signs of neuroaxonal injury in preeclampsia—a case control study. PLoS ONE 16, e0246786, 10.1371/journal.pone.0246786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP et al. (2014) Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood–brain barrier permeability. Physiol. Rep 2, e12134, 10.14814/phy2.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wallace K, Bean C, Bowles T, Spencer S-K, Randle W, Kyle PB et al. (2018) Hypertension, anxiety, and blood-brain barrier permeability are increased in postpartum severe preeclampsia/hemolysis, elevated liver enzymes, and low platelet count syndrome rats. Hypertension 72, 946–954, 10.1161/HYPERTENSIONAHA.118.11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duncan JW, Azubuike D, Booz GW, Fisher B, Williams JM, Fan F et al. (2020) Angiotensin II type 1 receptor autoantibody blockade improves cerebral blood flow autoregulation and hypertension in a preclinical model of preeclampsia. Hypertens. Pregnancy 39, 451–460, 10.1080/10641955.2020.1833215 [DOI] [PubMed] [Google Scholar]
- 120.Warrington JP, Fan F, Duncan J, Cunningham MW, LaMarca BB, Dechend R et al. (2019) The angiotensin II type I receptor contributes to impaired cerebral blood flow autoregulation caused by placental ischemia in pregnant rats. Biol. Sex Differ 10, 1–8, 10.1186/s13293-019-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones-Muhammad M and Warrington JP (2019) Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci 9, 224, 10.3390/brainsci9090224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guerci P, Vial F, Feugeas J, Pop M, Baka N-E, Bouaziz H et al. (2014) Cerebral oximetry assessed by near-infrared spectrometry during preeclampsia: an observational study impact of magnesium sulfate administration. Crit. Care Med 42, 2379–2386, 10.1097/CCM.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 123.Anumba DO, Ford GA, Boys RJ and Robson SC (1999) Stimulated nitric oxide release and nitric oxide sensitivity in forearm arterial vasculature during normotensive and preeclamptic pregnancy. Am. J. Obstet. Gynecol 181, 1479–1484, 10.1016/S0002-9378(99)70394-7 [DOI] [PubMed] [Google Scholar]
- 124.Knock GA and Poston L (1996) Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol 175, 1668–1674, 10.1016/S0002-9378(96)70123-0 [DOI] [PubMed] [Google Scholar]
- 125.Cockell AP and Poston L (1997) Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 30, 247–251, 10.1161/01.HYP.30.2.247 [DOI] [PubMed] [Google Scholar]
- 126.McCarthy AL, Woolfson RG, Raju SK and Poston L (1993) Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am. J. Obstet. Gynecol 168, 1323–1330, 10.1016/0002-9378(93)90389-Z [DOI] [PubMed] [Google Scholar]
- 127.Anumba DO, Robson SC, Boys RJ and Ford GA (1999) Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am. J. Physiol 277, H848–H854, 10.1152/ajpheart.1999.277.2.H848 [DOI] [PubMed] [Google Scholar]
- 128.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA and oude Egbrink MG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454, 345–359, 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Broekhuizen LN, Mooij HL, Kastelein JJ, Stroes ES, Vink H and Nieuwdorp M (2009) Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr. Opin. Lipidol 20, 57–62, 10.1097/MOL.0b013e328321b587 [DOI] [PubMed] [Google Scholar]
- 130.Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA et al. (2018) Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol 315, H531–H539, 10.1152/ajpheart.00104.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Machin DR, Gates PE, Vink H, Frech TM and Donato AJ (2017) Automated measurement of microvascular function reveals dysfunction in systemic sclerosis: a cross-sectional study. J. Rheumatol 44, 1603–1611, 10.3899/jrheum.170120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H et al. (2014) Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. (1985) 116, 998–1005, 10.1152/japplphysiol.00919.2013 [DOI] [PubMed] [Google Scholar]
- 133.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT and Vink H (2012) Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol 23, 1900–1908, 10.1681/ASN.2011121181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao Y, Rabodzey A and Dewey CF Jr (2007) Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am. J. Physiol. Heart Circ. Physiol 293, H1023–H1030, 10.1152/ajpheart.00162.2007 [DOI] [PubMed] [Google Scholar]
- 135.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO and Tarbell JM (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res 93, e136–e142, 10.1161/01.RES.0000101744.47866.D5 [DOI] [PubMed] [Google Scholar]
- 136.McClatchey PM, Schafer M, Hunter KS and Reusch JE (2016) The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am. J. Physiol. Heart Circ. Physiol 311, H168–H176, 10.1152/ajpheart.00132.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pries AR and Secomb TW (2005) Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart Circ. Physiol 289, H2657–H2664, 10.1152/ajpheart.00297.2005 [DOI] [PubMed] [Google Scholar]
- 138.Machin DR, Phuong TT and Donato AJ (2019) The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol 45, 66–71, 10.1016/j.coph.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van den Berg BM, Vink H and Spaan JA (2003) The endothelial glycocalyx protects against myocardial edema. Circ. Res 92, 592–594, 10.1161/01.RES.0000065917.53950.75 [DOI] [PubMed] [Google Scholar]
- 140.Dogne S and Flamion B (2020) Endothelial glycocalyx impairment in disease: focus on hyaluronan shedding. Am. J. Pathol 190, 768–780, 10.1016/j.ajpath.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 141.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ et al. (2008) Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J. Appl. Physiol 104, 845–852, 10.1152/japplphysiol.00440.2007 [DOI] [PubMed] [Google Scholar]
- 142.Lee DH, Dane MJ, van den Berg BM, Boels MG, van Teeffelen JW, de Mutsert R et al. (2014) Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 9, e96477, 10.1371/journal.pone.0096477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weissgerber TL, Garcia-Valencia O, Milic NM, Codsi E, Cubro H, Nath MC et al. (2019) Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. J. Am. Heart Assoc 8, e010647, 10.1161/JAHA.118.010647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gandley RE, Althouse A, Jeyabalan A, Bregand-White JM, McGonigal S, Myerski AC et al. (2016) Low soluble Syndecan-1 precedes preeclampsia. PLoS ONE 11, e0157608, 10.1371/journal.pone.0157608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kornacki J, Wirstlein P and Wender-Ozegowska E (2019) Levels of syndecan-1 and hyaluronan in early- and late-onset preeclampsia. Pregnancy Hypertens 18, 108–111, 10.1016/j.preghy.2019.08.165 [DOI] [PubMed] [Google Scholar]
- 146.Greeley ET, Rochelson B, Krantz DA, Xue X, Carmichael JB, Ashour S et al. (2020) Evaluation of Syndecan-1 as a novel biomarker for adverse pregnancy outcomes. Reprod. Sci 27, 355–363, 10.1007/s43032-019-00032-5 [DOI] [PubMed] [Google Scholar]
- 147.Kuessel L, Husslein H, Montanari E, Kundi M, Himmler G, Binder J et al. (2019) Dynamics of soluble syndecan-1 in maternal serum during and after pregnancies complicated by preeclampsia: a nested case control study. Clin. Chem. Lab. Med 58, 50–58, 10.1515/cclm-2019-0686 [DOI] [PubMed] [Google Scholar]
- 148.Kul S, Guvenc TS, Baycan OF, Celik FB, Caliskan Z, Cetin Guvenc R et al. (2020) Combined past preeclampsia and gestational diabetes is associated with a very high frequency of coronary microvascular dysfunction. Microvasc. Res 134, 104104, 10.1016/j.mvr.2020.104104 [DOI] [PubMed] [Google Scholar]
- 149.Ciftci FC, Caliskan M, Ciftci O, Gullu H, Uckuyu A, Toprak E et al. (2014) Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. J. Am. Soc. Hypertens 8, 820–826, 10.1016/j.jash.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 150.Clemmensen TS, Christensen M, Logstrup BB, Kronborg CJS and Knudsen UB (2020) Reduced coronary flow velocity reserve in women with previous pre-eclampsia: link to increased cardiovascular disease risk. Ultrasound Obstet. Gynecol 55, 786–792, 10.1002/uog.20407 [DOI] [PubMed] [Google Scholar]
- 151.Garcia RG, Celedon J, Sierra-Laguado J, Alarcon MA, Luengas C, Silva F et al. (2007) Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. Am. J. Hypertens 20, 98–103, 10.1016/j.amjhyper.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 152.Kamat R, Jain V and Bahl A (2011) Serial estimation of flow mediated dilatation in women at risk of hypertensive disorders of pregnancy. Int. J. Cardiol 149, 17–22, 10.1016/j.ijcard.2009.11.034 [DOI] [PubMed] [Google Scholar]
- 153.Noori M, Donald AE, Angelakopoulou A, Hingorani AD and Williams DJ (2010) Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122, 478–487, 10.1161/CIRCULATIONAHA.109.895458 [DOI] [PubMed] [Google Scholar]
- 154.Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P and Nicolaides KH (2003) Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 361, 1511–1517, 10.1016/S0140-6736(03)13177-7 [DOI] [PubMed] [Google Scholar]
- 155.Harville EW, Juonala M, Viikari JS, Kahonen M and Raitakari OT (2017) Vascular ultrasound measures before pregnancy and pregnancy complications: a prospective cohort study. Hypertens. Pregnancy 36, 53–58, 10.1080/10641955.2016.1237643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bellamy L, Casas JP, Hingorani AD and Williams DJ (2007) Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974, 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmed R, Dunford J, Mehran R, Robson S and Kunadian V (2014) Pre-eclampsia and future cardiovascular risk among women: a review. J. Am. Coll. Cardiol 63, 1815–1822, 10.1016/j.jacc.2014.02.529 [DOI] [PubMed] [Google Scholar]
- 158.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP et al. (2017) Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet. Gynecol 49, 143–149, 10.1002/uog.17343 [DOI] [PubMed] [Google Scholar]
- 159.Haukkamaa L, Salminen M, Laivuori H, Leinonen H, Hiilesmaa V and Kaaja R (2004) Risk for subsequent coronary artery disease after preeclampsia. Am. J. Cardiol 93, 805–808, 10.1016/j.amjcard.2003.11.065 [DOI] [PubMed] [Google Scholar]
- 160.Mongraw-Chaffin ML, Cirillo PM and Cohn BA (2010) Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 56, 166–171, 10.1161/HYPERTENSIONAHA.110.150078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alvarez-Alvarez B, Martell-Claros N, Abad-Cardiel M and Garcia-Donaire JA (2016) Hypertensive disorders during pregnancy: cardiovascular long-term outcomes. Hipertension y riesgo vascular [DOI] [PubMed] [Google Scholar]
- 162.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS and Olafsson O (2005) Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG 112, 286–292, 10.1111/j.1471-0528.2004.00396.x [DOI] [PubMed] [Google Scholar]
- 163.Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H and Sigfusson N (1995) Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet. Gynecol. Scand 74, 772–776, 10.3109/00016349509021195 [DOI] [PubMed] [Google Scholar]
- 164.Stanhewicz AE, Jandu S, Santhanam L and Alexander LM (2017) Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 70, 382–389, 10.1161/HYPERTENSIONAHA.117.09386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stanhewicz AE, Jandu S, Santhanam L and Alexander LM (2017) Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin. Sci. (Lond.) 131, 2777–2789, 10.1042/CS20171292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stanhewicz AE and Alexander LM (2019) Local Angiotensin 1–7 administration improves microvascular endothelial function in women who have had preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol 318, R148–R155, 10.1152/ajpregu.00221.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF and Seely EW (2010) Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55, 1239–1245, 10.1161/HYPERTENSIONAHA.109.147595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van der Graaf AM, Toering TJ, van der Wiel MWK, Frenay AS, Wallukat G, Dechend R et al. (2017) Angiotensin II responsiveness after preeclampsia: translational data from an experimental rat model and early-onset human preeclampsia. J. Hypertens 35, 2468–2478, 10.1097/HJH.0000000000001474 [DOI] [PubMed] [Google Scholar]
- 169.Stanhewicz AE (2018) Residual vascular dysfunction in women with a history of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R1062–R1071, 10.1152/ajpregu.00204.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


