Abstract
Coronavirus disease 2019 (COVID-19) has been associated with an increased risk of venous and arterial thrombotic disease. Although pulmonary embolism has been the most common thrombotic complication, there have been recent reports of COVID-19-associated large-vessel ischemic stroke, acute upper- and lower-limb ischemia, as well as infarctions of the abdominal viscera, including renal, splenic, and small bowel infarctions. Here, we describe a case of splenic infarction (SI) associated with aortic thrombosis, which evolved despite the prophylactic use of low-molecular-weight heparin (LMWH), in a 60-year-old female patient with COVID-19. The patient was treated clinically with a therapeutic dose of LMWH, followed by warfarin, and eventually presented a favorable outcome. We also present a review of the literature regarding SI in patients with COVID-19.
Key Indexing Terms: Spleen, Splenic infarction, Thrombosis, Covid-19, Aorta
Introduction
Thrombotic complications, mostly pulmonary complications, are common in patients with coronavirus disease 2019 (COVID-19) and contribute significantly to morbidity and mortality.1 In such patients, microvascular and macrovascular thromboembolic or in situ thrombotic complications have been observed with increasing frequency in the vasculature of the lungs, spleen, brain, gut, and limbs.2 Here, we describe a case of splenic infarction (SI) associated with aortic thrombosis that evolved in a patient with COVID-19, despite the prophylactic use of low-molecular-weight heparin (LMWH), and we review the literature on SI published before and during the current pandemic, emphasizing aspects related to its common risk factors, clinical presentation, diagnosis, and treatment.
Case presentation
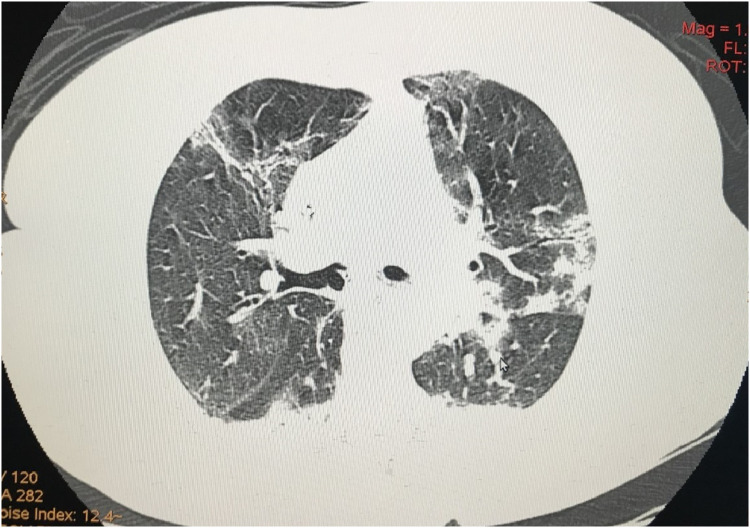
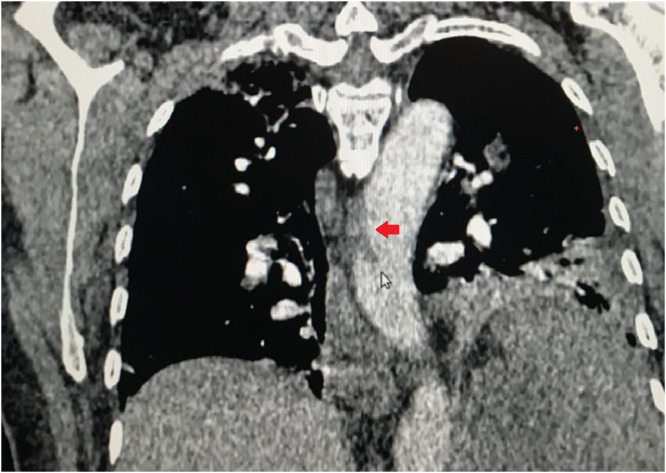
A 60-year-old female infected with severe acute respiratory syndrome coronavirus 2, as confirmed by reverse transcriptase-polymerase chain reaction, was admitted to our intensive care unit (ICU) because she presented with a 5-day history of fever, vomiting, abdominal discomfort, and mental confusion. The patient was previously healthy without any known risk factor for atherosclerotic cardiovascular disease (ASCVD) except for a body mass index of 28 kg/m2. Her calculated 10-year ASCVD risk was 5.2%, and she reported no history of hematologic disorders or vasculitis. At admission, she had a respiratory rate of 42 breaths/minute, a peripheral oxygen saturation of 88% on room air, a heart rate of 110 beats/minute, and a blood pressure of 98/50 mmHg. She was intubated and was started on volume expansion, antibiotics (ceftriaxone and azithromycin), and a vasopressor, as well as being given a prophylactic dose of enoxaparin (60 mg/day). The electrocardiogram showed only sinus tachycardia, and the patient did not have cardiac arrhythmia during her hospital stay. An initial computed tomography (CT) scan showed areas of consolidation interspersed with ground-glass opacities and central/peripheral septal thickening in all pulmonary lobes, affecting 50% of the parenchyma and accompanied by minimal left pleural effusion (Fig. 1). Within the first 24 hours after ICU admission, the patient showed rapid improvement and was extubated. However, she thereafter evolved to mild chest pain and continued to require oxygen. On day 10 after ICU admission, CT angiography showed consolidation in the left lung base, with minimal ipsilateral pleural effusion, together with multisegmental arterial filling defects in the right lung. The CT also showed marked splenomegaly with well-defined peripheral hypodense lesions (Fig. 2), highly suggestive of SI, and an area of hypodensity near the right wall of the descending aorta, consistent with a thrombus (Fig. 3). There were no other occurrences of ischemia (renal infarction or mesenteric artery occlusion). The patient was then started on a therapeutic dose of enoxaparin (60 mg BID), followed by warfarin. She responded favorably and was discharged on day 26 after ICU admission, without a prescription for oxygen therapy, although she was advised to continue the warfarin. Table 1 shows the laboratory test results obtained over the course of her hospitalization.
FIGURE 1.
Computed tomography of the chest, showing areas of consolidation interspersed with ground-glass opacities, together with central and peripheral septal thickening, in all pulmonary lobes, affecting 50% of the parenchyma and accompanied by minimal left pleural effusion.
FIGURE 2.
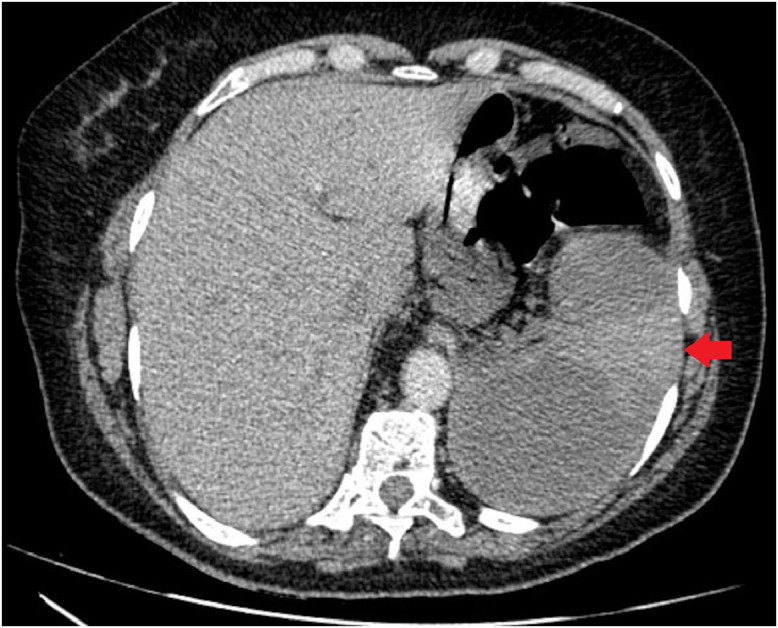
Computed tomography angiography of the abdomen, showing marked splenomegaly with well-defined, peripheral hypodense splenic lesions, highly suggestive of splenic infarction.
FIGURE 3.
Computed tomography angiography of the chest, showing a hypodense area near the right wall of the descending aorta, consistent with a thrombus (red arrow).
Table 1.
Laboratory test results at admission and throughout the hospital stay (April 20 to May 7, 2020).
| Variable | Normal range | Admission 04/20 |
04/21 | 04/22 | 04/23 | 04/29 | 04/30 | 05/05 | 05/06 | Discharge 05/07 |
|---|---|---|---|---|---|---|---|---|---|---|
| Leukocytes (cells/mm3) | 4000–11 000 | 25 400 | 15 400 | 15 400 | 6800 | 7900 | 9000 | |||
| Neutrophils (cells/mm3) | 1600–7000 | 23 622 | 12 166 | 12 166 | 4352 | 4424 | 3780 | |||
| Lymphocyte (cells/mm3) | 900–3400 | 1270 | 1694 | 2002 | 1700 | 2370 | 2340 | |||
| Platelets (cells/mm3) | 140 000–450 000 | 340 000 | 446 000 | 437 000 | 378 000 | 473 000 | 480 000 | |||
| CRP (mg/L) | 5–10 | 223 | 281 | 212 | 139 | 11 | < 5 | < 5 | < 5 | |
| CPK (U/ml) | 30–135 | 79 | 51 | 33 | 26 | < 20 | ||||
| LDH (U/L) | 120–246 | 346 | 348 | 471 | 493 | 455 | 262 | |||
| D-dimer (ng/ml) | < 500 | 3373 | 2408 | 5291 | 4057 | 4912 | 2103 | 838 | 853 | |
| Ferritin (ng/ml) | 30–400 | 1147 | 1479 | 719 | 725 | 578 | 438 | |||
| Fibrinogen (mg/dl) | 200–400 | 319 | ||||||||
| PT (INR) | 0,95-1,2 | 1.2 | ||||||||
| aPTT (seconds) | 28-40 | 29 |
CPK, creatine phosphokinase; CRP, C-reactive protein; LDH, lactate dehydrogenase; PT, prothrombin time; INR, international normalized ratio; aPTT, activated partial thromboplastin time.
Discussion
The cause of SI is occlusion of the splenic artery or of one or more of its branches, either by an embolus or by in situ thrombosis. Although that can result in global infarction of the organ, in most cases, only one segment of the spleen is affected.3 , 4 The clinical significance of SI depends mainly on the underlying conditions and complications, which, in rare cases, may include pseudocyst formation, hemorrhage, splenic rupture, and aneurysm. In some instances, the infarcted splenic tissue becomes infected and an abscess forms. Infarcted tissue may also undergo hemorrhagic transformation.5
The patient described in this case report presented with an infarct involving two segments of the spleen, and the filling defect in the right wall of the aorta suggested that the emboli originated from a thrombus in the aorta. That etiologic mechanism accounts for 19.0–26.6% of all thromboembolic SI.3 Although observations suggest that a significant proportion of arterial thromboses in COVID-19 patients occur in nondiseased or mildly diseased vessels,6 we cannot rule out the possibility of underlying ASCVD in this case. We also cannot rule out the possibility that COVID-19 exacerbated an aortic thrombus, which, because of the use of anticoagulants, detached from the aorta and caused the SI. Outside of the setting of COVID-19, SI is considered a rare event.
In a ten-year retrospective study conducted at a general teaching hospital, SI was diagnosed in 0.016% of admissions.3 In another study, there were 25 cases of SI among 168,572 admissions over a two-year period, corresponding to a quite similar prevalence of 0.015%.7 Among patients with COVID-19, the estimated prevalence of SI appears to be higher than that.
From March to July 2020, approximately 350 patients with COVID-19 were admitted to our hospital. Among those 350 patients, SI was diagnosed in one (0.3%). It is also likely that the incidence was underestimated, given that we did not perform abdominal CT scans in asymptomatic patients and that no autopsies that could have identified other cases were performed during the study period. In addition, the classic clinical presentation of discrete left upper quadrant pain, a finding suggestive of SI that could have prompted a diagnostic abdominal CT, might have been masked by the prevailing clinical presentations of patients with COVID-19 upon admission, which are characterized mainly by respiratory distress and multiorgan involvement.8
An apparent increase in the prevalence of SI, together with other manifestations of ischemic events involving the abdominal viscera, was also found among patients with COVID-19 in Italy and France.6 , 9 Of the first 460 patients with COVID-19 hospitalized in the province of Reggio Emilia in Italy, two (0.4%) were diagnosed with acute ischemic events involving abdominal viscera.9 In a retrospective study of patients with COVID-19 treated in three ICUs in France and Italy, splenic infarcts were diagnosed in three (1.4%) of the 209 patients treated.6
As detailed in Table 2 , a wide array of clinical conditions have been recognized as predisposing factors for SI.3 , 8 , 10 , 11 In a large recent case series, 40% of patients with SI were found to have had more than one plausible predisposing factor.8 Some of those predisposing factors, namely atrial fibrillation (AF) and hypercoagulability, are also frequently encountered in patients with COVID-19,2 , 12 , 13 which probably explains the increased risk of SI associated with COVID-19.
Table 2.
Main risk factors for splenic infarction.
| Structural heart disease with atrial fibrillation |
| Hypercoagulability |
| • Hematologic disease |
| • Nonhematologic malignancy |
| • Antiphospholipid syndrome |
| • Oral contraceptive use |
| Inflammation |
| • Pancreatitis |
| • Infective endocarditis |
| • Mononucleosis |
| • Cytomegalovirus |
| • Malaria |
| Trauma |
| Conditions with marked splenomegaly |
| Wandering Spleen |
Typically associated with SI caused by cardiogenic emboli,3 , 8 , 10 , 11 AF has been shown to be present in 19–21% of patients with COVID-19 in general and in up to 36% of those with a history of cardiovascular disease.12 This high prevalence of AF in COVID-19 could increase the likelihood of thromboembolic events, including splenic embolisms.
Hypercoagulability and hyperinflammation are hallmarks of the pathogenesis of COVID-19.2 , 14 Infection with SARS-CoV-2 induces a systemic inflammatory response, which is accompanied by excessive release of proinflammatory cytokines. The endothelial cell is a key target of cytokines. This hyperinflammation leads to endothelial activation and dysfunction, inducing endothelial inflammation, loss of vascular integrity, increased vascular permeability, activation of the coagulation pathway, and formation of a thrombus.15 A hypercoagulable state, which is common in patients with COVID-19, is strongly associated with mortality and often occurs even after prophylactic or therapeutic anticoagulation.2 , 13 In addition, although there have been relatively few autopsy studies related to the current pandemic, small case series of patients with COVID-19 have reported a high prevalence of macrovascular and microvascular thrombosis in the lungs, as well as in the heart, brain, kidneys, limbs, and spleen.2 It is noteworthy that the thrombotic complications in the case reported here also evolved despite the use of a prophylactic dose of LMWH.
Various authors have reported the presence of antiphospholipid (aPL) antibodies in patients with COVID-19 and have suggested that SARS-CoV-2 induces antiphospholipid syndrome, which is clinically characterized by arterial, venous, or microvascular thrombotic events, with or without obstetric complications (pregnancy loss), as well as by the serologically persistent presence of aPL, as evidenced by two positive aPL antibody test results at least 12 weeks apart.16 Unfortunately, aPL antibody tests were not available when our patient was admitted. The role that aPL plays in thrombotic complications of COVID-19 remains unclear. An analysis of aPL in a collective total of 250 patients with COVID-19 in 23 studies showed that lupus anticoagulant (LA), anticardiolipin, and anti-beta2-glycoprotein I antibodies were present in 64%, 9%, and 13% of the patients, respectively. None of those studies reported retesting for aPL antibodies after 12 weeks, and it is therefore unclear whether the presence of aPL antibodies in the COVID-19 patients was transient or persistent.17 In the only study in which aPL antibody testing was repeated (after 1 month), elevated aPL antibody levels were confirmed in 23 (74%) of the 31 patients evaluated, although nine of the 10 LA antibody-positive patients tested negative at that time.18 That supports the idea that LA positivity in isolation is common during the acute phase of COVID-19 infection, although such positivity is not clearly related to thrombotic complications.16 , 19
The classic clinical presentation of SI is left upper quadrant pain and tenderness, sometimes accompanied by nausea and vomiting. In up to 37.5% of cases, a chest X-ray at admission shows left pleural effusion, which is sometimes accompanied by compressive atelectasis.3 , 8
Table 3 summarizes the findings of five studies of SI in COVID-19,9 , 20, 21, 22, 23 in which a collective total of seven patients were evaluated. Of those seven patients, five presented with abdominal pain. The classic left upper quadrant pain, however, might not be reported by patients who are more severely ill, whose clinical condition might blunt their perception of discrete pain.8 This lack of the classic left upper quadrant pain presentation was reported in a recent study of SI, in which only 20% of the patients presented the symptom and, in those who did not, SI was typically an incidental finding on a CT scan performed for other purposes during critical illness.8
Table 3.
Clinical course, treatment, and outcomes of COVID-19-associated splenic infarction.
| Reference | Cases | Clinical presentation | Laboratory findings | Imaging | Associated ischemic event | Comorbidities | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Besutti et al5 | 53-year-old man | SARS-CoV-2 pneumonia presenting in day 6 of admission with severe left flank pain | Neutrophilia with increased LDH and CRP | CT angiography | Left kidney | Hypertension; previous mitral valve replacement | LMWH BID | Discharged |
| 72-year-old man | SARS-CoV-2 patient presenting in the second day of admission with severe abdominal pain | Neutrophilia with elevated LDH, CRP, and D-dimer | CT angiography | Small bowel | Stage 3 kidney disease; hypertension; type 2 diabetes; and previous myocardial infarction | Splenectomy and enterectomy plus continuous infusion of heparin | Discharged from the ICU and improving but still hospitalized at the time of reporting | |
| Hossri et al15 | 29-year-old woman | SARS-CoV-2 pneumonia presenting in admission with vomiting and abdominal pain | Leukopenia and lymphopenia with increased LDH, CRP, D-dimer and ferritin plus anti-cardiolipin positivity; IgM and anti-cardiolipin IgG phospholipid antibodies | CT angiography | Ischemic stroke | Hemoglobin sickle cell disease | Heparin in continuous infusion | Not reported |
| Karki et al14 | 32-year-old man | SARS-CoV-2 patient presenting in the third day of admission with severe periumbilical pain | Leucopenia and thrombocytopenia | CT angiography | None | None | Supportive care | Splenic laceration with hemoperitoneum; remained stable during ICU admission; outcome not reported |
| Santos Leite Pessoa et al13 | 67-year-old man | SARS-CoV-2 pneumonia presenting with ischemic stroke without abdominal symptoms | Not reported | CT angiography | Ischemic stroke plus pulmonary thromboembolism | Hypertension | Not reported | Not reported |
| 53-year-old woman | SARS-CoV-2 pneumonia presenting without abdominal symptoms | Not reported | Ultrasound + CT angiography | None | Rheumatoid arthritis | Not reported | Not reported | |
| Qasim Agha and Berryman16 | > 60-year-old man | SARS-CoV-2 pneumonia presenting in day 7 of admission with moderate, dull, left-side abdominal pain | Neutrophilia with increased CRP, D-dimer and ferritin | CT angiography | None | Asthma, obstructive sleep apnea, morbid obesity, IgG deficiency, and hypertension | Continuous infusion of heparin, followed by LMWH BID | Discharged on oral rivaroxaban |
CRP, C-reactive protein; ICU, intensive care unit; IgG, immunoglobulin G; IgM, immunoglobulin M; LDH, lactate dehydrogenase; LMWH, Low-molecular-weight heparin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The use of CT angiography of the spleen, ideally performed during the portal venous phase, is the cornerstone of the diagnosis of SI in the acute phase. The classic imaging pattern is a peripheral, wedge-shaped hypoenhancing lesion with the apex pointing to the hilum and the base parallel with the convex capsule. However, multiple infarcts typically appear as hypodense non-enhancing lesions interposed with normally enhancing splenic tissue.24
Conventional ultrasound, which is usually the first-line imaging method for evaluation of the splenic parenchyma, lacks sensitivity in the setting of SI because the region of the infarction is frequently isoechoic and difficult to characterize in the acute phase. The use of contrast enhancement can improve the sensitivity of ultrasound by making it easier to visualize the margins of the infarct.4 The potential advantages of contrast-enhanced ultrasound over CT are that it is faster and does not involve the use of radiation or nephrotoxic agents, as well as that it is portable, which can be especially useful in critically ill patients who cannot be moved.
The management of SI is usually conservative and is based primarily on the underlying causative disease state. In a noninfectious setting, SI may be treated with analgesics, hydration, antiemetics, and other means of supportive care. Surgery may be rarely necessary and is indicated for patients with persistent symptoms or complications such as abscess, hemorrhage, splenic rupture, and persistent pseudocyst. Among the seven cases of COVID-19-associated SI evaluated in our review of the literature, surgery was required in only one, who had a massive SI accompanied by mesenteric ischemia.9 In cases of COVID-19-associated SI, as well as in cases in which there is a hypercoagulable state and pronounced thrombosis, full anticoagulation is the cornerstone of the treatment.3 , 11
In conclusion, COVID-19 can be associated with thrombosis at unusual sites, even in patients receiving anticoagulation. Attending physicians should consider the possibility of infarction of abdominal viscera, and SI should be included in the differential diagnosis in every patient with COVID-19 who presents with severe abdominal pain. Nevertheless, the best diagnostic tool is CT angiography. When that is not feasible, bedside contrast-enhanced ultrasound can be performed.
Conflicts of Interest
The authors declare that they have no conflicts of interest to disclose.
Author contributions
JS and CMSM conceived and designed the work. LMCRB, LAR, JEV, and CFM collected the data. JS, LMCRB, LAR, and AFR contributed data or analysis tools. JS, LMCRB, and CMSM wrote the paper. JS, LMCRB, LAR, AFR, JEV, CFM, and CMSM approved the final version.
References
- 1.Aktaa S, Wu J, Nadarajah R, et al. Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: multi-sourced population-based health records cohort study. Thromb Res. 2021;202:17–23. doi: 10.1016/j.thromres.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanff TC, Mohareb AM, Giri J, et al. Thrombosis in COVID-19. Am J Hematol. 2020;95:1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schattner A, Adi M, Kitroser E, et al. Acute splenic infarction at an academic general hospital over 10 years presentation, etiology, and outcome. Med. 2015;94(36):1–6. doi: 10.1097/MD.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omar A, Freeman S. Contrast-enhanced ultrasound of the spleen. Ultrasound. 2016;24(1):41–49. doi: 10.1177/1742271X15617214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman J, Helm TA, Kahwaji CI. StatPearls Publishing; Treasure IslandFL: 2021 Jan. Splenic infarcts.https://www.ncbi.nlm.nih.gov/books/NBK430902/ Available at: [PubMed] [Google Scholar]
- 6.de Roquetaillade C, Chousterman BG, Tomasoni D, et al. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol. 2021;323:281–284. doi: 10.1016/j.ijcard.2020.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayaraghavan S, Thomas J. Clinical spectrum of splenic infarction—a South Indian perspective. Int Arch Med. 2016;9(126):1–6. [Google Scholar]
- 8.Brett AS, Azizzadeh N, Miller EM, et al. Assessment of clinical conditions associated with splenic infarction in adult patients. JAMA Intern Med. 2020;180(8):1125. doi: 10.1001/jamainternmed.2020.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besutti G, Bonacini R, Iotti V, et al. Abdominal visceral infarction in 3 patients with COVID-19. Emerg Infect Dis. 2020;26(8):1926–1928. doi: 10.3201/eid2608.201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence YR, Mbbs MA, Pokroy R, et al. Splenic Infarction : an update on William Osler ’ s observations. Isr Med Assoc J. 2010;12(6):362–365. [PubMed] [Google Scholar]
- 11.Antopolsky M, Hiller N, Salameh S, et al. Splenic infarction : 10 years of experience. Am J Emerg Med. 2009;27(3):262–265. doi: 10.1016/j.ajem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Gawałko M, Kapłon-Cieślicka A, Hohl M, et al. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Hear Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.England JT, Abdulla A, Biggs CM, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2021;45:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y, Ji W, Yang H, et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakota K, Perdan-Pirkmajer K, Hočevar A, et al. COVID-19 in association with development, course, and treatment of systemic autoimmune rheumatic diseases. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.611318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gkrouzman E, Barbhaiya M, Erkan D, et al. Reality check on antiphospholipid antibodies in COVID-19-associated aoagulopathy. Arthritis Rheumatol. 2021;73(1):173–174. doi: 10.1002/art.41472. [DOI] [PubMed] [Google Scholar]
- 18.Devreese KMJ, Linskens EA, Benoit D, et al. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost. 2020;18(9):2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGonagle D, Bridgewood C, Ramanan AV, et al. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos Leite Pessoa M, Franco Costa Lima C, Farias Pimentel AC, et al. Multisystemic Infarctions in COVID-19 : focus on the Spleen. Eur J Case Reports Intern Med. 2020;7 doi: 10.12890/2020_001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karki S, Rawal SB, Malla S, et al. A case report on spontaneous hemoperitoneum in COVID-19 patient. Int J Surg Case Rep. 2020;75:211–213. doi: 10.1016/j.ijscr.2020.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossri S, Shadi M, Hamarsha Z, et al. Clinically significant anticardiolipin antibodies associated with COVID-19. J Crit Care. 2020;59:32–34. doi: 10.1016/j.jcrc.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qasim Agha O, Berryman R. Acute splenic artery thrombosis and infarction associated with COVID-19 disease. Case Reports Crit Care. 2020;2020:1–4. doi: 10.1155/2020/8880143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller LA, Mirvis SE, Shanmuganathan K, et al. CT diagnosis of splenic infarction in blunt trauma: imaging features, clinical significance and complications. Clin Radiol. 2004;59(4):342–348. doi: 10.1016/j.crad.2003.09.005. [DOI] [PubMed] [Google Scholar]