Abstract
Purpose of Review
With the rising cost of cardiovascular clinical trials, there is interest in determining whether new technologies can increase cost effectiveness. This review focuses on current and potential uses of voice-based technologies, including virtual assistants, in cardiovascular clinical trials.
Recent Findings
Numerous potential uses for voice-based technologies have begun to emerge within cardiovascular medicine. Voice biomarkers, subtle changes in speech parameters, have emerged as a potential tool to diagnose and monitor many cardiovascular conditions, including heart failure, coronary artery disease, and pulmonary hypertension. With the increasing use of virtual assistants, numerous pilot studies have examined whether these devices can supplement initiatives to promote transitional care, physical activity, smoking cessation, and medication adherence with promising initial results. Additionally, these devices have demonstrated the ability to streamline data collection by administering questionnaires accurately and reliably. With the use of these technologies, there are several challenges that must be addressed before wider implementation including respecting patient privacy, maintaining regulatory standards, acceptance by patients and healthcare providers, determining the validity of voice-based biomarkers and endpoints, and increased accessibility.
Summary
Voice technology represents a novel and promising tool for cardiovascular clinical trials; however, research is still required to understand how it can be best harnessed.
Keywords: Voice technology, Virtual assistants, Clinical trials
Introduction
The organizational cost of running well-designed clinical trials to answer meaningful clinical questions has increased tremendously over the years, with a median cost of $21–35 million for phase III clinical trials conducted between 2010 and 2015 [1–3•]. These costs are driven by increasing trial sizes, globalization of recruitment, study monitoring, and administrative staff costs. Numerous initiatives have been proposed to combat these rising costs, such as a reduction in the complexity of trial design by reducing study visits and minimizing manual data collection [4] and by the development of innovative endpoint capture strategies [5••].
As we enter the digital era, voice-based technologies have permeated our daily lives and represent a novel data collection tool in cardiovascular clinical trials [6••]. In general, voice technologies utilize software applications or devices to record human speech, which is then uploaded to the cloud, and through an artificial intelligence-based algorithm, the recording is parsed into commands and then output is sent back to the user using a computer-generated message. Early forms of voice-based technologies took the form of interactive voice response systems, which are computer-operated phone systems that allow the user to interact with them through speech recognition or a touch-tone keypad on the phone. End users of the voice-based system can then proceed through a predetermined algorithm based on the responses of the user. Recently, several commercially available devices have been created that incorporate the use of interactive voice response technology, which are categorized as voice assistants. There are several voice assistants on the market today, including Amazon’s Alexa, Apple’s Siri, Microsoft’s Cortana, and Google Assistant. Such voice assistants are frequently embedded in smart speakers or smartphones. Currently, people can use voice assistants to answer basic questions and control home automation devices, media, and other daily tasks.
There is a potential to collect healthcare data using such devices given the new development of platforms that are compliant with health data regulators such as the US Health Insurance Portability and Accountability Act of 1996 (HIPAA) [7]. With this new development, voice-based technologies are beginning to spill over into the healthcare realm. Patients and healthcare providers in multiple fields and demographics have demonstrated a high degree of readiness for voice-based technology [8, 9•, 10•].
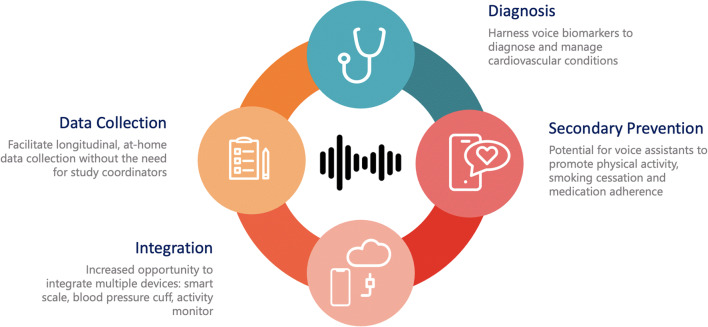
Voice-based technologies have the potential to critically impact the way we practice cardiovascular medicine and conduct clinical trials by assisting in diagnosing and monitoring disease states, supplementing cardiovascular risk modification strategies, promoting medication adherence, and collecting routine medical data (Fig. 1).
Fig. 1.
Potential Uses and Impact of Voice-Based Technologies in Cardiovascular Care and Clinical Trials
Current and Potential Uses of Voice-Based Technologies in Cardiovascular Medicine
Voice Analysis for Diagnosis and Monitoring
There has been interest in determining whether analysis of voice signal characteristics can be used to diagnose or monitor cardiovascular conditions. The biological plausibility of this phenomenon is that the cardiovascular system is dynamically associated with vocal cord function. Changes in heart rate; heart rate variability; blood pressure; and respiratory rate, depth, and regularity result in unique “vocal biomarkers” that can be used to identify pathologic states [11]. Recently, several of these vocal biomarkers have been found to be independently associated with coronary artery disease [12, 13•] and pulmonary hypertension [14•]. Similarly, vocal biomarkers have been found to be independently associated with mortality and hospitalization in patients with heart failure [15•]. There are several possible applications of this technology in the management of heart failure. As it has been observed that even small volume changes are able to produce significant phonetic variation [16, 17], vocal biomarkers may be able to detect heart failure-related edema earlier than traditional clinical signs and symptoms.
Should this technology demonstrate acceptable sensitivity and specificity to gold standard diagnostic testing, it is poised to revolutionize clinical care and clinical trials. Not only is such an approach noninvasive, but would also be incredibly cost effective. From an epidemiological perspective, a voice assistant in an individual’s home could continuously monitor voice biomarkers over decades with a one-time cost of the device. Compared to traditional epidemiology studies such as the Framingham study, large, long-term voice analysis studies could be conducted at a fraction of the cost due to reductions in patient visits, follow-up, and administrative costs. Similarly, we expect to see an increase in the exploration of voice analysis-guided heart failure therapy. Hypothetically, a voice assistant may monitor an individual’s vocal biomarkers on a daily basis and subsequently recommend a diuretic dose within a prescriber’s pre-specified margin. However, commercially available voice assistants function by converting live audio into text, which is subsequently programmed to interact with the software, rather than using the raw voice recording. Modifications to existing software, such as open access to the recordings, will need to be undertaken before raw vocal signal analysis can be incorporated with these technologies.
Similarly, there are numerous potential applications for the use of voice assistance in the post-hospital discharge setting. Recently, a pragmatic randomized controlled trial aimed to assess the impact of an interactive voice response augmented discharge strategy in 478 patients with heart failure and chronic obstructive pulmonary disease [18]. This telephone-based automated technology collected data on disease-specific red flags, provided customized patient education, and alerted a care transition nurse to any red flags. While the study did not demonstrate a reduction in their primary outcome of a 30-day readmission rate, they do demonstrate that it is feasible to integrate voice-based technologies in the transition to home process with >85% compliance. Further studies to identify innovative and specific “red flags” during the transition process will be of interest to future clinical trials attempting to demonstrate the clinical benefit of voice-based technologies in transitions of care.
Voice-based technologies also demonstrate the ability to facilitate communication between patients and their caregivers. A randomized controlled trial of 331 heart failure patients demonstrated that an interactive voice response (IVR) system could collect information about a participant’s overall health, heart failure symptoms, and self-care practices [19]. All participants received IVR telephone calls over a period of 12 months. Participants in the interventional arm additionally had a summary of their IVR responses sent by e-mail to a caregiver who lives outside of their homes. Participants in the interventional group demonstrated increased medication compliance and a reduction in symptoms of heart failure and depression. Whether such an intervention can improve clinical outcomes remains to be known.
Cardiovascular Risk Modification
Numerous studies have demonstrated that IVR technology can effectively supplement smoking cessation programs [20, 21]. While no clinical trials have been published regarding the use of newer voice-assistant smoking cessation, it is highly likely that such an approach would be more or as effective as smartphone-based interventions [22]. Similarly, IVR applications have been shown to be effective in multiple other lifestyle interventions including weight loss [23–25]; however, its impact is heterogenous and likely depends on the underlying messages and supplementary support [26]. While no large, virtual-assistant-aided clinical trials have been completed or are ongoing, it is plausible that a virtual assistant could be paired with WIFI- or Bluetooth-enabled weighing scales to offer individualized support to weight loss strategies.
A recently completed small clinical trial of 42 cancer survivors was randomized to one of three arms: [1] on-demand coaching using the Amazon Echo, [2] text messaging via mobile phone, or [3•] written information on the benefits of physical activity. Participants enrolled in the Amazon Echo arm were the only group to demonstrate an increase in physical activity, with an average of additional ~3500 steps per day [27•].
Numerous applications have recently been released that incorporate the use of virtual assistants in the management of diabetes and hypertension [28•, 29•, 30•, 31•, 32]; however, the clinical impact of these applications has not been assessed in clinical trials.
Medication Adherence
Whether in clinical trials or cohort studies, adherence to cardiovascular medications has consistently been demonstrated to be less than optimal [33, 34]. The use of IVR has been demonstrated to increase medication compliance in cardiovascular patients [35, 36•, 37•]. Recently, Amazon’s Alexa has added features to remind patients how and when to take their medication and the ability to refill their prescription in the United Kingdom [38]. Several other smart and voice-based devices have emerged on the market that claim to aid with improved medication adherence including RxPense by MediPENSE, Spenser by Spenser Health Solutions, and Mabu by Catalia Health and Pillo. However, none of these technologies has been tested in clinical trials.
Integration with Medical Devices
Theoretically, virtual assistants are able to pair with smart devices around an individual’s home [39•]. This may include a home blood pressure monitoring device, heart rate monitor, activity monitor, weigh scale, and smart medication dispensers, among many other possibilities. While such integrative monitoring and adaptive treatment applications are not currently commercially available, the technology to accomplish this exists. This creates the possibility to collect rich, meaningful data that can be used to provide personalized instructions, motivation, and guide therapy in conjunction with healthcare providers. Such data could be mined by artificial intelligence to detect early warning signs for deterioration of many cardiovascular disease states and alert healthcare providers of the same. Furthermore, in the context of clinical trials, IVR can direct and instruct participants to measure vital signs or parameters within an environment that is convenient and may reduce the need for in-person clinical trial specific follow-up.
Collecting Clinical Trial Endpoints
One major barrier in clinical trial enrollment is the high burden on participant time. Frequent hospital visits often reduce the feasibility for participants — an issue that is exacerbated among women, minorities, and individuals with lower socioeconomic status and/or digital health technology literacy [6]. Voice-based technologies have demonstrated the ability to streamline and automate medical data collection and may facilitate the collection of endpoints [40, 41]. CardioCube is a software that has been developed for Amazon’s Alexa, which has been programmed to collect medical history from patients directly and interfaces with an electronic medical record system. A feasibility study of this software in a small group of cardiovascular patients has demonstrated that it was able to collect cardiovascular risk factors and past medical history with a high degree of accuracy (97.5%) [42•].
Extrapolating this finding into the clinical trials context, intelligent voice assistants raise the possibility that baseline medical information and follow-up data points can be collected from the comfort of the participant’s home. While clinical trials are frequently limited by the intermittent nature of data collection that occurs only during study visits, the use of virtual assistants to collect information on symptoms and quality of life on any given day allows for a deeper understanding of day to day and add granularity to these important patient-reported outcomes. Provided proper validation of this endpoint capture method is performed, this continuously available real-world data could be transformed into real-world evidence. Such an approach also reduces the burden of traveling to a study site and the time commitment required from the participant. Conveniently, this approach would eliminate or reduce the administrative costs of administering questionnaires or case report forms.
Previous user experiences raise several concerns that limit the current use of voice assistants for data collection. Users of voice assistants note that voice assistants may struggle with their accents, misunderstand them, or require them to speak differently and that the ability to interpret interruptions was a desired feature [43•]. Additional field testing demonstrated that navigating through a long interaction with voice assistants can be challenging, with the inability to go back and forth between responses and timing out the device or making an error results in having to start the encounter from the beginning [44•]. These experiences highlight important considerations for future voice-based applications, as failure to do so may lead to user frustration and decreased compliance.
Use in the Clinical Environment
As highlighted previously, voice-based technologies have demonstrated the ability to collect and collate data using personalized questions based on responses to previous questions. This feature lends itself easily to the ambulatory and clinical settings, reducing the time required to collect routine information. However, the use of voice technologies extends beyond the clinic. The strength of these technologies is their connection to the internet and ability to store data that can be accessed easily. Pilot studies have demonstrated the feasibility of such uses in various settings including providing device recommendations in the operation room [45•], confirming surgical timeout data [46•], and predicting cardiac arrests based on 911 audio conversations [47•]. Hypothetical uses include confirming medication dosage and administration, facilitating communication between patients and hospital staff, and recording patient-reported outcomes.
Challenges and Barriers
Voice-based technologies have several obstacles to overcome before they become part of mainstream cardiovascular care and clinical trials. The first of these to consider involves health data protection regulatory compliance. Only recently has Amazon’s Alexa provided developer tools which are HIPAA compliant; however, these features are relatively limited, and other devices have yet to offer regulatory compliant alternatives. While HIPAA regulation may apply to the use of voice-based technologies in the United States, as standards around the world continue to evolve, software developers will need to ensure compliance with a wide variety of regulations if these technologies are to be widely used. Similarly, it is currently unclear how information collected from voice-based technologies can be transmitted and incorporated into existing electronic medical record systems while ensuring regulatory compliance. In studies exploring patient concerns, only a minority (~10%) reported any significant concerns regarding privacy or confidentiality [48•]. In surveys of both users and nonusers of voice-based technologies, we see that users have much lower concerns regarding security, suggesting that perceptions of security and privacy may prevent the widespread adoption of voice technologies in the general population [49, 50•].
Whereas voice assistants are increasing in popularity with 132 million individuals in the United States using at least one, there is still a large portion of the population which do not have access to a voice assistant [51]. From other digital technologies, we have observed disparities in the use of digital technologies across ethnicities and socioeconomic status [52, 53]. It is expected that for the foreseeable future, this digital divide would extend to voice-based technologies as well. To combat the digital divide, voice-based technology initiatives need to consider access to low-cost internet, equipment costs, and provision of basic digital skills [54]. The next issue that needs to be addressed is the quality of speech recognition and comprehension. Despite significant improvements in this field, voice assistants still struggle with complex and long phrases, limiting their use to short, simple responses. Connectivity remains an ongoing issue, with voice assistants requiring a continuous, reliable internet connection. While internet connectivity has improved across the world, issues regarding backup systems during power and internet outages remain a concern. From a medico-legal perspective, it is unclear to what extent device manufacturers and healthcare providers are responsible for the medical problems uncovered by such technologies lie and whether prompts for contacting emergency medical services should be built-in. Lastly, we should consider that implementing successful, outpatient, voice-assistant-directed therapies requires a robust infrastructure of technical support and healthcare providers that are available to manage voice-assistant-identified health concerns.
Ongoing Clinical Trials
Multiple ongoing and recently completed clinical trials will provide additional insight into the utility of voice-based technologies in cardiovascular medicine (Table 1). Studies examining telephone-based IVR are also ongoing, examining the impact on smoking cessation, weight loss, and medication adherence in acute coronary syndrome patients. With the recent release of the customizable HIPAA-compliant Alexa skills toolkit, numerous studies are ongoing examining the ability of Alexa to reliably administer and collect responses for COVID-19 questionnaires and monitor for symptoms of depression. Two ongoing cardiovascular studies have begun to determine if a voice-assistant-supplemented outpatient strategy can improve heart failure monitoring and adherence to the dietary approaches to stop hypertension diet. Over the next few years, as more devices develop health data regulatory-compliant services, we expect to see a rise in novel uses for voice assistants in the field of cardiovascular medicine.
Table 1.
Ongoing or recently completed studies utilizing voice-based technologies
| Identifier | Study type | No. of participants | Brief description |
|---|---|---|---|
| Interactive voice response | |||
| NCT04574518 | RCT | 2400 | Examining the use of interactive voice response to supplement a smoking cessation program in individuals identified to have a pulmonary nodule. Primary outcome is smoking cessation at 56 weeks. |
| NCT03869658 | Observational | 40 | Examining the use of a smartphone-based application that utilized interactive voice response technology to log self-recorded food intake. Primary outcome is feasibility. |
| NCT01940016 | RCT | 71 | Examining a telephone-based interactive voice response strategy to increase physical activity in overweight postmenopausal women. Primary outcome is feasibility and retention at 12 weeks. |
| NCT01260207 | RCT | 654 | Examining the impact of an interactive voice response follow-up intervention assessing medication management, smoking cessation, diet, exercise, and general cardiovascular education following an acute coronary syndrome. Primary outcome is compliance with best practice guidelines at 1 year. |
| Voice assistant | |||
| NCT03707275 | RCT | 50 | Examining the utilization of the Amazon Alexa to intermittently ask questions regarding signs and symptoms of heart failure for 3 months. Primary outcome is comfort using voice-assistant technology, and secondary outcomes include heart failure hospitalization and medication adherence at 3 months. |
| NCT04508972 | Observational | 200 | Examining the feasibility of using Amazon Alexa to administer a COVID-19 questionnaire to individuals entering a healthcare facility. Primary outcome is the correlation between voice assistant and manual data collection. |
| NCT04609267 | Randomized cross-over design | 40 | Examining the ability of Amazon Alexa to administer the Patient Health Questionnaire 9, a clinical tool to diagnose major depressive disorder. Primary outcome is the correlation between voice-assistant and manual responses. |
Future Directions
As the innovation behind voice-based technologies continues to develop, there will be an increasing need to validate the use of these technologies to accurately collect information from participants. In many instances, there may be the need to reformat questions into shorter, abbreviated questions. These modified data collection tools will need to be validated against traditional data collection tools. In order for voice-based technologies to permeate clinical practice, definitive randomized controlled trials will be required to demonstrate improved clinical benefits or patient-oriented outcomes, such as quality of life or convenience. Along with this, patients, the end users of this technology, will need to be seen as accepting of this alternative form of communication. While initial surveys of patient satisfaction are promising, various patient populations may have differing opinions and needs. The other end user to consider is healthcare providers who will need to observe that the use of these technologies improves workflow and facilitates care rather than creates a cumbersome obstacle. From a clinical trials perspective, study coordinators and investigators will need to assess and optimize how voice-based technologies can be utilized to facilitate data collection.
Conclusion
Voice-based technologies are still in their infancy. However, as the technology develops, there are several possible therapeutic uses in the management of cardiovascular patients and the organization of cardiovascular clinical trials.
Declarations
Conflict of Interest
A.S. reports receiving support from the Fonds de Recherche Santé Quebec (FRSQ) Junior 1 clinician scholars program, Alberta Innovates Health Solution, European Society of Cardiology young investigator grant, Roche Diagnostics, Boeringer-Ingelheim, Novartis, and Takeda. All authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Technology and Cardiovascular Health
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pishoy Gouda, Email: gouda@ualberta.ca.
Elie Ganni, Email: elie.ghamlouche@mail.mcgill.ca.
Peter Chung, Email: seok.chung@mail.mcgill.ca.
Varinder Kaur Randhawa, Email: randhav@ccf.org.
Guillaume Marquis-Gravel, Email: guillaume.marquis.gravel@umontreal.ca.
Robert Avram, Email: Robert.avram.md@gmail.com.
Justin A. Ezekowitz, Email: jae2@ualberta.ca
Abhinav Sharma, Email: Abhinav.sharma@mcgill.ca.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Martin L, Hutchens M, Hawkins C, Radnov A. How much do clinical trials cost? Nat Rev Drug Discov. 2017;16(6):381–382. doi: 10.1038/nrd.2017.70. [DOI] [PubMed] [Google Scholar]
- 2.Sertkaya A, Wong H-H, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clinical Trials. 2016;13(2):117–126. doi: 10.1177/1740774515625964. [DOI] [PubMed] [Google Scholar]
- 3.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015-2016. JAMA Intern Med. 2018;178(11):1451–1457. doi: 10.1001/jamainternmed.2018.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenstein EL, Lemons PW, Tardiff BE, Schulman KA, Jolly MK, Califf RM. Reducing the costs of phase III cardiovascular clinical trials. Am Heart J. 2005;149(3):482–488. doi: 10.1016/j.ahj.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Marquis-Gravel G, Roe MT, Turakhia MP, Boden W, Temple R, Sharma A, et al. Technology-enabled clinical trials: transforming medical evidence generation. Circulation. 2019;140(17):1426–1436. doi: 10.1161/CIRCULATIONAHA.119.040798. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Harrington RA, MB MC, Turakhia MP, Eapen ZJ, Steinhubl S, et al. Using digital health technology to better generate evidence and deliver evidence-based care. J Am Coll Cardiol. 2018;71(23):2680–2690. doi: 10.1016/j.jacc.2018.03.523. [DOI] [PubMed] [Google Scholar]
- 7.Amazon Alexa. Voice for healthcare [available from: https://developer.amazon.com/en-US/alexa/alexa-skills-kit/get-deeper/custom-skills/healthcare-skills
- 8.Seniors with CHF embrace monitoring system based on automatic voice technology Dis Manag Advis. 2001;7(3):40–44. [PubMed] [Google Scholar]
- 9.Kim S. Exploring how older adults use a smart speaker-based voice assistant in their first interactions: qualitative study. JMIR Mhealth Uhealth. 2021;9(1):e20427. doi: 10.2196/20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalska M, Gładyś A, Kalańska-Łukasik B, Gruz-Kwapisz M, Wojakowski W, Jadczyk T. Readiness for voice technology in patients with cardiovascular diseases: cross-sectional study. J Med Internet Res. 2020;22(12):e20456. doi: 10.2196/20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RMBd A, Barón-López FJ, Alguacil MD, Dawid-Milner MS. Interactions between voice fundamental frequency and cardiovascular parameters. Preliminary results and physiological mechanisms. Logopedics Phoniatrics Vocology. 2013;38(2):52–58. doi: 10.3109/14015439.2012.696140. [DOI] [PubMed] [Google Scholar]
- 12.Pareek V, Sharma R, editors. Coronary heart disease detection from voice analysis. In: 2016 IEEE Students’ Conference on Electrical: Electronics and Computer Science (SCEECS); 2016. IEEE.
- 13.• Maor E, Sara JD, Orbelo DM, Lerman LO, Levanon Y, Lerman A, editors. Voice signal characteristics are independently associated with coronary artery disease. In: Mayo Clinic Proceedings; 2018: Elsevier. Cross-sectional study of 101 patients with coronary angiographic data utilized a smartphone to record a series of voice recordings. Multiple voice “biomarkers” were identified that were independently associated with coronary artery disease (odds ratio of 4.01; confidence interval 1.25–12.84).
- 14.Sara JDS, Maor E, Borlaug B, Lewis BR, Orbelo D, Lerman LO, et al. Non-invasive vocal biomarker is associated with pulmonary hypertension. PLoS One. 2020;15(4):e0231441. doi: 10.1371/journal.pone.0231441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maor E, Perry D, Mevorach D, Taiblum N, Luz Y, Mazin I, et al. Vocal biomarker is associated with hospitalization and mortality among heart failure patients. J Am Heart Assoc. 2020;9(7):e013359. doi: 10.1161/JAHA.119.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdolini K, Min Y, Titze IR, Lemke J, Brown K, van Mersbergen M, et al. Biological mechanisms underlying voice changes due to dehydration. Journal of Speech, Language, and Hearing Research. 2002. [DOI] [PubMed]
- 17.Murton OM, Hillman RE, Mehta DD, Semigran M, Daher M, Cunningham T, Verkouw K, Tabtabai S, Steiner J, Dec GW, Ausiello D. Acoustic speech analysis of patients with decompensated heart failure: a pilot study. The Journal of the Acoustical Society of America. 2017;142(4):EL401–ELEL7. doi: 10.1121/1.5007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie CS, Houston TK, Richman JS, Sobko HJ, Berner ES, Taylor BB, Salanitro AH, Locher JL. The E-Coach technology-assisted care transition system: a pragmatic randomized trial. Transl Behav Med. 2016;6(3):428–437. doi: 10.1007/s13142-016-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, Fisher L, Aikens JE. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. J Med Internet Res. 2015;17(6):e142. doi: 10.2196/jmir.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigotti NA, Chang Y, Rosenfeld LC, Japuntich SJ, Park ER, Tindle HA, Levy DE, Reid ZZ, Streck J, Gomperts T, Kelley JHK, Singer DE. Interactive voice response calls to promote smoking cessation after hospital discharge: pooled analysis of two randomized clinical trials. J Gen Intern Med. 2017;32(9):1005–1013. doi: 10.1007/s11606-017-4085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Educ Couns. 2007;66(3):319–326. doi: 10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017;7(2):292–299. doi: 10.1007/s13142-017-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg DM, Levine EL, Lane I, Askew S, Foley PB, Puleo E, Bennett GG. Adherence to self-monitoring via interactive voice response technology in an eHealth intervention targeting weight gain prevention among Black women: randomized controlled trial. J Med Internet Res. 2014;16(4):e114. doi: 10.2196/jmir.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block G, Azar KM, Romanelli RJ, Block TJ, Hopkins D, Carpenter HA, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. doi: 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Educ Couns. 2008;72(1):34–41. doi: 10.1016/j.pec.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Svetkey LP, Batch BC, Lin P-H, Intille SS, Corsino L, Tyson CC, Bosworth HB, Grambow SC, Voils C, Loria C, Gallis JA, Schwager J, Bennett GB. Cell phone intervention for you (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity. 2015;23(11):2133–2141. doi: 10.1002/oby.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassoon A, Baig Y, Naimann D, Celentano D, Lansey D, Stearns V, et al. Abstract 54: addressing cardiovascular health using artificial intelligence: randomized clinical trial to increase physical activity in cancer survivors using intelligent voice assist (Amazon Alexa) for patient coaching. Circulation. 2020;141(Suppl_1):A54–A5A. doi: 10.1161/circ.141.suppl_1.54. [DOI] [Google Scholar]
- 28.• Cheng A, Raghavaraju V, Kanugo J, Handrianto YP, Shang Y, editors. Development and evaluation of a healthy coping voice interface application using the Google home for elderly patients with type 2 diabetes. In: 2018 15th IEEE Annual Consumer Communications & Networking Conference (CCNC); 2018. p. 12–5. Highlights the development of a voice interface application to help elderly adults with type two diabetes cope with their disease, monitor their glucose, medication reminders, increase physical activity, and promote healthy eating.
- 29.• Anastasiadou M, Alexiadis A, Polychronidou E, Votis K, Tzovaras D, editors. A prototype educational virtual assistant for diabetes management. In: 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE); 2020. p. 26–8. Description of a voice-assistant prototype that has been designed to improve diabetic education. The prototype was tested using 3266 questions of which only ~2% questions were not appropriately answered.
- 30.Tan S, Fatehi F. Sweet talking: voice technology and virtual assistants in clinical diabetes management. Stud Health Technol Inform. 2019;264:1787–1788. doi: 10.3233/SHTI190648. [DOI] [PubMed] [Google Scholar]
- 31.Balsa J, Félix I, Cláudio AP, Carmo MB, IC ES, Guerreiro A, et al. Usability of an intelligent virtual assistant for promoting behavior change and self-care in older people with type 2 diabetes. J Med Syst. 2020;44(7):1–12. doi: 10.1007/s10916-020-01583-w. [DOI] [PubMed] [Google Scholar]
- 32.Keith-Hynes P, Mize B, Robert A, Place J. The diabetes assistant: a smartphone-based system for real-time control of blood glucose. Electronics. 2014;3(4):609–623. doi: 10.3390/electronics3040609. [DOI] [Google Scholar]
- 33.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 34.Kolandaivelu K, Leiden BB, O'Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35(46):3267–3276. doi: 10.1093/eurheartj/ehu364. [DOI] [PubMed] [Google Scholar]
- 35.Sherrard H, Duchesne L, Wells G, Kearns SA, Struthers C. Using interactive voice response to improve disease management and compliance with acute coronary syndrome best practice guidelines: a randomized controlled trial. Canadian Journal of Cardiovascular Nursing. 2015;25(1). [PubMed]
- 36.Kamal AK, Khalid W, Muqeet A, Jamil A, Farhat K, SRA G, et al. Making prescriptions “talk” to stroke and heart attack survivors to improve adherence: results of a randomized clinical trial (The Talking Rx Study) PLoS One. 2018;13(12):e0197671. doi: 10.1371/journal.pone.0197671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassavou A, Sutton S. Automated telecommunication interventions to promote adherence to cardio-metabolic medications: meta-analysis of effectiveness and meta-regression of behaviour change techniques. Health Psychol Rev. 2018;12(1):25–42. doi: 10.1080/17437199.2017.1365617. [DOI] [PubMed] [Google Scholar]
- 38.Jiang R. New ways to manage your medications at home using Alexa 2019 [available from: https://www.aboutamazon.com/news/devices/new-ways-to-manage-your-medications-at-home-using-alexa
- 39.CKY N-T, Fritz RL. Health-assistive smart homes for aging in place: leading the way for integration of the Asian immigrant minority voice. Asian Pac Isl Nurs J. 2018;3(4):154–159. doi: 10.31372/20180304.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zan S, Agboola S, Moore SA, Parks KA, Kvedar JC, Jethwani K. Patient engagement with a mobile web-based telemonitoring system for heart failure self-management: a pilot study. JMIR mHealth uHealth. 2015;3(2):e33. doi: 10.2196/mhealth.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Park JB, Choi SW, Yoon YE, Park HE, Lee SE, Lee SP, Kim HK, Cho HJ, Choi SY, Lee HY, Choi J, Lee YJ, Kim YJ, Cho GY, Choi J, Sohn DW. Impact of a telehealth program with voice recognition technology in patients with chronic heart failure: feasibility study. JMIR Mhealth Uhealth. 2017;5(10):e127. doi: 10.2196/mhealth.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jadczyk T, Kiwic O, Khandwalla RM, Grabowski K, Rudawski S, Magaczewski P, et al. Feasibility of a voice-enabled automated platform for medical data collection: CardioCube. Int J Med Inform. 2019;129:388–393. doi: 10.1016/j.ijmedinf.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 43.• Dubiel M, Halvey M, Azzopardi L. A survey investigating usage of virtual personal assistants. arXiv preprint arXiv:180704606. 2018; Cross-sectional survey of 188 participants regarding their usage of voice assistants. Participants predominantly used the devices for easy tasks, with most features rarely being used. Of interest, satisfaction with the device was associated with higher usage. Most participants had some concerns regarding privacy.
- 44.Pradhan A, Lazar A, Findlater L. Use of intelligent voice assistants by older adults with low technology use. ACM Transactions on Computer-Human Interaction (TOCHI) 2020;27(4):1–27. doi: 10.1145/3373759. [DOI] [Google Scholar]
- 45.Seals K, Al-Hakim R, Mulligan P, Lehrman E, Fidelman N, Kolli K, et al. 03: 45 PM Abstract No. 38 the development of a machine learning smart speaker application for device sizing in interventional radiology. J Vasc Interv Radiol. 2019;30(3):S20. doi: 10.1016/j.jvir.2018.12.077. [DOI] [Google Scholar]
- 46.Yoo TK, Oh E, Kim HK, Ryu IH, Lee IS, Kim JS, et al. Deep learning-based smart speaker to confirm surgical sites for cataract surgeries: a pilot study. PLoS One. 2020;15(4):e0231322. doi: 10.1371/journal.pone.0231322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan J, Rea T, Gollakota S, Sunshine JE. Contactless cardiac arrest detection using smart devices. npj Digital Medicine. 2019;2(1):52. doi: 10.1038/s41746-019-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy O, Synnott J, McNaney R, Brito Zambrano P, Kernohan WG. Attitudes toward the use of voice-assisted technologies among people with Parkinson disease: findings from a web-based survey. JMIR Rehabil Assist Technol. 2021;8(1):e23006. doi: 10.2196/23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau J, Zimmerman B, Schaub F. Alexa, are you listening? Privacy perceptions, concerns and privacy-seeking behaviors with smart speakers. Proceedings of the ACM on Human-Computer Interaction. 2018;2(CSCW):1–31. doi: 10.1145/3274371. [DOI] [Google Scholar]
- 50.• Liao Y, Vitak J, Kumar P, Zimmer M, Kritikos K, editors. Understanding the role of privacy and trust in intelligent personal assistant adoption. In: International Conference on Information; 2019: Springer. Highlights that among nonusers of voice-assistants, there is a significant concern regarding the privacy of their data, which negates the potential convenience of such technologies.
- 51.Statista. Number of voice assistant users in the United States from 2017 to 2022 2020 [available from: https://www.statista.com/statistics/1029573/us-voice-assistant-users/.
- 52.Bender MS, Choi J, Arai S, Paul SM, Gonzalez P, Fukuoka Y. Digital technology ownership, usage, and factors predicting downloading health apps among Caucasian, Filipino, Korean, and Latino Americans: the digital link to health survey. JMIR mHealth uHealth. 2014;2(4):e43. doi: 10.2196/mhealth.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheerder A, van Deursen A, van Dijk J. Determinants of Internet skills, uses and outcomes. A systematic review of the second-and third-level digital divide. Telematics Inform. 2017;34(8):1607–1624. doi: 10.1016/j.tele.2017.07.007. [DOI] [Google Scholar]
- 54.Sheon AR, Bolen SD, Callahan B, Shick S, Perzynski AT. Addressing disparities in diabetes management through novel approaches to encourage technology adoption and use. JMIR Diabetes. 2017;2(2):e16. doi: 10.2196/diabetes.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]