Abstract
Introduction
Transcatheter aortic valve replacement (TAVR) has been established as a standard of care for patients with severe aortic stenosis. We aim to study the predictors of acute kidney injury (AKI) after TAVR from a contemporary analysis using the National Inpatient Sample (NIS) database.
Methods
We performed a national analysis using the NIS database to evaluate predictors of acute kidney injury (AKI) after TAVR. Our study period was from 2015 to 2018, and we identified TAVR patients in all procedure fields. Patients aged less than 18 years were excluded from the study.
Results
We report data of 173,760 TAVR patients, of which 20,045 (11.5%) had AKI and 153,715 (88.4%) did not. There were three principal findings of our study. First, mortality was higher in patients with AKI compared to patients who did not have AKI (8% vs. 0.8%; p<0.01). Second, patients with chronic kidney disease, weight loss, liver disease, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, metastatic cancer, and peripheral vascular disease had higher adjusted odds of AKI. Third, length of stay and cost of stay were significantly higher in patients who had AKI during the index admission.
Conclusion
Patients with AKI had higher in-hospital mortality. We also report that at baseline, chronic kidney disease, weight loss, liver disease, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, metastatic cancer, and peripheral vascular disease were important predictors of AKI in patients after TAVR. Length of stay and cost of stay were higher with AKI, which result in higher burden on the health care system due to increased resource utilization.
Keywords: tavr, tavi, aortic stenosis, transcatheter aortic valve replacement, transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve replacement has revolutionized the treatment of patients with severe aortic stenosis who are considered high risk for surgical aortic valve replacement [1-4]. The PARTNER (Placement of AoRTic TraNscathetER Valves) trials have led the way for broadening the indication of TAVR to low-risk surgical patients [3]. TAVR has led to a constant improvement in clinical outcomes with aortic stenosis [5]. In patients with aortic valve disease, TAVR, in the last 15 years has progressed from a last resort procedure in patients who were at high perioperative risk for major mortality and morbidity from surgical valve replacement to a viable and alternate option to surgery [6]. Although TAVR has shown great results, acute kidney injury (AKI) remains one of the major complications and is associated with mortality, increased adverse events, and resource use [7,8].
Rapid loss of kidney function occurring within hours or days and resulting in impaired electrolyte hemostasis, dysregulation of volume, and accumulation of waste product is defined as AKI [9]. AKI is a frequent complication seen in TAVR that remains associated with a dismal prognosis and was identified as one of the most common complications in the landmark PARTNER trials [2,3,10-12]. According to one estimate, AKI is seen in up to 30% of patients undergoing TAVR [13]. We know from prior literature that AKI in surgical aortic valve replacement was associated with up to a four-fold higher risk of mortality. During the procedure, there are multiple predisposing factors such as contrast use and hypotension episodes that predispose to acute renal injury [14]. As TAVR is mostly performed in elderly and frail patients, CKD has a wide prevalence and further predisposes patients to acute-on-chronic kidney injury [12,14-18].
Previous studies have looked at AKI in TAVR; however, studies have remained limited to case series and retrospective studies with a small sample size. Predictors of AKI after TAVR from a large sample size of patients and real-world experience of TAVR remain scarce. Hence, the aim of this study was to identify important predictors of AKI, mortality rate, and resource utilization after TAVR from a U.S. national database.
Materials and methods
We used the U.S. National Inpatient Sample (NIS) database to identify cases of TAVR performed from 2015 to 2018. The NIS data are available to the public and anonymized; hence, Institutional Review Board approval was not necessary for this study.
To perform a national analysis, we used the International Classification of Diseases (ICD)-9 and ICD-10 codes (3505, 3506, and O2RF3) to identify hospitalizations for TAVR. We queried all diagnosis fields to select TAVR patients. Similarly, ICD-9 and ICD-10 diagnosis codes were used to define and identify all baseline co-morbidities. We excluded patients who were less than 18 years of age.
We used weighted data based on discharge weights provided by the NIS. For categorical variables, we used the chi-square test. For continuous variables, testing of non-normality was used. Since continuous variables are not normally distributed, Mann-Whitney U test was used. We also developed a binary logistic model using entry method including demographic factors such as age, sex, race, median income, hospital location, baseline co-morbidities, obesity, weight loss, metastatic cancer, lymphoma, solid organ tumor, alcohol use, coagulopathy, hypothyroidism, chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CVA), congestive heart failure (CHF), coronary artery disease (CAD), diabetes mellitus, hypertension, liver disease, chronic kidney disease (CKD), and peripheral vascular disease (PVD). In compliance with the Healthcare Cost and Utilization Project guidelines, we did not report observations with less than 11 cases. Comparisons were two-sided, and p < 0.05 was considered statistically significant. All analyses were performed utilizing SPSS Version 27 (IBM Corp., Armonk, NY) and R Version 3.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
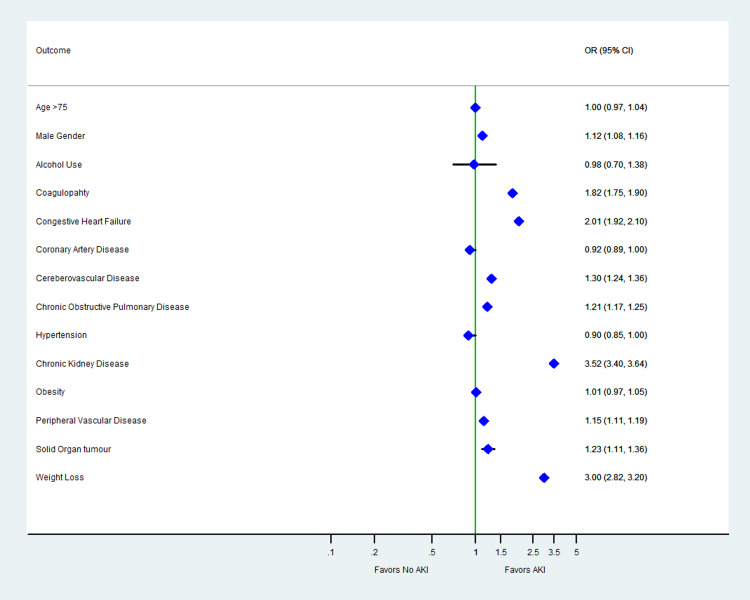
A total of 173,760 weighted hospitalizations for TAVR were included in the analysis. Of the patient undergoing the procedure, 20,045 (11.5%) had AKI and 153,715 (88.4%) did not. The detailed baseline characteristics are summarized in Table 1. At baseline, patients with CKD (OR: 3.52; 95% CI: 3.40-3.64), weight loss (OR: 3.01; 95% CI: 2.82-3.20), liver disease (OR: 2.29; 95% CI: 2.13-2.46), CHF (OR: 2.01; 95% CI: 1.92-2.10), CVA (OR: 1.30; 95% CI: 1.24-1.36), COPD (OR: 1.21; 95% CI: 1.17-1.25), metastatic cancer (OR: 1.16; 95% Cl: 0.95-1.41), and PVD (OR: 1.15; 95% CI: 1.11-1.19) had higher adjusted odds of AKI (Table 1, Figure 1). Mortality rate was higher with AKI (8% vs 0.8%; p<0.01). Patients who had AKI had a higher median cost of stay (US$63,110 vs. US$44,853; p<0.01) and length of stay (9 vs. 2 days; p<0.01) (Table 2).
Table 1. Baseline characteristics and predictors of AKI in patients after TAVR.
AKI, acute kidney injury; IQR, interquartile range; TAVR, transcatheter aortic valve replacement
| Variable | No AKI (153,715) | With AKI (20,045) | No AKI vs. AKI (multivariate analysis), OR (95% CI) |
| Age, median (IQR) | 82 (75-86) | 82 (74-87) | 1.00 (0.97-1.04) (reference age < 75 years) |
| Male gender | 81590 (53.1%) | 11585 (57.8%) | 1.12 (1.08-1.16) (reference to female) |
| Caucasian | 128800 (87.4%) | 15875 (83.4%) | Reference |
| African Americans | 6060 (4.1%) | 1005 (5.3%) | 0.74 (0.67-0.81) |
| Hispanics | 6855 (4.6%) | 1285 (6.7%) | 0.71 (0.63-0.80) |
| Chronic kidney disease | 33645 (21.9%) | 10575 (52.8%) | 3.52 (3.40-3.64) |
| Weight loss | 3870 (2.5%) | 1865 (9.3%) | 3.01 (2.82-3.20) |
| Liver disease | 4060 (2.6%) | 1405 (7.0%) | 2.29 (2.13-2.46) |
| Congestive heart failure | 110385 (71.8%) | 17205 (85.8%) | 2.01 (1.92-2.10) |
| Coagulopathy | 17270 (11.2%) | 4315 (21.5%) | 1.82 (1.75-1.90) |
| Cerebrovascular disease | 17320 (11.3%) | 2930 (14.6%) | 1.30 (1.24-1.36) |
| Solid organ tumor | 3655 (2.4%) | 605 (3.0%) | 1.23 (1.11-1.36) |
| Chronic obstructive pulmonary disease | 46145 (30.0%) | 7095 (35.4%) | 1.21 (1.17-1.25) |
| Metastatic cancer | 995 (0.6%) | 150 (0.7%) | 1.16 (0.95-1.41) |
| Peripheral vascular disease | 33015 (21.5%) | 5130 (25.6%) | 1.15 (1.11-1.19) |
| Obesity | 25870 (16.8%) | 3335 (16.6%) | 1.01 (0.97-1.05) |
| Lymphoma | 1060 (0.7%) | 125 (0.6%) | 0.99 (0.81-1.20) |
| Alcohol use | 240 (0.2%) | 45 (0.2%) | 0.98 (0.70-1.39) |
| Hypothyroidism | 31350 (20.4%) | 4050 (20.2%) | 0.98 (0.94-1.02) |
| Coronary artery disease | 106475 (69.3%) | 13950 (69.6%) | 0.92 (0.89-1.00) |
| Hypertension | 136605 (88.9%) | 18015 (89.9%) | 0.90 (0.85-1.0) |
| Diabetes mellitus | 25140 (16.4%) | 1990 (9.9%) | 0.76 (0.72-0.80) |
| Income | |||
| 0-25th percentile | 32110 (21.2%) | 4485 (22.7%) | Reference |
| 25th-50th percentile | 38530 (25.4%) | 5210 (26.4%) | 1.08 (1.03-1.13) |
| 50th-75th percentile | 40805 (26.9%) | 4955 (25.1%) | 1.05 (1.00-1.10) |
| 75th-100th percentile | 39990 (26.4%) | 5075 (25.7%) | 0.93 (0.89-0.97) |
| Urban | 1380 (0.9%) | 110 (0.5%) | Reference |
| Urban non-teaching | 14550 (9.5%) | 1625 (8.1%) | 0.56 (0.46-0.69) |
| Urban teaching | 137785 (89.6%) | 18310 (91.3%) | 0.85 (0.80-0.90) |
Table 2. In-hospital outcomes of patients with AKI in TAVR.
AKI, acute kidney injury; IQR, interquartile range; TAVR, transcatheter aortic valve replacement
| Outcome | No AKI | AKI | p-Value |
| Died during hospitalization, n (%) | 1285 (0.8%) | 1605 (8%) | <0.01 |
| Length of stay, median (IQR) | 2 (2-4) | 9 (5-15) | <0.01 |
| Cost of stay (in US dollars), median (IQR) | 44853 (35218-56473) | 63110 (47235-85623) | <0.01 |
Figure 1. Adjusted odds of predictors of in-hospital AKI in patients with TAVR.
AKI, acute kidney injury; TAVR, transcatheter aortic valve replacement
Discussion
The principal findings of our study are summarized as follows: (1) AKI occurs in about 11.5% of patients undergoing TAVR, (2) mortality is higher in patients with AKI compared to patients who do not have AKI (8% vs. 0.8%; p<0.01), (3) factors at baseline such as male sex, CVA, CHF, COPD, liver disease, CKD, metastatic cancer, weight loss, and PVD are important predictors of AKI in patients undergoing TAVR, and (4) patients who developed AKI after TAVR have a higher length of stay and cost of stay.
The reported incidence of post-TAVR AKI is 22.1% ± 11.2% based on the current Valve Academic Research Consortium (VARC)-2. Both persistent and transient AKI have been independently associated with higher mortality rates. Hence, our study aims to identify baseline characteristics that predispose a patient to AKI so that we can institute an approach of pre-TAVR intravenous fluid hydration and try to minimize contrast exposure in these patients [9]. In contrast to the previous literature, in our national analysis, we have reported an AKI rate of 13%. However, it is important to note that we used ICD codes to identify AKI, whereas prior literature has reported AKI in terms of VARC-2 criteria [12]. However, our study results were congruent with the findings of Bagur et al., who reported an AKI rate of 11.7%, which is very close to our estimate of 13%. In prior literature, mortality rate in TAVR was approximated to be 28%, which was significantly higher compared to our study findings (8%) [14]. One of the possible explanations is that our study was more recent and has looked at TAVR experience in recent times, which have undergone significant technological advancements and increased operator experience. Similarly, Aregger et al. reported data on a series of 54 patients and reported an AKI frequency of 28% [19]. In contrast, our study reported more new findings from a large sample size of 173,760 patients, which would be a better indicator of AKI complication rates in recent times.
According to one estimate, patients who developed AKI had a four-fold increase in mortality. Kidney disease is a well-known predictor of worse outcomes in patients undergoing TAVR [12,20,21]. Our study findings are consistent with prior literature, and we report that patients with CKD have a 3.5 times higher risk of developing AKI. Similarly, co-morbidities such as COPD have been reported in prior literature to cause AKI [22]. Our study reinforces these findings and reports a 1.12-fold higher risk of AKI with COPD. Similarly, our national analysis identifies CVA, liver disease, and PVD as significant predictors of this adverse event. Previous studies have reported hypertension to be an important predictor of AKI due to loss of kidney autoregulation. However, in our analysis, hypertension was not found to be significantly associated with an increased incidence of AKI [23].
Our study findings must be interpreted in light of the following limitations. This was a retrospective study hence association should not be misinterpreted as causation. Important data including medication use and laboratory data were not available in the NIS. Surgical risk scores such as EuroSCORE (European System for Cardiac Operative Risk Evaluation) and STS (Society of Thoracic Surgeons) score cannot be calculated using this database. We were only able to look at in-hospital data, and longer follow-up data were not available. NIS is a billing dataset that relies on ICD codes; hence, coding errors are a possibility. CKD in our study was defined as CKD-1 to CKD-IV based on ICD-9 and ICD-10 diagnosis codes. We used ICD codes whereas non-database studies used standardized definitions. However, we used well-validated ICD codes that reduce the chance of coding errors. We are not able to quantify the degree of AKI from the NIS database given its inherent limitations; in contrast, previous studies used VARC-2 criteria for AKI definition and were able to quantify AKI.
Conclusions
In conclusion, our study reported a contemporary analysis of 173,760 TAVR patients. The rate of AKI post-TAVR was 11.5%. We further reported that at baseline CKD, weight loss, liver disease, CHF, CVA, COPD, metastatic cancer, and PVD were important predictors of AKI in patients undergoing TAVR. AKI in TAVR is associated with increased burden on the healthcare system in the form of increased length of hospital stay and cost of hospitalization. It is of utmost importance to identify patients who are at high risk of developing complications as TAVR indication expands to a larger population. These data can also help clinicians in decision-making to optimize patients prior to the procedure and decrease the risk of AKI post-TAVR.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. N/A issued approval N/A. Given the deidentified nature of the NIS database, IRB approval is not required.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Principles of TAVR valve design, modelling, and testing. Rotman OM, Bianchi M, Ghosh RP, Kovarovic B, Bluestein D. Expert Rev Med Devices. 2018;15:771–779. doi: 10.1080/17434440.2018.1536427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transcatheter versus surgical aortic-valve replacement in high-risk patients. Smith CR, Leon MB, Mack MJ, et al. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. Mack MJ, Leon MB, Thourani VH, et al. N Engl J Med. 2019;380:1695–1670. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Clinical outcomes of renal and liver transplant patients undergoing transcatheter aortic valve replacement: analysis of national inpatient sample database. Ullah W, Sattar Y, Al-Khadra Y, et al. Expert Rev Cardiovasc Ther. 2021;19:363–368. doi: 10.1080/14779072.2021.1892489. [DOI] [PubMed] [Google Scholar]
- 5.How to make the TAVI pathway more efficient. Tchetche D, de Biase C, Brochado B, Mastrokostopoulos A. Interv Cardiol. 2019;14:31–33. doi: 10.15420/icr.2018.28.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TAVR procedural volumes and patient outcomes: analysis of recent data. Cormican D, Jayaraman A, Villablanca P, Ramakrishna H. J Cardiothorac Vasc Anesth. 2020;34:545–550. doi: 10.1053/j.jvca.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Acute kidney injury after transcatheter aortic valve replacement in the elderly: outcomes and risk management. Zaleska-Kociecka M, Dabrowski M, Stepinska J. Clin Interv Aging. 2019;14:195–201. doi: 10.2147/CIA.S149916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The "big five" complications after transcatheter aortic valve replacement: do we still have to be afraid of them? Grube E, Sinning JM. JACC Cardiovasc Interv. 2019;12:370–372. doi: 10.1016/j.jcin.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Acute kidney injury after transcatheter aortic valve implantation. Scherner M, Wahlers T. J Thorac Dis. 2015;7:1527–1535. doi: 10.3978/j.issn.2072-1439.2015.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Transcatheter aortic valve replacement: comprehensive review and present status. Arora S, Misenheimer JA, Ramaraj R. Tex Heart Inst J. 2017;44:29–38. doi: 10.14503/THIJ-16-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedside renal Doppler ultrasonography and acute kidney injury after TAVR. Peillex M, Marchandot B, Bayer S, et al. J Clin Med. 2020;9:905. doi: 10.3390/jcm9040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acute kidney injury after transcatheter aortic valve implantation: incidence, risk factors, and prognostic effects. Alassar A, Roy D, Abdulkareem N, Valencia O, Brecker S, Jahangiri M. Innovations (Phila) 2012;7:389–393. doi: 10.1177/155698451200700603. [DOI] [PubMed] [Google Scholar]
- 13.Acute kidney injury associated with cardiac surgery. Rosner MH, Okusa MD. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 14.Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Bagur R, Webb JG, Nietlispach F, et al. Eur Heart J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vascular complications associated with transcatheter aortic valve replacement. Sardar MR, Goldsweig AM, Abbott JD, Sharaf BL, Gordon PC, Ehsan A, Aronow HD. Vasc Med. 2017;22:234. doi: 10.1177/1358863X17697832. [DOI] [PubMed] [Google Scholar]
- 16.Expansion of TAVR into low-risk patients and who to consider for SAVR. Patel KV, Omar W, Gonzalez PE, Jessen ME, Huffman L, Kumbhani DJ, Bavry AA. Cardiol Ther. 2020;9:377–394. doi: 10.1007/s40119-020-00198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trends and predictors of transcatheter aortic valve implantation related in-hospital mortality (from the National Inpatient Sample Database) Ullah W, Zahid S, Hamzeh I, Birnbaum Y, Virani SS, Alam M. Am J Cardiol. 2021;143:97–103. doi: 10.1016/j.amjcard.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Predictors of in-hospital mortality in patients with end-stage renal disease undergoing transcatheter aortic valve replacement: a nationwide inpatient sample database analysis [Online ahead of print] Ullah W, Jafar M, Zahid S, et al. Cardiovasc Revasc Med. 2021 doi: 10.1016/j.carrev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, Frey FJ. Nephrol Dial Transplant. 2009;24:2175–2179. doi: 10.1093/ndt/gfp036. [DOI] [PubMed] [Google Scholar]
- 20.Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Anand IS, Chandrashekhar Y, Ferrari R, et al. Circulation. 1992;86:12–21. doi: 10.1161/01.cir.86.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Trends, predictors, and outcomes of major bleeding after transcatheter aortic valve implantation, from National Inpatient Sample (2011-2018) [Online ahead of print] Zahid S, Ullah W, Khan MU, et al. Expert Rev Cardiovasc Ther. 2021 doi: 10.1080/14779072.2021.1924678. [DOI] [PubMed] [Google Scholar]
- 22.Is transcatheter aortic valve replacement better than surgical aortic valve replacement in patients with chronic obstructive pulmonary disease? A Nationwide Inpatient Sample analysis. Ando T, Adegbala O, Akintoye E, et al. J Am Heart Assoc. 2018;7:0. doi: 10.1161/JAHA.117.008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The risk of acute renal failure in patients with chronic kidney disease. Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]