Abstract
This chapter reviews the relationship between stress and brain function in patients with neuropsychiatric disorders, with an emphasis on disorders that have most clearly been linked to traumatic stress exposure. These disorders, which have been described as trauma spectrum disorders, include posttraumatic stress disorder (PTSD), a subgroup of major depression, borderline personality disorder (BPD) and dissociative disorders; they share in common a neurobiological footprint, including smaller hippocampal volume, and are distinguished from other disorders that may share symptom similarities, like some of the anxiety disorders, but are not as clearly linked to stress. The relationship between environmental events such as stressors, especially in early childhood, and their effects on brain and neurobiology is important to understand in approaching these disorders as well as the development of therapeutic interventions. Addressing patients with stress-related disorders from multiple developmental (age at onset of trauma) as well as levels of analysis (cognitive, cultural, neurobiological) approaches will provide the most complete picture and result in the most successful treatment outcomes.
1. Lasting effects of traumatic stress
Traumatic stress can have lasting effects on the individual. Brain and physiological systems that helped us survive when we were wandering in small bands on the savannah are no longer as necessary in modern times. A sympathetic nervous system that allowed us to respond a split second faster to a predator even at the expense of long-term exaggerated responsiveness was favored in terms of survival and passing on genes to the next generation. Today, a hyperactive sympathetic nervous system may impair our ability to perform mundane office-based tasks with no real added value in a world essentially devoid of predators. The task of those in the mental health field is to optimize the health of our patients, which includes adjustment for the potentially crippling effects of neurobiological systems that previously were essential for survival but now may have detrimental effects.
Physiological response systems involved in survival underlie the pathophysiology of stress-related psychiatric disorders. Neural and physiological systems make us react quickly, fight back or run away. These stress response systems are preserved even when they are no longer as valuable. Reactions that once may have prevented death from an attack of a predator now simply make it impossible to function in daily life. Repeated episodes of stress lead to increased responsivity of these systems. This may have been useful in previous times, for example, the body shifting to an increased chronic presence of a band of lions in the region. In the modern age, stress can lead to psychiatric disorders with resultant pathology. We review here the relationship between stress, brain and physiology, and neuropsychiatric disorders. We focus on disorders with a clear link to stress, that have been described elsewhere as Trauma Spectrum Disorders (Bremner, 2002a). Those disorders include posttraumatic stress disorder (PTSD), a subgroup of major depression, Borderline Personality Disorder (BPD), and Dissociative Identity Disorder (DID). With the Diagnostic and Statistical Manual (DSM)-5, PTSD was moved from the category of Anxiety Disorders to Trauma- and Stressor-related Disorders (American Psychiatric Association, 2013). Included in this category is Acute Stress Disorder (ASD), which is seen as an early form of PTSD as opposed to a unique disorder (Bremner, 1999). Other disorders in this category include Adjustment Disorders, which have not been the subject of extensive research, and childhood attachment disorders that are beyond the scope of the current review. Neurobiological studies have shown that the other anxiety disorders, including Panic Disorder, Generalized Anxiety Disorder, and Obsessive Compulsive Disorder, in addition to lacking the clear link to stress, do not have the same neurobiological profile as the Trauma Spectrum Disorders (Bremner, 2002a; Heim, Bremner, & Nemeroff, 2006). These disorders are therefore not considered in detail here.
2. Trauma spectrum disorders
The model of Trauma Spectrum Disorders posits that a group of psychiatric disorders related to psychological trauma, usually in childhood, share an underlying neurobiological footprint (Bremner, 1999, 2002a, 2016). Although there is often symptom overlap, a common cause, such as childhood trauma, can lead to multiple outcomes, illustrating the principle of multifinality (Fig. 1) (Cicchetti & Rogosch, 1996). The differences in outcomes, which may be related to genetic, cultural, or concomitant environmental factors, can often be quite striking. For instance, patients with trauma spectrum disorders share a common neurobiological footprint in smaller volume of a brain area involved in memory called the hippocampus, although they will show differing physiological responses to different stimuli depending on the nature of their specific psychiatric disorder.
Fig. 1.
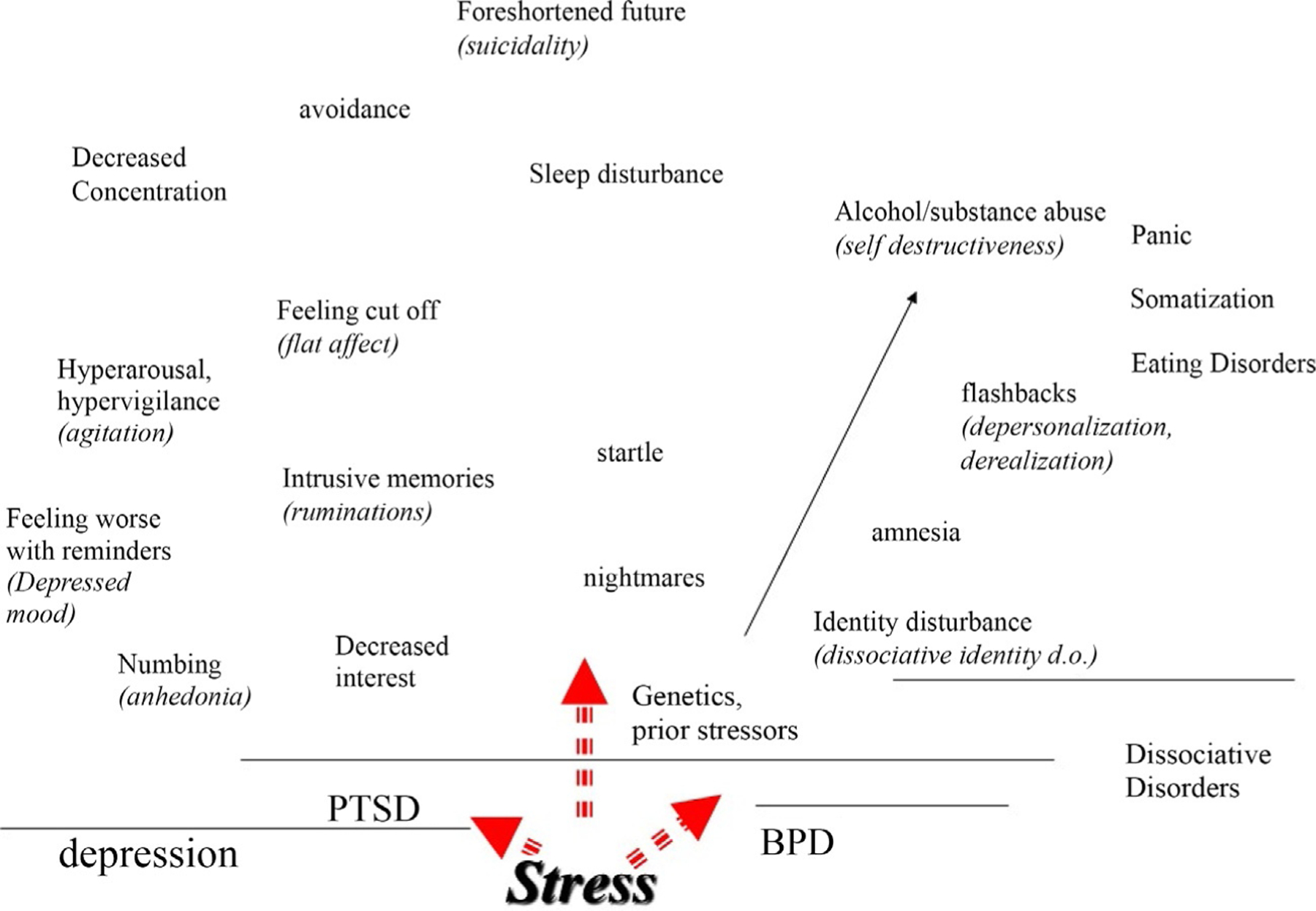
Stress can lead to multiple psychiatric outcomes. However many of the symptoms of these disorders overlap. The symptoms above the line over posttraumatic stress disorder (PTSD) include the symptoms of that disorder. However, it can be seen that they overlap with symptoms of depression, represented by the symptoms listed above the line over that disorder. Other outcomes include dissociative disorders, which overlap with Borderline Personality Disorder (BPD). Genetics and prior experience modulate how stress manifests itself in psychiatric symptoms.
In the trauma spectrum disorders model, patients with PTSD, BPD, DID and a subgroup of depression share common changes in brain and neurobiology that underlie the symptoms of these disorders. Although these are separate disorders, they also share a high degree of symptom overlap, and are often co-morbid with one another (Fig. 1), a fact that is not surprising given their overlapping neurobiological correlates (Bremner, 2002a, 2016). The DSM, the modern Bible of Psychiatry, was based on the medical disease model, and treats mental disorders as discrete entities (Bremner, 2006), forcing the use of terms like “high co-morbidity” to account for the overlapping nature of these disorders. The use of this term implies that these are discreet disorders, and their overlapping nature is an anomaly. The DSM attempts to side-step these issues by describing the same phenomena using differing terminology. For instance, psychomotor retardation and loss of interest in things you used to enjoy in major depression and feeling cut off from others and loss of pleasure in PTSD are all aspects of anhedonia that likely share altered function in mesocortical and mesolimbic dopamine pathways. Other symptoms are identical, like sleep disturbance and irritability. The utility of a model lies in its ability to predict and describe phenomena, therefore the trauma spectrum model is superior to the medical model in describing stress-related mental disorders as it more accurately describes these disorders as they actually exist in clinical practice and correctly predicts future behaviors. This likely accounts for the sparse use of DSM by clinicians engaged in actual clinical practice, outside of compliance with bureaucratic requirements.
The trauma spectrum model also has relevance on a neurobiological level. The model predicts that brain circuits and systems involved in the stress response and fear memory will be affected in these disorders, and that they will share a similar neurobiological footprint. A priori we identified a network of brain areas based on animal studies, a knowledge of functional neuroanatomy and findings from patients with specific neurological deficits, that were likely involved in the trauma spectrum disorders (Bremner, Krystal, Southwick, & Charney, 1995). These included the amygdala, hippocampus, thalamus, and medial prefrontal cortex (anterior cingulate). Close to a decade of research later, brain imaging studies in patients with PTSD and other stress-related psychiatric disorders corroborated the initial hypotheses (Bremner, 2003), and another decade of research replicated those initial studies (Campanella & Bremner, 2016). Neurohormonal systems like cortisol and norepinephrine also show similar alterations stress-related psychiatric disorders (Heim et al., 2006).
Hippocampal volume has been a particularly useful marker of the trauma spectrum disorders (Bremner & Vermetten, 2012). The hippocampus is sensitive to stress and plays a key role in declarative memory (Bremner & Vermetten, 2012). In a series of studies we and others showed smaller hippocampal volume as measured with magnetic resonance imaging (MRI) in patients with a history of childhood abuse and the diagnoses of PTSD (Bremner, Randall, et al., 1995), women with BPD and early life trauma (Driessen et al., 2000; Irle, Lange, & Sachsse, 2005; Schmahl, Vermetten, Elzinga, & Bremner, 2003), and women with abuse and DID (Vermetten, Schmahl, Lindner, Loewenstein, & Bremner, 2006). Women with early childhood sexual abuse and depression had smaller hippocampal volume compared to women with depression without early abuse, and non-abused non-depressed women (Vythilingam et al., 2002). Smaller hippocampal volume was not seen in patients with anxiety disorders, including panic disorder (Vythilingam et al., 2000), obsessive-compulsive disorder (OCD) or other anxiety disorders (Bremner, 2005a; Cannistraro & Rauch, 2003). These studies show a common correlate of stress-related mental disorders in a brain area known to be sensitive to stress that is specific to these disorders.
The amygdala is involved in the encoding of fear memories and also plays a role in some symptoms of stress-related psychiatric disorders. Women with abuse and BPD (Driessen et al., 2000; Schmahl, Vermetten, et al., 2003) and women with abuse and DID (Vermetten et al., 2006) showed smaller amygdala volume on MRI, which is seen in some studies of PTSD but not others (Bremner & Vermetten, 2012; Campanella & Bremner, 2016). Other neurobiological overlaps between PTSD and BPD associated with childhood abuse include alterations in function of the hypothalamic-pituitary-adrenal (HPA) axis and dysregulation of the prefrontal-limbic axis (Donegan et al., 2003; Driessen et al., 2004; Juengling et al., 2003; Lange, Kracht, Herholz, Sachsse, & Irle, 2005; Schmahl & Bremner, 2006; Schmahl, Elzinga, et al., 2003; Schmahl, McGlashan, & Bremner, 2002; Schmahl, Vermetten, Elzinga, & Bremner, 2004). In summary, findings are consistent with a common neurobiology and neurocircuitry underlying the trauma spectrum disorders, including PTSD, DID, BPD, and depression related to early abuse.
3. Psychologic trauma at various levels of development
A proper understanding of the effects of psychological trauma on neurobiology and the individual requires a knowledge of the developmental epoch at which the trauma occurred (Bremner, 2006, 2016). As the individual progresses through development from infancy to young adulthood and possibly moves from one physical and cultural environment to the next, changes are taking place on a neurological, social, individual, psychological, and cultural level (Feiring & Lewis, 1996). Some might start at different points with pathways that lead them to the same place (equifinality), or travel the same path with different ultimate outcomes (multipotentiality) (Cicchetti & Dawson, 2002; Cicchetti & Rogosch, 1996). The same childhood trauma may lead to two different outcomes, with one individual showing resilience and overcoming and perhaps thriving in spite of their environment while others succumb to psychopathology and dysfunction (Vythilingam et al., 2009). Childhood trauma is associated with an increase in risk for injected drug use (Anda et al., 2006; Dube et al., 2003), although not all patients with Opioid Use Disorders (OUDs) have a history of childhood trauma. Amongst women with depression, those with the “childhood trauma footprint” appear to respond better to combinations of psychotherapy and medication, illustrating the utility of the multi-level assessment in clinical practice (Richters & Cicchetti, 1993; Richters & Hinshaw, 1999).
4. A psycho-evolutionary view of stress, the brain, and neuropsychiatric disorders
Another factor to consider in reviewing stress, the brain, and neuropsychiatric disorders is the interaction between evolution and mental disorders. Fundamental to understanding stress-related psychiatric disorders is the role of neurophysiological systems involved in survival and their role in ancient and modern times. In the pre-agricultural age, humans roved the Savannah in small bands of hunter-gatherers, and evolution up to that point involved changes in the human brain that allowed individuals to run faster and fight back harder. Other advantages conveyed on humans included developing the capacity for abstract thought, language, and advanced social relationships. These processes required the development of a much larger frontal cortex than other animals, and along with that came side effects of anxiety and depression. Whether these are accidental by-products of evolution or served their own roles in specific situations is unclear. Taking an example from another area of medical science, the heterozygous gene for sickle cell anemia confers resistance to malaria, which is advantageous in African countries where this disease is common. Homozygous expression of the gene, however, confers sickle cell anemia. Thus a biological alteration that is advantageous in some situations is not in others. Similarly, depressive behaviors may be advantageous during sickness (in order to preserve energy) or during times of extreme threat (removing the individual from threat). In other situations, however, depressive behavior may be maladaptive. Similarly, avoidance and hyper responsiveness seen in PTSD may have allowed members of primitive bands to survive threats when groups of predators were in the vicinity. Whatever behaviors were helpful in these prehistoric periods, however, are probably lost in the transition to modern culture, where life in high rise buildings and days spent puzzling over financial accounts rather than chasing animals through the bush came to predominate.
In earlier times fear-related behaviors may have meant the difference between life and death. If there was a pack of tigers in an area where a band of hunter-gatherers was camped, hyper-vigilance could promote survival. These behaviors would be expected to persist, even after the threat had been removed. Children who exist in an environment of real or perceived threat respond in similar ways would sense the type of environment they were born into, and their developmental evolution would be adjusted accordingly. Children in a harsh, deprived, or non-supportive environment would grow up being mistrustful and socially withdrawn. This might have survival value in protecting the child from potentially harmful persons in the absence of a functional parent to protect them.
Symptoms of depression also probably have had a behavioral function at some point in time. They are a by-product of the development of the capacity in humans to form close social attachments. When those attachments are broken, through death or separation, there is a natural process of grieving where individuals recover from the disruption of the social connection and form the capacity to form new connections. If they do not go through this process, they may not go on to reproduce with others in the future. In addition, depression causes a social withdrawal and decrease in activity. Death of a partner or parent may put the survivor at greater risk without the added protection, so withdrawal from potentially dangerous situations would also have survival value. Similar behavioral effects occur with illnesses such as infections, which probably have similar survival value in allowing the conservation of energy and resources.
5. Neurohormonal responses to trauma
Trauma can have lasting effects on neurotransmitter and neurohormonal systems involved in the stress response (Bremner & Pearce, 2016). Cortisol and norepinephrine systems are altered in PTSD (Bremner, 2002b; Bremner & Vermetten, 2001; Yehuda, 2006) and underlie symptoms of PTSD and other stress-related mental disorders (Baker et al., 2005; Bremner, 2002a; Lindqvist et al., 2014; Neylan et al., 2005).
The hypothalamic-pituitary-adrenal (HPA) axis is activated by stress. Corticotropin-releasing factor (CRF) is released from the hypothalamus, with stimulation of adrenocorticotropic hormone (ACTH) release from the pituitary resulting in turn in cortisol release from the adrenal. CRF also acts centrally to create fear-related behaviors and triggers other neurochemical responses to stress, including the noradrenergic neurotransmitter system (Melia & Duman, 1991). Early life stress leads to long-term sensitization of the HPA axis and CRF to further stressors (Coplan et al., 1996; Levine, Weiner, & Coe, 1993; Makino, Smith, & Gold, 1995; Plotsky & Meaney, 1993; Stanton, Gutierrez, & Levine, 1988). Stress also leads to over activation of the norepinephrine system, which plays a critical role in the fight or flight response (Bremner, Krystal, Southwick, & Charney, 1996a, 1996b) (Fig. 2).
Fig. 2.
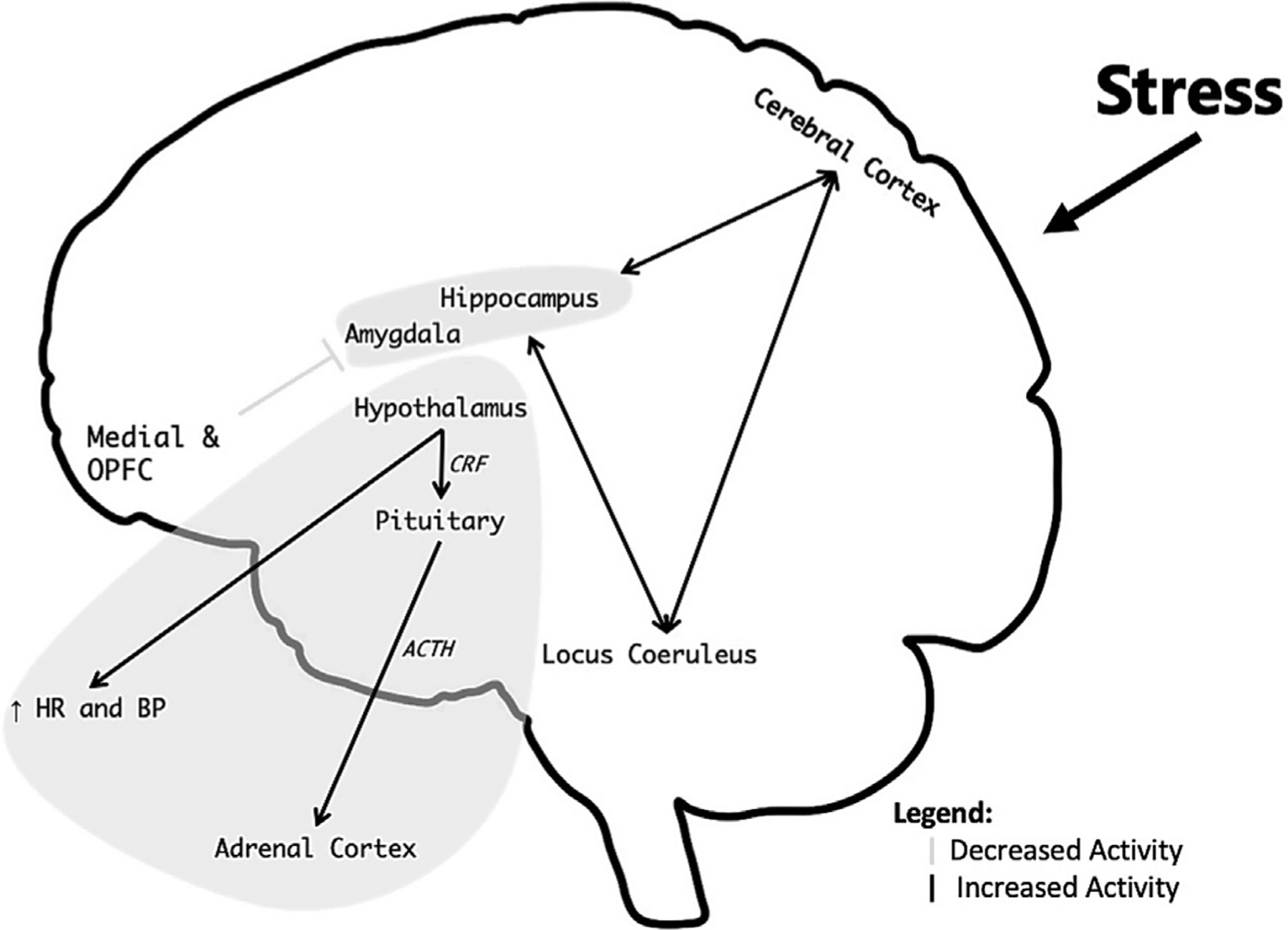
Model of how stress affects brain function. Brain areas involved in stress and memory including the hippocampus, amygdala, and medial and orbitofrontal cortex (OPFC) (including anterior cingulate) mediate symptoms of stress-related psychiatric disorders. Neurochemical systems also mediate the stress response and underlie psychiatric disorders. Most of the norepinephrine cell bodies are located in the locus coeruleus (pons) with neurons that distribute throughout the brain and activate the fight or flight response. Stress information is processed by the cerebral cortex with pathways through the hypothalamus that activate peripheral sympathetic and cardiovascular responses, including increased Heart Rate (HR) and Blood Pressure (BP), as well as the hypothalamic-pituitary-adrenal (HPA) axis, with activation of Corticotropin Releasing Factor (CRF) from the hypothalamus, which releases Adrenocorticotropic hormone (ACTH) from the pituitary, which in turn releases cortisol from the Adrenal Cortex. Norepinephrine and cortisol play critical roles in the stress response, and dysfunction of these systems underlie symptoms of stress-related psychiatric disorders.
As noted above, a network of brain areas involved in memory and fear, including the hippocampus, medial prefrontal cortex, and amygdala, interact with these neurochemical systems to mediate the brain’s response to stress (Bremner, 2005b, 2011; Rauch, Shin, & Phelps, 2006). The medial prefrontal cortex includes the anterior cingulate, orbitofrontal cortex, and adjacent areas. Inhibition of the amygdala by the medial prefrontal cortex represents the brain circuitry underlying extinction of fear responses (Milad & Quirk, 2002; Quirk, Garcia, & Gonzalez-Lima, 2006). This brain area, together with the hypothalamus, also modulates peripheral neurohormonal responses to stress via relay stations in the spinal cord (Diorio, Viau, & Meaney, 1993; Feldman, Conforti, & Weidenfeld, 1995; Frysztak & Neafsey, 1994).
Altered HPA axis function is associated with PTSD (Yehuda, 2002). Women with childhood sexual abuse-related PTSD had decreased baseline cortisol based on 24-h diurnal assessments of plasma with 10min sampling periods, a flattening of the normal diurnal cortisol curve, and increased pulsatility of cortisol reflecting dysregulation of CRF release, compared to women with abuse without PTSD, and women without abuse or PTSD (Bremner, Vermetten, & Kelley, 2007). Lower baseline cortisol was correlated with increased symptoms of PTSD in the PTSD group (Bremner et al., 2007). In a similar study of comprehensive sampling over 24h, combat veterans with PTSD showed lower plasma cortisol levels and a flattening of the normal diurnal curve (Yehuda, Teicher, Levengood, Trestman, & Siever, 1994), while other studies showed either normal or low cortisol measured in 24h urine collections (Yehuda, Boisoneau, Lowy, & Giller, 1995; Yehuda, Kahana, et al., 1995; Yehuda, Teicher, Trestman, Levengood, & Siever, 1996) and a blunting of the normal rise in cortisol after awakening in PTSD (de Kloet, Vermetten, Heijnen, et al., 2007; Neylan et al., 2005; Wessa, Rohleder, Kirschbaum, & Flor, 2006). Other findings included increased suppression of cortisol to low doses of dexamethasone, which together with other studies reflects altered sensitivity of glucocorticoid receptors in the pituitary and/or hippocampus (Bremner, Vythilingam, Vermetten, Newcomer, & Charney, 2004; de Kloet, Vermetten, Bikker, et al., 2007; de Kloet, Vermetten, Heijnen, et al., 2007; Goenjian et al., 1996; Stein, Yehuda, Koverola, & Hanna, 1997; Vermetten, 2008; Yehuda, Halligan, Grossman, Golier, & Wong, 2002; Yehuda, Levengood, et al., 1996; Yehuda, Lowy, Southwick, Shaffer, & Giller, 1991; Yehuda et al., 1993), PTSD patients showed increased cortisol in response to hearing personalized scripts of their childhood abuse (Elzinga, Schmahl, Vermetten, van Dyck, & Bremner, 2003) as well as with “neutral” mental stress tasks involving cognitive stressors like mental arithmetic (Bremner, Vythilingam, Vermetten, Adil, et al., 2003). Women with depression and a history of childhood abuse had increased cortisol response to a stressful cognitive challenge relative to controls (Heim et al., 2000) and a blunted ACTH response to CRF challenge (Heim, Newport, Bonsall, Miller, & Nemeroff, 2001). PTSD was associated with increases in CRF concentrations in the cerebrospinal fluid (CSF) (Baker et al., 2005, 1999; Bremner, Licinio, et al., 1997; Sautter et al., 2003) and plasma (de Kloet et al., 2008), and a blunted ACTH response to CRF challenge, suggesting decreased pituitary sensitivity to CRF (Smith et al., 1989).
Increased noradrenergic and peripheral sympathetic function is associated with PTSD (Bremner et al., 1996a, 1996b). Symptoms irritability, increased startle, hyperarousal and sleep disturbance, all indicate excessive sympathetic and noradrenergic function (Bremner et al., 1996b). Consistent with this, baseline concentrations of norepinephrine and its metabolites in urine and plasma in PTSD were found to be increased (De Bellis et al., 1999; De Bellis, Lefter, Trickett, & Putnam, 1994; Lemieux & Coe, 1995; Mason, Giller, & Kosten, 1988; Yehuda, Siever, & Teicher, 1998) or unchanged (Blanchard, Kolb, Prins, Gates, & McCoy, 1991; McFall, Veith, & Murburg, 1992; Mellman, Kumar, Kulick-Bell, Kumar, & Nolan, 1995; Pitman & Orr, 1990; Southwick et al., 1993) and increased in CSF (Geracioti et al., 2001). Increased sympathetic reactivity based on heart rate, blood pressure, and skin conductance (Blanchard, Kolb, Gerardi, Ryan, & Pallmeyer, 1986; Blanchard, Kolb, Pallmeyer, & Gerardi, 1982; Bremner et al., 1996b; Malloy, Fairbank, & Keane, 1983; McFall, Murburg, Ko, & Veith, 1990; Orr et al., 1998; Orr, Lasko, Shalev, & Pitman, 1995; Orr, Pitman, Lasko, & Herz, 1993; Orr & Roth, 2000), as well as increased norepinephrine and epinephrine in plasma (Blanchard et al., 1991; McFall et al., 1992), was seen in PTSD patients exposed to traumatic reminders in the form of combat slides and sounds or personalized trauma scripts, while administration of the alpha2 adrenergic receptor antagonist, yohimbine, which increases firing of noradrenergic neurons in the locus coeruleus, increased PTSD symptoms and anxiety, as well plasma concentrations of the norepinephrine metabolite, 3-methoxy-4-hydroxyphenylglycol (MHPG) (Southwick et al., 1997, 1993). PTSD patients had a decrease in brain function in the medial prefrontal cortex with yohimbine not seen in healthy controls (decreased frontal lobe function occurs with excessive release of norepinephrine in that brain area) (Bremner, Innis, et al., 1997). Increased heart rate in the emergency room after trauma predicted subsequent development of chronic PTSD (Shalev et al., 1998). These findings all support increased noradrenergic function in PTSD.
6. The neural circuitry of PTSD
Brain imaging studies have been consistent in supporting the model of a specific circuitry of PTSD (Bremner, 2011). Exposure to memories of childhood sexual abuse with personalized traumatic scripts in women with childhood sexual abuse-related PTSD resulted in decreased blood flow in the medial prefrontal cortex/anterior cingulate, including Brodmann’s area 25, or subcallosal gyrus, and area 32, as well as decreased blood flow in parietal cortex and inferior frontal gyrus (Bremner, Narayan, et al., 1999; Shin et al., 1999), hippocampus and visual association cortex (Bremner, Narayan, et al., 1999). Emotional memory tasks, such as retrieval of word pairs like “blood-stench” resulted in similar decreases in blood flow in this medial prefrontal cortex area, as well as hippocampus, and fusiform gyrus/inferior temporal gyrus, with increased activation in posterior cingulate, left inferior parietal cortex, left middle frontal gyrus, and visual association and motor cortex (Bremner et al., 2003b). Decreased medial prefrontal cortical/anterior cingulate activation was seen in women with abuse-related PTSD during performance of the emotional Stroop task (i.e., naming the color of a word such as “rape”) (Bremner, Vermetten, et al., 2004). Other studies replicated the finding of decreased medial prefrontal cortex/anterior function during exposure to traumatic reminders in the form of traumatic slides and/or sounds or traumatic scripts using PET, SPECT or fMRI (Bremner, Narayan, et al., 1999; Bremner, Staib, et al., 1999; Britton, Phan, Taylor, Fig, & Liberzon, 2005; Lanius et al., 2001, 2003; Liberzon et al., 1999; Lindauer et al., 2004; Phan, Britton, Taylor, Fig, & Liberzon, 2006; Semple et al., 2000; Shin et al., 1997, 1999, , 2004, 2001, 2005; Yang, Wu, Hsu, & Ker, 2004). Studies have also shown increased amygdala function with classical fear conditioning or other fear-related tasks (Armony, Corbo, Clement, & Brunet, 2005; Bremner et al., 2005; Liberzon et al., 1999; Pissiota et al., 2002; Protopopescu et al., 2005; Rauch et al., 1996, 2000; Shin et al., 2004, 2005; Vermetten, Schmahl, Southwick, & Bremner, 2007). Other findings in studies of traumatic reminder exposure include decreased function with exposure to traumatic reminders in hippocampus (Bremner, Narayan, et al., 1999), thalamus(Laniusetal.,2001,2003), visual association cortex (Bremner, Narayan, et al., 1999; Lanius et al., 2003; Shin et al., 1997, 2004), parietal cortex (Bremner, Narayan, et al., 1999; Rauch et al., 1996; Sakamoto et al., 2005; Shin et al., 1997, 1999), inferior frontal gyrus (Bremner, Narayan, et al., 1999; Lanius et al., 2003; Rauch et al., 1996; Sakamoto et al., 2005; Shin et al., 1997, 1999, 2001), and increased function in insula (Bruce et al., 2013; Nicholson et al., 2016), posterior cingulate (Bremner, Narayan, et al., 1999; Bremner, Staib, et al., 1999; Lanius et al., 2001; Shin et al., 1997), insula (Vermetten et al., 2007), and parahippocampal gyrus (Bremner, Narayan, et al., 1999; Bremner, Staib, et al., 1999; Liberzon et al., 1999).
Brain imaging and physiological assessments were used to test the hypothesis that a common network of brain areas mediated symptoms of trauma spectrum disorders. We studied women with a history of childhood abuse and the diagnosis of BPD, with half of them having a co-morbid diagnosis of PTSD, although BPD was felt to be the primary diagnosis. Exposure to scripts of a hypothetical situation where they were abandoned in a mall led to marked increases in sympathetic responses as measured by heart rate and electrodermal responses, whereas exposure to personalized scripts of traumatic events from their childhood led to a much less exaggerated response (Schmahl, Elzinga, & Bremner, 2002; Schmahl, Elzinga, et al., 2004). Women with PTSD related to childhood abuse without the diagnosis of BPD, on the other hand, had no response to abandonment scripts, but had pronounced physiological responses to the personalized traumatic scripts (Schmahl, Elzinga, et al., 2004). This was in spite of the fact that the severity of traumas between the two groups of women were comparable. Brain imaging showed that the women with PTSD and BPD had a similar footprint in neural responses (failure of response of the medial prefrontal cortex/anterior cingulate) to the particular script (abandonment/trauma) for which they were most reactive (Bremner, Narayan, et al., 1999; Schmahl, Elzinga, et al., 2003; Schmahl, Vermetten, et al., 2004). These studies illustrate how similar events lead to different outcomes, however brain circuits mediating the effects overlap, suggesting that other factors like prior experience or genetics overlap with neurobiology to determine outcome (Fig. 2).
Studies of structural MRI showed smaller hippocampal volume across the trauma spectrum disorders (Bremner, 2016), including abuse-related PTSD (Bremner, Randall et al., 1997, Bremner et al., 2003a), DID with early abuse (Vermetten et al., 2006), BPD with early abuse (Driessen et al., 2000; Schmahl, Vermetten, et al., 2003), and depression with early abuse (Vythilingam et al., 2002). Patients with another anxiety disorder, panic disorder, did not show a reduction in hippocampal volume (Vythilingam et al., 2000). Patients with trauma spectrum disorders exhibit other alterations in stress responsive neurohormonal systems. HPA axis dysfunction, and a functional dysregulation of the prefrontal-limbic axis, are seen in BPD that are similar to PTSD (Donegan et al., 2003; Driessen et al., 2004; Juengling et al., 2003; Lange et al., 2005; Schmahl & Bremner, 2006; Schmahl, Elzinga, et al., 2003; Schmahl, McGlashan, et al., 2002; Schmahl, Vermetten, et al., 2004). These findings are consistent with a common neurobiology and neurocircuitry underlying the trauma spectrum disorders, including PTSD, DID, BPD, and depression related to early abuse, as well as some differences. The partial overlap of neurobiology then parallels the partial overlap in symptomatology in patients with trauma-related mental disorders.
7. Conclusions
Stress can have effects on the brain that lead to the symptoms of many stress-related psychiatric disorders. Treatments that target the underlying neurobiology can be helpful in their treatment. For instance, serotonin is one of the neurochemical systems associated with symptoms of PTSD and depression, and treatment with selective serotonin reuptake inhibitors (SSRIs) can be helpful for many patients. Other non-medication treatments can be helpful as well, including psychotherapy and behavioral interventions like meditation training. Meditation adds a sense of control (Astin, 1997; Weissbecker et al., 2002), allowing trauma victims to gain control of their thoughts and memories in a non-emotional way, disrupting patterns of ruminative thinking (Teasdale, Segal, & Williams, 1995) which can be associated with pathological arousal (Teasdale et al., 2000). Interventions like these may modify the way traumatic memories are encoded and consolidated and therefore reduce risk of chronic symptoms of PTSD. Other interventions that block sympathetic arousal with reminders, like neuromodulation, may also be useful (Bremner & Rapaport, 2017). Research on neurobiological correlates of stress-related psychiatric disorders will be helpful in the development of new treatments for these disabling disorder.
Acknowledgment
The work presented in this review was supported by Grants from NIH R01 MH056120, R01 HL088726, K24 MH076955, and P01 HL101398.
References
- American Psychiatric Association. (2013). The diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Anda RF, Felitti VJ, Walker J, Whitfield C, Bremner JD, Perry BD, et al. (2006). The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Corbo V, Clement MH, & Brunet A (2005). Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. The American Journal of Psychiatry, 162(10), 1961–1963. [DOI] [PubMed] [Google Scholar]
- Astin J (1997). Stress reduction through mindfulness meditation:Effects on psychologicalsymptomatology, sense of control, and spiritual experiences. Psychotherapy and Psychosomatics, 66, 97–106. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, et al. (2005). Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry, 162, 992–994. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. (1999). Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry, 156, 585–588. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Gerardi RJ, Ryan P, & Pallmeyer TP (1986). Cardiac response to relevant stimuli as an adjunctive tool for diagnosing post-traumatic stress disorder in Vietnam veterans. Behavior Therapy, 17(5), 592–606. [Google Scholar]
- Blanchard EB, Kolb LC, Pallmeyer TP, & Gerardi RJ (1982). A psychophysiological study of post-traumatic stress disorder in Vietnam veterans. Psychiatric Quarterly, 54(4), 220–229. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, & McCoy GC (1991). Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. Journal of Nervous and Mental Disease, 179, 371–373. [DOI] [PubMed] [Google Scholar]
- Bremner JD (1999). Acute and chronic responses to stress: Where do we go from here? [Editorial]. The American Journal of Psychiatry, 156(3), 349–351. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2002a). Does stress damage the brain? Understanding trauma-related disorders from a mind-body perspective. New York: W.W. Norton. [Google Scholar]
- Bremner JD (2002b). Neuroimaging of childhood trauma. Seminars in Clinical Neuropsychiatry, 7, 104–112. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2003). Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacology Bulletin, 37(2), 6–25. [PubMed] [Google Scholar]
- Bremner JD (2005a). Brain imaging handbook. New York: W.W. Norton. [Google Scholar]
- Bremner JD (2005b). T he neurobiology of childhood sexual abuse in women with posttraumatic stress disorder. In Kendall-Tackett KA (Ed.), Handbook of women, stress and trauma (pp. 181–206). New York: Brunner-Routledge. [Google Scholar]
- Bremner JD (2006). Traumatic stress from a multiple-levels-of-analysis perspective. In Cicchetti D & Cohen DJ (Eds.), Vol. 2 Developmental psychopathology (pp. 656–676). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Bremner JD (2011). Stress and human neuroimaging studies. In Conrad CD (Ed.), The handbook of stress: Neuropsychological effects on the brain (pp. 446–462). Chicester, West Sussex, UK: Wiley-Blackwell Press. [Google Scholar]
- Bremner JD (2016). Traumatic stress from a multi-level developmental psychopathology perspective. In Cicchetti D (Ed.), Developmental psychopathology, Vol. 3: Maladaptation and psychopathology (3rd ed., pp. 386–424). Hoboken, N.J.: Wiley. [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, et al. (1997). PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Archives of General Psychiatry, 54, 246–256. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1995). Functional neuroanatomical correlates of the effects of stress on memory. Journal of Traumatic Stress, 8, 527–554. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1996a). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse, 23, 28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1996b). Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse, 23, 39–51. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens M, Southwick SM, et al. (1997). Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. The American Journal of Psychiatry, 154, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, & Charney DS (1999). Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. The American Journal of Psychiatry, 156, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, & Pearce B (2016). Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD. In Bremner JD (Ed.), Posttraumatic stress disorder: From neurobiology to treatment (pp. 181–232). Hoboken, New Jersey: Wiley-Blackwell. [Google Scholar]
- Bremner JD, Randall PR, Scott TM, Bronen RA, Delaney RC, Seibyl JP, et al. (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry, 152, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Mazure CM, et al. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: A preliminary report. Biological Psychiatry, 41, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, & Rapaport MH (2017). Vagus nerve stimulation: Back to the future. The American Journal of Psychiatry, 174(7), 609–610. 10.1176/appi.ajp.2017.17040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, & Charney DS (1999). Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry, 45, 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, & Vermetten E (2001). Stress and development: Behavioral and biological consequences. Development and Psychopathology, 13, 473–489. [DOI] [PubMed] [Google Scholar]
- Bremner JD, & Vermetten E (2012). The hippocampus and post-traumatic stress disorders. In Bartsch T (Ed.), The clinical neurobiology of the hippocampus: An integrative view (pp. 262–272): Oxford University Press. [Google Scholar]
- Bremner D, Vermetten E, & Kelley ME (2007). Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. Journal of Nervous and Mental Disease, 195(11), 919–927. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. (2005). Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychological Medicine, 35(6), 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga BE, et al. (2004). Neural correlates of the classical color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry, 55(6), 612–620. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, et al. (2003). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology, 28, 733–750. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Newcomer JW, & Charney DS (2004). Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (PTSD). Psychiatry Research, 129(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. (2003a). MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD). American Journal of Psychiatry, 160, 924–932. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib L, et al. (2003b). Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder (PTSD) related to early childhood sexual abuse. Biological Psychiatry, 53, 289–299. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, & Liberzon I (2005). Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry, 57(8), 832–840. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Buchholz KR, Brown WJ, Yan L, Durbin A, & Sheline YL (2013). Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. Neuroimage. Clinical, 2, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella C, & Bremner JD (2016). Neuroimaging of PTSD. In Bremner JD (Ed.), Posttraumatic stress disorder: From neurobiology to treatment (pp. 291–320). Hoboken, New Jersey: Wiley-Blackwell. [Google Scholar]
- Cannistraro PA, & Rauch SL (2003). Neural circuitry of anxiety: Evidence from structural and functional neuroimaging studies. Psychopharmacology Bulletin, 37(4), 8–25. [PubMed] [Google Scholar]
- Cicchetti D, & Dawson G (2002). Editorial: Multiple levels of analysis. Development and Psychopathology, 14, 417–420. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8, 597–600. [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. (1996). Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America, 93, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, et al. (1999). A.E. Bennett Research Award: Developmental traumatology: Part I: Biological stress systems. Biological Psychiatry, 45, 1259–1270. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Lefter L, Trickett PK, & Putnam FW (1994). Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child and Adolescent Psychiatry, 33, 320–327. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Bikker A, Meulman E, Geuze E, Kavelaars A, et al. (2007). Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Molecular Psychiatry, 12(5), 443–453. 10.1038/sj.mp.4001934. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, et al. (2008). Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Progress in Brain Research, 167, 287–291. 10.1016/s0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, & Westenberg HG (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology, 32(3), 215–226. 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, & Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13(9), 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. (2003). Amygdala hyperreactivity in borderline personality disorder: Implications for emotional dysregulation. Biological Psychiatry, 54, 1284–1293. [DOI] [PubMed] [Google Scholar]
- Driessen M, Beblo T, Mertens M, Piefke M, Rullkoetter N, Silva-Saavedra A, et al. (2004). Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biological Psychiatry, 55(6), 603–611. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. (2000). Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry, 57, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, & Anda RF (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111(3), 564–572. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CS, Vermetten E, van Dyck R, & Bremner JD (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology, 28(9), 1656–1665. [DOI] [PubMed] [Google Scholar]
- Feiring C, & Lewis M (1996). Finality in the eye of the beholder: Multiple sources, multiple time points, multiple paths. Development and Psychopathology, 8, 721–733. [Google Scholar]
- Feldman S, Conforti N, & Weidenfeld J (1995). Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neuroscience and Biobehavioral Reviews, 19(2), 235–240. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, & Neafsey EJ (1994). The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Research, 643, 181–193. [DOI] [PubMed] [Google Scholar]
- Geracioti TDJ, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. (2001). CSF norepinephrine concentrations in posttraumatic stress disorder. American Journal of Psychiatry, 158, 1227–1230. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, et al. (1996). Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. The American Journal of Psychiatry, 153, 929–934. [DOI] [PubMed] [Google Scholar]
- Heim C, Bremner JD, & Nemeroff CB (2006). Trauma spectrum disorders. In Runge MS & Patterson C (Eds.), Principles of molecular medicine (pp. 1203–1210). Totowa, N.J: Humana Press. [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, & Nemeroff CB (2001). Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry, 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association, 284, 592–597. [DOI] [PubMed] [Google Scholar]
- Irle E, Lange C, & Sachsse U (2005). Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biological Psychiatry, 57(2), 173–182. [DOI] [PubMed] [Google Scholar]
- Juengling F, Schmahl CG, Hesslinger B, Eberg D, Bremner JD, Gostomzyk M, et al. (2003). Positron emission tomography in female patients with borderline personality disorder. Journal of Psychiatric Research, 37, 109–115. [DOI] [PubMed] [Google Scholar]
- Lange C, Kracht L, Herholz K, Sachsse U, & Irle E (2005). Reduced glucose metabolism in temporo-parietal cortices of women with borderline personality disorder. Psychiatry Research: Neuroimaging, 139, 115–126. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. (2001). Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. The American Journal of Psychiatry, 158, 1920–1922. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, et al. (2003). Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biological Psychiatry, 53(3), 204–210. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, & Coe CL (1995). Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosomatic Medicine, 57, 105–115. [DOI] [PubMed] [Google Scholar]
- Levine S, Weiner SG, & Coe CL (1993). Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology, 4, 297–306. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, et al. (1999). Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry, 45, 817–826. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, et al. (2004). Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biological Psychiatry, 56(11), 853–861. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, et al. (2014). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain, Behavior, and Immunity, 42, 81–88. 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, & Gold PW (1995). Increased expression of corticotropin-releasing hormone and vasopressin messenger-ribonucleic acid (messenger RNA) in the hypothalamic paraventricular nucleus during repeated stress-association with reduction in glucocorticoid messenger-RNA levels. Endocrinology, 136, 3299–3309. [DOI] [PubMed] [Google Scholar]
- Malloy PF, Fairbank JA, & Keane TM (1983). Validation of a multimethod assessment of posttraumatic stress disorders in Vietnam veterans. Journal of Consulting and Clinical Psychology, 51(4), 488–494. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, & Kosten TR (1988). Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. Journal of Nervous and Mental Disease, 176, 498–502. [DOI] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, & Veith RC (1990). Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biological Psychiatry, 27, 1165–1175. [DOI] [PubMed] [Google Scholar]
- McFall ME, Veith RC, & Murburg MM (1992). Basal sympathoadrenal function in posttraumatic stress disorder. Biological Psychiatry, 31, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Melia KR, & Duman RS (1991). Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proceedings of the National Academy of Sciences of the United States of America, 88, 8382–8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Kumar A, Kulick-Bell R, Kumar M, & Nolan B (1995). Nocturnal/daytime urine norepinephrine measures and sleep in combat-related PTSD. Biological Psychiatry, 38, 174–179. [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420, 70–73. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. (2005). PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology, 30(4), 373–381. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RWJ, Théberge J, et al. (2016). Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Research: Neuroimaging, 250, 61–72. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Ahern CE, Berry NJ, & Pitman RK (1998). Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting and Clinical Psychology, 66(6), 906–913. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, & Pitman RK (1995). Physiological responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 104, 75–82. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, & Herz LR (1993). Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology, 102, 152–159. [DOI] [PubMed] [Google Scholar]
- Orr SP, & Roth WT (2000). Psychophysiological assessment: Clinical applications for PTSD. Journal of Affective Disorders, 61, 225–240. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, & Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry, 63(2), 184–192. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Fernandez M, Von Knorring L, Fischer H, & Fredrikson M (2002). Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. European Archives of Psychiatry and Clinical Neuroscience, 252, 68–75. [DOI] [PubMed] [Google Scholar]
- Pitman RK, & Orr SP (1990). Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biological Psychiatry, 27, 245–247. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, & Meaney MJ (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research, 18(3), 195–200. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. (2005). Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry, 57(5), 464–473. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, & Gonzalez-Lima F (2006). Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry, 60(4), 337–343. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biological Psychiatry, 60(4), 376–382. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. (1996). A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Archives of General Psychiatry, 53, 380–387. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry, 47(9), 769–776. [DOI] [PubMed] [Google Scholar]
- Richters JE, & Cicchetti D (1993). Mark Twain meets DSM-III-R: Conduct disorder, development, and the concept of harmful dysfunction. Development and Psychopathology, 5, 5–29. [Google Scholar]
- Richters JE, & Hinshaw SP (1999). The abduction of disorder in psychiatry. Journal of Abnormal Psychology, 108(3), 438–445. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. (2005). Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: A functional MRI study. NeuroImage, 26(3), 813–821. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. (2003). Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biological Psychiatry, 54(12), 1382–1388. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, & Bremner JD (2006). Neuroimaging in borderline personality disorder. Journal of Psychiatric Research, 40(5), 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, & Bremner JD (2002). Individual differences in psychophysiological reactivity in adults with childhood abuse. Clinical Psychology and Psychotherapy, 9, 271–276. [Google Scholar]
- Schmahl CG, Elzinga B, Ebner U, Haaf B, Sanislow C, McGlashan TH, et al. (2004). Psychophysiological reactivity to traumatic and abandonment scripts in borderline personality disorder and posttraumatic stress disorder: A preliminary report. Psychiatry Research, 126(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Vermetten E, Sanislow C, McGlashan TH, & Bremner JD (2003). Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biological Psychiatry, 54, 42–51. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, McGlashan T, & Bremner JD (2002). Neurobiological correlates of borderline personality disorder. Psychopharmacology Bulletin, 36, 69–87. [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, & Bremner JD (2003). Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research: Neuroimaging, 122, 193–198. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BE, & Bremner JD (2004). A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biological Psychiatry, 55(7), 759–765. [DOI] [PubMed] [Google Scholar]
- Semple WE, Goyer P, McCormick R, Donovan B, Muzic RF, Rugle L, et al. (2000). Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared to controls. Psychiatry, 63, 65–74. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, et al. (1998). A prospective study of heart rate responses following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry, 55, 553–559. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, et al. (1997). Visual imagery and perception in posttraumatic stress disorder: A positron emission tomographic investigation. Archives of General Psychiatry, 54, 233–237. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. (1999). Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American Journal of Psychiatry, 156, 575–584. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. (2004). Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry, 61(2), 168–176. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62(3), 273–281. [DOI] [PubMed] [Google Scholar]
- Smith MA, Davidson R, Ritchie JC, Kudler H, Lipper S, Chappell P, et al. (1989). The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biological Psychiatry, 26, 349–355. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, Nicolaou A, Nagy LM, et al. (1997). Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of General Psychiatry, 54, 749–758. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. (1993). Abnormal noradrenergic function in posttraumatic stress disorder. Archives of General Psychiatry, 50(4), 266–274. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, & Levine S (1988). Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience, 102, 692–700. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, & Hanna C (1997). Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biological Psychiatry, 42, 680–686. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal A, & Williams JMG (1995). How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behavior Research and Therapy, 33(1), 25–39. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, & Lau MA (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68, 615–623. [DOI] [PubMed] [Google Scholar]
- Vermetten E (2008). Epilogue: Neuroendocrinology of PTSD. Progress in Brain Research, 167, 311–313. 10.1016/s0079-6123(07)67030-7. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, & Bremner JD (2006). Hippocampal and amygdalar volumes in dissociative identity disorder. The American Journal of Psychiatry, 163, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, & Bremner JD (2007). Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacology Bulletin, 40(1), 8–30. [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Anderson E, Goddard A, Woods SW, Staib LH, Charney DS, et al. (2000). Temporal lobe volumes in panic disorder-a quantitative magnetic resonance imaging study. Psychiatry Research: Neuroimaging, 99, 75–82. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport CD, Miller AH, Vermetten E, Anderson E, et al. (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. The American Journal of Psychiatry, 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Nelson EE, Scaramozza M, Waldeck T, Hazlett G, Southwick SM, et al. (2009). Reward circuitry in resilience to severe trauma: An fMRI investigation of resilient special forces soldiers. Psychiatry Research, 172(1), 75–77. 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbecker I, Salmon P, Studts JL, Floyd AR, Dedert EA, & Sephton SE (2002). Mindfulness-based stress reduction and sense of coherence among women with fibromyalgia. Journal of Clinical Psychology in Medical Settings, 9, 297–307. [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, & Flor H (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology, 31(2), 209–215. [DOI] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, & Ker JH (2004). Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: A functional MRI study. Neuroscience Letters, 370(1), 13–18. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2002). Post-traumatic stress disorder. New England Journal of Medicine, 346, 108–114. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2006). Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences, 1071, 137–166. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, & Giller EL Jr. (1995). Dose response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Archives of General Psychiatry, 52, 583–593. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R, Golier JA, & Wong C (2002). The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biological Psychiatry, 52(2), 393–403. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick S, Mason JW, & Giller EL (1995). Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. The American Journal of Psychiatry, 152, 982–986. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Levengood RA, Schmeidler J, Wilson S, Guo LS, & Gerber D (1996). Increased pituitary activation following metyrapone administration in posttraumatic stress disorder. Psychoneuroendocrinology, 21, 1–16. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lowy MT, Southwick SM, Shaffer D, & Giller EL (1991). Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. The American Journal of Psychiatry, 148(4), 499–504. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, & Teicher MH (1998). Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biological Psychiatry, 44, 56–63. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner JD, Charney DS, & Mason J (1993). Enhanced suppression of cortisol with low dose dexamethasone in posttraumatic stress disorder. American Journal of Psychiatry, 150, 83–86. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Levengood RA, Trestman RL, & Siever LJ (1994). Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Annals of the New York Academy of Sciences, 746(1), 378–380. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH,Trestman RL, Levengood RA,& Siever LJ(1996). Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry, 40(2), 79–88. [DOI] [PubMed] [Google Scholar]


