Abstract
Da Costa originally described Soldier’s Heart in the 19th Century as a syndrome that occurred on the battlefield in soldiers of the American Civil War. Soldier’s Heart involved symptoms similar to modern day posttraumatic stress disorder (PTSD) as well as exaggerated cardiovascular reactivity felt to be related to an abnormality of the heart. Interventions were appropriately focused on the cardiovascular system. With the advent of modern psychoanalysis, psychiatric symptoms became divorced from the body and were relegated to the unconscious. Later, the physiology of PTSD and other psychiatric disorders was conceived as solely residing in the brain. More recently, advances in psychosomatic medicine led to the recognition of mind-body relationships and the involvement of multiple physiological systems in the etiology of disorders, including stress, depression PTSD, and cardiovascular disease, has moved to the fore, and has renewed interest in the validity of the original model of the Soldier’s Heart syndrome.
Keywords: Posttraumatic stress disorders, cardiovascular disease, coronary artery disease, stress, depression, depressive disorders, major depression, child abuse, child development, child development disorders, pervasive, neurobiology, dissociative disorders, myocardial ischemia
CONFEDERATES IN THE ATTIC: THE RETURN OF SOLDIER’S HEART
The American Civil War (1861–1865) was an epic conflict between the Union Army of the North and the Confederate States of America in the South. Many of the battles prefigured the bloody conflicts of the future First World War, and were associated with thousands of casualties. The Civil War saw the advent of trench warfare; the remains of the trenches where the Confederates dug in against the invading Union Army can be seen on the slopes of Kennesaw Mountain in Georgia, even today. Their failed attempt to halt the advance of the Union Army on Atlanta from the north resulted in Sherman’s March to the Sea and the eventual capitulation of the Confederates. Swords were traded for ploughshares with varying degrees of effort to move on from this divisive war. Years later, battle uniforms and mementos were often rediscovered in the attic by later generations, which led to the well-known phrase “Confederates in the Attic.” Political history, however, has the potential to shine a light upon the past in a way that illuminates the present. In a similar way, the history of medicine can shed light on modern conceptions of disease. Syndromes described in earlier eras once thought to have become irrelevant can be the subject of renewed interest in the light of new discoveries in a particular field.
One example of this is a medical diagnosis that came directly from the Civil War, namely, Soldier’s Heart or Da Costa’s syndrome. The syndrome was originally described by Jacob Mendes Da Costa, a physician in the Union Army (1833–1900) (Fig. 1). Da Costa, born into a small Sephardic Jewish community in St. Thomas, the US Virgin Islands (at that time a Danish colony), attended medical school at Jefferson University in Philadelphia, and was later on the faculty there (Clarke, 1903; Kobrin and Kobrin, 1999). During the Civil War, Da Costa worked at the Satterlee Hospital in Philadelphia, Pennsylvania, the largest hospital in the country serving the Union Army. There he observed over 400 patients with nonspecific cardiac complaints after exposure to the stress of combat. Da Costa published his observations on the syndrome in 1871 in the American Journal of Medical Sciences under the title “On Irritable Heart: A Clinical Study of a Form of Functional Cardiac Disorder and Its Consequences,” describing how soldiers following exposure to combat developed an array of symptoms including fatigue, shortness of breath, sighing respirations, palpitations, rapid heart rate, and sweating, with no observable cardiological abnormality (Da Costa, 1871).
FIGURE 1.

Jacob Mendes Da Costa, American Physician. Portrait by Thomas Eakins, Scanned from “Thomas Eakins: Volume II” by Lloyd Goodrich. Harvard University Press, 1982. ISBN 0674884906, Public Domain. Available at: https://commons.wikimedia.org/w/index.php?curid=6821493. Accessed February 12, 2019.
Soldier’s Heart was one of several syndromes found in the history books that identified somatic symptoms after exposure to psychological trauma (Bremner, 2006; Kirmayer et al., 1995; Micale and Lerner, 2001). These syndromes predated the current era, which is dominated by the Diagnostic and Statistical Manual (DSM) and its establishment of the posttraumatic stress disorder (PTSD) diagnosis in 1980 (American Psychiatric Association, 1981; Saigh and Bremner, 1999). For instance, in 1830, a syndrome called “railway spine” or “railway injuries” was first described in victims of train accidents in England. Many of the symptoms looked like PTSD, including exaggerated fear reactions, amnesia for aspects of the traumatic event, and anxiety, as well as symptoms of memory disturbance, confusion, and back pain (Micale and Lerner, 2001). The etiology was felt to be contusions of the spinal cord below the level of physical detection, and was described in a book by Erichsen called “On Railway and Other Injuries of the Nervous System” (Erichsen, 1867). About this time in Germany, a psychiatrist named Oppenheim described traumatic neurosis as a psychological stress or extreme shock that could lead to long-term changes in physiology in the absence of physical injury. World War I saw the development of the concept of “shell-shock,” which was originally thought to be a physical disorder caused by exploding shells, but later it was realized that it could develop in locations far from the blast (Micale and Lerner, 2001). Symptoms included confusion on the battlefield and forgetting one’s name or identity. After the war, Freud’s ideas about unconscious conflicts as the source of psychiatric symptoms became dominant. Combat-related reactions were called “combat neurosis” and thought to be caused by a suppressed urge to run from the battlefield (Crocq and Crocq, 2000). In other words, the environment was not the cause and there was no physical disorder, rather symptoms created by unconscious conflicts, similar to anxiety neurosis.
Recent advances in medicine have increased our knowledge of the effects of traumatic stress on the body (Mayer and Bushnell, 2009). This has led to a reappraisal of stress-related psychiatric disorders as more than just “above the neck” disorders and renewed appreciation for earlier concepts of the effects of stress on the individual as involving multiple physiological systems (Anda et al., 2006; Mayer and Bushnell, 2009; Vaccarino et al., 2016a). Stress has lasting effects on a number of physical systems, including the cardiovascular system (Vaccarino et al., 2016b). Accumulating evidence shows a strong relationship between stress-related psychiatric disorders, including both PTSD and depression, and cardiovascular disease (CVD) (Vaccarino and Bremner, 2013, 2015; Vaccarino et al., 2018).
POSTTRAUMATIC STRESS DISORDER
Posttraumatic stress disorder (PTSD) affects 8% of people in the United States at some point in their lives, with women affected twice as often as men (Kessler et al., 1995). PTSD is most commonly related to childhood sexual abuse in women and physical assault in men (Kessler et al., 1995), and is more common in civilians in the United States than in combat veterans (MacMillan et al., 1997). PTSD requires exposure to a traumatic event defined as a threat to life or self with associated symptoms of intrusive memories of the event, increased arousal with reminders, avoidance of reminders, negative cognitions, feeling cut off and emotionally numb, disturbed sleep and concentration, hyperarousal, and hypervigilance.
Bremner described a trauma-spectrum group of psychiatric disorders that held in common exposure to a traumatic event (Bremner, 1999a, 2002a). Trauma-spectrum disorders shared a common neurobiological mechanism with slight variations based on the interaction of stress with genetics, environment, and other factors (Bremner, 1999a, 2002a). A series of studies showed that the trauma-spectrum disorders share important neurobiological fingerprints that differentiate them from the non-PTSD anxiety disorders. Trauma-spectrum disorders of PTSD, major depression related to childhood abuse, borderline personality disorder, and dissociative identity disorder share in common smaller hippocampal volume (a brain area sensitive to stress that plays an important role in short term memory) as measured with magnetic resonance imaging, a finding not seen in other anxiety disorders (Bremner, 2004a, 2005a; Schmahl et al., 2003; Vermetten et al., 2006; Vythilingam et al., 2002). In fact, a number of the symptoms of trauma-spectrum disorders are overlapping or can be seen as a rephrasing of symptoms that are essentially identical (Bremner, 1999b, 2002a, 2004b). Grouping of psychiatric disorders by common biological or etiological framework was a departure from the pure descriptive format that underlay the development of the original versions of the DSM (Bremner, 2006). The descriptive approach was itself a conscious departure from the previous system, which was built around the theoretical formulation of repressed, unconscious conflicts as the root basis of psychiatric disorders (Bremner, 2006). The synthetic framework of the trauma-spectrum disorders involved a shift in perspective of psychiatric disorders as more than just what was happening “from the neck up” and instead incorporating whole-body etiologies. Stress-related psychiatric disorders in fact involve lasting alterations in peripheral autonomic, cardiovascular, and inflammatory systems, and are in fact much more than pure brain-based disorders (Bremner and Vaccarino, 2015; Vaccarino and Bremner, 2013; Vaccarino et al., 2018). It is alterations in these systems that are largely responsible for the symptoms of Soldier’s Heart.
THE ORIGINS OF SOLDIER’S HEART
Lasting disruptions of peripheral autonomic and cardiovascular function after exposure to extreme traumas such as the stress of war are best thought of from larger developmental and evolutionary perspectives (Bracha et al., 2005). Behaviors such as hypervigilance, hyperarousal, and light sleep that had survival value for primitive hunter gatherers thousands of years ago are no longer useful for humans who live in buildings with security systems and no longer have natural predators (Teicher et al., 2003). Symptoms of anxiety probably served specific purposes, for instance, if there was a pack of lions in the area where a band of hunter-gatherers was camped, this could lead to an increase in fear and vigilance, which may have made the difference between life and death. These symptoms would persist for long periods, possibly after the threat had been removed. The fight-or-flight response that was formerly critical to survival holds less importance in the modern age when we are rarely under actual physical threat. Activation of the fight-or-flight response involves the peripheral autonomic and cardiovascular systems, with the resultant symptoms of rapid heart rate and respiratory changes looking like Da Costa’s syndrome. If these responses become chronic, they can in fact lead to the development of CVD or acceleration of the disease process (Shively et al., 2009). Similarly, in combat arenas, soldiers develop behaviors such as being jumpy or easily startled that may have survival value, especially if there is an ongoing threat of unexpected attack by insurgents. It is only when the soldier returns from the battlefield and is unable to turn off the “combat mind” that he or she runs into trouble. Behaviors that were adaptive in one environment suddenly become not so in another. Chronic stress not adaptive in modern contexts can therefore lead to psychiatric symptoms in some individuals, in addition to progression of CVD (Shively et al., 2009).
NEUROBIOLOGY OF POSTTRAUMATIC STRESS DISORDER
Recent studies showed that PTSD and other stress-related psychiatric disorders are associated with an increase in CVD (Vaccarino et al., 2007, 2013). PTSD is also associated with a range of other medical disorders, including infectious diseases, diabetes, and asthma (Anda et al., 2008; Sareen et al., 2007; Zatzick et al., 2010). Childhood abuse is also linked to increase risk factors for CVD, including obesity, smoking, alcohol abuse, and drug abuse (Anda et al., 2006; Dube et al., 2003; Williamson et al., 2002).
PTSD is characterized by lasting changes in stress circuits and systems that can also underlie both the development of CVD and symptoms of PTSD (Bremner, 2002a, 2002b; Bremner and Charney, 2010; Bremner and Pearce, 2016; Bremner and Vermetten, 2001). The hypothalamic-pituitary-adrenal (HPA) axis plays an important role in the stress response. Early-life stress leads to long-term sensitization of the HPA axis (Coplan et al., 1996; Levine et al., 1993; Makino et al., 1995; Plotsky and Meaney, 1993; Stanton et al., 1988), and lasting alterations in this system are associated with PTSD. Stress also leads to overactivation of the norepinephrine system, which plays a critical role in the fight-or-flight response (Bremner et al., 1996a, 1996b).
A network of brain areas involved in memory and fear, including the hippocampus, medial prefrontal cortex, insula, and amygdala, is involved in the brain’s response to stress (Bremner, 2005b, 2011; Campanella and Bremner, 2016; Rauch et al., 2006). The hippocampus plays a critical role in memory and is also sensitive to stress. Studies in animals showed that stress resulted in damage to neurons in the CA3 region of the hippocampus (Gould et al., 1998; Magarinos et al., 1996; McEwen et al., 1992; Nibuya et al., 1995; Sapolsky, 1996; Sapolsky et al., 1990). High levels of glucocorticoids seen with stress were also associated with deficits in new learning (Diamond et al., 1996; Luine et al., 1994). The medial prefrontal cortex mediates extinction through inhibition of fear memories in the amygdala (Milad and Quirk, 2002; Quirk et al., 2006). Peripheral cardiovascular and neurohormonal responses to stress are also regulated by the medial prefrontal cortex (Diorio et al., 1993; Feldman et al., 1995; Frysztak and Neafsey, 1994). This brain area is also sensitive to early stress, which leads to decreased branching of neurons (Radley et al., 2004).
PTSD is associated with lasting alterations in HPA axis function (Yehuda, 2002). For instance, it is related to normal or low cortisol in 24-hour urine collections (Yehuda et al., 1995b, 1996b) and decreased baseline cortisol based on 24-hour diurnal assessments of plasma with 10-minute sampling periods, a flattening of the normal diurnal cortisol curve, and increased pulsatility of cortisol reflecting dysregulation of corticotropin-releasing factor (CRF) release (Bremner et al., 2007; Yehuda et al., 1994, 1996b). Other findings in PTSD include increased cortisol response to personalized scripts of traumatic events (Elzinga et al., 2003) and “neutral” mental stress tasks including mental arithmetic (Bremner et al., 2003), increased suppression of cortisol in response to low doses of dexamethasone (Goenjian et al., 1996; Stein et al., 1997; Yehuda et al., 1993, 1995a, 2002), increased glucocorticoid receptors (GRs) in peripheral lymphocytes (van Zuiden et al., 2011; Yehuda et al., 1991), hypersensitivity of pituitary response to metyrapone (Yehuda et al., 1996a) consistent with increased feedback sensitivity in PTSD, a blunted effect of glucocorticoids (dexamethasone) on declarative memory function, suggesting decreased sensitivity of GRs in the hippocampus (Bremner et al., 2004), blunted suppression response of T-cell proliferation in leukocytes to dexamethasone (de Kloet et al., 2007), and increased CRF in cerebrospinal fluid (CSF) (Baker et al., 1999, 2005; Bremner et al., 1997b; Sautter et al., 2003) and plasma (de Kloet et al., 2008). Dysregulation of the HPA axis likely contributes to immune system alterations in PTSD, including chronic low-grade inflammation, which is particularly relevant to cardiovascular pathophysiology (Bremner and Pearce, 2016; Michopoulos et al., 2016; Neigh and Ali, 2016; Speer et al., 2018). In summary, these findings are consistent with lasting alterations in HPA function in PTSD that may contribute to an increased risk of cardiovascular disorders.
Increased noradrenergic and peripheral sympathetic function is linked to PTSD (Bremner et al., 1996a, 1996b). Symptoms characteristic of increased noradrenergic function include irritability, increased startle, hyperarousal, and sleep disturbance (Bremner et al., 1996b). Baseline concentrations of norepinephrine and its metabolites were found to be elevated in plasma of PTSD patients (De Bellis et al., 1994, 1999; Lemieux and Coe, 1995; Mason et al., 1988; Yehuda et al., 1998) or normal (Blanchard et al., 1991; McFall et al., 1992; Mellman et al., 1995; Pitman and Orr, 1990; Southwick et al., 1993), while CSF concentrations were increased (Geracioti et al., 2001). Reminders of the original trauma in the form of pictures and sounds or scripts resulted in increased noradrenergic reactivity, shown by an increase in heart rate, blood pressure, and skin conductance, all markers of sympathetic function (Blanchard et al., 1982, 1986; Bremner et al., 1996b; Malloy et al., 1983; McFall et al., 1990; Orr et al., 1993, 1995, 1998; Orr and Roth, 2000), as well as increased norepinephrine and epinephrine in plasma (Blanchard et al., 1991; McFall et al., 1992). Increased baseline heart rate after trauma exposure predicted subsequent development of chronic PTSD (Shalev et al., 1998). Other findings include decreased binding to platelet alpha2 receptors (Perry et al., 1991), decreased activity of the second messenger, cyclic adenosine monophosphate (Lerer et al., 1990; Lerer et al., 1987), and decreased platelet monoamine oxidase activity (Davidson et al., 1985), all consistent with downregulation due to chronic elevations of norepinephrine. The administration of the alpha2 adrenergic receptor yohimbine, which increases firing of noradrenergic neurons in the locus coeruleus, increased PTSD symptoms and anxiety and concentrations of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol in plasma (Southwick et al., 1993, 1997). PTSD patients had a decrease in brain function in the medial prefrontal cortex with yohimbine not seen in healthy controls (decreased frontal lobe function occurs with excessive release of norepinephrine in that brain area) (Bremner et al., 1997a). Based on this study, clinical trials of the alpha-1 antagonist prazosin were conducted, with findings of efficacy for nightmares in PTSD (Raskind et al., 2000). These findings support increased noradrenergic function in PTSD.
Alterations in brain areas involved in the stress response are associated with PTSD (Bremner, 2010). Studies have shown smaller volume and/or altered function in anterior cingulate/medial prefrontal cortex, hippocampus, insula, and amygdala (Andersen et al., 2008; Campanella and Bremner, 2016; Kitayama et al., 2005; Shin et al., 2009; Smith, 2005). These brain areas have projections that regulate peripheral neurohormonal and autonomic nervous system (ANS) responses to stress that can promote the development of CVD (Soufer et al., 2009; Vaccarino and Bremner, 2015).
POSTTRAUMATIC STRESS DISORDER AND CARDIOVASCULAR DISEASE
PTSD is associated with an increased risk for CVD (Vaccarino et al., 2013), probably through multiple pathways including activation of neurohormonal or brain circuit pathways (Vaccarino, 2018) (Fig. 2). Stress-induced activation of norepinephrine and cortisol or neuroendocrine dysregulation associated with chronic PTSD may lead to an increase in atherosclerosis or vulnerability to cardiac arrhythmias or other consequences that increase the risk for CVD and/or adverse cardiac outcomes. PTSD may lead to adverse CVD outcomes directly or through an increase in risk factors for CVD or unhealthy behaviors including smoking, substance abuse, lack of exercise, or obesity (Beckham, 1999; Butterfield et al., 2000; Dube et al., 2003; Williamson et al., 2002). All of these factors contribute to the fact that an increased risk for CVD is seen in both depression (Carney and Freedland, 2003; Evans et al., 2005) and PTSD (Vaccarino and Bremner, 2013). Behaviors such as stress-induced anger were also associated with increased CVD risk (Boltwood et al., 1993; Burg et al., 1993; Gabbay et al., 1996; Mittleman et al., 1995; Strike et al., 2006; Strike and Steptoe, 2005). Anger and irritability, symptoms of PTSD, could therefore contribute to increased risk of CVD. PTSD is also associated with a decrease in heart rate variability (the normal increase and decrease in heart rate that occurs with respiration) (Shah et al., 2013), which increases the risk of sudden death from arrhythmia. Stress and emotion also have effects on myocardial electrical stability, increasing the risk for cardiac arrhythmias (Kop et al., 2004; Lampert et al., 2002; Lane et al., 2005).
FIGURE 2.
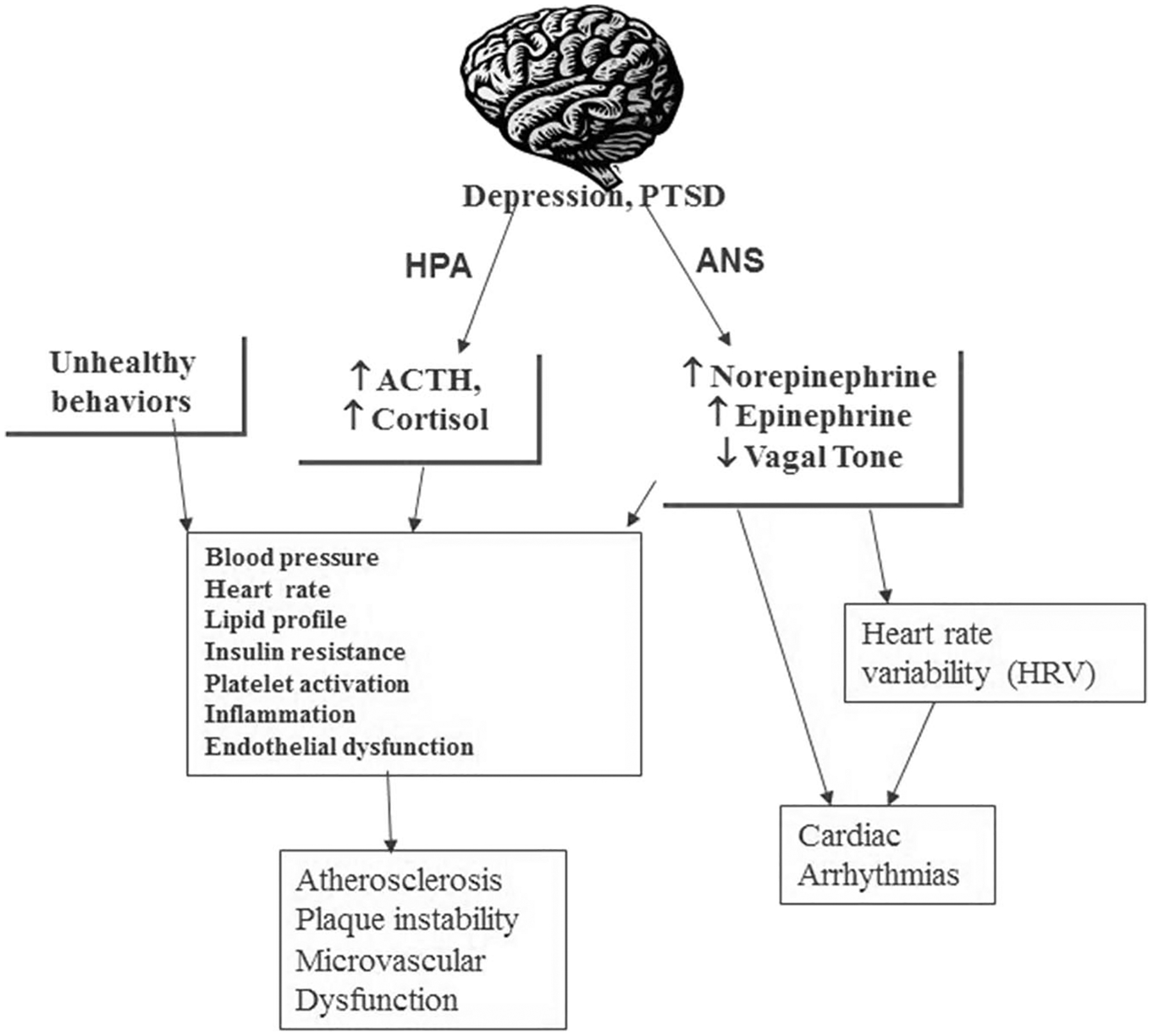
Model of how stress acts through the brain to affect CVD in patients with PTSD and depression. Stress acts through the brain to activate the HPA axis and ANS. This leads to enhanced cortisol release and sympathetic (norepinephrine) that affect electrical properties of the heart, activation of blood pressure and heart, and changes in inflammation, which lead changes in peripheral vascular reactivity, all of which increase the risk for CD. Adapted from Vaccarino V, Drugs of Today, 2000.
Stress can be standardized in laboratory protocols to understand its effects on CVD (Hammadah et al., 2017a). Mental stress involves the use of public speaking tasks or mental arithmetic (performing problem solving under time pressure with negative feedback) (Bremner et al., 2003; Hammadah et al., 2017a). These “neutral” mental stress tasks used in research on CVD are to be differentiated from trauma-specific stressor such as listening to personalized traumatic scripts often used in PTSD research (Elzinga et al., 2003). Studies have shown that mental stress can induce myocardial ischemia (MSI) in coronary artery disease (CAD) patients using these standardized protocols (Arri et al., 2016; Hammadah et al., 2017b; Ramadan et al., 2013; Vaccarino et al., 2014; Wei et al., 2014a; Wei et al., 2014b). Mental stress-induced MSI often occurs without pain and has been hypothesized to be related to coronary vasospasm (Deanfield et al., 1984; Lacy et al., 1995) and/or peripheral vasoconstriction during stress (Arri et al., 2016; Ramadan et al., 2013; Sullivan et al., 2018; Vaccarino et al., 2018). MSI occurs at lower heart rates than those required for physical stress-induced ischemia, often occurs in patients without physical stress-induced MSI (Krantz et al., 1991; LaVeau et al., 1989; Ramachandruni et al., 2006; Rozanski et al., 1988; Schang and Pepine, 1977), is more common in women than men, and is associated with worse outcomes, especially in younger women (Sullivan et al., 2018; Vaccarino et al., 2009, 2016b). MSI may represent the mechanism by which patients with stress-related mental disorders including PTSD and depression are at increased risk for CAD (Lima et al., 2018; Wei et al., 2014a).
Brain areas involved in memory and the stress response that have been implicated in PTSD, including the medial prefrontal cortex/anterior cingulate/orbitofrontal cortex, likely play a role in MSI (Campanella and Bremner, 2016). This region has been shown to modulate peripheral cardiovascular function, including heart rate variability (Thayer et al., 2009), and peripheral cardiovascular and neurohormonal responses to stress (Campanella and Bremner, 2016; Vaccarino and Bremner, 2017). MSI in CAD patients was associated with increased activation in anterior cingulate, in addition to other brain areas mediating stress, including inferior frontal gyrus, insula, and parietal (Bremner et al., 2018).
As noted previously, PTSD and depression are associated with increased sympathetic function, causing an increase in heart rate and blood pressure, especially with traumatic reminders (Bremner et al., 1996a, 1996b; Delgado and Moreno, 2000). PTSD and depression, as discussed previously, are also associated with increases in stress-induced cortisol release, especially related to specific traumatic reminders (Bremner et al., 2003; Elzinga et al., 2003; Heim et al., 2000). Stress-induced activation of norepinephrine may lead to damage to the endothelium or inner lining of the coronary arteries, as well as accelerated atherosclerosis (Kaplan et al., 1982, 1987; Troxler et al., 1977). An excess in cortisol levels with stress may also increase the risk for CVD by injuring the endothelium (Kemper et al., 1957; Nahas et al., 1958).
Traumatic stress may also mediate its effects through brain areas sensitive to stress that are affected by PTSD and depression, including amygdala, medial prefrontal cortex, and hippocampus. These brain circuits have direct or indirect outputs through the hypothalamus and medial prefrontal cortex to neurohormonal systems (cortisol, norepinephrine) that influence heart function. The medial prefrontal cortex and anterior cingulate are unique in directly mediating cortisol and sympathetic responses to stress via the brainstem (Diorio et al., 1993). The hippocampus and medial prefrontal cortex have inhibitory pathways to the amygdala (Milad and Quirk, 2002; Morgan and LeDoux, 1995; Morgan et al., 1993; Phillips and LeDoux, 1992) that activates the stress response (Davis, 1992; Hitchcock, 1986; Hitchcock et al., 1989; LeDoux, 1993; Miserendino et al., 1990; Rosen and Davis, 1988), including activation of the peripheral stress response through pathways through the lateral hypothalamus that activate cortisol and sympathetic pathways in the periphery (Hitchcock and Davis, 1991).
In addition to PTSD-mediating CVD, stress-related autonomic and cardiovascular alterations may influence symptoms of PTSD. For instance, stress-induced increases in blood pressure and/or heart rate correlate with activation in the right insula, cerebellum, and the anterior cingulate (Critchley et al., 2000). Studies have also implicated insula and somatosensory cortex in representation of peripheral autonomic function (Critchley et al., 2001). We found in patients with CAD that mental stress-induced MSI was associated with activation in the insula, anterior cingulate, inferior frontal gyrus, parietal, and somatosensory cortex (Bremner et al., 2018). These findings could be interpreted as stress-induced brain activations that lead to increased cardiovascular reactivity with associated MSI or as the brain’s response to peripheral autonomic changes with stress. We have also found that stress-induced MSI is associated with an increase in morbidity and mortality compared with exercise-induced MSI (Vaccarino et al., 1999, 2009, 2014). Stress-induced MSI is also more common in women than men, especially younger women (Vaccarino et al., 2016b, 2018), which is interesting given that stress-induced psychiatric disorders including PTSD and depression are twice as common in women as in men (Kessler et al., 1995). Overall the findings indicate that there is a subpopulation of individuals at increased risk for stress-induced MSI that parallels the increased vulnerability to psychiatric disorders, although the exact relationship between these entities requires further study.
Researchers have investigated the relationship between PTSD and depression and MSI. CAD patients with a history of traumatic stress and depression had increased MSI (Bremner et al., 2009). In two separate studies, depressed CAD patients showed altered anterior cingulate response to stress, with decreases in some areas related to nondepressed CAD patients and increases in those with MSI (Bremner et al., 2019a, 2019b). Increased heart disease mortality in patients with PTSD or depression could be mediated through “silent” MSI mediated by these brain pathways.
CONCLUSIONS AND FUTURE DIRECTIONS
Over 100 years after the death of Da Costa, his description of the effects of traumatic stress on behavior and cardiovascular function continue to be relevant. PTSD is associated with lasting effects on peripheral neurohormonal, immune, and ANS function. Changes in cortisol and norepinephrine function could increase the risk of CVD observed in PTSD patients, as well as with other stress-related mental disorders including depression. Alterations in autonomic function likely underlie symptoms of PTSD, including recurrent and intrusive thoughts about traumatic events. Emerging findings show that PTSD is no longer a pure brain-based disease and that alterations in whole-body physiological systems first observed by Da Costa need to be taken into account. This approach suggests that treatments focused on neuropsychopharmacology may have limitations. New approaches are needed, such as neuromodulatory interventions that have effects on peripheral physiology. Electrical stimulation of the vagus nerve, associated with decreased peripheral autonomic and sympathetic function and inflammatory function, as well as enhancement of parasympathetic tone, with modulatory effects on anterior cingulate and other brain regions involved in stress, represent some of the potential approaches (Bremner and Rapaport, 2017). Vagal nerve stimulation has been shown to have these salutary effects when paired with both mental stress and personalized traumatic scripts (Bremner et al., 2019b; Gurel et al., 2018, 2020). Future treatment developments should continue to target these peripheral stress physiological systems.
DISCLOSURE
The work presented in this review was supported by grants from the NIH R01 MH056120, R01 HL088726, K24 MH076955, P01 HL101398, T32 MH067547, K24 HL077506, R01 HL068630, R01 HL109413, K23 HL127251, R01 HL125246, S10 RR16917, DARPA Cooperative Agreement N66001-16-2-4054, the Department of Veterans Affairs, and the University of Alberta Research Fund.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH (2008) Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 34:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH (2006) The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 256:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1981) Diagnostic and statistical manual of mental disorders (3rd ed). Philadelphia, PA: American Psychiatric Association. [Google Scholar]
- Arri SS, Ryan M, Redwood SR, Marber MS (2016) Mental stress-induced myocardial ischaemia. Heart. 102:472–480. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD Jr. (2005) Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 162:992–994. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD (1999) Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 156:585–588. [DOI] [PubMed] [Google Scholar]
- Beckham JC (1999) Smoking and anxiety in combat veterans with chronic posttraumatic stress disorder: A review. J Psychoactive Drugs. 31:103–110. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Gerardi RJ, Ryan P, Pallmeyer TP (1986) Cardiac response to relevant stimuli as an adjunctive tool for diagnosing post-traumatic stress disorder in Vietnam veterans. Behav Ther. 17:592–606. [Google Scholar]
- Blanchard EB, Kolb LC, Pallmeyer TP, Gerardi RJ (1982) A psychophysiological study of post traumatic stress disorder in Vietnam veterans. Psychiatry Q. 54: 220–229. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC (1991) Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis. 179:371–373. [DOI] [PubMed] [Google Scholar]
- Boltwood MD, Taylor CB, Burke MB, Grogin H, Giacomini J (1993) Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am J Cardiol. 72:1361–1365. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Yoshioka DT, Masukawa NK, Stockman DJ (2005) Evolution of the human fear-circuitry and acute sociogenic pseudoneurological symptoms: The Neolithic balanced-polymorphism hypothesis. J Affect Disord. 88:119–129. [DOI] [PubMed] [Google Scholar]
- Bremner D, Vermetten E, Kelley ME (2007) Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 195:919–927. [DOI] [PubMed] [Google Scholar]
- Bremner JD (1999a) Acute and chronic responses to stress: Where do we go from here? Am J Psychiatry. 156:349–351. [DOI] [PubMed] [Google Scholar]
- Bremner JD (1999b) Does stress damage the brain? Biol Psychiatry. 45:797–805. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2002a) Does stress damage the brain? Understanding trauma-related disorders from a mind-body perspective. New York: W.W. Norton. [Google Scholar]
- Bremner JD (2002b) Neuroimaging of childhood trauma. Semin Clin Neuropsychiatry. 7:104–112. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2004a) Brain imaging in anxiety disorders. Expert Rev Neurother. 4:275–284. [DOI] [PubMed] [Google Scholar]
- Bremner JD (2004b) Does stress damage the brain? Understanding trauma-related disorders from a mind-body perspective. Can Child Adolesc Psychiatr Rev. 13:20. [Google Scholar]
- Bremner JD (2005a) Brain imaging handbook. New York: W.W. Norton. [Google Scholar]
- Bremner JD (2005b) The neurobiology of childhood sexual abuse in women with posttraumatic stress disorder. In Kendall-Tackett KA (Ed), Handbook of women, stress and trauma (pp 181–206). New York: Brunner-Routledge. [Google Scholar]
- Bremner JD (2006) Traumatic stress from a multiple-levels-of-analysis perspective. In Cicchetti D, Cohen DJ (Eds), Developmental psychopathology (Vol. 2, pp 656–676). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Bremner JD (2010) Imaging in CNS disease states: PTSD. In Borsook D, Beccera L, Bullmore E, Hargreaves RJ (Eds), Imaging in CNS Drug discovery and development: Implications for disease and therapy (pp 339–360). Basel, Switzerland: Springer. [Google Scholar]
- Bremner JD (2011) Stress and human neuroimaging studies. In Conrad CD (Ed), The handbook of stress: Neuropsychological effects on the brain (pp 446–462). Chichester, United Kingdom: Blackwell Press. [Google Scholar]
- Bremner JD, Campanella C, Khan Z, Fani N, Kasher N, Evans S, Reiff C, Mishra S, Ladd S, Nye JA, Raggi P, Vaccarino V (2019a) Brain mechanisms of stress and depression in coronary artery disease. J Psychiatr Res. 109:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V (2018) Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Charney DS (2010) Neural circuits in fear and anxiety. In Stein DJ, Hollander E, Rothbaum BO (Eds), Textbook of anxiety disorders (2nd ed, pp 55–71). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, Musselman DL, Ritchie JC, Faber T, Votaw JR, Nemeroff CB, Vaccarino V (2009) Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress Health. 25:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Fani N, Cheema FA, Ashraf A, Vaccarino V (2019b) Effects of a mental stress challenge on brain function in coronary artery disease patients with and without depression. Health Psychol. 38:910–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS (1997a) PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry. 54:246–256. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1996a) Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 23:28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1996b) Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 23:39–51. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS (1997b) Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 154:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Pearce B (2016) Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD. In Bremner JD (Ed), Posttraumatic stress disorder: From neurobiology to treatment (pp 181–232). Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Bremner JD, Rapaport MH (2017) Vagus nerve stimulation: Back to the future. Am J Psychiatry. 174:609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vaccarino V (2015) Neurobiology of early life stress in women. In Orth-Gomér K, Schneiderman N, Vaccarino V, Deter HC (Eds), Psychosocial stress and cardiovascular disease in women: Concepts, findings, future perspectives (pp 161–168). Springer International Publishing. [Google Scholar]
- Bremner JD, Vermetten E (2001) Stress and development: Behavioral and biological consequences. Dev Psychopathol. 13:473–489. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS (2003) Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 28:733–750. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Afzal N, Nazeer A, Newcomer JW, Charney DS (2004) Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (PTSD). Psychiatry Res. 129:1–10. [DOI] [PubMed] [Google Scholar]
- Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL (1993) Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 22:440–448. [DOI] [PubMed] [Google Scholar]
- Butterfield MI, Forneris CA, Feldman ME, Beckham JC (2000) Hostility and functional health status in women veterans with and without posttraumatic stress disorder: A preliminary study. J Trauma Stress. 13:735–741. [DOI] [PubMed] [Google Scholar]
- Campanella C, Bremner JD (2016) Neuroimaging of PTSD. In Bremner JD (Ed), Posttraumatic stress disorder: From neurobiology to treatment (pp 291–320). Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Carney RM, Freedland KE (2003) Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 54:241–247. [DOI] [PubMed] [Google Scholar]
- Clarke MA (1903) Memoir of J.M. Da Costa, M.D. Am J Med Sci. 125:318–329. [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB (1996) Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 93:1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000) Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol (Lond). 523:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001) Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci. 4:207–212. [DOI] [PubMed] [Google Scholar]
- Crocq MA, Crocq L (2000) From shell shock and war neurosis to posttraumatic stress disorder: A history of psychotraumatology. Dialogues Clin Neurosci. 2:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa JM (1871) On Irritable Heart: A clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci. 61:17–52. [Google Scholar]
- Davidson J, Lipper S, Kilts CD, Mahorney S, Hammett E (1985) Platelet MAO activity in posttraumatic stress disorder. Am J Psychiatry. 142:1341–1343. [DOI] [PubMed] [Google Scholar]
- Davis M (1992) The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 15:353–375. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND (1999) A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 45:1259–1270. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Lefter L, Trickett PK, Putnam FW (1994) Urinary catecholamine excretion in sexually abused girls. J Am Acad Child Adolesc Psychiatry. 33:320–327. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Bikker A, Meulman E, Geuze E, Kavelaars A, Westenberg HG, Heijnen CJ (2007) Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol Psychiatry. 12:443–453. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG (2008) Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 167:287–291. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP (1984) Silent myocardial ischaemia due to mental stress. Lancet. 2:1001–1005. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Moreno FA (2000) Role of norepinephrine in depression. J Clin Psychiatry. 61:5–12. [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM (1996) Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 110:661–672. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 13:3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF (2003) The impact of adverse childhood experiences on health problems: Evidence from four birth cohorts dating back to 1900. Prev Med. 37:268–277. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD (2003) Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 28:1656–1665. [DOI] [PubMed] [Google Scholar]
- Erichsen JE (1867) On railway and other injuries of the nervous system. Philadelphia, PA: Henry C. Lea. [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P Jr., Valvo WJ (2005) Mood disorders in the medically ill: Scientific review and recommendations. Biol Psychiatry. 58:175–189. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Weidenfeld J (1995) Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci Biobehav Rev. 19:235–240. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ (1994) The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 643:181–193. [DOI] [PubMed] [Google Scholar]
- Gabbay FH, Krantz DS, Kop WJ, Hedges SM, Klein J, Gottdiener JS, Rozanski A (1996) Triggers of myocardial ischemia during daily life in patients with coronary artery disease: Physical and mental activities, anger and smoking. J Am Coll Cardiol. 27:585–592. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr., Kasckow JW (2001) CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 158:1227–1230. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA (1996) Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. 153:929–934. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 95:3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MMH, Ko YA, Shallenberger L, Nye JA, Pearce B, Vaccarino V, Shah AJ, Bremner JD, Inan OT (2020) Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 13:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Mobashir HS, Bremner JD, Vaccarino V, Ladd SL, Shah A, Inan OT (2018) Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: A proof-of-concept study. In 2018 IEEE: 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN) (pp 78–81). Las Vegas, Nevada: IEEE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA (2017a) The Mental Stress Ischemia Prognosis Study: Objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Samman Tahhan A, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA (2017b) Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol. 243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB (2000) Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 284:592–597. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM (1986) Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 100:11–22. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M (1991) Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 105:826–842. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Sananes CB, Davis M (1989) Sensitization of the startle reflex by footshock: Blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behav Neurosci. 103:509–518. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB (1987) Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 76:1365–1372. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM (1982) Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 2:359–368. [DOI] [PubMed] [Google Scholar]
- Kemper JW, Baggenstoss AH, Slocumb CH (1957) The relationship of therapy with cortisone to the incidence of vascular lesions in rheumatoid arthritis. Ann Intern Med. 46:831–851. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 52:1048–1060. [DOI] [PubMed] [Google Scholar]
- Kirmayer LJ, Young A, Hayton BC (1995) The cultural context of anxiety disorders. Psychiatr Clin North Am. 18:503–521. [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD (2005) Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 88:79–86. [DOI] [PubMed] [Google Scholar]
- Kobrin NH, Kobrin JL (1999) J.M. da Costa, M.D—A tinok she-nishbah? An American Civil War converso physician. Shofra. 18:16–39. [Google Scholar]
- Kop WJ, Krantz DS, Nearing BD, Gottdiener JS, Quigley JF, O’Callahan M, DelNegro AA, Friehling TD, Karasik P, Suchday S, Levine J, Verrier RL (2004) Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation. 109:1864–1869. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A (1991) Cardiovascular reactivity and mental stress-induced myocardial ischemia in patients with coronary artery disease. Psychosom Med. 53:1–12. [DOI] [PubMed] [Google Scholar]
- Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB (1995) Coronary vasoconstriction induced by mental stress (simulated public speaking). Am J Cardiol. 75:503–505. [DOI] [PubMed] [Google Scholar]
- Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D (2002) Emotional and physical precipitants of ventricular arrhythmia. Circulation. 106: 1800–1805. [DOI] [PubMed] [Google Scholar]
- Lane RD, Laukes C, Marcus FI, Chesney MA, Sechrest L, Gear K, Fort CL, Priori SG, Schwartz PJ, Steptoe A (2005) Psychological stress preceding idiopathic ventricular fibrillation. Psychosom Med. 67:359–365. [DOI] [PubMed] [Google Scholar]
- LaVeau PJ, Rozanski A, Krantz DS, Cornell CE, Cattanach L, Zaret BL, Wackers FJ (1989) Transient left ventricular dysfunction during provocative mental stress in patients with coronary artery disease. Am Heart J. 118:1–8. [DOI] [PubMed] [Google Scholar]
- LeDoux JL (1993) Emotional memory: In search of systems and synapses. Ann N Y Acad Sci. 702:149–157. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL (1995) Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosom Med. 57:105–115. [DOI] [PubMed] [Google Scholar]
- Lerer B, Bleich A, Bennett ER, Ebstein RP, Balkin J (1990) Platelet adenylate cyclase and phospholipase C activity in posttraumatic stress disorder. Biol Psychiatry. 27:735–740. [DOI] [PubMed] [Google Scholar]
- Lerer B, Ebstein RP, Shestatsky M, Shemesh Z, Greenberg D (1987) Cyclic AMP signal transduction in posttraumatic stress disorder. Am J Psychiatry. 144:1324–1327. [DOI] [PubMed] [Google Scholar]
- Levine S, Weiner SG, Coe CL (1993) Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 4:297–306. [DOI] [PubMed] [Google Scholar]
- Lima BB, Hammadah M, Wilmot K, Pearce BD, Shah A, Levantsevych O, Kaseer B, Obideen M, Gafeer MM, Kim JH, Sullivan S, Lewis TT, Weng L, Elon L, Li L, Bremner JD, Raggi P, Quyyumi A, Vaccarino V (2018) Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav Immun. 75:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS (1994) Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 639:167–170. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Trocmé N, Boyle MH, Wong M, Racine YA, Beardslee WR, Offord DR (1997) Prevalence of child physical and sexual abuse in the community. Results from the Ontario Health Supplement. JAMA. 278:131–135. [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flügge G, Fluchs E (1996) Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 16:3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW (1995) Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: Association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 136:3299–3309. [DOI] [PubMed] [Google Scholar]
- Malloy PF, Fairbank JA, Keane TM (1983) Validation of a multimethod assessment of posttraumatic stress disorders in Vietnam veterans. J Consult Clin Psychol. 51:488–494. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Harkness L (1988) Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. J Nerv Ment Dis. 176:498–502. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC (2009) Functional pain syndromes: Presentation and pathophysiology. Seattle, WA: IASP Press. [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley CS (1992) Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 31:177–199. [DOI] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC (1990) Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biol Psychiatry. 27:1165–1175. [DOI] [PubMed] [Google Scholar]
- McFall ME, Veith RC, Murburg MM (1992) Basal sympathoadrenal function in posttraumatic distress disorder. Biol Psychiatry. 31:1050–1056. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B (1995) Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 38:174–179. [DOI] [PubMed] [Google Scholar]
- Micale MS, Lerner P (2001) Traumatic pasts: History, psychiatry, and trauma in the modern age, 1870–1930. Cambridge: Cambridge University Press. [Google Scholar]
- Michopoulos V, Vester A, Neigh G (2016) Posttraumatic stress disorder: A metabolic disorder in disguise? Exp Neurol. 284:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 420:70–73. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M (1990) Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 345:716–718. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE (1995) Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 92:1720–1725. [DOI] [PubMed] [Google Scholar]
- Morgan CA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 109:681–688. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Romanski LM, LeDoux JE (1993) Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 163:109–113. [DOI] [PubMed] [Google Scholar]
- Nahas GG, Brunson JG, King WM, Cavert HM (1958) Functional and morphologic changes in heart lung preparations following administration of adrenal hormones. Am J Clin Pathol. 34:717–729. [PMC free article] [PubMed] [Google Scholar]
- Neigh GH, Ali FF (2016) Co-morbidity of PTSD and immune system dysfunction: Opportunities for treatment. Curr Opin Pharmacol. 29:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 15:7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK (1998) Psychophysiological assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J Consult Clin Psychol. 66:906–913. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK (1995) Physiological responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol. 104:75–82. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR (1993) Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 102:152–159. [DOI] [PubMed] [Google Scholar]
- Orr SP, Roth WT (2000) Psychophysiological assessment: Clinical applications for PTSD. J Affect Disord. 61:225–240. [DOI] [PubMed] [Google Scholar]
- Perry BD, Southwick SM, Giller EJ (1991) Adrenergic receptor regulation in posttraumatic stress disorder. In Giller EJ (Ed), Biological assessment and treatment of posttraumatic stress disorder. Washington, DC: American Psychiatric Press. [Google Scholar]
- Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 106:274–285. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP (1990) Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 27:245–247. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 18:195–200. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 60:337–343. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH (2004) Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 125:1–6. [DOI] [PubMed] [Google Scholar]
- Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS (2006) Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 47:987–991. [DOI] [PubMed] [Google Scholar]
- Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA (2013) Myocardial ischemia during mental stress: Role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER (2000) The a1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: A report of 4 cases. J Clin Psychiatry. 61:129–133. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biol Psychiatry. 60:376–382. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Davis M (1988) Enhancement of acoustic startle by electrical stimulation of the amygdala. Behav Neurosci. 102:195–202. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS (1988) Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 318:1005–1012. [DOI] [PubMed] [Google Scholar]
- Saigh PA, Bremner JD (1999) The history of posttraumatic stress disorder. In Saigh PA, Bremner JD (Eds), Posttraumatic stress disorder: A comprehensive text (pp 1–17). Needham Heights, MA: Allyn & Bacon. [Google Scholar]
- Sapolsky RM (1996) Why stress is bad for your brain. Science. 273:749–750. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE (1990) Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 10:2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJ (2007) Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 69:242–248. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D (2003) Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 54:1382–1388. [DOI] [PubMed] [Google Scholar]
- Schang SJ Jr., Pepine CJ (1977) Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 39:396–402. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner JD (2003) Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 122:193–198. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V (2013) Post-traumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 73:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK (1998) A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry. 55:553–559. [DOI] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK (2009) Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 66:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB (2009) Social stress, visceral obesity, and coronary artery atherosclerosis: Product of a primate adaptation. Am J Primatol. 71:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME (2005) Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 15:798–807. [DOI] [PubMed] [Google Scholar]
- Soufer R, Jain H, Yoon AJ (2009) Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep. 11:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA 3rd, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS (1997) Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 54:749–758. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS (1993) Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 50:266–274. [DOI] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A (2018) Systemic low-grade inflammation in post-traumatic stress disorder: A systematic review. J Inflamm Res. 11:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S (1988) Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 102:692–700. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C (1997) Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 42:680–686. [DOI] [PubMed] [Google Scholar]
- Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A (2006) Triggering of acute coronary syndromes by physical exertion and anger: Clinical and socio-demographic characteristics. Heart. 92:1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Steptoe A (2005) Behavioral and emotional triggers of acute coronary syndromes: A systematic review and critique. Psychosom Med. 67:179–186. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V (2018) Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol. 38:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM (2003) The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 27:33–44. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH (2009) Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 37: 141–153. [DOI] [PubMed] [Google Scholar]
- Troxler RG, Sprague EA, Albanese RA, Fuchs R, Thompson AJ (1977) The association of elevated plasma cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis. 26:151–162. [DOI] [PubMed] [Google Scholar]
- Vaccarino V (2018) Psychiatric and behavioral aspects of cardiovascular disease. In Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF (Eds), Braunwald’s heart disease: A textbook of cardiovascular medicine (pp 1880–1889). Philadelphia, PA: Elsevier-Saunders. [Google Scholar]
- Vaccarino V, Bremner JD (2013) Traumatic stress is heartbreaking. Biol Psychiatry. 74:790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD (2015) Posttraumatic stress disorder and risk of cardiovascular disease. In Alvarenga M, Byrne D (Eds), Handbook of psychocardiology. Singapore: Springer. [Google Scholar]
- Vaccarino V, Bremner JD (2017) Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD (2013) Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. J Am Coll Cardiol. 62:97–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN, National Heart, Lung, and Blood Institute (2007) Depression, inflammation and incident cardiovascular disease in women with suspected coronary ischemia: The NHLBI-Sponsored WISE Study. J Am Coll Cardiol. 50:2044–2050. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Mayer E, Bremner JD (2016a) Stress and health. In Bremner JD (Ed), Posttraumatic stress disorder: From neurobiology to treatment. Hoboken, NJ: Wiley-Blackwell Press. [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM (1999) Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 341:217–225. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J (2009) Sex differences in mortality after acute myocardial infarction: Changes from 1994 to 2006. Arch Intern Med. 169:1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D’Marco L, Karohl C, Bremner JD, Raggi P (2014) Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 76:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P (2018) Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: Sex differences and mechanisms. Circulation. 137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, Quyyumi AA (2016b) Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 5:pii:e003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, Kavelaars A (2011) Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 168:89–96. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD (2006) Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 163:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD (2002) Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V (2014a) Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS One. 9:e102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V (2014b) Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 114:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V (2002) Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 26:1075–1082. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2002) Post-traumatic stress disorder. N Engl J Med. 346:108–114. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL Jr. (1995a) Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 52:583–593. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C (2002) The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol Psychiatry. 52:393–403. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL (1995b) Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 152:982–986. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Levengood RA, Schmeidler J, Wilson S, Guo LS, Gerber D (1996a) Increased pituitary activation following metyrapone administration in posttraumatic stress disorder. Psychoneuroendocrinology. 21:1–16. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL Jr. (1991) Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry. 148:499–504. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, Teicher MH (1998) Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 44:56–63. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW (1993) Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 150:83–86. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ (1994) Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Ann N Y Acad Sci. 746:378–380. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ (1996b) Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol Psychiatry. 40:79–88. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Rivara FP, Jurkovich GJ, Hoge CW, Wang J, Fan MY, Russo J, Trusz SG, Nathens A, Mackenzie EJ (2010) Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch Gen Psychiatry. 67:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


