Dear Editor,
We read with interest the study recently published by Capetti and colleagues showing one-year durability of anti-spike IgG after natural exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Although the antibody kinetics in symptomatic and asymptomatic patients is known,1 , 2 we still ignore how it evolve beyond 6 months in vaccinees and if and how the initial serological status of vaccinees might influence it.
To date, antibody kinetics data after vaccination remain fragmented. The study by Doria-Rose et al., showed persistence of antibodies 6 months after the second dose of mRNA-1273 vaccine in 33 participants included in the phase 1 follow-up of the Moderna study without knowing their initial serological status before the vaccination.3 Likewise, interim results from a phase 3 trial of the mRNA-1273 vaccine indicated 94.5% efficacy in preventing coronavirus disease 2019 (Covid-19).4 Since efficacy trials have focused on individuals without prior exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), little is known about the immune responses induced by mRNA-1273 in participants who have suffered from Covid-19. Finally, this large-scale, phase 3 study conducted by the firm Moderna was carried out from July 27 to October 23, 2020, away from the worrying spread of new SARS-CoV-2 variants.5, 6, 7
In our independent study, we compared the antibody response 2 weeks after the first injection (T1) (median time [± 95% CI]: 16 [± 2.26] days), 2 weeks after the second injection (T2) (median time [± 95% CI]: 14 [± 1.83] days) and 3 months after the first injection (T3) (median time [± 95% CI]: 86 [± 4.59] days) from 205 healthcare workers (HCWs) stratified according to their initial serological status. The quantitative analysis of the anti-SARS-CoV-2 IgG antibodies directed against the subunits (S1) and (S2) of the virus spike protein was carried out using the LIAISON®SARS-CoV-2 IgG kit (DiaSorin®, Saluggia, Italy) previously validated in our laboratory8 and also used by Capetti et al.1 Effectiveness of the mRNA-1273 vaccine was also assessed through a medical questionnaire. Participants were asked to declare any results of RT-qPCR tests regardless of the reason behind, even in asymptomatic situations, and any eventual Covid-19 infection after vaccination (including severity of symptoms). To better apprehend the observed efficacy, a comparison of the level of antibodies directed against the nucleocapsid was carried out on part of the cohort of seronegative participants at T0 and T3 with the Platelia® SARS-CoV-2 Total Ab test (Bio-Rad®, Marnes-la-Coquette, France) detecting total antibodies (IgM, IgA and IgG) (n = 86/161). Since only these antibodies are produced during a natural infection, their detection allows us to identify the participants who have been infected by SARS-CoV-2 since their vaccination.
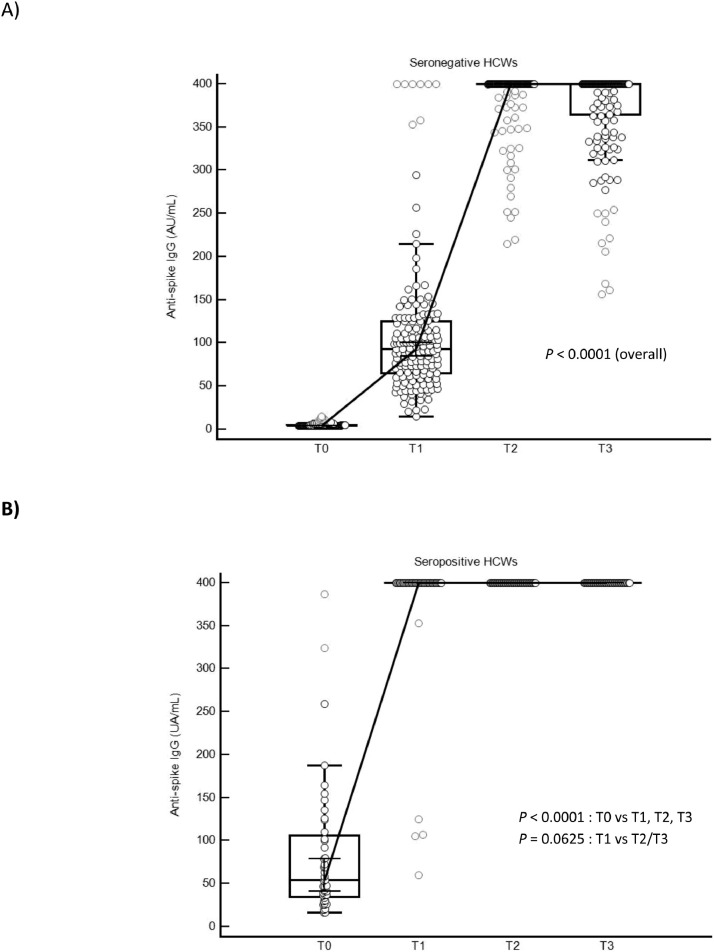
In the initially seronegative participants (n = 161), we observed a persistence of anti-S-antibody levels 3 months after vaccination with nevertheless a decrease in the antibody levels observed between T2 and T3 in 48 participants (Fig. 1 A). Conversely, an increase in antibody levels was observed in 15 seronegative HCWs. Interestingly, in seropositive people (n = 44), no drop in antibody was observed between T2 and T3. The measured levels are all above the maximum quantification value (> 400 AU/mL). Moreover, the administration of a second dose of vaccine in participants initially seropositive made it possible to catch-up the very few vaccinees (n = 5) with a weaker response at T1 by reaching the maximum level of antibodies at T2 (Fig. 1B).
Fig. 1.
Antibody responses in seronegative (A) and seropositive (B) HCWs after mRNA-1273 vaccination.
It shows the titers of SARS-CoV-2 IgG antibodies directed against the subunits (S1) and (S2) of the virus spike protein before (T0), 2 weeks after the first injection (T1), 2 weeks after the second injection (T2) and 3 months after the first infection (T3) according to the participant serological status (n = 205). The Box-and-Whisker plot represents the 25th and 75th percentiles. Inside the box, the horizontal line indicates the median (the 50th percentile). Discs in light grey represent far out values. A Wilcoxon test was used to assess the changes in IgG levels between T0, T1, T2 and T3 times within seronegative (n = 161) and seropositive subjects (n = 44). A P-value < 0.05 was considered significant.
Analysis of the clinical follow-up questionnaires revealed that none of the respondents reported thinking they had been infected (n = 167). Thirty-six of them had to undergo a RT-qPCR and all were negative. Finally, among the seronegative ones, only 2 participants developed antibodies directed against the nucleocapsid at T3 while all were negative at T0 (n = 86). Based on these results and given that none of the participants developed symptoms, the mRNA-1273 vaccine is effective at preventing Covid-19 illness. However, additional long-term serosurveillance studies based on larger cohorts will be necessary to confirm these observations. Monitoring of anti-nucleocapsid antibodies remains a complementary aid in detecting infections which are sometimes asymptomatic in vaccinated persons known to be initially seronegative.
Faced with an unprecedented global vaccine deployment, a close follow-up of vaccinees remains crucial to confirm both safety and long-lasting immune protection. In this study, we evaluated the immune response of the participants but also the effectiveness of the mRNA-1273 vaccine in a context different from the previous phase 1 and 3 studies by Moderna, under a higher virological pressure, and by categorizing the participants into 2 cohorts according to their serological status at initiation. Three months after vaccination, we confirm a very high efficacy and a persistence of anti-spike antibodies. However, the decrease observed in some seronegative participants argues for an additional dose of vaccine in the upcoming months for this specific subgroup.
Ethical approval
This study has been approved by the Ethical Committee of the HIS-IZZ (ethical agreement number: CEHIS/2021–7).
Funding
None declared.
CRediT authorship contribution statement
Marie Tré-Hardy: Writing – review & editing, Investigation, Formal analysis, Visualization, Supervision, Writing – original draft. Roberto Cupaiolo: Writing – review & editing, Investigation, Formal analysis, Visualization. Alain Wilmet: Writing – review & editing, Investigation. Ingrid Beukinga: Writing – review & editing, Investigation. Laurent Blairon: Writing – review & editing, Investigation, Formal analysis, Visualization, Supervision, Writing – original draft.
Conflicts of Competing Interest
The authors have no relevant competing interest to disclose in relation to this work.
Acknowledgment
The authors thank all the members of the clinical laboratory staff for technical assistance. We also thank the HCWs who participated in this study.
References
- 1.Capetti AF, Borgonovo F, Mileto D, Gagliardi G, Mariani C, Lupo A, et al. One-year durability of anti-spike IgG to SARS-CoV-2: Preliminary data from the anticrown prospective observational study one year durability of COVID-19 anti-spike IgG. J Infect. 2021 doi: 10.1016/j.jinf.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer B. Waning antibodies to SARS-CoV-2 - Don't panic. Lancet Reg Health Eur. 2021;4 doi: 10.1016/j.lanepe.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N Engl J Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021 doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan M, Ferguson ID, Miao W, Khavari PA. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tré-Hardy M, Wilmet A, Beukinga I, Dogné JM, Douxfils J, Blairon L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin Chem Lab Med. 2020;58(8):1357–1364. doi: 10.1515/cclm-2020-0594. [DOI] [PubMed] [Google Scholar]