Abstract
Background
Many countries/regions implemented strict border measures (e.g., 14-day quarantines) as a blanket policy to prevent COVID-19 importations, while proposed “travel bubbles” as an alternative to reduce the impact of border controls. We aim to examine the differential importation risks with departure origins and post-arrival controls.
Methods
We developed a Bayesian framework to model disease progress of COVID-19 and the effectiveness of travel measures and inferred the origin-specific disease prevalence among inbound travellers, using data on passengers arriving in Hong Kong and laboratory-confirmed imported cases. We estimated the origin-specific risks of releasing infectious travellers under different control strategies and traveller volumes. We also estimated the risk of having released infectious travellers when a resurgence occurs in departure locations with no imported cases during a certain period.
Findings
Under the then strict controls of 14-day quarantine and testing on day 12, the Philippines imposed the greatest importation risk among the studied countries/regions (95.8% of releasing at least one infectious traveller, 95% credible interval (CrI), 94.8-96.6%). This was higher than that from low prevalence countries/regions (e.g., 23.4%, 95% CrI, 21.6-25.3% for Taiwan) if controls relaxed (i.e., 7-day quarantine and test on day 5). Increased traveller volumes and resurgence in departure locations with low prevalence under relaxed controls did not impose a greater importation risk than high prevalence locations under stricter controls.
Interpretation
Moderate relaxation of control measures for travellers arriving from low prevalence locations did not impose higher risks of community outbreaks than strict controls on travellers from high prevalence locations.
Funding
Health and Medical Research Fund, Hong Kong.
Research in context.
Evidence before this study
The ongoing COVID-19 pandemic caused unprecedented interruption of international travel. Countries and regions adopted various travel control measures, including border closure, strict quarantine, post-arrival tests for COVID-19. Most of the measures were implemented as blanket policies for all inbound travellers regardless of the prevalence of COVID-19 in the departure origins, with some exemptions where countries broadly classified the departure locations into several categories of risk and relaxed measures to travellers from lower categories (e.g., “travel bubbles” launched by Singapore and Australia). However, no evidence was available to evaluate the origin-specific importation risks and the feasibility of these targeted travel measures.
We searched in PubMed for peer-reviewed studies and in Google for grey literatures about evaluating the origin-specific importation risks and feasibility of targeted control measures on 17 February, 2021 with no restrictions on publication time and language. We used terms of “COVID-19 OR SARS-CoV-2” AND “travel* AND international” AND “quarantine OR test* OR screen* OR restrict*” in title and abstract. Current evidence suggested that international travellers played critical role in seeding and sustaining local epidemics in destinations with low prevalence, while the early travel measures were insufficient to preventing from COVID-19 importations. We found no peer-reviewed study, one pre-print and one report directly examined the variations of importation risks between locations and different control measures, while none of those applied empirical data on travel and imported COVID-19 cases to infer the risks.
Added value of this study
We proposed an evaluation framework of origin-specific importation risks, which integrating the natural history of the disease, the effectiveness of control strategies and travel volumes and the estimated COVID-19 incidence of inbound travellers from a given departure origin. We compared the risks of releasing infectious travellers between countries or regions, control strategies and travel volumes. We found that relaxing quarantine to 7 days with a second PCR test on day 5 to travellers from low prevalence countries or regions would not impose greater importation risks than applying strict control measures to travellers from high prevalence. Such observations were still held when the travel volume double the current or when resurgence reoccurred in these low prevalence settings.
Implications of all the available evidence
The ongoing COVID-19 pandemic unprecedentedly interrupted the international travel, while many governments are seeking exit strategies to reduce the economic and societal impacts. Moderate relaxation of control measures (e.g., 7-day quarantine and secondary test on day 5) to inbound travellers from low prevalence locations does not appear to impose greater risks than that has already been imposed by travellers from high prevalence under the strict regimen, suggesting the feasibility of including low COVID-19 prevalence countries or regions into “travel bubbles”. Meanwhile, we are expecting an accelerated increasing of population immune to the virus with the mass vaccination, therefore relaxing control strategies to vaccinated travellers from different origins of departure can be further examined within this framework.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19) emerged in late 2019, in Wuhan, China [1]. The first confirmed case outside mainland China was identified on 13 January 2020 in a visitor to Bangkok from Wuhan [2]. In the following weeks, cases were identified in other locations around the world, and by the end of February 2020 cases had been reported in more than 50 countries or regions [2]. Imported cases were found to seed the local epidemics [3,4], indicating that current travel measures were insufficient to prevent the importation of COVID-19 by travellers [3]. There have been few evidence-based evaluations of the importation risks and the effectiveness of travel restrictions and controls.
Countries or regions adopted travel restrictions with various stringency, including closure their borders to non-residents and institution of post-arrival quarantine and isolation measures [5,6]. These measures have been implemented by some governments as a blanket policy for all inbound travellers, regardless of the travel volume and the disease prevalence at the origin of departure. Such policies fail to consider the substantially reduced risk of importation from locations which have achieved local elimination as compared to locations that have adopted a containment or mitigation strategy. Meanwhile, some governments adopted triage measures depending on the importation risks, though evidence-based risks assessments were rarely reported.
“Travel bubbles” (or triage models) have been proposed by several governments (e.g., New Zealand, Singapore and Australia) to reduce the economic impacts of border controls [7], [8], [9]. Key to this is the assessment of the risk an infectious traveller could escape from a series of control measures, which is determined by the volume of travellers, the disease prevalence among inbound travellers from a given origin of departure, and the effectiveness of post-arrival controls (Figure 1). Reliable estimation of the importation risk by origin of departure is critical for the safe implementation of “travel bubbles”.
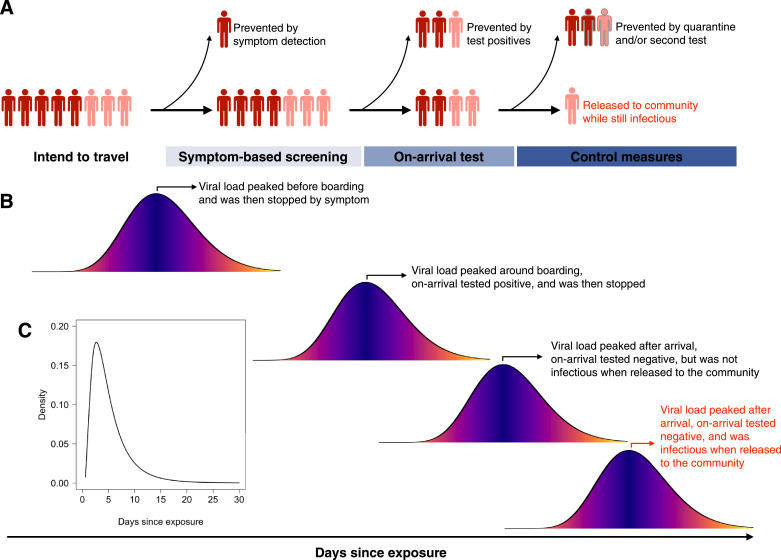
Figure 1.
Travel controls and the natural history of COVID-19. (A) Example travel control process and possible steps where infected travellers could be identified. Dark and light red indicate symptomatic (including who show symptoms post arrival) and asymptomatic individuals, respectively. Grey bordered figures indicate the infected individual is no longer infectious. The released infectious travellers were indicated as red texts (same for panel B), which are the central outcome that was modelled in this study. (B) Representative individual infectious profiles by peak viral load. We assumed symptoms onset coincides with peak viral load for symptomatic cases. (C) Incubation period of COVID-19. Data were derived from He et al.(12)
We hypothesized that relaxation of post-arrival quarantine requirements for travellers arriving from locations with low COVID-19 prevalence would pose a lower risk of community transmission than strict quarantine requirements for travellers arriving from high prevalence locations. We developed a comprehensive framework that considered disease prevalence, travel volume, control strategies and their effectiveness, and the natural history of disease to estimate the importation risks by the origin of departure. We illustrate the utility of this framework using Hong Kong as a case study and considering the importation risks from eight countries or regions of origin (hereafter referred to as origins) with different traveller volumes to Hong Kong and COVID-19 prevalence.
2. Methods
2.1. Study settings
Since 25 March 2020, non-Hong Kong residents from overseas were prohibited from entering Hong Kong. Those permitted to travel were required to undertake 14 days home/hotel quarantine and were tested on arrival (day 0) and day 12 of their quarantine period [10]. Individuals who reported symptoms during quarantine were arranged to have additional testing during the quarantine period. The majority received the results of their on-arrival test on the same day and received the results for the second test before their quarantine ended. Thus, we assumed all persons received their tests results before the end of quarantine. All confirmed cases were isolated in hospitals. Since late July 2020, travellers were also required to provide a pre-flight negative PCR test result [11]. On 25 December 2020, quarantine was extended to 21 days with an additional test on day 19 due to concerns around the higher transmissibility of some SARS-CoV-2 variants [11]. Therefore, we restricted our study period from 1 April to 31 July 2020 to avoid the impact of changing policies. However, we simulated scenarios for 21-day quarantine for comparison purposes.
We compared the importation risk of infectious inbound travellers from eight countries or regions that had high traveller volumes through direct flights to Hong Kong but with a different prevalence of COVID-19, including the Philippines, United Kingdom (UK), United States of America (USA), Japan, South Korea, Australia, Singapore and Taiwan.
2.2. Sources of data
We obtained monthly flight data on origin-specific arrivals from the Hong Kong International Airport and assumed that all passengers received a test on-arrival during the study period. We calculated the cumulative number of inbound travellers arriving from a given origin between April and July as the number of on-arrival tests performed.
We obtained data on laboratory-confirmed COVID-19 cases whose exposure risk was listed as travel outside Hong Kong from Department of Health. These data included the port of origin, date of arrival and self-reported occupation. Sources of importation were determined by the travel history in the past 14 days. Cases whose occupation were sea crew were excluded. For the simplicity and generalizability of our model and due to the limited information to distinguish cases identified on day 12 from those from additional symptomatic testing, we only fitted the model to cases confirmed by the on-arrival test. We calculated the cumulative number of imported cases from a departure origin during the study period as the origin-specific number of on-arrival test-positives.
2.3. Natural history of COVID-19
We assumed a mean time since exposure to peak viral load of 5.2 days, with a maximum of 14 days (Figure 1C) [1]. Symptomatic cases were assumed to show symptoms on the peak viral load day and for all cases, infectiousness peaked when viral load peaked (95% confidence interval (CI), -4.3 to 6.8 days) [12].
We assumed that test sensitivity changes over time (pp(t)), and was highest the day before peak viral load (Figure S1A) [13]. In sensitivity analysis, we used the time-varying test sensitivity from an alternative source [14] (Figure S1B) to repeat the estimations of COVID-19 prevalence among inbound travellers and the importation risks across different control regimens. In addition, we assumed test sensitivity for asymptomatic individuals was 62% of that for symptomatic individuals [15]. Test specificity was assumed to be 1.
2.4. Statistical model
2.4.1. Estimating the prevalence of COVID-19 infections among inbound travellers
To estimate the origin-specific number of potential travellers and average incidence during the study period, we formulated a Bayesian framework to model the disease history of infected travellers and the travel measures that were in force (i.e., pre-flight symptom screening and on-arrival RT-PCR test) for each departure of origin (Figure 1, Table S1; details in Appendix). Briefly, we assumed that infected travellers who intended to travel were in t days after exposure, distributed uniformly between 1 to 14 days. This information was subsequently used to impute the day of symptoms onset and peak viral load, test sensitivity and infectiousness. We assumed all healthy, asymptomatic cases (probability for asymptomatic: 19.5%, 95% CI, 9.6-29.4% [16]) and symptomatic cases who developed symptoms after travel would board and received tests on-arrival. We also assumed that 30% [15,17] of symptomatic cases who developed symptoms before travel would board, counter to travel requirements. Among infected individuals who received a test on-arrival, the probability of being test-positive was determined by 1) whether they are asymptomatic and 2) the test sensitivity that varies with the time since peak viral load.
We fitted the model to the data on origin-specific arrivals and imported cases in a Bayesian framework. We jointly estimated the number of potential travellers and prevalence using a Monte Carlo Markov Chain (MCMC) algorithm. To validate our model, we conducted a simulation study to illustrate that our estimation algorithm could provide unbiased estimates of parameters (details in Appendix). The model recovered the true values for travel volume and prevalence in 96% and 94% of simulations, respectively (Figure S2).
2.4.2. Estimating the importation risk for different origins
The importation risk was measured by the number of released infectious travellers and the probability of releasing at least one infectious traveller. We used these measurements to compare different control strategies. The first measure was about controlling the importation risk to contain or mitigate the local transmission, while the second measure was of interest for elimination of local cases.
To estimate these measurements, we simulated the disease history of infected travellers, symptom screening, requirements of COVID-19 tests and effectiveness of measures under different traveller volumes, disease prevalence and post-arrival travel control strategies using the number of potential travellers and prevalence of COVID-19 infection that were estimated from our data for each origin. Seven quarantine control strategies were considered, and all strategies required the arriving person to test negative on arrival:
-
1)
immediate release (NoQ)
-
2)
release after 7-day quarantine without a secondary test (Q7)
-
3)
release after 7-day quarantine with a negative test on day 5 (Q7/T5)
-
4)
release after 14-day quarantine without a secondary test (Q14);
-
5)
release after 14-day quarantine with a test-negative on day 12 (Q14/T12, which was the regimen in force in Hong Kong during the study period)
-
6)
release after 21-day quarantine without a secondary test (Q21); and
-
7)
release after 21-day quarantine with two negative tests on day 12 and 19 (Q21/T12/T19).
The probability of a positive second test and the probability of being infectious when released were determined by the individual exposure time, incubation period, infectious profile and time-varying test sensitivity. We calculated the number of released infectious travellers; i.e., test-negative but infectious when released. We calculated the risk of releasing at least one infectious traveller by dividing the number of episodes where infectious travellers were released by the number of simulations performed. We repeated this simulation but increased traveller volumes, to evaluate the importation risks when travellers grow in response to the relaxed control measures.
To inform the potential risks we may face if a resurgence occurs in origin of departure with no imported case during a certain period, we estimated the risks of infectious travellers who might have been released to the community when a new case occurs among the inbound travellers under different travel control strategies. Based on the developed model, we estimated the prevalence of COVID-19 infection when one on-arrival test-positive was identified under different traveller volumes, namely half of current (n = 5,000), double of current (n = 20,000) and pre-pandemic volume (n = 300,000). Then, we conducted simulations using the abovementioned approach to estimate the expected number of released infectious travellers and the risk of at least one infectious traveller being released to the community.
We reported the posterior distributions from 2,000 simulations and provided detailed parameterizations and distributions used in the posterior simulations in Appendix.
2.5. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
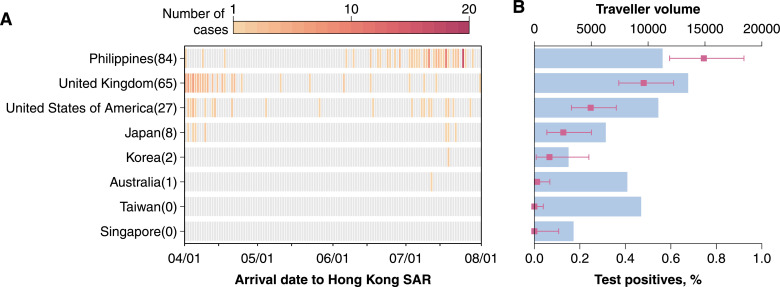
In total 187 COVID-19 cases were imported from the eight countries or regions considered between 1 April and 31 July 2020 (Figure 2A), accounting for 35% (187/535) of all importations during that period. The Philippines was the largest importation source (n = 84, 45%), followed by the UK (n = 65, 35%) and USA (n = 27, 14%), while Taiwan and Singapore introduced no cases (Figure 2A). The Philippines recorded the highest observed on-arrival test-positive rate (0.75%, 95% CI 0.59-0.92%) and the highest estimated COVID-19 prevalence among inbound travellers (32 per 1000, 95% credible interval (CrI), 24-41 per 1000 travellers).
Figure 2.
Observed temporal distribution of imported COVID-19 cases (A) and distribution of the on-arrival tests (B) from the eight representative countries and regions between April to July, 2020. In panel B, blue bars represent the number of inbound travellers arriving Hong Kong; the red points and red lines indicate the mean and 95% CI of inbound travellers that tested positive on arrival.
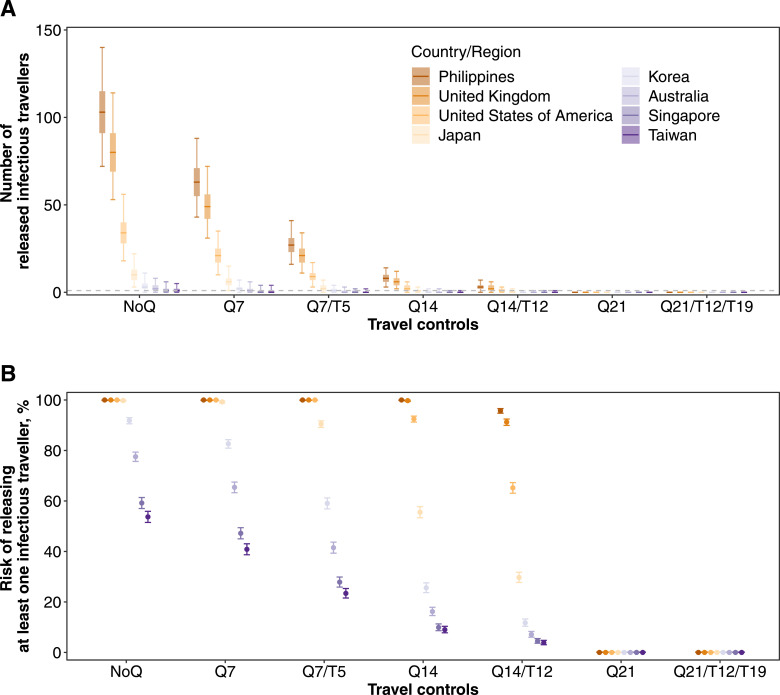
The risk of releasing at least one infectious traveller for each country or region and under each of the modelled quarantine arrangements is shown in Figure 3. Based on traveller volume, disease prevalence and quarantine requirements in place in Hong Kong between April and July 2020 (i.e., Q14/T12), the greatest importation risks were among travellers from the Philippines, the UK and the USA. Travellers from Singapore and Taiwan imposed the lowest risk, consistent with the low prevalence of disease among travellers from these countries or regions. We estimated there was a 96% (95% CrI, 95-97%) risk that at least one infectious traveller from the Philippines had been released into the community (Figure 3B), corresponding to an expected median of 5 infectious travellers (95% CrI, 0-7; Figure 3A). In contrast, this probability was 4% (95% CrI, 3-5%) for Taiwan corresponding to a median of 0 released cases (95% CrI, 0-1).
Figure 3.
Expected importation risks of infectious travellers under different quarantine control strategies. Traveller volumes and prevalence of COVID-19 among inbound travellers that were estimated from actual data were used in the simulations. (A) The number of infectious travellers who were released to the community. Median (thick horizontal tick), interquartile (shaded rectangles) and 95% quantiles (solid vertical lines) of 2,000 simulations were shown. (B) The risk of releasing at least one infectious traveller to the community. Mean (dots) and 95% CI (vertical lines) are shown.
The total number of estimated imported cases from these eight counties or regions was 7 (95% CrI, 3-14) under the Q14/T12 control strategy, compared with 237 (95% CrI, 184-305) under no quarantine strategy (NoQ), corresponding to 97% (95% CrI, 94-99%) effectiveness. Testing on-arrival, only, could prevent an average of 40-42% of infectious travellers at the airport from mixing with the community (Figure S3). Tightening the travel measures to 21-day quarantine would reduce the risk of releasing infectious travellers to 0 for all examined countries or regions (Figure 3).
Under a Q7/T5 regimen, the risk of releasing at least one infectious traveller from a low prevalence setting was still lower than under a Q14/T12 regimen for travellers from a high prevalence. For example, the risk of missing a case in a returned traveller from Singapore under a Q7/T5 regimen was 28% (95% CrI, 26-30%) and substantially lower than the risk for a traveller from the Philippines under the Q14/T12 regimen (96%, 95% CrI, 95-97%) (Figure 3).
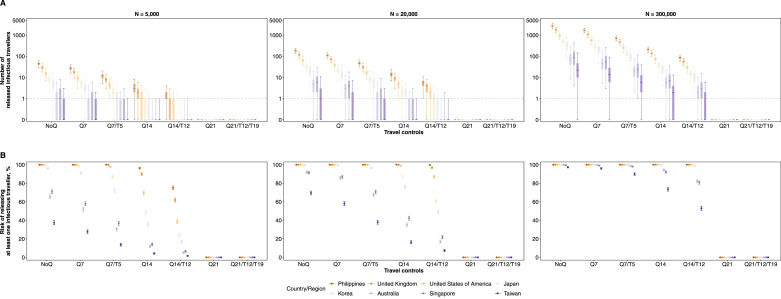
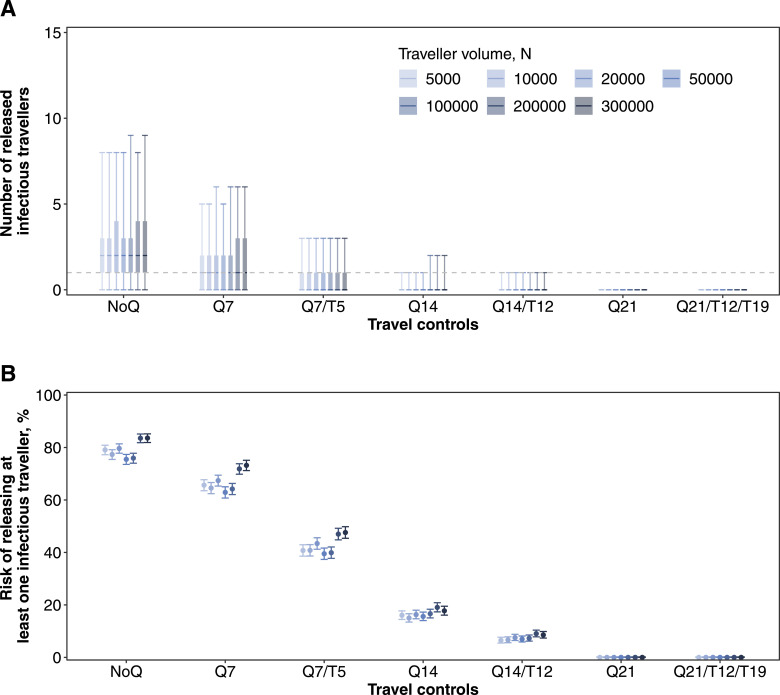
For low prevalence countries or regions, the risk of an infectious traveller escaping the Q7/T5 policy was greater than under a longer quarantine arrangement without testing (i.e., Q14) (Figure 3). However, the expected number of released infectious travellers from these low prevalence origins neared 0 so would be unlikely to result in any actual cases under the traveller volumes at the time (Figure 3A). Having said that, if traveller volumes increased (which might happen if people were incentivised by the reduced quarantine requirement), the risk of an importation would correspondingly increase (Figure 4). Taking Taiwan as an example, the risk of releasing at least one infectious traveller increased from 38% (95% CrI, 36-40%) to 90% (95% CrI, 89-91%) when the traveller volume increased from 20,000 to 300,000. This corresponded to an increase in the number of released infectious travellers from 0.1 (95% CrI, 0.1-3.0) to 6.0 (95% CrI, 0.1-39) (Figure 4). Nevertheless, once there was one on-arrival test-positive identified among travellers from low prevalence locations, the risk of at least one infectious traveller being released after Q7/T5 would increase only minimally from 43% (95% CrI, 41-46%) to 48% (95% CrI, 45-50%) (Figure 5). This is still lower than the risks from high prevalence origins under Q14/T12 (e.g., the Philippines in Figure 3).
Figure 4.
Expected importation risks of infectious travellers under quarantine control strategies and increasing traveller volumes. Prevalence of COVID-19 among inbound travellers between April to July 2020 that were estimated from actual data were used in the simulations. (A) The number of infectious travellers who were released to the community. Median (thick horizontal tick), interquartile (shaded rectangles) and 95% quantiles (solid vertical lines) of 2,000 simulations were shown, while grey dashed line indicates the number of 1. (B) The risk of releasing at least one infectious traveller to the community. Mean (dots) and 95% CI (vertical lines) are shown.
Figure 5.
Expected number of infectious travellers that were released to community (A) and risk of released at least one infectious traveller to the community (B) when one on-arrival test positive in different traveller volumes. In panel A, median (thick horizontal tick), interquartile (shaded rectangles) and 95% quantiles (solid vertical lines) of 2,000 simulations were shown, while grey dashed line indicates the number of. In panel B, mean (dots) and 95% CI (vertical lines) of the probability are shown.
If quarantine was a deterrent and traveller volumes reduced, the importation risk under a Q14/T12 regimen would decrease even for high prevalence countries or regions (Figure 3 and 4). For example, the median number of released infectious travellers from the Philippines was estimated to decrease from 3 (95% CrI, 0-7) to 1 (95% CrI, 0.1-4.0) if the travel volume decreased to about half the current volume (i.e., n = 5,000) (Figure 3 and 4).
In sensitivity analyses, we explored alternative temporal distributions of test sensitivity [14]. We observed consistent results for the relative introduction risks when comparing relaxed measures for low prevalence locations to stricter measures for high prevalence locations (Figure S2 and S4). The absolute introduction risks were higher than those in the main analysis, due to lower test sensitivity for symptomatic cases (Figure S1 and S4).
4. Discussion
In this study, we proposed a framework to evaluate the origin-specific importation risks of COVID-19 from inbound travellers under various travel measures. The speed with which the disease can spread, even amid reduced travel, was recently re-illustrated by the spread of the newly emerged B.1.1.7 variant from the UK [18,19]. Although travel was tightened to prevent circulation of this variant, it was generally too late to prevent introduction of infections into local communities, and local epidemics [20], underscoring the need for robust quarantine systems to prevent community transmission.
We found the importation risk of infectious COVID-19 cases to Hong Kong varied substantially across departure origins. Among the countries or regions studied, the risk was highest among arrivals from the Philippines. Based on the expected importation risk of infectious travellers from the Philippines under the then regimen (14-day quarantine and a secondary test on day 12), a more relaxed regimen (i.e., 7-day quarantine and a secondary test on day 5) for inbound travellers from low COVID-19 prevalence origins would not impose higher importation risks. This observation held when the travel volume was doubled or when resurgence occurred in locations with no introduction during the study period.
Although the risk of releasing infectious travellers arriving from low prevalence origins to the community under a relaxed regimen (e.g., Q7/T5) increased compared to that prior to the relaxation (i.e., Q14/T12), it was lower than that posed by travellers from high prevalence origins under the then effective measures between April and July. The increased importation risk may be overcome with proactive contact tracing, frequent post-arrival testing and prevention from mixing the quarantined individuals with the general population (e.g., within-hotel transmissions). Of note, our results only supported a moderate relaxation (i.e., Q7/T5) of the travel measures from low prevalence origins, while easing the quarantine or post-arrival test would still impose considerable risks even for travellers from those low prevalence countries or regions. The decision to relax travel restrictions for low prevalence origins should also consider economic and societal factors, such as the demands for essential travel (e.g., cross-border family reunion) between the two destinations. In addition, the successful and sustained implementation of “travel bubbles” also requires effective measures to control local transmission in both locations.
In this study, we estimated the origin-specific prevalence of COVID-19 from on-arrival test positives and actual traveller volumes during April to July 2020, which are largely consistent with the reported incidence. However, attack rates in the general population may not reflect attack rates among travellers. For example, between April and July 2020 Singapore had a higher incidence of COVID-19 than the UK (9.5 and 4.3 cases per 1000, respectively) [21], yet no cases were introduced to Hong Kong from Singapore. This was probably because the majority of confirmed COVID-19 cases in Singapore were migrant workers [22] who may be less likely to travel to Hong Kong. Therefore, estimating the prevalence among the inbound travellers using post-arrival tests results could provide more reliable evaluation of the importation risks than using reported disease incidence (which may be subject to various extents of underreporting, and could also be mediated by the travellers’ socioeconomic status) in the departure origins.
In this study, we used data between April and July 2020 from Hong Kong to illustrate our model. Regular monitoring of the importation risks from different departure origins is critical to inform the border controls, due to the rapid changing dynamics of the pandemic. Our proposed framework can be applied to data with various temporal (e.g., bi-weekly and monthly) and spatial (e.g., between state for countries with large areas) resolutions, depending on the data availability. Data on testing for travellers upon and after arrival become critical for the accurate assessments of the importation risks yet are not routinely reported in a systematic fashion. Benefiting from the mandatory requirement of on-arrival tests for all inbound travellers, we assumed the travel volumes reflect the number of on-arrival tests performed for origins without substantial indirect travels (e.g., Singapore and Japan disallowed transit by entering and transiting during the study period).
We performed the study during the period before the new variants of concern were identified [18]. It remains unclear whether the increased transmissibility was caused by increased transmission efficiency (e.g., increased successful transmissions per contact) or prolonged viral shedding duration. If the viral shedding duration prolonged for the variants [23], our model could underestimate the risk for policies of 21-day quarantines as the data we used for infection profiles were obtained from the wild type (e.g., incubation period very rarely longer than 14 days and cessation of infectiousness within 7 days after viral load peak [12,24]). Updated data on the new variants could help to refine the model estimates. If the increased transmissibility was due to the increased transmission efficiency without changes in the duration of viral shedding, our results will remain unchanged.
Our model implicitly assumed the presence of COVID-19 in all departure origins. When there were no imported cases and low traveller volume from a particular origin the importation risk may be overestimated (e.g., Singapore), especially when the traveller volumes increase. For these countries or regions, it may be more reasonable to use the importation risk from an infectious traveller who tested positive on arrival for evaluating the risks of relaxing travel restrictions.
We assumed complete adherence and effectiveness of the post-arrival quarantines, suggesting imperfect effectiveness of quarantines would further increase the importation risks of travellers from both low- and high-risk locations. Indeed, a fraction of inbound travellers arriving in Hong Kong could quarantine at home and infect family members who need not quarantine, while transmissions within quarantine hotels were also reported occasionally [25,26]. The leaking risk of quarantine could trigger new outbreaks in the local community, in particular in low COVID-19 prevalence locations. We were not able to model the leaking risk of quarantine due to lack of data on home-quarantine (which was no longer an option when the study was performed) and within-hotel transmission. Future studies that assessed the leaking risks of quarantine settings could better inform the importation risks across different control regimens.
Our simulations suggested that more than half of the infected travellers may be missed by the on-arrival test, due to the low sensitivity of COVID-19 tests (Figure S2). This is particularly true for infected individuals who were infected a few days before travel. Based on the disease progression, these individuals are more likely to show symptoms after arrival, escape the on-arrival test and pose an onward transmission risk when released [12]. Therefore, quarantine and subsequent testing after arrival are needed to prevent undetected infections from entering the communities while infectious. Additionally, the use of low-sensitivity tests, such as rapid antigen tests, may be unsuitable for on-arrival testing and triaging. Including a second test later in the quarantine period should reduce the risk of releasing infectious travellers, and is consistent with findings reported elsewhere [27], [28], [29]. More intensive testing with an affordable, high sensitivity test may also be able to enhance the overall detection sensitivity of infectious travellers, though individuals may suffer from false positives due to the decreased specificity [29,30].
Our study has several limitations. First, we did not consider the pre-flight negative result for nucleic acid test for SARS-CoV-2 as part of the travel control suite, which has been adopted by a number of countries or regions. A previous study found that pre-flight antigen tests had minimal effect on preventing infectious travellers from releasing to the community [15]. Moreover, a substantial black market has emerged, compromising the value of these tests [31]. Second, we assumed the test specificity as 1 in our model, which may overestimate the prevalence among inbound travellers. However, this was unlikely to affect our main conclusion as it would not affect the relative scales between our estimates. Thirdly, we assumed no in-flight transmission in our model, which risk was suggested to be low [32,33], especially with the mask on and low travel volumes and should not affect our main conclusions. Finally, our study only examined the importation risks imposed by travellers through flights, which could not be generalized to travel measures of importations via other travel routes (e.g., by sea).
We proposed a framework to regularly assess the origin-specific importation risks of infectious COVID-19 travellers by simultaneously accounting for the disease prevalence of travellers, traveller volumes and the effectiveness of control measures, which can be used to inform the safe reopening of the boarders, particularly for countries or regions with low local transmission. Our results suggested that moderate relaxation of control measures (i.e., 7-day quarantine and test on day 5) for travellers from low prevalence locations would not have posed a higher risk of onward transmission, assuming no further within-institution transmissions during quarantine. Our proposed framework can be further adapted to model various data resolution, travel measures, the natural history of the new variants and the immune status of inbound travellers for risks assessments as per demand.
Contributors
All authors meet the ICMJE criteria for authorship. The study was conceived by BY, PW, SGS and BJC. TKT, JYTW, YH, HG, FH and EHYL prepared the data. BY conducted the data analyses and wrote the first draft of the manuscript. All authors provided critical review and revision of the text and approved the final version.
Data Sharing Statement
Data on the imported cases are available from the Department of Health. Data on travel volumes are available from the Hong Kong International Airport on request.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of Competing Interest
BJC consults for Roche, GSK, Moderna, AstraZeneca and Sanofi Pasteur and is supported by the AIR@innoHK program of the Innovation and Technology Commission of the Hong Kong SAR Government. SGS reports unpaid consulting for Sanofi Pasteur. The authors report no other potential conflicts of interest.
Acknowledgments
We thank the Department of Health of the Food and Health Bureau of the Government of Hong Kong for conducting the outbreak investigation and providing the data for the analysis. We thank the Hong Kong International Airport for providing the statistics on arrival passengers for the analysis. This project was supported by the Health and Medical Research Fund, Food and Health Bureau, Government of the Hong Kong Special Administrative Region (grant no. COVID190118) and the National Foundation for Australia China Relations. The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100184.
Appendix. Supplementary materials
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Archived: WHO Timeline - COVID-19 [Internet]. 2020 [cited 2020 Dec 10]. Available from: https://www.who.int/news-room/detail/27-04-2020-who-timeline—covid-19?gclid=EAIaIQobChMI4MaewOeo6gIVyyMrCh2JRgUIEAAYASAAEgLo3_D_BwE.
- 3.Wells C.R., Sah P., Moghadas S.M., Pandey A., Shoukat A., Wang Y. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2002616117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell T.W., Wu J.T., Clifford S., Edmunds W.J., Kucharski A.J., Jit M. Lancet Public Heal; 2020. Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summers D.J., Cheng D.H.-Y., Lin P.H.-H., Barnard D.L.T., Kvalsvig D.A., Wilson P.N. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. Lancet Reg Heal - West Pacific. 2020;4(0) doi: 10.1016/j.lanwpc.2020.100044. https://linkinghub.elsevier.com/retrieve/pii/S2666606520300444 [Internet]Oct [cited 2020 Nov 3]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grépin K.A., Ho T.L., Liu Z., Marion S., Piper J., Worsnop C.Z. Evidence of the effectiveness of travel-related measures during the early phase of the COVID-19 pandemic: A rapid systematic review. BMJ Glob Heal. 2021;6(3) doi: 10.1136/bmjgh-2020-004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New Zealand safe travel zone 2020 COVID-19 and the border.
- 8.Designated flights under HK-Singapore Air Travel Bubble to begin on May 26 [Internet]. [cited 2021 Apr 29]. Available from: https://www.info.gov.hk/gia/general/202104/26/P2021042600252.htm.
- 9.Special webpage against Epidemics. URL: https://www.ssm.gov.mo/apps1/PreventCOVID-19/en.aspx#clg17458 Date of access: 2021-04-29.
- 10.Wu P., Tsang T., Wong J., Ng T., Ho F., Gao H. Suppressing COVID-19 Transmission in Hong Kong: An Observational Study of the First Four Months. SSRN Electron J [Internet] 2020 doi: 10.21203/rs.3.rs-34047/v1. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3627304 Jun 9; Available from: [DOI] [Google Scholar]
- 11.news.gov.hk [Internet]. [cited 2021 Jan 13]. Available from: https://www.news.gov.hk/eng/index.html.
- 12.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 13.Hellewell J., Russell T.W., Beale R., Kelly G., Houlihan C., Nastouli E., et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. medRxiv [Internet]. 2020; Available from: http://medrxiv.org/content/early/2020/11/24/2020.11.24.20229948.abstract. [DOI] [PMC free article] [PubMed]
- 14.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure [Internet] Annals of internal medicine. NLM (Medline) 2020;173:262–267. doi: 10.7326/M20-1495. https://www.acpjournals.org/doi/abs/10.7326/M20-1495 [cited 2020 Oct 13]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford S., Quilty B.J., Russell T.W., Liu Y., Chan Y.W.D., Pearson C.A.B., et al. Strategies to reduce the risk of SARS-CoV-2 reintroduction from international travellers. medRxiv [Internet]. 2020 Jul 25 [cited 2020 Oct 13]; Available from: https://www.medrxiv.org/content/10.1101/2020.07.24.20161281v2.
- 16.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gostic K.M., Gomez A.C.R., Mummah R.O., Kucharski A.J., Lloyd-Smith J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;9 doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Hopkins S., et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv [Internet]. 2021;2020.12.30.20249034. Available from: https://www.medrxiv.org/content/10.1101/2020.12.30.20249034v2%0Ahttps://www.medrxiv.org/content/10.1101/2020.12.30.20249034v2.abstract.
- 19.Du Z, Wang L, Yang B, Ali S, Tsang TK, Shan S, et al. Risk for International Importations of Variant SARS-CoV-2 Originating in the United Kingdom. Emerg Infect Dis. 2021;27(5):1527-1529. 10.3201/eid2705.210050. [DOI] [PMC free article] [PubMed]
- 20.Gonzalez-Reiche A.S., Hernandez M.M., Sullivan M.J., Ciferri B., Alshammary H., Obla A. Introductions and early spread of SARS-CoV-2 in the New York City area. Science (80-) 2020;369(6501):297–301. doi: 10.1126/science.abc1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han E., Tan M.M.J., Turk E., Sridhar D., Leung G.M., Shibuya K. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. The Lancet. 2020;396:1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kissler S.M., Fauver J.R., Mack C., Tai C.G., Watkins A.E., Samant R.M., et al. Densely sampled viral trajectories suggest longer duration of acute infection with B.1.1.7 1 variant relative to non-B.1.1.7 SARS-CoV-2 [Internet]. 2021 Feb [cited 2021 Feb 18]. Available from: https://dash.harvard.edu/handle/1/37366884
- 24.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. Scientific brief. 2020;09:1–10. July 2020. WHO. [Google Scholar]
- 25.More than a dozen COVID leaks in 6 months: to protect Australians, it's time to move quarantine out of city hotels [Internet]. [cited 2021 May 3]. Available from: https://theconversation.com/more-than-a-dozen-covid-leaks-in-6-months-to-protect-australians-its-time-to-move-quarantine-out-of-city-hotels-159808.
- 26.New Zealand hotel quarantine worker in Auckland tests positive for COVID-19 - ABC News [Internet]. [cited 2021 May 3]. Available from: https://www.abc.net.au/news/2021-04-08/new-zealand-quarantine-worker-positive-auckland/100055706.
- 27.Quilty B.J., Clifford S., Hellewell J., Russell T.W., Kucharski A.J., Flasche S. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Heal. 2021;6(3):e175–e183. doi: 10.1016/S2468-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.traQ Study: Transparent Risk Assessment of Quarantine | Burnet Institute [Internet]. [cited 2021 Feb 1]. Available from: https://burnet.edu.au/projects/466_traq_study_transparent_risk_assessment_of_quarantine.
- 29.Larremore D.B., Wilder B., Lester E., Shehata S., Burke J.M., Hay J.A. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1) doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mina M.J., Parker R., Larremore D.B. Rethinking Covid-19 Test Sensitivity — A Strategy for Containment. N Engl J Med. 2020;383(22):e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 31.Tourists are buying fake covid-19 test results on the black market to travel - The Washington Post [Internet]. [cited 2021 Mar 19]. Available from: https://www.washingtonpost.com/travel/2020/11/10/fake-tests-covid-flights/.
- 32.Freedman D.O., Wilder-Smith A. In-flight transmission of SARS-CoV-2: a review of the attack rates and available data on the efficacy of face masks. J Travel Med. 2020 doi: 10.1093/jtm/taaa178. https://academic.oup.com/jtm/article/doi/10.1093/jtm/taaa178/5910636 [Internet]Dec 23 [cited 2021 Apr 27];27(8). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu M., Wang J., Lin H., Ruktanonchai C.W., Xu C., Meng B., et al. Transmission risk of SARS-CoV-2 on airplanes and high-speed trains. medRxiv [Internet]. 2020 [cited 2021 Apr 27]. p. 2020.12.21.20248383. Available from: https://www.medrxiv.org/content/10.1101/2020.12.21.20248383v1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.