Abstract
Dysregulated crosstalk between different signaling pathways contributes to tumor development, including resistance to cancer therapy. In the present study, we found that the mitogen-activated extracellular signal-regulated kinase (MEK) inhibitor trametinib failed to suppress the proliferation of PANC-1 and MGC803 cells by activating the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway, while the JAK2 inhibitor fedratinib failed to inhibit the growth of the PANC-1 cells upon stimulation of extracellular signal-regulated kinase (ERK) signaling. In particular, the most prominent enhancement of the anti-proliferative effect resulted from the concurrent blockage of the JAK2/STAT3 and ERK signaling pathways. Furthermore, the combination of the two inhibitors resulted in a reduced tumor burden in mice. Our evidence suggests novel crosstalk between JAK2/STAT3 and ERK signaling in gastric cancer (GC) and pancreatic ductal adenocarcinoma (PDAC) cells and provides a therapeutic strategy to overcome potential resistance in gastrointestinal cancer.
Keywords: Gastrointestinal cancers, JAK2/STAT3 pathway, ERK pathway, Crosstalk, Apoptosis
1 Introduction
Gastrointestinal (GI) cancer is one of the foremost health concerns in the world. It includes gastric cancer (GC) and pancreatic cancer (PANC), both of which are very lethal malignant tumors (Nie et al., 2017). GC is the second leading cause of cancer-associated mortality (Pourhoseingholi et al., 2015), whereas PANC is the seventh most common cause of death from malignancies worldwide (Schizas et al., 2020). Our knowledge of GI cancer development and progression has been greatly improved by intensive study during the last decade. Currently, clinical trials directed at targets such as epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), hepatocyte growth factor (HGF)/scatter factor (SF)-MET proto-oncogene, receptor tyrosine kinase (MET), Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), and rat sarcoma virus (RAS)/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated extracellular signal-regulated kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling, as well as other molecules, are in progress (Chen et al., 2014; Samatar and Poulikakos, 2014; Mizukami et al., 2015; Liu et al., 2020).
Janus kinases (JAKs) and their downstream signal transducer and activator of transcription (STAT) represent a widely studied signaling pathway that responds to the stimulation of cytokines and growth factors (Xin et al., 2020). The JAK family consists of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), while seven family members of the transcriptional activator STATs have been identified (Pencik et al., 2016). JAKs, upon interactions between cytokine ligands and their receptors, become phosphorylated and activated. Sequentially, activated JAKs lead to the phosphorylation of STATs (Pencik et al., 2016), followed by nuclear translocation. A constitutively active mutation of JAK2 was found in the irregular proliferation of some myeloid cells and in leukaemia (Wu et al., 2018, 2019). In addition, the transcriptional activity of STAT3 increased due to the occurrence of STAT3 mutations in leukaemia (Andersson et al., 2016). Of note, accumulated evidence has demonstrated the link between the dysregulation of JAKs/STATs and tumors (Groner and von Manstein, 2017). It has been reported that the JAK/STAT signaling pathway is activated in dozens of human tumors and cancers, and persistent STAT3 signaling contributes to the development of cancers, including Ewing sarcoma and GC (Anderson et al., 2014; Ashrafizadeh et al., 2020). Thus, JAKs/STATs signaling has been viewed as an attractive therapeutic target for the treatment of cancer patients. Indeed, various inhibitors targeting JAKs or STATs have been tested in preclinical and clinical trials (Ashrafizadeh et al., 2020).
The RAS/RAF/MEK/ERK pathway is a well-known signaling cascade that controls important cellular events, including cell growth and differentiation, to maintain physiological homeostasis upon extracellular mitogen stimulation. The binding of various growth factors, such as epidermal growth factor (EGF), to their receptors on the cell surface triggers activation of receptor tyrosine kinase (RTK) and phosphorylates RAS to initiate (ERK) signal transduction (Degirmenci et al., 2020). A large number of studies revealed that constitutively active mutations within key molecules of the RAS/RAF/MEK/ERK signaling pathway are ubiquitously detected in different cancers. In particular, active mutants of RAS and RAF are present in 30% and 8% of human tumors, respectively (Samatar and Poulikakos, 2014). Furthermore, RAS and RAF perform an oncogenic function in tumorigenesis by driving ERK signaling in vitro and in vivo (Guo et al., 2020). In fact, specific inhibitors targeting RAF or MEK are used to treat patients with melanoma (Samatar and Poulikakos, 2014). Hence, RAS/RAF/MEK/ERK signaling offers therapeutic candidates.
Signal transduction plays an essential role in regulating cell behavior upon intrinsic and extrinsic signals. During the past two decades, our understanding of the molecular mechanism of cell signal transduction has broadened extensively. Accumulating evidence demonstrates that signals transmitted within mammalian cells are not limited to one individual specific pathway without interacting with other pathways (Chen et al., 2016; Zhang et al., 2016; Hua et al., 2019). In fact, different signaling pathways are integrated and form a complex network through crosstalk to direct different cell events, such as growth, differentiation, and apoptosis (Vert and Chory, 2011). Many oncogenes and tumor suppressors act as a nexus in signaling pathways. Mutations of oncogenes and loss-of-function mutations of tumor suppressors result in persistent oncogenic signaling to promote cancer development (Ezerskyte et al., 2018; Wang et al., 2018). These findings provide potential clinical biomarkers and therapeutic targets for cancer treatment. However, targeting a single molecule or signaling pathway in cancer therapies often results in unsatisfactory outcomes. With the awareness of tumor heterogeneity and signaling crosstalk, it is urgent to test combinational targets for the treatment of cancer.
The object of our present study was to identify novel crosstalk between the JAK2/STAT3 and MEK/ERK signaling pathways in GC and pancreatic ductal adenocarcinoma (PDAC) cells, and to demonstrate the effects of JAK2/STAT3 and MEK/ERK signaling inhibition in dramatically decreasing proliferation and increasing apoptosis, as well as in the prevention of tumor growth in a subcutaneous human gastric tumor model. This study will provide a potential target for combinational therapy for GI cancer treatment.
2 Materials and methods
2.1. Reagents and antibodies
Rabbit monoclonal antibodies against JAK2 (Cat. 3230), phospho-JAK2 (p-JAK2)-1007/1008 (Cat. 3771), STAT3 (Cat. 4904), p-STAT3-Tyr705 (Cat. 9145), poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP; Cat. 9532), cleaved caspase-3 (Cat. 9664), caspase-3 (Cat. 14220), cleaved caspase-9 (Cat. 20750) and ERK (Cat. 4695), and mouse monoclonal antibodies against caspase-9 (Cat. 9508) and p-ERK (Cat. 5726) were purchased from Cell Signalling Technology (Danvers, MA, USA). A mouse monoclonal anti-β-actin (Cat. A5441) antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). JAK2 inhibitor fedratinib (Cat. S2736) and MEK inhibitor trametinib (Cat. S2673) were provided by Selleck (Shanghai, China).
2.2. Cell culture
The human GC cell line MGC803 and human PDAC cell lines PANC-1 and MIA PaCa-2 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). MGC803 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Cat. R5886, Sigma-Aldrich). PANC-1 and MIA PaCa-2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Cat. 51435C, Sigma-Aldrich). The culture media were supplemented with 10% heat-inactivated fetal bovine serum (Cat. 12483020, Thermo Fisher Scientific, Waltham, MA, USA), 1% (0.01 g/mL) L-glutamine (Cat 25030081, Thermo Fisher Scientific), penicillin (100 U/mL), and streptomycin (100 µg/mL; Cat. 15140122, Thermo Fisher Scientific). Cells were incubated at 37 ℃ in 5% CO2.
2.3. Cell proliferation analysis
Cells (8000 cells/well) were seeded into 96-well plates and cultured for 16 h, after which trametinib was added to the growth medium at concentrations of 5 and 10 μmol/L and incubated for 24 to 72 h. Then, the solution from the Cell Counting Kit-8 (CCK-8; Cat. CK04, Dojindo, Japan) was added to the cells to measure the number of viable cells. Briefly, the cells were incubated with 10 μL CCK-8 solution per well for 13 h. The absorbance was measured at 450 nm using a multiplate reader (Thermo Varioskan Flash, Waltham, MA, USA). The results were calculated as mean±standard deviation (SD) of values from at least three individual experiments.
2.4. Apoptosis assay
Cells (1.6×105 cells/mL) were seeded into six-well plates and were treated with inhibitors for 24 h. Then, the cells were detached with ethylene diamine tetraacetic acid (EDTA)-free trypsin and centrifuged for collection. Next, the cell pellets were washed twice with cold phosphate-buffered saline (PBS). In accordance with the manufacturer's instructions, the resuspended cells in binding buffer were sequentially incubated with propidium iodide (PI) and annexin-V-fluorescein isothiocyanate (FITC) using the Apoptosis Detection Kit (Cat. 556570, Becton Dickinson, Franklin Lakes, NJ, USA). Double-stained cells were analyzed with a FACS CantoTMII (Becton Dickinson) and CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ, USA).
2.5. Western blotting analysis
Whole-cell lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitors (Cat. 11697498001, Roche, Indianapolis, IN, USA) and phosphatase inhibitors (Cat. 524631, Merck, Temecula, CA, USA). Next, the protein concentrations were determined using a DCTM Protein Assay Kit (Cat. 5000-0111, Bio-Rad, Hercules, CA, USA). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 5% to 10% (1%=0.01 g/mL)) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). Then, the filter was immersed into blocking buffer, followed by incubation with primary and secondary antibodies. Finally, the protein signals were visualized by using an enhanced chemiluminescence (ECL) reagent (Cat. 1705061, Bio-Rad).
2.6. Subcutaneous xenograft assays
BALB/c nu/nu mice (4‒6-week-old) were ordered from the Zhejiang Academy of Medicine (Hangzhou, China). Subcutaneous xenografts were administered by injecting MGC803 cells (4×106 cells in 200 μL of RPMI-1640) into the right axillary regions of 6-week-old female BALB/c nu/nu mice (one implantation site per animal). Tumor size in three dimensions was measured twice a week using a calliper after tumor appearance. The mice were randomly divided into groups treated with solvent only, fedratinib (30 mg/kg), trametinib (0.75 mg/kg), or a combination of these two inhibitors, respectively (eight animals per group) as the tumor volume reached approximately 100 mm3. Three weeks later, tumor tissues from the sacrificed mice were collected and weighed. Finally, the tumor tissues were removed from the animals and frozen in liquid nitrogen for western blotting analysis.
2.7. Statistical analysis
All data are expressed as mean±standard deviation (SD) from three independent experiments. For statistical analysis, one-way analysis of variance (ANOVA) with Dunnett's post-hoc test was performed using SPSS 19.0 software. The differences were considered significant when P<0.05.
3 Results
3.1. Abrogation of MEK/ERK signaling upregulates tyrosine phosphorylation of JAK2 and STAT3, blocking anti-proliferative effects in GC and PDAC cancer cells
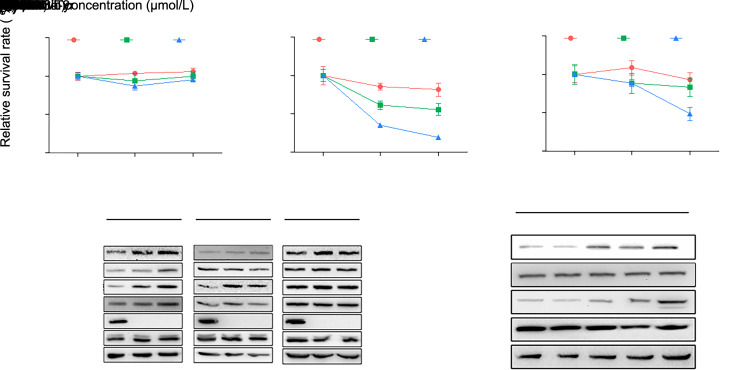
The MEK inhibitor trametinib has been used to treat patients with melanoma. We first tested the effect of inhibition of MEK/ERK signaling on the proliferation of GC and PDAC cells. Cell proliferation was measured using a CCK-8 assay. There was no effect of trametinib on the proliferation of MGC803 cells (Fig. 1a and Table S1). The proliferation of MIA PaCa-2 was decreased by trametinib at 5 and 10 μmol/L at 48 and 72 h (Fig. 1b and Table S1). However, PANC-1 cells were dramatically suppressed only by 10 μmol/L trametinib at 72 h (Fig. 1c and Table S1). Next, we found that the application of trametinib in human MGC803, MIA PaCa-2, and PANC-1 cells resulted in a nearly total loss of tyrosine phosphorylation of ERK while prominently enhancing the tyrosine phosphorylation levels of JAK2 and STAT3 proteins in a dose-dependent manner (Fig. 1d). Additional time-course analysis showed that the tyrosine phosphorylation levels of JAK2 and STAT3 proteins in MGC803 cells were gradually elevated with a prolonged period of treatment with trametinib (Fig. 1e). These data suggested that the inhibition of the anti-proliferative effect by trametinib in GC and PDAC cells may be related to the activation of the JAK2/STAT3 pathway.
Fig. 1. Mitogen-activated extracellular signal-regulated kinase (MEK) inhibitor trametinib fails to inhibit the viability of human gastric cancer (GC) cells and pancreatic ductal adeno carcinoma (PDAC) cells sufficiently due to activation of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling. MGC803 (a), MIA PaCa-2 (b), and PANC-1 (c) cells were exposed to trametinib at the concentrations of 5 and 10 μmol/L for 24, 48, or 72 h. Cell survival was measured using a Cell Counting Kit-8 (CCK-8) assay. (d) Trametinib was added to MGC803, MIA PaCa-2, and PANC-1 cells at different concentrations for 8 h, after which the states of the JAK2/STAT3 and extracellular signal-regulated kinase (ERK) pathways were measured by western blot. (e) MGC803 cells were treated with 10 μmol/L trametinib at the indicated time points, and the tyrosine phosphorylation levels of JAK2 and STAT3 were measured by western blot. The results are presented as mean±standard deviation (SD) from three independent experiments. ** P<0.01 and *** P<0.001 vs. control (no trametinib).
3.2. Inhibition of JAK2/STAT3 signaling upregulates the tyrosine phosphorylation levels of EGFR and ERK in GC and PDAC cells, respectively
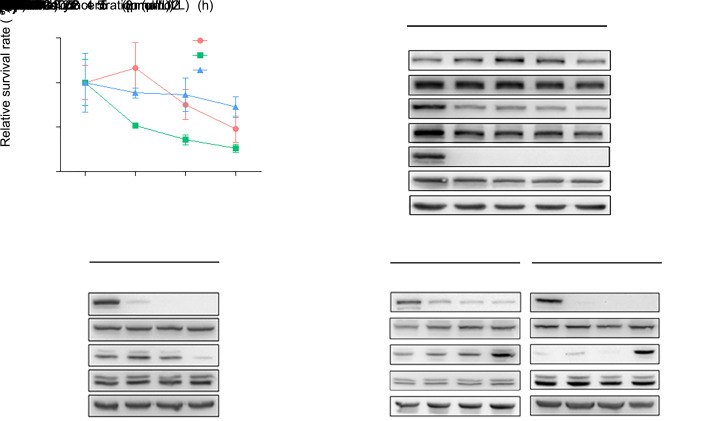
Some malignancies have been proven to benefit from fedratinib, a novel selective JAK2 antagonist (Pardanani et al., 2015). Based on the above evidence, we wondered whether fedratinib would promote GC and PDAC cell proliferation more effectively by inhibiting JAK2/STAT3 signaling. First, MGC803, PANC-1, and MIA PaCa-2 cells were treated with fedratinib at different concentrations (0, 1, 2, and 5 μmol/L) for 24 h. The relative survivals of MGC803 and MIA PaCa-2 cells were only significantly decreased by treatment with fedratinib at 5 μmol/L, which had no effect on the viability of PANC-1 cells (Fig. 2a and Table S2). Then, fedratinib was added initially to MGC803 cells in a time course (0, 2, 4, 8, and 12 h), and total protein expression and tyrosine phosphorylation levels of JAK2, STAT3, ERK, and EGFR were analyzed by immunoblotting after exposure to different concentrations (0, 1, 2, and 5 μmol/L) of fedratinib. As expected, addition of fedratinib had no effect on the total protein expression level of JAK2 or STAT3 but resulted in a dose- and time-dependent decrease in the tyrosine phosphorylation of JAK2 and STAT3 (Fig. 2b). Interestingly, we found that the tyrosine phosphorylation of EGFR was increased by fedratinib treatment after 2 and 4 h and was then reduced thereafter (Fig. 2b). Surprisingly, tyrosine phosphorylation of ERK was increased at low concentrations of fedratinib (1 and 2 μmol/L) for 4 h in MGC803 cells (Fig. 2c). However, a striking elevation in the phosphorylation of ERK was observed in MIA PaCa-2 and PANC-1 cells treated with fedratinib at different concentrations for 8 h, accompanied by a strong inhibition of tyrosine phosphorylation of STAT3 (Fig. 2d). Taken together, these results suggested that an alternative signaling switch between the JAK2/STAT3 and ERK pathways could be triggered due to inactivation of either one of them in GC and PDAC cells.
Fig. 2. Janus kinase 2 (JAK2) inhibitor fedratinib causes a loss in the ability to suppress the survivals of MGC803, MIA PaCa-2, and PANC-1 cells via the inducement of extracellular signal-regulated kinase (ERK) signaling. (a) Fedratinib was added to MGC803, MIA PaCa-2, and PANC-1 cells at the indicated concentrations for 24 h. Cell viability was measured using a Cell Counting Kit-8 (CCK-8) assay. (b) MGC803 cells were treated with 5 μmol/L fedratinib at the indicated time points, and the tyrosine phosphorylation levels of EGFR, JAK2, and STAT3 were measured by western blot. (c) Fedratinib was added to MGC803 cells at different concentrations for 4 h, and the tyrosine phosphorylation levels of signal transducer and activator of transcription 3 (STAT3) and ERK were examined by western blot. (d) MIA PaCa-2 and PANC-1 cells were treated with fedratinib at different concentrations for 8 h, and the tyrosine phosphorylation levels of STAT3 and ERK were examined by western blot. The results are presented as the mean±standard deviation (SD) from three independent experiments. * P<0.05 and ** P<0.01 vs. control (no fedratinib).
3.3. Proliferation of GC and PDAC cells is effectively retarded by inhibition of JAK2/STAT3 and ERK signaling
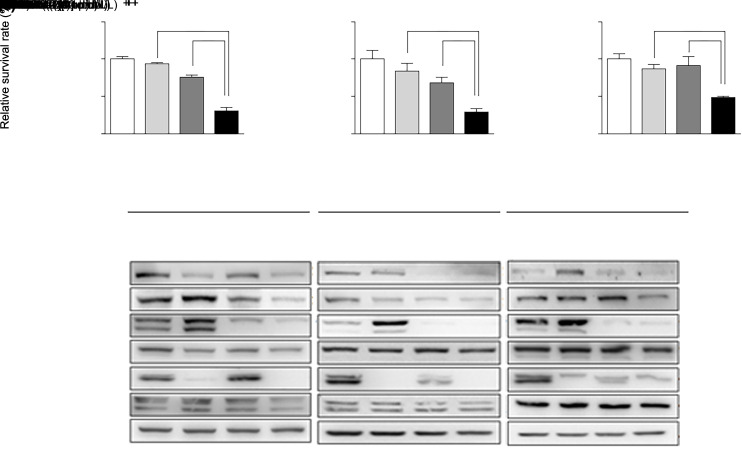
The results above demonstrate that there is crosstalk between the JAK2/STAT3 and ERK signaling pathways in GC and PDAC cells. We then tried to identify how JAK2/STAT3 and ERK signaling pathways act on the biological function of cancer cells. We examined the effect on the proliferation of GC and PDAC cells by interrupting both JAK2/STAT3 and ERK signaling. Treatment with 10 μmol/L trametinib alone could not inhibit the viability of MGC803, MIA PaCa-2, or PANC-1 cells (Figs. 3a‒3c and Table S3). The survival rates of MGC803 and MIA PaCa-2 cells were slightly decreased by treatment with 2 μmol/L fedratinib for 24 h (Figs. 3a‒3c and Table S3). Finally, when fedratinib and trametinib were combined for treatment, the proliferation of the three cell lines was significantly suppressed (Figs. 3a‒3c and Table S3). Twenty-four hours after treatment, the relative survival rates of MGC803, MIA PaCa-2, and PANC-1 cells in combination group were approximately (30.63±4.59)%, (28.99±4.57)%, and (48.21±1.73)%, respectively (Figs. 3a–3c and Table S3). Furthermore, the most dramatic suppression of tyrosine phosphorylation levels of JAK2, STAT3, and ERK proteins in MGC803, PANC-1, and MIA PaCa-2 cells was observed by the combined treatment with fedratinib (2 μmol/L) and trametinib (10 μmol/L) for 24 h (Fig. 3d). These data indicated that concomitant inactivation of JAK2/STAT3 and ERK signaling is able to lead to a synergistic effect to suppress the proliferation of cancer cells.
Fig. 3. Combination of trametinib and fedratinib results in an anti-proliferative effect in MGC803, MIA PaCa-2, and PANC-1 cells through a decrease in the tyrosine phosphorylation levels of extracellular signal-regulated kinase (ERK) and Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) simultaneously. MGC803 (a), MIA PaCa-2 (b), and PANC-1 (c) cells were treated with a combination of trametinib (10 μmol/L) and fedratinib (2 μmol/L) for 24 h. (d) Cell lysates were collected for western blot analysis. The results are presented as mean±standard deviation (SD) from three independent experiments. * P<0.05 and *** P<0.001 vs. control; && P<0.01 and &&& P<0.001 vs. 10 μmol/L trametinib treatment; ## P<0.01 and ### P<0.001 vs. 2 μmol/L fedratinib treatment.
3.4. Interference with JAK2/STAT3 and ERK signaling triggers apoptosis in MGC803 GC cells
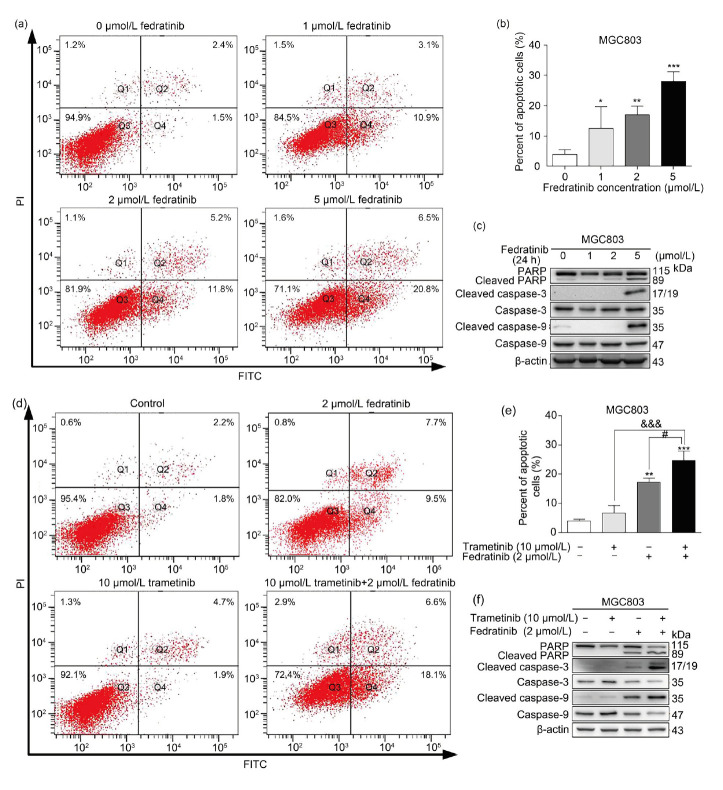
After having observed an anti-proliferative effect on MGC803, PANC-1, and MIA PaCa-2 cells through disruption of the JAK2/STAT3 and ERK signaling pathways, we investigated whether administration of fedratinib alone or combined with trametinib could induce apoptosis in MGC803 GC cells. We first added fedratinib to MGC803 GC cells at different concentrations (0, 1, 2, and 5 μmol/L) for 24 h, after which apoptotic cells were quantified by double staining with annexin-V-FITC and PI, followed by FACS analysis. As shown in Figs. 4a, 4b and Table S4, the number of cells that underwent apoptosis increased with the increase in fedratinib concentration. We further examined the proteolytic processing of PARP, caspase-3, and caspase-9. In support of the FACS analysis, MGC803 cells responded to fedratinib at 5 μmol/L 24 h after treatment. The cleaved fragments of PARP, caspase-3, and caspase-9 were increased in MGC803 cells (Fig. 4c). A very much higher percentage of apoptosis in MGC803 GC cells was observed with the addition of fedratinib and trametinib combined (Figs. 4d and 4e). It is notable that trametinib induced a minor apoptotic effect in MGC803 GC cells, consistent with its marginal effect on the proliferation in MGC803 cells. In addition, combinational treatment with fedratinib and trametinib in MGC803 GC cells produced substantially stronger intensities of cleaved bands of PARP, caspase-3, and caspase-9 than fedratinib alone, whereas trametinib did not trigger the breakdown of PARP, caspase-3, and caspase-9 in MGC803 GC cells, consistent with the FACS results (Fig. 4f).
Fig. 4. Fedratinib alone or in combination with trametinib induces apoptosis in human MGC803 gastric cancer (GC) cells. (a, b) Apoptotic level of MGC803 cells treated with fedratinib at the indicated concentrations for 24 h was examined by FACS analysis. (c) Expression of the apoptotic markers, including total caspase-3, caspase-9, poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP), and their cleaved forms, was detected by western blot in MGC803 cells exposed to fedratinib alone. (d, e) Apoptotic level of MGC803 cells treated with trametinib and fedratinib was examined by FACS analysis. (f) Expression of caspase-3, caspase-9, PARP, and their cleaved forms in MGC803 cells treated with trametinib and fedratinib was tested by western blot. The results are presented as mean±standard deviation (SD) from three independent experiments. * P<0.05, ** P<0.01, and *** P<0.001 vs. control; &&& P<0.001 vs. 10 μmol/L trametinib treatment; # P<0.05 vs. 2 μmol/L fedratinib treatment. PI, propidium iodide; FITC, fluorescein isothiocyanate.
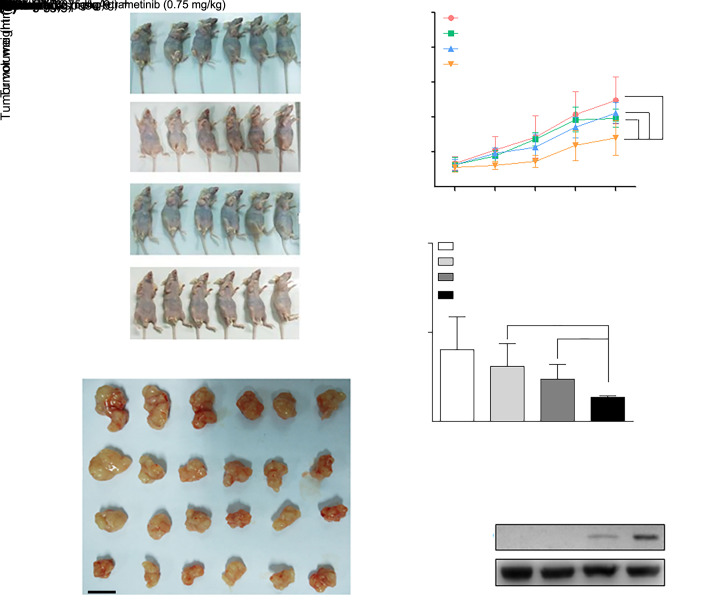
3.5. Disturbance of JAK2/STAT3 and ERK signaling profoundly impairs tumor growth in mice by inducing apoptosis
To evaluate the roles of JAK2/STAT3 and ERK signaling in GC cell growth in physiological conditions, MGC803 GC cells were injected subcutaneously into nude mice. After tumor formation, fedratinib and trametinib were delivered individually or together into the mice. The tumor volume was measured during the 11-d follow-up. The difference in tumor growth between the control and the fedratinib groups was negligible, whereas the trametinib or combination group displayed a retardation of tumor growth compared with the control group (Figs. 5a‒5c and Table S5). In particular, treatment with a combination of fedratinib and trametinib led to remarkable tumor growth suppression in mice (Figs. 5a‒5c and Table S5). Of note, dosages of the two inhibitors received by the mice in the experiment did not affect the weight of the mice (Fig. S1). The delay in tumor growth was further assessed by tumor weight after the mice were sacrificed. Tumor weight was decreased by (23.06±25.51)%, (41.20±16.39)%, and (66.48±1.89)% in the fedratinib, trametinib, and combination groups, respectively (Fig. 5d and Table S6). To address whether apoptosis involving caspases also occurred in vivo, tumor tissue lysates from control, fedratinib, trametinib, and combination groups were subjected to immunoblotting; cleaved caspase-9 was detected in the trametinib group and was especially prominently augmented in the combination group (Fig. 5e).
Fig. 5. Fedratinib combined with trametinib suppresses tumor growth and induces apoptosis in MGC803 gastric cancer (GC) cell xenograft tumors in vivo. (a) Mice with tumors formed by subcutaneous injection of MGC803 GC cells received fedratinib and trametinib alone or in combination (n=6). (b) Resected tumor tissues after the in vivo experiment are shown (n=6). (c) Tumor growth curve was documented based on tumor volume on the indicated following days. (d) Weights of tumors exposed to fedratinib and trametinib alone or in combination were examined on the indicated days (n=6). (e) Apoptotic level was analyzed using immunoblotting of cleaved caspase-9 in tumor tissue lysates. The results are presented as mean±standard deviation (SD). * P<0.05 and ** P<0.01 vs. control; & P<0.05 vs. 0.75 mg/kg trametinib treatment; # P<0.05 and ## P<0.01 vs. 30 mg/kg fedratinib treatment.
4 Discussion
Therapy targeting key components in oncogenic signaling is an attractive treatment for cancer. After nearly three decades of efforts in basic research and clinical trials, a milestone drug in targeted cancer therapy, imatinib (known by its brand name Gleevec), was developed and approved for clinical application in patients with haematologic malignancy in 2001 (Thambi and Sausville, 2002; Gerber, 2008). Since then, dozens of targeted agents have entered into clinical practice and fundamentally changed the treatment for many types of cancers, including breast, ovarian, cervical, colorectal, and lung cancers, as well as melanoma, lymphoma, and leukaemia over the past fifteen years. However, the efficiency of a targeted cancer therapy using a monoagent is generally transient and becomes invalidated after a couple of months (Holohan et al., 2013; Colmegna et al., 2018). Patients with cancers lose their response to a single targeted agent, and tumor burden relapses invariably. This, which is characterized by a more aggressive phenotype. Hence, resistance is the major challenge faced by targeted therapy. Intensive studies have been carried out to address the molecular mechanisms of therapy resistance. Among the mechanisms being reported, one of the major reasons leading to resistance has been attributed to malfunction of crosstalk between different signaling pathways. Investigations have revealed that inhibition of a pivotal component in a specific signaling pathway using a single targeted agent could activate other parallel signaling pathways (Chatterjee and Bivona, 2019).
Several JAK2 inhibitors, such as ruxolitinib and fedratinib, have been developed. In particularly, ruxolitinib is clinically applied to treat myelofibrosis (Verstovsek et al., 2017). Resistance to JAK2 inhibitors has been explored, and occurrence of an extra mutation in JAK2 leads to loss of response to JAK2 inhibitors in cancer cells (Bose and Verstovsek, 2017; Meyer, 2017). Trametinib, the MEK inhibitor tested in our study, has been successfully approved to treat patients with melanoma characterized typically by an active RAF mutation (Grimaldi et al., 2017). Recent studies have revealed resistance to RAF and MEK inhibitors. RAF/MEK/ERK signaling drives the inhibitory regulation of upstream RTKs, such as EGFR. Upon treatment with RAF and/or MEK inhibitors, activation of EGFR signaling is triggered and initiates other oncogenic signaling pathways, such as phosphoinositide 3-kinase (PI3K)/serine-threonine-protein kinase (AKT) (Meierjohann, 2017). In this study, we found that the inhibition of the JAK2/STAT3 or ERK signaling pathway caused their reciprocal activation in PDAC and GC cells. Our present study provides a novel finding that administration of JAK2 inhibitors resulted in hyperactivation of EGFR/ERK signaling in GI cancer cells. More interestingly, the parallel JAK2/STAT3 and ERK signaling pathways could be mutually activated upon treatment with fedratinib and trametinib, respectively. Our results disclose a new mechanism that induces resistance to JAK2 and MEK inhibitors.
In our study, addition of either JAK2 or MEK inhibitor caused a significantly repressed proliferation in PDAC cells but not in GC cells. Notably, human PANC-1 and MIA PaCa-2 PDAC cells harbor active mutations of Kirsten rat sarcoma viral oncogene homolog (KRAS) (Deer et al., 2010) that are not detected in MGC803 GC cells. This may suggest that both RAS/RAF/MEK/ERK and JAK2/STAT3 signaling could sustain the proliferation of PANC-1 and MIA PaCa-2 PDAC cells. Thus, PANC-1 and MIA PaCa-2 PDAC cells become sensitive to either fedratinib or trametinib, whereas MGC803 GC cells are more responsive to fedratinib than to trametinib. This indicates that JAK2/STAT3 might be the major signaling pathway to transduce proliferative signaling in MGC803 GC cells. Nevertheless, due to tumor heterogeneity and molecular mechanisms mentioned above, such as the complex crosstalk between different signaling pathways, resistance to targeted therapy is inevitably induced. Combinational therapy is therefore recommended to reduce resistance based on its effectiveness and cost savings (Mokhtari et al., 2017). A combination of targeted therapies, such as combining trametinib and dabrafenib, a B-Raf proto-oncogene, serine/threonine kinase (BRAF) inhibitor, has been approved in clinical practice (Meierjohann, 2017). In support of this recommendation, we found that the simultaneous inactivation of the JAK2/STAT3 and ERK signaling pathways using a combination of fedratinib and trametinib achieved a very greater antitumor effect in vitro and in vivo than that using the single addition of either fedratinib or trametinib. Furthermore, it is noteworthy that fedratinib has been viewed as a potential therapeutic agent for patients with myelofibrosis because of the promising results in clinical trials (Blair, 2019). However, a clinical trial of fedratinib was halted due to adverse effects on the nervous system. Thus, agent toxicity also hampers the further clinical application and encourages combination therapy to decrease the dosage of the agents and attenuate the various side effects. Indeed, a combination of fedratinib and trametinib at a lower concentration produced a more significant effect than the addition of fedratinib or trametinib alone with higher doses and could thus contribute to a reduction in the dose of drugs required.
Of note, the anticancer function of fedratinib was more dramatic than that of trametinib in our study in vitro. In contrast, treatment with fedratinib in vivo showed a less suppressive effect on tumor growth than trametinib. The discrepancy between the in vitro and in vivo results reflects the more complicated physiological microenvironment. During the past ten years, the tumor microenvironment has increasingly been recognized as an essential player in tumorigenesis (Hirata and Sahai, 2017). Within the tumor microenvironment, tumor cells reciprocally interact with stromal components, such as extracellular matrix molecules (ECMs) and stromal cells. ECMs not only maintain the three-dimensional structure of the tumor but also initiate cell signaling by binding to their receptors on the cancer cell surface. Surrounding stromal cells including fibroblasts, adipocytes, and immune cells can be educated by tumor cells through paracrine and direct cell–cell contact to promote cancer development (Hirata and Sahai, 2017; Ramamonjisoa and Ackerstaff, 2017). Therefore, the efficiency of the targeted therapy would be considerably affected by the tumor microenvironment.
5 Conclusions
The reactivation of one signaling pathway after treatment with the inhibitor of another leads to a limited survival benefit. Therefore, a novel combination therapy in order to overcome the interaction among the possible signaling pathways is needed. Our study discovered that trametinib failed to restrain tumor growth by reactivating the JAK2/STAT3 signaling pathway, whereas the effect of the JAK2 inhibitor on PADC and GC cells was not able to suppress proliferation due to the excitation of ERK signaling. Combinational therapy with trametinib and fedratinib in our study exhibited a significant inhibition in cell survival and induction of apoptosis. These results may provide a potential therapeutic strategy for the treatment of GI cancers.
Supplementary information
Acknowledgments
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LQ18H280005 and LY21H030002), the National Natural Science Foundation of China (Nos. 81770535, 81600595, 81503297, 81603340, 81773945, and 81803775), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Nos. 2019RC228, 2019RC229, and 2019RC113), and the Open Foundation from Chinese and Western Integrative Medicine in the Most Important Subjects of Zhejiang (No. ZXYJH2018002), China.
Author contributions
Xi WANG, Chunyan DAI, Yifei YIN, and Yufei FU performed the experimental research and data analysis. Xi WANG, Chunyan DAI, and Zhe CHEN wrote and edited the manuscript. Lin WU and Weiyang JIN performed the establishment of animal models. Ke HAO and Bin LU contributed to the study design, data analysis, writing and editing of the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Xi WANG, Chunyan DAI, Yifei YIN, Lin WU, Weiyang JIN, Yufei FU, Zhe CHEN, Ke HAO, and Bin LU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed. Approval for the animal experiments was obtained from the Experimental Animal Committee of the Provincial Government of Zhejiang (China), and the animal protocols fulfilled the institutional guidelines on the Protection of Animals used for Scientific or Educational Purposes.
References
- Anderson JL, Titz B, Akiyama R, et al. , 2014. Phosphoproteomic profiling reveals IL6-mediated paracrine signaling within the Ewing sarcoma family of tumors. Mol Cancer Res, 12(12): 1740-1754. 10.1158/1541-7786.MCR-14-0159 [DOI] [PubMed] [Google Scholar]
- Andersson E, Kuusanmäki H, Bortoluzzi S, et al. , 2016. Activating somatic mutations outside the SH2-domain of STAT3 in LGL leukemia. Leukemia, 30(5): 1204-1208. 10.1038/leu.2015.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M, Zarrabi A, Orouei S, et al. , 2020. STAT3 pathway in gastric cancer: signaling, therapeutic targeting and future prospects. Biology (Basel), 9(6): 126. 10.3390/biology9060126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HA, 2019. Fedratinib: first approval. Drugs, 79: 1719-1725https://doi.org/10.1007/s40265- 019-01205-x [DOI] [PubMed] [Google Scholar]
- Bose P, Verstovsek S, 2017. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood, 130(2): 115-125. 10.1182/blood-2017-04-742288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Bivona TG, 2019. Polytherapy and targeted cancer drug resistance. Trends Cancer, 5(3): 170-182. 10.1016/j.trecan.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WB, Wu JH, Shi H, et al. , 2014. Hepatic stellate cell coculture enables sorafenib resistance in Huh7 cells through HGF/c-Met/Akt and Jak2/Stat3 pathways. BioMed Res Int, 2014: 764981. 10.1155/2014/764981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chien LH, Huang BM, et al. , 2016. Aqueous extracts of Toona sinensis leaves inhibit renal carcinoma cell growth and migration through JAK2/stat3, Akt, MEK/ERK, and mTOR/HIF-2α pathways. Nutr Cancer, 68(4): 654-666. 10.1080/01635581.2016.1158292 [DOI] [PubMed] [Google Scholar]
- Colmegna B, Morosi L, D'Incalci M, 2018. Molecular and pharmacological mechanisms of drug resistance: an evolving paradigm. Handb Exp Pharmacol, 249: 1-12. 10.1007/164_2017_20 [DOI] [PubMed] [Google Scholar]
- Deer EL, González-Hernández J, Coursen JD, et al. , 2010. Phenotype and genotype of pancreatic cancer cell lines. Pancreas, 39(4): 425-435. 10.1097/MPA.0b013e3181c15963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci U, Wang M, Hu JC, 2020. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells, 9(1): 198. 10.3390/cells9010198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezerskyte M, Paredes JA, Malvezzi S, et al. , 2018. O 6-methylguanine-induced transcriptional mutagenesis reduces p53 tumor-suppressor function. Proc Natl Acad Sci USA, 115(18): 4731-4736. 10.1073/pnas.1721764115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber DE, 2008. Targeted therapies: a new generation of cancer treatments. Am Fam Physician, 77(3): 311-319. [PubMed] [Google Scholar]
- Grimaldi AM, Simeone E, Festino L, et al. , 2017. MEK inhibitors in the treatment of metastatic melanoma and solid tumors. Am J Clin Dermatol, 18(6): 745-754. 10.1007/s40257-017-0292-y [DOI] [PubMed] [Google Scholar]
- Groner B, von Manstein V, 2017. Jak Stat signaling and cancer: opportunities, benefits and side effects of targeted inhibition. Mol Cell Endocrinol, 451: 1-14. 10.1016/j.mce.2017.05.033 [DOI] [PubMed] [Google Scholar]
- Guo YJ, Pan WW, Liu SB, et al. , 2020. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp Ther Med, 19(3): 1997-2007. 10.3892/etm.2020.8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata E, Sahai E, 2017. Tumor microenvironment and differential responses to therapy. Cold Spring Harb Perspect Med, 7(7): a026781. 10.1101/cshperspect.a026781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohan C, van Schaeybroeck S, Longley DB, et al. , 2013. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer, 13(10): 714-726. 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- Hua F, Li K, Shang S, et al. , 2019. Immune signaling and autophagy regulation. Adv Exp Med Biol, 1206: 551-593. 10.1007/978-981-15-0602-4_26 [DOI] [PubMed] [Google Scholar]
- Liu QQ, Zeng XL, Guan YL, et al. , 2020. Verticillin A inhibits colon cancer cell migration and invasion by targeting c-Met . J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(10): 779-795. 10.1631/jzus.B2000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierjohann S, 2017. Crosstalk signaling in targeted melanoma therapy. Cancer Metastasis Rev, 36(1): 23-33. 10.1007/s10555-017-9659-z [DOI] [PubMed] [Google Scholar]
- Meyer SC, 2017. Mechanisms of resistance to JAK2 inhibitors in myeloproliferative neoplasms. Hematol Oncol Clin North Am, 31(4): 627-642. 10.1016/j.hoc.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Mizukami T, Togashi Y, Sogabe S, et al. , 2015. EGFR and HER2 signals play a salvage role in MEK1-mutated gastric cancer after MEK inhibition. Int J Oncol, 47(2): 499-505. 10.3892/ijo.2015.3050 [DOI] [PubMed] [Google Scholar]
- Mokhtari RB, Homayouni TS, Baluch N, et al. , 2017. Combination therapy in combating cancer. Oncotarget, 8(23): 38022-38043. 10.18632/oncotarget.16723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie YZ, Wu KC, Yu J, et al. , 2017. A global burden of gastric cancer: the major impact of China. Expert Rev Gastroenterol Hepatol, 11(7): 651-661. 10.1080/17474124.2017.1312342 [DOI] [PubMed] [Google Scholar]
- Pardanani A, Harrison C, Cortes JE, et al. , 2015. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol, 1(5): 643-651. 10.1001/jamaoncol.2015.1590 [DOI] [PubMed] [Google Scholar]
- Pencik J, Pham HTT, Schmoellerl J, et al. , 2016. JAK-STAT signaling in cancer: from cytokines to non-coding genome. Cytokine, 87: 26-36. 10.1016/j.cyto.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhoseingholi MA, Vahedi M, Baghestani AR, 2015. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench, 8(1): 19-27. [PMC free article] [PubMed] [Google Scholar]
- Ramamonjisoa N, Ackerstaff E, 2017. Characterization of the tumor microenvironment and tumor‒stroma interaction by non-invasive preclinical imaging. Front Oncol, 7: 3. 10.3389/fonc.2017.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samatar AA, Poulikakos PI, 2014. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov, 13(12): 928-942. 10.1038/nrd4281 [DOI] [PubMed] [Google Scholar]
- Schizas D, Charalampakis N, Kole C, et al. , 2020. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev, 86: 102016. 10.1016/j.ctrv.2020.102016 [DOI] [PubMed] [Google Scholar]
- Thambi P, Sausville EA, 2002. STI571 (imatinib mesylate): the tale of a targeted therapy. Anticancer Drugs, 13(2): 111-114. 10.1097/00001813-200202000-00001 [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Gotlib J, Mesa RA, et al. , 2017. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol, 10: 156. 10.1186/s13045-017-0527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Chory J, 2011. Crosstalk in cellular signaling: background noise or the real thing? Dev Cell, 21(6): 985-991. 10.1016/j.devcel.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Wu CF, Rajasekaran N, et al. , 2018. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem, 51(6): 2647-2693. 10.1159/000495956 [DOI] [PubMed] [Google Scholar]
- Wu QY, Ma MM, Fu L, et al. , 2018. Roles of germline JAK2 activation mutation JAK2 V625F in the pathology of myeloproliferative neoplasms. Int J Biol Macromol, 116: 1064-1073. 10.1016/j.ijbiomac.2018.05.120 [DOI] [PubMed] [Google Scholar]
- Wu QY, Ma MM, Zhang S, et al. , 2019. Disruption of R867 and Y613 interaction plays key roles in JAK2 R867Q mutation caused acute leukemia. Int J Biol Macromol, 136: 209-219. 10.1016/j.ijbiomac.2019.06.068 [DOI] [PubMed] [Google Scholar]
- Xin P, Xu XY, Deng CJ, et al. , 2020. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol, 80: 106210. 10.1016/j.intimp.2020.106210 [DOI] [PubMed] [Google Scholar]
- Zhang JY, Tian XJ, Xing JH, 2016. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J Clin Med, 5(4): 41. 10.3390/jcm5040041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang Y, Diamond S, et al. , 2016. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke’s encephalopathy. Drug Metabo Dispos, 42(10): 1656-1662. 10.1124/dmd.114.058883 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.