Abstract
目的
研究木犀草素(LUT)对镉(Cd)诱导的人肺上皮Beas-2B细胞损伤的保护作用。
方法
用不同浓度的木犀草素(0~ 160 μmol/L)或镉(0 ~40 μmol/L)处理Beas-2B细胞24 h,用MTT法检测细胞活性。用木犀草素(0.25、0.50和0.75 μmol/L)和/或镉(5 μmol/L)处理Beas-2B细胞24 h,用MTT法检测细胞活性;用LD-L微板法检测细胞乳酸脱氢酶(LDH)活性;用Hoechst荧光染色法观察细胞核形态变化;用DCFH-DA法测定细胞ROS水平;用WST-8法测定细胞SOD水平;用TBA法测定细胞GSH水平;用DTNB法测定细胞MDA水平,用Western blot检测细胞Akt、p-Akt和核因子E2相关因子2(Nrf2)蛋白的表达水平。
结果
木犀草素在一定浓度范围内(0~80 μmol/L)不影响Beas-2B细胞的存活率(P>0.05),镉在5 μmol/L浓度水平时即可显著抑制Beas-2B细胞的生长(P < 0.05),半数抑制浓度为24.6 μmol/L。木犀草素干预可不同程度地减轻镉处理引起的Beas-2B细胞活力的降低(P < 0.05),减少镉处理组细胞LDH的释放量(P < 0.05),改善镉处理组细胞的凋亡情况,抑制镉处理组细胞ROS水平的升高(P < 0.05),增强镉处理组细胞SOD活性(P < 0.05)和GSH含量(P < 0.05),减少镉处理组细胞MDA的产生(P < 0.05)。此外,木犀草素(0.5和0.75 μmol/L)干预可上调镉处理组细胞p-Akt和Nrf2的蛋白表达水平(P < 0.05)。
结论
木犀草素可显著减轻镉诱导的Beas-2B细胞的损伤,其机制可能与p-Akt和Nrf2蛋白表达水平的上调有关。
Keywords: 木犀草素, 镉, 肺细胞, 氧化应激
Abstract
Objective
To investigate the protective effect of luteolin against cadmium (Cd)-induced injury in human lung epithelial Beas-2B cells.
Methods
Beas-2B cells were treated with different concentrations of luteolin (0-160 μmol/L) or Cd (0-40 μmol/L) for 24 h, and the cell viability was examined using MTT assay. After treatment with luteolin (0.25, 0.5 and 0.75 μmol/L) with or without Cd (5 μmol/L) for 24 h, the cells were examined for viability, lactate dehydrogenase (LDH) activity and morphological changes of the cell nuclei using Hoechst fluorescent staining. The levels of ROS, SOD, GSH and MDA in the treated cells were detected, and the expression levels of Akt, p-Akt and nuclear factor E2-related factor 2 (Nrf2) proteins were determined using Western blotting.
Results
Luteolin within the concentration range of 0-80 μmol/L did not significantly affect the survival rate of Beas-2B cells (P>0.05), but Cd at 5 μmol/L significantly decreased the cell viability (P < 0.05) with an IC50 of 24.6 μmol/L. In Cd-treated cells, treatment with luteolin significantly mitigated the decrease of cell viability, reduced LDH release and cell apoptosis, enhanced SOD activity and GSH content, and inhibited the production of MDA and ROS (all P < 0.05). Luteolin also significantly up-regulated the expression levels of p-Akt and Nrf2 protein in Cd-treated Beas-2B cells (P < 0.05).
Conclusion
Luteolin has a significant protective effect against Cd-induced injury in Beas-2B cells, and the effects are probably mediated, at least in part, by promoting the activation of Akt and Nrf2.
Keywords: luteolin, cadmium, lung cells, oxidative stress
近年来,随着各种工业废物排放量的增加,以及化肥和农药的广泛使用,空气、水和土壤等环境污染问题层出不穷。镉(Cd)是广泛存在于环境中的一种有毒重金属,可通过多种途径进入人体,并蓄积于人体的肺、肝脏和肾脏等组织和器官,在体内的生物半衰期长达15~ 30年[1]。吸烟和镉污染的室内可吸入颗粒物吸入是人类接触镉的主要途径之一,吸入的镉主要经肺吸收,肺组织更易受到含镉气溶胶的损害[2]。镉暴露可诱导小鼠肺炎症发生,诱导大鼠肺原代细胞炎症细胞因子IL-6和IL-8分泌的增加[3-4]。镉在肺组织的蓄积可导致多种具有慢性炎症特征的肺部疾病的发生,如慢性阻塞性肺病、肺气肿和肺癌等[5-7]。
目前,对于镉中毒的处理主要是采用螯合疗法,但是螯合剂本身存在一些安全性和有效性问题,对机体的副作用大[8]。寻找预防镉中毒的方法以大幅度降低镉中毒的发生率成为迫切需要解决的一个问题。天然植物因其所具有的高效、低毒和易得性成为近年来的一个研究热点。有人尝试用从植物中提取得到的天然活性成分对镉暴露引起的肺部损伤进行预防。如姜黄素可阻止镉诱导的人体气道上皮HBE细胞IL-6和IL-8的分泌,预防镉吸入引起的气道炎症的发生[9]。葫芦巴叶提取物可以保护大鼠肝上皮细胞免受镉诱导的损伤[10]。水飞蓟素可以改善小鼠体内镉诱导的氧化应激,并且增强小鼠的抗氧化防御能力[11]。适量添加维生素C可对大鼠镉暴露所致的肺组织损伤产生较强的保护作用[12]。适量摄入具有抗炎、抗凋亡、抗癌活性的天然植物活性成分预防镉诱导的肺部损伤的发生发展将是一个可行的策略。
木犀草素(LUT)是一种黄酮类化合物,广泛存在于药用植物、蔬菜和水果中,比如金银花、菊花、芹菜、花椰菜、卷心菜、胡萝卜和石榴等,具有抗氧化、抗癌、抗炎、抗凋亡和神经保护等多种药理作用[13-16],多用于心血管疾病、神经系统疾病和癌症等疾病的治疗,应用前景广阔[17-18]。有研究显示,木犀草素可减轻过氧化氢诱导的PC12细胞活力丧失、氧化应激增加和细胞凋亡[19],缓解LPS诱导的小鼠支气管肺炎损伤[20],显著下调包括镉在内的重金属混合物所诱导的HL7702肝细胞的死亡[21]。基于此,LUT干预应该能够用来预防镉暴露引起的肺部损伤,但用LUT干预预防镉暴露所致肺损伤的研究至今还未见报道。因此,本研究以永生化的人肺上皮Beas-2B细胞为研究对象,观察LUT干预在镉暴露诱导的肺细胞损伤中发挥的作用,并对其作用机制进行初步分析,为研发安全可靠的防治镉致肺损伤的方法提供一定的理论依据。
1. 材料和方法
1.1. 材料和试剂
永生化人支气管肺上皮Beas-2B细胞株(ATCC);6孔、96孔细胞培养板和10 cm细胞培养皿(Corning Costar)。DMEM细胞培养基(Gibco)和胎牛血清(Clark)。木犀草素和氯化镉(sigma)。MTT和DMSO (Solarbio)。乳酸脱氢酶(LDH)测定试剂盒(建成生物)。活性氧检测试剂盒、总SOD活性检测试剂盒(WST-8法)、GSH和GSSG检测试剂盒、脂质氧化(MDA)检测试剂盒和细胞凋亡-Hoechst染色试剂盒(碧云天)。单克隆抗体Akt和p-Akt(CST);Nrf2 (abcam);β-actin(proteintech);辣根过氧化物酶(HRP) 耦联的羊抗兔IgG和羊抗鼠IgG二抗(CST)。Western及IP细胞裂解液、苯甲基磺酰氟(PMSF)、BCA蛋白浓度测定试剂盒和超特敏ECL化学发光试剂盒(碧云天)。
1.2. 细胞培养
Beas-2B细胞在37 ℃,5% CO2条件下,用含5% 胎牛血清+1% 抗生素(100 U/mL青霉素+100 µg/mL链霉素)的DMEM培养基培养。
1.3. 细胞增殖实验
将处于对数生长期的细胞接种于96孔板中(3×104/mL),培养24 h后,用不同浓度的镉(0、1.25、2.5、5、10、20和40 µmol/L)和LUT(0、10、20、40、80和160 µmol/L)处理24h,或用不同浓度的木犀草素(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L的镉处理24 h,每个浓度设6个平行孔。终止培养前每孔加入10 μL MTT溶液(5 mg/mL),继续培养4 h后吸去上清液,每孔加入100 μL DMSO,于漩涡振荡器上振摇10 min,用酶标仪测量490 nm波长处的吸光度(A)值,并计算各处理组细胞的相对存活率。细胞相对存活率=实验组A值/对照组A值×100%[22]。实验重复3次。
1.4. 乳酸脱氢酶活性的测定
取对数生长期细胞接种于96孔板中(3×104/mL),培养24 h后,用不同浓度的LUT(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L镉处理24 h。收集各处理组细胞的上清,按LD-L微板法进行乳酸脱氢酶(LDH) 活性检测。
1.5. Hoechst 33258染色
取对数生长期细胞接种于6孔板中(1×106/mL),培养过夜,待细胞贴壁,用不同浓度的LUT(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L的镉处理24 h。弃去原培养液,每孔加入1 mL固定液固定10 min;弃去固定液,每孔加入1 mL Hoechst 33258染色液染色5 min,于倒置荧光显微镜下以40倍镜观察各处理组细胞细胞核染色情况并拍照。
1.6. 活性氧(ROS)检测
将处于对数生长期的细胞接种于6孔板中(1× 106/mL),培养24 h后,用不同浓度的木犀草素(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L镉处理24 h。用DCFH-DA法测定细胞中的ROS。即去除细胞培养液,每孔加入1 mL稀释好的DCFH-DA(1 μL DCFH-DA:1000 μL无血清培养液),37 ℃细胞培养箱内孵育20 min;用无血清细胞培养液洗涤细胞3次,胰酶消化,收集各处理组细胞,300目筛网过滤后于流式细胞仪处检测ROS。
1.7. 抗氧化能力检测
将处于对数生长期的细胞接种于6孔板中(1× 106/mL),培养24 h后,用不同浓度的木犀草素(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L镉处理24 h。分别用WST-8法、DTNB法和TBA法测定各处理组细胞中超氧化物歧化酶(SOD)、还原型谷胱甘肽(GSH)和丙二醛(MDA)的水平。
1.8. Western blot实验
将处于对数生长期的细胞以合适密度接种于10 cm培养皿中,培养24 h后,用不同浓度的LUT(0、0.25、0.5和0.75 µmol/L)和/或5 µmol/L镉处理24 h。弃去原培养液,PBS清洗2遍,加入适量冰上溶解的混有PMSF的Western及IP细胞裂解液(1∶10),用细胞刮刀收集各理组细胞,冰上裂解30 min,4 ℃,12 000 r/min离心,30 min。取上清液,用BCA蛋白定量法测定各处理组蛋白的浓度。每泳道取40 μg总蛋白上样,经10% 的SDSPAGE凝胶垂直电泳分离蛋白,然后将其转移到PVDF膜上。封闭液室温封闭2 h后,将膜用Akt(1∶1000)、p-Akt (1∶1000)、Nrf2(1∶500)和β-actin(1∶20 000)抗体4 ℃孵育过夜。TBST充分洗膜后,用HRP标记的羊抗兔IgG和羊抗鼠IgG二抗(1∶2000)室温孵育1 h,TBST充分洗膜后用化学发光仪器(BioRad)曝光并拍照,实验重复3次。条带灰度值用Image J软件分析。
1.9. 统计分析
采用SPSS 25.0软件进行统计学分析,所得实验数据用均数±标准差表示,组间比较采用单因素方差分析,两两比较用LSD法,以P < 0.05为差异有统计学意义。
2. 结果
2.1. LUT抑制镉诱导的Beas-2B细胞活力降低
同对照组相比,5 µmol/L及以上浓度的镉显著降低了细胞存活率(5 µmol/L处理组P=0.003,10、20、40 µmol/L处理组均P < 0.001,图 1A),且呈浓度依赖性。镉作用于Beas-2B细胞24 h的半数抑制浓度(IC50) 为24.6 µmol/L。Beas-2B细胞对LUT的毒性不敏感,同对照组相比,0 ~80 µmol/L LUT单独处理Beas-2B细胞24 h不影响细胞活力(P>0.05,图 1B)。用不同浓度的LUT(0.25、0.5和0.75 µmol/L)同镉(5 µmol/L)共同处理Beas-2B细胞24 h,同对照组相比,镉处理组细胞存活率明显下降(P < 0.001)。与镉处理组相比,LUT干预组(LUT同镉共同处理组)的细胞活力得到提升,分别提高了(1.9±3.2)%、(4.8±3.7)% 和(3.6±2.2)%,表明LUT干预可以减轻镉对Beas-2B细胞活性的抑制(图 1C)。
1.

镉和LUT对Beas-2B细胞活性的影响
The effect of Cd and luteolin (LUT) on viability of Beas-2B cells. A: Effect of different concentrations of Cd on Beas-2B cell viability; B: Effect of different concentrations of LUT on Beas-2B cell viability; C: Effect of Cd and LUT, alone or in combination, on Beas-2B cell viability. **P < 0.01, ***P < 0.001 vs control group, #P < 0.05 vs Cd group.
2.2. LUT抑制镉诱导Beas-2B细胞LDH活性的升高
同对照组相比,镉处理组细胞LDH活性明显升高(P < 0.001),而LUT单独处理组细胞的LDH活性相对较低(P < 0.001)。与镉处理组相比,LUT干预组细胞LDH活性显著下降(P < 0.001,图 2)。
2.

Luteolin对镉诱导Beas-2B细胞LDH活性的影响
Effect of Luteolin and/ or Cd on LDH activity in Beas-2B cells. ***P < 0.001 vs control group, ###P < 0.001 vs Cd group.
2.3. LUT抑制镉诱导Beas-2B细胞凋亡
经Hoechst 33258染色后,对照组(图 3A)细胞形态完整,细胞核被染成均一的淡蓝色。与对照组相比,镉处理组(图 3E)细胞核被染为亮蓝色,出现了新月形的细胞核,并且伴随有细胞核固缩、碎裂,染色质凝聚的现象;而单独用LUT处理组(图 3B~D)细胞核形态未见改变。与镉处理组相比,LUT干预组内(图 3F~H)细胞核形态改变情况得到了不同程度的改善,且细胞核固缩和染色质凝聚的现象随着LUT浓度的增加明显减少。
3.
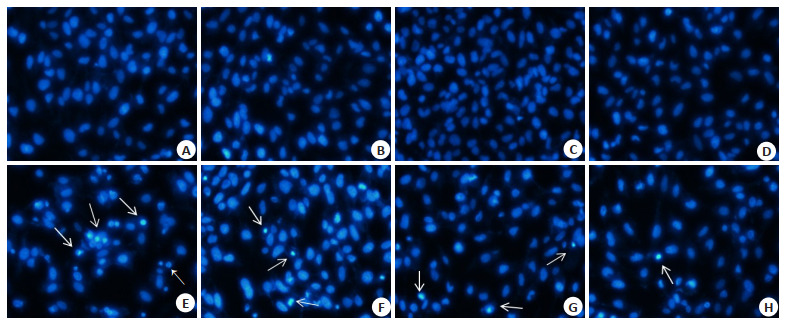
Luteolin对镉诱导Beas-2B细胞凋亡的影响
Effect of Luteolin and/ or Cd on apoptosis of Beas-2B cells (Original magnification: × 400). A: control group; B: 0.25 µmol/L Luteolin group; C: 0.5 µmol/L Luteolin group; D: 0.75 µmol/L Luteolin group; E: 5 µmol/L Cd group; F: 0.25 µmol/L Luteolin+5 µmol/L Cd group; G: 0.5 µmol/L Luteolin+5 µmol/L Cd group; H: 0.75 µmol/L Luteolin+5 µmol/L Cd group. White arrow: Nucleus pycnosis.
2.4. LUT抑制镉诱导Beas-2B细胞产生ROS
数据分析结果如图 4B所示,与对照组相比,0.75 µmol/L LUT处理组细胞内ROS水平略有上升(P= 0.003),镉处理组细胞内ROS水平显著升高(P < 0.001)。与镉处理组相比,LUT干预组内ROS水平明显下降(P < 0.001)。
4.

Luteolin对镉诱导Beas-2B细胞ROS的影响
Effect of Luteolin and/ or Cd on ROS content in Beas-2B cells measured by DCFH-DA. ***P < 0.001 vs control group, **P < 0.01 vs control group, ###P < 0.001 vs Cd group.
2.5. LUT对镉诱导Beas-2B细胞抗氧化能力的影响
镉处理组细胞SOD活性和GSH水平明显低于对照组(P=0.008,P=0.009),MDA水平明显高于对照组(P < 0.001);LUT单独处理组细胞SOD活性有上升的趋势(P=0.03,P < 0.001,P=0.04),GSH水平显著升高(P= 0.001,P < 0.001,P=0.004)。同镉处理组相比,LUT干预组细胞内SOD活性和GSH水平均明显增高(SOD:P < 0.001,P=0.002,P=0.02;GSH:P=0.02,P=0.003,P < 0.001),MDA水平显著下降(P < 0.001)。
5.

Luteolin对镉诱导Beas-2B细胞抗氧化物质的影响
Effect of Luteolin and/ or Cd on SOD activity (A), GSH content (B) and MDA level (C) in Beas-2B cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs Cd group.
2.6. LUT对镉诱导Beas-2B细胞内Akt、p-Akt和Nrf2蛋白质表达水平的调控
镉和LUT处理Beas-2B细胞可以引起胞内p-Akt和Nrf2蛋白表达水平发生改变。如图 6所示,同对照组相比,镉可引起Nrf2蛋白表达水平的下调,p-Akt/Akt比值的下降。与镉处理组相比,LUT干预组细胞内p-Akt和Nrf2蛋白表达水平出现上调。
6.

Luteolin对镉诱导Beas-2B细胞Akt、p-Akt和Nrf2蛋白表达的影响
Effect of Luteolin on Akt, p-Akt (A) and Nrf2 (B) protein expression in Cd-treated Beas-2B cells. *P < 0.05, **P < 0.01 vs control group, #P < 0.05, ##P < 0.01 vs Cd group.
3. 讨论
通过饮食摄入天然来源(水果和蔬菜)的生物活性化合物对包括癌症在内的多种人类疾病的预防作用已经得到公认[13]。具有高效低毒等特点的黄酮类化合物是一类备受关注的天然活性产物[23]。研究表明,某些类黄酮可拮抗多种疾病的进展[24-25]。LUT是一种常见的膳食黄酮类化合物,广泛分布于多种植物中,药理研究表明其具有多种有益活性[14-16]。有研究报道,LUT可抑制多种重金属(锌、铅、铜、镉、汞、镍)对HL7702肝细胞的联合毒性,减少细胞死亡,抑制细胞凋亡[21]。本研究利用肺上皮Beas-2B细胞为研究对象,也得出了类似的结果。为了探讨LUT对镉致Beas-2B细胞损伤的影响,本研究先分别检测了镉和LUT对Beas-2B细胞活性的影响。发现浓度水平在5 µmol/L的镉即可明显抑制Beas-2B细胞的活性,而LUT的浓度水平高达160 µmol/L时才会对Beas-2B细胞的存活率产生影响。在这里,本研究选取了低浓度水平的LUT(0.25、0.5和0.75 µmol/L)和刚能引起Beas-2B细胞损伤的浓度水平的镉(5 µmol/L) 同时作用于细胞,发现LUT可明显改善镉对Beas-2B细胞活性的抑制。
LDH是细胞膜完整性的重要指标,当细胞破裂或受损时,细胞膜结构遭到破坏,细胞内的LDH会被释放出来,细胞培养基中LDH活性增加[26]。本研究检测结果表明,镉可显著增加Beas-2B细胞培养液中的LDH活性,LUT干预能显著降低镉处理所致的LDH活性增加。这表明LUT处理可保护Beas-2B细胞不受镉诱导的损伤,使得细胞膜的完整性得以维持。
细胞凋亡在多细胞生物体的发育中起着重要的调控作用。有研究显示,镉可诱导多种细胞的凋亡[10, 27]。本研究通过Hoechst 33258荧光染色发现,镉处理组细胞中出现许多细胞核被染为亮蓝色的细胞,并有细胞核固缩、碎裂,染色质凝聚现象。用LUT干预后,随着干预浓度的增加,干预组细胞的细胞核形态改变情况得到了不同程度的改善,细胞核固缩和染色质凝聚的现象明显减少。这表明LUT干预缓解了镉诱导的Beas-2B细胞凋亡情况,降低了镉诱导的细胞毒性。
外部因素的刺激可引起机体活性氧水平的增加,从而打破机体氧化还原状态的平衡,导致氧化应激的发生[11, 28]。体内外研究表明,氧化应激可能是镉发挥损伤作用的一个关键步骤[11, 29-30]。与此类似,本研究通过流式细胞术实验发现,相比对照组,镉可诱导Beas-2B细胞内ROS水平显著增高。此外,LUT干预组细胞内的ROS水平与镉处理组相比均有显著下降,这表明LUT可能是通过抑制镉诱导的细胞ROS水平升高发挥的细胞保护作用。本研究进一步分析了各处理组细胞中抗氧化物SOD、GSH水平及脂质过氧化产物MDA水平的变化,结果显示,镉降低了SOD活性和GSH含量,并促进了MDA的合成,而LUT干预处理则可显著增加SOD活性和还原性GSH的含量,并减少MDA水平。这暗示了LUT在预防镉诱导的氧化应激中的重要作用,但是具体机制有待进一步研究。
PI3K/Akt信号通路在细胞增殖、分化、凋亡和氧化应激等生物学功能的调节中起着至关重要的作用[31-32]。本研究结果显示,镉处理能显著下调p-Akt的表达,降低p-Akt/Akt的比值。0.5 µmol/L和0.75 µmol/L的LUT干预处理均可减轻镉处理所致的p-Akt表达的抑制,提升p-Akt/Akt的比值。这与Abdelfatteh等和Nazima Bashir等的研究一致[33-34]。核因子E2相关因子2 (Nrf2)是存在于肝脏、肾脏和肺等器官和组织的调节细胞抗氧化酶和多种凋亡蛋白的重要转录因子[35]。Nrf2激活是细胞防御氧化应激的重要调节机制,对人类健康具有重要意义[36]。有研究发现,PKC、PI3K和MAPK (p38、ERK1/2和JNK1/2)信号通路同Nrf2信号通路之间存在交叉激活[28]。Nazima Bashir等[34]有研究也显示葡萄籽原花青素通过激活PI3K/Akt通路调节Nrf2的表达。本研究结果显示,镉可下调Beas-2B细胞中Nrf2蛋白的表达水平;0.5 µmol/L和0.75 µmol/L的LUT处理均可上调镉处理组细胞的Nrf2蛋白表达水平,即LUT处理可缓解镉对细胞Nrf2蛋白表达的抑制。这表明LUT对镉所致Beas-2B细胞损伤的保护作用可能与PI3K/Akt通路和Nrf2通路的激活有关。
综上所述,镉可以促进Beas-2B细胞的氧化损伤和细胞凋亡,而LUT可能通过上调p-Akt和Nrf2蛋白的表达,减轻镉诱导的细胞氧化应激水平,从而缓解镉诱导的Beas-2B细胞损伤。
Biography
储娜,硕士,E-mail: cn1786586642@163.com
Funding Statement
国家自然科学基金(32001159);安徽省高校自然科学研究项目(KJ2019A0316,KJ2019A0332);蚌埠医学院研究生科研创新项目(Byycx1907)
Supported by National Natural Science Foundation of China (32001159)
Contributor Information
储 娜 (Na CHU), Email: cn1786586642@163.com.
王 允 (Yun WANG), Email: wy_sunnyday@126.com.
References
- 1.Odewumi C, Latinwo LM, Sinclair A, et al. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol Med Rep. 2015;12(5):6422–6. doi: 10.3892/mmr.2015.4316. [Odewumi C, Latinwo LM, Sinclair A, et al. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells[J]. Mol Med Rep, 2015, 12(5): 6422-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odewumi CO, Latinwo LM, Ruden ML, et al. Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. Environ Toxicol. 2016;31(11):1612–9. doi: 10.1002/tox.22165. [Odewumi CO, Latinwo LM, Ruden ML, et al. Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells[J]. Environ Toxicol, 2016, 31(11): 1612-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kundu S, Sengupta S, Chatterjee S, et al. Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach. J Inflamm: Lond. 2009;6:19. doi: 10.1186/1476-9255-6-19. [Kundu S, Sengupta S, Chatterjee S, et al. Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach[J]. J Inflamm: Lond, 2009, 6: 19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lag M, Rodionov D, Ovrevik J, et al. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: Expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol Lett. 2010;193(3):252–60. doi: 10.1016/j.toxlet.2010.01.015. [Lag M, Rodionov D, Ovrevik J, et al. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: Expression and release of cytokines in fibroblasts, epithelial cells and macrophages[J]. Toxicol Lett, 2010, 193(3): 252-60.] [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, Liu Y, Xie C, et al. Cadmium induces histone H3 lysine methylation by inhibiting histone demethylase activity. Toxicol Sci. 2015;145(1):80–9. doi: 10.1093/toxsci/kfv019. [Xiao C, Liu Y, Xie C, et al. Cadmium induces histone H3 lysine methylation by inhibiting histone demethylase activity[J]. Toxicol Sci, 2015, 145(1): 80-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson D. Cadmium lung adsorption, citrullination and an enhanced risk of COPD. Eur Respir Rev. 2018;27(149):180054. doi: 10.1183/16000617.0054-2018. [Hutchinson D. Cadmium lung adsorption, citrullination and an enhanced risk of COPD[J]. Eur Respir Rev, 2018, 27(149): 180054.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33(4):681–703. doi: 10.1016/j.ccm.2012.07.001. [Field RW, Withers BL. Occupational and environmental causes of lung cancer[J]. Clin Chest Med, 2012, 33(4): 681-703.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, et al. Cadmium toxicity and treatment: an update. http://www.ncbi.nlm.nih.gov/pubmed/28932363. Caspian J Intern Med. 2017;8(3):135–45. doi: 10.22088/cjim.8.3.135. [Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, et al. Cadmium toxicity and treatment: an update[J]. Caspian J Intern Med, 2017, 8(3): 135-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rennolds J, Malireddy S, Hassan F, et al. Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium. Biochem Biophys Res Commun. 2012;417(1):256–61. doi: 10.1016/j.bbrc.2011.11.096. [Rennolds J, Malireddy S, Hassan F, et al. Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium[J]. Biochem Biophys Res Commun, 2012, 417(1): 256-61.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odewumi C, Latinwo LM, Lyles RL, et al. Comparative whole genome transcriptome analysis and fenugreek leaf extract modulation on cadmium-induced toxicity in liver cells. http://www.ncbi.nlm.nih.gov/pubmed/29749534. Int J Mol Med. 2018;42(2):735–44. doi: 10.3892/ijmm.2018.3669. [Odewumi C, Latinwo LM, Lyles RL, et al. Comparative whole genome transcriptome analysis and fenugreek leaf extract modulation on cadmium-induced toxicity in liver cells[J]. Int J Mol Med, 2018, 42(2): 735-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farjad E, Momeni HR. Silymarin ameliorates oxidative stress and enhances antioxidant defense system capacity in cadmium-treated mice. http://europepmc.org/abstract/MED/29845797. Cell J. 2018;20(3):422–6. doi: 10.22074/cellj.2018.5355. [Farjad E, Momeni HR. Silymarin ameliorates oxidative stress and enhances antioxidant defense system capacity in cadmium-treated mice[J]. Cell J, 2018, 20(3): 422-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Refaiy AI, Eissa FI. Histopathology and cytotoxicity as bio-markers in treated rats with cadmium and some therapeutic agents. Saudi J Biol Sci. 2013;20(3):265–80. doi: 10.1016/j.sjbs.2013.02.004. [El-Refaiy AI, Eissa FI. Histopathology and cytotoxicity as bio-markers in treated rats with cadmium and some therapeutic agents[J]. Saudi J Biol Sci, 2013, 20(3): 265-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review[J]. Biomed Pharmacother, 2019, 112: 108612.] [DOI] [PubMed] [Google Scholar]
- 14.Aziz N, Kim MY, Cho JY. Antiinflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–58. doi: 10.1016/j.jep.2018.05.019. [Aziz N, Kim MY, Cho JY. Antiinflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies[J]. J Ethnopharmacol, 2018, 225: 342-58.] [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Shang P, Li D. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol. 2017;8:692. doi: 10.3389/fphar.2017.00692. [Luo Y, Shang P, Li D. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms[J]. Front Pharmacol, 2017, 8: 692.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SC, Chen PJ, Chang SH, et al. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem Pharmacol. 2018;154:384–96. doi: 10.1016/j.bcp.2018.06.003. [Yang SC, Chen PJ, Chang SH, et al. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity[J]. Biochem Pharmacol, 2018, 154: 384-96.] [DOI] [PubMed] [Google Scholar]
- 17.Maaliki D, Shaito AA, Pintus G, et al. Flavonoids in hypertension: a brief review of the underlying mechanisms. Curr Opin Pharmacol. 2019;45:57–65. doi: 10.1016/j.coph.2019.04.014. [Maaliki D, Shaito AA, Pintus G, et al. Flavonoids in hypertension: a brief review of the underlying mechanisms[J]. Curr Opin Pharmacol, 2019, 45: 57-65.] [DOI] [PubMed] [Google Scholar]
- 18.Wu HT, Lin J, Liu YE, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and meta-stasis by epigenetic regulation of MMP9 expression via the AKT/ mTOR signaling pathway. Phytomedicine. 2021;81:153437. doi: 10.1016/j.phymed.2020.153437. [Wu HT, Lin J, Liu YE, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and meta-stasis by epigenetic regulation of MMP9 expression via the AKT/ mTOR signaling pathway[J]. Phytomedicine, 2021, 81: 153437.] [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Xu P, Yu H, et al. Luteolin protects PC-12 cells from H2O2-induced injury by up-regulation of microRNA-21. Biomed Pharmacother. 2019;112:108698. doi: 10.1016/j.biopha.2019.108698. [Zhang Z, Xu P, Yu H, et al. Luteolin protects PC-12 cells from H2O2-induced injury by up-regulation of microRNA-21[J]. Biomed Pharmacother, 2019, 112: 108698.] [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Meng J. Luteolin alleviates LPS-induced bronchopneumonia injury in vitro and in vivo by down-regulating microRNA-132 expression. Biomed Pharmacother. 2018;106:1641–9. doi: 10.1016/j.biopha.2018.07.094. [Liu X, Meng J. Luteolin alleviates LPS-induced bronchopneumonia injury in vitro and in vivo by down-regulating microRNA-132 expression[J]. Biomed Pharmacother, 2018, 106: 1641-9.] [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Su H, Song X, et al. Luteolin inhibits multi-heavy metal mixture-induced HL7702 cell apoptosis through downregulation of ROS-actived mitochondrial pathway. http://www.ncbi.nlm.nih.gov/pubmed/?term=Luteolin+inhibits+multi-heavy+metal+mixture-induced+HL7702+cell+apoptosis+through+downregulation+of+ROS-activated+mitochondrial+pathway. Int J Mol Med. 2018;41(1):233–41. doi: 10.3892/ijmm.2017.3219. [Wang Y, Su H, Song X, et al. Luteolin inhibits multi-heavy metal mixture-induced HL7702 cell apoptosis through downregulation of ROS-actived mitochondrial pathway[J]. Int J Mol Med, 2018, 41 (1): 233-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Wu F, Jin W, et al. Theabrownin inhibits cell cycle progression and tumor growth of lung carcinoma through c-myc-related mechanism. http://pubmedcentralcanada.ca/pmcc/articles/PMC5326752/ Front Pharmacol. 2017;8:75. doi: 10.3389/fphar.2017.00075. [Zhou L, Wu F, Jin W, et al. Theabrownin inhibits cell cycle progression and tumor growth of lung carcinoma through c-myc-related mechanism[J]. Front Pharmacol, 2017, 8: 75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Disco-very and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–614. doi: 10.1016/j.biotechadv.2015.08.001. [Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Disco-very and resupply of pharmacologically active plant-derived natural products: a review[J]. Biotechnol Adv, 2015, 33(8): 1582-614.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozłowska A, Szostak-Wegierek D. Flavonoids: food sources and health benefits. http://europepmc.org/abstract/med/25272572. Roczniki Panstwowego Zakl Higieny. 2014;65(2):79–85. [Kozłowska A, Szostak-Wegierek D. Flavonoids: food sources and health benefits[J]. Roczniki Panstwowego Zakl Higieny, 2014, 65 (2): 79-85.] [PubMed] [Google Scholar]
- 25.Kumar AD, Bevara GB, Kaja LK, et al. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H2O2 induced cytotoxicity in lung and liver cells. BMC Complement Altern Med. 2016;16(1):376. doi: 10.1186/s12906-016-1354-z. [Kumar AD, Bevara GB, Kaja LK, et al. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H2O2 induced cytotoxicity in lung and liver cells[J]. BMC Complement Altern Med, 2016, 16(1): 376.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkharashi NAO, Periasamy VS, Athinarayanan J, et al. Cadmium triggers mitochondrial oxidative stress in human peripheral blood lymphocytes and monocytes: Analysis using in vitro and system toxicology approaches. J Trace Elem Med Biol. 2017;42:117–28. doi: 10.1016/j.jtemb.2017.04.014. [Alkharashi NAO, Periasamy VS, Athinarayanan J, et al. Cadmium triggers mitochondrial oxidative stress in human peripheral blood lymphocytes and monocytes: Analysis using in vitro and system toxicology approaches[J]. J Trace Elem Med Biol, 2017, 42: 117-28.] [DOI] [PubMed] [Google Scholar]
- 27.Hossain S, Liu HN, Nguyen M, et al. Cadmium exposure induces mitochondria-dependent apoptosis in oligodendrocytes. http://europepmc.org/abstract/med/19523979. Neuro-toxicology. 2009;30(4):544–54. doi: 10.1016/j.neuro.2009.06.001. [Hossain S, Liu HN, Nguyen M, et al. Cadmium exposure induces mitochondria-dependent apoptosis in oligodendrocytes[J]. Neuro-toxicology, 2009, 30(4): 544-54.] [DOI] [PubMed] [Google Scholar]
- 28.Tu W, Wang H, Li S, et al. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10(3):637–51. doi: 10.14336/AD.2018.0513. [Tu W, Wang H, Li S, et al. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases[J]. Aging Dis, 2019, 10(3): 637-51. .] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeer RS, Soliman D, Kassab RB, et al. Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: Involvement of the Nrf2 pathway. Int J Mol Sci. 2018;19(12):3979. doi: 10.3390/ijms19123979. [Almeer RS, Soliman D, Kassab RB, et al. Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: Involvement of the Nrf2 pathway[J]. Int J Mol Sci, 2018, 19(12): 3979.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SH, Li P, Yu LH, et al. Sulforaphane protect against cadmium-induced oxidative damage in mouse leydigs cells by activating Nrf2/ ARE signaling pathway. Int J Mol Sci. 2019;20(3):630. doi: 10.3390/ijms20030630. [Yang SH, Li P, Yu LH, et al. Sulforaphane protect against cadmium-induced oxidative damage in mouse leydigs cells by activating Nrf2/ ARE signaling pathway[J]. Int J Mol Sci, 2019, 20(3): 630.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang A, Hausenloy DJ, Mocanu MM, et al. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95(3):230–2. doi: 10.1161/01.RES.0000138303.76488.fe. [Tsang A, Hausenloy DJ, Mocanu MM, et al. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway[J]. Circ Res, 2004, 95 (3): 230-2.] [DOI] [PubMed] [Google Scholar]
- 32.Zhang BX, Zhao ZS, Meng XY, et al. Hydrogen ameliorates oxidative stress via PI3K-Akt signaling pathway in UVB-induced HaCaT cells. http://europepmc.org/abstract/MED/29532858. Int J Mol Med. 2018;41(6):3653–61. doi: 10.3892/ijmm.2018.3550. [Zhang BX, Zhao ZS, Meng XY, et al. Hydrogen ameliorates oxidative stress via PI3K-Akt signaling pathway in UVB-induced HaCaT cells[J]. Int J Mol Med, 2018, 41(6): 3653-61.] [DOI] [PubMed] [Google Scholar]
- 33.El Omri A, Han J, Kawada K, et al. Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res. 2012;1437:16–25. doi: 10.1016/j.brainres.2011.12.019. [El Omri A, Han J, Kawada K, et al. Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways[J]. Brain Res, 2012, 1437: 16-25.] [DOI] [PubMed] [Google Scholar]
- 34.Bashir N, Shagirtha K, Manoharan V, et al. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced Testes-toxicity in rat model: implication of PI3K/Akt/Nrf-2 signaling. Biosci Rep. 2019;39(1):BSR20180515. doi: 10.1042/BSR20180515. [Bashir N, Shagirtha K, Manoharan V, et al. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced Testes-toxicity in rat model: implication of PI3K/Akt/Nrf-2 signaling[J]. Biosci Rep, 2019, 39 (1): BSR20180515.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med. 2013;57:119–31. doi: 10.1016/j.freeradbiomed.2012.12.014. [Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance[J]. Free Radic Biol Med, 2013, 57: 119-31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. http://europepmc.org/abstract/med/15706085. Antioxid Redox Signal. 2005;7(3/4):385–94. doi: 10.1089/ars.2005.7.385. [Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation[J]. Antioxid Redox Signal, 2005, 7(3/4): 385-94.] [DOI] [PubMed] [Google Scholar]


