Abstract
目的
探讨DJ-1(Park7)在脑缺血再灌注损伤中的抗氧化作用及相关机制。
方法
构建SD大鼠大脑缺血再灌注损伤模型,将SD大鼠随机分为假手术组、MCAO组、Scramble组和DJ-1 siRNA组、NC组和Overexpression组。通过DJ-1 siRNA干扰片段干扰DJ-1的表达,以及DJ-1过表达腺相关病毒载体过表达DJ-1的表达。通过测定MCAO/R后各组神经功能学评分和脑含水量,同时应用HE和Nissl染色法评估脑组织的形态学变化和皮层梗死区神经元的损伤情况,评价DJ-1对大鼠脑缺血再灌注损伤的神经保护作用。用超氧化物歧化酶(SOD)和丙二醛(MDA)分析脑组织氧化应激状态。免疫印迹法检测脑组织中DJ-1,Nrf2,HO-1以及NQO1的表达水平,以及免疫荧光染色法观察Nrf2的表达及核转位情况,探讨DJ-1减轻大鼠脑缺血再灌注氧化应激损伤的可能机制。
结果
与MCAO组相比,DJ-1 siRNA组的神经功能学评分(P <0.001)、脑含水量(P <0.001)均明显增加; HE和Nissl染色显示脑缺血区神经元损伤进一步加重; SOD含量进一步降低,MDA含量进一步增加(P <0.001);干扰DJ-1后,其蛋白水平明显降低(P=0.003),同时Nrf2及其下游的HO-1和NQO1也明显降低(P <0.001)。而过表达DJ-1以后,其DJ-1 (P=0.006)、Nrf2(P=0.006)及其下游的HO-1(P=0.004)和NQO1明显增加(P=0.014)。
结论
DJ-1作为体内重要的神经保护因子,可以减少大鼠脑缺血再灌注氧化应激损伤,并可能是通过激活Nrf2信号通路来实现的。
Keywords: DJ-1, Nrf2信号通路, 脑缺血-再灌注, 氧化应激
Abstract
Objective
To investigate the antioxidant effect of DJ-1 (Park7) in rats with cerebral ischemia/reperfusion (IR) injury and its potential mechanism.
Methods
A total of 108 SD rats were randomly divided into sham-operated group, middle cerebral artery occlusion (MCAO) group, Scramble group, DJ-1 siRNA group, negative control (NC) group and DJ-1 overexpression group. Except for those in the sham group, all the rats were subjected to MCAO to establish models of cerebral IR injury. In DJ-1 siRNA and DJ-1 overexpression group, a DJ-1 siRNA and an adeno-associated virus vector carrying DJ-1 gene was injected into the lateral ventricle of the rats, respectively. In each group, neurological scores and brain water content were determined after the operation, and pathological changes of the brain tissue and neuronal injury in the cortical infarction area were assessed using HE and Nissl staining. Oxidative stress in the brain tissues was analyzed by detecting superoxide dismutase (SOD) and malondialdehyde (MDA). The expression levels of DJ-1, Nrf2, Ho-1 and NQO1 in the brain tissue were detected with Western blotting, and the expression and nucleation of Nrf2 was determined by immunofluorescence staining.
Results
Compared with those in MCAO group, the neurological scores (P < 0.001) and brain water content (P < 0.001) were significantly increased in DJ-1 siRNA group. Intracerebral injection of DJ-1 siRNA following MCAO obviously aggravated neuron injury in cerebral ischemia region, further reduced SOD activity and increased MDA content (P < 0.001), and significantly lowered the expression levels of Nrf2 and its downstream proteins HO-1 and NQO1 (P < 0.001). Intracerebral injection of the adenoviral vector for DJ-1 (P=0.003) overexpression significantly upregulated the levels of Nrf2 (P=0.006) and its downstream proteins HO-1 (P=0.004) and NQO1 (P=0.014).
Conclusion
As an important neuroprotective factor, DJ-1 alleviates oxidative stress induced by cerebral IR injury in rats by activating the Nrf2 pathway.
Keywords: DJ-1, Nrf2 pathway, cerebral ischemia-reperfusion, oxidative stress
脑缺血再灌注损伤的发生发展是一个复杂的病理生理过程,其中,氧化应激反应与脑缺血再灌注损伤继发性神经细胞死亡密切相关[1-2]。机体内源性抗氧化系统的激活对脑缺血再灌注损伤后神经细胞的存活有至关重要的作用[3-4]。DJ-1(又称PARK7,或者park7),是通过进化选择的多功能蛋白之一,在抗氧化应激、抗凋亡以及信号转导、基因转录、分子伴侣等多方面发挥重要的作用,具高度保守性,在原核细胞和真核细胞中均有表达。DJ-1蛋白以同源二聚体的形式存在,单体呈螺旋片层螺旋的三明治结构,属于Thij/PfpI家族[5-6]。近年来,人们发现DJ-1依靠其良好的抗氧化作用在保护神经细胞方面发挥重要功能[7-8]。DJ-1与帕金森疾病的发病机制密切相关,DJ-1在帕金森疾病中能够保护神经元免受氧化应激损伤[9-10]。最近,DJ-1在缺血性脑卒中的保护作用受到了广泛的研究,但具体的机制仍不清楚。转录因子NF -E2相关因子2(Nrf2)是转录因子家族成员之一,它通过与抗氧化反应元件(ARE)结合作为机体抗氧化防御系统的主调节器[11-13]。
我们之前的研究也已经证实,Nrf2/ARE作为体内重要的抗氧化信号通路,上调Nrf2可以一定程度保护缺血后神经损伤。DJ-1作为抗氧化蛋白,在Nrf2活性调控中发挥重要作用[14-15]。然而,DJ-1是通过上调Nrf2发挥抗氧化保护作用还是通过调节Nrf2的亚细胞定位,促进Nrf2核转移,激活其抗氧化功能,目前尚未有研究报道。本研究旨在探讨DJ-1在脑缺血再灌注氧化应激损伤中的作用及其相关调控机制,为进一步防治脑缺血再灌注氧化应激损伤提供理论依据。
1. 材料和方法
1.1. 试剂
DJ-1干扰片段及其乱序片段均合成于上海吉玛公司。兔抗DJ-1单克隆抗体(1∶2000,Abcam)、兔抗Nrf2多克隆抗体(1∶200,Santa Cruz)、兔抗Nrf2单克隆抗体(1∶50,Abcam)、兔抗HO-1单克隆抗体(1∶500,Cell Signaling Technology)、兔抗NQO1单克隆抗体(1∶500,Abcam)、β-actin抗体(Proteintech)、二抗山羊抗兔IgG和二抗山羊抗鼠IgG(碧云天)。PVDF膜和化学发光液ECL(Millpore)、RIPA裂解液(强)和1%PMSF(碧云天)、SOD和MD检测试剂盒(南京建成)。
1.2. 实验动物及分组
成年雄性SPF级Sprague-Dawley大鼠108只,由重庆医科大学实验动物中心提供,体质量250~280 g。将所有大鼠随机分为假手术组(Sham组,18只)、脑缺血再灌注模型组(MCAO组,30只)、乱序siRNA + MCAO组(Scramble组,18只)、DJ-1 siRNA+MCAO组(DJ-1 siRNA组,18只)、对照AAV+MCAO组(NC组,12只)、DJ-1 AAV+MCAO组(Overexpression组,12只)。其中Sham组仅做手术切口,不插入线栓; MCAO组进行手术造模; Scramble组和DJ-1 siRNA组分别侧脑室注射10 μL的乱序siRNA和DJ-1 siRNA再手术建模; NC组和Overexpression组分别侧脑室注射2 μL的对照AVV和DJ-1 AVV再手术建模。
所有实验均重庆医科大学医学研究动物伦理委员会授权,并按照《实验动物的护理和使用指南》进行操作。所选择的动物品种、等级、数量、规格合适。实验中善待动物,给予麻醉和镇痛处理,实验后给予安乐死,死后动物无害化处理,没有对环境带来危害,实验符合动物实验研究伦理标准。
1.3. 中脑动脉栓塞(MCAO)模型的建立
将SD大鼠麻醉后按既往文献报道[9]的方法进行中脑动脉栓塞(MCAO)模型构建。用3.5%水合氯醛腹腔注射麻醉(350 mg/kg)大鼠。将大鼠仰卧位固定,消毒,颈部正中切口,按照解剖结构依次分离出颈总动脉(CCA)、颈内动脉(ICA)及颈外动脉(ECA)。结扎颈总动脉近心端和颈外动脉,动脉夹暂时夹闭颈内动脉。在颈内动脉远心端剪一小口,插入尼龙线栓,当线栓越过颈总动脉分叉处,继续进线约18~20 mm,可明显感到阻力时,说明线栓已经到位。1 h后,缓慢拔出线栓,恢复血流灌注。Sham组仅做手术切口并不插入线栓。
1.4. 大鼠侧脑室注射干扰片段
MCAO模型前24进行左侧侧脑室注射siRNA片段,其干扰DJ- 1的siRNA片段碱基序列sense:5- CCCAUUGGCUAAGGACAAATT-3;antisense:5-UU UGUCCUUAGCCAAUGGGTT-3,乱序的siRNA片段碱基序列:sense:5-UUCUCCGAACGUGUCACGUT T-3;antisense:5-ACGUGACACGUUCGGAGAATT-3,大鼠腹腔麻醉后用脑立体定位仪固定,以其大脑前囟缝为基准,向后1 mm、向左2 mm定位左侧侧脑室。钻孔,微量针吸取10 μL片段,进针,进针深度为3.5 mm,缓慢注射后再留针10 min。Sham组和MCAO组均只定位及颅骨钻孔,不注射片段。
1.5. 大鼠左侧脑皮质注射DJ-1过表达腺相关病毒载体
MCAO模型前1月进行左侧侧脑室注射DJ-1过表达的腺相关病毒(AAV)载体和不包含序列的腺相关病毒(对照)载体。CDS序列(NM001277249)被用于在大鼠体内过表达DJ-1基因。大鼠腹腔麻醉后用脑立体定位仪固定,以其大脑前囟缝为基准,向前1.0 mm、向左2.0 mm和向后3.0 mm、向左1.5 mm两处定位左侧脑皮质。钻孔,微量针吸取2 μL腺相关病毒,进针,进针深度为1.2 mm,缓慢注射后再留针10 min。
1.6. 神经功能评分
再灌注24 h后,参照Longa[9]的5级4分为评分标准:0分:无明显的神经功能缺损; 1分:大鼠不能完全伸长右侧前肢; 2分行走时向对侧打圈; 3分:行走时向对侧倾倒; 4分:不能自发行走,意识丧失。
1.7. 脑含水量测定
再灌注24 h后,将大鼠处死后断头取脑,去掉小脑和脑干,分离出整个大脑,称质量得湿重。120 ℃恒温干燥箱烘干至重量恒定,称重得干重。脑含水量=(湿重- 干重)/湿重×100%。
1.8. 石蜡切片制备
再灌注24 h后,将大鼠麻醉后用4%多聚甲醛进行心脑灌注,四肢和躯干僵直后,快速断头取脑,将大脑置于4%甲醛中固定。脑组织做冠状切块,将带有脑皮质梗死区的脑块常规脱水后包埋。制作厚度为5 μm的石蜡切片。
1.9. HE染色和Nissl染色
HE染色:脱蜡,进入苏木精染室温下染色10 min,自来水冲洗,盐酸酒精分化10 s,饱和碳酸锂返蓝3 min,反复洗涤。伊红复染,水洗,脱水,透明,封片。Nissl染色:脱蜡,进入10%亚甲蓝溶液室温下染色20 min,蒸馏水洗涤,95%酒精分化,脱水,透明,封片。置于普通光学显微镜观察拍片,计数完整神经元数。
1.10. MDA和SOD测定
再灌注24 h后,生理盐水灌注后断头取脑,分离出大脑皮质缺血区域,称重后放入玻璃匀浆器中,加入9倍量预冷的生理盐水,匀浆充分后2000 r/min低温离心15 min,取上清液。按试剂盒说明书操作并计算。组织中的MDA含量计算公式:组织中的MDA含量(nmol/mg prot)=(测定管吸光度-测定空白管吸光度)/(标准管吸光度-标准测定管吸光度)×标准品浓度(10 nmol/mL)÷待测样本蛋白浓度(mg prot/mL)。组织匀浆中SOD活力计算公式:组织匀浆中SOD活力(U/mgprot)=(对照管吸光度-测定管吸光度)/对照管吸光度÷50%×反应液总体积/取样量(mL)÷组织蛋白含量(mg prot/mL)。
1.11. Western blot检测
再灌注24 h后,快速断头取脑,提取组织总蛋白,采用BCA法测定蛋白浓度。根据所测蛋白浓度计算上样量。配胶SDS凝胶、上样、电泳、切胶、转膜、封闭后,加入稀释好的目的蛋白一抗4 ℃孵育过夜,TBST洗膜后,加入稀释好的目的蛋白的二抗,室温孵育2 h,TBST洗膜后,ECL化学发光检测。运用ImageJ软件分析,目的蛋白的相对表达量=目的蛋白条带吸光度值/内参β-actin条带吸光度值。
1.12. 免疫荧光染色
再灌注24 h后,快速断头取脑,将大脑脱水后制作成冰冻切片。将冰冻切片用预冷4%多聚甲醛固定20 min,PBS漂洗3次,每次5 min。滴加封闭血清(5% FBS+0.01% Triton X-100溶于PBS),37 ℃孵育60 min。封闭完成后,加入稀释好的Nrf2(1∶50,Abcam),4 ℃湿盒过夜,PBS漂洗3次,每次5 min。避光加入红色荧光二抗(1∶200),37 ℃孵育30 minPBS漂洗3次,每次5 min。含DAPI的防荧光淬灭封片剂封片,荧光显微镜下观察。
1.13. 统计学分析
实验结果用均数±标准差表示,采用Prism软件进行统计学分析。组间比较用单因素方差分析,并结合Tukey's检验分析,P < 0.05表示差异具有统计学意义。
2. 结果
2.1. 干扰DJ-1加重脑缺血再灌注后神经功能缺损和脑水肿
大鼠脑MCAO模型后进行神经功能学评分。Sham组无神经功能缺损,评分为0分。与Sham组相比,MCAO组大鼠神经功能学评分增加(P <0.001),干扰DJ-1后,大鼠神经功能学评分进一步增加(P <0.001)。同样,与Sham组相比,MCAO组大鼠脑含水量增加(P <0.001),与MCAO组相比,DJ-1 siRNA组的脑含水量显著加重(P <0.001,图 1)。
1.
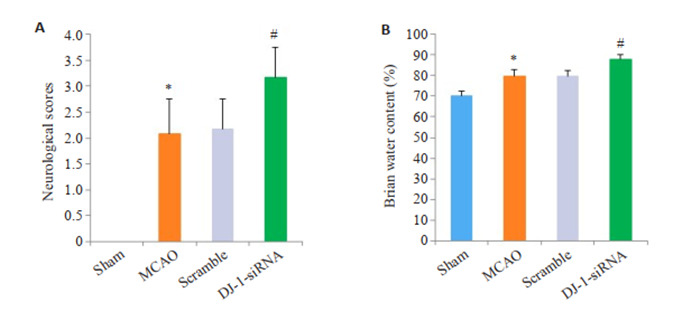
干扰DJ-1基因对神经功能缺损和脑水肿的影响
Effect of DJ-1 interference on neurological deficit scores and brain water content (n=6). A: Neurological deficit scores. B: Brain water content. *P < 0.05 vs Sham group; #P < 0.05 vs MCAO group.
2.2. 干扰DJ-1加重脑I/R后神经细胞损伤
HE染色结果显示,Sham组中大鼠脑组织结构完整,排列规则紧密。与Sham组比较,MCAO组和Scramble组脑组织水肿疏松、细胞排列紊乱,神经元出现坏死、核固缩(P < 0.001)。而DJ-1 siRNA组脑组织水肿更加严重,大量空泡形成,大多数细胞出现核碎裂、核固缩,核溶解,完整细胞数量明显减少(P < 0.001)。Nissl染色结果显示,sham组神经细胞结构清晰,在胞质中尼氏小体均匀分布,呈蓝色颗粒状; MCAO组和Scramble组脑组织水肿疏松,神经元胞浆浓缩,核固缩,尼氏体染色不清,完整神经元数量减少(P < 0.001);干扰DJ-1后,脑组织结构更加疏松,神经元萎缩,核固缩、碎裂,完整神经元数量极少,尼氏体也几乎消失殆尽(P < 0.001)。
2.3. 干扰DJ-1加重脑I/R后脑组织氧化应激水平
MDA和SOD用于评价脑I/R后脑组织氧化应激水平,如图 2所示,与Sham组相比,MCAO组MDA含量明显增加,而SOD活性显著降低(P < 0.001)。Scramble组和MCAO组的SOD和MDA无统计学差异。与MCAO组比,DJ-1 siRNA组的MDA含量进一步增加,而SOD活性进一步降低(P < 0.001)。
2.
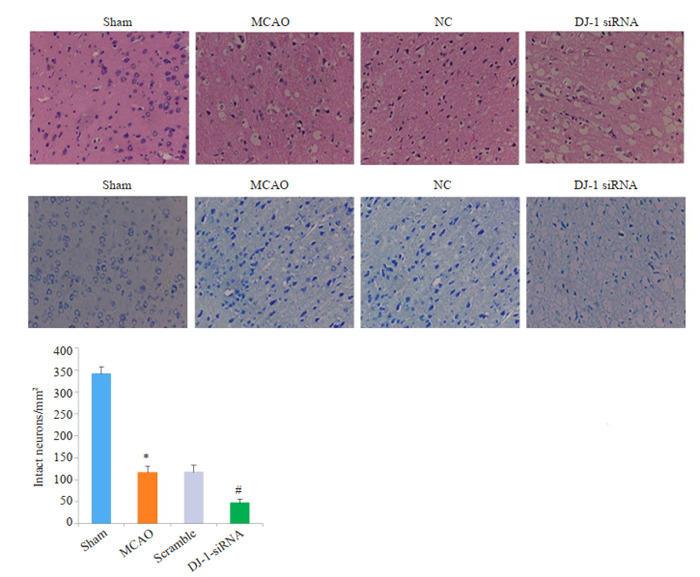
干扰DJ-1基因对脑组织形态学的影响
Histological assessment of the effect of DJ-1 interference using HE staining and Nissl staining (Original magnification: ×400). *P < 0.001 vs Sham group (n=6); #P < 0.001 vs MCAO group (n=6).
2.4. DJ-1促进脑I/R后Nrf2的表达
与Sham组相比,MCAO组的DJ-1表达明显增加,干扰DJ-1后,DJ-1蛋白表达明显下调(P=0.003,图 3A,C)。与Sham组相比,MCAO组的Nrf2蛋白水平明显增加,与MCAO组相比,DJ-1siRNA组的Nrf2蛋白水平明显降低(P <0.001)。与MCAO组相比,Overexpression组的DJ-1表达明显增加(P=0.006,图 3B、E)。同样,过表达DJ-1后,Nrf2蛋白水平明显增加(P=0.006)。
3.
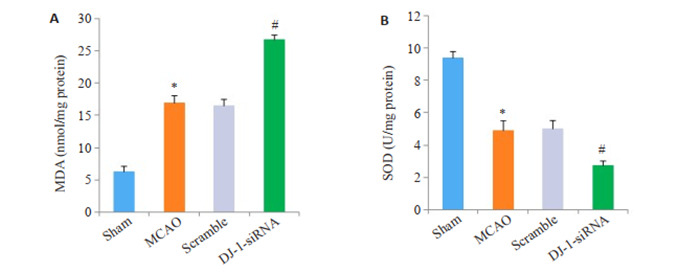
干扰DJ-1基因对MDA含量和SOD活性的影响
Effects of DJ-1 interference on MDA content (A) and SOD activity (B). *P < 0.001 vs Sham group (n=6); #P < 0.001 vs MCAO group (n=6).
2.5. DJ-1明显降低脑I/R后Nrf2入核
与Sham组相比,MCAO组的Nrf2表达明显增加,与MCAO组相比,DJ-1 siRNA组的Nrf2表达明显降低(P <0.001,图 4)。与Sham组相比,MCAO组的Nrf2表达以及入核明显增加,与MCAO组相比,Overexpression组的Nrf2表达以及入核明显增加(P <0.001,图 4C)。
4.
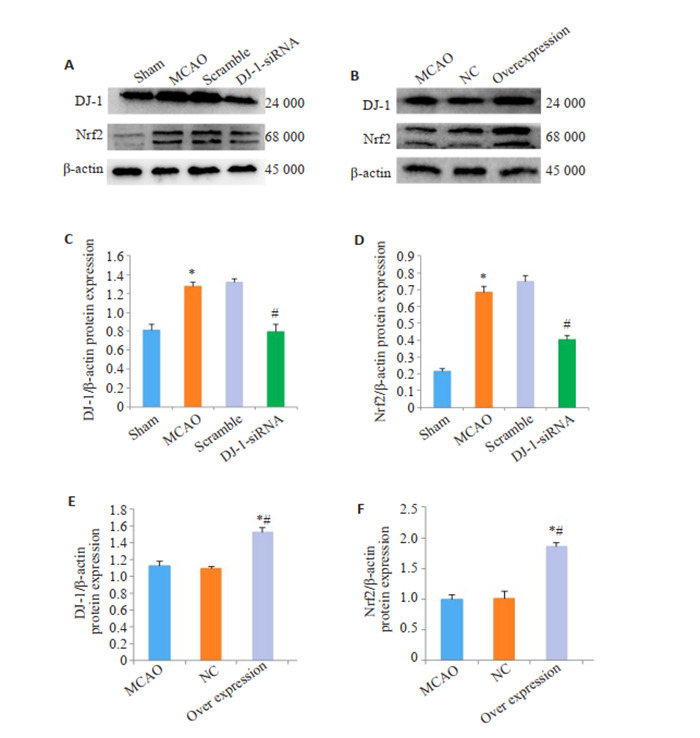
DJ-1对Nrf2表达的影响
Effects of DJ-1 overexpression on expression of Nrf2. A, B: Western blotting of DJ- 1 and Nrf2. C, E: Relative expression level of DJ-1 protein. D, F: Relative expression level of Nrf2 protein. *P < 0.001 vs Sham group; #P < 0.001 vs MCAO group; *#P < 0.001 vs MCAO group.
2.6. DJ-1促进脑I/R后HO-1和NQOI的表达
与Sham组相比,脑I/R损伤后HO-1和NQOI的蛋白水平明显上调(P <0.001)。干扰DJ-1可以抑制HO-1和NQOI的蛋白水平。用DJ-1过表达腺相关病毒过表达DJ-1后,与MCAO组相比,Overexpression组的HO-1 (P <0.004)和NQOI (P=0.014)的蛋白水平明显增加(图 4)。
5.
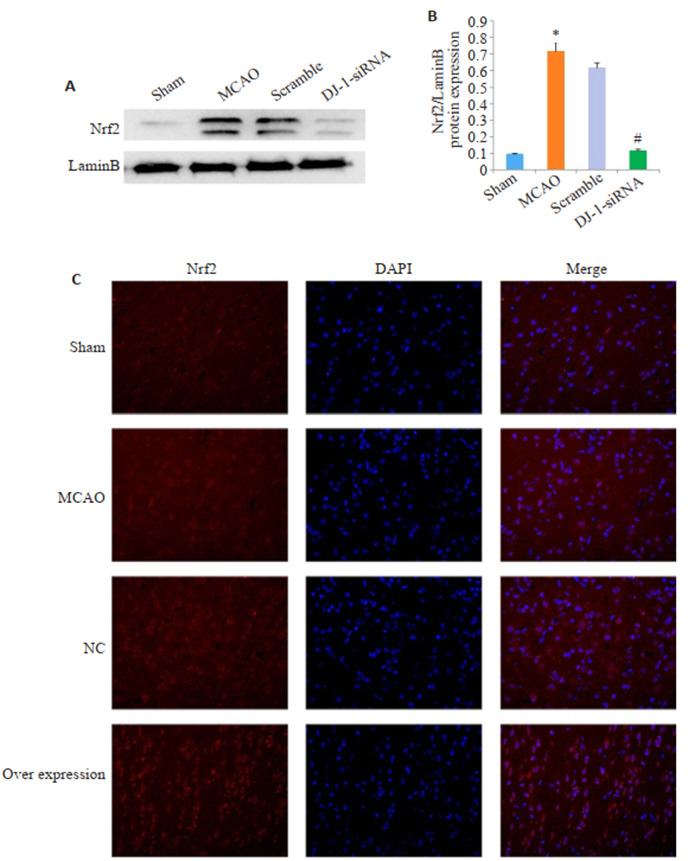
DJ-1对Nrf2入核的影响
Effect of DJ-1 expression on Nrf2 transfer into the nucleus. A: Western blotting of Nrf2 in the nucleus. B: Relative expression level of Nrf2 in the nucleus. C: Immunocytochemistry (Original magnification: ×400). *P < 0.001 vs Sham group (n=6); #P < 0.001 vs MCAO group (n=6).
3. 讨论
脑缺血再灌注损伤触发的氧化应激反应可损害神经元,导致其死亡或凋亡[16-17]。氧化应激通过对神经元死亡-生存机制的调节,成为脑缺血再灌注损伤关键病理生理环节[1-2]。在本研究中,我们通过构建大鼠脑MCAO模型模拟大鼠脑缺血再灌注损伤,发现缺血再灌注损伤后,出现大鼠神经功能缺失,以及脑水肿和神经元死亡。同时,MDA含量增加,SOD活性降低。这些结果表明脑缺血再灌注损伤触发的氧化应激反应是神经元损伤或死亡的关键原因。因此,寻找抵抗脑缺血再灌注氧化应激损伤的关键分子是本研究的目的。DJ-1是具有神经保护作用的重要抗氧化蛋白之一,既往对其的功能及机制的研究多集中在帕金森等慢性神经退行性疾病上。虽然,DJ-1在脑缺血再灌注氧化应激损伤方面的功能及机制尚不清楚。但我们有理由推测DJ-1可能是抵抗脑缺血再灌注氧化应激损伤的关键分子。有研究表明,DJ-1在缺血刺激下会诱导其表达水平增高[18]。预实验结果及相关文献[19]显示脑缺血再灌注损伤后24 h时DJ-1蛋白表达最高,因此本研究选取这一时间点来研究DJ-1在脑缺血再灌注损伤中的功能及相关机制。
为了探讨DJ-1在脑缺血再灌注损伤中的神经保护功能。本研究应用小分子干扰RNA(siRNA)技术干扰DJ-1的表达。siRNA因其特异性、高效性在科学研究中广泛使用[20-21]。在我们的研究中,DJ-1 siRNA处理后,脑缺血再灌注损伤所引起的神经行为学损伤加重,证实了DJ-1在缺血性脑卒中的神经保护作用。脑缺血再灌注损伤后会触发机体氧化应激反应发生。SOD和MDA的含量是衡量脑组织氧化应激的重要指标,SOD能清除机体内自由基,体内MDA含量不但直接反映脂质过氧化反应的水平和程度,而且间接反映机体清除自由基的能力。脑缺血再灌注损伤后,脑组织SOD含量降低,MDA含量增加,且干扰DJ-1后两者改变增加更显著。这些结果表明DJ-1能够减轻脑缺血再灌注氧化应激损伤。
Nrf2是转录因子家族成员之一,是调控机体氧化应激反应的一个关键核转录因子[22-23]。在我们之前的研究中,均证实在脑缺血再灌注损伤中Nrf2发挥了重要的神经保护作用。DJ-1和Nrf2同抗氧化应激的关键分子,两者之间的关系需要进一步去研究。Clements等发现,DJ-1能够影响Nrf2的稳定性和转录功能[24]。DJ-1通过抑制Nrf2泛素化,阻止其与Keap1的结合,促进Nrf2向细胞核的转运[25-26]。因此,DJ-1可能作为Nrf2的上游信号分子,在Nrf2活性调控扮演着重要的角色[14]。并且,DJ-1将通过怎样的方式来调控Nrf2还有待进一步明确。
我们观察到干扰DJ-1能够降低脑组织Nrf2的表达并阻止了Nrf2的核积累。同样,免疫荧光染色结果提示过表达DJ-1促进了Nrf2的表达和核累计。这些结果表明,DJ-1可能是通过上调Nrf2的表达并促进Nrf2向细胞核的转运,来调控机体氧化应激反应。
6.
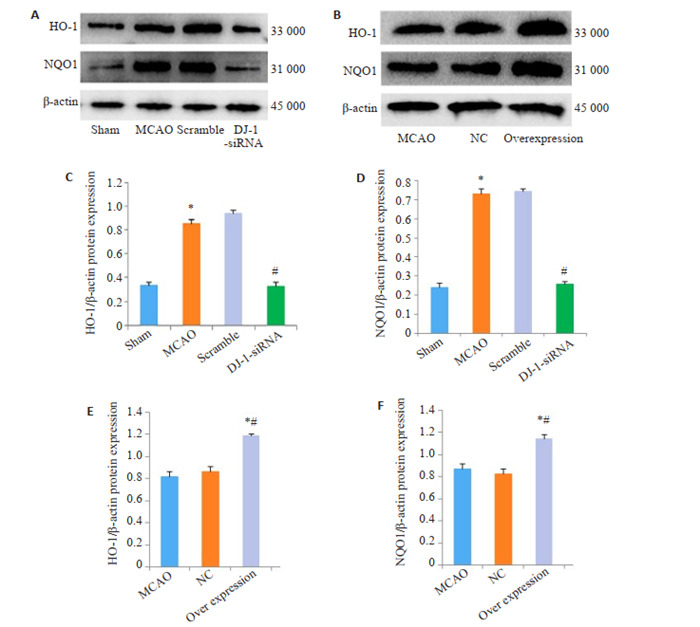
DJ-1对HO-1和NQO1表达的影响
Effect of DJ-1 expression on expression of HO-1 and NQO1. A, B: Western blotting of HO-1 and NQO1. C, E: Relative expression level of HO-1 protein; D, F: Relative expression level of NQO1 protein. *P < 0.001 vs Sham group; #P < 0.001 vs MCAO group; *#P < 0.001 vs MCAO group (n=6).
正常情况下,Nrf2与细胞浆中的Keap1蛋白相结合,使Nrf2的活性受到抑制。当受到氧化应激反应刺激时,Nrf2转移至细胞核内与抗氧化反应元件(ARE)结合,介导抗氧化酶基因的转录激活,如:血红素加氧酶-1 (HO-1)、醌氧化还原酶1(NQO1)谷胱甘肽S-转移酶(GST)家族等[27-28]。Nrf2通常是通过Nrf2/ARE途径调节phaseII解毒酶和抗氧化酶(如HO-1、NQO1等)表达的中枢信号开关[29-30]。那么,DJ-1诱导的核转运的Nrf2可能与ARE结合,并通过转录激活下游的抗氧化酶来对抗氧化应激引起的损伤。因此,我们检测了HO-1、NQO1的表达。我们发现干扰DJ-1后,HO-1和NQO1蛋白水平降低。过表达DJ-1明显上调HO-1和NQO1蛋白水平。表明DJ-1能够通过正向调控Nrf2信号通路调节抗氧化酶HO-1和NQO1的表达。
综上所述,DJ-1作为体内重要的神经保护因子,可以减少MCAO后大鼠氧化应激损伤,并且这种保护功能可能与其对细胞重要的核转录因子Nrf2及下游信号通路的激活密切相关。
Biography
李莉,主治医师,E-mail: 443157498@qq.com
Funding Statement
国家自然科学基金(81271460)
Supported by National Natural Science Foundation of China (81271460)
Contributor Information
李 莉 (Li LI), Email: 443157498@qq.com.
赵 涌 (Yong ZHAO), Email: zhaoyong668@cqmu.edu.cn.
References
- 1.Su XT, Wang L, Ma SM, et al. Mechanisms of acupuncture in the regulation of oxidative stress in treating ischemic stroke. http://www.researchgate.net/publication/346576407_Mechanisms_of_Acupuncture_in_the_Regulation_of_Oxidative_Stress_in_Treating_Ischemic_Stroke. Oxid Med Cell Longev. 2020:7875396. doi: 10.1155/2020/7875396. [Su XT, Wang L, Ma SM, et al. Mechanisms of acupuncture in the regulation of oxidative stress in treating ischemic stroke[J]. Oxid Med Cell Longev, 2020: 7875396.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malko P, Jiang LH. TRPM2 channel-mediated cell death: an important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox Biol. 2020;37:101755. doi: 10.1016/j.redox.2020.101755. [Malko P, Jiang LH. TRPM2 channel-mediated cell death: an important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions[J]. Redox Biol, 2020, 37: 101755.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitimus DM, Popescu MR, Voiculescu SE, et al. Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules. 2020;10(9):E1211. doi: 10.3390/biom10091211. [Chitimus DM, Popescu MR, Voiculescu SE, et al. Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease[J]. Biomolecules, 2020, 10(9): E1211.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kletkiewicz H, Klimiuk M, Woźniak A, et al. How to improve the antioxidant defense in asphyxiated newborns-lessons from animal models. Antioxidants (Basel) 2020;9(9):E898. doi: 10.3390/antiox9090898. [Kletkiewicz H, Klimiuk M, Woźniak A, et al. How to improve the antioxidant defense in asphyxiated newborns-lessons from animal models[J]. Antioxidants (Basel), 2020, 9(9): E898.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang O. Role of oxidative stress in Parkinson's disease. Exp Neurobiol. 2013;22(1):11–7. doi: 10.5607/en.2013.22.1.11. [Hwang O. Role of oxidative stress in Parkinson's disease[J]. Exp Neurobiol, 2013, 22(1): 11-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puno MR, Patel NA, Møller SG, et al. Structure of Cu(I)-bound DJ-1 reveals a biscysteinate metal binding site at the homodimer interface: insights into mutational inactivation of DJ-1 in Parkinsonism. JAm Chem Soc. 2013;135(43):15974–7. doi: 10.1021/ja406010m. [Puno MR, Patel NA, Møller SG, et al. Structure of Cu(I)-bound DJ-1 reveals a biscysteinate metal binding site at the homodimer interface: insights into mutational inactivation of DJ-1 in Parkinsonism[J]. JAm Chem Soc, 2013, 135(43): 15974-7.] [DOI] [PubMed] [Google Scholar]
- 7.Andreeva A, Bekkhozhin Z, Omertassova N, et al. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals. J Biol Chem. 2019;294(49):18863–72. doi: 10.1074/jbc.RA119.011237. [Andreeva A, Bekkhozhin Z, Omertassova N, et al. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals[J]. J Biol Chem, 2019, 294(49): 18863-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Kim KS, Iyirhiaro GO, et al. DJ-1 modulates the unfolded protein response and cell death via upregulation of ATF4 following ER stress. Cell Death Dis. 2019;10(2):135. doi: 10.1038/s41419-019-1354-2. [Yang J, Kim KS, Iyirhiaro GO, et al. DJ-1 modulates the unfolded protein response and cell death via upregulation of ATF4 following ER stress[J]. Cell Death Dis, 2019, 10(2): 135.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahfeldt T, Ordureau A, Bell C, et al. Pathogenic pathways in earlyonset autosomal recessive Parkinson's disease discovered using isogenic human dopaminergic neurons. Stem Cell Reports. 2020;14(1):75–90. doi: 10.1016/j.stemcr.2019.12.005. [Ahfeldt T, Ordureau A, Bell C, et al. Pathogenic pathways in earlyonset autosomal recessive Parkinson's disease discovered using isogenic human dopaminergic neurons[J]. Stem Cell Reports, 2020, 14(1): 75-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giangrasso DM, Furlong TM, Keefe KA. Characterization of striatum-mediated behavior and neurochemistry in the DJ-1 knockout rat model of Parkinson's disease. Neurobiol Dis. 2020;134:104673. doi: 10.1016/j.nbd.2019.104673. [Giangrasso DM, Furlong TM, Keefe KA. Characterization of striatum-mediated behavior and neurochemistry in the DJ-1 knockout rat model of Parkinson's disease[J]. Neurobiol Dis, 2020, 134: 104673.] [DOI] [PubMed] [Google Scholar]
- 11.Li WJ, Sun K, Hu F, et al. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: a mini review. J Biochem Mol Toxicol. 2021;35(3):e22658. doi: 10.1002/jbt.22658. [Li WJ, Sun K, Hu F, et al. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: a mini review[J]. J Biochem Mol Toxicol, 2021, 35(3): e22658] [DOI] [PubMed] [Google Scholar]
- 12.Ahn YJ, Lim JW, Kim H. Docosahexaenoic acid induces expression of NAD(P)H: quinone oxidoreductase and heme oxygenase- 1 through activation of Nrf2 in cerulein-stimulated pancreatic acinar cells. Antioxidants (Basel) 2020;9(11):E1084. doi: 10.3390/antiox9111084. [Ahn YJ, Lim JW, Kim H. Docosahexaenoic acid induces expression of NAD(P)H: quinone oxidoreductase and heme oxygenase- 1 through activation of Nrf2 in cerulein-stimulated pancreatic acinar cells[J]. Antioxidants (Basel), 2020, 9(11): E1084.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanas C, Ziros PG, Chartoumpekis DV, et al. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update. Antioxidants (Basel) 2020;9(11):1082. doi: 10.3390/antiox9111082. [Thanas C, Ziros PG, Chartoumpekis DV, et al. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update[J]. Antioxidants (Basel), 2020, 9(11): 1082.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimhan KKS, Jayakumar D, Velusamy P, et al. Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/Nrf2/ARE signaling pathway. http://www.researchgate.net/publication/332822995_Morinda_citrifolia_and_Its_Active_Principle_Scopoletin_Mitigate_Protein_Aggregation_and_Neuronal_Apoptosis_through_Augmenting_the_DJ-1Nrf2ARE_Signaling_Pathway. Oxid Med Cell Longev. 2019;2019:2761041. doi: 10.1155/2019/2761041. [Narasimhan KKS, Jayakumar D, Velusamy P, et al. Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/Nrf2/ARE signaling pathway[J]. Oxid Med Cell Longev, 2019, 2019: 2761041.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng L, Zhao YP, Li YX, et al. Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury. J Mol Med (Berl) 2019;97(2):189–99. doi: 10.1007/s00109-018-1719-5. [Peng L, Zhao YP, Li YX, et al. Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury[J]. J Mol Med (Berl), 2019, 97(2): 189-99.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy. 2014;10(10):1692–701. doi: 10.4161/auto.36076. [Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells[J]. Autophagy, 2014, 10(10): 1692-701.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura-Ohba S, Yang Y. Oxidative DNA damage mediated by intranuclear MMP activity is associated with neuronal apoptosis in ischemic stroke. http://pubmedcentralcanada.ca/pmcc/articles/PMC4748094/ Oxid Med Cell Longev. 2016:6927328. doi: 10.1155/2016/6927328. [Kimura-Ohba S, Yang Y. Oxidative DNA damage mediated by intranuclear MMP activity is associated with neuronal apoptosis in ischemic stroke[J]. Oxid Med Cell Longev, 2016, 6927328.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2009;29(2):125–31. doi: 10.1111/j.1440-1789.2008.00955.x. [Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age[J]. Neuropathology, 2009, 29(2): 125-31.] [DOI] [PubMed] [Google Scholar]
- 19.Yanagida T, Tsushima J, Kitamura Y, et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid Med Cell Longev. 2009;2(1):36–42. doi: 10.4161/oxim.2.1.7985. [Yanagida T, Tsushima J, Kitamura Y, et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury[J]. Oxid Med Cell Longev, 2009, 2(1): 36-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JH, Hua CL, Fang YY, et al. The dual edge of RNA silencing suppressors in the virus-host interactions. Curr Opin Virol. 2016;17:39–44. doi: 10.1016/j.coviro.2015.12.002. [Zhao JH, Hua CL, Fang YY, et al. The dual edge of RNA silencing suppressors in the virus-host interactions[J]. Curr Opin Virol, 2016, 17: 39-44.] [DOI] [PubMed] [Google Scholar]
- 21.Wang JQ, Mi P, Lin G, et al. Imaging-guided delivery of RNAi for anticancer treatment. Adv Drug Deliv Rev. 2016;104:44–60. doi: 10.1016/j.addr.2016.01.008. [Wang JQ, Mi P, Lin G, et al. Imaging-guided delivery of RNAi for anticancer treatment[J]. Adv Drug Deliv Rev, 2016, 104: 44-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–91. doi: 10.3390/molecules15107266. [Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants[J]. Molecules, 2010, 15(10): 7266-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piotrowska M, Swierczynski M, Fichna J, et al. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol Res. 2021;163:105243. doi: 10.1016/j.phrs.2020.105243. [Piotrowska M, Swierczynski M, Fichna J, et al. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases[J]. Pharmacol Res, 2021, 163: 105243.] [DOI] [PubMed] [Google Scholar]
- 24.Clements CM, McNally RS, Conti BJ, et al. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103(41):15091–6. doi: 10.1073/pnas.0607260103. [Clements CM, McNally RS, Conti BJ, et al. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2[J]. Proc Natl Acad Sci USA, 2006, 103(41): 15091-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31(6):967–77. doi: 10.1111/j.1460-9568.2010.07138.x. [Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1[J]. Eur J Neurosci, 2010, 31(6): 967- 77.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuevas S, Yang Y, Konkalmatt P, et al. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension. 2015;65(6):1251–7. doi: 10.1161/HYPERTENSIONAHA.114.04525. [Cuevas S, Yang Y, Konkalmatt P, et al. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1[J]. Hypertension, 2015, 65(6): 1251-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke BX, Zhang TM, An TY, et al. Soy isoflavones ameliorate the cognitive dysfunction of Goto-Kakizaki rats by activating the Nrf2- HO-1 signalling pathway. Aging (Albany NY) 2020;12(21):21344–54. doi: 10.18632/aging.103877. [Ke BX, Zhang TM, An TY, et al. Soy isoflavones ameliorate the cognitive dysfunction of Goto-Kakizaki rats by activating the Nrf2- HO-1 signalling pathway[J]. Aging (Albany NY), 2020, 12(21): 21344-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waz S, Heeba GH, Hassanin SO, et al. Nephroprotective effect of exogenous hydrogen sulfide donor against cyclophosphamideinduced toxicity is mediated by Nrf2/HO-1/NF-κB signaling pathway. Life Sci. 2021;264:118630. doi: 10.1016/j.lfs.2020.118630. [Waz S, Heeba GH, Hassanin SO, et al. Nephroprotective effect of exogenous hydrogen sulfide donor against cyclophosphamideinduced toxicity is mediated by Nrf2/HO-1/NF-κB signaling pathway[J]. Life Sci, 2021, 264: 118630.] [DOI] [PubMed] [Google Scholar]
- 29.Li N, Sun YR, He LB, et al. Amelioration by Idesia polycarpa maxim. var. vestita Diels. of oleic acid-induced nonalcoholic fatty liver in HepG2 cells through antioxidant and modulation of lipid metabolism. http://www.researchgate.net/publication/346351784_Amelioration_by_Idesia_polycarpa_Maxim_var_vestita_Diels_of_Oleic_Acid-Induced_Nonalcoholic_Fatty_Liver_in_HepG2_Cells_through_Antioxidant_and_Modulation_of_Lipid_Metabolism. Oxid Med Cell Longev. 2020:1208726. doi: 10.1155/2020/1208726. [Li N, Sun YR, He LB, et al. Amelioration by Idesia polycarpa maxim. var. vestita Diels. of oleic acid-induced nonalcoholic fatty liver in HepG2 cells through antioxidant and modulation of lipid metabolism[J]. Oxid Med Cell Longev, 2020: 1208726.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warpsinski G, Smith MJ, Srivastava S, et al. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox Biol. 2020;37:101708. doi: 10.1016/j.redox.2020.101708. [Warpsinski G, Smith MJ, Srivastava S, et al. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation[J]. Redox Biol, 2020, 37: 101708.] [DOI] [PMC free article] [PubMed] [Google Scholar]


