Abstract
Abstract
The morbidity and mortality caused by invasive fungal infections are increasing across the globe due to developments in transplant surgery, the use of immunosuppressive agents, and the emergence of drug-resistant fungal strains, which has led to a challenge in terms of treatment due to the limitations of three classes of drugs. Hence, it is imperative to establish effective strategies to identify and design new antifungal drugs. Drug repurposing is a potential way of expanding the application of existing drugs. Recently, various existing drugs have been shown to be useful in the prevention and treatment of invasive fungi. In this review, we summarize the currently used antifungal agents. In addition, the most up-to-date information on the effectiveness of existing drugs with antifungal activity is discussed. Moreover, the antifungal mechanisms of existing drugs are highlighted. These data will provide valuable knowledge to stimulate further investigation and clinical application in this field.
Key points
• Conventional antifungal agents have limitations due to the occurrence of drug-resistant strains.
• Non-antifungal drugs act as antifungal agents in various ways toward different targets.
• Non-antifungal drugs with antifungal activity are demonstrated as effective antifungal strategies.
Keywords: Drug repurposing, Antifungal therapy, Antifungal mechanism, Clinical application, Antifungal agent
Introduction
Fungal infection has become a significant event leading to over 1.5 million deaths annually worldwide (Deaguero et al. 2020). To date, the most common fungal infections related to human mortality and morbidity are caused by Cryptococcus, Candida, and Aspergillus (Boral et al. 2018). The impact of mycoses has increased, especially in patients with immunodeficiency disorders who have undergone transplant surgery, chemoradiotherapy, hemodialysis, or the treatment with immunosuppressive agents (Drgona et al. 2014). Hence, antifungal therapy represents a challenging problem for clinicians. In addition, the limited number of antifungal agents in the clinic can induce side effects and a great number of drug-resistant or multidrug-resistant strains have emerged. Candida auris, a multidrug-resistant fungus, has shown a global increase in recent years. Importantly, some of these infections are resistant to almost all current antifungal agents (Du et al. 2020). In New Delhi, it was reported that 15 COVID-19 patients had secondary candidiasis in the intensive care unit (ICU), two-thirds of which were caused by C. auris, and the mortality rate was up to 60 % (Chowdhary et al. 2020). Lomentospora prolificans is another classic fungus with intrinsic resistance to nearly all existing antifungal agents (Pellon et al. 2018). In A. fumigatus, the emergence of azole resistance has made the treatment of aspergillosis a global public health problem, especially in Australia, China, the USA, and parts of Europe (Meis et al. 2016). More than 25 % of A. fumigatus has been found to be resistant to the triazole antifungal agents in an ICU in the Netherlands (Lestrade et al. 2016). More concerningly, patients with invasive aspergillosis caused by azole-resistant A. fumigatus have mortality rates ranging from 50 to 100 % (Lestrade et al. 2019).
Currently, the first-line antifungal agents for invasive fungal infections are amphotericin B, echinocandins, isavuconazole, itraconazole, posaconazole, and voriconazole (Zhao et al. 2016). However, due to the existence of toxicity and drug-resistant strains, the present antifungal options have become more restricted. A variety of approaches have been employed to conduct antifungal therapies, such as the synthesis of new substances, the use of extracts from organisms, changing of the administration methods or forms of old drugs to treat fungal diseases, and an association between known antifungal drugs and non-antifungal agents (Robbins et al. 2016). Moreover, drug repurposing is a potential strategy for the treatment of invasive fungal infections, owing to the excellent antifungal activity of these drugs. Several agents have recently been confirmed to serve as antifungal candidates in the treatment of mycoses. The purpose of this review is to present a series of known drugs that have been investigated for their application in the treatment of fungal infections. Firstly, the strategies, mechanisms, and challenges of current antifungal drugs are described. Secondly, the extensive application and antifungal mechanisms of drugs with antifungal activity that is used in the clinic to treat non-mycotic infections are highlighted.
Current antifungal drugs used in clinics
Since the first active antimycotic griseofulvin was recognized in 1939, a multitude of antifungal agents have been used clinically. Polyenes, azoles, echinocandins, and flucytosine are currently the main treatments for invasive fungal infections in clinical settings. In fungi, ergosterol, located in the cell membrane, regulates membrane structure permeability, mobility, and substance transportation by making direct linkages with the phospholipid membrane (Anderson et al. 2014). The representative polyene drug is amphotericin B, which can bind to ergosterol from lipid bilayers and form large and extramembranous aggregates (Anderson et al. 2014). These extramembranous aggregates lead to the formation of transmembranal pores, which can leak cellular components. This results in the death of pathogenic fungi (Anderson et al. 2014). As the “gold standard” for combating invasive fungal infections for decades, amphotericin B has a relatively broad spectrum of antifungal activity against yeasts and molds (Ostrosky-Zeichner et al. 2003). For instance, an investigation of 78 Candida sp. clinical strains showed that all examined free-living cells were susceptible to amphotericin B (Prazynska and Gospodarek 2014). The MIC90 of amphotericin B for common yeasts in clinical settings ranges from 0.25 to 2 μg/mL and is 1–4 μg/mL for clinically important molds (Ellis 2002). In addition, amphotericin B has been used as an alternative therapy for invasive aspergillosis (Patterson et al. 2016). In vitro, amphotericin B combined with caspofungin and voriconazole has synergistic anti-Aspergillus species effects (O'Shaughnessy et al. 2006), but these findings have not been validated in vivo (Baddley and Pappas 2005; Haidar and Singh 2018). Moreover, the clinical applications of amphotericin B have been limited due to toxicity, which includes nephrotoxicity and infusion-related reactions such as chest pain, dyspnea, hypoxia, flushing, and urticaria (Roden et al. 2003). To resolve this problem, lipid formations of amphotericin B, including liposomal amphotericin B (LAmB), AmpB lipid complex (ABLC), and AmpB colloid dispersion (ABCD), were developed (Ostrosky-Zeichner et al. 2003). Toxicity was greatly reduced using these formulations; however, they are inefficient in penetrating certain tissues, such as the kidney, and thus fail to reach therapeutic concentrations (Smitherman 2016).
Due to the safety and wide availability, azoles (including fluconazole, isavuconazole, itraconazole, posaconazole, and voriconazole) are the most widely used antifungal agents. Azoles can be used against the majority of fungi, as they inhibit the cytochrome P450-dependent enzyme 14α demethylase (Cyp51) (Emami et al. 2017). Lanosterol conversion into ergosterol is blocked when azoles inhibit Cyp51. Azoles have excellent therapeutic effects on molds as well as yeasts (Meis et al. 2016; Patterson et al. 2016; Robbins et al. 2017). For example, in a randomized trial, voriconazole was used in therapy for invasive aspergillosis and achieved a 52.8 % curative rate (Haidar and Singh 2018). However, the extensive use of azoles has subsequently led to the emergence of acquired azole resistance, particularly in Aspergillus species (Wiederhold 2017). A resistance rate of 26 % was found in A. fumigatus culture–positive patients in the ICU (Paassen et al. 2016). Azoles can also bind to the human cytochrome P450 enzyme system (CYP450), leading to reduced antifungal efficiency (Wiederhold 2018).
Echinocandins are a group of effective antifungal agents which, according to the current Infectious Society of America (IDSA) and European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) guidelines, are useful for the primary treatment of invasive candidiasis (Chang et al. 2017). Anidulafungin, caspofungin, and micafungin are representatives of this group (Emri et al. 2013). Compared with the azole antifungal agents, echinocandins rarely cause resistance, have a good safety profile, have better clinical outcomes, and have been used for two decades. The mechanism of echinocandins mainly involves inhibition of cell wall synthesis by inhibiting β-1,3-D-glucan synthase, a key cell wall component of pathogenic fungi. Following treatment with echinocandins, the buried glucan cell wall architecture can be exposed, which induces abnormal morphology and growth limitation. The time-kill methodology of C. albicans biofilms, treated with caspofungin, displayed at least 99 % killing at physiological concentrations. Investigations in AIDS patients, who were unable to tolerate amphotericin B, have also shown that caspofungin is an efficient therapeutic option for azole-resistant Candida infections (Ramage et al. 2002; Garbino 2004). Anidulafungin is an excellent therapeutic choice against Aspergillus and Candida species, including those resistant to either fluconazole or amphotericin B. A literature review mentioned that it was a superior option to fluconazole in the treatment of oesophageal candidiasis and candidemia (Vazquez 2005). Micafungin also exhibits excellent activity on Candida spp. resistant to multiple azoles, and 8 μg/mL of micafungin demonstrated a 57 % antifungal rate in C. parapsilosis (Gil-Alonso et al. 2015). Nevertheless, probably due to the differences in cell wall composition or structure, this class is largely inactive in most filamentous fungi including Zygomycetes and Fusarium species (Perlin 2020). In addition, C. neoformans is naturally resistant to echinocandins, which are completely ineffective in treating cryptococcosis (Denning 2003). Additionally, due to their characteristic large molecular weight, low oral bioavailability, and limited absorption in the gastrointestinal tract, these drugs are only administered intravenously.
Flucytosine is also an important antifungal agent that inhibits the synthesis of DNA and RNA and is mainly used to treat cryptococcosis and candidiasis (Bennet 1977). Because of the poor efficacy of monotherapy due to the prevalence of intrinsically resistant strains (approximately 10 % C. albicans isolates) and the frequent development of resistance during treatment (Vermes et al. 2000), flucytosine is always used in combination with another antifungal agent (such as amphotericin B). These combination treatments have shown promising clinical effects. For example, 49 HIV-infected patients with cryptococcosis treated with combination flucytosine and amphotericin B were reported to have a higher survival rate than patients receiving amphotericin B alone (Chuck and Sande 1989). For candidiasis, several similar trials have shown that this combined regimen also has synergic activity against a variety of Candida spp. (Medoff et al. 1971; Montgomerie et al. 1975; Smego Jr et al. 1984). Currently, flucytosine combined with amphotericin B is the standard induction therapy for Cryptococcal and Candida meningitis, because it has lower rates of treatment failure and mortality than other alternative approaches (Bridges et al. 2017; Greene et al. 2020).
Azole resistance among Candida species, such as C. auris and Aspergillus species, as well as echinocandin resistance in C. glabrata is an alarming problem (Wiederhold 2017). In addition, other molds such as Scedosporium and Fusarium species have reduced susceptibility to clinically available antifungal drugs (Wiederhold 2017). Although amphotericin B is recognized to have excellent antifungal activity, there are still fungi, such as C. lusitaniae and A. terreus that show intrinsic resistance to amphotericin B (Arendrup et al. 2012). Consequently, the development of new antifungals is needed to resolve this issue.
Drug repurposing strategy
Compared with the development of new drugs, drug repurposing has several advantages, as mainly reflected in decreased cost and security risk (Cha et al. 2018). The average new drug development time savings has been estimated to reach 5–7 years, and the failure rate for problems related to safety or toxicity is less than 50 % (Ashburn and Thor 2004). Drug repurposing involves tapping into new applications for existing drugs and thus requires an innovative clinical in-depth investigation of the pharmacological mechanisms. For example, aspirin, a traditional drug with analgesic and antipyretic activity initially, and is now gradually being used in the prevention and treatment of multi-system diseases including cardiovascular disease, stroke, and digestive tract cancers (Theken and Grosser 2018; Bosetti et al. 2020; Burn et al. 2020; Johnston et al. 2020). Vitamin D is widely used to regulate calcium and phosphate metabolism for bone health (Ng et al. 2019). Recent studies have found that vitamin D deficiency is associated with an increased risk of cardiovascular disease, diabetes, hypercholesterolemia, and even COVID-19 (Kouvari et al. 2020; Meltzer et al. 2020). Metformin, originally used to treat diabetes, has so far more than a dozen potential new functions including the treatment and prevention of cancer, cardiovascular disease, and mental illness such as infantile autism and cognitive disorder (Tseng 2015; Dy et al. 2018; Shiers et al. 2018; Arrieta et al. 2019; Cheung et al. 2019; Lee et al. 2019; Mohan et al. 2019).
In addition, drug repurposing is also very significant in the development of new antifungal drugs, the effectiveness of existing drugs with antifungal activity have been associated with exciting non-antifungal agents chiefly consisting of antibacterial drugs, immunosuppressants, statins, antiarrhythmic drugs, antipsychotic drugs, antidepressant drugs, and non-steroidal anti-inflammatory drugs (NSAIDs).
Antibacterial drugs
The discovery of antibacterial drugs has been the greatest invention of medical science in reducing morbidity and mortality. Traditionally, antibacterial drugs were commonly used as medical treatments for infectious diseases caused by gram-negative bacteria, gram-positive bacteria, mycoplasma, chlamydia, rickettsia, as well as spirochaetes. Antibacterial drugs with antifungal activity mainly include tetracyclines (e.g., demeclocycline, doxycycline, minocycline, and tigecycline), aminoglycosides (e.g., gentamicin, neomycin, paromomycin, ribostamycin, streptomycin, and tobramycin), macrolides (e.g., azithromycin and clarithromycin), quinolone polypeptides (e.g., ciprofloxacin, gatifloxacin, levofloxacin, moxifloxacin, norfloxacin, and trovafloxacin), polypeptides (e.g., polymyxin B), and others such as linezolid and rifampicin. Many studies in vitro and in animal models have revealed that antibacterial drugs have broad-spectrum antifungal activity (Table 1). In general, they are commonly used alone or in combination to regulate the gene expression levels of adhesion, hypha, or biofilm formation, to decrease extracellular glycan level and cell surface hydrophobicity, and even to inhibit efflux pump activity. In Fusarium spp., tobramycin combined with amphotericin B and voriconazole has an 80 % and 76 % synergistic effect, respectively, in increasing the permeability of the cell wall and cell membrane (Venturini et al. 2016). Minocycline, a tetracycline, has been verified to exhibit broad-spectrum antifungal activity against A. fumigatus, A. flavus, Fusarium solani, and F. oxysporum with MICs in the range of 0.125–4 μg/mL. In addition, a series of studies on the antifungal activity of minocycline combined with antifungal agents (amphotericin B, fluconazole, itraconazole, posaconazole, and voriconazole) have been reported (Lew et al. 1977; Shi et al. 2010; Loreto et al. 2014; Gao et al. 2020). Similar findings have also been confirmed for polymyxin B which mainly combines with ketoconazole, micafungin, and amphotericin B in C. albicans to alter the permeability of the cell membrane (Moneib 1995). Moreover, polymyxin B can bind anionic lipids on the fungal membrane, both alone, with the MIC100 range from 8 to 256 μg/mL, and combined with fluconazole for Fusarium spp., C. neoformans, Rhizopus oryzae, and A. fumigatus, which destroys the membrane integrity (Zhai et al. 2010; Venturini et al. 2016). Many animal models have also been used to verify the antifungal activity of antibacterial agents. In the murine model of candidiasis, a combination of cefoperazone–sulbactam, colistin, or meropenem with caspofungin has been found to result in lower fungal burden in the kidneys, than that after treatment with caspofungin alone (Ozcan et al. 2006; Zeidler et al. 2013; Keçeli et al. 2014). Histopathological results indicated that the degree of inflammation was 25 % less in the group treated with caspofungin and meropenem than caspofungin monotherapy (Ozcan et al. 2006). In mice, quinolones combined with fluconazole and amphotericin B also have synergistic effects against invasive candidiasis (Sugar et al. 1997; Sasaki et al. 2000). β-Lactam derivatives show broad-spectrum antifungal activity in vitro against Candida spp. and Aspergillus spp. (Gowri et al. 2016; O'Driscoll et al. 2008; Mohamadzadeh et al. 2020). However, studies have indicated that β-lactam antibiotics are unable to inhibit the growth of Candida species, and amoxicillin can increase the virulence of Candida krusei and Candida tropicalis in Caenorhabditis elegans (Aguiar Cordeiro et al. 2018).
Table 1.
Summary of antibacterial drugs with antifungal activity
| Antibiotics | Fungus | In vitro (MIC: μg/mL) | In vivo | In combination (synergistic effects) | Relevant molecular mechanism | Ref. |
|---|---|---|---|---|---|---|
| Tobramycin | Fusarium spp. | > 64 | - | VRC, AmB | Probably increases permeability of the cell wall and cell membrane. | Venturini et al. (2016) |
| Gentamicin | Resistant-azole C. albicans | > 512 | G. mellonella | FLC |
(I) Suppresses overexpression of the efflux pump. (II) Reduces phospholipase activity of resistant C. albicans. |
Lu et al. (2018) |
| Clarithromycin | C. tropicalis | - | - | AmB, ANI | - | Fernández-Rivero et al. (2017) |
| C. parapsilosis, C. glabrata, C. albicans | No effects | - | AmB | - | Del Pozo et al. (2011) | |
| Pythium insidiosum | 0.25–8 | - | - | - | Loreto et al. (2014) | |
| Azithromycin | Pythium insidiosum | 1–16 | - | - | - | Loreto et al. (2014) |
| Aspergillus spp. | No effects | - | AmB | Probably inhibits mitochondrial protein synthesis. | Nguyen et al. (1997) | |
| Norfloxacin | C. albicans | - | - | MIA | - | Moneib (1995) |
| Levofloxacin | C. albicans, A. fumigatus | + | - | AmB, CAS | Probably inhibits fungal DNA replication by binding to fungal topoisomerase. | Stergiopoulou et al. (2009) |
| Gatifloxacin | Candida spp. | + | - | - | - | Ozdek et al. (2006) |
| Moxifloxacin | C. albicans | + | - | Liposomal AmB, CAS | Probably inhibits fungal DNA replication by binding to fungal topoisomerase. | Ozdek et al. (2006); Stergiopoulou et al. (2009); Deren et al. (2010) |
| Ciprofloxacin | C. albicans | + | - | AmB | Probably inhibits fungal DNA replication by binding to C. albicans topoisomerase. | Stergiopoulou et al. (2009) |
| A. fumigatus | + | - | AmB, CAS, ARC | |||
| Trovafloxacin | C. albicans | No effects | Murine | FLC, AmB | - | Sugar et al. (1997) |
| C. tropicalis, C. neoformans | No effects | - | FLC, AmB | |||
| Tetracycline | C. albicans | 320–2560 | G. mellonella | AmB, FLC | - | Lew et al. (1977); Gu et al. (2018) |
| Demeclocycline | C. albicans | 640 | - | AmB | - | Lew et al. (1977) |
| Doxycycline | C. parapsilosis, C. krusei, C. glabrata | No effects | - | AmB | Probably inhibits protein synthesis. | El-Azizi (2007) |
| C. albicans | 640–1280 | G. mellonella | AmB, FLC, CAS |
(I) Inhibits FLC-inducible efflux pump gene overexpression. (II) Disturbs calcium homeostasis. (III) Disturbs iron homeostasis. |
Lew et al. (1977); Miceli et al. (2009); Fiori and Van Dijck (2012); Gao et al. (2013); Gao et al. (2014); Gu et al. (2018) | |
| Minocycline | Resistant-FLC C. albicans | 256–512 | - | AmB, FLC | Disturbs calcium homeostasis. | Lew et al. (1977); Shi et al. (2010) |
| A. fumigatus | 0.125–4 | G. mellonella | ITR, VRC, POS | Probably interferes with the balance of cellular electrolytes and loss of mitochondrial function. | Loreto et al. (2014); Gao et al. (2020) | |
| A. flavus, F. solani, F. oxysporum | 0.125–4 | - | ITR, VRC, POS | |||
| Tigecycline | C. albicans | 2048 | - | AmB, FLC, CAS | - | Ku et al. (2010) |
| Fusarium spp. | 0.25–4 | - | VRC, AmB | Inhibits the synthesis of protein. | Loreto et al. (2014); Venturini et al. (2016) | |
| Polymyxin B | C. albicans | - | - | AmB, KET, MIA | Probably alters cell membrane permeability. | Moneib (1995) |
| Fusarium spp. | 4–16 | - | VRC, AmB | Probably disturbs the synthesis of ergosterol. | Venturini et al. (2016) | |
| C. neoformans | 8–256 | - | FLC | Probably through binding anionic lipids on fungal membrane and destroys membrane integrity. | Zhai et al. (2010) | |
| Rhizopus oryzae | 32 | - | - | |||
| A. fumigatus | 28–56 | - | - | |||
| Rifampicin | C. tropicalis | - | - | AmB, ANI | - | Fernández-Rivero et al. (2017) |
| C. parapsilosis, C. glabrata, C. albicans, C. krusei | No effects | - | AmB | Probably disturbs RNA synthesis in the presence of AmB. | El-Azizi (2007); Loreto et al. (2014) | |
| Linezolid | Pythium insidiosum | 1–32 | - | - | Inhibits protein synthesis. | Loreto et al. (2014) |
| C. neoformans | > 64 | - | AmB | - | Rossato et al. (2015) | |
| C. albicans | > 512 | G. mellonella | FLC, ITR, VRC | Probably inhibits mitochondrial protein synthesis and interferes with the induction of stress-response mitochondrial chaperones. | Lu et al. (2019) |
Note: VRC, voriconazole; AmB, amphotericin B; FLC, fluconazole; ITR, itraconazole; CTZ, clotrimazole; POS, posaconazole; ANI, anidulafungin; MIA, micafungin; CAS, caspofungin; KET, ketoconazole; -, no studies were mentioned in the corresponding references; +, the drug has antifungal effect, but no specific data in the corresponding references
Biofilms represent one of the major virulence factors in pathogenic fungi, which develop on the surfaces of stents, shunts, prostheses, implants, endotracheal tubes, pacemakers, and various types of catheters (Sardi Jde et al. 2014). Compared to planktonic cells, the interior biofilm cells display severe resistance to a wide variety of clinical antifungal agents. A small subset of yeast cells in C. albicans biofilms were found to be highly resistant to amphotericin B. This resistance was independent of the upregulation of efflux pumps and cell membrane composition (Sardi Jde et al. 2014). When combined with clarithromycin, the permeability barrier of the biofilm matrix was altered, which resulted in increased penetration of amphotericin B (Del Pozo et al. 2011). In addition, doxycycline, tigecycline, and rifampicin were also found to enhance the activity of amphotericin B to suppress biofilm formation (Lew et al. 1977; El-Azizi 2007; Miceli et al. 2009; Ku et al. 2010; Del Pozo et al. 2011; Venturini et al. 2016; Fernández-Rivero et al. 2017).
Thus, antibacterial drugs have potential antifungal value due to their good antifungal activity. However, human health is based on the balance of microbiota (Limon et al. 2017; Liu et al. 2020). If antibacterial drugs are approved for the treatment of invasive fungal infections, many problems still need to be considered. For instance, antibiotic treatment will reduce the composition of colonizing microbiota (Sam et al. 2017). Moreover, many antimicrobial drugs can promote fungal growth and enhance fungal pathogenicity indirectly by disrupting the microbiome and eliminating anaerobic bacteria, which might inhibit fungi, especially in the gut (Sam et al. 2017). Our previous investigation (unpublished article) also showed that the antifungal applications of antibiotics interfere with the homeostasis of symbiotic bacteria and fungi in the body. Moreover, dysbiosis of microbiota is responsible for the occurrence of many other diseases in humans such as cardiovascular, cancer, allergy, and the microbiota also affect the human immune system and the synthesis of nutrients (Thomas et al. 2017; Ran et al. 2020). In addition, the pharmacokinetics of antibiotics with antifungal activity in vivo require further investigation.
Immunosuppressants
Immunosuppressants are another example of drug repurposing due to their antifungal activity. It is known that immunosuppressants mainly include calcineurin inhibitors (e.g., cyclosporine; pimecrolimus; and tacrolimus, FK506), target of rapamycin inhibitors (e.g., rapamycin), anti-metabolic agents (e.g., mizoribine, MZP; mycophenolic acid, MPA), and glucocorticoids (e.g., budesonide, dexamethasone, and hydrocortisone). Initially, rapamycin was found to be an antifungal agent and inhibited yeasts including C. albicans, dermatophytes, Microsporum gypseum, and Trichophyton granulosum (Vézina et al. 1975). Rapamycin, probably acting on the recombinant FK506 binding protein (FKBP) complex, showed activity on clinical isolates of Candida in vitro, on the basis of a randomized controlled trial of rapamycin versus candicidin, nystatin, and amphotericin B. The results demonstrated that rapamycin has antifungal activity superior to that of the other tested compounds (Sehgal et al. 1975). The immunosuppressants found to have antifungal ability are briefly summarized in Table 2.
Table 2.
Summary of immunosuppressant drugs with antifungal activity
| Immunosuppressants | Fungus | In vitro (MIC: μg/mL) | In vivo | In combination (synergistic effects) | Relevant molecular mechanism | Ref. |
|---|---|---|---|---|---|---|
| Cyclosporine | Aspergillus spp. | 1–25 | - | CAS, ISA | Inhibits the calcineurin pathways. | Marchetti et al. (2000a, 2000b); Kontoyiannis et al. (2003) |
| C. albicans | >10 | Rat | FLC, VRC, CAS, AmB | Inhibits the expression of genes related to hyphal development, adhesion, biofilm formation, and drug transporter in C. albicans (Jia et al. 2016). | Uppuluri et al. (2008); Shinde et al. (2012) | |
| Rhizopus spp., Lichtheimia spp., Mucor spp., Rhizomucor spp. | 1–16 | - | ISA | Schwarz et al. (2019) | ||
| Tacrolimus | Trichosporon asahii | > 64 | - | AmB, CAS | Inhibits the activity of calcineurin pathway. | Kubiça et al. (2016) |
| Aspergillus spp. | 0.25–16 | Mice | CAS | High and Washburn (1997); Steinbach et al. (2004); Kubiça et al. (2016); Schwarz and Dannaoui (2020) | ||
| Fusarium spp. | 1–40 | - | CAS | Shalit et al. (2009) | ||
| C. albicans | No effects. | - | FLC, ITR, VRC | Uppuluri et al. (2008); Sun et al. (2008) | ||
| C. dubliniensis | > 4 | - | CAS, FLC, POS | Zhang et al. (2012) | ||
| Rhizopus spp., Lichtheimia spp., Mucor spp., Rhizomucor spp. | 1–8 | - | ISA | Schwarz et al. (2019) | ||
| Pimecrolimus | Malassezia spp. | 16–64 | - | - | - | Sugita et al. (2006) |
| Rapamycin | C. albicans | < 0.09 - > 100 | - | - | Inhibits the TOR pathways via FKBP12-Rapa complex. | Cruz et al. (2001) |
| C. neoformans | 0.39 - >100 | - | - | Cruz et al. (2001) | ||
| Mucor spp. | 8 | G.mellonella | ISA | Bastidas et al. 2012; Schwarz et al. (2019) | ||
| Rhizopus spp., Lichtheimia spp., Rhizomucor spp. | 8 | ISA | Schwarz et al. (2019) | |||
| Phycomyces blakesleeanus | 6.3-200 | - | CAS, ISA | Bastidas et al. (2012) | ||
| Aspergillus spp. | 16 | Mice | ISA | High and Washburn (1997); Kontoyiannis et al. (2003); Schwarz and Dannaoui (2020) | ||
| Mycophenolic acid | C. neoformans | 30 | Nematode | AmB | Disrupts de novo GTP biosynthesis. | Morrow et al. (2012); Banerjee et al. (2014) |
| C. albicans | 0.25 | - | - | Köhler et al. (2005) | ||
| Mizoribine | C. albicans | - | - | - | Disrupt de novo GTP biosynthesis. | Köhler et al. (2005) |
| Dexamethasone | Resistant-azole C. albicans | No effects. | G. mellonella | FLC | Inhibits the drug efflux pump and reduces the activity of extracellular phospholipases. | Sun et al. (2017) |
| Budesonide | Resistant-azole C. albicans | 16- > 128 | G. mellonella | FLC |
(I) Inhibits the function of drug transporters. (II) Reduces the activity of extracellular phospholipases and the formation of biofilm. (III) Promotes apoptosis by the accumulation of ROS. |
Li et al. (2016) |
| Hydrocortisone | A. fumigatus | - | - | ITR | - | Ramondenc et al. (1998) |
Note: VRC, voriconazole; AmB, amphotericin B; FLC, fluconazole; ITR, itraconazole; POS, posaconazole; CAS, caspofungin; ISA, isavuconazole; -, no studies were mentioned in the corresponding references
Inosine monophosphate dehydrogenase (IMPDH) is a crucial enzyme in de novo guanine nucleotide biosynthesis and plays an important role in the rapid proliferation of cells (Cuny et al. 2017). A recent investigation found that benzo[b]thiophene 1,1-dioxide, an IMPDH inhibitor, can weaken the virulence of C. neoformans or kill it entirely (Kummari et al. 2018). In addition, MPA and MZP, other types of IMPDH inhibitors, were confirmed to have significant antifungal effects in C. albicans and C. neoformans by disrupting de novo GTP biosynthesis (Köhler et al. 2005; Morrow et al. 2012; Banerjee et al. 2014). Ribavirin, an antiviral agent, is also a type of IMPDA inhibitor. Interestingly, it was found that ribavirin displayed potent antifungal activity in C. albicans in monotherapy or in combination with fluconazole, itraconazole, and posaconazole in vitro and in vivo. The antifungal mechanism involves disruption of vacuolar function and the reduction of extracellular phospholipase activity (Yousfi et al. 2019; Zhang et al. 2020).
Calcineurin, a conserved serine-threonine specific phosphatase, consists of a catalytic subunit and a regulatory subunit (Bandyopadhyay et al. 2004; Sun et al. 2016). It is one of the important mediators in calcium signals and is involved in hyphal/mycelium formation in C. neoformans and A. fumigatus, and virulence in C. tropicalis (Zhang et al. 2012; Chen et al. 2014). Calcineurin inhibitors (cyclosporin A and FK506) have been shown to improve the survival of mice infected with A. fumigatus (High and Washburn 1997). Clinical experience has also suggested that calcineurin inhibitors (cyclosporin A and FK506) can decrease the mortality of invasive Aspergillosis by forming protein-drug complexes (Torre-Cisneros et al. 1991; Singh et al. 2003). Moreover, cyclosporin A and FK506 combined with fluconazole have highly synergistic effects on C. albicans biofilms both in vitro and in rat venous catheter biofilm models (Uppuluri et al. 2008). Glucocorticoids, such as hydrocortisone, budesonide, and dexamethasone, are immunosuppressants with antifungal activity (Ramondenc et al. 1998; Li et al. 2016; Sun et al. 2017). However, the antifungal activity of glucocorticoids remains controversial. For instance, an investigation found that hydrocortisone enhanced the growth of Aspergillus spp. (Ng et al. 1994). Betamethasone, one of the glucocorticoids, is also capable of promoting hyphal formation, stimulating extracellular phospholipase production, and decreasing the anti–C. albicans activity of amphotericin B and nystatin (Jakab et al. 2015). Moreover, an in vivo investigation also found that glucocorticoids increased fungal burden in the gastrointestinal tract in rats (Myerowitz 1981) and enhanced the frequency of fungal translocation in mice (Maraki et al. 1999). Thus, the practical application of glucocorticoids in fungal infections requires further investigation.
Immunosuppressant-treated fungal cells show phenotypes consistent with inhibition of planktonic cell growth, morphological transformation, and biofilm formation (Uppuluri et al. 2008; Jia et al. 2016). However, at present, the exact mechanisms of immunosuppressant synergism with various antifungal drugs have not been delineated. In particular, the TOR as a representative target may be a promising antifungal approach. To date, there are no relevant fungal-specific inhibitors available (Cordeiro et al. 2014). On the other hand, the use of immunosuppressants can inhibit the host immune response, which increases the risk of fungal infection. According to a large number of in vitro antifungal experiments, immunosuppressants do have potent antifungal effects. However, there are no clinical studies to demonstrate whether immunosuppressants have antifungal effects in humans.
Statins
Statins are firstly known as lipid-lowering and cholesterol-lowering drugs as they inhibit HMG-CoA reductase (an essential enzyme in cholesterol biosynthesis) (Esfahani et al. 2019) and are classified according to their hydrophobicity into hydrophilic statins (pravastatin and rosuvastatin) and lipophilic statins (atorvastatin, cerivastatin, fluvastatin, lovastatin, pitavastatin, and simvastatin). It has been confirmed that statins exert a broad spectrum of anti-fungal effects on Candida spp., Aspergillus spp., and Zygomycetes (Callegari et al. 2010). Table 3 shows a brief summary of the statins with antifungal ability. The antifungal mechanism of statins is focused primarily on the biofilm. For example, the changes in the main components of the biofilm or genes associated with fungal biofilm formation following monotherapy or/and synergism of antifungal agents have been verified. Inexplicably, antifungal activity findings are inconsistent. For instance, pravastatin has synergistic effects with fluconazole by inhibiting farnesol production against C. albicans (Tashiro et al. 2012), and in contrast, no synergy was found between pravastatin and fluconazole in vitro in another investigation (Nash et al. 2002). In addition, a study even reported that pravastatin did not inhibit the growth of Candida spp. (Brilhante et al. 2015). The reason for this may be due to differences in fungi strains and different methodology. These contradictory findings require clarification in further investigations.
Table 3.
Summary of statin drugs with antifungal activity
| Statins | Fungus | In vitro (MIC: μg/mL) | In vivo | In combination (synergistic effects) | Relevant molecular mechanism | Ref. |
|---|---|---|---|---|---|---|
| Lovastatin | Paecilomyces variotii | 64 | - | - | - | Chamilos et al. (2006); Nyilasi et al. (2010); Zhou et al. (2018) |
| Rhizopus oryzae | 128 | - | - | - | ||
| C. albicans | 50–64 | - | FLC, ITR | Inhibits ergosterol synthesis. | ||
| C. glabrata | 128 | - | FLC | - | ||
| A. fumigatus | 25 | - | FLC | - | ||
| Zygomycetes | 32–56 | - | VRC | - | ||
| Simvastatin | C. glabrata | 16–32 | - | - | (I) Inhibits ergosterol synthesis; (II) Causes the loss of mtDNA. | Chamilos et al. (2006); Nyilasi et al. (2010); Westermeyer and Macreadie (2007) |
| C. albicans | 8 | - | MIA | - | Nyilasi et al. (2010) | |
| C. utilis | 200 | - | MIA | Inhibits ergosterol synthesis. | Cabral et al. (2013) | |
| Cryptococcus spp. | 62.5–1000 | - | AmB, ITR, FLC | Inhibits ergosterol synthesis. | Silva et al. (2020) | |
| Saccharomyces cerevisiae | 40 | - | CTZ, ITR, MIA | Inhibits ergosterol synthesis. | Cabral et al. (2013) | |
| Paecilomyces variotii | 8 | - | - | - | Nyilasi et al. (2010) | |
| Rhizopus oryzae | 64 | - | - | - | Nyilasi et al. (2010) | |
| A. fumigatus | 6.25 | - | FLC | - | Nyilasi et al. (2010) | |
| Pravastatin | C. albicans | No effects. | Mice | FLC | Inhibits farnesol production. | Nash et al. (2002) |
| Atorvastatin | C. albicans | 16–256 | G. mellonella | MIA | Inhibits ergosterol synthesis. | Nyilasi et al. (2010); Ajdidi et al. (2019); Esfahani et al. (2019) |
| C. glabrata | 4–64 | - | FLC | - | Nyilasi et al. (2010); Esfahani et al. (2019); Lima et al. (2019) | |
| C. utilis | 200 | - | - | - | Silva et al. (2020) | |
| C. krusei | 8 | - | - | - | Esfahani et al. (2019) | |
| C. kefyr | 4-16 | - | - | - | Esfahani et al. (2019) | |
| Cryptococcus gattii | ≥ 256 | Mice | FLC | (I) Inhibits ergosterol synthesis; (II) Induces the production of ROS. | Chin et al. (1997); Ribeiro et al. (2017) | |
| Saccharomyces cerevisiae | 40 | - | CTZ, ITR, MIA | - | Cabral et al. (2013) | |
| C. stellatoidea | 16 | - | - | - | Esfahani et al. (2019) | |
| Rhizopus oryzae | 32 | - | ITR, KET | - | Nyilasi et al. (2010) | |
| Paecilomyces variotii | 32 | - | - | - | Nyilasi et al. (2010) | |
| Aspergillus flavus | > 128 | - | ITR | - | Nyilasi et al. (2010) | |
| A. fumigatus | 64 | - | ITR, FLC, MIA, KET |
(I) Stimulates oxidative stress response; (II) Inhibits ergosterol synthesis. |
Nyilasi et al. (2010; Ajdidi et al. (2020) | |
| Fluvastatin | C. albicans | 25 | No effects in mice | FLC | - | Nyilasi et al. (2010); Lima et al. (2019) |
| C. glabrata | 32–64 | - | - | - | Nyilasi et al. (2010); Lima et al. (2019) | |
| C. tropicalis | 128 | - | - | - | Lima et al. (2019) | |
| C. dubliniensis | 16–512 | - | - | - | Lima et al. (2019) | |
| Cryptococcus spp. | 64- >128 | - | ITR, FLC | - | Chin et al. (1997) | |
| Paecilomyces variotii | 25 | - | - | - | Nyilasi et al. (2010) | |
| Rhizopus oryzae | 2–3.125 | - | KET, ITR | - | Nyilasi et al. (2010) | |
| A. fumigatus | 2 | - | - | - | Nyilasi et al. (2010) | |
| A. flavus | 128 | - | KET, MIA, ITR | - | Nyilasi et al. (2010) | |
| Rosuvastatin | C. albicans | 8–128 | - | MIA | - | Nyilasi et al. (2010); Lima et al. (2019) |
| C. glabrata | 32–128 | - | - | - | Nyilasi et al. (2010; Lima et al. (2019) | |
| C. utilis | 200 | - | MIA | - | Cabral et al. (2013) | |
| Paecilomyces variotii | 32 | - | - | - | Nyilasi et al. (2010) | |
| Saccharomyces cerevisiae | 40 | - | CTZ, ITR, MIA | - | Cabral et al. (2013) | |
| Rhizopus oryzae | > 128 | - | KET, ITR | - | Nyilasi et al. (2010) | |
| A. fumigatus | 128 | - | ITR | - | Nyilasi et al. (2010) | |
| A. flavus | > 128 | - | - | - | Nyilasi et al. (2010) | |
| Pitavastatin | C. albicans, C. glabrata, C. auris | 8 | Caenorhabditis elegans | ITR, VRC, FLC | - | Eldesouky et al. (2020) |
Note: VRC, voriconazole; AmB, amphotericin B; FLC, fluconazole; ITR, itraconazole; CTZ, clotrimazole; ANI, anidulafungin; MIA, micafungin; -, no relevant studies were mentioned in the corresponding references
Antiarrhythmic drugs
Antiarrhythmic drugs, which are used in the prevention and treatment of tachycardia, bradycardia, or arrhythmia, include sodium channel antagonists, β-receptor blockers, potassium channel blockers (PCRs), and calcium channel blockers (CCBs). Trials have shown that amiodarone, PCRs, and CCBs exhibit favorable antifungal activity when administered alone or combined with conventional antifungals. CCBs, as the name suggests, prevent calcium ions from entering cells and maintain metabolic processes. Verapamil (verapamil hydrochloride), a phenylalkylamine CCB, mainly combats C. albicans by affecting hyphal development, adhesion, gastrointestinal colonization, or increasing strain susceptibility to oxidative stress (Yu et al. 2014a, 2014b). In addition, verapamil enhances the antifungal activity of tunicamycin or fluconazole against C. albicans during biofilm formation and pre-formed biofilms (Yu et al. 2013). In A. fumigatus, the drug efflux pump was blocked and ergosterol content was decreased following treatment with verapamil and itraconazole simultaneously (Zeng et al. 2019). Other CCBs, such as diltiazem, nicardipine, and nifedipine, have shown antifungal activity against C. albicans, C. glabrata, Ascomycetous, and Mucoralean fungi, either alone or in combination with antifungals (Bulatova and Darwish 2008; Homa et al. 2017; Alnajjar et al. 2018). Amiodarone is known to block potassium, sodium, and calcium channels and is commonly used to treat and prevent tachyarrhythmia (Kodama et al. 1999; Nattel and Singh 1999; Dorian 2000). In pathogens, amiodarone mainly disrupted calcium homeostasis to elicit high levels of cytoplasmic calcium, leading to cell death in Cryptococcus spp., Aspergillus spp., Fusarium oxysporum, C. albicans, C. tropicalis, and Saccharomyces cerevisiae (Courchesne 2002; Afeltra et al. 2004; Courchesne et al. 2009; Knorre et al. 2009; Gamarra et al. 2010; Bagar and Benčina 2012; Silva et al. 2013). In addition, amiodarone displayed potent fungicidal effects at a low dose combined with fluconazole and miconazole (Gupta et al. 2003). Hence, calcium channels may be potential targets in the therapy of fungal-related infections.
Antipsychotic drugs
Antipsychotic drugs include benzamides, butyrophenones, dibenzoxazepine, phenothiazines, and thioxanthene. Phenothiazines are the first generation of antipsychotics and are mainly used to treat schizophrenia and mania. In addition, phenothiazines possess multiple effects such as altering the metabolism of cyclic nucleotides, modifying the structure of membranes, binding to calmodulin and they participate in many intracellular responses (Kim et al. 2019), which may explain the antifungal action of phenothiazines (Wood and Nugent 1985. 1985; Rossato et al. 2016). Chlorpromazine and trifluoperazine are representative phenothiazines. Both block the central dopamine D2 receptor to improve symptoms in mentally ill patients. They have excellent activity against Candida spp. and C. neoformans either alone or in combination with ketoconazole and amphotericin B (Wood and Nugent 1985; Ben-Gigi et al. 1988; Sharma et al. 2001; Galgóczy et al. 2011; Rossato et al. 2016). Trifluoperazine also has fungicidal effects on C. neoformans, especially on melanized cells (Wang and Casadevall 1996). In addition, chlorpromazine and trifluoperazine have fungicidal effects on Zygomycetes when concentrations reach 25–200 μg/mL. Moreover, it has synergistic effects with amphotericin B (Galgóczy et al. 2009). The minimum fungicidal concentration of chlorpromazine and trifluoperazine in Aspergillus spp., Scedosporium, and Pseudallescheria ranged between 10 and 64 μg/mL (Vitale et al. 2007; Homa et al. 2015). Flunarizine is a difluorinated derivative of piperazine as well as a potent CCB. It has the same structure as phenothiazines. Flunarizine also exhibits broad-spectrum antifungal effects against Candida spp., Cryptococcus spp., and Zygosaccharomyces spp. alone or jointly with ketoconazole in vitro probably by inhibiting calmodulin activity and increasing the penetration of ketoconazole through cell walls (Krajewska-Kułak and Niczyporuk 1993).
Compared to the traditional antifungal drugs, the main advantage of phenothiazines is that they can cross the blood-brain barrier and improve bioavailability. Moreover, the levels achievable in the brain with antipsychotic therapeutic doses range from 50 to 100 μg/mL; however, the range in plasma is only between 0.5 and 1 μg/mL (Homa et al. 2015).
Antidepressant drugs
There are currently many types of antidepressant drugs used in clinics. These mainly include monoamine oxidase inhibitors (e.g., phenelzine), tricyclic (e.g., amitriptyline and doxepin), tetracyclic (e.g., maprotiline), and selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine and paroxetine). Of these, SSRIs are first-line antidepressant drugs with low side effects. They not only have anti-depression activity but also have anti-anxiety activity (Latendresse et al. 2017). SSRIs effectively inhibit the uptake of serotonin by neurons from synaptic spaces. This increases the availability of this neurotransmitter in these spaces and improves emotional states. As such, SSRIs can be used to treat depressive mental disorders (Bandelow et al. 2017). Encouragingly, SSRIs have been shown to have positive antifungal activity in various studies. An antifungal experiment showed that fluoxetine could kill some azole-resistant Candida sp. strains in vitro with or without fluconazole. Moreover, they also improved the survival rate of Galleria mellonella. The antifungal mechanism involves inhibition of extracellular phospholipase activity by down-regulated SAP1-4 genes in resistant C. albicans (Oliveira et al. 2014; Gu et al. 2016). The SAP genes encode secreted aspartyl proteinases (SAP), which are key virulence factors and play an important role in the growth, development, and pathogenicity of Candida spp. (Naglik et al. 2003). In addition, fluoxetine exhibited synergistic effects against C. albicans biofilms and relieved oral candidiasis in infected mice when combined with caspofungin (Jiang et al. 2020). Sertraline, another type of SSRI, also showed positive antifungal activity alone or in combination with antifungal agents. It can reduce the fungal burden, improve survival rate, and impair tissue damage in mice and G. mellonella (Treviño-Rangel Rde et al. 2016; Treviño-Rangel et al. 2019). Similar results showed that sertraline had fungistatic or fungicidal effects in Candida spp., Coccidioides immitis, C. neoformans, Trichosporon asahii, and A. fumigatus (Cong et al. 2016; Paul et al. 2016; Treviño-Rangel Rde et al. 2016; Oliveira et al. 2018; Treviño-Rangel et al. 2019; Gowri et al. 2020).
Non-steroidal anti-inflammatory drugs
NSAIDs act mainly by inhibiting the activity of cyclooxygenase to reduce the production of prostaglandins (PGs) and thus have antipyretic, analgesic, anti-inflammatory, and other functions (Fitzpatrick 2004). NSAIDs, especially aspirin, etodolac, diclofenac, celecoxib, nimesulide, ibuprofen, meloxicam, ketoprofen, tenoxicam, and ketorolac exhibited favorable anti–C. albicans effects by inhibiting the synthesis of fungal PGs, which play an important role in biofilm development, adhesion, and morphogenesis in C. albicans (Alem and Douglas 2004; Abdelmegeed and Shaaban 2013). In addition, most of these drugs had synergistic or additive (ketorolac) activity with fluconazole against C. albicans (Abdelmegeed and Shaaban 2013). Subsequently, the antifungal mechanisms of aspirin and ibuprofen have become clearer. They can, by activating the high-osmolarity glycerol pathway, induce the accumulation of reactive oxygen species (ROS) and then simultaneously damage the integrity of cell membranes leading to the death of Cryptococcus cells (Ogundeji et al. 2016). In addition, aspirin and ibuprofen combined with fluconazole, caspofungin, and amphotericin B have effects on fungi (Ogundeji et al. 2016; Yang et al. 2016). Ibuprofen also has anti-Sporothrix activity singly (median MIC of 256 μg/mL) or in combination with antifungal agents including amphotericin B, itraconazole, and terbinafine (Borba-Santos et al. 2021). Diclofenac sodium can downregulate the expression of Ef-1 gene, which is involved in cellular RNA transport, cell cycle, and apoptosis (Alves et al. 2015), thus resulting in a reduction in the formation of A. fumigatus filaments (Nargesi and Rezaie 2018).
Conclusion
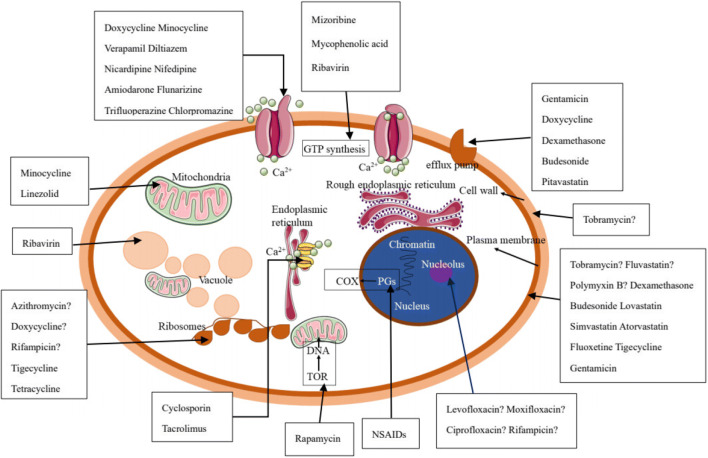
In this review, we summarized the antifungal effects of a number of non-antifungal agents. In addition, some antitumor agents such as miltefosine (Wu et al. 2020), tamoxifen (Hai et al. 2019), methotrexate (Yang et al. 2019), and antiepileptic drugs (Kathwate et al. 2015) have been reported to have antifungal effects. The use of drug repurposing strategies in the discovery of novel antifungal agents is a revelation in the identification of new antifungal drugs through structural readjustment. With regard to their related antifungal targets, there are still many antifungal mechanisms of the above-mentioned drugs which are unclear (Fig. 1). To reveal the precise targets, further investigations should be performed using transcriptome analysis and molecular techniques, which will lay the foundation for the development of novel antifungal drugs for example using target design. In addition, these antifungal experiments have only focused on either in vitro studies or animal model experiments. There is not enough clinical evidence to prove their practical use in the clinic. Moreover, many factors, such as changes in medium composition will perhaps lead to different or completely opposite results. In in vivo studies, differences between animal models or homogeneous animal models and differences in pharmacokinetic and pharmacodynamic parameters of compounds in these models, and the effects of host-derived serum and, cellular factors should be clarified. Hence, it is essential to use systematic and standard research approaches and to collect more clinical data to evaluate the antifungal effectiveness of these agents.
Fig. 1.
The antifungal targets of the above-mentioned non-antifungal agents. “?”, the target is not clear; COX, cyclooxygenase; PGs, prostaglandins; NSAIDs, non-steroidal anti-inflammatory drugs
Author contribution
ZYS and YYM designed the study. QZ and FYL drafted the manuscript. MZ contributed to the revision of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was supported financially by the Sichuan Province Administration of Traditional Chinese Medicine (2020JC0129), the Technology Strategic Cooperation Project of Luzhou municipal people's government-Southwest Medical University (2020LZXNYDJ38, 2020LZXNYDJ23), and the Foundation of Southwest Medical University (No. 2020ZRQNA023, No. 2020ZRQNA039, No. 2020ZRQNB066).
Declarations
Ethics statement
This article does not contain any studies on human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qian Zhang, Email: Zq1729174802@163.com.
Fangyan Liu, Email: liufangyan1989@163.com.
Meng Zeng, Email: zengmeng19881026@163.com.
Yingyu Mao, Email: tianya1000@126.com.
Zhangyong Song, Email: szy83529@163.com.
References
- Abdelmegeed E, Shaaban MI (2013) Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of fluconazole resistant Candida albicans. J Microbiol 51(5):598–604. 10.1007/s12275-013-3052-6 [DOI] [PubMed]
- Afeltra J, Vitale RG, Mouton JW, Verweij PE. Potent synergistic in vitro interaction between nonantimicrobial membrane-active compounds and itraconazole against clinical isolates of Aspergillus fumigatus resistant to itraconazole. Antimicrob Agents Chemother. 2004;48(4):1335–1343. doi: 10.1128/aac.48.4.1335-1343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar Cordeiro R, de Jesus Evangelista AJ, Serpa R, Colares de Andrade AR, Leite Mendes PB, Silva Franco JD, de Oliveira JS, de Alencar LP, Sales JA, Carneiro Câmara LM, Souza Collares Maia Castelo-Branco D, Nogueira Brilhante RS, Costa Sidrim JJ, Gadelha Rocha MF. β-lactam antibiotics & vancomycin increase the growth & virulence of Candida spp. Future Microbiol. 2018;13:869–875. doi: 10.2217/fmb-2018-0019. [DOI] [PubMed] [Google Scholar]
- Ajdidi A, Sheehan G, Abu Elteen K, Kavanagh K. Assessment of the in vitro and in vivo activity of atorvastatin against Candida albicans. J Med Microbiol. 2019;68(10):1497–1506. doi: 10.1099/jmm.0.001065. [DOI] [PubMed] [Google Scholar]
- Ajdidi A, Sheehan G, Kavanagh K. Exposure of Aspergillus fumigatus to atorvastatin leads to altered membrane permeability and induction of an oxidative stress response. J Fungi (Basel) 2020;6(2):42. doi: 10.3390/jof6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alem MA, Douglas LJ. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother. 2004;48(1):41–47. doi: 10.1128/aac.48.1.41-47.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnajjar LM, Bulatova NR, Darwish RM. Evaluation of four calcium channel blockers as fluconazole resistance inhibitors in Candida glabrata. J Glob Antimicrob Resist. 2018;14:185–189. doi: 10.1016/j.jgar.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Alves LR, Oliveira C, Goldenberg S. Eukaryotic translation elongation factor-1 alpha is associated with a specific subset of mRNAs in Trypanosoma cruzi. BMC Microbiol. 2015;15:104. doi: 10.1186/s12866-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol. 2014;10(5):400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup MC, Cuenca-Estrella M, Lass-Flo¨rl C, Hope WW. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect. 2012;18(7):E248–E250. doi: 10.1111/j.1469-0691.2012.03890.x. [DOI] [PubMed] [Google Scholar]
- Arrieta O, Barrón F, Padilla MS, Avilés-Salas A, Ramírez-Tirado LA, Arguelles Jiménez MJ, Vergara E, Zatarain-Barrón ZL, Hernández-Pedro N, Cardona AF, Cruz-Rico G, Barrios-Bernal P, Yamamoto Ramos M, Rosell R. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):e192553. doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Baddley JW, Pappas PG. Antifungal combination therapy: clinical potential. Drugs. 2005;65(11):1461–1480. doi: 10.2165/00003495-200565110-00002. [DOI] [PubMed] [Google Scholar]
- Bagar T, Benčina M. Antiarrhythmic drug amiodarone displays antifungal activity, induces irregular calcium response and intracellular acidification of Aspergillus niger - amiodarone targets calcium and pH homeostasis of A. niger. Fungal Genet Biol. 2012;49(10):779–791. doi: 10.1016/j.fgb.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–107. doi: 10.31887/DCNS.2017.19.2/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay J, Lee J, Bandyopadhyay A. Regulation of calcineurin, a calcium/calmodulin-dependent protein phosphatase, in C. elegans. Mol Cell. 2004;18(1):10–16. [PubMed] [Google Scholar]
- Banerjee D, Burkard L, Panepinto JC. Inhibition of nucleotide biosynthesis potentiates the antifungal activity of amphotericin B. PLoS One. 2014;9(1):e87246. doi: 10.1371/journal.pone.0087246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas RJ, Shertz CA, Lee SC, Heitman J, Cardenas ME. Rapamycin exerts antifungal activity in vitro and in vivo against Mucor circinelloides via FKBP12-dependent inhibition of Tor. Eukaryot Cell. 2012;11(3):270–281. doi: 10.1128/EC.05284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Gigi G, Polacheck I, Eilam Y. In vitro synergistic activity of ketoconazole with trifluoperazine and with chlorpromazine against medically important yeasts. Chemotherapy. 1988;34(2):96–100. doi: 10.1159/000238554. [DOI] [PubMed] [Google Scholar]
- Bennet JE. Flucytosine. Ann Intern Med. 1977;86(3):319–321. doi: 10.7326/0003-4819-86-3-319. [DOI] [PubMed] [Google Scholar]
- Boral H, Metin B, Döğen A, Seyedmousavi S, Ilkit M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, and Exophiala dermatitidis. Fungal Genet Biol. 2018;111:92–107. doi: 10.1016/j.fgb.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Borba-Santos LP, Nucci M, Ferreira-Pereira A, Rozental S. Anti-Sporothrix activity of ibuprofen combined with antifungal. Braz J Microbiol. 2021;52(1):101–106. doi: 10.1007/s42770-020-00327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31(5):558–568. doi: 10.1016/j.annonc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Bridges KJ, Li R, Fleseriu M, Cetas JS. Candida meningitis after transsphenoidal surgery: a single-institution case series and literature review. World Neurosurg. 2017;108:41–49. doi: 10.1016/j.wneu.2017.08.115. [DOI] [PubMed] [Google Scholar]
- Brilhante RS, Caetano EP, Oliveira JS, Castelo-Branco Dde S, Souza ER, Alencar LP, Cordeiro Rde A, Bandeira Tde J, Sidrim JJ, Rocha MF. Simvastatin inhibits planktonic cells and biofilms of Candida and Cryptococcus species. Braz J Infect Dis. 2015;19(5):459–465. doi: 10.1016/j.bjid.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulatova NR, Darwish RM. Effect of chemosensitizers on minimum inhibitory concentrations of fluconazole in Candida albicans. Med Princ Pract. 2008;17(2):117–121. doi: 10.1159/000112964. [DOI] [PubMed] [Google Scholar]
- Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, Möslein G, McRonald FE, Bertario L, Evans DG, Gerdes AM, Ho JWC, Lindblom A, Morrison PJ, Rashbass J, Ramesar R, Seppälä T, Thomas HJW, Pylvänäinen K, Borthwick GM, Mathers JC, Bishop DT, CAPP2 Investigators Cancer prevention with aspirin in hereditary colorectal cancer (lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–1863. doi: 10.1016/S0140-6736(20)30366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral ME, Figueroa LI, Fariña JI. Synergistic antifungal activity of statin-azole associations as witnessed by Saccharomyces cerevisiae - and Candida utilis - bioassays and ergosterol quantification. Rev Iberoam Micol. 2013;30(1):31–38. doi: 10.1016/j.riam.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Callegari S, McKinnon RA, Andrews S, de Barros Lopes MA. Atorvastatin-induced cell toxicity in yeast is linked to disruption of protein isoprenylation. FEMS Yeast Res. 2010;10(2):188–198. doi: 10.1111/j.1567-1364.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, Papapetropoulos S, Grossman I, Laifenfeld D. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175(2):168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G, Lewis RE, Kontoyiannis DP. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob Agents Chemother. 2006;50(1):96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Slavin MA, Chen SCA. New developments and directions in the clinical application of the echinocandins. Arch Toxicol. 2017;91(4):1613–1621. doi: 10.1007/s00204-016-1916-3. [DOI] [PubMed] [Google Scholar]
- Chen YL, Yu SJ, Huang HY, Chang YL, Lehman VN, Silao FG, Bigol UG, Bungay AA, Averette A, Heitman J. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot Cell. 2014;13(7):844–854. doi: 10.1128/EC.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Metformin use and gastric cancer risk in diabetic patients after helicobacter pylori eradication. J Natl Cancer Inst. 2019;111(5):484–489. doi: 10.1093/jnci/djy144. [DOI] [PubMed] [Google Scholar]
- Chin NX, Weitzman I, Della-Latta P. In vitro activity of fluvastatin, a cholesterol-lowering agent, and synergy with fluconazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41(4):850–852. doi: 10.1128/AAC.41.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26(11):2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321(12):794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- Cong L, Liao Y, Yang S, Yang R. In vitro antifungal activity of sertraline and synergistic effects in combination with antifungal drugs against planktonic forms and biofilms of clinical Trichosporon asahii isolates. PLoS One. 2016;11(12):e0167903. doi: 10.1371/journal.pone.0167903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro RA, Macedo RB, Teixeira CEC, Marques FJF, Bandeira TJPG, Moreira JLB, Brilhante RSN, Rocha MFG, Sidrim JJC. The calcineurin inhibitor cyclosporin A exhibits synergism with antifungals against Candida parapsilosis species complex. J Med Microbiol. 2014;63(Pt 7):936–944. doi: 10.1099/jmm.0.073478-0. [DOI] [PubMed] [Google Scholar]
- Courchesne WE. Characterization of a novel, broad-based fungicidal activity for the antiarrhythmic drug amiodarone. J Pharmacol Exp Ther. 2002;300(1):195–199. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- Courchesne WE, Tunc M, Liao S. Amiodarone induces stress responses and calcium flux mediated by the cell wall in Saccharomyces cerevisiae. Can J Microbiol. 2009;55(3):288–303. doi: 10.1139/w08-132. [DOI] [PubMed] [Google Scholar]
- Cruz MC, Goldstein AL, Blankenship J, Del Poeta M, Perfect JR, McCusker JH, Bennani YL, Cardenas ME, Heitman J. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother. 2001;45(11):3162–3170. doi: 10.1128/AAC.45.11.3162-3170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny GD, Suebsuwong C, Ray SS. Inosine-5'-monophosphate dehydrogenase (IMPDH) inhibitors: a patent and scientific literature review (2002-2016) Expert Opin Ther Pat. 2017;27(6):677–690. doi: 10.1080/13543776.2017.1280463. [DOI] [PubMed] [Google Scholar]
- Deaguero IG, Huda MN, Rodriguez V, Zicari J, Al-Hilal TA, Badruddoza AZM, Nurunnabi M. Nano-vesicle based anti-fungal formulation shows higher stability, skin diffusion, biosafety and anti-fungal efficacy in vitro. Pharmaceutics. 2020;12(6):516. doi: 10.3390/pharmaceutics12060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Francés ML, Hernáez S, Serrera A, Alonso M, Rubio MF. Effect of amphotericin B alone or in combination with rifampicin or clarithromycin against Candida species biofilms. Int J Artif Organs. 2011;34(9):766–770. doi: 10.5301/ijao.5000023. [DOI] [PubMed] [Google Scholar]
- Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362(9390):1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- Deren YT, Ozdek S, Kalkanci A, Akyürek N, Hasanreisoğlu B. Comparison of antifungal efficacies of moxifloxacin, liposomal amphotericin B, and combination treatment in experimental Candida albicans endophthalmitis in rabbits. Can J Microbiol. 2010;56(1):1–7. doi: 10.1139/w09-112. [DOI] [PubMed] [Google Scholar]
- Dorian P. Mechanisms of action of class III agents and their clinical relevance. Europace. 2000;1(Suppl C):C6–C9. [PubMed] [Google Scholar]
- Drgona L, Khachatryan A, Stephens J, Charbonneau C, Kantecki M, Haider S, Barnes R. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014;33(1):7–21. doi: 10.1007/s10096-013-1944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy ABC, Tassone F, Eldeeb M, Salcedo-Arellano MJ, Tartaglia N, Hagerman R. Metformin as targeted treatment in fragile X syndrome. Clin Genet. 2018;93(2):216–222. doi: 10.1111/cge.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Azizi M (2007) Enhancement of the in vitro activity of amphotericin B against the biofilms of non-albicans Candida spp. by rifampicin and doxycycline. J Med Microbiol 56(Pt 5):645–649. 10.1099/jmm.0.46952-0 [DOI] [PubMed]
- Eldesouky HE, Salama EA, Li X, Hazbun TR, Mayhoub AS, Seleem MN. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci Rep. 2020;10(1):7525. doi: 10.1038/s41598-020-64571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother. 2002;49(Suppl 1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- Emami S, Tavangar P, Keighobadi M. An overview of azoles targeting sterol 14α-demethylase for antileishmanial therapy. Eur J Med Chem. 2017;135:241–259. doi: 10.1016/j.ejmech.2017.04.044. [DOI] [PubMed] [Google Scholar]
- Emri T, Majoros L, Tóth V, Pócsi I. Echinocandins: production and applications. Appl Microbiol Biotechnol. 2013;97(8):3267–3284. doi: 10.1007/s00253-013-4761-9. [DOI] [PubMed] [Google Scholar]
- Esfahani AN, Golestannejad Z, Khozeimeh F, Dehghan P, Maheronnaghsh M, Zarei Z. Antifungal effect of atorvastatin against Candida species in comparison to fluconazole and nystatin. Med Pharm Rep. 2019;92(4):368–373. doi: 10.15386/mpr-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rivero ME, Del Pozo JL, Valentín A, de Diego AM, Pemán J, Cantón E. Activity of amphotericin B and anidulafungin combined with rifampicin, clarithromycin, ethylenediaminetetraacetic acid, N-acetylcysteine, and farnesol against Candida tropicalis biofilms. J Fungi (Basel) 2017;3(1):16. doi: 10.3390/jof3010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori A, Van Dijck P. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob Agents Chemother. 2012;56(7):3785–3796. doi: 10.1128/AAC.06017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004;10(6):577–588. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- Galgóczy L, Papp T, Kovács L, Ordögh L, Vágvölgyi C. In vitro activity of phenothiazines and their combinations with amphotericin B against Zygomycetes causing rhinocerebral zygomycosis. Med Mycol. 2009;47(3):331–335. doi: 10.1080/13693780802378853. [DOI] [PubMed] [Google Scholar]
- Galgóczy L, Bácsi A, Homa M, Virágh M, Papp T, Vágvölgyi C. In vitro antifungal activity of phenothiazines and their combination with amphotericin B against different Candida species. Mycoses. 2011;54(6):e737–e743. doi: 10.1111/j.1439-0507.2010.02010.x. [DOI] [PubMed] [Google Scholar]
- Gamarra S, Rocha EM, Zhang YQ, Park S, Rao R, Perlin DS. Mechanism of the synergistic effect of amiodarone and fluconazole in Candida albicans. Antimicrob Agents Chemother. 2010;54(5):1753–1761. doi: 10.1128/AAC.01728-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang C, Lu C, Liu P, Li Y, Li H, Sun S. Synergistic effect of doxycycline and fluconazole against Candida albicans biofilms and the impact of calcium channel blockers. FEMS Yeast Res. 2013;13(5):453–462. doi: 10.1111/1567-1364.12048. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li H, Liu S, Zhang X, Sun S. Synergistic effect of fluconazole and doxycycline against Candida albicans biofilms resulting from calcium fluctuation and downregulation of fluconazole - inducible efflux pump gene overexpression. J Med Microbiol. 2014;63(Pt 7):956–961. doi: 10.1099/jmm.0.072421-0. [DOI] [PubMed] [Google Scholar]
- Gao L, Sun Y, Yuan M, Li M, Zeng T. In vitro and in vivo study on the synergistic effect of minocycline and azoles against pathogenic fungi. Antimicrob Agents Chemother. 2020;64(6):e00290–e00220. doi: 10.1128/AAC.00290-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbino J. Caspofungin--a new therapeutic option for oropharyngeal candidiasis. Clin Microbiol Infect. 2004;10(3):187–189. doi: 10.1111/j.1198-743x.2004.00823.x. [DOI] [PubMed] [Google Scholar]
- Gil-Alonso S, Jauregizar N, Eraso E, Quindós G. Postantifungal effect of micafungin against the species complexes of Candida albicans and Candida parapsilosis. PLoS One. 2015;10(7):e0132730. doi: 10.1371/journal.pone.0132730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowri M, Sofi Beaula W, Biswal J, Dhamodharan P, Saiharish R, Rohan prasad S, Pitani R, Kandaswamy D, Raghunathan R, Jeyakanthan J, Rayala SK, Venkatraman G. β-lactam substituted polycyclic fused pyrrolidine/pyrrolizidine derivatives eradicate C. albicans in an ex vivo human dentinal tubule model by inhibiting sterol 14-α demethylase and cAMP pathway. Biochim Biophys Acta. 2016;1860(4):636–647. doi: 10.1016/j.bbagen.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Gowri M, Jayashree B, Jeyakanthan J, Girija EK. Sertraline as a promising antifungal agent: inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J Appl Microbiol. 2020;128(2):426–437. doi: 10.1111/jam.14490. [DOI] [PubMed] [Google Scholar]
- Greene RA, Adams KK, Rogers RD, Berard-Collins C, Lorenzo MP. Pharmacokinetics of flucytosine in a critically ill patient on continuous venovenous hemodiafiltration. Am J Health Syst Pharm. 2020;77(8):609–613. doi: 10.1093/ajhp/zxaa034. [DOI] [PubMed] [Google Scholar]
- Gu W, Guo D, Zhang L, Xu D, Sun S. The synergistic effect of azoles and fluoxetine against resistant Candida albicans strains is attributed to attenuating fungal virulence. Antimicrob Agents Chemother. 2016;60(10):6179–6188. doi: 10.1128/AAC.03046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Yu Q, Yu C, Sun S. In vivo activity of fluconazole/tetracycline combinations in Galleria mellonella with resistant Candida albicans infection. J Glob Antimicrob Resist. 2018;13:74–80. doi: 10.1016/j.jgar.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Gupta SS, Ton VK, Beaudry V, Rulli S, Cunningham K, Rao R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J Biol Chem. 2003;278(31):28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- Hai TP, Van AD, Ngan NTT, Nhat LTH, Lan NPH, Vinh Chau NV, Thwaites GE, Krysan D, Day JN. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses. 2019;62(9):818–825. doi: 10.1111/myc.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar G, Singh N. How we approach combination antifungal therapy for invasive aspergillosis and mucormycosis in transplant recipients. Transplantation. 2018;102(11):1815–1823. doi: 10.1097/TP.0000000000002353. [DOI] [PubMed] [Google Scholar]
- High KP, Washburn RG. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin) J Infect Dis. 1997;175(1):222–225. doi: 10.1093/infdis/175.1.222. [DOI] [PubMed] [Google Scholar]
- Homa M, Galgóczy L, Tóth E, Tóth L, Papp T, Chandrasekaran M, Kadaikunnan S, Alharbi NS, Vágvölgyi C. In vitro antifungal activity of antipsychotic drugs and their combinations with conventional antifungals against Scedosporium and Pseudallescheria isolates. Med Mycol. 2015;53(8):890–895. doi: 10.1093/mmy/myv064. [DOI] [PubMed] [Google Scholar]
- Homa M, Hegedűs K, Fülöp Á, Wolfárt V, Kadaikunnan S, Khaled JM, Alharbi NS, Vágvölgyi C, Galgóczy L. In vitro activity of calcium channel blockers in combination with conventional antifungal agents against clinically important filamentous fungi. Acta Biol Hung. 2017;68(3):334–344. doi: 10.1556/018.68.2017.3.10. [DOI] [PubMed] [Google Scholar]
- Jakab Á, Emri T, Sipos L, Kiss Á, Kovács R, Dombrádi V, Kemény-Beke Á, Balla J, Majoros L, Pócsi I. Betamethasone augments the antifungal effect of menadione--towards a novel anti-Candida albicans combination therapy. J Basic Microbiol. 2015;55(8):973–981. doi: 10.1002/jobm.201400903. [DOI] [PubMed] [Google Scholar]
- Jia W, Zhang H, Li C, Li G, Liu X, Wei J. The calcineruin inhibitor cyclosporine a synergistically enhances the susceptibility of Candida albicans biofilms to fluconazole by multiple mechanisms. BMC Microbiol. 2016;16(1):113. doi: 10.1186/s12866-016-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zheng L, Sun KA, Zhou P, Xu R, Gu J, Wei X. In vitro and in vivo evaluation of the antifungal activity of fluoxetine combined with antifungals against Candida albicans biofilms and oral candidiasis. Biofouling. 2020;36(5):537–548. doi: 10.1080/08927014.2020.1777401. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y, THALES investigators Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383(3):207–217. doi: 10.1056/NEJMc2027491. [DOI] [PubMed] [Google Scholar]
- Kathwate GH, Shinde RB, Karuppayil SM. Antiepileptic drugs inhibit growth, dimorphism, and biofilm mode of growth in human pathogen Candida albicans. Assay Drug Dev Technol. 2015;13(6):307–312. doi: 10.1089/adt.2015.29007.ghkdrrr. [DOI] [PubMed] [Google Scholar]
- Keçeli SA, Willke A, Tamer GS, Boral OB, Sonmez N, Cağatay P. Interaction between caspofungin or voriconazole and cefoperazone-sulbactam or piperacillin-tazobactam by in vitro and in vivo methods. APMIS. 2014;122(5):412–417. doi: 10.1111/apm.12159. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chan KL, Cheng LW, Tell LA, Byrne BA, Clothier K, Land KM. High efficiency drug repurposing design for new antifungal agents. Methods Protoc. 2019;2(2):31. doi: 10.3390/mps2020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorre DA, Krivonosova TN, Markova OV, Severin FF. Amiodarone inhibits multiple drug resistance in yeast Saccharomyces cerevisiae. Arch Microbiol. 2009;191(8):675–679. doi: 10.1007/s00203-009-0493-8. [DOI] [PubMed] [Google Scholar]
- Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84(9A):20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- Köhler GA, Gong X, Bentink S, Theiss S, Pagani GM, Agabian N, Hedstrom L. The functional basis of mycophenolic acid resistance in Candida albicans IMP dehydrogenase. J Biol Chem. 2005;280(12):11295–11302. doi: 10.1074/jbc.M409847200. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J Antimicrob Chemother. 2003;51(2):313–316. doi: 10.1093/jac/dkg090. [DOI] [PubMed] [Google Scholar]
- Kouvari M, Panagiotakos DB, Chrysohoou C, Yannakoulia M, Georgousopoulou EN, Tousoulis D, Pitsavos C, ATTICA study investigators Dietary vitamin D intake, cardiovascular disease and cardiometabolic risk factors: a sex-based analysis from the ATTICA cohort study. J Hum Nutr Diet. 2020;33(5):708–717. doi: 10.1111/jhn.12748. [DOI] [PubMed] [Google Scholar]
- Krajewska-Kułak E, Niczyporuk WW. Anticandidal activity of flunarizine. Mater Med Pol. 1993;25(3-4):143–144. [PubMed] [Google Scholar]
- Ku TS, Palanisamy SK, Lee SA. Susceptibility of Candida albicans biofilms to azithromycin, tigecycline and vancomycin and the interaction between tigecycline and antifungals. Int J Antimicrob Agents. 2010;36(5):441–446. doi: 10.1016/j.ijantimicag.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Kubiça TF, Denardi LB, Azevedo MI, Oliveira V, Severo LC, Santurio JM, Alves SH. Antifungal activities of tacrolimus in combination with antifungal agents against fluconazole-susceptible and fluconazole-resistant Trichosporon asahii isolates. Braz J Infect Dis. 2016;20(6):539–545. doi: 10.1016/j.bjid.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummari LK, Butler MS, Furlong E, Blundell R, Nouwens A, Silva AB, Kappler U, Fraser JA, Kobe B, Cooper MA, Robertson AAB. Antifungal benzo[b]thiophene 1,1-dioxide IMPDH inhibitors exhibit pan-assay interference (PAINS) profiles. Bioorg Med Chem. 2018;26(20):5408–5419. doi: 10.1016/j.bmc.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Latendresse G, Elmore C, Deneris A. Selective serotonin reuptake inhibitors as first-line antidepressant therapy for perinatal depression. J Midwifery Womens Health. 2017;62(3):317–328. doi: 10.1111/jmwh.12607. [DOI] [PubMed] [Google Scholar]
- Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, Grossman EA, Hart PC, Kang C, Sanderson SM, Andrade J, Nomura DK, Bonini MG, Locasale JW, Rosner MR. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019;568(7751):254–258. doi: 10.1038/s41586-019-1005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestrade PP, Meis JF, Arends JP, van der Beek MT, de Brauwer E, van Dijk K, de Greeff SC, Haas PJ, Hodiamont CJ, Kuijper EJ, Leenstra T, Muller AE, Oude Lashof AM, Rijnders BJ, Roelofsen E, Rozemeijer W, Tersmette M, Terveer EM, Verduin CM, Wolfhagen MJ, Melchers WJ, Verweij PE. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses. 2016;59(2):101–107. doi: 10.1111/myc.12440. [DOI] [PubMed] [Google Scholar]
- Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect. 2019;25(7):799–806. doi: 10.1016/j.cmi.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Lew MA, Beckett KM, Levin MJ. Antifungal activity of four tetracycline analogues against Candida albicans in vitro: potentiation by amphotericin B. J Infect Dis. 1977;136(2):263–270. doi: 10.1093/infdis/136.2.263. [DOI] [PubMed] [Google Scholar]
- Li X, Yu C, Huang X, Sun S. Synergistic effects and mechanisms of budesonide in combination with fluconazole against resistant Candida albicans. PLoS One. 2016;11(12):e0168936. doi: 10.1371/journal.pone.0168936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima WG, Alves-Nascimento LA, Andrade JT, Vieira L, de Azambuja Ribeiro RIM, Thomé RG, Dos Santos HB, Ferreira JMS, Soares AC. Are the statins promising antifungal agents against invasive candidiasis? Biomed Pharmacother. 2019;111:270–281. doi: 10.1016/j.biopha.2018.12.076. [DOI] [PubMed] [Google Scholar]
- Limon JJ, Skalski JH, Underhill DM. Commensal fungi in health and disease. Cell Host Microbe. 2017;22(2):156–165. doi: 10.1016/j.chom.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ran Z, Wang F, Xin C, Xiong B, Song Z. Role of pulmonary microorganisms in the development of chronic obstructive pulmonary disease. Crit Rev Microbiol. 2020;47(1):1–12. doi: 10.1080/1040841X.2020.1830748. [DOI] [PubMed] [Google Scholar]
- Loreto ES, Tondolo JS, Pilotto MB, Alves SH, Santurio JM. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob Agents Chemother. 2014;58(12):7534–7537. doi: 10.1128/AAC.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Yu C, Cui X, Shi J, Yuan L, Sun S. Gentamicin synergises with azoles against drug-resistant Candida albicans. Int J Antimicrob Agents. 2018;51(1):107–114. doi: 10.1016/j.ijantimicag.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Lu M, Yang X, Yu C, Gong Y, Yuan L, Hao L, Sun S. Linezolid in combination with azoles induced synergistic effects against Candida albicans and protected Galleria mellonella against experimental candidiasis. Front Microbiol. 2019;9:3142. doi: 10.3389/fmicb.2018.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraki S, Hajiioannou I, Anatoliotakis N, Plataki M, Chatzinikolaou I, Zoras O, Tselentis Y, Samonis G. Ceftriaxone and dexamethasone affecting yeast gut flora in experimental mice. J Chemother. 1999;11(5):363–366. doi: 10.1179/joc.1999.11.5.363. [DOI] [PubMed] [Google Scholar]
- Marchetti O, Entenza JM, Sanglard D, Bille J, Glauser MP, Moreillon P. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother. 2000;44(11):2932–2938. doi: 10.1128/aac.44.11.2932-2938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000;44(9):2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G, Comfort M, Kobayashi GS. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proc Soc Exp Biol Med. 1971;138(2):571–574. doi: 10.3181/00379727-138-35943. [DOI] [PubMed] [Google Scholar]
- Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE (2016) Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond Ser B Biol Sci 371(1709):20150460. 10.1098/rstb.2015.0460 [DOI] [PMC free article] [PubMed]
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli MH, Bernardo SM, Lee SA. In vitro analyses of the combination of high-dose doxycycline and antifungal agents against Candida albicans biofilms. Int J Antimicrob Agents. 2009;34(4):326–332. doi: 10.1016/j.ijantimicag.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Zarei M, Vessal M. Synthesis, in vitro biological evaluation and in silico molecular docking studies of novel β-lactam-anthraquinone hybrids. Bioorg Chem. 2020;95:103515. doi: 10.1016/j.bioorg.2019.103515. [DOI] [PubMed] [Google Scholar]
- Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, Baig F, Hussain MS, Bhalraam U, Khan F, Choy AM, Matthew S, Houston JG, Struthers AD, George J, Lang CC. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. 2019;40(41):3409–3417. doi: 10.1093/eurheartj/ehz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneib NA. In-vitro activity of commonly used antifungal agents in the presence of rifampin, polymyxin B and norfloxacin against Candida albicans. J Chemother. 1995;7(6):525–529. doi: 10.1179/joc.1995.7.6.525. [DOI] [PubMed] [Google Scholar]
- Montgomerie JZ, Edwards JE, Jr, Guze LB. Synergism of amphotericin B and 5-fluorocytosine for Candida species. J Infect Dis. 1975;132(1):82–86. doi: 10.1093/infdis/132.1.82. [DOI] [PubMed] [Google Scholar]
- Morrow CA, Valkov E, Stamp A, Chow EW, Lee IR, Wronski A, Williams SJ, Hill JM, Djordjevic JT, Kappler U, Kobe B, Fraser JA. De novo GTP biosynthesis is critical for virulence of the fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2012;8(10):e1002957. doi: 10.1371/journal.ppat.1002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz RL. Gastrointestinal and disseminated candidiasis. An experimental model in the immunosuppressed rat. Arch Pathol Lab Med. 1981;105(3):138–143. [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400–428. doi: 10.1128/mmbr.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargesi S, Rezaie S. Investigation an antifungal activity of diclofenac sodium against hyphae formation in Aspergillus fumigatus with attention to the expression of Ef-1 gene. Iran J Public Health. 2018;47(5):770–772. [PMC free article] [PubMed] [Google Scholar]
- Nash JD, Burgess DS, Talbert RL. Effect of fluvastatin and pravastatin, HMG-CoA reductase inhibitors, on fluconazole activity against Candida albicans. J Med Microbiol. 2002;51(2):105–109. doi: 10.1099/0022-1317-51-2-105. [DOI] [PubMed] [Google Scholar]
- Nattel S, Singh BN. Evolution, mechanisms, and classification of antiarrhythmic drugs: focus on class III actions. Am J Cardiol. 1999;84(9A):11R–19R. doi: 10.1016/s0002-9149(99)00697-9. [DOI] [PubMed] [Google Scholar]
- Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology (Reading) 1994;140(Pt 9):2475–2479. doi: 10.1099/13500872-140-9-2475. [DOI] [PubMed] [Google Scholar]
- Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, Rubinson DA, Schrag D, Miksad R, Bullock AJ, Allen J, Zuckerman D, Chan E, Chan JA, Wolpin BM, Constantine M, Weckstein DJ, Faggen MA, Thomas CA, Kournioti C, Yuan C, Ganser C, Wilkinson B, Mackintosh C, Zheng H, Hollis BW, Meyerhardt JA, Fuchs CS. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019;321(14):1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MH, Clancy CJ, Yu YC, Lewin AS. Potentiation of antifungal activity of amphotericin B by azithromycin against Aspergillus species. Eur J Clin Microbiol Infect Dis. 1997;16(11):846–848. doi: 10.1007/BF01700417. [DOI] [PubMed] [Google Scholar]
- Nyilasi I, Kocsubé S, Krizsán K, Galgóczy L, Pesti M, Papp T, Vágvölgyi C. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol Lett. 2010;307(2):175–184. doi: 10.1111/j.1574-6968.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- O'Driscoll M, Greenhalgh K, Young A, Turos E, Dickey S, Lim DV. Studies on the antifungal properties of N-thiolated beta-lactams. Bioorg Med Chem. 2008;16(16):7832–7837. doi: 10.1016/j.bmc.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogundeji AO, Pohl CH, Sebolai OM. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob Agents Chemother. 2016;60(8):4799–4808. doi: 10.1128/AAC.02810-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AS, Gaspar CA, Palmeira-de-Oliveira R, Martinez-de-Oliveira J, Palmeira-de-Oliveira A. Anti-Candida activity of fluoxetine alone and combined with fluconazole: a synergistic action against fluconazole -resistant strains. Antimicrob Agents Chemother. 2014;58(7):4224–4226. doi: 10.1128/AAC.02623-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AS, Martinez-de-Oliveira J, Donders GGG, Palmeira-de-Oliveira R, Palmeira-de-Oliveira A. Anti-Candida activity of antidepressants sertraline and fluoxetine: effect upon pre-formed biofilms. Med Microbiol Immunol. 2018;207(3-4):195–200. doi: 10.1007/s00430-018-0539-0. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy EM, Meletiadis J, Stergiopoulou T, Demchok JP, Walsh TJ. Antifungal interactions within the triple combination of amphotericin B, caspofungin and voriconazole against Aspergillus species. J Antimicrob Chemother. 2006;58(6):1168–1176. doi: 10.1093/jac/dkl392. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: time for a new "gold standard". Clin Infect Dis. 2003;37(3):415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- Ozcan SK, Budak F, Willke A, Filiz S, Costur P, Dalcik H. Efficacies of caspofungin and a combination of caspofungin and meropenem in the treatment of murine disseminated candidiasis. APMIS. 2006;114(12):829–836. doi: 10.1111/j.1600-0463.2006.apm_450.x. [DOI] [PubMed] [Google Scholar]
- Ozdek SC, Miller D, Flynn PM, Flynn HW. In vitro antifungal activity of the fourth generation fluoroquinolones against Candida isolates from human ocular infections. Ocul Immunol Inflamm. 2006;14(6):347–351. doi: 10.1080/09273940600976953. [DOI] [PubMed] [Google Scholar]
- Paassen J, Russcher A, In’t Veld-van Wingerden AW, Verweij PE, Kuijper EJ (2016) Emerging aspergillosis by azole-resistant Aspergillus fumigatus at an intensive care unit in the Netherlands, 2010 to 2013. Euro Surveill 21(30). 10.2807/1560-7917.ES.2016.21.30.30300 [DOI] [PubMed]
- Patterson TF, Thompson GR, 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Mortimer RB, Mitchell M. Sertraline demonstrates fungicidal activity in vitro for Coccidioides immitis. Mycology. 2016;7(3):99–101. doi: 10.1080/21501203.2016.1204368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellon A, Ramirez-Garcia A, Buldain I, Antoran A, Martin-Souto L, Rementeria A, Hernando FL. Pathobiology of Lomentospora prolificans: could this species serve as a model of primary antifungal resistance? Int J Antimicrob Agents. 2018;51(1):10–15. doi: 10.1016/j.ijantimicag.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Perlin DS. Cell wall-modifying antifungal drugs. Curr Top Microbiol Immunol. 2020;425:255–275. doi: 10.1007/82_2019_188. [DOI] [PubMed] [Google Scholar]
- Prazynska M, Gospodarek E. In vitro effect of amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis biofilm formation. Mycopathologia. 2014;177(1-2):19–27. doi: 10.1007/s11046-014-9727-7. [DOI] [PubMed] [Google Scholar]
- Ramage G, VandeWalle K, Bachmann SP, Wickes BL, López-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46(11):3634–3636. doi: 10.1128/aac.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramondenc I, Pinel C, Ambroise-Thomas P, Grillot R. Does hydrocortisone modify the in vitro susceptibility of Aspergillus fumigatus to itraconazole and amphotericin B? Med Mycol. 1998;36(2):69–73. doi: 10.1080/02681219880000121. [DOI] [PubMed] [Google Scholar]
- Ran Z, Liu J, Wang F, Xin C, Shen X, Zeng S, Song Z, Xiong B. Analysis of pulmonary microbial diversity in patients with advanced lung cancer based on high-throughput sequencing technology. Zhongguo Fei Ai Za Zhi. 2020;23(12):1031–1038. doi: 10.3779/j.issn.1009-3419.2020.103.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro NQ, Costa MC, Magalhães TFF, Carneiro HCS, Oliveira LV, Fontes ACL, Santos JRA, Ferreira GF, Araujo GRS, Alves V, Frases S, Paixão TA, de Resende Stoianoff MA, Santos DA. Atorvastatin as a promising anticryptococcal agent. Int J Antimicrob Agents. 2017;49(6):695–702. doi: 10.1016/j.ijantimicag.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Robbins N, Wright GD, Cowen LE (2016) Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr 4(5). 10.1128/microbiolspec.FUNK-0002-2016 [DOI] [PubMed]
- Robbins N, Caplan T, Cowen LE. Molecular evolution of antifungal drug resistance. Annu Rev Microbiol. 2017;71:753–775. doi: 10.1146/annurev-micro-030117-020345. [DOI] [PubMed] [Google Scholar]
- Roden MM, Nelson LD, Knudsen TA, Jarosinski PF, Starling JM, Shiflett SE, Calis K, DeChristoforo R, Donowitz GR, Buell D, Walsh TJ. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis. 2003;36(10):1213–1220. doi: 10.1086/374553. [DOI] [PubMed] [Google Scholar]
- Rossato L, Loreto ÉS, Venturini TP, Azevedo MI, Weiblen C, Botton SA, Santurio JM, Alves SH. In vitro interaction of antifungal and antibacterial drugs against Cryptococcus neoformans var. grubii before and after capsular induction. Med Mycol. 2015;53(8):885–889. doi: 10.1093/mmy/myv059. [DOI] [PubMed] [Google Scholar]
- Rossato L, Loreto ÉS, Zanette RA, Chassot F, Santurio JM, Alves SH. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol (Praha) 2016;61(5):399–403. doi: 10.1007/s12223-016-0449-8. [DOI] [PubMed] [Google Scholar]
- Sam QH, Chang MW, Chai LY. The fungal mycobiome and its interaction with gut bacteria in the host. Int J Mol Sci. 2017;18(2):330. doi: 10.3390/ijms18020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi Jde C, Pitangui Nde S, Rodríguez-Arellanes G, Taylor ML, Fusco-Almeida AM, Mendes-Giannini MJ. Highlights in pathogenic fungal biofilms. Rev Iberoam Micol. 2014;31(1):22–29. doi: 10.1016/j.riam.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Maesaki S, Miyazaki Y, Yanagihara K, Tomono K, Tashiro T, Kohno S. Synergistic effect of ofloxacin and fluconazole against azole-resistant Candida albicans. J Infect Chemother. 2000;6(3):151–154. doi: 10.1007/s101560070014. [DOI] [PubMed] [Google Scholar]
- Schwarz P, Dannaoui E. In vitro interaction between isavuconazole and tacrolimus, cyclosporin A, or sirolimus against Aspergillus species. J Fungi (Basel) 2020;6(3):103. doi: 10.3390/jof6030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz P, Schwarz PV, Felske-Zech H, Dannaoui E. In vitro interactions between isavuconazole and tacrolimus, cyclosporin A or sirolimus against Mucorales. J Antimicrob Chemother. 2019;74(7):1921–1927. doi: 10.1093/jac/dkz102. [DOI] [PubMed] [Google Scholar]
- Sehgal SN, Baker H, Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Shalit I, Shadkchan Y, Mircus G, Osherov N. In vitro synergy of caspofungin with licensed and novel antifungal drugs against clinical isolates of Fusarium spp. Med Mycol. 2009;47(5):457–462. doi: 10.1080/13693780802232910. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kaur H, Khuller GK. Cell cycle effects of the phenothiazines: trifluoperazine and chlorpromazine in Candida albicans. FEMS Microbiol Lett. 2001;199(2):185–190. doi: 10.1111/j.1574-6968.2001.tb10672.x. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen Z, Chen X, Cao L, Liu P, Sun S. The combination of minocycline and fluconazole causes synergistic growth inhibition against Candida albicans: an in vitro interaction of antifungal and antibacterial agents. FEMS Yeast Res. 2010;10(7):885–893. doi: 10.1111/j.1567-1364.2010.00664.x. [DOI] [PubMed] [Google Scholar]
- Shiers S, Pradhan G, Mwirigi J, Mejia G, Ahmad A, Kroener S, Price T. Neuropathic pain creates an enduring prefrontal cortex dysfunction corrected by the type II diabetic drug metformin but not by gabapentin. J Neurosci. 2018;38(33):7337–7350. doi: 10.1523/JNEUROSCI.0713-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde RB, Chauhan NM, Raut JS, Karuppayil SM. Sensitization of Candida albicans biofilms to various antifungal drugs by cyclosporine A. Ann Clin Microbiol Antimicrob. 2012;11:27. doi: 10.1186/1476-0711-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CR, de Andrade Neto JB, Sidrim JJ, Angelo MR, Magalhães HI, Cavalcanti BC, Brilhante RS, Macedo DS, de Moraes MO, Lobo MD, Grangeiro TB, Nobre Júnior HV. Synergistic effects of amiodarone and fluconazole on Candida tropicalis resistant to fluconazole. Antimicrob Agents Chemother. 2013;57(4):1691–1700. doi: 10.1128/AAC.00966-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva THS, Araújo CV, Santos KMDC, Alves NDS, Gomes THS, Silva AKFE, Silva NCLDS, Damasceno Júnior ECB, Carvalho AMA, Mendes MGA, Caminha HB, Daboit TC, Ferreira TB, Andrade-Silva LE, Silva-Vergara ML, Ferreira-Paim K, Fonseca FM. Synergic effect of simvastatin in combination with amphotericin B against environmental strains of Cryptococcus neoformans from northeastern Brazil: a prospective experimental study. Sao Paulo Med J. 2020;138(1):40–46. doi: 10.1590/1516-3180.2019.0107.R2.16092019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, Tollemar JG, Dominguez EA, Yu CM, Paterson DL, Husain S, Kusne S, Linden P. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36(1):46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- Smego RA, Jr, Perfect JR, Durack DT. Combined therapy with amphotericin B and 5-fluorocytosine for Candida meningitis. Rev Infect Dis. 1984;6(6):791–801. doi: 10.1093/clinids/6.6.791. [DOI] [PubMed] [Google Scholar]
- Smitherman L. In brief: antifungal drugs. Pediatr Rev. 2016;37(6):267–268. doi: 10.1542/pir.2015-0171. [DOI] [PubMed] [Google Scholar]
- Steinbach WJ, Schell WA, Blankenship JR, Onyewu C, Heitman J, Perfect JR. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother. 2004;48(5):1664–1669. doi: 10.1128/aac.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulou T, Meletiadis J, Sein T, Papaioannidou P, Tsiouris I, Roilides E, Walsh TJ. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus fumigatus. J Antimicrob Chemother. 2009;63(2):343–348. doi: 10.1093/jac/dkn473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar AM, Liu XP, Chen RJ. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob Agents Chemother. 1997;41(11):2518–2521. doi: 10.1128/AAC.41.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T, Tajima M, Tsubuku H, Tsuboi R, Nishikawa A. A new calcineurin inhibitor, pimecrolimus, inhibits the growth of Malassezia spp. Antimicrob Agents Chemother. 2006;50(8):2897–2898. doi: 10.1128/AAC.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Li Y, Guo Q, Shi C, Yu J, Ma L. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother. 2008;52(2):409–417. doi: 10.1128/AAC.01070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoying JB, Deymier PA, Zhang DD, Wong PK. Cellular architecture regulates collective calcium signaling and cell contractility. PLoS Comput Biol. 2016;12(5):e1004955. doi: 10.1371/journal.pcbi.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Wang D, Yu C, Huang X, Li X, Sun S. Strong synergism of dexamethasone in combination with fluconazole against resistant Candida albicans mediated by inhibiting drug efflux and reducing virulence. Int J Antimicrob Agents. 2017;50(3):399–405. doi: 10.1016/j.ijantimicag.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Kimura S, Tateda K, Saga T, Ohno A, Ishii Y, Izumikawa K, Tashiro T, Kohno S, Yamaguchi K. Pravastatin inhibits farnesol production in Candida albicans and improves survival in a mouse model of systemic candidiasis. Med Mycol. 2012;50(4):353–360. doi: 10.3109/13693786.2011.610037. [DOI] [PubMed] [Google Scholar]
- Theken KN, Grosser T. Weight-adjusted aspirin for cardiovascular prevention. Lancet. 2018;392(10145):361–362. doi: 10.1016/S0140-6736(18)31307-2. [DOI] [PubMed] [Google Scholar]
- Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, Sela DA, Muller AJ, Mullin JM, Albert K, Gilligan JP, DiGuilio K, Dilbarova R, Alexander W, Prendergast GC. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 2017;77(8):1783–1812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Cisneros J, Mañez R, Kusne S, Alessiani M, Martin M, Starzl TE. The spectrum of aspergillosis in liver transplant patients: comparison of FK506 and cyclosporine immunosuppression. Transplant Proc. 1991;23(6):3040–3041. [PMC free article] [PubMed] [Google Scholar]
- Treviño-Rangel Rde J, Villanueva-Lozano H, Hernández-Rodríguez P, Martínez-Reséndez MF, García-Juárez J, Rodríguez-Rocha H, González GM. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol. 2016;54(3):280–286. doi: 10.1093/mmy/myv109. [DOI] [PubMed] [Google Scholar]
- Treviño-Rangel RJ, Villanueva-Lozano H, Méndez-Galomo KS, Solís-Villegas EM, Becerril-García MA, Montoya AM, Robledo-Leal ER, González GM. In vivo evaluation of the antifungal activity of sertraline against Aspergillus fumigatus. J Antimicrob Chemother. 2019;74(3):663–666. doi: 10.1093/jac/dky455. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Metformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2015;31(6):619–626. doi: 10.1002/dmrr.2649. [DOI] [PubMed] [Google Scholar]
- Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52(3):1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez JA. Anidulafungin: a new echinocandin with a novel profile. Clin Ther. 2005;27(6):657–673. doi: 10.1016/j.clinthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Venturini T, Rossato L, Chassot F, Tairine Keller J, Baldissera Piasentin F, Morais Santurio J, Hartz Alves S. In vitro synergistic combinations of pentamidine, polymyxin B, tigecycline and tobramycin with antifungal agents against Fusarium spp. J Med Microbiol. 2016;65(8):770–774. doi: 10.1099/jmm.0.000301. [DOI] [PubMed] [Google Scholar]
- Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;6(2):171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Vitale RG, Afeltra J, Meis JF, Verweij PE. Activity and post antifungal effect of chlorpromazine and trifluopherazine against Aspergillus, Scedosporium and zygomycetes. Mycoses. 2007;50(4):270–276. doi: 10.1111/j.1439-0507.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob Agents Chemother. 1996;40(3):541–545. doi: 10.1128/AAC.40.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer C, Macreadie IG. Simvastatin reduces ergosterol levels, inhibits growth and causes loss of mtDNA in Candida glabrata. FEMS Yeast Res. 2007;7(3):436–441. doi: 10.1111/j.1567-1364.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017;10:249–259. doi: 10.2147/IDR.S124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold NP. The antifungal arsenal: alternative drugs and future targets. Int J Antimicrob Agents. 2018;51(3):333–339. doi: 10.1016/j.ijantimicag.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Wood NC, Nugent KM. Inhibitory effects of chlorpromazine on Candida species. Antimicrob Agents Chemother. 1985;27(5):692–694. doi: 10.1128/aac.27.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Totten M, Memon W, Ying C, Zhang SX. In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen Candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob Agents Chemother. 2020;64(2):e02063–e02019. doi: 10.1128/AAC.02063-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Liao Y, Cong L, Lu X, Yang R. In vitro interactions between non-steroidal anti-inflammatory drugs and antifungal agents against planktonic and biofilm forms of Trichosporon asahii. PLoS One. 2016;11(6):e0157047. doi: 10.1371/journal.pone.0157047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Gao L, Yu P, Kosgey JC, Jia L, Fang Y, Xiong J, Zhang F. In vitro synergy of azole antifungals and methotrexate against Candida albicans. Life Sci. 2019;235:116827. doi: 10.1016/j.lfs.2019.116827. [DOI] [PubMed] [Google Scholar]
- Yousfi H, CASsagne C, Ranque S, Rolain JM, Bittar F. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob Agents Chemother. 2019;63(10):e00263–e00219. doi: 10.1128/AAC.00263-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Ding X, Xu N, Cheng X, Qian K, Zhang B, Xing L, Li M. In vitro activity of verapamil alone and in combination with fluconazole or tunicamycin against Candida albicans biofilms. Int J Antimicrob Agents. 2013;41(2):179–182. doi: 10.1016/j.ijantimicag.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Yu Q, Ding X, Zhang B, Xu N, Jia C, Mao J, Zhang B, Xing L, Li M. Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res. 2014;14(4):633–641. doi: 10.1111/1567-1364.12150. [DOI] [PubMed] [Google Scholar]
- Yu Q, Xiao C, Zhang K, Jia C, Ding X, Zhang B, Wang Y, Li M. The calcium channel blocker verapamil inhibits oxidative stress response in Candida albicans. Mycopathologia. 2014;177(3-4):167–177. doi: 10.1007/s11046-014-9735-7. [DOI] [PubMed] [Google Scholar]
- Zeidler U, Bougnoux ME, Lupan A, Helynck O, Doyen A, Garcia Z, Sertour N, Clavaud C, Munier-Lehmann H, Saveanu C, d'Enfert C. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J Antimicrob Chemother. 2013;68(6):1285–1296. doi: 10.1093/jac/dks538. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zhang Z, Chen P, Long N, Lu L, Sang H. In vitro and in vivo efficacy of a synergistic combination of itraconazole and verapamil against Aspergillus fumigatus. Front Microbiol. 2019;10:1266. doi: 10.3389/fmicb.2019.01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B, Zhou H, Yang L, Zhang J, Jung K, Giam CZ, Xiang X, Lin X. Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J Antimicrob Chemother. 2010;65(5):931–938. doi: 10.1093/jac/dkq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Heitman J, Chen YL. Comparative analysis of calcineurin signaling between Candida dubliniensis and Candida albicans. Commun Integr Biol. 2012;5(2):122–126. doi: 10.4161/cib.18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yan H, Lu M, Wang D, Sun S. Antifungal activity of ribavirin used alone or in combination with fluconazole against Candida albicans is mediated by reduced virulence. Int J Antimicrob Agents. 2020;55(1):105804. doi: 10.1016/j.ijantimicag.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Khoo AL, Tan G, Teng M, Tee C, Tan BH, Ong B, Lim BP, Chai LY. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob Agents Chemother. 2016;60(1):376–386. doi: 10.1128/AAC.01985-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang H, Zhou X, Luo H, Tang F, Yang J, Alterovitz G, Cheng L, Ren B. Lovastatin synergizes with itraconazole against planktonic cells and biofilms of Candida albicans through the regulation on ergosterol biosynthesis pathway. Appl Microbiol Biotechnol. 2018;102(12):5255–5264. doi: 10.1007/s00253-018-8959-8. [DOI] [PubMed] [Google Scholar]