Abstract
Background:
Interstitial lung disease (ILD) is a category of chronic lung diseases with more than 200 subtypes. Idiopathic interstitial pneumonia (IIP), systemic sclerosis (SSc) ILD, and familial interstitial pneumonia (FIP) are three major groups of lung diseases with different causes or with unknown causes. Mucin5B (MUC5B) belongs to the mucin family, which contribute to the lubricating and viscoelastic properties of the whole saliva, normal lung mucus, and cervical mucus. The association between MUC5B rs35705950 and ILDs risks has been widely studied. However, the results were inconclusive and inconsistent.
Methods:
In the present meta-analysis, the database PubMed, Embase, Cochrane Central Register of Controlled Trials, CNKI and Chinese Biomedical Literature Database were searched till Aug 20th, 2018. Overall 16 publications with 28 studies, 76345 cases and 18402 controls were included.
Results:
The results indicated a significant increase of overall IIP risk for TT genotype and T allele of the rs35705950 in all genetic models (TT vs GG, OR=9.11; TT vs GT+TT, OR=5.80; GT+TT vs GG, OR=4.34; T vs G, OR=4.03. P<0.0001). Subgroup analysis by subtypes of IIP revealed higher risks of TT genotype and T allele for IPF and iNSIP (P<0.05). A significant increase of FIP risk was also found for the TT genotype and T allele of the rs35705950 (TT vs GG, OR=17.08; GT+TT vs GG, OR=6.02; T vs G, OR=1.64.P<0.05).
Conclusion:
No significant relations existed between the rs35705950 and SSc-ILD risks. MUC5B rs35705950 might be a predictor for the susceptibility of IIP and FIP.
Keywords: Idiopathic interstitial pneumonia, Familial interstitial pneumonia, Polymorphism, Meta-analysis
Introduction
Interstitial lung disease (ILD) is a category of chronic lung diseases characterized by the scarring and/or inflammation of the lungs. ILD includes over 200 disorders (1, 2). Among them, idiopathic interstitial pneumonia (IIP) is a group of diffuse parenchymal lung diseases of unknown cause. IIP is characterized by cellular infiltration of the interstitial compartment of the lung and with various patterns of inflammation and fibrosis. According to the 2013 American Thoracic Society/European Respiratory Society Statement, the IIPs were divided into major IIP, rare IIP and Unclassifiable IIP (3). The major IIP included idiopathic pulmonary fibrosis (IPF), idiopathic nonspecific interstitial pneumonia (iNSIP), respiratory bronchiolitis–interstitial lung disease (RB-ILD), desquamative interstitial pneumonia (DIP), cryptogenic organizing pneumonia (COP), and acute interstitial pneumonia (AIP). Rare idiopathic interstitial pneumoniaincludes idiopathic lymphoid interstitial pneumonia (ILP), and idiopathic pleuroparenchymal fibroelastosis (PPFE) (3). Systemic sclerosis ILD (SSc-ILD) is a lung disease caused by systemic sclerosis (SSc). As reported previously, 40% of the SSc patients were present ILD. ILD has been the major cause of death during SSc (4). Familial interstitial pneumonia (FIP) is an inherited lung disease, in which at least two or more primary biological family members have a clinical classification of IPF (3). Many evidence indicated that IIP, SSc-ILD, FIP have a genetic basis.
Mucin 5B (MUC5B) belongs to the mucin family of proteins, which contribute to the lubricating and viscoelastic properties of the whole saliva, normal lung mucus and cervical mucus. Rs35705950 is an important mutation on the MUC5B promoter. The association between MUC5B mutations and IIP, FIP, SSc-ILD risks have been widely studied (5–8). However, the results were inconclusive and inconsistent. A comprehensive meta-analysis of the published studies was needed to give a more precise estimation of the association between MUC5B rs35705950 and IIP, SSc-ILD, FIP.
In the present meta-analysis, a comprehensive analysis was performed to reveal whether rs35705950 is related to IIP, SSc-ILD, and FIP.
Methods
Search methods and inclusion criteria
In order to include all the related literature, a comprehensive search was conducted via the electronic databases Pubmed, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Chinese National Knowledge Infrastructure (CNKI) and Chinese Biomedical Literature Database (CBM). The terms used in the present searching were (“MUC5B” or “mucin5b”) and (“idiopathic interstitial pneumonias” or “idiopathic pulmonary fibrosis” or “idiopathic non-specific interstitial pneumonia” or “respiratory bronchiolitis–interstitial lung disease” or “desquamative interstitial pneumonia” or “cryptogenic organizing pneumonia” and “acute interstitial pneumonia” or “idiopathic lymphoid interstitial pneumonia”, or “idiopathic pleuroparenchymal fibroelastosis” or “familial interstitial pneumonia” or “systemic sclerosis interstitial lung disease”) and (“polymorphism” or “mutation” or “single nucleotide polymorphism”). The deadline for publication search was Oct 20, 2018. For the retrieved results, the duplications were removed first. The title and abstract were screened secondly to determine whether they met the inclusion criteria. Then, the full text was retrieved if the title and abstract met the requirements. Also, the references of included studies were checked for potentially relevant studies.
The included studies should meet the following criteria: case-control studies, studies demonstrated the association of MUC5B polymorphism and IIP, SSc-ILD or FIP. If the studies are abstracts, dissertations, letter to the editor, reviews or not meet the inclusion criteria, they will be excluded.
Data extraction
All the data from each included studies were extracted manually by two independent investigators. If discrepancies happened during data extraction, a consensus was conducted by the third author to solve it. The information extracted from each involved study are first author, published year, country, ethnicity, genotype method, subjects number, age, gender, odds ratio (OR) and confidence interval (CI).
Statistical Methods
Meta-analysis, sensitivity analysis, publication bias, heterogeneity were conducted on the STATA 12.0 software (StataCorp, College Station, TX, USA).
The pooled OR and 95% CI were used to evaluate the association between MUC5B rs35705950 and IIP, SSc-ILD, FIP. Subtypes of IIP, including IPF, iNSIP were analyzed. The Z test was used to evaluate the statistical significance of the association, with P<0.05 considered to be statically significant. Genetic models such as additive model (TT vs GG), recessive model (TT vs GT+GG), dominant model (TT+TG vs GG) and allelic model (T vs G) were analyzed.
The heterogeneity of included studies was determined by the I2 test.I2>50% was considered to be with obvious heterogeneity, while I2<50% not. When I2>50%, the random-effects model (the DerSimonian and Laird method) was used for the meta-analysis, otherwise the fixed-effect model (the Mantel-Haenszel method) was used.
The publication bias was evaluated by Begg’s funnel plot and Egger’s linear regression method.
The influence of each individual study on pooled ORs and 95% CIs were performed by omitting one study at one time using STATA 12.0 software in the sensitivity analysis.
Results
Literature search and characteristics of included studies
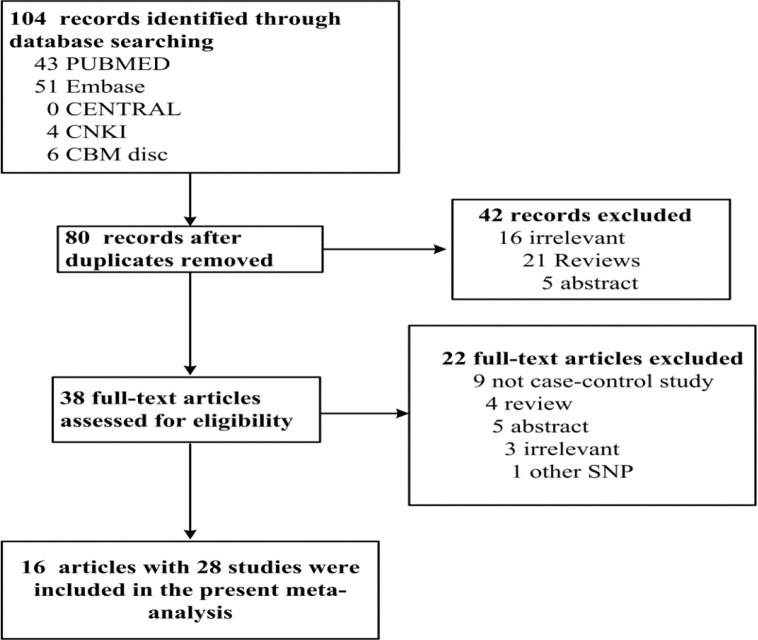
After a comprehensive searching of relevant studies, totally 80 studies were found without duplications. Among them, 19 were irrelevant, 25 were reviews, 9 were not case-control studies, 10 were abstract, and 1 with other mutation. At last, 16 publications with 28 studies, 76345 cases and 18402 controls were included. The PRISMA flowchartwas shown in Fig. 1, and the detailed information of the included studies was presented in Table 1.
Fig. 1:
PRISMA flow chart for the association between MUC5B rs35705950 and IIP, SSc-ILD, and FIP
Table 1:
Characteristics of eligible studies included in the meta-analysis
| Author | Year | Country | Ethnicity | Disease | Genotyping Methods | Sample Size (Case/Control) | Odds Ratio | LCI | UCI | HWE of Control |
|---|---|---|---|---|---|---|---|---|---|---|
| Borie R [1](9) | 2013 | France | Caucasian | IPF | Taqman | 142/1383 | 5.22 | 3.99 | 6.81 | 0.284 |
| Coghlan MA(10) | 2014 | USA | Caucasian | IPF | Taqman | 132/192 | 2.68 | 1.78 | 4.01 | N.A. |
| Fingerlin TA(11) | 2013 | UK/TX/etc | Non-hispanic |
|||||||
| White | IPF | Taqman | 2492/6573 | 4.51 | 3.91 | 5.21 | N.A. | |||
| Helling BA(12) | 2017 | USA | Caucasian | IPF | Taqman | 202/139 | 3.11 | 2.03 | 4.77 | 0.125 |
| Horimasu Y [1](13) | 2015 | Germany | Caucasian | IPF | Taqman | 71/35 | 11.05 | 3.3 | 36.99 | 0.791 |
| Horimasu Y [2](13) | 2015 | Japan | Asian | IPF | Taqman | 44/310 | 4.34 | 1.02 | 18.49 | 0.886 |
| Jiang H(14) | 2015 | China | Asian | IPF | Taqman | 187/250 | 1.93 | 1.32 | 2.82 | 0.01 |
| Kishore A [1](15) | 2016 | Czech | Caucasian | IPF | Taqman | 41/96 | 3.77 | 1.9 | 7.47 | N.A. |
| Kishore A [2](15) | 2016 | Germany | Caucasian | IPF | Taqman | 33/96 | 4.83 | 2.39 | 9.79 | N.A. |
| Kishore A [3](15) | 2016 | Greek | Caucasian | IPF | Taqman | 40/96 | 5.46 | 2.76 | 10.82 | N.A. |
| Kishore A [4](15) | 2016 | France | Caucasian | IPF | Taqman | 51/96 | 6.77 | 3.62 | 12.65 | N.A. |
| Noth I et al(16) | 2013 | USA | Caucasian | IPF | Taqman | 1410/1931 | 2.43 | 2.13 | 2.77 | N.A. |
| Seibold MA [1](5) | 2011 | USA | Caucasian | IPF | Taqman | 492/322 | 8.3 | 5.8 | 11.9 | N.A. |
| Stock CJ [1](17) | 2013 | UK | Caucasian | IPF | Taqman | 110/416 | 4.9 | 3.42 | 7.03 | N.A. |
| van der Vis JJ[1](18) | 2015 | Holland | Caucasian | IPF | Taqman | 115/249 | 3.63 | 2.38 | 5.55 | 0.426 |
| Wang C [1](19) | 2014 | China | Asian | IPF | Taqman | 165/1013 | 4.33 | 1.99 | 9.42 | 0.800 |
| Wei R [1](20) | 2014 | USA | Caucasian | IPF | Taqman | 84/689 | 3.2 | 2.21 | 4.63 | 0.556 |
| Zhang Y, et al(21) | 2011 | USA | Caucasian | IPF | Taqman | 341/802 | 4.18 | 3.35 | 5.22 | 0.447 |
| Borie R [2](9) | 2013 | France | Caucasian | Ssc- ILD | Taqman | 346/1383 | 1.05 | 0.8 | 1.37 | 0.284 |
| Borie R [3](9) | 2013 | Italy | Caucasian | Ssc- ILD | Taqman | 207/494 | 1.18 | 0.84 | 1.66 | 0.342 |
| Peljto A (22) | 2012 | USA | Caucasian | Ssc- ILD | Taqman | 109/122 | 1.02 | 0.55 | 1.91 | N.A. |
| Stock CJ [2](17) | 2013 | UK | Caucasian | Ssc- ILD | Taqman | 229/416 | 1.24 | 0.86 | 1.79 | N.A. |
| Horimasu Y [3](13) | 2015 | Germany | Caucasian | NSIP | Taqman | 31/35 | 8.44 | 2.34 | 30.47 | 0.791 |
| Horimasu Y [4](13) | 2015 | Japan | Asian | NSIP | Taqman | 30/310 | 2.08 | 0.24 | 18.14 | 0.886 |
| van der Vis JJ [3](18) | 2015 | Holland | Caucasian | NSIP | Taqman | 43/249 | 2.85 | 1.58 | 5.17 | 0.426 |
| Seibold MA [2](5) | 2011 | USA | Caucasian | FIP | Taqman | 83/322 | 6.2 | 3.7 | 10.4 | N.A. |
| van der Vis JJ [2](18) | 2015 | Holland | Caucasian | FIP | Taqman | 55/249 | 4.31 | 2.59 | 7.18 | 0.426 |
| Johnson C [2](23) | 2017 | USA | Caucasian | IIP | Taqman | 60/134 | 4.1 | 2.17 | 7.74 | N.A. |
IPF: idiopathic pulmonary fibrosis; ssc-ILD: systemic sclerosis-interstitial lung diseases; iNSIP: idiopathic nonspecific interstitial pneumonia; FIP: Familial interstitial pneumonia; IIP: idiopathic interstitial pneumonia; LCI: low confidence interval; UCI: upper confidence interval; HWE: Hardy-Weinberg equilibrium
Results of meta-analysis
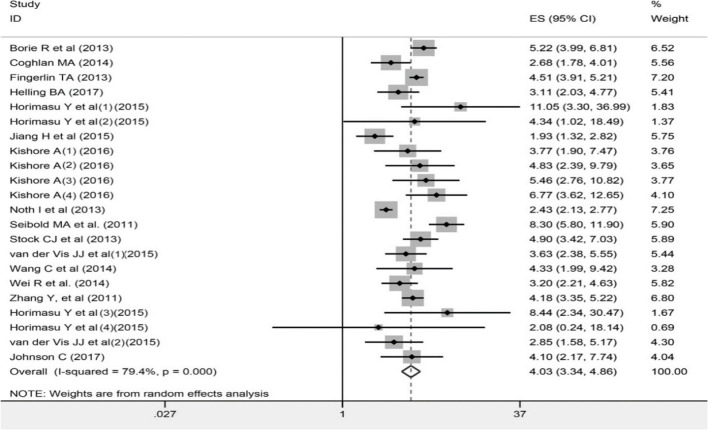
As shown in Table 2 and Fig. 2–4, a total of 28 studies, with 76345 cases and 18402 controls, were included in the allelic models (T vs G). Overall 16 studies were involved in additive (TT vs GG), recessive (TT vs TG+GG) and dominant model (TT+TG vs GG) respectively. Significant increase of overall IIP risks were found for TT genotype or T allele of rs35705950 in all genetic models (TT vs GG, OR=9.11, 95% CI=6.06–13.70, P<0.0001; TT vs GT+TT, OR=5.80, 95% CI=3.95–8.52, P<0.0001; GT+TT vs GG, OR=4.34, 95% CI=3.22–5.84, P<0.0001; T vs G, OR=4.03, 95% CI= 3.34–4.86, P<0.0001). Subgroup analysis by ethnicity demonstrated significant increases of IIP risks for TT genotype and T allele of rs35705950 in both Asians and Caucasians (Asians: TT vs GG, OR = 4.09, 95% CI=1.67–10.00, P=0.002; TT vs GT+TT, OR=3.93, 95% CI=1.61–9.55, P=0.003; GT+TT vs GG, OR=4.95, 95% CI=3.85–6.35, P<0.0001; T vs G, OR=2.64, 95% CI= 1.60–4.34, P<0.0001.
Table 2:
Pooled ORs and 95% CIs of the association between MUC5B promoter mutation (rs35705950) and interstitial lung diseases
| Genetic model | Subgroup | N | Model | OR | P value | I2% |
|---|---|---|---|---|---|---|
| TT vs GG | Overall IIP | 13 | F | 9.11 [6.06, 13.70] | <0.0001 | 18% |
| Asian | 4 | F | 4.09 [1.67, 10.00] | 0.002 | N.A. | |
| Caucasian | 9 | F | 11.72 [7.46, 18.41] | <0.0001 | 0% | |
| IPF | 10 | F | 9.00 [5.93, 13.64] | <0.0001 | 35% | |
| iNSIP | 3 | F | 12.29 [1.61, 93.86] | 0.02 | 0% | |
| Ssc-ILD | 2 | F | 0.94 [0.43, 2.08] | 0.88 | 0 | |
| FIP | 1 | N.A. | 17.08 [1.49, 195.49] | 0.02 | N.A. | |
| TT vs GT+ GG | Overall IIP | 13 | F | 5.80 [3.95, 8.52] | <0.0001 | 0 |
| Asian | 4 | F | 3.93 [1.61, 9.55] | 0.003 | N.A | |
| Caucasian | 9 | F | 6.42 [4.20, 9.82] | <0.0001 | 0 | |
| IPF | 10 | F | 5.71 [3.86, 8.46] | <0.0001 | 0 | |
| iNSIP | 3 | F | 8.41 [1.11, 63.80] | 0.04 | 0 | |
| Ssc-ILD | 2 | F | 0.92 [0.42, 2.02] | 0.83 | 0 | |
| FIP | 1 | N.A. | 9.36 [0.83, 105.11] | 0.07 | N.A. | |
| GT+TT vs GG | Overall IIP | 13 | R | 4.34 [3.22, 5.84] | <0.0001 | 69% |
| Asian | 4 | F | 2.09 [1.44, 3.05] | 0.0001 | 46% | |
| Caucasian | 9 | R | 4.95 [3.85, 6.35] | <0.0001 | 52% | |
| IPF | 10 | R | 4.39 [3.17, 6.10] | <0.0001 | 75% | |
| iNSIP | 3 | F | 3.96 [2.23, 7.03] | <0.0001 | 23% | |
| Ssc-ILD | 2 | F | 1.11 [0.90, 1.38] | 0.34 | 0 | |
| FIP | 1 | N.A. | 6.02 [3.22, 11.24] | <0.0001 | N.A. | |
| T vs G | Overall IIP | 22 | R | 4.03 [3.34–4.86] | <0.0001 | 79.4% |
| Asian | 4 | F | 2.64 [1.60–4.34] | <0.0001 | 26.9% | |
| Caucasian | 18 | R | 4.22 [3.47–5.14] | <0.0001 | 81.2% | |
| IPF | 18 | R | 4.06 [3.31–4.97] | <0.0001 | 82.9% | |
| iNSIP | 3 | F | 3.35 [1.99–5.65] | <0.0001 | 18.8% | |
| Ssc-ILD | 4 | F | 1.12 [0.94–1.34] | 0.197 | 0.0% | |
| FIP | 2 | F | 1.64 [1.28–2.00] | <0.0001 | 0.0% |
PF: idiopathic pulmonary fibrosis; ssc-ILD: systemic sclerosis-interstitial lung diseases; iNSIP: idiopathic nonspecific interstitial pneumonia; FIP: Familial interstitial pneumonia; IIP: idiopathic interstitial pneumonia; LCI: low confidence interval; UCI: upper confidence interval; HWE: Hardy-Weinberg equilibrium; F: Fixed model; R: random model
Fig. 2:
Forest plot of ORs for the association between rs35705950 and overall IIP in the allelic model (T vs G)
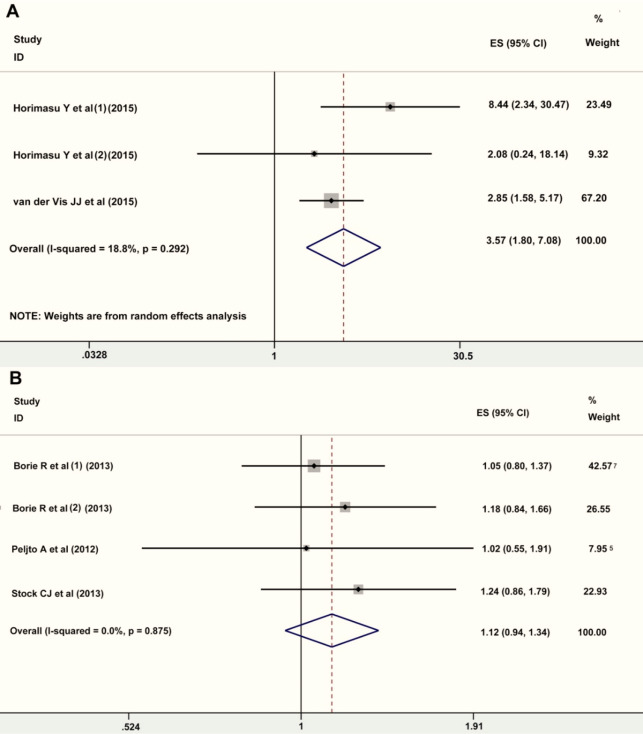
Fig. 4:
Forest plot of ORs for the association between rs35705950 and iNSIP and ssc-ILD in the allelic model (T vs G). A, iNSIP; B, ssc-ILD
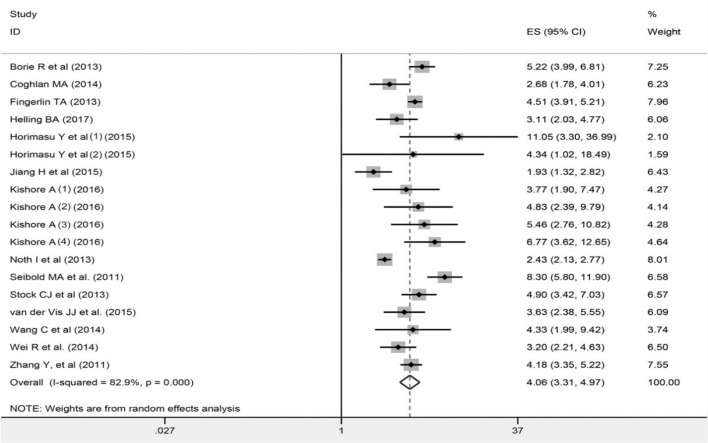
Caucasians: TT vs GG, OR=11.72, 95% CI=7.46–18.41, P<0.0001; TT vs GT+TT, OR=6.42, 95% CI= 4.20–9.82, P<0.0001; GT+TT vs GG, OR=2.68, 95% CI=1.39–5.16, P=0.0001; T vs G, OR=4.22, 95% CI= 3.47–5.14, P<0.0001). When analyzed by subtypes of IIP, higher risk of IPF and NSIP was found for the TT genotype or T allele of rs35705950 in all genetic models (IPF: TT vs GG, OR=9.00, 95% CI=5.93–13.64, P<0.0001; TT vs GT+TT, OR=5.71, 95% CI=3.86–8.46, P<0.0001; GT+TT vs GG, OR=4.39, 95% CI=3.17–6.10, P<0.0001; T vs G, OR=4.06, 95% CI= 3.31–4.97, P<0.0001; iNSIP: TT vs GG, OR=2.29, 95% CI=1.61–93.86, P=0.02; TT vs GT+TT, OR=8.41, 95% CI=1.11–63.80, P=0.04; GT+TT vs GG, OR=3.96, 95% CI=2.23–7.03, P<0.0001; T vs G, OR=3.35, 95% CI=1.99–5.65, P<0.0001).Dramatic increase of FIP risks were found for the TT genotype and T allele of rs35705950 in additive, dominant, and allelic model (TT vs GG, OR=17.08, 95% CI=1.49–195.49, P = 0.02; GT+TT vs GG, OR=6.02, 95% CI=3.22–11.24, P<0.0001; T vs G, OR=1.64, 95% CI= 1.28–2.00, P<0.0001).No significant relations existed between rs35705950 and ssc-ILD risks (TT vs GG, OR=0.94, 95% CI=0.43–2.08, P = 0.88; TT vs GT+TT, OR=0.92, 95% CI = 0.42–2.02, P =0.83; GT+TT vs GG, OR=1.11, 95% CI=0.90–1.38, P=0.34; T vs G, OR=1.12, 95% CI= 0.94–1.34, P=0.34).
Publication Bias
To assess the publication bias, funnel plots with the Egger’s and Begg’s tests were used in the present meta-analysis. As shown in Table 3, Egger’s and Begg’s tests did not find any significant bias either.
Table 3:
P-value of publication bias tests of included studies
| Test | IIP | IPF | NSIP | SSc-ILD | FIP |
|---|---|---|---|---|---|
| Begg’s | 0.218 | 0.880 | 1.000 | 0.734 | 1.000 |
| Egger’s | 0.910 | 0.211 | 0.773 | 0.931 | N.A |
Sensitivity analysis
The sensitivity analysis was performed by evaluating the influence of each individual study on pooled ORs and 95% CIs. The STATA software was used to omit each individual study at one time and evaluate the influences. The results showed no obvious influence on pooled ORs by each individual study.
Source of heterogeneity
Obvious heterogeneity was found between the studies involved for IIP in the dominant model (GT+TT vs GG) and the allelic model (T vs G)(69.0% and 79.4% respectively). To identify the sources of the heterogeneity, subgroup analysis by disease subtypes (iNSIP and IPF) and ethnicity were conducted. The results showed no significant heterogeneity existed between studies involved for iNSIP, but a high heterogeneity between studies involved for IPF, indicating the disease subtype partly responsible for the heterogeneity. Further subgroup analysis by ethnicity found a relatively high heterogeneity existed in both Asians and Caucasians in the dominant model, and high heterogeneity in Caucasians in the allelic model, indicating the ethnicity may not be the source.
After checking each individual study carefully, we found one study deviated from HWE (14), and one was with relative high influence on overall pooled ORs (16). After adjustment by excluding these two studies, the obvious heterogeneity was removed (Table 4).
Table 4:
Comparison of pooled ORs and heterogeneity after adjustment
| Genetic model | Subgroup | N | OR | P value | I2% | OR adjusted* | P-value adjusted* | I2% adjusted* |
|---|---|---|---|---|---|---|---|---|
| GT+TT vs GG | Overall IIP | 13 | 4.34 [3.22, 5.84] | <0.0001 | 69% | 5.13[4.41,5.97] | <0.0001 | 37% |
| Asian | 4 | 2.09 [1.44, 3.05] | <0.0001 | 46% | 4.13[2.15,7.95] | <0.0001 | 0% | |
| Caucasian | 9 | 4.95 [3.85, 6.35] | <0.0001 | 52% | 4.95 [3.85, 6.35] | <0.0001 | 52% | |
| IPF | 10 | 4.39 [3.17, 6.10] | <0.0001 | 75% | 5.22[4.46, 8.10] | <0.0001 | 42% | |
| T vs G | Overall IIP | 22 | 4.03 [3.34–4.86] | <0.0001 | 79% | 4.38[3.82,5.03] | <0.0001 | 34% |
| Asian | 4 | 2.64 [1.60–4.34] | <0.0001 | 27% | 4.05[2.11, 7.78] | <0.0001 | 0.40 | |
| Caucasian | 18 | 4.22 [3.47–5.14] | <0.0001 | 81% | 4.43[4.07, 4.83] | <0.0001 | 33.89 | |
| IPF | 18 | 4.06 [3.31–4.97] | <0.0001 | 83% | 4.47[4.10, 4.87] | <0.0001 | 30.71 |
Note:
adjusted by removing the data of Jiang H et al. and Noth I et al
Discussion
In the present meta-analysis, significant increases risks of overall IIP, and IIP subtypes, IPF and NSIP, were found for TT genotype and T allele of rs35705950 in all genetic models (Table 2 and Fig. 2, 3). A significant increase of FIP risk also existed for the TT genotype and T allele of rs35705950 in additive, dominant, and allelic model (Table 2). No significant relation between the rs35705950 and SSc-ILD risks.
Fig. 3:
Forest plot of ORs for the association between rs35705950 and IPF in the allelic model (T vs G)
ILDs were a group of diseases with a high death rate and low survival expectancy. Studies have shown MUC5B played important roles in the pathogenesis of ILD. MUC5B was a gene encodes a protein named mucin 5B, a member of the mucin family. Mucin was a family of high glycosylated macromolecular proteins, which was a major component of mucus secretion. Their gel-forming characteristics made them essential for the functions of lubrication, cell signal transduction and chemical barrier (24). Mucin5bwas a principle mucin secreted by submucosal glands and epithelial secretory cells on airway (25). The function of mucin5b in the airway was responsible for the clearance of inhaled particles (26). Loss of mucin5b may lead to the overwhelming of inhaled particles over normal clearance mechanisms. However, the mutation on the MUC5B promoter, rs35705950, actually increased the expression of mucin5b (5). And this over-expression of mucin5b mainly presented in terminal bronchioles, where normally didn’t express mucin5b. The increased mucin5b in distal airway cells led to the increase of cell turnover and repaire of mesenchymal cell proliferation and fibrosis, which was partly responsible for the ILD (27). The associations between rs35705950 and ILDs, such as IPF, NISP, and FIP have been identified by several reports (5, 18). Our meta-analysis confirmed the increased risks of IIP and FIP for TT genotype or T allele of MUC5B.
However, we didn’t find any associations between rs35705950 and SSc-ILD. SSc-ILD is a disease-specific phenotype of systemic sclerosis. It is a lung disease with different pathological process compared to IIP (28). Other SSc related factors, such as IRF5 rs20046640, STAT4 rs7574865 may contribute to SSc-ILD (29).
Obvious heterogeneity existed in the present meta-analysis in dominant model (GT+TT vs GG) and allelic model (T vs G) for overall IIP. Subgroup analysis by IIP subtypespartly illustrated the source of this heterogeneity. However, we found two studies may responsible for this heterogeneity, one deviated from HWE and another had a high influence on pooled ORs (14, 16). After adjustment by excluding these two studies, the obvious heterogeneity has been removed.
The present meta-analysis should be interpreted with caution because of the following limitations: First, most of the studies were conducted in Caucasians. We included 28 studies, and only 4 studies were conducted in Asians. Second, the heterogeneity between included studies in dominant and allelic models was significant. Third, more subtypes of IIP were not studied yet. The ILDs included more than 130 subtypes, our meta-analysis only studied IIP, FIP and SSc-ILD because of lacking information from original studies. Fourth, ILDs were influenced by both environmental and genetic factors. However, most of the included studies were without the information on environmental exposure and multiple SNPs in haplotypes.
Conclusion
The current meta-analysis suggested a significantly higher risk of overall IIP, IIP subtypes (IPF and NSIP), and FIP in TT genotype and T allele of MUC5B rs35705950. No associations were found between rs35705950 and SSc-ILD. However, the results of the present meta-analysis should be interpreted with caution because of the ethnicity and heterogeneity. Further studies in Asians, and with environmental exposure were required.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The present study was supported by the Hunan Key Laboratory Cultivation Base of the Research and Development of Novel Pharmaceutical Preparations (No. 2016TP1029); The Hunan Provincial Innovation Platform and Talents Program (No. 2018RS3105); and the Application Characteristic Discipline of Hunan Province.
The National Sciences Foundation of China (Grant No. 81670427); Hunan Provincial Natural Science Foundation (No. 2018JJ2463, 2019JJ40330 and 2018JJ2462) and Foundation of Hunan Educational Committee (No. 17B035, 19A055).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.King TE. (2005). Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med, 172: 268–279. [DOI] [PubMed] [Google Scholar]
- 2.Marsha A, Mouna M. (2020). Interstitial lung disease. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, et al. (2013). An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med, 188(6): 733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steen VD, Medsger TA. (2007). Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis,66 (7): 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seibold MA, Wise AL, Speer MC, et al. (2011). A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med,364(16): 1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borie R, Kannengiesser C, Crestani B. (2012). Familial forms of nonspecific interstitial pneumonia/idiopathic pulmonary fibrosis: Clinical course and genetic background. Curr Opin Pulm Med, 18(5): 455–461. [DOI] [PubMed] [Google Scholar]
- 7.Horimasu Y, Hattori N, Ishikawa N, et al. (2013). MUC5B promoter polymorphism is significantly associated with idiopathic interstitial pneumonia in German but not in Japanese patients. Eur Respir J, 42 (Suppl 57) P2332. [Google Scholar]
- 8.Johnson C, Giles JT, Bathon J, Danoff SK. (2013). Exploration Of The Muc5b Polymorphism Frequency In Rheumatoid Arthritis Interstitial Lung Disease. Am J Respir Crit Care Med, 187: A5982. [Google Scholar]
- 9.Borie R, Crestani B, Dieude P, et al. (2013). The MUC5B Variant Is Associated with Idiopathic Pulmonary Fibrosis but Not with Systemic Sclerosis Interstitial Lung Disease in the European Caucasian Population. PLoS One,8(8): e70621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coghlan MA, Shifren A, Huang HJ, et al. (2014). Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir Res, 1(1): e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingerlin TE, Murphy E, Zhang W, et al. (2013). Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet, 45(6): 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helling BA, Gerber AN, Kadiyala V, et al. (2017). Regulation of MUC5B Expression in Idiopathic Pulmonary Fibrosis. Am J Respir Cell Mol Biol, 57(1): 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horimasu Y, Ohshimo S, Bonella F, et al. (2015). MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology, 20(3): 439–44. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Hu Y, Shang L, Li Y, Yang L, Chen Y. (2015). Association between MUC5B polymorphism and susceptibility and severity of idiopathic pulmonary fibrosis. Int J Clin Exp Pathol,8(11): 14953–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Kishore A, Žižková V, Kocourková L, et al. (2016). Association study for 26 candidate loci in idiopathic pulmonary fibrosis patients from four European populations. Front Immunol, 7: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noth I, Zhang Y, Ma SF, et al. (2013). Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med, 1(4): 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock CJ, Sato H, Fonseca C, et al. (2013). Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax, 68(5): 436–41. [DOI] [PubMed] [Google Scholar]
- 18.van der Vis JJ, Snetselaar R, Kazemier KM, et al. (2016). Effect of Muc5b promoter polymorphism on disease predisposition and survival in idiopathic interstitial pneumonias. Respirology, 21(4): 712–7. [DOI] [PubMed] [Google Scholar]
- 19.Wang CL, Zhuang Y, Guo WW, et al. (2014). Mucin 5B Promoter Polymorphism Is Associated with Susceptibility to Interstitial Lung Diseases in Chinese Males. PLoS One,9(8): e104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei R, Li C, Zhang M, et al. (2014). Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl Res, 163(5): 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Noth I, Garcia JG, et al. (2011). A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med, 364(16): 1576–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peljto AL, Steele MP, Fingerlin TE, et al. (2012). The pulmonary fibrosis-associated MUC5B promoter polymorphism does not influence the development of interstitial pneumonia in systemic sclerosis. Chest, 142(6): 1584–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson C, Rosen P, Lloyd T, et al. (2017). Exploration of the MUC5B promoter variant and ILD risk in patients with autoimmune myositis. Respir Med, 130: 52–54. [DOI] [PubMed] [Google Scholar]
- 24.Marin F, Luquet G, Marie B, et al. (2008). Molluscan shell proteins: primary structure, origin, and evolution. Curr Top Dev Biol, 80: 209–76. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DC. (2011). Airway mucus function and dysfunction. N Engl J Med, 364(10): 978. [DOI] [PubMed] [Google Scholar]
- 26.Roy MG, Livraghi-Butrico A, Fletcher AA, et al. (2014). Muc5b is required for airway defence. Nature,505(7483): 412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickey BF, Whitsett JA. (2017). Understanding Interstitial Lung Disease: It’s in the Mucus. Am J Respir Cell Mol Biol, 57(1): 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldin JG, Lynch DA, Strollo DC, et al. (2008). High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest,134(2): 358–367. [DOI] [PubMed] [Google Scholar]
- 29.Dieudé P, Guedj M, Wipff J, et al. (2009). Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: a new perspective for pulmonary fibrosis. Arthritis Rheum,60(1): 225–33. [DOI] [PubMed] [Google Scholar]