Abstract
The occurrence and development of cardiovascular-related diseases are associated with structural and functional changes in gut microbiota (GM). The accumulation of beneficial gut commensals contributes to the improvement of cardiovascular-related diseases. The cardiovascular-related diseases that can be relieved by Lactobacillus supplementation, including hypercholesterolemia, atherosclerosis, myocardial infarction, heart failure, type 2 diabetes mellitus, and obesity, have expanded. As probiotics, lactobacilli occupy a substantial part of the GM and play important functional roles through various GM-derived metabolites. Lactobacilli ultimately have a beneficial impact on lipid metabolism, inflammatory factors, and oxidative stress to relieve the symptoms of cardiovascular-related diseases. However, the axis and cellular process of gut commensal Lactobacillus in improving cardiovascular-related diseases have not been fully elucidated. Additionally, Lactobacillus strains produce diverse antimicrobial peptides, which help maintain intestinal homeostasis and ameliorate cardiovascular-related diseases. These strains are a field that needs to be further investigated immediately. Thus, this review demonstrated the mechanisms and summarized the evidence of the benefit of Lactobacillus strain supplementation from animal studies and human clinical trials. We also highlighted a broad range of lactobacilli candidates with therapeutic capability by mining their metabolites. Our study provides instruction in the development of lactobacilli as a functional food to improve cardiovascular-related diseases.
Keywords: cardiovascular-related diseases, lactobacilli, gut microbiota, antimicrobials, therapeutic use
Introduction
Cardiovascular diseases and related diseases, such as hypercholesterolemia, hypertension, atherosclerosis, obesity, and diabetes, are the leading causes of death worldwide and continue to be an economic and health burden (1–3). Alterations in the composition and function of the gut microbiota (GM), known as dysbiosis, are linked to the occurrence and development of cardiovascular-related diseases. Tang et al. (4) summarized the GM composition of patients suffering from atherosclerosis, hypertension, obesity, and type 2 diabetes mellitus (T2DM) with high Firmicutes/Bacteroides ratio, trimethylamine-N-oxide (TMAO), short-chain fatty acids (SCFAs), and bile acids (BAs), as well as lipopolysaccharide (LPS) alterations. Recent research has demonstrated that the function of GM dysbiosis on cardiovascular-related diseases includes the following four aspects: inflammatory response due to the release of intestinal bacterial endotoxin, lipid metabolism abnormality, in vivo oxidative stress reaction, and tryptophan metabolism abnormality (5, 6).
Probiotic supplementation could lead to a reduction in cardiovascular risk (4, 7). Probiotics may act through different mechanisms, including establishing intestinal balance, affecting nutrient absorption and immune functions, and competing with pathogens (8). Among gut probiotics, the genus Lactobacillus is a Gram-positive probiotic classified as lactic acid bacteria (9). Various Lactobacillus strains have been widely studied for their interventions in cardiovascular-related diseases via the modulation of lipid cholesterol metabolism, immune-inflammatory response, oxidative stress response, and the involvement of GM-derived metabolites, including TMAO, SCFAs, LPS, and BAs (10–13). The metabolites produced by Lactobacillus, especially antimicrobial substances, can inhibit the growth of pathogens and regulate GM disorder (14, 15). They act as the protectors of GM and are the major contribution of lactobacilli in the improvement of cardiovascular-related diseases. However, as an important therapy for the improvement of cardiovascular-related diseases, a wide range of Lactobacillus can also be mined and visualized with a clear function. The discovery of antimicrobials by genome mining is feasible and worthwhile because the subsequent identification and isolation of known and putative molecules can attract pharmacological interest (16). The specific alteration of Lactobacillus through these mediators in the host physiology for the remedy of cardiovascular-related diseases should also be analyzed for future studies. Developing more Lactobacillus strains is beneficial for clinical application.
China issued the list of bacteria, including 13 Lactobacillus species, which are recognized as safe ingredients and widely used in the production of food products. The list was supplemented in the form of an announcement by the National Health Commission of the People's Republic of China [http://www.nhc.gov.cn, (2010) No. 65]. From 2005 to 2021, more than 20 strains among nine Lactobacillus species have been classified as “generally recognized as safe” by the U.S. Food and Drug Administration (https://www.accessdata.fda.gov). Besides, the European Food Safety Authority provides the assessment for probiotics, and 37 Lactobacillus species had been recommended for the Qualified Presumption of Safety list until 2021 (17–20). Among these species, L. acidophilus, L. casei, L. crispatus, L. curvatus, L. delbrueckii, L. fermentum, L. gasseri, L. helveticus, L. johnsonii, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, and L. salivarius were certified by at least two organizations (Supplementary Table 1). L. murinus has made a remarkable contribution to the prevention and treatment of hypertension (21). This review studied 38 Lactobacillus species (Supplementary Figure 1), updated the findings of the relationship between Lactobacillus and cardiovascular-related diseases, and provided a rich candidate of Lactobacillus strains that lessen cardiovascular risks.
How do Lactobacilli as GM Commensal Alleviate Cardiovascular-Related Diseases?
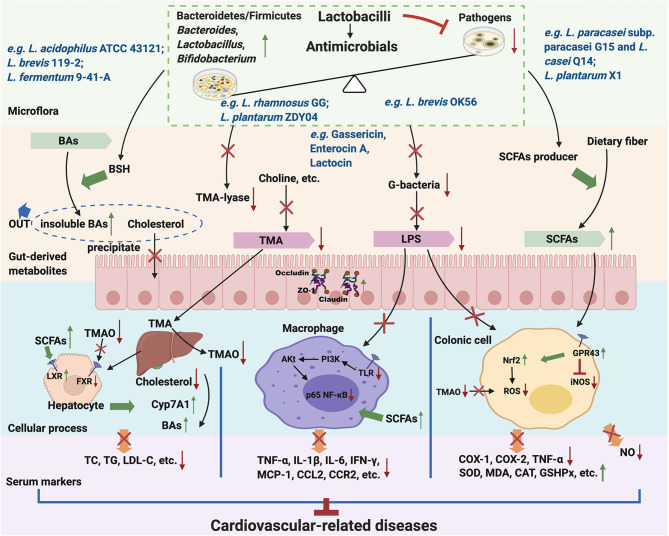
In recent years, our perception of the microbiome has evolved from a group of inert microorganisms into a true “endocrine organ” (22). The GM-dependent mechanism of Lactobacillus has also attracted widespread attention. A few reports revealed the evidence of lactobacilli on modulating GM composition, including only L. acidophilus, L. brevis, L. casei, L. fermentum, L. johnsonii, L. mucosae, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, L. sakei, L. salivarius. Nonetheless, the primary beneficial effect of lactobacilli starts from restoring GM abundance and species diversity. Lactobacillus colonization in the intestinal tract directly affects intestinal homeostasis and reduces gut permeability by inhibiting pathogens because of their antimicrobial products. Lactobacillus can modulate gut-derived metabolites and further decrease the level of serum cholesterol and reduce inflammation and oxidant damage. Many studies have revealed the relevant relationships between gut-derived mechanisms and the development of cardiovascular-related diseases (Figure 1).
Figure 1.
Mechanisms of lactobacilli on prevention and treatment of cardiovascular-related diseases through the GM. Green↑, Increase/Promote; Red↓/×/⊥, Decrease/Inhibit.
Lactobacillus Corrects Risk-Associated Microflora and Metabolites
Characteristic shifts in GM structure due to Lactobacillus intervention are manifested by corrections of vital gut dysbiosis parameters, such as decreased Firmicutes/Bacteroidetes ratio and abundant Bacteroides, Lactobacillus, and Bifidobacterium (Table 1). Lactobacillus further affects itself and other intestinal microbes that metabolize the host digestive products into various metabolites, such as trimethylamine (TMA), SCFAs, LPS, and BAs, which are involved in the progression of cardiovascular-related diseases (48). Lactobacillus reduces the putative cardiovascular risk mediator TMAO, which is produced by TMA oxidation in the liver, to prevent atherosclerosis and hypertension (11, 49). However, the possible mode of action of lactobacilli on TMAO is still unclear because a paucity of literature is available on the subject, and supporting clinical data is limited. Genomic data of GM showed that 37 bacterial species belonging to the phyla Firmicutes, Proteobacteria, and Actinobacteria harbor genes involved in TMA production (50). In consideration of the role of GM in TMAO metabolism, lactobacilli might inhibit gut microbes that produce key enzymes that catalyze TMA production. Lactobacillus supplementation promotes the SCFAs-producing bacteria Roseburia, Ruminococcus, and Eubacterium to facilitate the dietary fiber-fermented byproducts SCFAs (51, 52), which play critical roles in maintaining healthy cardiovascular functions. As the major component of the outer membrane of Gram-negative bacteria, LPS plays a key part in the pathogenesis of hypertension, obesity, and T2DM. Lactobacillus has the ability to reduce the LPS concentration in serum (53, 54). The potential of lactobacilli to inhibit Gram-negative is discussed in this paper. Disorders in BAs metabolism cause dyslipidemia, cardiovascular diseases, and diabetes. Lactobacilli have a major function in BAs biotransformation by promoting the activity of microbial bile salt hydrolase (BSH), regenerating primary free BAs, and facilitating the microbial formation of secondary BAs, as well as a range of intermediates (55, 56).
Table 1.
A variety of lactobacilli with therapeutic effects on cardiovascular-related diseases.
| Lactobacillus | Strain | Via Cellular process | Via Gut Microbiota (GM) | Cardiovascular-related diseases | Model | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol-lowering | Anti-Inflammation | Anti-oxidative stress | Metabolite | GM variety | |||||
| L. acidophilus | La5 | • | ° | ° | ° | Dyslipidemia | Type 2 diabetic adults | (23) | |
| L. amylovorus | CP1563 | • | ° | ° | ° |
Roseburia and Lachnospiraceae↑ Collinsella↓ |
Obesity | Obese class I adults; pre-obese healthy adults | (24, 25) |
| L. casei | 01 | ° | ° | ° | ° | Type 2 diabetes mellitus | Type 2 diabetic adults | (26) | |
| Shirota | • | ° | ° | SCFAs↑ | Bifidobacterium, Bacteroides fragilis group, Atopobium cluster, Lactobacillus gasseri group↑ | Obesity | Obese children | (27) | |
| L. fermentum | ME-3 | • | • | • | ° | Cardiovascular and diabetes risk | Asymptomatic adults | (13) | |
| L. gasseri | BNR17 | ° | ° | ° | ° | Obesity | Overweight and obese adults | (28) | |
| SBT2055(LG 2055) | ° | ° | ° | ° | Obesity | Healthy adults with large visceral fat areas | (29, 30) | ||
| • | ° | ° | ° | Obesity and type 2 diabetes mellitus | Hypertriacylglycerolemic adults | (31) | |||
| L. helveticus | LBK-16H | ° | ° | ° | ° | Hypertension | Hypertensive adults | (32, 33) | |
| CM4 | ° | ° | ° | ° | Hypertension | Hypertensive adults | (34) | ||
| L. murinus | ° | ° | ° | ° | Salt-sensitive hypertension | In vitro trial (TH17 cells) healthy men, HSD-fed FVB/N mice | (21) | ||
| L. plantarum | Lp299v | ° | • | • | ° | Coronary artery disease | Men with stable coronary artery disease | (35) | |
| ECGC13110402 | • | • | ° | ° | Hypercholesterolemia | Hypercholesterolemic adults | (36) | ||
| OLL2712 | • | • | ° | ° | Obesity | Overweight adults | (37) | ||
| Dad-13 | ° | ° | ° | ° | Bifidobacteria and Lactobacilli↑ Enterobacteriaceae and Staphylococcus↓ |
Obesity | Overweight adults | (38) | |
| L. reuteri | NCIMB30242 | • | • | ° | ° | Hypercholesterolemia | Hypercholesterolemic adults | (7) | |
| DSM17938 | • | ° | ° | BAs↑ | Diversity↑ | Insulin sensitivity | Type 2 diabetic adults | (39) | |
| ADR-1 or ADR-3 | • | ° | ° | ° | Lactobacillus and Bifdobacterium↑ | Type 2 diabetes mellitus | Type 2 diabetic adults | (40) | |
| V3401 | ° | • | ° | ° | Verrucomicrobia↑ | Metabolic syndrome | Adults with metabolic syndrome | (41) | |
| L. rhamnosus | ° | ° | ° | ° | Myocardial infarction | Post-myocardial infarction adults | (42) | ||
| GG | ° | ° | ° | ° | Type 2 diabetes mellitus | Middle age and older adults | (43) | ||
| GG | ° | • | ° | LPS↓ | Coronary artery diseases | Adults with coronary artery diseases | (44) | ||
| ° | • | • | ° | Myocardial infarction | Post-myocardial infarction adults | (45) | |||
| L. sakei | CJLS03 | ° | ° | ° | ° | Obesity | Obese adults | (46) | |
| L. salivarius | Ls-33 | ° | ° | ° | ° | Bacteroides-Prevotella-Porphyromonas group/Firmicutes↑ | Obesity | Obese adolescents | (47) |
°, Non-significant effect between groups/Not applicable; •, Observed effect; ↑, Increase/Promote; ↓, Decrease/Inhibit.
Lactobacillus Inhibits Pathogenic Bacteria and Reduces Gut Permeability
The destruction of the intestinal barrier function in patients with cardiovascular-related diseases is the main reason for the excessive proliferation of related pathogens and the increase in plasma endotoxin concentration. These conditions further aggravate gut permeability, promote inflammation, and increase cardiovascular risks (57). Lactobacillus exerts strong antimicrobial activity against pathogens and reinforces the intestinal barrier (58). Although antimicrobial production by lactobacilli has been regarded as a beneficial trait for some time, the full extent of the benefits of antimicrobials in the gut is only beginning to be appreciated. A comparison of L. salivarius UCC118 (with bacteriocin Abp118) with the non-bacteriocin-producing strain, L. salivarius UCC118 (knock out Abp118), showed that antimicrobial production resulted in an increase in Bacteroidetes and a reduction in the proportions of Actinobacteria in the GM of diet-induced obesity mice (59). Further clinical research demonstrated that the favorable effects of the UCC118 strain possibly rely on positive alterations in gut permeability and microbiota (60). An animal study showed that L. plantarum ZLP001 pretreatment alleviated the reduction in junction proteins (claudin-1, occludin, and ZO-1) (61), which are the key contributors to establishing an effective intestinal barrier (62). Several in vitro experiments also showed that lactobacilli positively affect gut permeability, as incubation of Caco-2 cells with different Lactobacillus strains was found to restore impaired intestinal barrier (63, 64).
Of note, LPS produced by Gram-negative bacteria in the gut is the main component of endotoxin. Gassericin, enterocin A, paracin 1.7, lactocin, bacteriocin TSU4, and bacteriocin 217 greatly inhibit Gram-negative bacteria (such as Escherichia coli) (65–74). Therapeutic manipulation of microbiota using different antimicrobial strategies may be a useful approach for the management of cardiovascular-related diseases. Our extended list of antimicrobial compounds demonstrates that lactobacilli are excellent antimicrobial producers (Supplementary Table 2). L. paracasei, L. sakei, L. plantarum, L. casei, L. gasseri, L. alimentarius, L. coryniformis, L. panis, L. crispatus, L. johnsonii, L. amylovorus, L. curvatus, L. rhamnosus, and L. helveticus can now readily be tapped experimentally for improving cardiovascular-related diseases.
Lactobacillus Presents Cholesterol-Lowering, Anti-Inflammation, and Anti-Oxidative Stress Effects
By expressing BSH, lactobacilli have certain advantages in the intestine that result in the deconjugation of free bile salts, which combine with cholesterol to form a precipitate and are more easily excreted via feces (10, 75–77). Lactobacilli induce the catabolism of cholesterol to BAs by cytochrome P450 a1 (Cyp7A1) by inhibiting farnesoid X receptor (FXR) (78, 79) and inhibit the reabsorption of BAs into the enterohepatic circulation (80). SCFAs are transported to the liver to modulate the hepatic metabolism of lipids and cholesterol by elevating the transcriptional activity of liver X receptor (LXR) alpha and upregulating Cyp7A1 (81). By contrast, TMAO regulates changes in BAs synthesis to accelerate aortic lesion formation by activating FXR and small heterodimer partner to suppress BAs synthetic enzyme expression and decrease BAs transporters in the liver but not in the gut (82).
Lactobacillus has anti-inflammatory activity and exerts protective effects on damage by inhibiting PI3K/Akt, NF-κB activation, and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, and monocyte chemotactic protein 1 (MCP-1). Lactobacillus promotes SCFAs production that activates the G protein-coupled receptor 43 (GPR43) pathway (51, 83), and downregulates the expression and activation of NF-κB, interferon-γ, Toll-like receptor 2, TNF-α, and other cytokines/chemokines involved in inflammatory responses (84). Lactobacillus presents an anti-oxidative effect by blocking LPS-induced nitric oxide (NO) production; decreasing the expression of cyclooxygenase (COX)-1, COX-2, inducible nitric oxide synthase (iNOS), and TNF-α mRNA (85). In addition, Lactobacillus promotes Nrf-2-induced antioxidative activity in mice to reduce cardiovascular risk (86). Other serum markers of oxidative stress, including thiobarbituric acid-reactive substances (TBARS), superoxide dismutase (SOD), malondialdehyde, catalase (CAT), glutathione peroxidase (GSH-Px), norepinephrine (NE), and prostacyclin, were also altered by Lactobacillus intervention. Butyrate promotes endothelial nitric oxide synthase (eNOS) expression and NO bioavailability in vascular smooth muscle cells (87). TMAO induces reactive oxygen species (ROS) generation, particularly mitochondrial ROS through the suppression of SOD2 activity and sirtuin-3 in human umbilical vein endothelial cells and aortas from apolipoprotein E (ApoE)-deficient mice.
Without a doubt, our review is limited by the available studies. A few metabolites such as indole derivatives, polyamines, and taurine are not deeply discussed in the function of lactobacilli treatment. Furthermore, some new regulatory mechanisms of vascular-related diseases should be concerned, such as activation of aromatic hydrocarbon receptor, rather than classical pathways.
Lactobacillus Strains Worthy of Attention and Their Therapeutic Use
Various lactobacilli strains play roles in reducing cardiovascular risk factors, balancing metabolic disorders, and altering health-related microflora metabolite production. However, little is known about the role of these supplements as important dietary components in preventing or treating cardiovascular-related disease. Still, some reports and clinical studies were conducted, offering new ways of treatment. In addition, some lactobacilli species, such as L. acidophilus, L. gasseri, and L. rhamnosus, have been associated with a wide range of purported health benefits, such as anti-infectious activity; immunomodulation; anti-allergenic effects; and tumor suppression. Below are the selected lactobacilli that improve cardiovascular-related diseases. Eleven Lactobacillus species take effect on extensive cardiovascular risks, 10 species act on a single disease, and the effects of the other 17 species on cardiovascular-related diseases have not been reported (Supplementary Figure 1). Due to the strain specificity and individual physical differences, a personalized clinical evaluation and intake recommendation should be developed.
Lactobacillus acidophilus
L. acidophilus, which is one of the most important resident microorganisms in the small intestine, has a cholesterol-lowering function for the improvement of hyperlipidemia, hypercholesterolemia, atherosclerosis, coronary heart disease (CHD), T2DM, and obesity (75, 88–92). Strain ATCC 43121 can reduce cholesterol metabolism by increasing insoluble BAs (lithocholic acid) in hypercholesterolemia rats (75). L. acidophilus ATCC 4356 reduced the expression of the Niemann-Pick C1-Like 1 and glucose transporter 2 gene and inhibited the cellular uptake of micelle cholesterol and glucose in Caco-2 cells (89, 93). Further animal studies (90, 94) found that the administration of strain ATCC 4356 can prevent atherosclerosis by inhibiting the absorption of intestinal cholesterol and enhancing the abundance of Lactobacillus and Bifidobacterium in the gut. In addition to reducing total cholesterol (TC), L. acidophilus SJLH001 isolated from fermented food can also reduce blood glucose in high-fat diet (HFD)-induced obese mice by regulating the key genes involved in the glucose transport, ion channels, and immune response of the bacterium (95) and affect the structure of intestinal microbiota. Strain LA5 improved saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila (96). Besides, L. acidophilus KLDS1.0901 administration showed antidiabetic and antioxidant activity in T2DM mice induced by HFD and intraperitoneal injection of streptozotocin (STZ) (97). L. acidophilus is known for the mixed-use with Bifidobacteria in probiotic dairy foods and effectively used in clinical practice (23). Since L. acidophilus is safe in humans, it is likely a potential drug for improving cardiovascular health. Therefore, clinical studies are warranted to explore the beneficial effects of this bacterium.
Lactobacillus brevis
L. brevis is a microaerophilic, obligately heterofermentative lactic acid bacterium isolated from various natural environments with relieving effects on hypercholesterolemia, atherosclerosis, obesity, and hypertension. The potential mechanism of the cholesterol-lowering effect of L. brevis 119-2 was the inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase activity by insulin-induced gene (Insig) protein and the induction of the catabolism of cholesterol to BAs by Cyp7A1 (78). L. brevis OK56 ameliorated HFD-induced obesity in mice by inhibiting NF-κB activation and gut microbial LPS production (98). L. brevis OPK-3 inhibited the induction of inflammation in adipose tissue along with preventing weight gain (99). L. brevis presented an anti-hypertensive effect by blocking LPS-induced NO production and decreasing the expression of COX-1, COX-2, iNOS, and TNF-α mRNA (85). Gamma-aminobutyric acid-producing strains, L. brevis DPC6108, and L. brevis DSM32386, had the potential to improve metabolic profiles in mice with metabolic dysfunction (100). There are few studies on the clinical practicability and suggestions for L. brevis, and extensive studies are expected.
Lactobacillus casei
L. casei is a transient bacterium in the human body and can relieve hypocholesterolemia, atherosclerosis, and hypertension in mice/rats through a cholesterol-lowering mechanism attributed to the improvement of BAs, SCFAs, and TMAO (10, 101–103). L. casei 01 supplementation significantly decreased dietary intake and body weight through improving serum fetuin-A and sirtuin1 levels and glycemic response in T2DM patients (26). L. casei can also effectively treat T2DM and hyperglycemia by suppressing GM-mediated inflammation (104–106). L. casei prevented T2DM possibly via a microbiota-based BA-chloride exchange mechanism by upregulating chloride ion-dependent genes (ClC1-7, GlyRα1, SLC26A3, SLC26A6, GABAAα1, Bestrophin-3, and CFTR) (104). L. casei CCFM419 had a potential ability to ameliorate insulin resistance and hyperglycemia in T2DM mice through underlying PI3K/Akt signaling pathway and gut flora-SCFAs-inflammation/GLP-1 mechanism (105, 106). L. casei strain Shirota as a dietary intervention not only played a role in controlling childhood obesity and improving lipid metabolism through an apparent increase in acetic acid concentration (27). The common points of these lactobacilli in modulating GM were the increase of Lactobacillus and Bifidobacterium. L. casei C1 supplementation in hypertensive rats increased serum glutathione (GSH) and NO levels; thus, the strain worked through antioxidant function and increased NO levels (induced vasodilation) for attenuating hypertension (107). Besides, L. casei has a significant effect on the treatment of liver injury (108), indicating that this bacterium is suitable for patients with the above risks.
Lactobacillus delbrueckii
L. delbrueckii subsp. bulgaricus (L. bulgaricus) is widely used in the dairy industry and can treat hypercholesterolemia, ischemic heart disease, and diabetes. L. bulgaricus NS12 can reduce serum TC, low-density lipoprotein (LDL), apolipoprotein B, and free fatty acid levels and increase apolipoprotein A-I levels in rats with high cholesterol diet. Simultaneously, this strain remarkably reduced liver damage and liver lipid deposition by regulating the mRNA expression levels of liver enzymes related to cholesterol metabolism (109). Besides, L. bulgaricus had a certain protective effect on the heart. L. bulgaricus 51 remarkably reduced rapid arrhythmia after reperfusion in ischemic rats, reduced the release of NE and prostacyclin in the first minute of reperfusion, and improved the heart function of ischemic rats. The protective effect is related to the activation of CAT and the expression of heat shock protein 70 (110, 111). L. delbrueckii subsp. lactis PTCC1057 treatment decreased the fasting blood glucose and fetuin-A level and increased the serum sestrin 3 level in the STZ-induced diabetic mice (112). However, whether L. delbrueckii is an effective supplement or not in clinical cohorts is still being debated. Nonetheless, this bacterium has shown promising results in clinical trials for patients with respiratory or vaginal infections (113, 114).
Lactobacillus fermentum
L. fermentum colonizes the gut and plays an important role in intestinal health. Clinical and animal experiments have proven its preventive and therapeutic effects on hypercholesterolemia, hyperlipidemia, hyperglycemia, atherosclerosis, obesity, and hypertension. L. fermentum has diverse regulatory mechanisms in addition to cholesterol-lowering metabolism together through SCFAs and BAs regulation (76, 115–120). Adverse physiological alterations, including considerably higher levels of serum TC, low-density lipoprotein cholesterol (LDL-C), triacylglycerols (TG), atherogenic index, coronary artery risk index, hepatic lipids, lipid peroxidation, mRNA expression of inflammatory cytokines (TNF-α and IL-6) in the liver, and anti-oxidative enzyme activities (CAT, SOD, and GSH-Px) in the liver and kidney, improved after the supplementation of L. fermentum MTCC: 5898-fermented buffalo milk (2.5% fat) in rats fed with cholesterol-enriched diet (118). L. fermentum can also play a role in fat metabolism. Fecal cholesterol and BAs levels considerably increased after L. fermentum 9-41-A administration (121). Intestinal Lactobacillus and Bifidobacterium colonies increased whereas Escherichia coli colonies decreased. L. fermentum strains can effectively inhibit HFD-induced obesity through modulation of the PPAR-α signaling pathway, oxidative phosphorylation in adipose tissue, and gut microbiome (122–124). L. fermentum CECT5716 can also restore vascular redox status and improve eNOS coupling to prevent hypertension and endothelial dysfunction caused by tacrolimus (125). In clinical trials, the use of L. fermentum ME-3 positively affected blood lipoprotein, oxidative stress, and inflammatory profile (13). However, L. fermentum may destroy the intestinal barrier, so further safety evaluation should be carried out (126).
Lactobacillus gasseri
Obesity is one of the common cardiovascular-related diseases, and research on new probiotic therapy has good application prospects. As a type of Lactobacillus found in the gastrointestinal tract, L. gasseri presents an anti-obesity effect by inhibiting lipid absorption. The effect of L. gasseri SBT2055 (LG2055) on fat hydrolysis was measured by measuring the activity of pancreatic lipase and the in vitro properties of the fat emulsion. The results showed that LG2055 increased the size of fat emulsion droplets and therefore inhibited lipase-mediated fat hydrolysis and promoted human fecal fat excretion (127). Similarly, skimmed milk fermented by LG2055 remarkably reduced average adipocyte size and reduced leptin and cholesterol in rats (128). LG2055 also has an anti-inflammatory function. A 24-week study found that the supplemental feeding of strain LG2055 to mice fed with a 10% fat diet could reduce the expression of pro-inflammatory genes, such as CCL2 and CCR2, and prevent weight gain and fat accumulation (129). Consumption of probiotic LG2055 can reduce serum non-esterified fatty acid levels after meals and on an empty stomach, indicating that it may help reduce the risk of obesity and T2DM (29–31, 130). LG2055 and L. gasseri BNR17 also have a good anti-obesity effect on overweight and obese adults, and healthy adults with large visceral fat areas (28, 30, 130, 131). The cholesterol-lowering effect of L. gasseri SBT0270 in hypercholesterolemia rats is attributed to the inhibition of BA reabsorption into the enterohepatic circulation and the enhancement of the excretion capacity of acidic steroids in the feces, which can effectively reduce heart vascular risk (80). These findings warrant a subsequent longer-term prospective clinical investigation with a larger obese population.
Lactobacillus helveticus
L. helveticus commonly used for dairy fermentation has long-term hypotensive effects in patients with hypertension (32). Two tripeptides (Val-Pro-Pro and Ile-Pro-Pro) that inhibit the activity of angiotensin I converting enzyme are produced in fermented milk and do not cause adverse effects while lowering blood pressure (34). Long-term intervention can reduce arterial stiffness in patients with hypertension (33). L. helveticus KII 13 isolated from fermented milk can produce hypotensive peptides, reduce serum cholesterol and, increase the expression of LDL receptor and SREBF2 genes related to cholesterol metabolism in the liver in mice in the HFD group (132). Moreover, L. helveticus can absorb a certain degree of cholesterol for biotransformation in vitro (133). Also, the effectiveness study of L. helveticus on patients with hypertension and major depressive disorder requires further study (134).
Lactobacillus paracasei
L. paracasei, a gastrointestinal tract bacterium, plays multiple roles in the improvement of cardiovascular-related diseases, including hypercholesterolemia, hyperlipidemia, atherosclerosis, T2DM, obesity, and hypertension. The cholesterol-lowering effect of L. paracasei NTU101 resulted in the increased abundance of Allobaculum and Clostridium XIVa (135). Supplementation with L. paracasei NTU101 considerably reduced the ratio of LDL-C to high-density lipoprotein cholesterol (HDL-C), SOD activity, and total antioxidant status of the blood and relieve the degree of TBARS; hence, this strain can effectively prevent hyperlipidemia-induced oxidative stress and atherosclerosis (136). L. paracasei NTU101 can also act in hypertension treatment through substances, such as angiotensin-converting enzyme inhibitors (ACEI) and aminobutyric acid (γ-aminobutyric acid) (137), and exert neuroprotection in the brain (138). L. paracasei can regulate cholesterol metabolism, BAs homeostasis, as well as the LXR/inflammatory axis of LPS-stimulated alveolar macrophages, in animals fed with HFD (139–142). L. paracasei also protects the cardiovascular system by regulating blood glucose, insulin sensitivity, and fat metabolism (143). Researchers evaluated the α-glucosidase inhibitory activity of eight L. paracasei strains in vitro, and L. paracasei TD062, which has a high α-glucosidase inhibitory activity (31.9%), showed excellent antidiabetic ability. Further in vivo study showed that L. paracasei TD062 had a positive effect on the antioxidant capacity and the expression levels of genes that were related to glucose metabolism and the PI3K/Akt pathway in diabetic mice (144). Similarly, the studies are in animal models, and extrapolation in humans needs further studies, especially patients associated with allergic disease (145).
Lactobacillus plantarum
L. plantarum is a widespread member of the genus Lactobacillus and is commonly found in fermented food products and anaerobic plant matter. L. plantarum has important effects in preventing hypercholesterolemia, hyperlipidemia, atherosclerosis, T2DM, obesity, and hypertension. L. plantarum can absorb cholesterol directly from the culture medium and was thus selected as a probiotic that potentially reduced cholesterol levels in mice/rats (86, 146–154). L. plantarum HT121 improved serum lipid profiles, restored beneficial gut microbes, and regulated BAs metabolism (155). L. plantarum DR7 reduced cholesterol via the phosphorylation of AMPK, which downregulated the mRNA expression of 3-hydroxy-3-methyl glutaryl coenzyme A reductase in hepatic (HepG2) and intestinal (HT-29) cells (146). In vivo experiments confirmed that L. plantarum CAI6 and SC4 can regulate lipid metabolism and Nrf-2-induced oxidative defense in hyperlipidemic mice to reduce cardiovascular risk (86). L. plantarum ZDY04 remarkably reduced serum TMAO levels and TMAO-induced atherosclerosis by modulating the relative abundance of the families Lachnospiraceae, Erysipelotrichaceae, and Bacteroidaceae and the genus Mucispirillum in mice (11). L. plantarum strains can also exert anti-obesity effect by ameliorating lipid accumulation, oxidative damage, inflammation, and gut dysbiosis (156–158). In vitro, the cell-free supernatant of L. plantarum X1 can inhibit α-glucosidase activity and show potential antidiabetic ability. L. plantarum X1 can partially enhance antioxidant capacity and improve the secretion of cytokines and pancreatic damage in T2DM mice. In addition, this strain remarkably restored the acetic acid level and increased the butyric acid level in the feces of diabetic mice; thus, the ability of L. plantarum to lower blood sugar was closely related to exercise and fatty acid and intestinal flora composition changes (159). L. plantarum can also regulate blood pressure by inhibiting ACEI activity and promoting NO production and therefore improved learning and memory in rats with hypertension-induced vascular dementia induced by deoxycorticosterone salt (160). The expression of TNF-α, IL-6, MCP-1, vascular cell adhesion molecule, intercellular adhesion molecule, and E-selectin were remarkably downregulated in L. plantarum Lp91-fed LPS-induced mice compared with the control group; hence, its anti-inflammation effect might be involved in cardiovascular-related diseases (161). Besides, beneficial effects of L. plantarum, inulin, or their combination on GM, cardiac apoptosis, and diabetes have been studied (162–164). In the clinic, strain Lp299v improved vascular endothelial function and decreased systemic inflammation, and ECGC 13110402 exerted lipids reduction function (35, 36). Heat-treated L. plantarum OLL2712 reduced abdominal fat accumulation and chronic inflammation in overweight adults (37). GM analysis indicated that L. plantarum Dad-13 intervention in obese adults decreased the Firmicutes population and increased the Bacteroidetes population (38). Circulating gut-derived metabolites TMAO and SCFAs likely contribute to these improvements by L. plantarum and merit further study.
Lactobacillus reuteri
L. reuteri has been reported to exist naturally in the intestines of all vertebrates and mammals and is associated with most cardiovascular-related diseases. L. reuteri has cholesterol-lowering effects in the body and can effectively reduce TC, TG, and LDL values (165–169). Similar to L. plantarum DR7, L. reuteri NCIMB30242 exerted cholesterol-lowering properties along the AMPK pathway (146). In clinical trials, L. reuteri NCIMB30242 can best meet the dietary requirements for therapeutic lifestyle changes (TLC), which remarkably reduced LDL-C and TC and improved other CHD risk factors, such as inflammatory biomarkers (7). A high-calorie diet led to heart damage and promoted heart failure, whereas oral L. reuteri GMNL-263 treatment can regulate plasma lipids and reduce high-calorie-induced cardiac inflammation, hypertrophy, and fibrosis (170, 171). In addition, L. reuteri GMNL-263 can treat obesity in high-energy diet-induced obese rats by improving serum levels of pro-inflammatory factors and antioxidant enzymes and remodeling white adipose tissue (WAT) energy metabolism (172). L. reuteri strain ATCC PTA 4659 may partially prevent diet-induced obesity through a previously unknown mechanism that induced the expression of carnitine palmitoyltransferase 1a in the liver (173). L. reuteri GMN-32 treatment can reduce the impact of diabetes on the heart, decrease blood glucose levels, inhibit caspase 8-mediated apoptosis, promote heart function, and prevent diabetic cardiomyopathy (174). L. reuteri strains ADR-1 and ADR-3 ameliorated symptoms of T2DM patients, and increased intestinal level of L. reuteri to further up-regulate Lactobacillus and Bifidobacterium, and decrease Bacteroidetes (40). Oral L. reuteri DSM17938 supplementation for 12 weeks did not affect glycated hemoglobin but improved insulin sensitivity and increased serum secondary BA deoxycholic acid levels in patients with insulin-treated T2DM (39). Furthermore, L. reuteri V3401 can reduce inflammatory markers in patients with metabolic syndrome, including obesity, and improve microbial intestinal composition, especially Verrucomicrobia, for improving metabolic syndrome and reducing cardiovascular risk mechanism (41). L. reuteri has achieved remarkable results in the treatment of osteoporosis (175), suggesting that it might improve bone and cardiovascular health by modulating the GM.
Lactobacillus rhamnosus
L. rhamnosus is one of the most studied beneficial bacteria in the gut and has a beneficial effect on hyperlipidemia, obesity, T2DM, and damage after myocardial infarction (MI). Studies have highlighted the potential of L. rhamnosus to reduce HFD-related metabolic disorders by cholesterol-lowering mechanism (176–179). Notably, L. rhamnosus GR-1 reduced the development of oxidative stress and chronic inflammation through the NF-κB signaling pathway and therefore reduced the formation of atherosclerotic plaque in ApoE-deficient mice fed with HFD (176). L. rhamnosus effectively regulated the abundance and diversity of intestinal flora in rats and zebrafish HFD models by increasing the abundance of Bacteriodetes. L. rhamnosus GG, a well-established probiotic strain, has a direct anti-obesity effect through the regulation of intestinal microbiota, particularly by decreasing the Firmicutes/Bacteroidetes ratio (180). In addition, L. rhamnosus GG can protect dyslipidemia and improve insulin sensitivity by inhibiting FXR and fibroblast growth factor 15 signaling and upregulating hepatic Cyp7A1 (79, 177, 181–183). L. rhamnosus GG supplementation was also found to be associated with stable HbA1c levels in healthy individuals (43). The cardioprotective effect of L. rhamnosus GG against high-fat high-fructose diet-induced obesity was associated with up-regulation of Nrf2-mediated antioxidant pathways (184). This strain also mitigated the development of obstructive sleep apnea-induced hypertension caused by a high-salt diet by regulating TMAO level and CD4+ T cell induced-type I inflammation (49). Compared with L. rhamnosus GG, L. rhamnosus NCDC17 can improve oral glucose tolerance and biochemical parameters; oxidative stress (TBARS); and CAT, SOD, and GSH-Px activities in the blood and liver and decrease the proportion of propionic acid in the cecum (185). Tissue weight assessment and atrial natriuretic peptide gene expression in MI rats given L. rhamnosus showed a substantial attenuation of left ventricular hypertrophy (LVH), and various ultrasound indicators reflected the improvement of left ventricular function (186). A clinical study also proved the effectiveness and safety of Lactobacillus supplementation in preventing cardiac remodeling after MI (42). Supplementation of L. rhamnosus in patients with coronary artery disease had beneficial effects on depression, anxiety, and inflammatory biomarkers (44, 45). In addition to the bacteria itself, the p75 protein isolated from L. rhamnosus GG (187) considerably reduced infarcts in rat cardiac tissues in a dose-dependent manner (188). Thus, the protein produced by lactobacilli also had a direct cardioprotective effect on ischemic damage and was no longer restricted to improving cardiovascular-related diseases through dietary intake. L. rhamnosus seems to be suitable for various people, we should dig out more strains like L. rhamnosus GG through clinical research.
Other Lactobacilli
Eating habits are related to human health. For example, a high-salt diet can cause hypertension and other cardiovascular-related diseases. The intestinal flora perspective proved that a high-salt diet reduced the abundance of Lactobacillus in the gut, especially the consumption of L. murinus. Treatment with L. murinus prevented the salt-induced aggravation of actively induced experimental autoimmune encephalomyelitis and salt-sensitive hypertension in mice by modulating TH17 cells. In line with these findings, a moderate high-salt challenge in a pilot study in humans reduced the intestinal survival of Lactobacillus spp. and increased TH17 cells and blood pressure (21). High-salt intake is linked to the intestinal immune axis, and the intestinal microflora is an important condition against potential therapeutic targets for salt sensitivity that helps reduce the incidence of hypertension and reduces the risk of cardiovascular-related diseases. In vitro studies showed the cholesterol-lowering effect of strains with BSH activity, including L. alimentarius, L. paraplantarum, and L. pentosus (189–191). Lactobacillus supplementation with L. buchneri, L. johnsonii, and L. mucosae decreased serum cholesterol levels to prevent hypercholesterolemia (77, 192–195). Specifically, L. johnsonii BS15 markedly enhanced the population of Bacteroidetes and Lactobacillus spp. Moreover, the probiotic reduced the population of Enterobacteriaceae and the Firmicutes/Bacteroidetes ratio (192). The anti-obesity effect has been observed in L. amylovorus, L. sakei, and L. salivarius. The supplementation of L. amylovorus CP1563 had improved the anthropometric measurements and markers related to lipid and glucose metabolism and reduced the body fat of overweight and mildly obese individuals (24). Continuous ingestion of the fragmented CP1563 containing 10-hydroxyoctadecanoic acid also modulated the GM in pre-obese healthy subjects (25). L. amylovorus LKU4 exerted an anti-obesity effect on mice through facilitating browning of white adipocytes and increasing lactate levels (196). L. sakei CJLS03 treatment caused weight loss in people with obesity (46). L. sakei OK67 ameliorated HFD-induced blood glucose intolerance and obesity in mice by reducing inflammation and increasing the expression of colon tight junction proteins in mice (12, 197). Various obesity-associated biomarkers in the GM were also beneficially influenced by L. sakei administration (198, 199). L. salivarius Ls-33 modified fecal microbiota by increasing the Bacteroides–Prevotella–Porphyromonas group/Firmicutes ratio in obese adolescents (47). Viable probiotics (Lactobacillus and Bifidobacterium) had a cardioprotective effect on infarct-like myocardial injury by suppressing TNF-α and oxidative stress damage in a rat model; this effect had not been reported in the single treatment of L. casei, L. bulgaricus, and L. acidophilus (200). The co-supplementation of viable probiotics may be used as a new option for patients at risk of heart disease in the future. Despite evidence on the beneficial effects of Lactobacillus on the cardiovascular system has emerged, the impact of the other 17 Lactobacillus species on the management of cardiovascular-related diseases has not been elucidated.
Candidate Lactobacillus Species for Relieving the Symptoms of Cardiovascular-Related Diseases
From the perspective of disease prevention, L. casei, L. plantarum, L. fermentum, L. rhamnosus, L. reuteri, and L. paracasei can treat the most cardiovascular-related diseases, and their corresponding mechanisms are the most extensive among the Lactobacillus species. This review provides guidance for the refinement of lactobacilli to treat several diseases effectively. Most lactobacilli prevent hyperlipidemia, hypercholesterolemia, and atherosclerosis through cholesterol-lowering mechanism via changes in BAs and TMAO. L. acidophilus, L. amylovorus, L. brevis, L. casei, L. fermentum, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, and L. sakei are examples of special bacteria for obesity that regulate lipid metabolism; inflammation; glucose metabolism; WAT energy metabolism; signaling pathways, including NF-κB and PPAR-α; and metabolites, including BAs and LPS. L. casei, L. fermentum, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, and L. sakei are special bacteria for T2DM and hyperglycemia that regulate glucose metabolism; inflammation; insulin sensitivity; pathways, including GPR43 and PI3K/Akt; and metabolites, including LPS, BAs, and SCFAs. L. brevis, L. casei, L. fermentum, L. helveticus, L. murinus, L. paracasei, L. plantarum, and L. rhamnosus are special bacteria for hypertension that regulate NO levels, ACEI activity, and LPS production.
As a primary and important factor in maintaining GM balance, the anti-pathogenic activity of lactobacilli has received a lot of attention. The massive numbers of bacteria with whole-genome sequence data have made possible the identification of an informative set of putative metabolite genes/gene clusters that encode antimicrobials across the genomes (Supplementary Figure 1). L. paracasei, L. sakei, L. plantarum, L. casei, L. gasseri, L. alimentarius, L. coryniformis L. panis, L. crispatus, L. johnsonii, L. amylovorus, L. curvatus, L. rhamnosus, and L. helveticus have been identified by genome mining as the most capable species for antimicrobial peptide production (Supplementary Table 2). Lactobacilli have the ability to produce different kinds of exopolysaccharides (EPSs) with a wide diversity of structures. EPS biosynthesis genes have been identified in most species within the Lactobacillus genus, such as L. fermentum, L. reuteri, L. sakei, and L. plantarum (201, 202). These species also have the potential to prevent and treat cardiovascular-related diseases by decreasing serum cholesterol, reducing the inflammatory response, and modulating GM composition (203–205). The predicted therapeutic candidates for cardiovascular-related diseases extend the existing reports of the Lactobacillus genus and are ready for experimental verification. Although numerous bacteria that lower cardiovascular risk are found in the literature, most bacteria have not been sequenced. Thus, genome mining cannot be performed. The possible disease treatment mechanism, target, and related products, which provide additional possibilities for the current research, can be speculated.
Challenges and Future Directions of Lactobacillus Species in Therapeutic Research
The Effectiveness and Safety of the Strains Is Still a Critical Issue
A proper evaluation of the products is essential before bringing Lactobacillus into routine usage. Animal and human studies have attempted to correct intestinal disorders by using probiotics, such as L. rhamnosus (180, 206) and L. reuteri (207), which were administered to animals under clinical and obesity factors, to target intestinal flora. Animal studies are consistent but occasionally fail to verify the results of human clinical studies. Animal experiments showed that L. gasseri BNR17 supplementation reduced body weight and white fat weight (208). By contrast, clinical trials showed that supplementation with L. gasseri BNR17 did not remarkably reduce body weight and waist and hip circumferences (209). L. paracasei had been used in animal experiments to reduce body weight and fat accumulation but did not affect metabolism in clinical studies (210). This discrepancy in outcome maybe because the current work is still in its early stages. Thus, apart from simple correlations, additional meaningful conclusions can be drawn from the data and lead to the transformation of animal experiments into human experiments. Animal experiment results that are consistent with clinical ones are reported. Moreover, most of the above studies are performed in strictly controlled animal models, which limits their potential applications in human subjects. Researchers must also need to consider lifestyle, age, genetic factors, different dietary conditions, and changes in the environment that affect the microbial composition. Therefore, clinical research is crucial. Some strains, such as L. acidophilus La5, L. amylovorus CP1563, L. casei Shirota, 01, L. fermentum ME-3, L. gasseri BNR17, SBT2055, L. helveticus LBK-16H, CM4, L. plantarum Lp299v, ECGC 13110402, OLL2712, Dad-13, L. reuteri NCIMB30242, DSM17938, ADR-1, ADR-3, V3401, L. rhamnosus GG, L. sakei CJLS03, and L. salivarius Ls-33 have been proven by clinical trials to be functional in improving cardiovascular-related diseases (Table 1). The scope of the topics covering legal edible bacteria can promote the study of clinical bacteria therapy.
Mining Into the Bacteria to Explore Their Mechanism
Studies have shown that certain strains in a clearly balanced state are related to cardiovascular-related diseases. However, possible microbial communities that may be causally related to these diseases remain unknown. Various kinds of bacteria exist in the intestinal flora, and any intervention may lead to flora fluctuation. Therefore, finding the target bacteria is difficult, and the technology of knocking out some bacteria in vivo research must be developed (59, 211). However, gene-editing technology invalidates the hypothetical target metabolites to verify its effect. Researchers can also attempt to separate the metabolites directly for animal clinical trials with the development of the isolation and purification of bacterial metabolites. The identification of the normal metabolic pathway given in this review or screening the target protein or pathway by high-throughput screening technologies, such as microarray, transcriptomic, and metabonomic technology, and then verifying the results by laboratory experiments for further mechanism research will be popular.
Therapeutic Potential of Natural Products by Targeting Lactobacilli
Polysaccharides, saponins, and flavonoids, as the main active components in daily food, are difficult to be directly digested and absorbed by the human body. They play remarkable pharmacological roles depending on the transformation by intestinal microorganisms. Lactobacilli produce various substances, such as β-glucosidase and β-galactosidase, making them useful as fermentation tools and influence the function and activity of natural products. The metabolic pathway of ginsenoside bioconversion using enzymes and microbial fermentation has been reviewed, and L. rhamnosus GG, L. delbrueckii, L. acidophilus, L. plantarum, and L. brevis play a major role (212). For instance, compound K (C-K; 20-O-D-glucopyranosyl-20(S)-protopanaxadiol) is a novel ginsenoside metabolite, with anti-inflammatory, anti-atherosclerosis, and anti-diabetic activity, formed by intestinal lactobacilli β-glucosidase and does not occur naturally in ginseng (213). L. rhamnosus C6 strain showed higher β-glucosidase activity as well as biotransformation of isoflavones from glycones (daidzin and genistin) to aglycones (daidzein and genistein), which indicated an inverse relationship between the incidence of cardiovascular diseases (214). A series of novel phenolic galactosides with high antioxidant capacity was achieved by β-galactosidase from L. bulgaricus L3 (215). Lactobacilli participate in producing SCFAs by the fermentation of polysaccharide, which is generated during the glycolytic pathway (216). Thus, the activity of lactobacilli in the intestines has a significant effect on the digestion of food and medicine, and prevents the occurrence and development of cardiovascular-related diseases.
Conclusions
The World Health Organization reports that 30% of deaths worldwide are caused by cardiovascular-related diseases and predicts that these diseases will remain the leading cause of death in the next 20 years. These diseases will introduce considerable physical and economic burden on humans (https://www.who.int/health-topics/cardiovascular-diseases); thus, additional attention has been provided to cardiovascular-related diseases. Studies have shown that the development of cardiovascular-related diseases is closely related to the structure and function of GM (4). The concept that probiotics could improve cardiovascular-related diseases is emerging. In recent years, changes in intestinal microbial community, metabolites, and their link to cardiovascular-related diseases have made GM a potential new target for treatment. Therapeutic approaches with Lactobacillus could directly maintain GM homeostasis and regulate functional metabolites, including TMAO, SCFAs, BAs, and LPS, which further help to reduce the risks of high lipid cholesterol, immune inflammation, and oxidative stress.
The category of beneficial Lactobacillus strains referred to in this review provides experimental data for clinical use. Lactobacilli have promising metabolites; thus, a wide range of Lactobacillus candidates for future research on the improvement of cardiovascular-related diseases have been figured out. In addition to current therapeutic interventions, including fecal microbial transplantation, dietary interventions, and prebiotic and antibiotic interventions (81, 217–219), Lactobacillus therapy still represents an exciting frontier in the prevention and treatment of cardiovascular-related diseases.
Author Contributions
XZha and XZho collected the literature's data and wrote the manuscript. XL helped draw mechanism's figures. XW and XG supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitors
- AM
Alveolar macrophages
- Apo A-I
Apolipoprotein A-I
- Apo B
Apolipoprotein B
- BAs
Bile acids
- BSH
Bile salt hydrolase
- CHD
Coronary heart disease
- Cpt1a
Carnitine palmitoyl transferase 1a
- Cyp7A1
Cytochrome P450 a1
- DC
Diabetic cardiomyopathy
- DCA
Deoxycholic acid
- DOCA
Deoxycorticosterone
- EFSA
European Food Safety Authority
- FGF
Fibroblast growth factor
- FXR
Farnesoid X receptor
- GM
Gut microbiota
- GRAS
Generally recognized as safe
- GSH
Glutathione
- HbA1c
Glycated hemoglobin
- HDL-C
High density lipoprotein cholesterol
- HF
Heart failure
- HFD
High-fat diet
- HMG-CoA
3-hydroxy-3-methyl glutaryl coenzyme A
- ICAM
Intercellular adhesion molecule
- Insig
Insulin induced gene
- LDL
Low density lipoprotein
- LDL-C
Low density lipoprotein cholesterol
- LDL-R
Low density lipoprotein receptor
- LPS
Lipopolysaccharide
- LVH
Left ventricular hypertrophy
- LXR
Liver-X-receptors
- MCP-1
Monocyte chemotactic protein-1
- MI
Myocardial infarction
- NE
Norepinephrine
- NO
Nitric oxide
- NPC1L1
Niemann-Pick C1-like 1
- QPS
Qualified presumption of safety
- SCFAs
Short chain fatty acids
- SOD
Superoxide dismutase
- TAS
Total antioxidant status
- TBARS
Thiobarbituric acid reactive substances
- TC
Total cholesterol
- T2DM
Type 2 diabetes
- TG
Triacylglycerols
- TLC
Therapeutic lifestyle changes
- TMAO
Trimethylamine N-oxide
- TSST-1
Toxic shock syndrome toxin-1
- VaD
Vascular dementia
- VCAM
Vascular cell adhesion molecule
- WAT
White adipose tissue
- WHO
World Health Organization.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China under Grant No. 81830112 and Tianjin Natural Science Foundation under Grant No. 20JCQNJC00160.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.693412/full#supplementary-material
References
- 1.Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, et al. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. J Am Coll Cardiol. (2016) 68:818–33. 10.1016/j.jacc.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilahur G, Badimon JJ, Bugiardini R, Badimon L. Perspectives: the burden of cardiovascular risk factors and coronary heart disease in Europe and worldwide. Eur Heart J Suppl. (2014) 16:A7–11. 10.1093/eurheartj/sut003 [DOI] [Google Scholar]
- 3.Lennon R, Claussen K, Kuersteiner K. State of the heart: an overview of the disease burden of cardiovascular disease from an epidemiologic perspective. Primary Care. (2018) 45:1–15. 10.1016/j.pop.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circul Res. (2017) 120:1183–96. 10.1161/circresaha.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogler G, Rosano G. The heart and the gut. Eur Heart J. (2013) 35:426–30. 10.1093/eurheartj/eht271 [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Yang Z. Research progress on correlation between intestinal flora and cardiovascular diseases. Chin Pharmacol Bull. (2018) 34:1037–41. 10.3969/j.issn.1001-1978.2018.08.001 [DOI] [Google Scholar]
- 7.DiRienzo DB. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr Rev. (2014) 72:18–29. 10.1111/nure.12084 [DOI] [PubMed] [Google Scholar]
- 8.Tang C, Lu Z. Health promoting activities of probiotics. J Food Biochem. (2019) 43:e112944. 10.1111/jfbc.12944 [DOI] [PubMed] [Google Scholar]
- 9.Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol. (1999) 65:3763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo CF, Li JY. Hypocholesterolaemic action of Lactobacillus casei F0822 in rats fed a cholesterol-enriched diet. Int Dairy J. (2013) 32:144–9. 10.1016/j.idairyj.2013.04.001 [DOI] [Google Scholar]
- 11.Qiu L, Tao X, Xiong H, Yu J, Wei H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. (2018) 9:4299–309. 10.1039/C8FO00349A [DOI] [PubMed] [Google Scholar]
- 12.Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. (2016) 36:337–48. 10.1016/j.nutres.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Kullisaar T, Zilmer K, Salum T, Rehema A, Zilmer M. The use of probiotic L. fermentum ME-3 containing Reg'Activ cholesterol supplement for 4 weeks has a positive influence on blood lipoprotein profiles and inflammatory cytokines: an open-label preliminary study. Nutr J. (2016) 15:93. 10.1186/s12937-016-0213-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biot. (2016) 100:2939–51. 10.1007/s00253-016-7343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy EF, Clarke SF, Marques TM, Hill C, Stanton C, Ross RP, et al. Antimicrobials: strategies for targeting obesity and metabolic health? Gut Microbes. (2013) 4:48–53. 10.4161/gmic.22328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Kuipers OP. Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics. (2016) 17:882. 10.1186/s12864-016-3224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Gironés R, et al. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. (2017) 15:177. 10.2903/j.efsa.2017.4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazards EPOB. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J. (2013) 10:2497. 10.2903/j.efsa.2012.3020 [DOI] [Google Scholar]
- 19.Hazards EPOB, Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, et al. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017-2019). EFSA J. (2020) 18:e05966. 10.2903/j.efsa.2020.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazards EPOB, Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 13: suitability of taxonomic units notified to EFSA until September 2020. EFSA J. (2021) 19:e06377. 10.2903/j.efsa.2021.6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. (2017) 551:585–9. 10.1038/nature24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Tang W. Contributory role of gut microbiota and their metabolites toward cardiovascular complications in chronic kidney disease. Semin Nephrol. (2018) 38:193–205. 10.1016/j.semnephrol.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. (2011) 94:3288–94. 10.3168/jds.2010-4128 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura F, Ishida Y, Aihara K, Sawada D, Ashida N, Sugawara T, et al. Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: a randomized controlled trial. Microb Ecol Health Dis. (2016) 27:30312. 10.3402/mehd.v27.30312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugawara T, Sawada D, Yanagihara S, Aoki Y, Takehara I, Sugahara H, et al. Daily intake of paraprobiotic Lactobacillus amylovorus CP1563 improves pre-obese conditions and affects the gut microbial community in healthy pre-obese subjects: a double-blind, randomized, placebo-controlled study. Microorganisms. (2020) 8:304. 10.3390/microorganisms8020304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalili L, Alipour B, Asghari Jafarabadi M, Hassanalilou T, Mesgari Abbasi M, Faraji I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr. (2019) 11:5. 10.1186/s13098-019-0400-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Nagata S, Chiba Y, Wang C, Yamashiro Y. The effects of the Lactobacillus casei strain on obesity in children: a pilot study. Benef Microbes. (2017) 8:535–43. 10.3920/bm2016.0170 [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: a randomized, double-blind, placebo-controlled trial. J Med Food. (2018) 21:454–61. 10.1089/jmf.2017.3937 [DOI] [PubMed] [Google Scholar]
- 29.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr. (2013) 110:1696–703. 10.1017/s0007114513001037 [DOI] [PubMed] [Google Scholar]
- 30.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. (2010) 64:636–43. 10.1038/ejcn.2010.19 [DOI] [PubMed] [Google Scholar]
- 31.Ogawa A, Kadooka Y, Kato K, Shirouchi B, Sato M. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis. (2014) 13:36. 10.1186/1476-511x-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure–lowering effect in hypertensive subjects. Am J Clin Nutr. (2003) 77:326–30. 10.1093/ajcn/77.2.326 [DOI] [PubMed] [Google Scholar]
- 33.Jauhiainen T, Ronnback M, Vapaatalo H, Wuolle K, Kautiainen H, Groop PH, et al. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur J Clin Nutr. (2010) 64:424–31. 10.1038/ejcn.2010.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr. (2005) 24:257–65. 10.1080/07315724.2005.10719473 [DOI] [PubMed] [Google Scholar]
- 35.Malik M, Suboc TM, Tyagi S, Salzman N, Wang J, Ying R, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. (2018) 123:1091–102. 10.1161/circresaha.118.313565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, et al. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE. (2017) 12:e0187964. 10.1371/journal.pone.0187964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toshimitsu T, Gotou A, Sashihara T, Furuichi K, Hachimura S, Shioya N, et al. Ingesting yogurt containing Lactobacillus plantarum OLL2712 reduces abdominal fat accumulation and chronic inflammation in overweight adults in a randomized placebo-controlled trial. Curr Dev Nutr. (2021) 5:nzab006. 10.1093/cdn/nzab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahayu ES, Mariyatun M, Putri Manurung NE, Hasan PN, Therdtatha P, Mishima R, et al. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J Gastroenterol. (2021) 27:107–28. 10.3748/wjg.v27.i1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. (2017) 19:579–89. 10.1111/dom.12861 [DOI] [PubMed] [Google Scholar]
- 40.Hsieh MC, Tsai WH, Jheng YP, Su SL, Wang SY, Lin CC, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. (2018) 8:16791. 10.1038/s41598-018-35014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenorio-Jimenez C, Martinez-Ramirez MJ, Del Castillo-Codes I, Arraiza-Irigoyen C, Tercero-Lozano M, Camacho J, et al. Lactobacillus reuteri V3401 reduces inflammatory biomarkers and modifies the gastrointestinal microbiome in adults with metabolic syndrome: the PROSIR study. Nutrients. (2019) 11:1761. 10.3390/nu11081761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moludi J, Alizadeh M, Davari M, Golmohammadi A, Maleki V. The efficacy and safety of probiotics intervention in attenuating cardiac remodeling following myocardial infraction: literature review and study protocol for a randomized, double-blinded, placebo controlled trial. Contemp Clin Trials Commun. (2019) 15:100364. 10.1016/j.conctc.2019.100364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanborn VE, Azcarate-Peril MA, Gunstad J. Lactobacillus rhamnosus GG and HbA1c in middle age and older adults without type 2 diabetes mellitus: a preliminary randomized study. Diabetes Metab Syndr. (2020) 14:907–9. 10.1016/j.dsx.2020.05.034 [DOI] [PubMed] [Google Scholar]
- 44.Moludi J, Khedmatgozar H, Nachvak SM, Abdollahzad H, Moradinazar M, Sadeghpour Tabaei A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. (2021) 28:1–10. 10.1080/1028415x.2021.1889451 [DOI] [PubMed] [Google Scholar]
- 45.Moludi J, Alizadeh M, Mohammadzad MHS, Davari M. The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind cinical trial. Psychosom Med. (2019) 81:770–7. 10.1097/psy.0000000000000749 [DOI] [PubMed] [Google Scholar]
- 46.Lim S, Moon JH, Shin CM, Jeong D, Kim B. Effect of Lactobacillus sakei, a probiotic derived from kimchi, on body fat in Koreans with obesity: a randomized controlled study. Endocrinol Metab. (2020) 35:425–34. 10.3803/EnM.2020.35.2.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, et al. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. (2013) 32:935–40. 10.1016/j.clnu.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 48.Jin M, Qian Z, Yin J, Xu W, Zhou X. The role of intestinal microbiota in cardiovascular disease. J Cell Mol Med. (2019) 23:2343–50. 10.1111/jcmm.14195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Li T, Wu H, Shi H, Bai J, Zhao W, et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4+ T cell induced-type I inflammation. Biomed Pharmacother. (2019) 112:108580. 10.1016/j.biopha.2019.01.041 [DOI] [PubMed] [Google Scholar]
- 50.Falony G, Vieira-Silva S, Raes J. Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Annu Rev Microbiol. (2015) 69:305–21. 10.1146/annurev-micro-091014-104422 [DOI] [PubMed] [Google Scholar]
- 51.Li K, Tian P, Wang S, Lei P, Qu L, Huang J, et al. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J Funct Foods. (2017) 38:561–70. 10.1016/j.jff.2017.09.049 [DOI] [Google Scholar]
- 52.Wang X, Zhang M, Chen S, Sun Y, Ren F, Tong Q. Effects of Lactobacillus paracasei L9 on the content of short-chain fatty acids in the intestine of mice. Food Sci. (2017) 13:238–43. 10.7506/spkx1002-6630-201713039 [DOI] [Google Scholar]
- 53.Lim SM, Jang HM, Jeong JJ, Han M, Kim DH. Lactobacillus johnsonii CJLJ103 attenuates colitis and memory impairment in mice by inhibiting gut microbiota lipopolysaccharide production and NF-κB activation. J Funct Foods. (2017) 34:359–68. 10.1016/j.jff.2017.05.016 [DOI] [Google Scholar]
- 54.Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M, et al. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. (2020) 11:997–1014. 10.1080/19490976.2020.1734423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prete R, Long SL, Gallardo AL, Gahan CG, Corsetti A, Joyce SA. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci Rep. (2020) 10:1165. 10.1038/s41598-020-58069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. (2006) 47:241–59. 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 57.Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. (2020) 12:2982. 10.3390/nu12102982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Ch. (1987) 31:1231–3. 10.1128/aac.31.8.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. (2013) 62:220–6. 10.1136/gutjnl-2011-300705 [DOI] [PubMed] [Google Scholar]
- 60.Axelrod CL, Brennan CJ, Cresci G, Paul D, Hull M, Fealy CE, et al. UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans. Physiol Rep. (2019) 7:e14276. 10.14814/phy2.14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, et al. Probiotic Lactobacillus plantarum promotes intestinal barrier function by srengthening the epithelium and modulating gut microbiota. Front Microbiol. (2018) 9:1953. 10.3389/fmicb.2018.01953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. (2020) 21:6402. 10.3390/ijms21176402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng Z, Wei B, Huang T, Liu Z, Guan Q, Xie M, et al. Screening, safety evaluation, and mechanism of two Lactobacillus fermentum strains in reducing the translocation of staphylococcus aureus in the Caco-2 monolayer model. Front Microbiol. (2020) 11:566473. 10.3389/fmicb.2020.566473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eun CS, Kim YS, Han DS, Choi JH, Lee AR, Park YK. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS. (2011) 119:49–56. 10.1111/j.1600-0463.2010.02691.x [DOI] [PubMed] [Google Scholar]
- 65.Lozo J, Vukasinovic M, Strahinic I, Topisirovic L. Characterization and antimicrobial activity of bacteriocin 217 produced by natural isolate Lactobacillus paracasei subsp. paracasei BGBUK2-16. J Food Prot. (2004) 67:2727–34. 10.4315/0362-028x-67.12.2727 [DOI] [PubMed] [Google Scholar]
- 66.Sahoo TK, Jena PK, Patel AK, Seshadri S. Purification and molecular characterization of the novel highly potent bacteriocin TSU4 produced by Lactobacillus animalis TSU4. Appl Biochem Biotechnol. (2015) 177:90–104. 10.1007/s12010-015-1730-z [DOI] [PubMed] [Google Scholar]
- 67.Jiménez J, Diep D, Borrero J, Gútiez L, Arbulu S, Herranz C, et al. Cloning strategies for heterologous expression of the bacteriocin enterocin A by Lactobacillus sakei Lb790, Lb. plantarum NC8 and Lb. casei CECT475. Microb Cell Fact. (2015) 14:166. 10.1186/s12934-015-0346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoyancheva G, Marzotto M, Dellaglio F, Torriani S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol. (2014) 196:645–53. 10.1007/s00203-014-1003-1 [DOI] [PubMed] [Google Scholar]
- 69.Zhu WM, Liu W, Wu DQ. Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J Appl Microbiol. (2000) 88:877–86. 10.1046/j.1365-2672.2000.01027.x [DOI] [PubMed] [Google Scholar]
- 70.Castellano P, Raya R, Vignolo G. Mode of action of lactocin 705, a two-component bacteriocin from Lactobacillus casei CRL705. Int J Food Microbiol. (2003) 85:35–43. 10.1016/S0168-1605(02)00479-8 [DOI] [PubMed] [Google Scholar]
- 71.Hu Y, Liu X, Shan C, Xia X, Wang Y, Dong M, et al. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: purification, identification and antimicrobial characteristics. Food Control. (2017) 77:290–7. 10.1016/j.foodcont.2017.02.007 [DOI] [Google Scholar]
- 72.Lü X, Yi L, Dang J, Dang Y, Liu B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control. (2014) 46:264–71. 10.1016/j.foodcont.2014.05.028 [DOI] [Google Scholar]
- 73.Shan C, Hu Y, Xia X, Liu X, Li Y, Wang Y, et al. Purification and characterization of the bacteriocin produced by Lactobacillus panis C-M_2. Food Sci. (2017) 38:20–6. 10.7506/spkx1002-6630-201720004 [DOI] [Google Scholar]
- 74.Ge J, Ping W, Song G, Du C, Ling H, Sun X, et al. Paracin 1.7, a bacteriocin produced by Lactobacillus paracasei HD1.7 isolated from Chinese cabbage sauerkraut, a traditional Chinese fermented vegetable food. Wei Sheng Wu Xue Bao. (2009) 49:609–16. 10.13343/j.cnki.wsxb.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 75.Park YH, Kim JG, Shin YW, Kim SH, Whang KY. Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J Microbiol Biotechnol. (2007) 17:655–62. 10.1007/s10295-006-0202-4 [DOI] [PubMed] [Google Scholar]
- 76.Pereira DI, McCartney AL, Gibson GR. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl Environ Microbiol. (2003) 69:4743–52. 10.1128/AEM.69.8.4743-4752.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sridevi N, Vishwe P, Prabhune A. Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res Int. (2009) 42:516–20. 10.1016/j.foodres.2009.02.016 [DOI] [Google Scholar]
- 78.Watanabe S, Katsube T, Hattori H, Sato H, Ishijima T, Nakai Y, et al. Effect of Lactobacillus brevis 119-2 isolated from Tsuda kabu red turnips on cholesterol levels in cholesterol-administered rats. J Biosci Bioeng. (2013) 116:45–51. 10.1016/j.jbiosc.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 79.Kim B, Park K-Y, Ji Y, Park S, Holzapfel W, Hyun C-K. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Bioph Res Co. (2016) 473:530–6. 10.1016/j.bbrc.2016.03.107 [DOI] [PubMed] [Google Scholar]
- 80.Usman HA. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J Dairy Sci. (2000) 83:1705–11. 10.3168/jds.s0022-0302(00)75039-9 [DOI] [PubMed] [Google Scholar]
- 81.Zhao Y, Liu J, Hao W, Zhu H, Liang N, He Z, et al. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. J Agric Food Chem. (2017) 65:10984–92. 10.1021/acs.jafc.7b04666 [DOI] [PubMed] [Google Scholar]
- 82.Ding L, Chang M, Guo Y, Zhang L, Xue C, Yanagita T, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. (2018) 17:286. 10.1186/s12944-018-0939-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. (2013) 145:396–406.e1–10. 10.1053/j.gastro.2013.04.056 [DOI] [PubMed] [Google Scholar]
- 84.Elce A, Amato F, Zarrilli F, Calignano A, Troncone R, Castaldo G, et al. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef Microbes. (2017) 8:841–7. 10.3920/bm2016.0197 [DOI] [PubMed] [Google Scholar]
- 85.Chang V, Chiu TH, Fu SC. In vitro anti-inflammatory properties of fermented pepino (Solanum muricatum) milk by γ-aminobutyric acid-producing Lactobacillus brevis and an in vivo animal model for evaluating its effects on hypertension. J Sci Food Agric. (2016) 96:192–8. 10.1002/jsfa.7081 [DOI] [PubMed] [Google Scholar]
- 86.Wang LX, Liu K, Gao DW, Hao JK. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol. (2013) 19:3150–6. 10.3748/wjg.v19.i20.3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan X, Feng L, Huang X, Yang Y, Yang C, Gao Y. Histone deacetylase inhibitors promote eNOS expression in vascular smooth muscle cells and suppress hypoxia-induced cell growth. J Cell Mol Med. (2017) 21:2022–35. 10.1111/jcmm.13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song M, Park S, Lee H, Min B, Jung S, Kim E, et al. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J Dairy Sci. (2015) 98:1492–501. 10.3168/jds.2014-8586 [DOI] [PubMed] [Google Scholar]
- 89.Huang Y, Zheng Y. The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br J Nutr. (2010) 103:473–8. 10.1017/s0007114509991991 [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, Wang J, Quan G, Wang X, Yang L, Zhong L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl Environ Microbiol. (2014) 80:7496–504. 10.1128/AEM.02926-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reamtong O, Thiangtrongjit T, Kosoltanapiwat N, Panbangred W, Prangthip P. Potential benefits of L. acidophilus in dyslipidemic rats. Sci Rep. (2021) 11:6115. 10.1038/s41598-021-85427-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan F, Li N, Shi J, Li H, Yue Y, Jiao W, et al. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. (2019) 10:5804–15. 10.1039/c9fo01062a [DOI] [PubMed] [Google Scholar]
- 93.Primec M, Škorjanc D, Langerholc T, Mičetić-Turk D, Gorenjak M. Specific Lactobacillus probiotic strains decrease transepithelial glucose transport through GLUT2 downregulation in intestinal epithelial cell models. Nutr Res. (2021) 86:10–22. 10.1016/j.nutres.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 94.Chen L, Liu W, Li Y, Luo S, Liu Q, Zhong Y, et al. Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int Immunopharmacol. (2013) 17:108–15. 10.1016/j.intimp.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 95.Sun Q, Zhang Y, Li Z, Yan H, Li J, Wan X. Mechanism analysis of improved glucose homeostasis and cholesterol metabolism in high-fat-induced obese mice treated with La-SJLH001 via transcriptomics and culturomics. Food Funct. (2019) 10:3556–66. 10.1039/C9FO00205G [DOI] [PubMed] [Google Scholar]
- 96.Ondee T, Pongpirul K, Visitchanakun P, Saisorn W, Kanacharoen S, Wongsaroj L, et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci Rep. (2021) 11:6367. 10.1038/s41598-021-85449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan F, Li N, Yue Y, Wang C, Zhao L, Evivie SE, et al. Screening for potential novel probiotics with dipeptidyl peptidase IV-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front Microbiol. (2019) 10:2855. 10.3389/fmicb.2019.02855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim KA, Jeong JJ, Kim DH. Lactobacillus brevis OK56 ameliorates high-fat diet-induced obesity in mice by inhibiting NF-κB activation and gut microbial LPS production. J Funct Foods. (2015) 13:183–91. 10.1016/j.jff.2014.12.045 [DOI] [Google Scholar]
- 99.Park JE, Oh SH, Cha YS. Lactobacillus brevis OPK-3 from kimchi prevents obesity and modulates the expression of adipogenic and pro-inflammatory genes in adipose tissue of diet-induced obese mice. Nutrients. (2020) 12:604. 10.3390/nu12030604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patterson E, Ryan PM, Wiley N, Carafa I, Sherwin E, Moloney G, et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. (2019) 9:16323. 10.1038/s41598-019-51781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Z, Zhu H, Xia M, Hou M. Effect of dietary Lactobacillus casei on cholesterol metabolism in apoE-deficient mice. Wei Sheng Yan Jiu. (2009) 38:406–8. 10.19813/j.cnki.weishengyanjiu.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 102.Wang G, Huang W, Xia Y, Xiong Z, Ai L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. (2019) 10:1684–95. 10.1039/c8fo02181c [DOI] [PubMed] [Google Scholar]
- 103.Hsu CN, Hou CY, Chan JYH, Lee CT, Tain YL. Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients. (2019) 11:2908. 10.3390/nu11122908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Guo X, Guo J, He Q, Li H, Song Y, et al. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci Rep. (2014) 4:5654. 10.1038/srep05654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X, Wang E, Yin B, Fang D, Chen P, Wang G, et al. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes. (2017) 8:421–32. 10.3920/BM2016.0167 [DOI] [PubMed] [Google Scholar]
- 106.Wang G, Li X, Zhao J, Zhang H, Chen W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. (2017) 8:3155–64. 10.1039/C7FO00593H [DOI] [PubMed] [Google Scholar]
- 107.Yap WB, Ahmad FM, Lim YC, Zainalabidin S. Lactobacillus casei strain C1 attenuates vascular changes in spontaneously hypertensive rats. Korean J Physiol Pharmacol. (2016) 20:621–8. 10.4196/kjpp.2016.20.6.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, Liu Y, Guo X, Ma Y, Zhang H, Liang H. Effect of Lactobacillus casei on lipid metabolism and intestinal microflora in patients with alcoholic liver injury. Eur J Clin Nutr. (2021). 10.1038/s41430-020-00852-8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu X, Wang T, Li W, Jin F, Wang L. Effects of NS Lactobacillus strains on lipid metabolism of rats fed a high-cholesterol diet. Lipids Health Dis. (2013) 12:67. 10.1186/1476-511X-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oxman T, Shapira M, Klein R, Avazov N, Rabinowitz B. Oral administration of Lactobacillus induces cardioprotection. J Altern Complement Med. (2001) 7:345–54. 10.1089/107555301750463224 [DOI] [PubMed] [Google Scholar]
- 111.Oxman T, Shapira M, Diver A, Klein R, Avazov N, Rabinowitz B. A new method of long-term preventive cardioprotection using Lactobacillus. Am J Physiol Heart C. (2000) 278:H1717–H24. 10.1152/ajpheart.2000.278.5.H1717 [DOI] [PubMed] [Google Scholar]
- 112.Hallajzadeh J, Eslami RD, Tanomand A. Effect of Lactobacillus delbrueckii subsp. lactis PTCC1057 on serum glucose, fetuin-A, and sestrin 3 levels in streptozotocin-induced diabetic mice. Probiotics Antimicrob Proteins. (2021) 13:383–9. 10.1007/s12602-020-09693-0 [DOI] [PubMed] [Google Scholar]
- 113.Laue C, Papazova E, Liesegang A, Pannenbeckers A, Arendarski P, Linnerth B, et al. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women - a double-blind, randomised, controlled clinical pilot trial. Benef Microbes. (2018) 9:35–50. 10.3920/bm2017.0018 [DOI] [PubMed] [Google Scholar]
- 114.Gelardi M, La Mantia I, Drago L, Meroni G, Aragona SE, Cupido G, et al. A probiotic mixture in patients with upper respiratory diseases: the point of view of the otorhinolaringologist. J Biol Regul Homeost Agents. (2020) 34:5–10. [PubMed] [Google Scholar]
- 115.Yan YT, Pan DD. Screening and hypocholesterolemic effect of Lactobacillus fermentum. Food Sci. (2010) 31:224–8. 10.1017/S0004972710001772 [DOI] [Google Scholar]
- 116.Tomaro-Duchesneau C, Saha S, Malhotra M, Jones M, Rodes L, Prakash S. Lactobacillus fermentum NCIMB 5221 and NCIMB 2797 as cholesterol-lowering probiotic biotherapeutics: in vitro analysis. Benef Microbes. (2015) 6:861–9. 10.3920/BM2015.0021 [DOI] [PubMed] [Google Scholar]
- 117.Tomaro-Duchesneau C, Saha S, Malhotra M, Jones ML, Labbé A, Rodes L, et al. Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: an in vivo analysis using ZDF rats. Appl Microbiol Biotechnol. (2014) 98:115–26. 10.1007/s00253-013-5252-8 [DOI] [PubMed] [Google Scholar]
- 118.Yadav R, Khan SH, Mada SB, Meena S, Kapila R, Kapila S. Consumption of probiotic Lactobacillus fermentum MTCC: 5898-fermented milk attenuates dyslipidemia, oxidative stress, and inflammation in male rats fed on cholesterol-enriched diet. Probiotics Antimicrob Proteins. (2019) 11:509–18. 10.1007/s12602-018-9429-4 [DOI] [PubMed] [Google Scholar]
- 119.Robles-Vera I, Toral M, de la Visitación N, Sánchez M, Gómez-Guzmán M, Romero M, et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol Nutr Food Res. (2020) 64:e1900616. 10.1002/mnfr.201900616 [DOI] [PubMed] [Google Scholar]
- 120.Russo M, Marquez A, Herrera H, Abeijon-Mukdsi C, Saavedra L, Hebert E, et al. Oral administration of Lactobacillus fermentum CRL1446 improves biomarkers of metabolic syndrome in mice fed a high-fat diet supplemented with wheat bran. Food Funct. (2020) 11:3879–94. 10.1039/d0fo00730g [DOI] [PubMed] [Google Scholar]
- 121.Xie N, Cui Y, Yin Y, Zhao X, Yang J, Wang Z, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complem Altern Med. (2011) 11:53. 10.1186/1472-6882-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu K, Tan F, Mu J, Yi R, Zhou X, Zhao X. Anti-obesity effects of Lactobacillus fermentum CQPC05 isolated from Sichuan pickle in high-fat diet-induced obese mice through PPAR-α signaling pathway. Microorganisms. (2019) 7:194. 10.3390/microorganisms7070194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoon Y, Kim G, Noh MG, Park JH, Jang M, Fang S, et al. Lactobacillus fermentum promotes adipose tissue oxidative phosphorylation to protect against diet-induced obesity. Exp Mol Med. (2020) 52:1574–86. 10.1038/s12276-020-00502-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Molina-Tijeras JA, Diez-Echave P, Vezza T, Hidalgo-García L, Ruiz-Malagón AJ, Rodríguez-Sojo MJ, et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol Res. (2021) 167:105471. 10.1016/j.phrs.2021.105471 [DOI] [PubMed] [Google Scholar]
- 125.Toral M, Romero M, Rodríguez-Nogales A, Jiménez R, Robles-Vera I, Algieri F, et al. Lactobacillus fermentum improves Tacrolimus-induced hypertension by restoring vascular redox state and improving eNOS coupling. Mol Nutr Food Res. (2018) 62:1800033. 10.1002/mnfr.201800033 [DOI] [PubMed] [Google Scholar]
- 126.Anderson RC, Young W, Clerens S, Cookson AL, McCann MJ, Armstrong KM, et al. Human oral isolate Lactobacillus fermentum AGR1487 reduces intestinal barrier integrity by increasing the turnover of microtubules in Caco-2 cells. PLoS One. (2013) 8:e78774. 10.1371/journal.pone.0078774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ogawa A, Kobayashi T, Sakai F, Kadooka Y, Kawasaki Y. Lactobacillus gasseri SBT2055 suppresses fatty acid release through enlargement of fat emulsion size in vitro and promotes fecal fat excretion in healthy Japanese subjects. Lipids Health Dis. (2015) 14:20. 10.1186/s12944-015-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sato M, Uzu K, Yoshida T, Hamad E, Hiroaki M, Abd El Gawad I, et al. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Brit J Nutr. (2008) 99:1013–7. 10.1017/S0007114507839006 [DOI] [PubMed] [Google Scholar]
- 129.Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr. (2014) 53:599–606. 10.1007/s00394-013-0568-9 [DOI] [PubMed] [Google Scholar]
- 130.Niibo M, Shirouchi B, Umegatani M, Morita Y, Ogawa A, Sakai F, et al. Probiotic Lactobacillus gasseri SBT2055 improves insulin secretion in a diabetic rat model. J Dairy Sci. (2019) 102:997–1006. 10.3168/jds.2018-15203 [DOI] [PubMed] [Google Scholar]
- 131.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. (2010) 48:712–4. 10.1007/s12275-010-0363-8 [DOI] [PubMed] [Google Scholar]
- 132.Damodharan K, Palaniyandi SA, Yang SH, Suh JW. Functional probiotic characterization and in vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow milk. J Microbiol Biotechnol. (2016) 26:1675–86. 10.4014/jmb.1603.03005 [DOI] [PubMed] [Google Scholar]
- 133.Ahire JJ, Bhat AA, Thakare JM, Pawar PB, Zope DG, Jain RM, et al. Cholesterol assimilation and biotransformation by Lactobacillus helveticus. Biotechnol Lett. (2012) 34:103–7. 10.1007/s10529-011-0733-2 [DOI] [PubMed] [Google Scholar]
- 134.Karakula-Juchnowicz H, Rog J, Juchnowicz D, Łoniewski I, Skonieczna-Zydecka K, Krukow P, et al. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. (2019) 18:50. 10.1186/s12937-019-0475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin CH, Chen YH, Tsai TY, Pan TM. Effects of deep sea water and Lactobacillus paracasei subsp. paracasei NTU 101 on hypercholesterolemia hamsters gut microbiota. Appl Microbiol Biot. (2017) 101:321–9. 10.1007/s00253-016-7868-y [DOI] [PubMed] [Google Scholar]
- 136.Tsai TY, Chu LH, Lee CL, Pan TM. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk– soymilk supplemented with Momordica charantia. J Agric Food Chem. (2009) 57:2065–71. 10.1021/jf802936c [DOI] [PubMed] [Google Scholar]
- 137.Liu CF, Tung YT, Wu CL, Lee BH, Hsu WH, Pan TM. Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. J Agric Food Chem. (2011) 59:4537–43. 10.1021/jf104985v [DOI] [PubMed] [Google Scholar]
- 138.Cheng MC, Pan TM. Prevention of hypertension-induced vascular dementia by Lactobacillus paracasei subsp. paracasei NTU 101-fermented products. Pharm Biol. (2017) 55:487–96. 10.1080/13880209.2016.1253109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trasino SE, Dawson HD, Urban JF, Jr, Wang TT, Solano-Aguilar G. Feeding probiotic Lactobacillus paracasei to ossabaw pigs on a high-fat diet prevents cholesteryl-ester accumulation and LPS modulation of the Liver X receptor and inflammatory axis in alveolar macrophages. J Nutr Biochem. (2013) 24:1931–9. 10.1016/j.jnutbio.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 140.Lv XC, Chen M, Huang ZR, Guo WL, Ai LZ, Bai WD, et al. Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res Int. (2021) 139:109956. 10.1016/j.foodres.2020.109956 [DOI] [PubMed] [Google Scholar]
- 141.Dehkohneh A, Jafari P, Fahimi H. Effects of probiotic Lactobacillus paracasei TD3 on moderation of cholesterol biosynthesis pathway in rats. Iran J Basic Med Sci. (2019) 22:1004–9. 10.22038/ijbms.2019.33933.8073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tarrah A, Dos Santos Cruz BC, Sousa Dias R, da Silva Duarte V, Pakroo S, Licursi de Oliveira L, et al. Lactobacillus paracasei DTA81, a cholesterol-lowering strain having immunomodulatory activity, reveals gut microbiota regulation capability in BALB/c mice receiving high-fat diet. J Appl Microbiol. (2021). 10.1111/jam.15058. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeng Z, Yuan Q, Yu R, Zhang J, Ma H, Chen S. Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol Nutr Food Res. (2019) 63:e1900457. 10.1002/mnfr.201900457 [DOI] [PubMed] [Google Scholar]
- 144.Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R, et al. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. (2018) 9:3630–9. 10.1039/C8FO00081F [DOI] [PubMed] [Google Scholar]
- 145.Cassard L, Sperber K, Buivan TP, Cotillard A, Bourdet-Sicard R, Albert ML, et al. Basophils from allergic patients are neither hyperresponsive to activation signals nor hyporesponsive to inhibition signals. J Allergy Clin Immunol. (2018) 142:1548–57. 10.1016/j.jaci.2017.11.053 [DOI] [PubMed] [Google Scholar]
- 146.Lew LC, Choi SB, Khoo BY, Sreenivasan S, Ong KL, Liong MT. Lactobacillus plantarum DR7 reduces cholesterol via phosphorylation of AMPK that down-regulated the mRNA wxpression of HMG-CoA reductase. Korean J Food Sci Anim. (2018) 38:350–61. 10.5851/kosfa.2018.38.2.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bosch M, Fuentes MC, Audivert S, Bonachera MA, Peiro S, Cune J. Lactobacillus plantarum CECT 7527, 7528 and 7529: probiotic candidates to reduce cholesterol levels. J Sci Food Agric. (2014) 94:803–9. 10.1002/jsfa.6467 [DOI] [PubMed] [Google Scholar]
- 148.Nguyen T, Kang J, Lee M. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol. (2007) 113:358–61. 10.1016/j.ijfoodmicro.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 149.Zhang L, Zhang X, Liu C, Li C, Li S, Li T, et al. Manufacture of Cheddar cheese using probiotic Lactobacillus plantarum K25 and its cholesterol-lowering effects in a mice model. World J Microbiol Biotechnol. (2013) 29:127–35. 10.1007/s11274-012-1165-4 [DOI] [PubMed] [Google Scholar]
- 150.Salaj R, Štofilová J, Soltesová A, Hertelyová Z, Hijová E, Bertková I, et al. The effects of two Lactobacillus plantarum strains on rat lipid metabolism receiving a high fat diet. Sci World J. (2013) 2013:135142. 10.1155/2013/135142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim S, Huang E, Park S, Holzapfel W, Lim S-D. Physiological characteristics and anti-obesity effect of Lactobacillus plantarum K10. Korean J Food Sci Anim. (2018) 38:554. 10.5851/kosfa.2018.38.3.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gan Y, Chen H, Zhou XR, Chu LL, Ran WT, Tan F, et al. Regulating effect of Lactobacillus plantarum CQPC03 on lipid metabolism in high-fat diet-induced obesity in mice. J Food Biochem. (2020) 44:e13495. 10.1111/jfbc.13495 [DOI] [PubMed] [Google Scholar]
- 153.Zheng ZY, Cao FW, Wang WJ, Yu J, Chen C, Chen B, et al. Probiotic characteristics of Lactobacillus plantarum E680 and its effect on Hypercholesterolemic mice. BMC Microbiol. (2020) 20:239. 10.1186/s12866-020-01922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yao C, Tian W, Song J, Wang J. Antihyperlipidaemic effect of microencapsulated Lactobacillus plantarum LIP-1 on hyperlipidaemic rats. J Sci Food Agric. (2020) 100:2007–17. 10.1002/jsfa.10218 [DOI] [PubMed] [Google Scholar]
- 155.Li X, Xiao Y, Song L, Huang Y, Chu Q, Zhu S, et al. Effect of Lactobacillus plantarum HT121 on serum lipid profile, gut microbiota, and liver transcriptome and metabolomics in a high-cholesterol diet-induced hypercholesterolemia rat model. Nutrition. (2020) 79–80:110966. 10.1016/j.nut.2020.110966 [DOI] [PubMed] [Google Scholar]
- 156.Choi WJ, Dong HJ, Jeong HU, Ryu DW, Song SM, Kim YR, et al. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci Rep. (2020) 10:869. 10.1038/s41598-020-57615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Long X, Zeng X, Tan F, Yi R, Pan Y, Zhou X, et al. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. (2020) 11:5460–72. 10.1039/d0fo00519c [DOI] [PubMed] [Google Scholar]
- 158.Cai H, Wen Z, Li X, Meng K, Yang P. Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl Microbiol Biotechnol. (2020) 104:5959–72. 10.1007/s00253-020-10620-0 [DOI] [PubMed] [Google Scholar]
- 159.Li X, Wang N, Yin B, Fang D, Zhao J, Zhang H, et al. Lactobacillus plantarum X1 with α-glucosidase inhibitory activity ameliorates type 2 diabetes in mice. RSC Advances. (2016) 6:63536–47. 10.1039/C6RA10858J [DOI] [Google Scholar]
- 160.Liu TH, Chiou J, Tsai TY. Effects of Lactobacillus plantarum TWK10-fermented soymilk on deoxycorticosterone acetate-salt-induced hypertension and associated dementia in rats. Nutrients. (2016) 8:260. 10.3390/nu8050260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aparna Sudhakaran V, Panwar H, Chauhan R, Duary RK, Rathore RK, Batish VK, et al. Modulation of anti-inflammatory response in lipopolysaccharide stimulated human THP-1 cell line and mouse model at gene expression level with indigenous putative probiotic lactobacilli. Genes Nutr. (2013) 8:637–48. 10.1007/s12263-013-0347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rahimiyan-Heravan M, Roshangar L, Karimi P, Sefidgari-Abrasi S, Morshedi M, Saghafi-Asl M, et al. The potential therapeutic effects of Lactobacillus plantarum and inulin on serum and testicular reproductive markers in diabetic male rats. Diabetol Metab Syndr. (2020) 12:53. 10.1186/s13098-020-00560-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sefidgari-Abrasi S, Karimi P, Roshangar L, Morshedi M, Bavafa-Valenlia K, Saghafi-Asl M, et al. Lactobacillus plantarum and inulin: therapeutic agents to enhance cardiac Ob receptor expression and suppress cardiac apoptosis in type 2 diabetic rats. J Diabetes Res. (2020) 2020:4745389. 10.1155/2020/4745389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sefidgari-Abrasi S, Roshangar L, Karimi P, Morshedi M, Rahimiyan-Heravan M, Saghafi-Asl M. From the gut to the heart: L. plantarum and inulin administration as a novel approach to control cardiac apoptosis via 5-HT2B and TrkB receptors in diabetes. Clin Nutr. (2021) 40:190–201. 10.1016/j.clnu.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 165.Singh TP, Malik RK, Katkamwar SG, Kaur G. Hypocholesterolemic effects of Lactobacillus reuteri LR6 in rats fed on high-cholesterol diet. Int J Food Sci Nutr. (2015) 66:71–5. 10.3109/09637486.2014.953450 [DOI] [PubMed] [Google Scholar]
- 166.Taranto M, Medici M, Perdigon G, Holgado AR, Valdez G. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. (1998) 81:2336–40. 10.3168/jds.s0022-0302(98)70123-7 [DOI] [PubMed] [Google Scholar]
- 167.Tomaro-Duchesneau C, Jones M, Shah D, Jain P, Saha S, Prakash S. Cholesterol assimilation by Lactobacillus probiotic bacteria: an in vitro investigation. BioMed Res Int. (2014) 2014:380316. 10.1155/2014/380316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiang J, Feng N, Zhang C, Liu F, Zhao J, Zhang H, et al. Lactobacillus reuteri A9 and Lactobacillus mucosae A13 isolated from Chinese superlongevity people modulate lipid metabolism in a hypercholesterolemia rat model. FEMS Microbiol Lett. (2019) 366:fnz254. 10.1093/femsle/fnz254 [DOI] [PubMed] [Google Scholar]
- 169.Sun Y, Tang Y, Hou X, Wang H, Huang L, Wen J, et al. Novel Lactobacillus reuteri HI120 affects lipid metabolism in C57BL/6 obese mice. Front Vet Sci. (2020) 7:560241. 10.3389/fvets.2020.560241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liao PH, Kuo WW, Kuo CH, Yeh YL, Shen CY, Chen YH, et al. Lactobacillus reuteri GMNL-263 reduces hyperlipidaemia and the heart failure process in high-calorie diet-fed induced heart dysfunction in rats. J Funct Foods. (2016) 20:226–35. 10.1016/j.jff.2015.11.009 [DOI] [Google Scholar]
- 171.Ting WJ, Kuo WW, Hsieh JD, Yeh YL, Day CH, Chen YH, et al. Heat killed Lactobacillus reuteri GMNL-263 reduces fibrosis effects on the liver and heart in high fat diet-hamsters via TGF-beta suppression. Int J Mol Sci. (2015) 16:25881–96. 10.3390/ijms161025881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen LH, Chen YH, Cheng KC, Chien TY, Chan CH, Tsao SP, et al. Antiobesity effect of Lactobacillus reuteri 263 associated with energy metabolism remodeling of white adipose tissue in high-energy-diet-fed rats. J Nutr Biochem. (2018) 54:87–94. 10.1016/j.jnutbio.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 173.Fak F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS ONE. (2012) 7:e46837. 10.1371/journal.pone.0046837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin CH, Lin CC, Shibu MA, Liu CS, Kuo CH, Tsai FJ, et al. Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Br J Nutr. (2014) 111:598–605. 10.1017/S0007114513002791 [DOI] [PubMed] [Google Scholar]
- 175.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. (2018) 284:307–17. 10.1111/joim.12805 [DOI] [PubMed] [Google Scholar]
- 176.Fang Y, Chen H, Zhang X, Zhang H, Xia J, Ding K, et al. Probiotic administration of Lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE-/-mice fed with a high-fat diet. Eur Rev Med Pharmacol Sci. (2019) 23:3533–41. 10.26355/eurrev_201904_17722 [DOI] [PubMed] [Google Scholar]
- 177.Kumar M, Rakesh S, Nagpal R, Hemalatha R, Ramakrishna A, Sudarshan V, et al. Probiotic Lactobacillus rhamnosus GG and aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition. (2013) 29:574–9. 10.1016/j.nut.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 178.Chen D, Yang Z, Chen X, Huang Y, Yin B, Guo F, et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complement Altern Med. (2014) 14:386. 10.1186/1472-6882-14-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Falcinelli S, Rodiles A, Hatef A, Picchietti S, Carnevali O. Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci Rep. (2017) 7:5512. 10.1038/s41598-017-05147-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ji YS, Kim HN, Park HJ, Lee JE, Yeo SY, Yang JS, et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef Microbes. (2012) 3:13–22. 10.3920/BM2011.0046 [DOI] [PubMed] [Google Scholar]
- 181.Kim SW, Park KY, Kim B, Kim E, Hyun CK. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Bioph Res Co. (2013) 431:258–63. 10.1016/j.bbrc.2012.12.121 [DOI] [PubMed] [Google Scholar]
- 182.Wa Y, Yin B, He Y, Xi W, Huang Y, Wang C, et al. Effects of single probiotic- and combined probiotic-fermented milk on lipid metabolism in hyperlipidemic rats. Front Microbiol. (2019) 10:1312. 10.3389/fmicb.2019.01312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T, et al. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J Nutr Biochem. (2020) 75:108256. 10.1016/j.jnutbio.2019.108256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu H, Wang J, Cai J, Feng W, Wang Y, Liu Q, et al. Protective effect of Lactobacillus rhamnosus GG and its supernatant against myocardial dysfunction in obese mice exposed to intermittent hypoxia is associated with the activation of Nrf2 pathway. Int J Biol Sci. (2019) 15:2471–83. 10.7150/ijbs.36465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singh S, Sharma RK, Malhotra S, Pothuraju R, Shandilya UK. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef Microbes. (2016) 8:243–55. 10.3920/BM2016.0090 [DOI] [PubMed] [Google Scholar]
- 186.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ-Heart Fail. (2014) 7:491–9. 10.1161/CIRCHEARTFAILURE.113.000978 [DOI] [PubMed] [Google Scholar]
- 187.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. (2007) 132:562–75. 10.1053/j.gastro.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. (2014) 5:719–28. 10.4161/19490976.2014.983775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ge P, Liu Y, Wang D, Ding M, Zhou C. Physiological characteristics of Lactobacillus alimentarius SR10 in fermented sour meat & its cholesterol-lowering effect. Food Ferment Indust. (2013) 039:52–6. 10.13995/j.cnki.11-1802/ts.2013.10.027 [DOI] [Google Scholar]
- 190.Liu C, Liu Q, Jiang B. Acid and bile tolerance and cholesterol reduction ability of Lactobacillus paraplantarum. Wei Sheng Wu Xue Bao. (2009) 49:1176–9. 10.1016/S1003-6326(09)60084-4 [DOI] [PubMed] [Google Scholar]
- 191.Bendali F, Kerdouche K, Hamma-Faradji S, Drider D. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benef Microbes. (2017) 8:271–80. 10.3920/bm2016.0121 [DOI] [PubMed] [Google Scholar]
- 192.Wang H, Ni X, Qing X, Zeng D, Luo M, Liu L, et al. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front Microbiol. (2017) 8:1073. 10.3389/fmicb.2017.01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.London LE, Kumar AH, Wall R, Casey PG, Stanton C. Exopolysaccharide-producing probiotic lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J Nutr. (2014) 144:1956. 10.3945/jn.114.191627 [DOI] [PubMed] [Google Scholar]
- 194.Ryan PM, Stolte EH, London LEE, Wells JM, Long SL, Joyce SA, et al. Lactobacillus mucosae DPC 6426 as a bile-modifying and immunomodulatory microbe. BMC Microbiol. (2019) 19:33. 10.1186/s12866-019-1403-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang T, Wu H, Yang X, Li Y, Zhang Z, Chen F, et al. Lactobacillus mucosae strain promoted by a high-fiber diet in genetic obese child alleviates lipid metabolism and modifies gut microbiota in ApoE(-/-) mice on a western diet. Microorganisms. (2020) 8:1225. 10.3390/microorganisms8081225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park SS, Lee YJ, Kang H, Yang G, Hong EJ, Lim JY, et al. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci Rep. (2019) 9:20152. 10.1038/s41598-019-56817-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jang HM, Han SK, Kim JK, Oh SJ, Jang HB, Kim DH. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation. Mol Nutr Food Res. (2019) 63:e1800978. 10.1002/mnfr.201800978 [DOI] [PubMed] [Google Scholar]
- 198.Ji Y, Park S, Chung Y, Kim B, Park H, Huang E, et al. Amelioration of obesity-related biomarkers by Lactobacillus sakei CJLS03 in a high-fat diet-induced obese murine model. Sci Rep. (2019) 9:6821. 10.1038/s41598-019-43092-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Won SM, Chen S, Lee SY, Lee KE, Park KW, Yoon JH. Lactobacillus sakei ADM14 induces anti-obesity effects and changes in gut microbiome in high-fat diet-induced obese mice. Nutrients. (2020) 12:3703. 10.3390/nu12123703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sadeghzadeh J, Vakili A, Sameni H, Shadnoush M, Bandegi A, zahedi khorasani M. The effect of oral consumption of probiotics in prevention of heart injury in a rat myocardial infarction model: a histopathological, hemodynamic and biochemical evaluation. Iran Biomed J. (2017) 21:174–81. 10.18869/acadpub.ibj.21.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jiang Y, Yang Z. A functional and genetic overview of exopolysaccharides produced by Lactobacillus plantarum. J Funct Foods. (2018) 47:229–40. 10.1016/j.jff.2018.05.060 [DOI] [Google Scholar]
- 202.Zannini E, Waters D, Coffey A, Arendt E. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl Microbiol Biot. (2016) 100:1121–35. 10.1007/s00253-015-7172-2 [DOI] [PubMed] [Google Scholar]
- 203.Wang Y, Fei Y, Liu L, Xiao Y, Pang Y, Kang J, et al. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats. Int J Mol Sci. (2018) 19:3587. 10.3390/ijms19113587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xiong Q, Zhu L, Zhang F, Li H, Wu J, Liang J, et al. Protective activities of polysaccharides from Cipangopaludina chinensis against high-fat-diet-induced atherosclerosis via regulating gut microbiota in ApoE-deficient mice. Food Funct. (2019) 10:6644–54. 10.1039/c9fo01530b [DOI] [PubMed] [Google Scholar]
- 205.Benkeblia N. Polysaccharides (natural fibers in food and nutrition). In: Benkeblia N, editor. Barley β-Glucan: Natural Polysaccharide for Managing Diabetes and Cardiovascular Diseases. Boca Raton, FL: CRC Press; (2014). p. 233–58. [Google Scholar]
- 206.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. (2006) 1761:736–44. 10.1016/j.bbalip.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 207.Chung HJ, Yu J, Lee IA, Liu MJ, Shen YF, Sharma S, et al. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio. (2016) 6:64–76. 10.1002/2211-5463.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE. (2013) 8:e54617. 10.1371/journal.pone.0054617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jung SP, Lee KM, Kang JH, Yun SI, Park HO, Moon Y, et al. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med. (2013) 34:80. 10.4082/kjfm.2013.34.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Blædel T, et al. Dietary modulation of the gut microbiota–a randomised controlled trial in obese postmenopausal women. Br J Nutr. (2015) 114:406–17. 10.1017/S0007114515001786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao W, Liu Y, Giometti C, Tollaksen S, Khare T, Wu L, et al. Knock-out of SO1377 gene, which encodes the member of a conserved hypothetical bacterial protein family COG2268, results in alteration of iron metabolism, increased spontaneous mutation and hydrogen peroxide sensitivity in Shewanella oneidensis MR-1. BMC Genomics. (2006) 7:76. 10.1186/1471-2164-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee NK, Paik HD. Bioconversion using lactic acid bacteria: ginsenosides, gABA, and phenolic compounds. J Microbiol Biotechnol. (2017) 27:869–77. 10.4014/jmb.1612.12005 [DOI] [PubMed] [Google Scholar]
- 213.Jin Y, Jung SY, Kim YJ, Lee DY, Min JW, Wang C, et al. Microbial ketonization of ginsenosides F1 and C-K by Lactobacillus brevis. Antonie Van Leeuwenhoek. (2014) 106:1215–21. 10.1007/s10482-014-0291-4 [DOI] [PubMed] [Google Scholar]
- 214.Hati S, Vij S, Singh BP, Mandal S. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J Sci Food Agric. (2015) 95:216–20. 10.1002/jsfa.6743 [DOI] [PubMed] [Google Scholar]
- 215.Lu L, Xu L, Guo Y, Zhang D, Qi T, Jin L, et al. Glycosylation of phenolic compounds by the site-mutated β-galactosidase from Lactobacillus bulgaricus L3. PLoS ONE. (2015) 10:e0121445. 10.1371/journal.pone.0121445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. (2012) 2:86. 10.3389/fcimb.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tang TW, Chen HC, Chen CY, Yen CY, Lin CJ, Prajnamitra RP, et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. (2019) 139:647–59. 10.1161/CIRCULATIONAHA.118.035235 [DOI] [PubMed] [Google Scholar]
- 218.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. New Engl J Med. (2018) 378:e34. 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 219.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. (2005) 82:559–67. 10.1093/ajcn.82.3.559 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.