Abstract
Ribonucleotide reductase (RNR) is the key enzyme that catalyzes the production of deoxyribonucleotides (dNTPs) for DNA replication and it is also essential for cancer cell proliferation. As the RNR inhibitor, Gemcitabine is widely used in cancer therapies, however, resistance limits its therapeutic efficacy and curative potential. Here, we identified that mTORC2 is a main driver of gemcitabine resistance in non-small cell lung cancers (NSCLC). Pharmacological or genetic inhibition of mTORC2 greatly enhanced gemcitabine induced cytotoxicity and DNA damage. Mechanistically, mTORC2 directly interacted and phosphorylated RNR large subunit RRM1 at Ser 631. Ser631 phosphorylation of RRM1 enhanced its interaction with small subunit RRM2 to maintain sufficient RNR enzymatic activity for efficient DNA replication. Targeting mTORC2 retarded DNA replication fork progression and improved therapeutic efficacy of gemcitabine in NSCLC xenograft model in vivo. Thus, these results identified a mechanism through mTORC2 regulating RNR activity and DNA replication, conferring gemcitabine resistance to cancer cells.
Keywords: Ribonucleotide reductase, mTORC2, Gemcitabine, DNA replication stress
Abbreviations: NSCLC, Non-small cell lung cancer; RNR, Ribonucleotide reductase; DSB, Double-strand break; dNTP, deoxyribonucleotide triphosphates; EGFR, Epidermal growth factor receptor
Introduction
Gemcitabine, a deoxycytidine analog, is widely employed for treatment of various cancer types including non-small cell lung cancer (NSCLC), pancreatic cancer and breast cancer [1], [2], [3]. As a terminal nucleoside analogue, gemcitabine could be directly incorporated into nascent DNA strand to inhibit DNA replication and cancer cell growth [4]. Gemcitabine blocks DNA replication progression could also through covalent binding and inactivating ribonucleotide reductase M1 (RRM1) to prevent deoxynucleoside triphosphate (dNTPs) synthesis [5]. Intrinsic or acquired gemcitabine resistance limits its clinical utility to a subset of patients [6]. Increased capacity for DNA repair and replication fork restart was considered as the main causes of gemcitabine resistance [7]. Although a group of effectors have been identified as predictive markers of gemcitabine resistance including RRM1, XRCC1, POLA2, most of these studies lacked molecular mechanisms and therapeutic relevance [8], [9], [10].
DNA replication is an essential process for all dividing cells and is tightly regulated to ensure genomic integrity. DNA replication fork is often impeded by encountering DNA lesions or obstacles, and this results in DNA replication stress, which subsequently activates cascades of signaling transduction to repair and restart the replication fork progression [11]. As an inhibitor of ribonucleotide reductase (RNR), the central controller for de novo synthesis of dNTPs building blocks of DNA replication, gemcitabine is commonly used to induce replication stress [12]. Therefore, identifying genes that dictate gemcitabine sensitivity could lead to discover novel regulators for DNA replication as well as therapeutic targets to optimize gemcitabine treatment in cancer patients [12].
The mammalian target of rapamycin(mTOR) is a serine/threonine protein kinase and functions via two distinct protein complexes, mTORC1 and mTORC2 [13]. mTORC1 is composed of mTOR, Raptor, GβL, and DEPTOR and is inhibited by rapamycin [14]. While, mTORC2 consists of mTOR, Rictor, mLST8 and mSin1 and is insensitive to rapamycin [15]. Rictor is an obligate regulatory component of mTORC2, and Rictor deficiency results in inactivation of mTORC2. RICTOR gene is amplified in 13% of lung cancer patients, and plays important roles in cancer cell growth [16]. Meanwhile, mTORC2 is frequently overactivated in cancer cells due to various oncogenic mutations. For instance, EGFR mutation is observed in 10% to 30% of NSCLC patients and stimulates mTORC2 activity [17]. mTORC2 promotes cell survival through phosphorylation of Akt at Ser 473 [18]. Besides, mTORC2 could also regulate a variety of other physiological functions through phosphorylating different substrates, including glycolytic enzyme pyruvate dehydrogenase kinase 1 (PDHK1), cystine-glutamate antiporter xCT, protein kinase Cζ (PKCζ) and so on [19], [20], [21]. Through a chemicogenetic screening in budding yeast, mTORC2 was found to be crucial for maintaining genome integrity [22], suggesting that it may have a role in DNA replication. In this study, we showed that mTORC2 has a critical role in RNR activity regulation and DNA replication. Consequently, inhibition of mTORC2 causes DNA replication stress and greatly potentiates gemcitabine efficacy on NSCLC cells.
Materials and methods
Materials
Rictor CRISPR/Cas9 KO plasmid (sc-400710-KO-2) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rictor shRNAs (#1853 and #1854) were obtained from Addgene (Cambridge, MA). Flag-RRM1 plasmid was purchased from Genscript (Nanjing, China). Anti-RRM1(sc-377415), anti-RRM2(sc-398294) anti-β-Actin(sc-47778) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p-Akt substrate (RXXS/T) (#9614), BrdU (#5292), Ki67(#9027), Cleaved caspase-3 (#9579) and pAkt S473 (#4060) antibodies were purchased from Cell Signaling Technology (Danvers, MA). γH2AX (05-636) was purchased from Millipore (Billerica, MA). Rictor (A300-459A), pRPA2 S33 (A300-246A), RPA2 (A300-244A) were purchased from Bethyl Laboratories (Montgomery, TX). Gemcitabine, PP242 and Rapamycin were obtained from Selleckchem (Houston, TX).
Cell culture and transfection
H1299, H157 and H460 cells were obtained from American Type Culture Collection and cultured in RPMI 1640 medium supplemented with 10% FBS (ExCell Bio, Shanghai, China). All the cells have been authenticated and tested for mycoplasma contamination every two months. Cells were transfected with indicated plasmids using ExFect2000 transfection reagent (Vazyme Biotech, Nanjing, China) following the manufacturer's instructions.
DNA fiber assay
DNA fiber spreads were performed as previously described [23]. Briefly, cells were first labeled with 5’chlorodeoxyuridine (CldU, 200 μM) for 20 min, and then labeled with iododeoxyuridine (IdU, 100 μM) for another 20 min. After labeling, cells were collected in cold PBS with density of 106 cells/ml and spotted 2 μl onto a microscope slide and mixed with 12 μl lysis buffer (0.5% SDS, 50 mM EDTA, 200 mM Tris-Cl) for 10 min at room temperature. Then, tilt slides to 15° to allow cell lysates to spread along the slide. After drying, DNA fibers on the slider were fixed with methanol and acetic acid (3:1), and then treated with 2.5 M HCl for 80 min to denature DNA. After extensively washing with PBS, slides were blocked with 10% goat serum for at least 1 hr, and then incubated with primary antibodies against IdU (mouse anti-BrdU clone B44) and CldU (rat anti-BrdU BU1/75(ICR1)) and secondary antibodies (Alexa Fluor 488 (green) goat anti-mouse and Alexa Fluor (red) 555 goat anti-rat). Images were collected by a Zeiss Axioplan2 microscope (Axioplan, Zeiss) and analyzed by Zeiss AxioVision software. The DNA track length was calculated as 2.59 kb/μm and the fork rate (kb/min) was calculated from the length of DNA track (kb) divided by the time of the pulse as described previously.
Ribonucleotide reductase activity assay
Indicated cell lysates (500 µg) were incubated with the reaction mixture contained 50 mM Hepes buffer pH 7.2,10 mM DTT, 20 μM FeCl3, 5 mM magnesium acetate, 50 μM CDP, 300 μM C14-CDP and 2 mM ATP at 37°C for 1 hr in a final volume of 50 µl. After incubation, 4 μl of 10 M perchloric acid was added to stop the reaction. After centrifugation, the supernatant was transferred to a new tube and boiled for 20 min. The supernatant containing the formed C14-dCDP and substrate C14-CDP were spotted on TLC (thin-layer chromatography) plate and separated by TLC. TLC plates were exposed to X-ray film and the dots of C14-CDP and C14-dCDP were quantified by Image J. The RNR activity was calculated as C14-dCDP/ (C14 -CDP+C14-dCDP).
Immunoprecipitation
Cells were harvested in EBC buffer (0.5% NP-40, 50 mM Tris-HCl pH 7.6, 1 mM EDTA, and 1 mM β-Mercaptoethanol) supplemented with protease inhibitor cocktail (Santa Cruz, CA) and lysed by sonication. After centrifugation at 14,000 × g for 10 min, the supernatant was incubated with indicated antibodies and protein A/G agarose beads overnight at 4°C. After washing with EBC buffer for 3 times, beads were boiled in 30 μl 2 × SDS-PAGE loading buffer and subjected to western blot analysis.
Cytometric analysis of γH2AX
After treatment, cells were collected in PBS, and fixed in 4% paraformaladehyde for 15 min on ice. Cells were then washed twice with PBS, and re-fixed with cold 70% ethanol overnight at 4°C. After washing, cells were resuspended in 5% goat serum in PBS for 1 hr at room temperature to block non-specific antibody binding. Then, cells were incubated with γH2AX antibody (1:500 dilution) in 5% goat serum overnight at 4°C. After washing, cells were then incubated with Alexa Fluor 488 secondary antibody (1:1000 dilution) at room temperature for 1 hr in dark. After washing, DNA contents were stained with propidium iodide (20 µg/ml) and 2.5 RNase A (15 µg/ml) before acquiring and analyzing by flow cytometry.
Immunofluorescence analysis
H1299 cells grown in the chamber slider were treated with 20 µM gemcitabine in presence of 10 µM Bromodeoxyuridine (BrdU) in the medium for 1 hour. After treatment, cells were washed with PBS and fixed using 4% paraformaldehyde for 10 min at room temperature. Cells were then permeabilized in 0.3% Triton-100 for 10 min, followed DNA hydrolysis by incubation with 2N HCl for 30 min at 37°C. After extensively washing with PBS, cells were incubated with primary antibodies including BrdU (1:200) and γH2AX (1:500) overnight at 4°C. After washing, cells were incubated with Alexa Fluor secondary antibodies (1:1000) at room temperature for 1 hr. Then, cells were washed and mounted with DAPI before image acquisition.
Cancer xenografts study
Lung cancer xenografts were generated as previously described [23]. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Jinan University and Hunan Cancer Hospital of Central South University. Briefly, Six-week-old female nude mice were purchased from GemPharmatech Co., Ltd. (Nanjing, China) and housed under pathogen-free conditions. 1 × 107 of H460 parental cells or Rictor KO cells were subcutaneously implanted into mouse flanks. When tumor volume reached around 100 mm3, mice were intraperitoneal (i.p) injected gemcitabine (80 mg/kg) 2 time per week. For combination treatment, tumor bearing mice were randomly divided into 6 groups and treated intraperitoneally (i.p) with gemcitabine (80 mg/kg), PP242 (20 mg/kg), rapamycin (2 mg/kg) or their combination. Tumor growth were monitored and tumor volumes were measured by caliper once every 5 days and calculated with the formula: V = (L × W2)/2 (L, length; W, width) as described [18].
Mass spectrometry analysis of RRM1 phosphorylation
RRM1 S631 phosphorylation was identified using liquid chromatography/tandem mass spectrometry (LC-MS/MS) by PTM-Biolabs Inc. (Hangzhou, China). First, Flag-RRM1 was overexpressed in H1299 cells and immunoprecipitated by Flag, and was subjected to SDS-PAGE, followed by cutting the Flag-RRM1 band. Then, the in-gel tryptic digestion was performed and peptides were extracted, dried, and resuspended in 2% acetonitrile /0.1% formic acid. The peptides were then analyzed by Q-ExactiveTM plus hybrid quadrupole-Orbitrap mass spectrometer (ThermoFisher Scientific). The resulting MS/MS data were processed using mascot search engine (v.2.3.0) and tandem mass spectra were searched against RRM1.
Measurement of dNTP concentrations
Cellular dNTP levels were analyzed by high performance liquid chromatography (HPLC) as previously described [24]. Briefly, cells were harvested, and cellular nucleotides were extracted with 0.4 N perchloric acid and neutralized with potassium hydroxide. dNTPs were separated from NTP using a boronic acid resin column (Thermo Fisher Scientific, Waltham, MA). Then, dNTPs were analyzed by HPLC through adjusting with dNTPs standard.
Statistical analysis
Data are shown from one representative experiment of at least three independent experiments and are expressed as mean ± SD. The statistical significance of difference between groups were analyzed with two-sided Student's T-test or Mann-Whitney U test. Results were considered statistically significant at P < 0.05.
Results
Inhibition of mTORC2 sensitizes NSCLC cells to gemcitabine
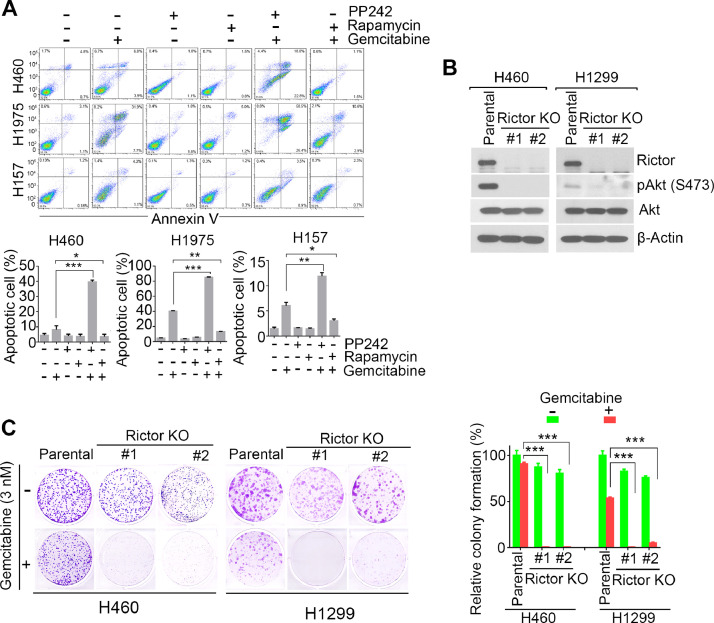
mTOR signaling has been implicated in cancer drug resistance and targeting mTOR is a promising strategy in cancer therapies [25]. To investigate whether mTOR signaling pathway is involved in gemcitabine sensitivity, we treated H460, H1975 and H157 cells with gemcitabine in combination with Rapamycin (the mTORC1 specific inhibitor) or PP242 (the dual mTORC1/2 inhibitor) [26], and used annexin V apoptosis assay to measure apoptotic cell death. Annexin V has high affinity to bind to phosphatidylserine, which is exposed on the outer plasma membrane during apoptosis, and Annexin V positive staining cells have been considered as apoptotic cells. By this approach, we found that although Rapamycin or PP242 treatment alone did not cause cell apoptosis, PP242 greatly potentized gemcitabine induced apoptosis (from 8.2% to 39.6% in H460 cells; from 40.1% to 85.2% in H1975 cells; from 6.0% to 11.8% in H157 cells), whereas, Rapamycin abated gemcitabine induced apoptosis (from 8.2% to 3.5% in H460 cells; from 40.1% to 13.3% in H1975 cells; from 6.0% to 3.0% in H157 cells) (Fig. 1A).
Fig. 1.
Inhibition of mTORC2 sensitizes NSCLC cells to gemcitabine treatment. (A) H460, H1975 and H157 cells were treated with 0.5 µM gemcitabine, 1 µM Rapamycin, 1 µM PP242 or their combination as indicated. Cell apoptosis were analyzed by Annexin V staining at 48 hrs after treatment. (B) Knockout (KO) of Rictor in H1299 and H460 cells using CRISPR/Cas9, and western blot analysis of expression of indicated proteins. (C) Colony formation survival analysis of H460 and H1299 parental or Rictor KO cells in presence or absence of 3 nM gemcitabine. Relative number of colonies were quantified and normalized to untreated parental cells (right) and representative colony formations were shown (left). * P < 0.05, **P < 0.01 and *** P < 0.001 by two-tailed t-test.
This result showed that inhibition of mTORC1/2, but not only mTORC1, enhanced therapeutic efficacy of gemcitabine against NSCLC cells, and indicating mTORC2 signaling pathway plays critical roles in sensitivity of NSCLC cells to gemcitabine therapy.
To confirm whether mTORC2 drive gemcitabine resistance in NSCLC cells, we first knocked down the essential component of mTORC2 complex, Rictor, by two different shRNAs in NSCLC cells including H1299, H460 and H157 cells. Consistent with pharmacologic inhibition, clonogenic survival assay revealed that knockdown of Rictor significantly enhanced the cytotoxicity induced by gemcitabine (Supplementary Fig.S1). To further validate the effects of mTORC2 on gemcitabine resistance, we knocked out Rictor in H460 and H1299 cells using CRISPR/Cas9 technique (Fig. 1B). Knockout of Rictor resulted in disappearance of Akt phosphorylation on S473 (Fig. 1B), a well-known mTORC2 substrate [19], indicating Rictor deficiency could abolish mTORC2 activity. Meanwhile, Rictor knockout H460 and H1299 cells were more sensitive to gemcitabine treatment in colony formation assay (Fig. 1C). These results confirmed that mTORC2 deficiency enhanced gemcitabine therapeutic efficacy in vitro.
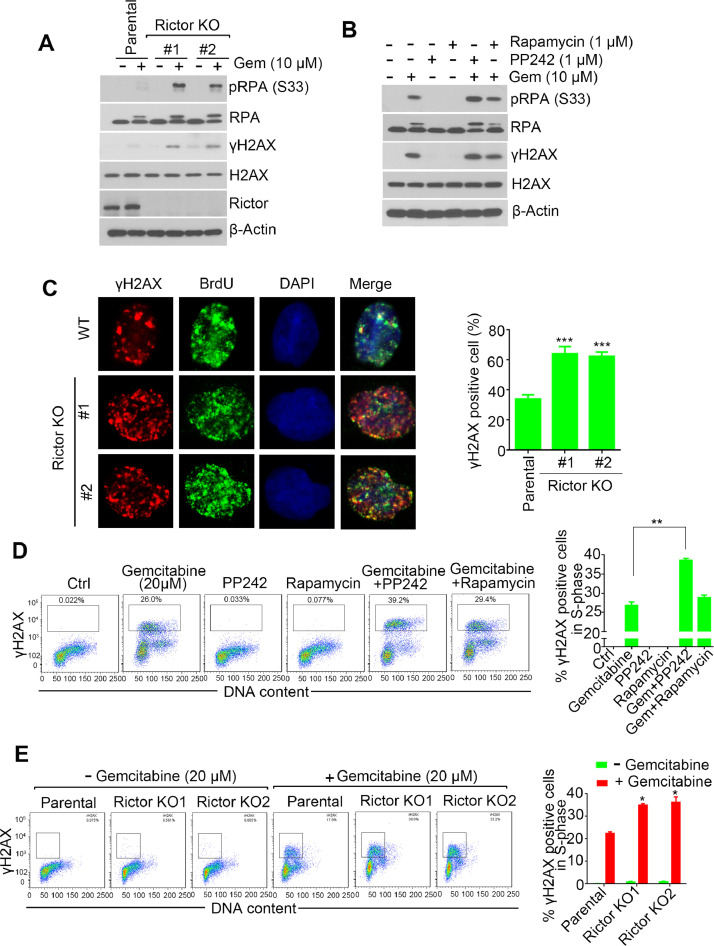
Rictor depletion enhances gemcitabine induced DNA damage
Given that gemcitabine kills cancer cells through inducing DNA replication stress and subsequent formation of DNA double-strand breaks (DSBs) [12]. To test whether mTORC2 loss leads to replication stress hypersensitivity in response to gemcitabine, we evaluated the level of RPA2 phosphorylation on Ser 33 (pRPA2-S33), a target of ATR and represents a hallmark of DNA replication stress [27], after gemcitabine treatment. As shown in Fig. 2A, we observed significant increase of pRPA2-S33 level in Rictor KO cells after gemcitabine treatment. Meanwhile, we also detected more γH2AX, which is the phosphorylation of H2AX at Ser 139 and recognized as a marker of DNA DSB [28], in Rictor KO cells after gemcitabine treatment (Fig. 2A, Supplementary Fig.S2A). Similarly, Combination treatment of gemcitabine with mTORC1/2 inhibitor PP242, but not mTORC1 inhibitor rapamycin induced increase of pRPA2 S33 and γH2AX (Fig. 2B, Supplementary Fig.S2B). To verify whether the DSBs were taken place in replicating cells, we labeled nascent DNA with BrdU before adding gemcitabine, the occurrence of DSBs in DNA replication sites were evaluated by co-staining of γH2AX and BrdU. As shown in Fig. 2C, γH2AX foci were exclusively co-localized with BrdU, and the γH2AX levels were significant elevated in Rictor KO cells. ds
Fig. 2.
Inhibition of mTORC2 potentiates gemcitabine induced DNA damage. (A) H1299 parental or Rictor KO cells were treated with or without 10 µM gemcitabine for 12 hrs, followed by western blot analysis with indicated antibodies. (B) Western blot analysis of cell lysates derived from H1299 cells treated as indicated for 12 hrs. (C) Immunostaining analysis of gemcitabine-induced γH2AX in parental or Rictor KO H1299 cells. (D) H1299 cells were treated with 10 µM gemcitabine, 1 µM Rapamycin, 1 µM PP242 or their combination for 12 hrs, followed by analysis of S-phase associated γH2AX formation by flow cytometry. (E) Flow cytometry analysis of γH2AX levels in H1299 parental or Rictor KO cells treated with or without 10 µM gemcitabine for 12 hrs. * P < 0.05, **P < 0.01 and *** P < 0.001 by two-tailed t-test.
We next employed flow cytometry to simultaneous analysis of γH2AX and propidium iodide (PI) labeled DNA to further analyze the γH2AX formation in S-phase cells. Consistent with immunofluorescence assay, gemcitabine-induced γH2AX mainly arose in S phase and Rictor depletion or PP242 treatment significantly increased γH2AX level in response to gemcitabine treatment (Fig. 2D–E), suggesting that enhanced formation of DNA DSBs induced by Rictor depletion were resulted from loss of mTORC2 activity.
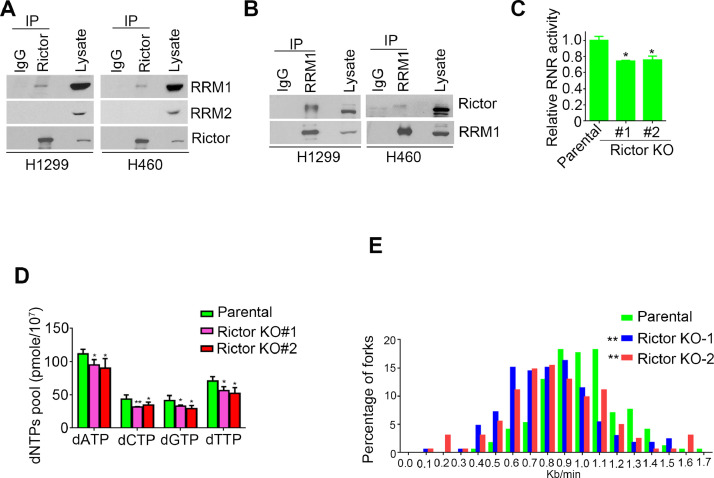
mTORC2 interacts with RRM1 and regulates DNA replication
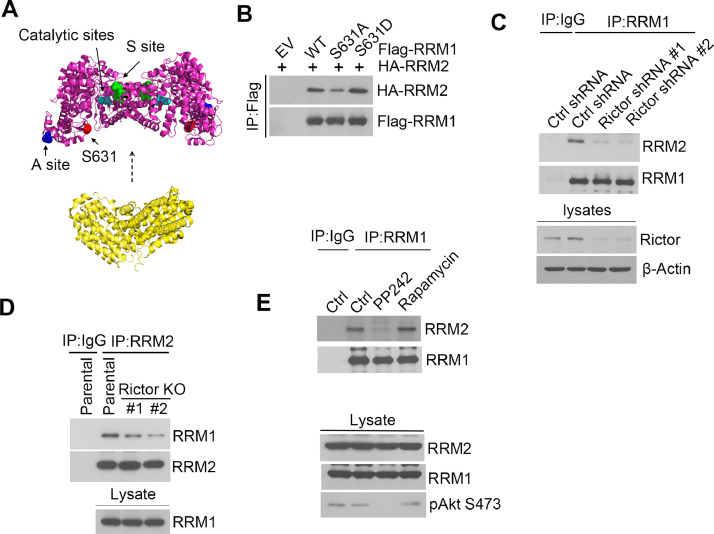
Gemcitabine induces replication stress and DNA damage though targeting RNR [5]. To test whether RNR is involved in mTORC2 mediated gemcitabine resistance, we performed co-immunoprecipitation (Co-IP) assay using Rictor antibody and found RNR large subunit interacts with Rictor (Fig. 3A). Besides, we also detected RRM1/Rictor interaction by IP with RRM1 antibody (Fig. 3B). Meanwhile, we found RRM1 also interacted with SIN1, another mTORC2 specific component, as wells as mTOR protein (Supplementary Fig.S3), indicating RRM1 is associated with mTORC2 complex.
Fig. 3.
mTORC2 interacts RRM1 and regulates RNR activity. (A-B) Co-immunoprecipitation (Co-IP) analysis of interaction between Rictor and RRM1 using anti-Rictor antibody (A) or anti-RRM1 antibody (B) in H1299 and H460 cells. (C) RNR activities in H1299 parental or Rictor KO cells were assessed as described in “Materials and methods”. (D) Intracellular dNTPs pool size in H1299 parental or Rictor KO cells were measured by High Performance Liquid Chromatography (HPLC). (E) H1299 parental or Rictor KO cells were labeled sequentially with CldU and IdU for 20 min each, the DNA replication progressions were visualized by DNA fiber assay using specific antibodies against CIdU (red) or IdU (green). P value was calculated using two-tailed t-test (C, D) and Mann–Whitney U test (E). * P < 0.05, **P < 0.01 when compared with parental.
Moreover, we detected a significant decrease of RNR enzymatic activity in Rictor KO cells (Fig. 3C). Consistent with Rictor depletion, treatment of mTORC1/2 inhibitor PP242, but not mTORC1 inhibitor rapamycin, also reduced RNR activity in H1299 cells (Supplementary Fig.S4A). These results demonstrated that mTORC2 interacts with RRM1 and regulates RNR activity. RNR impairment causes DNA replication stress, characterized by slowdown of replication fork progression [24]. Therefore, we measured DNA replication fork speed by DNA fiber assay [23], and we detected a slower replication fork speed in Rictor KO cells (Fig. 3E). Meanwhile, we obtained a similar result in H1299 cells treated with PP242, but not rapamycin (Supplementary Fig.S4B).
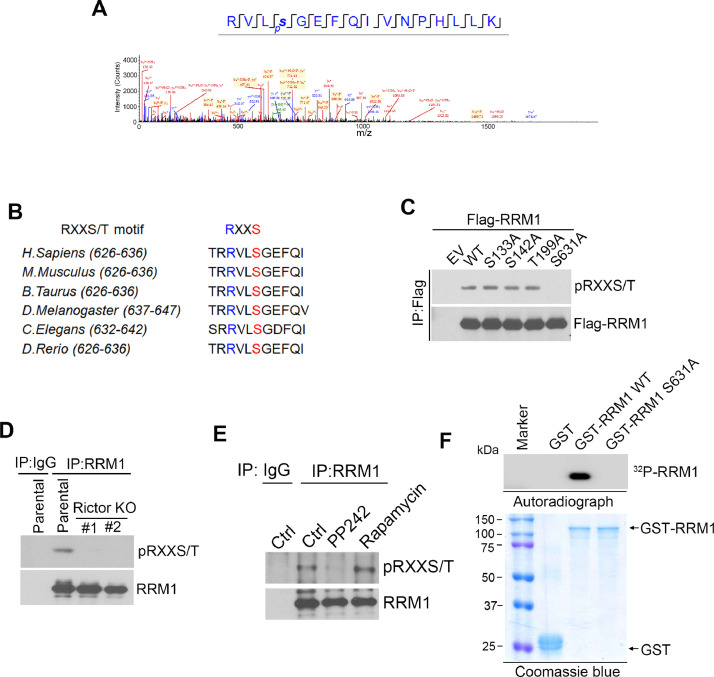
mTORC2 phosphorylates RRM1 at Ser631
To test whether mTORC2 could phosphorylate RRM1, we first examined RRM1 phosphorylation using mass spectrometry (LC-MS/MS) and identified S631 is phosphorylated (Fig. 4A). Through sequence analysis, we found that S631 of RRM1 belongs to RXXS/T motif and is conserved across species (Fig. 4B). RXXS/T motif is the substrate site which could be recognized by the broad category of AGC kinase family and it could also be phosphorylated by mTORC2 [19]. RRM1 contains three RXXS/T sites including S133, S142, T199 and S631. We then transfected H1299 cells with flag tagged WT or its phosphorylation defective mutants (SA/TA) RRM1, followed by analyzing RRM1 phosphorylation through IP using anti-flag agarose bead and western blot with phospho-RXXS/T-antibody. And we detected a clear phosphorylation on WT, S133A, S142A or T199A mutant RRM1, but we failed to observe RRM1 phosphorylation on S631A mutant RRM1 (Fig. 4C), suggesting S631 is the only RXXS/T phosphorylation site. Since, mTORC2 could affect RNR activity, thus, we speculated that mTORC2 might be the upstream kinase responsible for phosphorylating S631 on RRM1.
Fig. 4.
mTORC2 phosphorylates RRM1 at Ser 631. (A) H1299 cells were transfected with Flag-RRM1, followed by flag immunoprecipitated and analysis of LC-MS/MS peptide spectra of RRM1 phosphorylation. (B) Sequence alignment of S631 phosphorylation site in RRM1 from different species. (C) H1299 cells were transfected with indicated flag tagged RRM1 variants, followed by analysis of RRM1 phosphorylation through IP with flag and western blot with anti-pRXXS/T antibody. Analysis of RRM1 phosphorylation in H1299 Rictor KO cells (E) or H1299 cells treated with 1 µM PP242 or 1 µM rapamycin for 4 hrs (F) through RRM1 IP and western blot with anti-pRXXS/T antibody. (G) Active mTORC2 was immunoprecipitated from HEK293T cells with purified GST-tagged WT or S631A mutant RRM1 protein in kinase buffer containing [γ-32P] ATP. RRM1 phosphorylation was analyzed by autoradiography.
To test our hypothesis, we analyzed RRM1 S631 phosphorylation (pRRM1-S631) in parental or Rictor KO cells and detected pRRM1-S631 in parental cells, but not in Rictor KO cells (Fig. 4D). Meanwhile, PP242 treatment, but not rapamycin treatment, abolished RRM1 phosphorylation (Fig. 4E). To further validate mTORC2 phosphorylating RRM1, we performed an in vitro kinase assay using purified GST-RRM1 WT or S631A recombinant protein as the substrate. As shown in Fig. 4F, mTORC2 phosphorylated WT RRM1, but could not phosphorylate S631A mutant, indicating mTORC2 indeed phosphorylates RRM1 on S631.
RRM1 phosphorylation at S631 enhances its interaction with RRM2
As the regulatory subunit of RNR, RRM1 bears overall enzymatic activity regulating site (activity site, A-site) and substrate specificity regulating site (specificity site, S-site). The binding of dATP to the A-site could inhibit its overall activity, whereas, ATP binding to the A-site stimulates its activity. Meanwhile, dATP and ATP binding to the S-site could increase CDP and UDP reduction, whereas dTTP promotes GDP reduction and dGTP enhances ADP reduction. RNR makes the dNTP pool balance through this allosteric binding of nucleotides to S-site. To explore the function of RRM1 phosphorylation at S631, we examined RRM1 structure and found that S631 is not localized in proximity of catalytic site and the regulatory sites including activity site (A site) and specificity site (S site) (Fig. 5A). Whereas, we found S631 is localized at the interface of the interaction between RRM1 and RRM2 using docking program on ClusPro web server (https://cluspro.org/) (Fig. 5A), suggesting S631 phosphorylation might affect its interaction with RRM2.
Fig. 5.
RRM1 phosphorylation at S631 enhances its interaction with RRM2. (A) Computational modeling based on human RRM1 and human RRM2 structure, which were obtained from PDB database (RRM1, PDB ID: 3HNE) and (RRM2, PDB ID:3OLJ), showing that S631 is localized at interface of RRM1/RRM2 interaction. The active site (A site), specificity site (S site), catalytic site and S631 on RRM1 were indicated as blue, green, cyan and red respectively. (B) H1299 cells were co-transfected with HA-RRM2 along with empty vector (EV), WT, S631A or S631D flag-RRM1, followed by IP with anti-flag affinity beads and western blot analysis with indicated antibodies. (C-D) Co-IP analysis of RRM1/RRM2 interaction in Rictor silence (C) or Rictor KO H1299 cells (D) through IP with anti-RRM1 antibody and western blot using indicated antibodies. (E) H1299 cells were treated with 1 µM PP242 or 1 µM Rapamycin for 4 hrs, followed by analysis of RRM1/RRM2 interaction through RRM1 IP.
To analyze the effects of S631 phosphorylation on RRM1/RRM2 association, HA-RRM2 was co-transfected into H1299 cells along with Flag tagged WT, phosphorylation defective mutant (S631A), or constitutive phosphorylation mutant (S631D) RRM1, followed by IP analysis with anti-Flag antibody. The result revealed that less level of HA-RRM2 bound S631A RRM1 and increased HA-RRM2 bound with S631D RRM1(Fig. 5B, Supplementary Fig.S5A). Moreover, silence of Rictor could reduce RRM1/RRM2 association in H1299 cells (Fig. 5C, Supplementary Fig.S5B). We also obtained similar result of decreased RRM1/RRM2 interaction in Rictor KO cells (Fig. 5D, Supplementary Fig.S5C). In addition, inhibition of mTORC1/2 by PP242, not inhibition of mTORC1 by rapamycin could inhibit RRM1 interacts with RRM2 (Fig. 5E, Supplementary Fig.S5D), indicating mTORC2 mediated RRM1 phosphorylation on S631 promotes its interaction with RRM2. RNR enzymatic activity depends on its intact heterotetramer complex and disruption of RRM1/RRM2 interaction leads to impaired enzymatic activity. Thus, these results indicating that mTORC2 regulation of RNR activity might through phosphorylation of RRM1.
Targeting mTORC2 sensitize NSCLC to gemcitabine in vivo
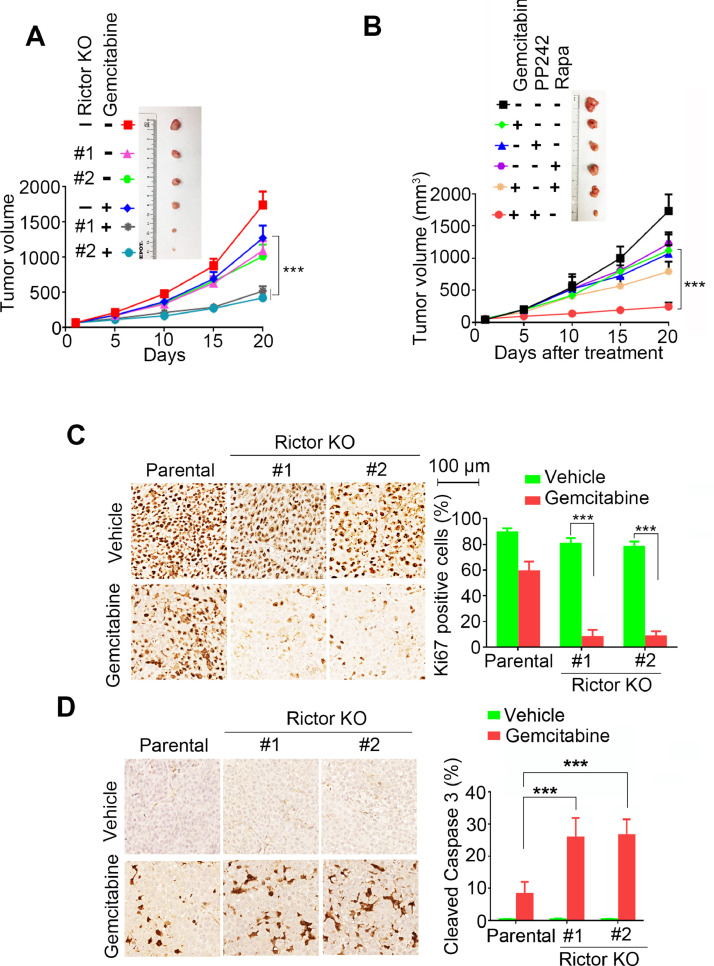
To test whether mTORC2 regulates gemcitabine sensitivity of NSCLC in vivo, We generated H1299 xenograft model using parental or Rictor KO H460 cells and treated mice with or without 80 mg/kg gemcitabine to study the therapeutic efficacy in vivo. As shown in Fig. 6A, tumors with Rictor deficiency were hypersensitive to gemcitabine treatment compared with parental tumors. Similarly, gemcitabine treatment in combination with PP242, but not Rapamycin, exerted marked synergistic anti-tumor effects in H460 xenografts (Fig. 6B). Meanwhile, combination treatment of PP242 and gemcitabine did not cause obvious loss of body weight compared with gemcitabine treatment alone (Supplementary Fig.S6), indicating mTORC1/2 inhibitor PP242 enhances gemcitabine therapeutic efficacy without toxicity. In addition, consistent with tumor growth curve (Fig. 6A), less level of Ki67 proliferation marker (Fig. 6C) and more cleaved caspase 3 (apoptosis marker) expression (Fig. 6D) were detected in gemcitabine treated Rictor KO tumors compared with parental tumors.
Fig. 6.
Rictor deficiency leads to hypersensitivity to gemcitabine treatment. (A) Mice bearing H460 parental or Rictor KO xenografts were treated with or without 80 mg/kg gemcitabine 2 times per week. Tumor volumes were measured, tumor growth curve (A) was shown. (B) Mice bearing H1299 xenografts were divided into 6 groups and each group were received 80 mg/kg gemcitabine, 20 mg/kg PP242, 2 mg/kg Rapamycin alone, or their combination, tumor volumes were measured every 5 days. Immunohistochemistry (IHC) analysis of Ki67 (C) and cleaved caspase 3 (D) in the tumors derived from vehicle or gemcitabine treated mice. ***P < 0.001 by 2-tailed t-test.
Discussion
As one of the most common chemotherapeutic agents, gemcitabine is widely used in treatment of pancreatic, lung and breast cancers. In this study, we identified that mTORC2 is a gemcitabine resistant gene and further found that genetic deletion or pharmacological inhibition of mTORC2 resulted in hypersensitivity to gemcitabine treatment and significantly increased gemcitabine-induced DNA damage and replication stress. Gemcitabine targets RNR to interfere with de novo production of dNTPs and subsequently inhibits DNA replication and cell proliferation [29]. Accumulating evidence showed that RNR activity plays a crucial role in determining gemcitabine sensitivity, and overexpression of RNR subunits including RRM1, RRM2 or RRM2B could confer resistance to gemcitabine treatment in cancer cells [29, 30]. Our study showed that mTORC2 stimulates RNR activity through phosphorylation of RRM1, and combination treatment of mTORC2 inhibitor and gemcitabine leads to lethal RNR inhibition and cancer cell death. Conversely, gemcitabine treatment in presence of active mTORC2 might result in residual RNR activity and resistance to cell death. Therefore, our finding suggests a mechanistic basis by which mTORC2 direct cancer cells to evade gemcitabine-induced cell death.
As the rapamycin-sensitive mTOR protein complex, mTORC1 has been extensively investigated and its roles in protein synthesis, autophagy and cell growth are well-established [31]. In contrast, the functions of mTORC2 are still poorly understood. Here, we showed that mTORC2 regulates DNA replication through modulating dNTPs pool balance (Fig. 3D). Consistent with our study, mTORC2 has been found to exert important functions in maintaining genomic integrity and inhibition of mTORC2 accentuates genotoxic agent induced DNA damage [22]. In line with previous study, our data (Fig. 3) provided an evidence that mTORC2 directly regulates dNTPs pool supply, which is the key factor in maintaining genomic stability and DNA replication. Therefore, our finding uncovered a novel function of mTORC2 and a mechanistic basis for mTORC2’s role in genomic integrity.
RNR catalyzes the rate-limiting reaction for de novo production of dNTPs and it is the only enzyme could catalyze the formation of deoxyribonucleotides from ribonucleotides [23]. In light of the importance of precise DNA replication in genomic stability, RNR overall activity is tightly regulated by allosteric regulation through binding of ribonucleotide to the RRM1. Binding of ATP to RRM1 stimulates RNR activity, while, dATP binding inhibits RNR activity [32]. Our study found that phosphorylation of RRM1 on Ser631 promotes its association with RRM2 and assembly of RNR, thus, enhances RNR activity. Our finding provides a novel mechanism to regulate RNR activity through post-translational phosphorylation of RRM1 by mTORC2.
Hyperactivation of mTORC2 has been observed in various types of cancers and plays important roles in cancer progression [15, 33, 34]. As the downstream of growth factor signaling, mTORC2 hyperactivation might be resulted from oncogenic EGFR, PIK3CA and KRAS mutations, and these mutations are frequently observed in NSCLC [17, 35]. In addition, overexpression of Rictor could directly stimulate mTORC2 activation and RICTOR amplification has been detected in 13% of NSCLC patients [16]. Thus, mTORC2 is highly activated in NSCLC and is a feasible target for NSCLC therapies. In our present study, we found mTORC2 activity regulates dNTPs biosynthesis and DNA replication fork speed (Fig. 3D–E), which is tightly associated with cell cycle progression and cell proliferation. Therefore, our finding provides data to support a novel mechanism through which mTORC2 promotes cancer cell growth.
Finally, our identification of mTORC2 could lead to the development of novel therapeutic approaches for gemcitabine-based cancer therapies. NSCLC cells with impaired mTORC2 activity through pharmacological inhibition or genetic deletion were more susceptible to gemcitabine treatment. These results suggesting that mTORC2 activity could serve as a molecular biomarker to predict gemcitabine sensitivity for personalized gemcitabine treatment in NSCLC patients.
Conclusions
In summary, our finding demonstrates that mTORC2 is a novel regulator to dictate RNR activity and DNA replication, therefore, it could serve as a molecular biomarker to predict gemcitabine sensitivity, as well as a potential target to sensitize NSCLC to gemcitabine therapy.
Author Contributions
Conceptualization and design: G. C.; Formal analysis of data: L.T., C. C., Y. G.; Funding acquisition: G. C.; Investigation: L.T., C. C., Y. G., F. Z., J. M., Q. F., S. L., J. T.; Data curation: N. X.; Methodology: Not applicable; Supervision: M.C., C.L., J. F., Y. Z., G. C.; Validation: L. T., F. Z.; Visualization: G. C.; Project administration: L. Y. and Y. C.; Resources: J. F., Y. Z. and G. C.; Software: Not applicable; Writing the original draft: G. C.; Review & Editing the manuscript: J. F..
Footnotes
Funding: The study was supported by the National Natural Science Foundation of China (No. 82073042 to G. Chen), Guangzhou Municipal Science and Technology Project (202002030451 to G. Chen), Natural Science Foundation of Guangdong Province of China (2019A1515011247 to G. Chen), Fundamental Research Funds for the Central Universities (21620421) and Open research funds from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (to G. Chen).
Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.05.007.
Contributor Information
Caiyong Lai, Email: lcy2015@jnu.edu.cn.
Jun Fan, Email: fanjun@jnu.edu.cn.
Yongchang Zhang, Email: zhangyongchang@csu.edu.cn.
Guo Chen, Email: gchen84@jnu.edu.cn.
Appendix. Supplementary materials
Reference
- 1.Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J, Sajjakulnukit P. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 2019;29:1390–1399. doi: 10.1016/j.cmet.2019.02.001. e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Shaughnessy J, Schwartzberg L, Danso MA, Miller KD, Rugo HS, Neubauer M, Robert N, Hellerstedt B, Saleh M, Richards P. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Schwerbrock NM, Rogers AB, Kim WY, Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013;21:1559–1569. doi: 10.1038/mt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeckemeier L, Kraehenbuehl R, Keszthelyi A, Gasasira MU, Vernon EG, Beardmore R, Vagbo CB, Chaplin D, Gollins S, Krokan HE. Mre11 exonuclease activity removes the chain-terminating nucleoside analog gemcitabine from the nascent strand during DNA replication. Sci Adv. 2020;6:eaaz4126. doi: 10.1126/sciadv.aaz4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Lohman GJ, Stubbe J. Enhanced subunit interactions with gemcitabine-5′-diphosphate inhibit ribonucleotide reductases. Proc Natl Acad Sci U S A. 2007;104:14324–14329. doi: 10.1073/pnas.0706803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raoof M, Zhu C, Cisneros BT, Liu H, Corr SJ, Wilson LJ, Curley SA. Hyperthermia inhibits recombination repair of gemcitabine-stalled replication forks. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh V, Kwan HY, Tan WL, Mah TL, Yong WP. Knockdown of POLA2 increases gemcitabine resistance in lung cancer cells. BMC Genomics. 2016;17:1029. doi: 10.1186/s12864-016-3322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao WY, Shih JY, Chang GC, Cheng YK, Yang JC, Chen YM, Yu CJ. Genetic polymorphism of XRCC1 Arg399Gln is associated with survival in non-small-cell lung cancer patients treated with gemcitabine/platinum. J Thorac Oncol. 2012;7:973–981. doi: 10.1097/JTO.0b013e31824fe98c. [DOI] [PubMed] [Google Scholar]
- 10.Mlak R, Krawczyk P, Ciesielka M, Koziol P, Homa I, Powrozek T, Prendecka M, Milanowski J, Malecka-Massalska T. The relationship between RRM1 gene polymorphisms and effectiveness of gemcitabine-based first-line chemotherapy in advanced NSCLC patient. Clin Transl Oncol. 2016;18:915–924. doi: 10.1007/s12094-015-1461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Primo LMF, Teixeira LK. DNA replication stress: oncogenes in the spotlight. Genet Mol Biol. 2019;43 doi: 10.1590/1678-4685-GMB-2019-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SC, Petrova AV, Madden MZ, Wang H, Pan Y, Warren MD, Hardy CW, Liang D, Liu EA, Robinson MH. A gemcitabine sensitivity screen identifies a role for NEK9 in the replication stress response. Nucleic Acids Res. 2014;42:11517–11527. doi: 10.1093/nar/gku840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guri Y, Colombi M, Dazert E, Hindupur SK, Roszik J, Moes S, Jenoe P, Heim MH, Riezman I, Riezman H. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–823. doi: 10.1016/j.ccell.2017.11.011. e812. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H, Zou Y, Ross JS, Wang K, Liu X, Halmos B, Ali SM, Liu H, Verma A, Montagna C. RICTOR amplification defines a novel subset of patients with lung cancer who may benefit from treatment with mTORC1/2 inhibitors. Cancer Discov. 2015;5:1262–1270. doi: 10.1158/2159-8290.CD-14-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J. Oncogenic EGFR signaling activates an mTORC2-NF-kappaB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1:524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu K, Chen G, Li X, Wu X, Chang Z, Xu J, Zhu Y, Yin P, Liang X, Dong L. MFN2 suppresses cancer progression through inhibition of mTORC2/Akt signaling. Sci Rep. 2017;7:41718. doi: 10.1038/srep41718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Y, Albuquerque CP, Braas D, Zhang W, Villa GR, Bi J, Ikegami S, Masui K, Gini B, Yang H. mTORC2 regulates amino acid metabolism in cancer by phosphorylation of the cystine-glutamate antiporter xCT. Mol Cell. 2017;67:128–138. doi: 10.1016/j.molcel.2017.05.030. e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Zhang X, Li M, Chen P, Zhang B, Guo H, Cao W, Wei X, Cao X, Hao X. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 2010;70:9360–9370. doi: 10.1158/0008-5472.CAN-10-0207. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Jia L, Liu T, Yip YL, Tang WC, Lin W, Deng W, Lo KW, You C, Lung ML. mTORC2-mediated PDHE1alpha nuclear translocation links EBV-LMP1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38:4669–4684. doi: 10.1038/s41388-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimada K, Filipuzzi I, Stahl M, Helliwell SB, Studer C, Hoepfner D, Seeber A, Loewith R, Movva NR, Gasser SM. TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol Cell. 2013;51:829–839. doi: 10.1016/j.molcel.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Luo Y, Warncke K, Sun Y, Yu DS, Fu H, Behera M, Ramalingam SS, Doetsch PW, Duong DM. Acetylation regulates ribonucleotide reductase activity and cancer cell growth. Nat Commun. 2019;10:3213. doi: 10.1038/s41467-019-11214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poli J, Tsaponina O, Crabbe L, Keszthelyi A, Pantesco V, Chabes A, Lengronne A, Pasero P. dNTP pools determine fork progression and origin usage under replication stress. EMBO J. 2012;31:883–894. doi: 10.1038/emboj.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murugan AK. mTOR: role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111. doi: 10.1016/j.semcancer.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Sturgill D, Sebastian R, Khurana S, Tran AD, Edwards GB, Kruswick A, Burkett S, Hosogane EK, Hannon WW. Replication stress shapes a protective chromatin environment across fragile genomic regions. Mol Cell. 2018;69:36–47. doi: 10.1016/j.molcel.2017.11.021. e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–2498. doi: 10.1093/nar/gkv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerqueira NM, Fernandes PA, Ramos MJ. Understanding ribonucleotide reductase inactivation by gemcitabine. Chemistry. 2007;13:8507–8515. doi: 10.1002/chem.200700260. [DOI] [PubMed] [Google Scholar]
- 30.Boukovinas I, Papadaki C, Mendez P, Taron M, Mavroudis D, Koutsopoulos A, Sanchez-Ronco M, Sanchez JJ, Trypaki M, Staphopoulos E. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One. 2008;3:e3695. doi: 10.1371/journal.pone.0003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72–82. doi: 10.1016/j.ceb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozman Grinberg I, Lundin D, Hasan M, Crona M, Jonna VR, Loderer C, Sahlin M, Markova N, Borovok I, Berggren G. Novel ATP-cone-driven allosteric regulation of ribonucleotide reductase via the radical-generating subunit. Elife. 2018;7 doi: 10.7554/eLife.31529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driscoll DR, Karim SA, Sano M, Gay DM, Jacob W, Yu J, Mizukami Y, Gopinathan A, Jodrell DI, Evans TR. mTORC2 signaling drives the development and progression of pancreatic cancer. Cancer Res. 2016;76:6911–6923. doi: 10.1158/0008-5472.CAN-16-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gkountakos A, Pilotto S, Mafficini A, Vicentini C, Simbolo M, Milella M, Tortora G, Scarpa A, Bria E, Corbo V. Unmasking the impact of Rictor in cancer: novel insights of mTORC2 complex. Carcinogenesis. 2018;39:971–980. doi: 10.1093/carcin/bgy086. [DOI] [PubMed] [Google Scholar]
- 35.Kovalski JR, Bhaduri A, Zehnder AM, Neela PH, Che Y, Wozniak GG, Khavari PA. The functional proximal proteome of oncogenic ras includes mTORC2. Mol Cell. 2019;73:830–844. doi: 10.1016/j.molcel.2018.12.001. e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.