Abstract
Introduction
Several kinds of researches are available on preterm mortality in the East Africa continent; however, it is inconsistent and inconclusive, which requires the pooled evidence to recognize the burden in general.
Purpose
To collect and synthesis evidence on preterm mortality and identify factors in the East Africa continent.
Methods
PubMed, Google Scholar, Hinary, Cochrane library, research gate, and institutional repositories were retrieved to identity eligible articles through Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. The articles were selected if the publication period is between 2010-2021 G.C. Data were extracted by a standardized JBI data extraction format for mortality rate and stratified the associated factors. Then exported to STATA 14 for further analysis. I2 and Egger's tests were employed to estimate the heterogeneity and publication bias respectively. Subgroup analysis based on country, study design, year of publication, and the sample size was also examined.
Result
This meta-analysis included 32 articles with a total of 21,405 study participants. The pooled mortality rate among preterm in the East Africa continent was found to be 19.2% (95% CI (confidence interval (16.0–22.4)). Regarding the study design, the mortality rate was found to be 18.1%, 19.4%, and 19.7% concerning the prospective cohort, retrospective cohort, and cross-sectional studies. The pooled odds of mortality among preterm with respiratory distress syndrome decreased survival by nearly three folds [AOR (Adjusted odds ratio = 3.2; 95% CI: 22, 4.6)] as compared to their counterparts. Similarly, preterm neonates presented with birth asphyxia were nearly three times higher in death as compared with preterm without birth asphyxia [AOR = 2.6; 95% CI: 1.9, 3.4].
Conclusion
Preterm mortality was found to be unacceptably high in Eastern Africa continent.
Fortunately, the main causes of death were found to be respiratory distress syndrome and birth asphyxia which are preventable and treatable hence early detection and timely management of this problem are highly recommended to improve preterm survival.
Keywords: Preterm, Respiratory distress syndrome, Asphyxia, Impact, Systematic review, Meta-analysis, East Africa
Preterm, Respiratory distress syndrome, Asphyxia, Impact, Systematic Review, Meta-analysis, East Africa.
1. Introduction
Preterm refers to a baby born before 37 weeks of pregnancy has been completed [1]. Preterm birth can be further sub-divided based on gestational age: extremely preterm (<28 weeks), very preterm (28 - <32 weeks) and moderate preterm (32 - <37 completed weeks of gestation) [2].
The Neonatal period is the most vulnerable time for a child's survival [3]. In 2016, 2.6 million deaths, or roughly 46% of all under-five deaths, occurred during this period [4]. Neonatal mortality (NM) is a major public health challenge worldwide [5, 6]. In 2019, approximately 17 deaths per 1,000 live births had been reported worldwide [3]. Of these, approximately 70% of the neonatal mortalities in resource-limited setting predominantly in East Africa [5, 6, 7].
Worldwide, neonatal mortality due to preterm accounts for 15 to 36, percent [8]. However, in low to middle-income countries neonatal mortality contributed by preterm ranges from 34–40%, and preterm is the second leading cause of under-five mortality [9, 10].
Globally, 13 million preterm were born annually and the highest percentage resource-limited setting [11], and eastern Africa countries have a lion sharing of this burden [10, 12, 13, 14]. Besides, a global action report 2020 showed that the preterm birth rate was 11%, and millions of children died due to preterm birth before the age of 5 years. Meanwhile, preterm birth is the leading cause of death among children [15, 16]. In 2019, 47% of all under-5 deaths occurred in the newborn period, and close to 75 % dying within the first week of life due to preterm birth [17, 17]. Even though premature babies can be saved with feasible and cost-effective care [9, 18], still it is a leading cause of infant mortality in developing countries [9, 10, 19].
In Sub-Saharan Africa had the highest neonatal mortality rate in 2019 at 27 deaths per 1,000 live births, followed by Central and Southern Asia with 24 deaths per 1,000 live births [18]. Besides, in Sub-Saharan Africa, East Africa countries accounts the highest number of neonatal mortality, 2019 [19,20].
Globally, the burden of preterm birth is disproportionately concentrated in East Africa and Asia, which account for 85% of all preterm births [21]. Preterm birth is a significant challenge in developing countries due to the rapid increase in their incidence and their disproportionate contribution to increased neonatal mortality rates [22].
According to Ethiopia Demographic Health Survey (EDHS) 2019, neonatal mortality was increased to 30 death per 1000 live births from 29 per 1000 in 2016 and preterm is the second leading factor [23], which is 10 folds of the mortality rate in the developed nations [3].
Different research conducted on preterm mortality in east Africa with a huge discrepancy range from 4.4 %- to 41% in Zambia and Sudan [24, 25] respectively. Besides, preterm mortality has been interlinked with different contributing factors including birth asphyxia, feeding difficulties, hypothermia, hypoglycemia, respiratory syndrome disease, jaundice, and necrotizing enter colitis [26, 27, 28]. Thus, this inconsistency, inconclusive, and uncertain requires pooled evidence to recognize the burden of Eastern African countries. In addition to this, the findings from this review will be utilized to guide the development of guidelines and to enhance the efforts of stakeholders towards the improvement of preterm survival. Therefore, the present study aimed to collect and synthesis evidence on preterm mortality and identify factors in the East Africa continent.
2. Methods
2.1. Design and search strategy
2.1.1. Review question
The review questions of this systematic review and meta-analysis were what is the pooled mortality rate among preterm neonates in the East Africa continent? In addition, what are the factors associated with preterm mortality in the East Africa continent?
2.1.2. Reporting
The review protocol has been sent to the PROSPERO database for registration Standard Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist was used to present the results of the review (Additional file).
2.2. Inclusion and exclusion criteria
Both published and unpublished cross-section, case-control, and cohort studies that reported the mortality rate or survival rate and factors associated with it among neonates in East African country context were included f. East African countries include (Sudan, South Sudan, Kenya, Uganda, Djibouti, Eritrea, Ethiopia, Somalia, Tanzania, Rwanda, Burundi, Comoros, Mauritius, Seychelles, Mozambique, Madagascar, Zambia, Malawi, Zimbabwe, Reunion, and Mayotte). The articles were selected if their publication period is between 2010-2021 G.C. However, studies with no abstracts, case series, case reports, and qualitative studies were excluded from the study.
2.3. Search strategies
This review identified studies that provide information on the mortality rate or survival rate in the continent of Eastern Africa. In the searching engine, PubMed, Google Scholar, Hinary, Cochrane library, research gate, and institutional repositories were retrieved. The search linked keywords were combined with Boolean operators “AND and OR`` in the context of population, exposures/intervention, comparison, and condition/outcome (PICO) format. Besides, the related articles for the references of the relevant articles were also searched and investigated. Those search terms or phrases included were: “preterm”, “term”, “neonate”, “newborn”, “small gestation age”, “low birth weight”, “mortality”, “survival”, and Eastern Africa. Using those key terms, the following search map was applied: (prevalence OR magnitude OR epidemiology) AND (predictor OR risk factor OR determinate OR associated factors) AND (preterm [MeSH Term] OR term OR neonate OR newborn OR small gestational age OR low birth weight) AND (mortality [MeSH Terms] OR survival) AND (Eastern Africa) OR developing country on PubMed database. These search terms were further paired with the names of each East African country including Sudan, South Sudan, Kenya, Uganda, Djibouti, Eritrea, Ethiopia, Somalia, Tanzania, Rwanda, Burundi, Comoros, Mauritius, Seychelles, Mozambique, Madagascar, Zambia, Malawi, Zimbabwe, Reunion, and Mayotte.
2.4. Study selection and screening
All titles and abstracts were screened exhaustively by independent authors (ESC and BMB.) to identify potentially relevant articles. Whenever further information is needed, we made some efforts to communicate via authors by email. The retrieved studies were exported to the citation manager (Zotero) and then duplicate articles were excluded. Disagreements were discussed during a consensus meeting with other reviewers (FAG, HSH, and TM) for the final selection of studies to be included in the systematic review and meta-analysis.
2.5. Data extraction and quality assessment
Two authors (WNA and DKM) independently and then in collaboration extracted all relevant information by using a standardized JBI data extraction format. For each included article, the following data were extracted: authors’ name, year of publications, study region, sample size, study design, and study setting. Besides, factors associated with mortality were recorded in a standardized JBI data abstraction format. Any disagreements between authors were resolved through discussions with the third and fourth authors when required. The retrieved data was crosschecked with the included papers, then modifications and editions of mistyping data were made when required.
2.6. Statistical analysis
The authors were edited, cleaned, and checked for completeness of the extracted data in an excel sheet, then exported into STATA 14 for further analysis. Pooled overall mortality rate and classified factors associated with mortality were estimated by a random effect meta-analysis model. Heterogeneity between the studies was assessed using the I2 statistic and the value of I2, they show the overall variation across the studies presented by low, moderate, and high with the percentage of 25, 50, and 75%, respectively [29]. Publication bias was estimated through a funnel plot and the Egger test [30]. Moreover, subgroup analysis was done by the study country, study design, sample size, and year of publication. Sensitivity analysis was piloted to examine the effect of a single study on the overall estimation.
3. Results
3.1. Search result
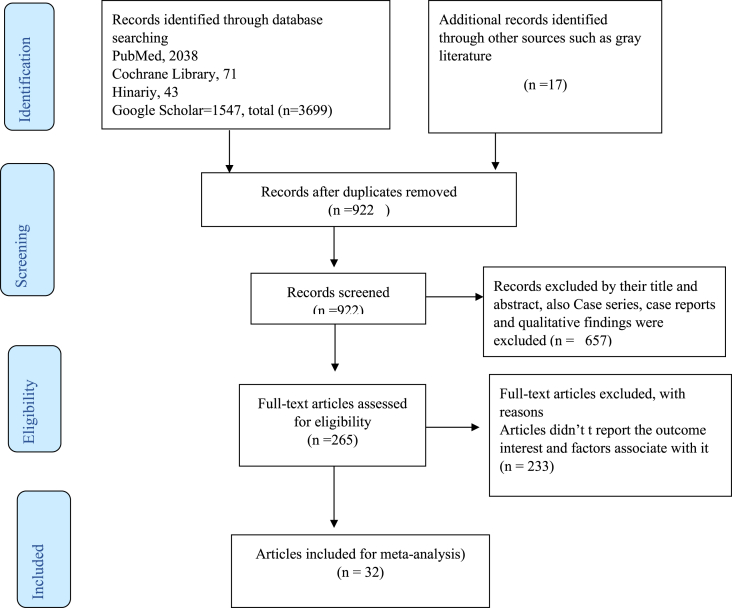
Our search yielded a total of 3699 records, 2038 from PubMed, 71 from Cochrane Library, 41 from Hinariy, 1547 from Google Scholar, 11 from CINAHL, and 06 from Scopus sources. After removal of duplicates, we screened the titles and abstracts of 922 records articles. Finally, a full-text review was conducted for 32 studies with a sample size of 21,405 participants were included to assess the overall mortality rate among preterm births from 11 East African countries including Ethiopia, Tanzania, Kenya, Burundi, Eritrea, Uganda, Tanzania, Malawi, Uganda, Zambia, Sudan, Mozambique, and Zimbabwe (Figure 1).
Figure 1.
The PRISMA flow chart that shows the searching process in East Africa from January 2012–December 2020.
3.2. Characteristics of the included articles
This meta-analysis included 32 different studies covering a total of 21,405 preterms. The studies were conducted from 2012 to 2020 among more than 11 Eastern Africa countries including 12 from Ethiopia represented by 14 studies [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44]. Tanzania [14, 45, 46, 47] and Uganda [12, 48, 49, 50] represented by 04 studies, Besides, Burundi [51, 52] and Eretria [53, 54] represented by 02 studies. While Malawi [55], Kenya [56], Zambia [24], Sudan [25], Mozambique [57], and Zimbabwe [58] are represented by 01 studies. Regarding study design, 13 studies employed retrospective cohort [12, 14, 37, 38, 39, 40, 41, 42, 43, 44, 45, 56, 57], whereas 07 prospective cohort studies [31, 32, 46, 47, 48, 49, 50], 01 case-control type [36], and 11 cross-sectional design [24, 25, 33, 34, 35, 51, 52, 53, 54, 55, 58]. All studies included in this review were observational studies which were conducted in the hospital with sample sizes ranging from 100 participants reported from a study in Ethiopia and Sudan [25, 36], and 29811 from Uganda [12] respectively (Table 1).
Table 1.
Distribution of mortality among preterm in East Africa from January 2012–December 2020.
| First Author/year | Country | Study design | Study type | Sample size | Magnitude (%) | Quality status |
|---|---|---|---|---|---|---|
| Yehuala et al. (2015) | Ethiopia | Retrospective Cohort | Hospital-based | 485 | 25 | Low risk |
| Tamene et al. (2020) | Ethiopia | cross-sectional study | Hospital-based | 686 | 36 | Low risk |
| Yismaw et al. (2019) | Ethiopia | Retrospective Cohort | Hospital-based | 516 | 29 | Low risk |
| Abebe et al. (2019) | Ethiopia | cross-sectional study | Hospital-based | 415 | 26 | Low risk |
| Mengist et al. (2020) | Ethiopia | Prospective Cohort | Hospital-based | 774 | 19 | Low risk |
| Chengo et al. (2020) | Tanzania | Prospective Cohort | Hospital-based | 311 | 15 | Low risk |
| Muchem et al. (2018) | Kenya | Retrospective Cohort | Hospital-based | 2080 | 6 | Low risk |
| Mmbaga et al. (2016) | Tanzania | Retrospective Cohort | Hospital-based | 1178 | 18 | Low risk |
| Seid et al. (2019) | Ethiopia | cross-sectional study | Hospital-based | 1488 | 9 | Low risk |
| Zuniga et al. (2013) | Burundi | cross-sectional | Hospital-based | 153 | 20 | Low risk |
| Shah et al. (2012) | Eritrea | cross-sectional | Hospital-based | 1502 | 6 | Low risk |
| Ndelem et al. (2016) | Burundi | cross-sectional | Hospital-based | 437 | 28 | Low risk |
| Andegio et al. (2020) | Eritrea | cross-sectional | Hospital-based | 242 | 17 | Low risk |
| Namazzi et al. (2020) | Uganda | Prospective Cohort | Hospital-based | 242 | 21 | Low risk |
| Moshiro et al. (2019) | Tanzania | Prospective Cohort | Hospital-based | 241 | 21 | Low risk |
| Orsido et al. (2019) | Ethiopia | Retrospective Cohort | Hospital-based | 212 | 40 | Low risk |
| Roro et al. (2019) | Ethiopia | Retrospective Cohort | Hospital-based | 206 | 14 | Low risk |
| van den et al. (2015) | Malawi | cross-sectional study | Hospital-based | 449 | 22 | Low risk |
| Egesa et al. (2020) | Uganda | Prospective Cohort | Hospital-based | 311 | 36 | Low risk |
| Dessu et al. (2018) | Ethiopia | Retrospective Cohort | Hospital-based | 107 | 35 | Low risk |
| Opio et al. (2019) | Uganda | Prospective Cohort | Hospital-based | 128 | 8 | Low risk |
| Sania et al. (2013) | Tanzania | Retrospective Cohort | Hospital-based | 1032 | 3 | Low risk |
| Dessu et al. (2020) | Ethiopia | Prospective Cohort | Hospital-based | 216 | 8 | Low risk |
| Farah et al. (2018) | Ethiopia | Retrospective Cohort | Hospital-based | 432 | 7 | Low risk |
| Paul et al. (2020) | Uganda | Retrospective Cohort | Hospital-based | 2981 | 5 | Low risk |
| Gudayu (2012) | Ethiopia | Retrospective Cohort | Hospital-based | 1733 | 33 | Low risk |
| Endalam et al. (2020) | Ethiopia | Retrospective Cohort | Hospital-based | 535 | 31 | Low risk |
| Miyoshi et al. (2019) | Zambia | cross-sectional study | Hospital-based | 1704 | 4 | Low risk |
| Kolobo et al. (2020) | Ethiopia | case-control | Hospital-based | 100 | - | Low risk |
| Salih et al. (2013) | Sudan | cross-sectional study | Hospital-based | 100 | 41 | Low risk |
| Garcia- et al. (2017) | Mozambique | Retrospective Cohort | Hospital-based | 147 | 12 | Low risk |
| Nyakan et al. (2019) | Zimbabwe | cross-sectional study | Hospital-based | 262 | 14 | Low risk |
3.3. Meta-analysis
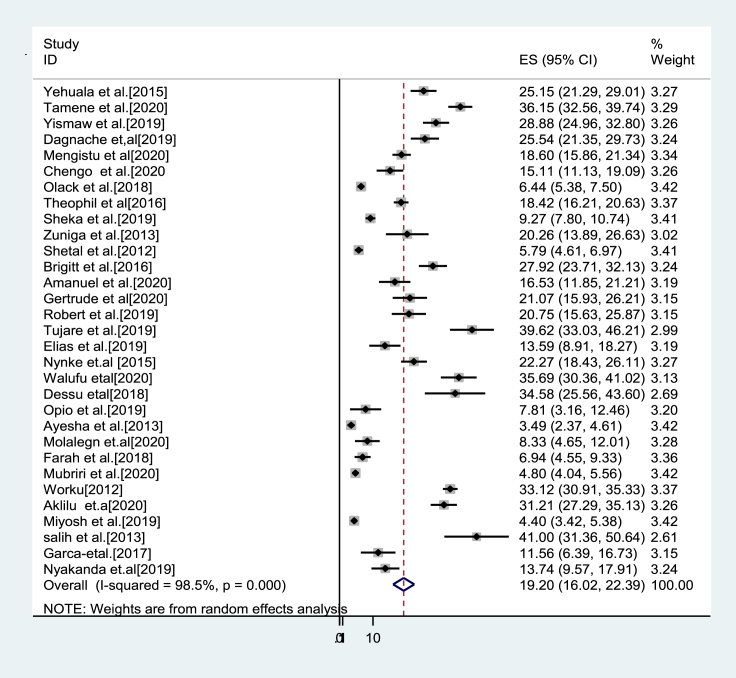
The pooled magnitude of mortality: Most of the studies (n = 31) have reported a mortality rate among preterm neonates. The mortality rate was ranged from highest reported from a study in Sudan 41% [25], and the least was from a study in Zambia 4.4% [24]. The pooled mortality rate among preterm neonates in East Africa using random-effects model analysis was found to be 19.2% (95%CI; 16.0–22.4); I2 = 98.5%; p < 0.001) (Table 2 &Figure 2).
Table 2.
The pooled mortality rate of preterm from 32 studies in East Africa from January 2012–December 2020.
| Study | ES [95% Conf. Interval] | % Weight | ||
|---|---|---|---|---|
| Yehuala et al. (2015) | 25.150 | 21.289 | 29.011 | 3.27 |
| Tamene et al. (2020) | 36.150 | 32.563 | 39.737 | 3.29 |
| Yismaw et al. (2019) | 28.880 | 24.960 | 32.800 | 3.26 |
| Abebe et al. (2019) | 25.540 | 21.346 | 29.734 | 3.24 |
| Mengistu et al. (2020) | 18.600 | 15.856 | 21.344 | 3.34 |
| Chengo et al. (2020) | 15.110 | 11.131 | 19.089 | 3.26 |
| Muchem et al. (2018) | 6.440 | 5.382 | 7.498 | 3.42 |
| Mmbaga et al. (2016) | 18.420 | 16.205 | 20.635 | 3.37 |
| Seid et al. (2019) | 9.270 | 7.800 | 10.740 | 3.41 |
| Zuniga et al. (2013) | 20.260 | 13.890 | 26.630 | 3.02 |
| Shah et al. (2012) | 5.790 | 4.614 | 6.966 | 3.41 |
| Ndelema et al. (2016) | 27.920 | 23.706 | 32.134 | 3.24 |
| Andegior et al. (2020) | 16.530 | 11.846 | 21.214 | 3.19 |
| Namazzi et al. (2020) | 21.070 | 15.935 | 26.205 | 3.15 |
| Moshiro et al. (2019) | 20.750 | 15.634 | 25.866 | 3.15 |
| Orsido et al. (2019) | 39.620 | 33.035 | 46.205 | 2.99 |
| Roro et al. (2019) | 13.590 | 8.906 | 18.274 | 3.19 |
| van den et al. (2015) | 22.270 | 18.428 | 26.112 | 3.27 |
| Egesa et al. (2020) | 35.690 | 30.359 | 41.021 | 3.13 |
| Dessu et al. (2018) | 34.580 | 25.564 | 43.596 | 2.69 |
| Opio et al. (2019) | 7.810 | 3.165 | 12.455 | 3.20 |
| Sania et al. (2013) | 3.490 | 2.373 | 4.607 | 3.42 |
| Dessu et al. (2020) | 8.330 | 4.645 | 12.015 | 3.28 |
| Farah et al. (2018) | 6.940 | 4.549 | 9.331 | 3.36 |
| Paul et al. (2020) | 4.800 | 4.036 | 5.564 | 3.42 |
| Gudayu (2012) | 33.120 | 30.905 | 35.335 | 3.37 |
| Endalam et al. (2020) | 31.210 | 27.290 | 35.130 | 3.26 |
| Miyosh et al. (2019) | 4.400 | 3.420 | 5.380 | 3.42 |
| Salih et al. (2013) | 41.000 | 31.357 | 50.643 | 2.61 |
| Garca- et al. (2017) | 11.560 | 6.386 | 16.734 | 3.15 |
| Nyakan et al. (2019) | 13.740 | 9.565 | 17.915 | 3.24 |
| D + L po ES | 19.203 | 16.019 | 22.387 | 100.00 |
Heterogeneity chi-squared = 1947.64 (d.f. = 30) p = 0.000.
I-squared (variation in ES attributable to heterogeneity) = 98.5%.
Test of ES = 0: z = 11.82 p = 0.000.
Figure 2.
Forest plot showing the pooled estimate of mortality among preterm in East Africa, from January 2012–December 2020.
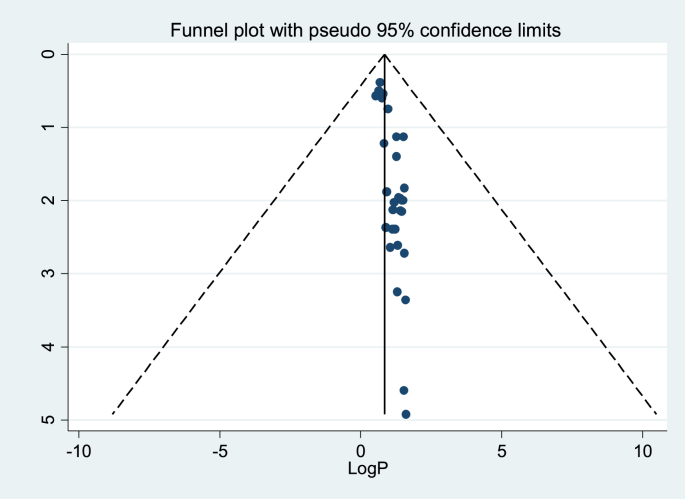
3.4. Publication bias
Egger's regression test value showed that there is a statistically significant publication bias (p < 0.000). Besides, a funnel plot showed an asymmetrical distribution which indicated the presence of publication bias (Table 3 & Figure 3).
Table 3.
Egger's test of the study involved 32 studies on preterm mortality in East Africa from January 2012–December 2020.
| Std_Eff | Coef. | Std. Err. | T | P > t | [95% Conf. Interval] | |
|---|---|---|---|---|---|---|
| Slope | .5574478 | .0524259 | 10.63 | 0.000 | .4502247 | .6646708 |
| Bias | .3443868 | .0500503 | 6.88 | 0.000 | .2420225 | .4467511 |
Figure 3.
Funnel plot to test the publication bias of the 32 studies, log proportion (x-axis) with a standard error of log proportion (y-axis).
Sensitivity analysis: The results of sensitivity analyses using the random effect model suggested that there is no single study that influenced the overall estimation significantly. Besides, the sensitivity analysis is displayed graphically (Table 4).
Table 4.
Summary of the sensitivity on mortality rate among preterm in Eastern Africa from January 2012–December 2020.
| Study omitted | Estimate | [95% Conf. Interval] | |
|---|---|---|---|
| Yehuala et al. (2015) | 18.99383 | 15.792513 | 22.195148 |
| Tamene et al. (2020) | 18.584991 | 15.508882 | 21.6611 |
| Yismaw et al. (2019) | 18.861759 | 15.687027 | 22.036489 |
| Abebe et al. (2019) | 18.983465 | 15.779122 | 22.187807 |
| Mengistu et al. (2020) | 19.223606 | 15.987537 | 22.459675 |
| Chengo et al. (2020) | 19.343513 | 16.096169 | 22.59086 |
| Muchem et al. (2018) | 19.698416 | 16.247002 | 23.149832 |
| Mmbaga et al. (2016) | 19.229933 | 15.993988 | 22.465878 |
| Seid et al. (2019) | 19.580208 | 16.217562 | 22.942852 |
| Zuniga et al. (2013) | 19.169939 | 15.939093 | 22.400784 |
| Shah et al. (2012) | 19.711596 | 16.308544 | 23.114645 |
| Ndelema et al. (2016) | 18.900389 | 15.710692 | 22.090086 |
| Andegior et al. (2020) | 19.292683 | 16.05171 | 22.533653 |
| Namazzi et al. (2020) | 19.141171 | 15.912008 | 22.370335 |
| Moshiro et al. (2019) | 19.151745 | 15.921703 | 22.381784 |
| Orsido et al. (2019) | 18.561008 | 15.38846 | 21.733557 |
| Roro et al. (2019) | 19.390844 | 16.14489 | 22.636799 |
| van den et al. (2015) | 19.095337 | 15.876361 | 22.314314 |
| Egesa et al. (2020) | 18.654905 | 15.487803 | 21.822006 |
| Dessu et al. (2018) | 18.77424 | 15.567248 | 21.981234 |
| Opio et al. (2019) | 19.582634 | 16.333736 | 22.83153 |
| Sania et al. (2013) | 19.783962 | 16.425308 | 23.142618 |
| Dessu et al. (2020) | 19.576698 | 16.319117 | 22.834278 |
| Farah et al. (2018) | 19.640337 | 16.356947 | 22.923727 |
| Paul et al. (2020) | 19.767809 | 16.256323 | 23.279293 |
| Gudayu (2012) | 18.614647 | 15.72389 | 21.505405 |
| Endamaw et al. (2020) | 18.777998 | 15.623595 | 21.9324 |
| Miyosh et al. (2019) | 19.764885 | 16.34239 | 23.18738 |
| salih et al. (2013) | 18.614325 | 15.418252 | 21.810398 |
| Garca- et al. (2017) | 19.453926 | 16.208763 | 22.699091 |
| Nyakan et al. (2019) | 19.389067 | 16.140238 | 22.637897 |
| Kolobo et al. (2020) | 19.203331 | 16.019365 | 22.387295 |
| Combined | 19.203331 | 16.019366 | 22.387296 |
3.5. Subgroup analysis of preterm mortality rate in Eastern Africa
The subgroup analysis was employed to estimate the pooled mortality rate by stratifying the studies into different categories. In this regard, the studies were stratified by country, study design, year of publication, and sample size. The mortality rate regarding by country including Ethiopia, Tanzania, Burundi, Eritrea, and Uganda was found to be 23.7%,14.3%,24.5%,10.9%, and 17.2% respectively. Besides, the mortality rate by study design was found to be 18.1%, 19.4%, and 19.7% for a prospective cohort, retrospective cohort, and cross-sectional study respectively. Based on the year of publication, the mortality rate was found to be 19.2% in the study conducted in between 2012-2018. Similarly, the mortality rate was 19.3% in the study period from 2019-2020. Moreover. the mortality rate was found to be19.2 and 17.9% with the sample size <350 and ≥350, respectively (Table 5).
Table 5.
Summary of subgroup analysis on mortality rate among preterm in Eastern Africa by country, design, year of publication, and sample size from January 2012–December 2020.
| Variable | Characteristics | Pooled prevalence % (95%CI) |
I2, (p-value) |
|---|---|---|---|
| Country | Ethiopia | 23.7 (17.2–30.3) | 98.2% (<0.001) |
| Tanzania | 14.3 (4.5–24.1) | 98.4% (<0.001) | |
| Burundi | 24.5 (17.0–31.9) | 74.1% (<0.049) | |
| Eritrea | 10.9 (0.4–21.4) | 94.7% (<0.001) | |
| Uganda | 17.2 (3.8–30.6) | 98.1% (<0.001) | |
| By study design | Prospective cohort | 18.1 (12.0–24.2) | 93.3% (<0.001) |
| Retrospective Cohort | 19.4 (14.1–24.8) | 99.0% (<0.001) | |
| Cross-sectional | 19.7 (14.3–25.1) | 98.3% (<0.001) | |
| By the year of publication | 2012–2018 | 19.2 (13.8–24.6) | 98.7% (<0.001) |
| 2019–2020 | 19.3 (15.0–23.7) | 98.3% (<0.001) | |
| By sample size | <350 | 19.2 (16.0–22.4) | 92.8% (<0.001) |
| ≥350 | 17.9 (13.9–22.0) | 99.0% (<0.001) |
3.6. The impact of respiratory distress syndrome on the survival of preterm neonates
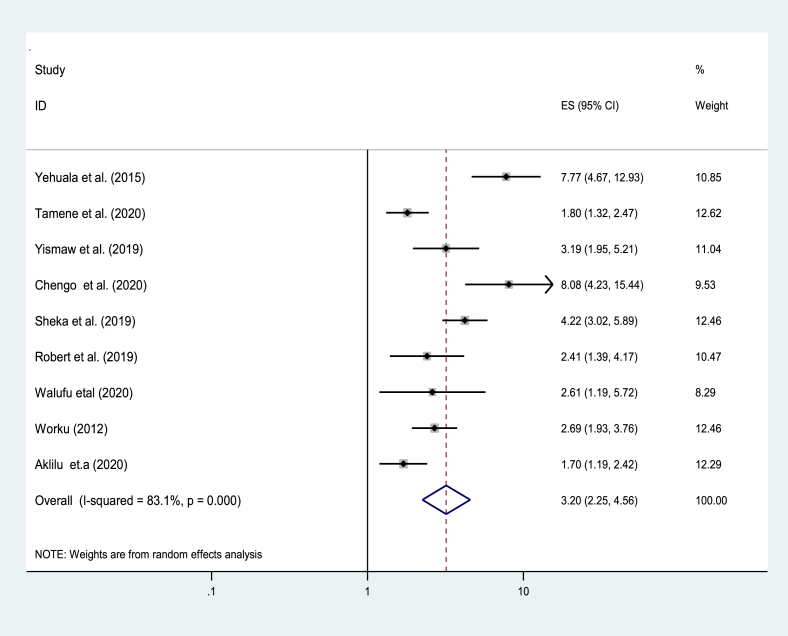
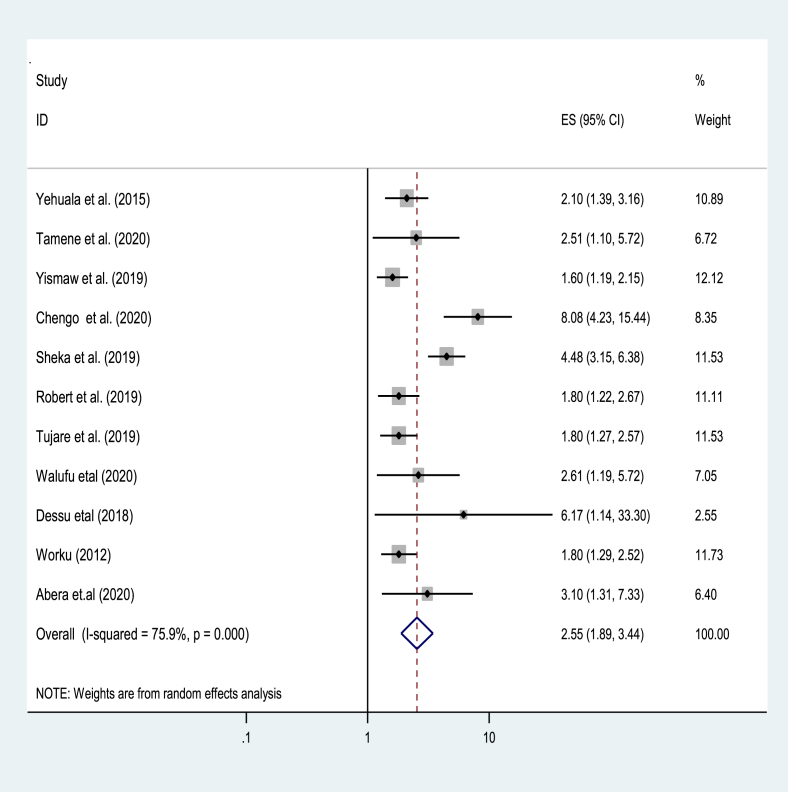
Seven studies from 32 studies reported that respiratory distress syndrome has an impact on the survival of preterm [33, 35, 37, 38, 46, 47, 49]. In this regard, the pooled effect of respiratory distress syndrome on the survival of preterm neonates showed that respiratory distress syndromes were 3.2 times increase risk of death in preterm neonates as compared to preterm neonates without respiratory distress syndrome [AOR = 3.2; 95% CI: 22, 4.6] (Figure 4).
Figure 4.
The pooled effect of respiratory distress syndrome on the pooled estimate of mortality among preterm in East Africa, from January 2012–December 2020.
3.7. The impact of birth asphyxia on the survival of preterm neonates
Nine studies from 32 studies reported that birth asphyxia had a significant association with preterm mortality [33, 35, 36, 37, 38, 39, 41, 47, 49]. In this concern, the pooled effect of birth asphyxia on the survival of preterm neonates showed that preterm presented with birth asphyxia nearly 2.6 times [AOR = 2.6; 95% CI: 1.9, 3.4] had a higher risk of death as compared to preterm without birth asphyxia (Figure 5).
Figure 5.
The pooled effects of birth asphyxia on the pooled estimate of mortality among preterm in East Africa, from January 2012–December 2020.
4. Discussion
In this systematic review and meta-analysis, the pooled mortality rate among preterm in East Africa was found to be 19.2%. This is consistent with the studies conducted in Asia, India, and Nigeria [48, 49, 51]. However, The mortality rate reported in this study is higher than studies reported from Germany [59], Iran [60], Australian [61], China [62], Libya [63], Mexico [64], Spain [65], and France 4.9% [66]. Moreover, the mortality rate of preterm associated with respiratory distress syndrome and asphyxia in this study is much higher than studies conducted in global, western Europe, Eastern Europe & Central Asia, and North America [67, 68]. In contrast, the mortality in this study was lower than the study conducted in Ghana, Brazil, and Pakistan [9, 69, 70].
The discrepancy might be due to sampling size, study settings, study period, and/or characteristics of study participants. Moreover, in developing countries, there is low skilled care at birth, antenatal care visits, low advancement of medical care, and, delayed health-seeking behavior than industrialized countries. Moreover, few women and newborns stay in the health facility for the recommended 24 h after birth in a resource limited-setting, which is the most critical time when complications can present [7, 71].
Subgroup analysis of mortality rate by country includes Ethiopia, Tanzania, Burundi, Eritrea, and Uganda was found to be 23.7%, 14.3%, 24.5%, 10.9%, and 17.2%. This variation can be explained by socioeconomic and cultural variation between the countries, the health status of the mother or father of the neonate, sample size, and study period.
Subgroup analysis regarding the study design showed that the mortality rate was found to be 18.1%, 19.4%, and 19.7% for the prospective cohort, retrospective cohort, and cross-sectional respectively. The reason could be a cross-sectional study including prevalence and incidence, while cohort studies only considered the incidence cases. Besides, subgroup analysis based on the year of publication advocated that the mortality rate was found to be 19.2% in studies conducted from 2012-2018. Similarly, it was 19.3% from 2019-2020. Moreover, the mortality rate regarding sample size subgroup analysis was found to be 19.2% and 17.9% with sample size <350 and ≥350 respectively.
The odds of mortality were higher nearly by three folds [AOR = 3.2; 95% CI: 22, 4.6] among preterm neonates with respiratory distress syndrome as compared to preterm neonates without respiratory distress syndrome. This is also supported by the findings in other developing countries [10, 35, 37, 46].
Respiratory distress syndrome is more fatal and prevalent in preterm neonates predominantly in resource-limited settings [72]. Besides, the treatment modalities, including antenatal corticosteroids, surfactants, and advanced respiratory care of the neonate are limited in Easter African countries [71]. The odds of mortality were increased 2.6 times among preterm with birth asphyxia when compared to their counterparts [AOR = 2.6; 95% CI: 1.9, 3.4]. Similar findings were also reported from previous studies [10, 73, 74]. Indeed, preterm neonates with birth asphyxia usually present hypoxic-ischemic encephalopathy, seizures, and cerebral palsy due to hypoxia. In this regard, respiratory distress syndrome and birth asphyxia.
4.1. Limitations of the study
In this study, only quantitative observational studies published in English were included in the analysis, and case series, case reports, and qualitative findings were excluded. Besides, we determined the subgroup analysis in different strata, heterogeneity was observed in some stratified groups. Thus, these are the limitations of the studies that the reader advised to be considered.
5. Conclusion
Preterm mortality was found to be unacceptably high in Eastern Africa continent.
Fortunately, the main causes of death were found to be respiratory distress syndrome and birth asphyxia which are preventable and treatable, hence early detection and timely management of this problem are highly recommended to improve preterm survival.
Declarations
Author contribution statement
Ermias Sisay Chanie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abebew Yeshambel Alemu, Demewoze Kefale Mekonen, Biruk Demissie Melese, Binyam Minuye, Habtamu Shimels Hailemeskel and Worku Necho Asferie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Wubet Alebachew Bayih, Tigabu Munye, Tekalign Amera Birlie, Abraham Tsedalu Amare, Nigusie Selomon Tibebue, Chalie Marew Tiruneh, Getasew Legas, Fisha Alebel Gebre Eyesus, and Demeke Mesfin Belay: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank all authors of the studies included in this systematic review and meta-analysis.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.What Is a Preterm Baby [Internet] 2020. https://www.who.int/news-room/q-a-detail/what-is-a-preterm-baby [cited 2020 Oct 16]. Available from:
- 2.Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.-B. Born Too Soon: the global epidemiology of 15 million preterm births. Reprod. Health. 2013 Nov 15;10(1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neonatal Mortality [Internet] UNICEF DATA; 2020. https://data.unicef.org/topic/child-survival/neonatal-mortality/ [cited 2020 Oct 16]. Available from: [Google Scholar]
- 4.WHO . WHO. World Health Organization; 2020. Neonatal Mortality [Internet]http://www.who.int/gho/child_health/mortality/neonatal_text/en/ [cited 2020 Oct 16]. Available from: [Google Scholar]
- 5.Tekelab T., Akibu M., Tagesse N., Tilhaun T., Yohanes Y., Nepal S. Neonatal mortality in Ethiopia: a protocol for systematic review and meta-analysis. Syst. Rev. 2019 Apr 26;8(1):103. doi: 10.1186/s13643-019-1012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mekonnen Y., Tensou B., Telake D.S., Degefie T., Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC Publ. Health. 2013 May 17;13(1):483. doi: 10.1186/1471-2458-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sankar M.J., Natarajan C.K., Das R.R., Agarwal R., Chandrasekaran A., Paul V.K. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. J. Perinatol. 2016 May;36(Suppl 1):S1–11. doi: 10.1038/jp.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn J.E., Blencowe H., Oza S., You D., Lee A.C.C., Waiswa P. Every Newborn: progress, priorities, and potential beyond survival. Lancet Lond. Engl. 2014 Jul 12;384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 9.Jehan I., Harris H., Salat S., Zeb A., Mobeen N., Pasha O. Neonatal mortality, risk factors and causes: a prospective population-based cohort study in urban Pakistan. Bull. World Health Organ. 2009 Feb;87(2):130–138. doi: 10.2471/BLT.08.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhe L.M., McClure E.M., Nigussie A.K., Mekasha A., Worku B., Worku A. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob. Health. 2019 Aug;7(8):e1130–e1138. doi: 10.1016/S2214-109X(19)30220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn J.E., Gravett M.G., Nunes T.M., Rubens C.E., Stanton C., the GAPPS Review Group Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010 Feb 23;10(1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.P M, H N, D K, E B, G N, N S Birthweight and gestational age-specific neonatal mortality rate in tertiary care facilities in Eastern Central Uganda. Health Sci. Rep. 2020 Oct 28;3(4):e196. doi: 10.1002/hsr2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimba E., Kinney M.V., Kachale F., Waltensperger K.Z., Blencowe H., Colbourn T. Newborn survival in Malawi: a decade of change and future implications. Health Pol. Plann. 2012 Jul;27(Suppl 3):iii88–103. doi: 10.1093/heapol/czs043. [DOI] [PubMed] [Google Scholar]
- 14.Sania A., Spiegelman D., Rich-Edwards J., Okuma J., Kisenge R., Msamanga G. The contribution of preterm birth and intrauterine growth restriction to infant mortality in Tanzania. Paediatr. Perinat. Epidemiol. 2014 Jan;28(1):23–31. doi: 10.1111/ppe.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PMNCH . WHO. World Health Organization; 2021. Born Too Soon: The Global Action Report on Preterm Birth [Internet]http://www.who.int/pmnch/knowledge/publications/preterm_birth_report/en/ [cited 2021 Jan 5]. Available from: [Google Scholar]
- 16.WHO . WHO. World Health Organization; 2021. Neonatal Cause-Of-Death Estimates for the Early and Late Neonatal Periods for 194 Countries: 2000–2013 [Internet]https://www.who.int/bulletin/volumes/93/1/14-139790/en/ [cited 2021 Jan 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walani S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020;150(1):31–33. doi: 10.1002/ijgo.13195. [DOI] [PubMed] [Google Scholar]
- 18.Newborns: Improving Survival and Well-Being [Internet] 2021. https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality [cited 2021 Jan 5]. Available from: [Google Scholar]
- 19.Glass H.C., Costarino A.T., Stayer S.A., Brett C.M., Cladis F., Davis P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015 Jun;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . WHO. World Health Organization; 2021. Newborn Death and Illness [Internet]https://www.who.int/pmnch/media/press_materials/fs/fs_newborndealth_illness/en/ [cited 2021 Jan 5]. Available from: [Google Scholar]
- 21.WHO . WHO. World Health Organization; 2020. The Worldwide Incidence of Preterm Birth: a Systematic Review of Maternal Mortality and Morbidity [Internet]https://www.who.int/bulletin/volumes/88/1/08-062554/en/ [cited 2020 Oct 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel Razeq N.M., Khader Y.S., Batieha A.M. The incidence, risk factors, and mortality of preterm neonates: a prospective study from Jordan (2012-2013) Turk. J. Obstet. Gynecol. [Internet] 2017;14(1):28–36. doi: 10.4274/tjod.62582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5558315/ Mar [cited 2020 Oct 16]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ethiopia: Mini Demographic and Health Survey 2019 - Key Indicators (English) [Internet] 2020. https://dhsprogram.com/publications/publication-pr120-preliminary-reports-key-indicators-reports.cfm [cited 2020 Oct 16]. Available from: [Google Scholar]
- 24.Miyoshi Y., Matsubara K., Takata N., Oka Y. Baby survival in Zambia: stillbirth and neonatal death in a local hospital setting. BMC Pregnancy Childbirth. 2019 Mar 12;19(1):90. doi: 10.1186/s12884-019-2231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salih S.A., Gadir YS A./ Early outcome of pre-term neonates delivered at Soba University Hospital. Sudan J. Paediatr. 2013;13(2):37–44. [PMC free article] [PubMed] [Google Scholar]
- 26.Children: Improving Survival and Well-Being [Internet] 2021. https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality [cited 2021 Jan 5]. Available from: [Google Scholar]
- 27.Foley K.L., Balázs P., Grenczer A., Rákóczi I. Factors associated with quit attempts and quitting among eastern Hungarian women who smoked at the time of pregnancy. Cent. Eur. J. Publ. Health. 2011 Jun 1;19(2):63–66. doi: 10.21101/cejph.a3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO . WHO. World Health Organization; 2021. Neonatal Mortality within 24 Hours of Birth in Six Low- and Lower-Middle-Income Countries [Internet]http://www.who.int/bulletin/volumes/94/10/15-160945/en/ [cited 2021 Jan 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Re: Meta-Analyses; what Exactly Does a High I2 Statistic Mean? [Internet] ResearchGate; 2020. https://www.researchgate.net/post/Re_meta-analyses_what_exactly_does_a_high_I2_statistic_mean [cited 2020 Nov 6]. Available from: [Google Scholar]
- 30.Bias in Meta-Analyses (Funnel Plots and Tests) - StatsDirect [Internet] 2020. https://www.statsdirect.com/help/meta_analysis/bias_detection.htm [cited 2020 Nov 6]. Available from: [Google Scholar]
- 31.Dessu S., Habte A., Mesele M. The Kaplan Meier estimates of mortality and its predictors among newborns admitted with low birth weight at public hospitals in Ethiopia. PloS One. 2020 Sep 11;15(9) doi: 10.1371/journal.pone.0238629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mengistu B.A., Yismaw A.E., Azene Z.N., Mihret M.S. Incidence and predictors of neonatal mortality among neonates admitted in Amhara regional state referral hospitals, Ethiopia: prospective follow up study. BMC Pediatr. 2020 Apr 1;20(1):142. doi: 10.1186/s12887-020-02031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seid S.S., Ibro S.A., Ahmed A.A., Akuma A.O., Reta E.Y., Haso T.K. Vol. 10. Dove Press; 2019. Causes and factors associated with neonatal mortality in Neonatal Intensive Care Unit (NICU) of Jimma University Medical Center, Jimma, South West Ethiopia [Internet] pp. 39–48.https://www.dovepress.com/causes-and-factors-associated-with-neonatal-mortality-in-neonatal-inte-peer-reviewed-article-PHMT (Pediatric Health, Medicine and Therapeutics). [cited 2021 Jan 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abebe T., Gebremariam M. Ababa University; 2019 Sep 13. Survival of Preterm Neonates and its Determinants in Teaching Hospitals of Addis. [Google Scholar]
- 35.Tamene A., Abeje G., Addis Z. Survival and associated factors of mortality of preterm neonates admitted to Felege Hiwot specialized hospital, Bahir Dar, Ethiopia. SAGE Open Med. 2020;8 doi: 10.1177/2050312120953646. 2050312120953646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolobo H.A., Chaka T.E., Kassa R.T. Determinants of neonatal mortality among newborns admitted to neonatal intensive care unit Adama, Ethiopia: a case–control study. J. Clin. Neonatol. 2019 Oct 1;8(4):232. [Google Scholar]
- 37.Yehuala S., Ayalew S., Teka Z. Survival analysis of premature infants admitted to Neonatal Intensive Care Unit (NICU) in Northwest Ethiopia using Semi-Parametric Frailty model. J. Biometrics Biostat. 2015;6(1) [Google Scholar]
- 38.Yismaw A.E., Gelagay A.A., Sisay M.M. Survival and predictors among preterm neonates admitted at University of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. Ital. J. Pediatr. 2019 Jan 7;45(1):4. doi: 10.1186/s13052-018-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orsido T.T., Asseffa N.A., Berheto T.M. Predictors of Neonatal mortality in Neonatal intensive care unit at referral Hospital in Southern Ethiopia: a retrospective cohort study. BMC Pregnancy Childbirth. 2019 Feb 28;19(1):83. doi: 10.1186/s12884-019-2227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roro E.M., Tumtu M.I., Gebre D.S. Predictors, causes, and trends of neonatal mortality at Nekemte Referral Hospital, east Wollega Zone, western Ethiopia (2010–2014). Retrospective cohort study. PloS One. 2019 Oct 9;14(10) doi: 10.1371/journal.pone.0221513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessu S., Gebremeskel F., Alemu G., Seman B. Southern Ethiopia; 2018. Survival Status and Predictors of Neonatal Mortality Among Neonates Who Were Admitted in Neonatal Intensive Care Unit at Arba Minch General Hospital. [Google Scholar]
- 42.Farah A.E., Abbas A.H., Ahmed A.T. Trends of admission and predictors of neonatal mortality: a hospital based retrospective cohort study in Somali region of Ethiopia. PloS One. 2018 Sep 14;13(9) doi: 10.1371/journal.pone.0203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endalamaw A., Minuye B., Alemu B., Alemu A.Y., Assefa N. 2019. Mortality of Preterm Neonates and its Predictors in the Northwest Part of Ethiopia: A Retrospective Cohort Study. [Google Scholar]
- 44.Gudayu T.W., Zeleke E.G., Lakew A.M. Vol. 10. Dove Press; 2020. Time to death and its predictors among neonates admitted in the intensive care unit of the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia [Internet] pp. 1–10.https://www.dovepress.com/time-to-death-and-its-predictors-among-neonates-admitted-in-the-intens-peer-reviewed-fulltext-article-RRN (Research and Reports in Neonatology). [cited 2021 Jan 12]. Available from: [Google Scholar]
- 45.Mmbaga B.T., Lie R.T., Olomi R., Mahande M.J., Kvåle G., Daltveit A.K. Cause-specific neonatal mortality in a neonatal care unit in Northern Tanzania: a registry based cohort study. BMC Pediatr. 2012 Aug 7;12(1):116. doi: 10.1186/1471-2431-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortality Rate and Associated Factors Among Preterm Babies Born in Moshi, north – Tanzania: A Prospective Cohort Study. 2019 Aug 4. https://www.researchsquare.com/article/rs-3165/v1 [cited 2021 Jan 12]; Available from: [Google Scholar]
- 47.Moshiro R., Perlman J.M., Mdoe P., Kidanto H., Kvaløy J.T., Ersdal H.L. Potential causes of early death among admitted newborns in a rural Tanzanian hospital. PloS One. 2019 Oct 2;14(10) doi: 10.1371/journal.pone.0222935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Namazzi G., Tumwine J.K., Hildenwall H., Ndeezi G., Mubiri P., Hanson C. Neurodevelopmental outcomes of preterm babies during infancy in Eastern Uganda: a prospective cohort study. Glob. Health Action [Internet] 2021;13(1) doi: 10.1080/16549716.2020.1820714. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7580792/ [cited 2021 Jan 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egesa W.I., Odong R.J., Kalubi P., Yamile E.A.O., Atwine D., Turyasiima M. Vol. 11. Dove Press; 2020. Preterm neonatal mortality and its determinants at a tertiary hospital in western Uganda: a prospective cohort study [Internet] pp. 409–420.https://www.dovepress.com/preterm-neonatal-mortality-and-its-determinants-at-a-tertiary-hospital-peer-reviewed-article-PHMT (Pediatric Health, Medicine and Therapeutics). [cited 2021 Jan 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opio C., Malumba R., Kagaayi J., Ajumobi O., Kamya C., Mukose A. Survival time and its predictors among preterms in the neonatal period post-discharge in Busoga region-Uganda June – July 2017. J. Interv. Epidemiol. Publ. Health [Internet] 2019 Sep 26;2(9) https://www.afenet-journal.net/content/article/2/9/full/ [cited 2021 Jan 5]. Available from: [Google Scholar]
- 51.Zuniga I., Van den Bergh R., Ndelema B., Bulckaert D., Manzi M., Lambert V. Characteristics and mortality of neonates in an emergency obstetric and neonatal care facility, rural Burundi. Public Health Action. 2013 Dec 21;3(4):276–281. doi: 10.5588/pha.13.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ndelema B., Van den Bergh R., Manzi M., van den Boogaard W., Kosgei R.J., Zuniga I. Low-tech, high impact: care for premature neonates in a district hospital in Burundi. A way forward to decrease neonatal mortality. BMC Res. Notes. 2016 Jan 16;9(1):28. doi: 10.1186/s13104-015-1666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah S., Zemichael O., Meng H.D. Factors associated with mortality and length of stay in hospitalised neonates in Eritrea, Africa: a cross-sectional study. BMJ Open. 2012 Jan 1;2(5) doi: 10.1136/bmjopen-2011-000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andegiorgish A.K., Andemariam M., Temesghen S., Ogbai L., Ogbe Z., Zeng L. Neonatal mortality and associated factors in the specialized neonatal care unit Asmara, Eritrea. BMC Publ. Health. 2020 Jan 6;20(1):10. doi: 10.1186/s12889-019-8118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Broek N., Ntonya C., Kayira E., White S., Neilson J.P. Preterm birth in rural Malawi: high incidence in ultrasound-dated population. Hum. Reprod. Oxf. Engl. 2005 Nov;20(11):3235–3237. doi: 10.1093/humrep/dei208. [DOI] [PubMed] [Google Scholar]
- 56.Muchemi O.M., Echoka E., Makokha A. Factors associated with low birth weight among neonates born at Olkalou District Hospital, Central Region, Kenya. Pan. Afr. Med. J. [Internet] 2015 May 2;20(108) doi: 10.11604/pamj.2015.20.108.4831. https://www.panafrican-med-journal.com/content/article/20/108/full/ [cited 2021 Jan 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.García-Basteiro A.L., Quintó L., Macete E., Bardají A., González R., Nhacolo A. Infant mortality and morbidity associated with preterm and small-for-gestational-age births in Southern Mozambique: a retrospective cohort study. PloS One. 2017 Feb 17;12(2) doi: 10.1371/journal.pone.0172533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nyakanda M.I., Madziyire M.G., Magwali T.L., Guzha B.T. The prevalence of preterm births and their perinatal outcomes in two Harare teaching hospitals: a prospective cross sectional survey. Cent. Afr. J. Med. 2019 Sep 6;65(1–3):5–9. [Google Scholar]
- 59.E J, A B, C G, T B, G H, Hd H. Mortality and major morbidity of very-low-birth-weight infants in Germany 2008-2012: a report based on administrative data. Front. Pediatr. [Internet] 2016 Mar 22;4:23. doi: 10.3389/fped.2016.00023. https://europepmc.org/article/pmc/4801886 [cited 2020 Oct 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haghighi L., Nojomi M., Mohabbatian B., Najmi Z. Survival predictors of preterm neonates: hospital based study in Iran (2010-2011) Iran J. Reprod. Med. [Internet] 2013 Dec;11(12):957–964. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3941403/ [cited 2020 Oct 17]. Available from: [PMC free article] [PubMed] [Google Scholar]
- 61.Abdel-Latif M.E., Nowak G., Bajuk B., Glass K., Harley D. Variation in hospital mortality in an Australian neonatal intensive care unit network. Arch. Dis. Child. Fetal Neonatal Ed. [Internet] 2018 Jul;103(4):F331–F336. doi: 10.1136/archdischild-2017-313222. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6047145/ [cited 2020 Oct 31]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H., Dai Q., Xu Y., Gong Z., Dai G., Ding M. Time trends and risk factor associated with premature birth and infants deaths due to prematurity in Hubei Province, China from 2001 to 2012. BMC Preg. Childbirth [Internet] 2015 Dec 10;15(1):329. doi: 10.1186/s12884-015-0767-x. [cited 2020 Oct 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neonatal-and-perinatal-mortality-rates-in-neonatal-intensive-care-unit-ofmisurata-teaching-hospital--Libya2013-2329-8790-1000194.pdf [Internet] https://www.longdom.org/open-access/neonatal-and-perinatal-mortality-rates-in-neonatal-intensive-care-unit-ofmisurata-teaching-hospital--libya2013-2329-8790-1000194.pdf [cited 2020 Nov 7]. Available from:
- 64.2018 - Neonatal Mortality and Associated Factors in newbo.Pdf [Internet] 2020. https://www.sap.org.ar/uploads/archivos/files_ao_lonareyes_i_8-1pdf_1514999751.pdf [cited 2020 Nov 7]. Available from: [Google Scholar]
- 65.García-Reymundo M., Demestre X., Calvo M.J., Ginovart G., Jiménez A., Hurtado J.A. Late preterm infants in Spain: experience of the 34–36 neonatal group. An. Pediatría Engl. Ed. [Internet] 2018 May 1;88(5):246–252. doi: 10.1016/j.anpedi.2017.05.006. http://www.sciencedirect.com/science/article/pii/S2341287918300528 [cited 2020 Nov 7]; Available from: [DOI] [PubMed] [Google Scholar]
- 66.Godeluck . 2020. 2019 - Mortality and Severe Morbidity of Very Preterm inf.Pdf [Internet]https://www.hal.inserm.fr/inserm-02454046/document [cited 2020 Nov 7]. Available from: [Google Scholar]
- 67.Table 3 Cause of Death in Periviable Neonates ≤500 G. 2021. https://www.nature.com/articles/s41598-020-59566-3/tables/3 [cited 2021 Apr 21]; Available from: [Google Scholar]
- 68.Neonatal Mortality [Internet] UNICEF DATA; 2021. https://data.unicef.org/topic/child-survival/neonatal-mortality/ [cited 2021 Apr 21]. Available from: [Google Scholar]
- 69.Santos S.L.D., Santos L.B., Campelo V., da Silva A.R.V., Santos S.L.D., Santos L.B. Factors associated with infant mortality in a Northeastern Brazilian capital. Rev. Bras. Ginecol. Obstet. 2016 Oct;38(10):482–491. doi: 10.1055/s-0036-1584686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Annan G.N., Asiedu Y. Vol. 2018. Hindawi; 2018. Predictors of neonatal deaths in Ashanti region of Ghana: a cross-sectional study [Internet] p. e9020914.https://www.hindawi.com/journals/aph/2018/9020914/ (Advances in Public Health). [cited 2021 Jan 5]. Available from: [Google Scholar]
- 71.Neal S., Falkingham J. Vol. 2014. Hindawi; 2014. Neonatal death and national income in developing countries: will economic growth reduce deaths in the first month of life? [Internet] p. e989485.https://www.hindawi.com/journals/ijpr/2014/989485/ (International Journal of Population Research). [cited 2021 Jan 5]. Available from: [Google Scholar]
- 72.Yadav S., Lee B., Kamity R. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Neonatal respiratory distress syndrome.http://www.ncbi.nlm.nih.gov/books/NBK560779/ [cited 2020 Nov 7]. Available from: [PubMed] [Google Scholar]
- 73.Ambient Air Pollutant PM10 and Risk of Preterm Birth in Lanzhou, China. Abstract - Europe PMC [Internet]; 2021. https://europepmc.org/article/pmc/pmc4526148 [cited 2021 Jan 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parappil H., Rahman S., Salama H., Al Rifai H., Parambil N.K., El Ansari W. Outcomes of 28+1 to 32+0 weeks gestation babies in the state of Qatar: finding facility-based cost effective options for improving the survival of preterm neonates in low income countries. Int. J. Environ. Res. Publ. Health. 2010 Jun;7(6):2526–2542. doi: 10.3390/ijerph7062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.