Abstract
Background
There are limited data on the occurrence, associations and outcomes of pericardial effusions and pericarditis on or after treatment with immune checkpoint inhibitors (ICIs).
Methods
This was a retrospective study at a single academic center that compared 2842 consecutive patients who received ICIs with 2699 age- and cancer-type matched patients with metastatic disease who did not receive ICI. A pericardial event was defined as a composite outcome of pericarditis and new or worsening moderate or large pericardial effusion. The endpoints were obtained through chart review and were blindly adjudicated. To identify risk factors associated with a pericardial event, we compared patients who developed an event on an ICI with patients treated with an ICI who did not develop a pericardial event. Cox proportional-hazard model and logistical regression analysis were performed to study the association between ICI use and pericardial disease as well as pericardial disease and mortality. An additional 6-week landmark analysis was performed to account for lead-time bias.
Results
There were 42 pericardial events in the patients treated with ICI (n=2842) over 193 days (IQR: 64–411), yielding an incidence rate of 1.57 events per 100 person-years. There was a more than fourfold increase in risk of pericarditis or a pericardial effusion among patients on an ICI compared with controls not treated with ICI after adjusting for potential confounders (HR 4.37, 95% CI 2.09 to 9.14, p<0.001). Patients who developed pericardial disease while on an ICI had a trend for increased all-cause mortality compared with patients who did not develop a pericardial event (HR 1.53, 95% CI 0.99 to 2.36, p=0.05). When comparing those who developed pericardial disease after ICI treatment with those who did not, a higher dose of corticosteroid pre-ICI (>0.7 mg/kg prednisone) was associated with increased risk of pericardial disease (HR 2.56, 95% CI 1.00 to 6.57, p=0.049).
Conclusions
ICI use was associated with an increased risk of development of pericardial disease among patients with cancer and a pericardial event on an ICI was associated with a trend towards increase in mortality.
Keywords: immunotherapy
Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target and inhibit negative immune regulators and thereby activate immune responses against tumor cells. The approved indications for ICIs are increasing rapidly, with ICIs currently indicated for the treatment of at least 16 cancer types with over 100 ongoing trials.1 2 The use of ICIs may lead to toxicities involving a range of organ systems collectively described as immune-related adverse events (irAEs).3 The best described cardiovascular irAE is myocarditis, which may be fulminant and is frequently fatal.4–6 However, evolving data suggest that cardiac toxicities occur beyond myocarditis.7 These expanded toxicities include arrhythmias, heart failure, and atherosclerosis-related cardiovascular events.8–10 Among patients with cancer, pericardial effusions often occur due to the cancer itself, but may also develop secondary to treatment with traditional cytotoxic chemotherapy, radiation therapy, or targeted therapies.11–14 There are limited data on the occurrence of pericarditis or pericardial effusions among patients on an ICI. There have been case reports describing pericardial disease in patients treated with ICI.15 16 Additionally, in a study leveraging irAEs reported to the WHO VigiBase, pericardial disease, defined as pericarditis and pericardial effusions, represented 0.36% of all reported toxicities; this risk of pericardial disease was more than threefold higher with an ICI and was more common among patients with lung cancer.17 While this methodologic approach benefits from the large sample size, interpretation is limited by the lack of a control group or descriptive demographics to allow for adjustment due to confounders. In this work, we aimed to add to the limited evidence base linking ICI therapy to pericardial disease. We were specifically interested in whether pericarditis or pericardial diseases were increased among those on an ICI compared with controls and aimed to identify the risk factors for pericardial disease or pericarditis among those on an ICI.
Methods
Study design, setting and population
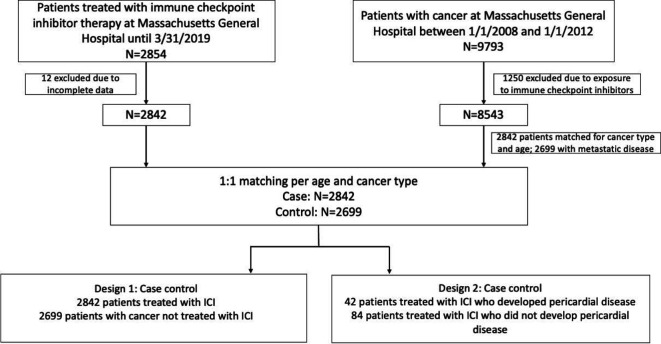
This was a single-center, retrospective cohort study. A total of 2842 consecutive patients who have received treatment with ICIs from July 2010 to March 2019 at Massachusetts General Hospital, Boston, Massachusetts were included in the study. Study design is outlined in figure 1. Two analyses were performed to evaluate the association between ICI use with pericardial disease and to identify risk factors associated with disease incidence. To evaluate whether the rates of pericardial disease (defined, as per prior studies, as pericarditis or pericardial effusion) were increased with an ICI, we performed a cohort study comparing incidences with a cohort of 2699 control patients with metastatic cancer who were not treated with ICIs (design 1).8 For this approach, we selected controls from all patients treated for cancer at Massachusetts General Hospital between January 1, 2008 and January 1, 2012. There were 9793 patients who met this criterion; of those, 1250 patients were excluded as they received ICIs later in their treatment course. Of the residual 8543 patients, we randomly selected controls with a 1:1 ratio to match cases for age and cancer type as in previous study using the same dataset.8 We further excluded patients without metastatic disease. In the second approach, we used a case–control study in which ICI recipients who developed pericardial disease were compared with those who did not (design 2), to identify the factors associated with the development of pericardial disease. Controls (2:1 to cases) were derived from randomly selected age- and cancer-type matched patients who did not develop pericarditis or pericardial effusion while on an ICI.
Figure 1.
Consolidated Standards of Reporting Trials diagram. ICI, immune checkpoint inhibitor.
Covariates
Demographics, medical history and medications were obtained through Research Patient Data Registry (RPDR). History of corticosteroid use, including timing and dose, was obtained through chart review in those treated with ICI. Recent corticosteroid was defined as corticosteroid treatment from 30 days prior to start of ICI treatment (online supplemental table 1). Oncologic data including chemotherapy history, radiation therapy, and specifics of ICI therapy were also derived from RPDR. Non-cardiac irAEs were captured via International Classification of Diseases, 9th and 10th Revisions diagnosis codes. A history of cardiovascular events was defined as a composite of myocardial infarction, stroke, or coronary revascularization.
jitc-2021-002771supp001.pdf (34.1KB, pdf)
Outcome measures: clinical study
The primary study endpoint was the occurrence of pericardial disease, defined as a composite of new or worsening pericardial effusion of at least moderate size or the occurrence of pericarditis. Pericarditis was defined as at least two out of four following criteria: typical chest pain, ECG changes consistent with pericarditis, pericardial rub on examination, new or worsening pericardial effusion. One individual who developed concurrent myocarditis and pericarditis was included. These events were searched through RPDR, confirmed through review of electronic medical records, and were blindly adjudicated by members of the study team.
Statistical analysis
Descriptive statistics were used to assess the distribution of variables. Categorical demographic variables were described as counts and percentage. Continuous, normally distributed demographic variables were summarized as mean and SD. Continuous, non-normally distributed variables were described as median and IQRs. For the cohort study of design 1 (figure 1), we performed Cox proportional-hazard models to evaluate the association of ICI treatment with pericardial disease. As pericardial disease is measured in the presence of competing risks, cause-specific HRs were used.18 Crude HRs with 95% CIs were calculated in addition to HRs adjusted for potential confounders. Individuals were right-censored if they did not experience pericardial disease by the end of follow-up, or were lost to follow-up, or died during the follow-up period. In survival analysis, patients who developed pericardial disease while on an ICI were compared with patients who did not develop pericardial disease; proportional-hazard analysis was performed to evaluate the association between pericardial disease and all-cause mortality. A landmark analysis was performed by excluding patients who died within six weeks of start date.19 For this, individuals were right-censored if they did not experience death by the end of follow-up or were lost to follow-up. For the case–control study of design 2 (figure 1), logistic regression was used to assess the relationship between predictors of interest and pericardial disease. Proportional-hazard analysis was performed to evaluate association between corticosteroid use and pericardial disease. All statistical analyses were performed using SAS V.9.4 (SAS Institute). P values of <0.05 were considered statistically significant and all testing was two sided.
Results
Baseline characteristics, comorbidities and oncological data
Cohort study of design 1: patients treated with ICI versus patients not treated with ICI
Demographics and clinical characteristics are summarized in table 1. Overall, those who were treated with ICI and those who were not treated with ICI were not different in terms of age and cancer type. Those who were treated with ICI were more likely to be male (57.4% vs 52.9%, p=0.001) and had lower body mass indices (27.0 vs 27.6, p=0.004). The ICI group had lower rates of hypertension (49.2% vs 54.5%, p<0.001), diabetes mellitus (16.4% vs 18.6%, p=0.005), prior myocardial infarction (4.7% vs 6.1%, p=0.02) and a history of any cardiovascular event (11.3% vs 13.0%, p=0.04). In contrast, those treated with ICI were more likely to have had a history of pericardial disease (1.3% vs 0.5%, p=0.02). Those who were on an ICI were more like to have received radiation therapy (20.7% vs 10.6%, p<0.001), 5-fluorouracil (10.4% vs 5.7%, p<0.001) and platinum-based chemotherapy (37.5% vs 21.7%, p<0.001). There was no difference between the two groups with regard to the use of anthracycline or tyrosine kinase inhibitors. Patients who received ICI were less likely to be on ACE inhibitor or angiotensin II receptor blocker (22.7% vs 27.0%, p<0.001), beta blockers (23.2% vs 33.1%, p<0.001), diuretics (24.8% vs 29.9%, p<0.001), and anti-platelet therapies including aspirin (aspirin: 21.4% vs 25.2%, p=0.002; other antiplatelet therapies: 2.4% vs 4.2%, p<0.001). Those who received an ICI were more likely to have received corticosteroids prior to the study period (17.8% vs 5.0%, p<0.001).
Table 1.
Baseline characteristics of patients treated with and without immune checkpoint inhibitors (ICIs)
| Patients treated with ICIs | Patients not treated with ICIs | P value | |
| Demographics | |||
| Number of patients | 2842 | 2699 | |
| Male | 1632/2842 (57.4%) | 1429/2699 (52.9%) | 0.001 |
| Age (years) | 64.4±13.1 | 64.0±13.2 | 0.33 |
| Race or ethnic group | <0.001 | ||
| White | 2475/2700 (91.7%) | 2443/2608 (93.7%) | |
| Hispanic | 29/2700 (1.1%) | 39/2608 (1.5%) | |
| Asian | 96/2700 (3.6%) | 43/2608 (1.7%) | |
| Black or African American | 57/2700 (2.1%) | 64/2608 (2.5%) | |
| Other | 43/2700 (1.6%) | 19/2608 (0.7%) | |
| Clinical variables | |||
| Body mass index (kg/m2) | 27.0±6.4 | 27.6±5.7 | 0.004 |
| Systolic blood pressure (mm Hg) | 127.6±18.6 | 127.4±16.9 | 0.72 |
| Diastolic blood pressure (mm Hg) | 75.7±9.7 | 74.7±10.3 | <0.001 |
| Baseline comorbidities | |||
| Hypertension | 1354/2752 (49.2%) | 1469/2695 (54.5%) | <0.001 |
| Diabetes mellitus | 432/2752 (16.4%) | 502/2695 (18.6%) | 0.005 |
| Smoking current or prior | 429/2753 (15.6%) | 398/2695 (14.8%) | 0.41 |
| History of any cardiovascular event | 320/2842 (11.3%) | 352/2699 (13.0%) | 0.04 |
| History of myocardial infarction | 134/1842 (4.7%) | 165/2699 (6.1%) | 0.02 |
| History of coronary revascularization | 121/2842 (4.3%) | 135/2699 (5.0%) | 0.20 |
| History of ischemic stroke | 83/2842 (2.9%) | 100/2699 (3.7%) | 0.11 |
| History of pericarditis or pericardial effusion | 36/2842 (1.3%) | 13/2699 (0.5%) | 0.02 |
| Chronic kidney disease | 325/2752 (11.8%) | 322/2695 (11.9%) | 0.90 |
| Cardiovascular medications | |||
| ACE inhibitor or angiotensin II receptor blocker | 612/2700 (22.7%) | 632/2340 (27.0%) | <0.001 |
| Beta blockers | 627/2700 (23.2%) | 775/2340 (33.1%) | <0.001 |
| Calcium channel blockers | 395/2700 (14.6%) | 355/2340 (15.2%) | 0.61 |
| Diuretics | 670/2700 (24.8%) | 699/2340 (29.9%) | <0.001 |
| Aspirin | 578/2700 (21.4%) | 589/2340 (25.2%) | 0.002 |
| Other anti-platelet therapies | 66/2700 (2.4%) | 97/2340 (4.2%) | <0.001 |
| Pre-ICI corticosteroid | 417/2338 (17.8%) | 67/1331 (5.0%) | <0.001 |
| Cancer types | |||
| Lung | 817/2842 (28.8%) | 815/2699 (30.2%) | 0.33 |
| Melanoma | 798/2842 (28.1%) | 654/2699 (24.2%) | |
| Head and neck | 343/2842 (12.1%) | 342/2699 (12.7%) | |
| Renal and genitourinary | 182/2842 (6.4%) | 182/2699 (6.7%) | |
| Breast | 118/2842 (4.2%) | 119/2699 (4.4%) | |
| Gastrointestinal | 116/2842 (4.1%) | 116/2699 (4.3%) | |
| Gynecologic | 110/2842 (3.9%) | 110/2699 (4.1%) | |
| Hepatobiliary | 101/2842 (3.6%) | 101/2699 (3.7%) | |
| Lymphoma | 82/2842 (2.9%) | 81/2699 (3.0%) | |
| Other | 99/2842 (3.5%) | 99/2699 (3.7%) | |
| Prior cancer treatment | |||
| Radiation therapy | 570/2752 (20.7%) | 285/2695 (10.6%) | <0.001 |
| 5-fluorouracil | 282/2719 (10.4%) | 148/2615 (5.7%) | <0.001 |
| Anthracyclines | 151/2719 (5.6%) | 153/2615 (5.9%) | 0.70 |
| Tyrosine kinase inhibitors | 61/2719 (2.2%) | 59/2615 (2.3%) | 1.00 |
| Platinum-based chemotherapy | 1019/2719 (37.5%) | 568/2615 (21.7%) | <0.001 |
| ICI type | |||
| Monotherapy | |||
| PD-L1 | 2139/2842 (75.3%) | ||
| CTLA-4 | 223/2842 (7.8%) | ||
| PD-1 | 282/2842 (9.9%) | ||
| CTLA-4 or PD-1 | 3/2842 (0.1%) | ||
| Combination therapy | |||
| CTLA-4 and PD-1 | 195/2842 (6.9%) | ||
| Number of cycles of ICI, no (IQR) | 5 (2–11) | ||
| Immune-mediated adverse events after ICI start | |||
| Gastrointestinal | 500/2745 (18.2%) | ||
| Skin | 429/2745 (15.6%) | ||
| Pulmonary | 189/2745 (6.9%) | ||
| Hepatic | 179/2745 (6.5%) | ||
| Endocrine | 175/2745 (6.4%) | ||
| Renal | 120/2745 (4.4%) | ||
| Neuromuscular | 98/2745 (3.6%) | ||
| Pancreas | 61/2745 (2.2%) | ||
| Any of the above adverse events | 1179/2745 (43.0%) | ||
| Immune-mediated adverse events treated with corticosteroid | |||
| Among the entire cohort | 734/2745 (17.8%) | ||
| Among those with immune-mediated adverse events | 734/1179 (62.3%) | ||
ACE, angiotensin-converting enzyme; CTLA-4, cytotoxic-T lymphocyte-associated protein 4; PD-1, programmed death protein 1; PD-L1, programmed death-ligand 1.
Treatment history with ICIs is also summarized in table 1. The most commonly used ICIs were monotherapy with antibodies against programmed death protein 1 (PD-1) (75.3%), followed by antibodies against programmed death-ligand 1 (PD-L1) (9.9%); the median number of treatment cycles was 5 (IQR: 2–11). Out of 2842 patients included in the study, 1179 developed irAEs and 734 of those patients received corticosteroids for those irAEs (62.3%).
Over a median follow-up time of 193 days (IQR: 64–411), 42 out of 2842 patients treated with ICIs developed pericardial disease with an incidence rate of 1.57 events per 100 person-years. Of these, 9 events were pericarditis (7 out of 9 had a pericardial effusion) and 33 events were new or worsening moderate to large pericardial effusions. Of the 40 patients with an effusion, 13 (32.5%) were diagnosed based on CT, 26 (65%) based on transthoracic echocardiography. One patient had a large pericardial effusion documented in the medical record based on imaging at an outside facility with no description of what imaging type was performed. Among the patients who developed effusions, 20 developed cardiac tamponade, and 15 received pericardiocentesis (5 patients did not receive pericardiocentesis given critical illness and goals of care that were not consistent with invasive procedures). Of those with pericardiocentesis, 8 patients had a malignant effusion on cytology. Among 12 patients with cell count data, three had neutrophilic predominance, three had lymphocytic predominance, three had unspecified cells and three had macrophage predominance. The median time from ICI initiation to onset of pericardial disease was 153 days (IQR: 42–288). Overall, the treatment of pericardial disease included observation and surveillance (n=23), corticosteroid therapy (n=2), colchicine (n=1), non-steroidal anti-inflammatory drugs (n=2) and pericardiocentesis (n=15). In comparison, out of 2699 patients not treated with ICIs, 21 developed pericardial disease over a median follow-up of 1841 days (IQR: 466–3993) with an incidence rate of 0.14 events per 100 person-years. Of the 21 events in patients who were not on an ICI, 3 developed pericarditis (1 out of 3 with evidence of a pericardial effusion) and 18 had a new or worsening pericardial effusion that was at least moderate or larger in size. Of the 19 patients with an effusion, 7 (36.8%) were detected on CT and 12 (63.2%) on echocardiography.
In a univariate Cox proportional-hazard model, patients who were treated with ICIs had a sevenfold increase in the risk of developing pericardial disease in comparison with patients not treated with ICI (HR 7.05, 95% CI 3.73 to 13.35, p<0.001, table 2 and figure 2). All baseline characteristics were examined via univariate Cox proportional-hazard model and factors associated with higher risks of developing pericardial disease included baseline corticosteroid use, history of lung cancer, history of prior pericardial disease and a history of platinum-based therapy (online supplemental table 2). There was a trend towards lower risk of pericardial disease among those with a history of cardiovascular events (online supplemental table 2). When adjusting for baseline risk factors including history of lung cancer, prior radiation, history of cardiovascular events, history of pericardial disease, history of platinum-based chemotherapy, and baseline corticosteroid use with a multivariate regression model, treatment with ICI was associated with a greater than fourfold increase in the risk of developing pericardial disease (HR 4.37, 95% CI 2.09 to 9.14, p<0.001, table 2). A history of lung cancer significantly increased the risk of developing pericardial disease (HR 5.46, 95% CI 2.96 to 10.10, p<0.001) but the associations between pericardial disease with baseline corticosteroid use and history of cardiovascular events did not reach statistical significance (p=0.06 and p=0.06, respectively, table 2). Given the difference in follow-up time between the patients who were treated with ICIs and those who were not on an ICI, we repeated the above analysis with follow-up time limited to 2 years with similar findings (online supplemental table 3).
Figure 2.
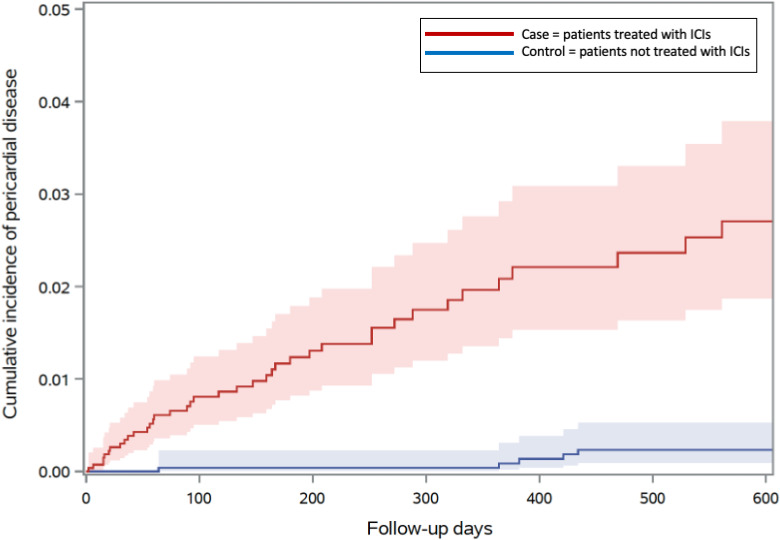
Cumulative incidence of pericardial disease in patients treated with ICI (n=2842) versus those not treated with ICI (n=2699), Gray’s test p<0.001. ICIs, immune checkpoint inhibitors.
Table 2.
Association with pericardial disease
| HR for pericardial disease | Χ2 | P value | |
| Treatment with ICI | 7.05 (3.73 to 13.35) | 36.02 | <0.001 |
| Multivariate model | |||
| Treatment with ICI | 4.37 (2.09 to 9.14) | 15.41 | <0.001 |
| Lung cancer | 5.46 (2.96 to 10.10) | 29.35 | <0.001 |
| Prior radiation | 0.75 (0.32 to 1.79) | 0.42 | 0.52 |
| Prior pericardial disease | 2.56 (0.62 to 10.72) | 1.67 | 0.20 |
| History of cardiovascular events | 0.15 (0.02 to 1.08) | 3.56 | 0.06 |
| Platinum-based therapy | 1.17 (0.64 to 2.14) | 0.27 | 0.60 |
| Pre-ICI corticosteroid use | 1.99 (0.97 to 4.09) | 3.50 | 0.06 |
Risks of pericardial disease in the 2842 patients treated with ICI were compared with reference group including 2699 age-type and cancer-type matched patients with metastatic disease who did not receive ICI treatment (design 1).
ICI, immune checkpoint inhibitor.
Among the 42 patients who were treated with ICI and developed pericardial disease, 26 patients (62%) died. In comparison, the mortality rate in the group that did not develop pericardial disease post-treatment was 41% (984 out of 2800 patients). Patients who developed pericardial disease while on an ICI (n=42) had an increased risk of death compared with those who did not develop pericardial disease (n=2800) (HR 1.53, 95% CI 1.04 to 2.26, p=0.03, table 3). Patients who were not on ICI did not have increased mortality with the development of a pericardial event (HR 1.21, 95% CI 0.71 to 2.05, p=0.48, table 3). The most common cause of death was disease progression in both groups (65.4% in patients on an ICI vs 42.9% in patients not on an ICI, online supplemental table 4). To address a potential lead-time bias, we performed a landmark analysis including patients who were alive at six weeks. In this, association between pericardial disease and death in patients on an ICI was reduced to a trend (HR 1.53, 95% CI 0.99 to 2.36, p=0.05, table 3).
Table 3.
Association between pericardial disease and mortality in those treated with and without ICI
| Deaths (n) | Time to death days (IQR) |
HR | P value | ||
| Patients treated with ICI (n=2842) | No pericardial disease (n=2800) | 1143 | 134 (54–276) | Reference | |
| Pericardial disease (n=42) | 26 | 148 (56–356) | 1.53 (1.04 to 2.26) | 0.03 | |
| Patients not treated with an ICI (n=2699) | No pericardial disease (n=2678) | 1401 | 843 (278– 1839) | Reference | |
| Pericardial disease (n=21) | 14 | 857 (642–2981) | 1.21 (0.71 to 2.05) | 0.48 | |
| Patients treated with ICI who were alive at 6 weeks (n=2621) | No pericardial disease (n=2584) | 927 | 187 (92–325) | Reference | |
| Pericardial disease (n=37) | 21 | 186 (83–256) | 1.53 (0.99 to 2.36) | 0.05 | |
| Patients not on an ICI who were alive at 6 weeks (n=2640) | No pericardial disease (n=2619) | 1342 | 893 (330–1882) | Reference | |
| Pericardial disease (n=21) | 14 | 857 (643–2981) | 1.26 (0.74 to 2.13) | 0.39 | |
ICI, immune checkpoint inhibitor.
Case–control design 2: patients treated with ICI who developed pericardial disease versus patients treated with ICI who did not develop pericardial disease
To identify potential risk factors for the development of pericardial disease, we compared patients who developed pericardial disease after ICI initiation (n=42) with age-type and cancer-type matched controls who did not develop pericardial disease after ICI initiation (n=84). Baseline characteristics including age, sex, and body mass index did not differ between those who developed pericardial disease while on an ICI versus those who did not have pericardial disease while on an ICI. Baseline cardiovascular risk factors and the use of cardiovascular medications were also similar between the two groups (table 4). Cancer type, the class of ICI used, combination ICI therapy, the number of cycles of ICI treatment received or the rate of other irAEs were not different between the two groups. The association between baseline corticosteroids with pericardial disease in proportional-hazard analysis (online supplemental table 2) was reduced to a trend when comparing those with and without a pericardial event while on an ICI (p=0.06, table 4). We further examined if dosing of corticosteroid was an effect modifier; a higher dose of corticosteroid (>0.7 mg/kg prednisone) but not lower dose corticosteroid (<0.7 mg/kg prednisone) was associated with the development of pericardial disease (p=0.049 vs p=0.70, table 5). There was no association between baseline corticosteroid use and all-cause mortality (table 5).
Table 4.
OR of baseline characteristics of patients who developed pericardial disease while on an ICI and age-type and cancer-type matched patients who did not develop pericardial disease while on an ICI
| Those who developed pericardial disease | Those who did not develop pericardial disease | OR | P value | |||
| Demographic, no (%) | ||||||
| Number of patients | 42 | 84 | ||||
| Male | 20 | 47.6 | 40 | 47.6 | 1.00 (0.48 to 2.10) | 1.00 |
| Age >65 | 20 | 47.6 | 40 | 47.6 | 1.00 (0.48 to 2.10) | 1.00 |
| Overweight (BMI 25–29.9) | 9 | 21.4 | 27 | 32.1 | 0.58 (0.24 to 1.37) | 0.21 |
| Obesity (BMI ≥30) | 8 | 19.1 | 13 | 15.5 | 1.29 (0.49 to 3.39) | 0.61 |
| Race or ethnic group, no (%) | ||||||
| White | 37 | 88.10 | 76 | 90.48 | 0.78 (0.24 to 2.55) | 0.68 |
| Asian | 5 | 11.90 | 2 | 2.38 | 5.54 (1.03 to 29.88) | 0.046 |
| Black or African American | 0 | 0.00 | 3 | 3.57 | N/A | |
| Hispanic | 0 | 0.00 | 0 | 0.00 | N/A | |
| Other | 0 | 0.00 | 1 | 1.19 | N/A | |
| Clinical variables, mean (SD) | ||||||
| BMI (kg/m2) | 26.61 | 4.48 | 26.24 | 4.80 | N/A | |
| Systolic blood pressure (mm Hg) | 124.43 | 13.33 | 130.44 | 19.54 | N/A | |
| Diastolic blood pressure (mm Hg) | 75.59 | 7.02 | 75.77 | 9.29 | N/A | |
| Cardiovascular risk factors, no (%) | ||||||
| Hypertension | 22 | 52.38 | 41 | 48.81 | 1.15 (0.55 to 2.42) | 0.71 |
| Diabetes mellitus | 4 | 9.52 | 17 | 20.24 | 0.42 (0.13 to 1.32) | 0.14 |
| Smoking current or prior | 6 | 14.29 | 22 | 26.19 | 0.47 (0.17 to 1.27) | 0.14 |
| Hyperlipidemia | 1 | 2.38 | 1 | 1.19 | 2.02 (0.12 to 33.19) | 0.62 |
| Cardiovascular diagnoses, no (%) | ||||||
| History of any cardiovascular event | 1 | 2.38 | 8 | 9.52 | 0.23 (0.028 to 1.928) | 0.18 |
| History of myocardial infarction | 0 | 0 | 6 | 7.14 | N/A | |
| History of coronary revascularization (PCI) | 1 | 2.38 | 4 | 4.76 | 1.17 (0.094 to 14.52) | 0.90 |
| History of ischemic stroke | 0 | 0.00 | 2 | 2.38 | N/A | |
| Cardiovascular medications, no (%) | ||||||
| ACE inhibitor or angiotensin II receptor blocker | 11 | 26.19 | 22 | 26.19 | 1.00 (0.43 to 2.32) | 1.00 |
| Beta blockers | 11 | 26.19 | 15 | 17.86 | 1.63 (0.67 to 3.96) | 0.28 |
| Calcium channel blockers | 3 | 7.14 | 9 | 10.71 | 0.64 (0.16 to 2.51) | 0.52 |
| Statins | 14 | 33.33 | 28 | 33.33 | 1.00 (0.46 to 2.19) | 1.00 |
| Diuretics | 11 | 26.19 | 25 | 29.76 | 0.84 (0.36 to 1.92) | 0.68 |
| Aspirin | 9 | 21.43 | 16 | 19.05 | 1.16 (0.46 to 2.90) | 0.75 |
| Other anti-platelet therapies | 0 | 0 | 2 | 2.38 | N/A | |
| Other medical comorbidities, no (%) | ||||||
| Chronic kidney disease | 6 | 14.29 | 9 | 10.71 | 1.39 (0.46 to 4.20) | 0.56 |
| Cancer types, no (%) | ||||||
| Lung | 27 | 64.29 | 57 | 67.86 | 0.85 (0.39 to 1.86) | 0.68 |
| Melanoma | 8 | 19.05 | 16 | 19.05 | 1.00 (0.39 to 2.57) | 1.00 |
| Head and neck | 2 | 4.76 | 4 | 4.76 | 1.00 (0.18 to 5.59) | 1.00 |
| Renal and genitourinary | 0 | 0 | 0 | 0 | N/A | |
| Breast | 1 | 2.38 | 0 | 0 | N/A | |
| Gastrointestinal | 0 | 0 | 0 | 0 | N/A | |
| Gynecologic | 2 | 4.76 | 4 | 4.76 | 1.00 (0.18 to 5.69) | 1.00 |
| Hepatobiliary | 0 | 0 | 1 | 1.19 | N/A | |
| Lymphoma | 0 | 0 | 0 | 0 | N/A | |
| Other | 2 | 4.76 | 2 | 2.38 | 2.05 (0.28 to 15.09) | 0.48 |
| Prior potentially cardiotoxic cancer therapies, no (%) | ||||||
| Radiation therapy | 6 | 14.29 | 13 | 15.48 | 0.91 (0.312 to 2.59) | 0.51 |
| 5-fluorouracil | 1 | 2.38 | 2 | 2.38 | 1.00 (0.088 to 11.35) | 1.00 |
| Anthracyclines | 0 | 0 | 0 | 0 | N/A | |
| Tyrosine kinase inhibitors | 1 | 2.38 | 1 | 1.19 | 2.02 (0.12 to 33.19) | 0.62 |
| Platinum-based therapy | 19 | 45.24 | 38 | 45.24 | 1.00 (0.48 to 2.11) | 1.00 |
| ICI type, no (%) | ||||||
| Programmed death-ligand 1 | 5 | 11.90 | 9 | 10.71 | 1.12 (0.35 to 3.60) | 0.84 |
| Cytotoxic-T lymphocyte-associated protein 4 | 2 | 4.76 | 4 | 4.76 | 1.00 (0.17 to 5.69) | 1.00 |
| Programmed death protein 1 | 33 | 78.57 | 66 | 78.57 | 1.00 (0.41 to 2.47) | 1.00 |
| Cytotoxic-T lymphocyte-associated protein 4 or programmed death protein 1 | 0 | 0 | 0 | 0 | N/A | |
| Combination therapy | ||||||
| Cytotoxic-T lymphocyte-associated protein 4/programmed death protein 1 | 2 | 4.76 | 5 | 5.95 | 1.54 (0.33 to 7.21) | 0.58 |
| Number of cycles of ICI, no (IQR) | 5 | (2–10) | 5 | (3–10) | N/A | |
| Greater than 5 cycles of treatment | 15 | 35.7 | 27 | 32.1 | 1.17 (0.54 to 2.56) | 0.68 |
| Immune-mediated adverse events after ICI start, no (%) | ||||||
| Gastrointestinal | 4 | 9.52 | 16 | 19.05 | 0.45 (0.14 to 1.44) | 0.18 |
| Skin | 7 | 16.67 | 8 | 9.52 | 1.90 (0.638 to 5.65) | 0.25 |
| Pulmonary | 6 | 14.29 | 6 | 7.14 | 2.17 (0.65 to 7.18) | 0.31 |
| Hepatic | 2 | 4.76 | 5 | 5.95 | 0.79 (0.15 to 4.25) | 0.78 |
| Endocrine | 5 | 11.90 | 4 | 4.76 | 2.70 (0.69 to 10.64) | 0.16 |
| Renal | 3 | 7.14 | 4 | 4.76 | 1.54 (0.33 to 7.21) | 0.51 |
| Neuromuscular | 1 | 2.38 | 0 | 0 | N/A | |
| Pancreas | 1 | 2.38 | 3 | 3.57 | 0.66 (0.066 to 6.53) | 0.72 |
| Any of the above adverse events | 22 | 52.38 | 34 | 40.28 | 1.618 (0.767 to 3.41) | 0.21 |
| Immune-mediated adverse events treated with corticosteroids, no (%) | ||||||
| Baseline corticosteroids (prior to ICI) | 7 | 16.67 | 5 | 5.96 | 3.16 (0.94 to 10.65) | 0.06 |
| Among those with immune-mediated adverse events | 14 | 33.33 | 17 | 20.24 | 1.97 (0.86 to 4.54) | 0.11 |
ACE, angiotensin-converting enzyme; BMI, body mass index; ICI, immune checkpoint inhibitor; N/A, not available; PCI, percutaneous coronary intervention.
Table 5.
Associations of pre-ICI corticosteroid use with pericardial disease and all-cause mortality
| N | Pericardial disease | All-cause mortality | |||
| Number | HR | Number | HR | ||
| Entire cohort | 126 | 42 | 65 | ||
| Corticosteroid prior to initiation of ICI | 12 | 7 | 2.11 (0.94 to 4.76), p=0.07 | 7 | 0.96 (0.44 to 2.12), p=0.93 |
| Higher dose (>0.7 mg/kg prednisone) | 7 | 5 | 2.56 (1.00 to 6.57), p=0.049 | 3 | 0.62 (0.19 to 1.98), p=0.42 |
| Lower dose (<0.7 mg/kg prednisone) | 5 | 2 | 1.33 (0.32 to 5.49), p=0.70 | 4 | 1.62 (0.59 to 4.48), p=0.35 |
Associations of pre-ICI corticosteroid use with pericardial disease and all-cause mortality in 42 patients who developed pericardial disease after ICI treatment vs 84 age-type and cancer-type matched control patients who did not develop pericardial disease after ICI treatment (design 2).
ICI, immune checkpoint inhibitor.
Discussion
In this large cohort study, the adjusted risk of pericardial disease was more than fourfold higher in patients treated with ICI compared with a control group not treated with ICI. The median time of onset of pericardial disease was approximately 6 months and those who developed pericardial disease on an ICI experienced a 1.5-fold increased risk of mortality. Finally, in patients treated with ICI, baseline high-dose corticosteroid use was associated with the occurrence of future pericardial disease.
Myocarditis is the most commonly described cardiac toxicity with ICI use.4–6 9 20 In contrast, despite a high reported mortality, data on the association between ICI use and pericardial disease are limited.17 Multiple case reports have highlighted the occurrence of pericardial effusion or tamponade in patients treated with an ICI.15 16 For example, three patients with non-small cell lung cancer treated with anti-PD-L1 therapy or combination therapy of anti-cytotoxic-T lymphocyte-associated protein 4 and anti-PD-1 monoclonal antibodies developed cardiac tamponade at 78, 98 and 131 days after ICI initiation.15 In a study leveraging the WHO VigiBase Database, a global database of individual case safety reports, pericardial disease comprised 0.36% of all ICI-related adverse events, representing a 3.8-fold higher risk of reporting pericardial disease with ICI use compared with the rest of the database. Notably, the majority (56%) of pericardial disease developed in those with lung cancer.17 Further, in a single-institution retrospective study of 3966 patients treated with ICI, compared with 82,157 patients who received a cancer treatment other than ICI, the rate of pericardiocentesis was 0.38% in the ICI patients and 0.11% in the non-ICI group (p<0.001).21 In our study, we identified a rate of 0.53% for pericardiocentesis and a rate of 1.5% for all pericardial events. Under-recognition and subsequent under-reporting of ICI cardiac toxicities has been noted in the past. For example, in a pharmacovigilance study, ICI myocarditis occurred in 0.41%.17 In contrast, in a multicenter cohort, the rate of myocarditis was reported to be closer to 1% and in a recent prospective study a rate of 1.4% was noted.9 22 We also considered whether the definitions of the endpoints were different. In the pharmacovigilance study, any pericardial effusion or pericarditis was applied as the endpoint. We restricted our analysis to only moderate or larger pericardial effusion or pericarditis; thus, if we had used the same definition, we would likely have had even more pericardial disease events, emphasizing overestimation in prior studies compared with that currently highlighted.
In our study, we observed that pericardial disease occurred 153 days (IQR: 42–288) after ICI initiation. However, the prior study using the WHO VigiBase noted a median onset of 30 days (IQR: 9–90).17 Prior case reports including a total of 12 patients reported the median time to onset, in terms of ICI cycles, as five cycles of treatment with an IQR of 1–35 cycles; with most ICI cycles 2–3 weeks in length, this corresponds to a median onset time of 14–105 days.16 The shorter time to onset observed in these studies might highlight the under-recognition and thus the under-reporting of pericardial disease associated with ICI use. As there is no definitive test for whether pericardial effusion or pericarditis is directly related to ICI use, pericardial disease that occurred later in the treatment course might be attributed to advanced malignancy rather than ICI use. This is likely for some but not all patients. In our study, cancer progression likely contributed to increased risk of pericardial disease in 22% of our cohort as these patients had malignant cells on pericardial fluid pathology. Our results are consistent with prior reports indicating that irAEs most commonly develop during the first few weeks to months after ICI therapy initiation, but can occur at later time points, and even after cessation of therapy.23 24
Although the exact mechanisms of cardiac irAEs remain incompletely understood, several theories exist. These include a loss of peripheral tolerance of self-reactive T cells due to ICI-mediated disruption of negative regulators of immune activation.23 Corticosteroids suppress immune activation via transcriptional and non-transcriptional pathways. Treatment with corticosteroids is recommended principally for grade 3 and 4, and occasionally grade 2 irAEs.3 25 They are also indicated in ICI-related cardiovascular toxicities.7 For example, for patients with cardiovascular toxicities, including myocarditis and vasculitis, high-dose corticosteroids are recommended.3 Unexpectedly, we observed that the use of corticosteroids prior to the initiation of ICIs increased the risk of subsequent pericardial disease. This may be mechanistically linked to the well-established increased risk of recurrence of non-ICI pericarditis after treatment with corticosteroids.11 26 27
This study has several limitations. First, this was a retrospective study which limited our ability to control for confounding. However, our cohort of patients treated with ICI is significantly larger than any prior publications on the same subject and the association of ICI use with pericardial disease persisted after adjusting for confounders. Second, our study did not investigate whether the increase in pericardial disease after ICI therapy reflected immune-mediated mechanisms or was due to progression of cancer. To try to control for cancer-specific factors, we matched controls for age, cancer type and only included those with metastatic disease; our control group was derived from years preceding the emergence of ICI therapy since patients not on an ICI once ICIs were available would likely have different baseline comorbidities and different severity of cancer. Patients who were on an ICI were followed up for a shorter period of time compared with those who did not receive ICI. There are several possible explanations for the difference: (1) patients who were on an ICI had treatment refractory disease, while those not on an ICI had less aggressive disease and the difference in follow-up time is a reflection of survival; (2) patients who were on an ICI were more likely to have been lost to follow-up due to the fact that some patients received cancer care elsewhere and were only referred for ICI treatment at a tertiary care center; (3) those on an ICI experienced pericardial events occurred much earlier at a higher rate, which again was reflected as shorter follow-up time; (4) patients who were not on ICI were captured between 2008 and 2012 per study design and thus were followed up for longer time. To account for (1) and (2), we performed competing risk analysis and patients who were lost to follow-up were right-censored. We also performed sensitivity analysis by including follow-up time only up to 2 years and showed similar findings.
In conclusion, ICI use among patients with cancer was associated with increased risk of pericardial disease and this increase in pericardial disease translated into a trend towards higher mortality. Our findings further highlight that pericardial disease is an under-recognized toxicity with ICI use. Increased awareness and close surveillance for pericardial disease are warranted for those treated with ICI, especially among those with lung cancer.
Acknowledgments
We gratefully acknowledge the Cardiovascular Imaging Research Center (CIRC) research team for providing feedback on the study design and interpretation. We also thank Dr. Hang Lee for statistical support. The CIRC is a combined effort from the Division of Cardiology and the Department of Radiology at Massachusetts General Hospital.
Footnotes
Twitter: @Gong2Jingyi, @zsofidrobni, @AZafar_MD
Contributors: TGN had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JG, TGN, ZDD, KLR and DZ drafted the study protocol and analysis plan. JG, ZDD, AZ, TQ, SH, HKG, VKR, CG, MES, RMA, LZ, AN, RS, KLR, DZ and TGN helped with the data acquisition, analysis and interpretation of the data. All authors contributed to the data collection, and the design, analysis, interpretation and drafting of the manuscript.
Funding: TGN is supported by a gift from A Curt Greer and Pamela Kohlberg; and grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (grants R01HL130539, R01HL137562, K24HL150238), and National Institutes of Health/Harvard Center for AIDS Research (grant P30 AI060354). RMA, VKR and AZ are supported by the US National Institutes of Health/National Heart, Lung, and Blood Institute (grant T32HL076136). LZ is supported by a gift from Mr Gordan Pugh and Dr Christine Olsen.
Competing interests: TGN has been a consultant to and received fees from Intrinsic Imaging, H3-Biomedicine, Amgen, Roche, and AbbVie, outside of the current work. TGN also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and grant funding from AstraZeneca. RS has been a consultant to Asana, Bristol Myers Squibb, Merck, Replimune; and received research funding from Amgen and Merck, all outside of the current work. KLR has received research funding from Project Datasphere. AN receives research support from Amgen and is a consultant for Takeda Oncology, AstraZeneca and Boehringer Ingelheim.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data used for the study are de-identified patient data.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020;12. 10.3390/cancers12030738. [Epub ahead of print: 20 03 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367. 10.1126/science.aax0182. [Epub ahead of print: 31 01 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drobni ZD, Zafar A, Zubiri L, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc 2020;9:e018306. 10.1161/JAHA.120.018306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–43. 10.1093/eurheartj/ehaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neilan TG, Rothenberg ML, Amiri-Kordestani L, et al. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist 2018;23:874–8. 10.1634/theoncologist.2018-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Zlotoff DA, Awadalla M, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–4. 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020;142:2299–311. 10.1161/CIRCULATIONAHA.120.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Souza M, Nielsen D, Svane IM, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 2021;42:1621–31. 10.1093/eurheartj/ehaa884 [DOI] [PubMed] [Google Scholar]
- 11.Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Rev Esp Cardiol 2015;68:1126. 10.1016/j.rec.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 12.Imazio M, Colopi M, De Ferrari GM. Pericardial diseases in patients with cancer: contemporary prevalence, management and outcomes. Heart 2020;106:569–74. 10.1136/heartjnl-2019-315852 [DOI] [PubMed] [Google Scholar]
- 13.Imazio M, Demichelis B, Parrini I, et al. Relation of acute pericardial disease to malignancy. Am J Cardiol 2005;95:1393–4. 10.1016/j.amjcard.2005.01.094 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen KM, Offersen BV, Nielsen HM, et al. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol 2017;40:255–61. 10.1002/clc.22634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altan M, Toki MI, Gettinger SN, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol 2019;14:1102–8. 10.1016/j.jtho.2019.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saade A, Mansuet-Lupo A, Arrondeau J, et al. Pericardial effusion under nivolumab: case-reports and review of the literature. J Immunother Cancer 2019;7:266. 10.1186/s40425-019-0760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem J-E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–89. 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ. The robust inference for the COX proportional hazards model. J Am Stat Assoc 1989;84:1074–8. 10.1080/01621459.1989.10478874 [DOI] [Google Scholar]
- 19.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awadalla M, Mahmood SS, Groarke JD, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint Inhibitor-Related myocarditis. J Am Coll Cardiol 2020;75:467–78. 10.1016/j.jacc.2019.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palaskas N, Morgan J, Daigle T, et al. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol 2019;123:1351–7. 10.1016/j.amjcard.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 22.Waliany S, Neal JW, Reddy S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC CardioOncol 2021;3:137–9. 10.1016/j.jaccao.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 24.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 25.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation 2008;118:667–71. 10.1161/CIRCULATIONAHA.107.761064 [DOI] [PubMed] [Google Scholar]
- 27.Artom G, Koren-Morag N, Spodick DH, et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi-centre all-case analysis. Eur Heart J 2005;26:723–7. 10.1093/eurheartj/ehi197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002771supp001.pdf (34.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data used for the study are de-identified patient data.