Abstract
Background: Currently, the association between sodium-glucose cotransporter 2 inhibitor (SGLT-2i) and malignancy risk has yet to be fully elucidated. This meta-analysis aimed to determine the relationship between SGLT-2i and malignancy risk in type 2 diabetes (T2D) patients.
Methods: We searched PubMed, ScienceDirect, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science to identify randomized controlled trials (RCTs) published up to August 2020 related to T2D patients treated with SGLT-2i vs. placebo or other hypoglycemic agents. The meta-analysis's primary outcome was malignancies' incidence, and the results were evaluated using risk ratio (RR) and 95% confidence interval (CI).
Results: We reviewed 76 articles (77 RCTs), comprising 45,162 and 43,811 patients in SGLT-2i and control groups, respectively. Compared with the control group, SGLT-2i had no significant association with augmented overall malignancy risk in T2D patients (RR = 1.05, 95% CI = 0.97–1.14, P = 0.20), but ertugliflozin may upsurge the risk (RR = 1.80, 95% CI = 1.02–3.17, P = 0.04). Compared with active hypoglycemic agents, dapagliflozin may increase (RR = 2.71, 95% CI = 1.46–6.43, P = 0.02) and empagliflozin may decrease (RR = 0.67, 95% CI = 0.45–0.98, P = 0.04) the malignancy risk. Compared with placebo, empagliflozin may exhibit risk increase (RR = 1.25, 95% CI = 1.05–1.49, P = 0.01), primarily in digestive system (RR = 1.48, 95% CI = 0.99–2.21, P = 0.05).
Conclusions: Our results proposed that in diverse comparisons, ertugliflozin and dapagliflozin seemed to increase the malignancy risk in T2D patients. Empagliflozin may cause malignancy risk reduction compared with active hypoglycemic agents but increase overall risk primarily in the digestive system compared with placebo. In short, the relationship between SGLT-2i and malignancy in T2D patients remains unclear.
Keywords: SGLT-2i, type 2 diabetes, malignant tumor, meta-analysis, RCT
Introduction
The incidence of diabetes rises annually, with about 463 million people living with the disease today and an estimated 578 million by 2030 (1). Poor blood sugar control in diabetics may cause blindness, kidney failure and lower limb amputations (2). In recent decades, type 2 diabetes (T2D) has become a global public health crisis with a severe impact on human health (3), accounting for about 90% of people with diabetes, and the second leading global death reason is cancer, representing one sixth (4). Diabetes is evidenced to associate with a potential malignancy, risk with diabetics having a 10–20% higher risk of malignancy than non-diabetics (5). Studies have demonstrated that T2D significantly increases specific cancers' risk, such as liver and pancreatic cancer (6). The tumor-causing mechanisms of T2D may include hyperinsulinemia, insulin resistance, hyperglycemia, oxidative stress, and chronic inflammation (7).
Sodium-glucose cotransporter 2 inhibitor (SGLT-2i) can selectively inhibit glucose renal reabsorption and increase urine glucose excretion, independent of insulin action to reduce the blood sugar level of drug (8). In addition to reducing blood sugar and weight and lowering blood pressure, studies have revealed that SGLT-2i is beneficial in slowing the progression of cardiovascular and kidney diseases (9, 10). Based on the above advantages, SGLT-2i has a great application prospect. Evidence proposes that SGLT-2i is not significantly associated with increased overall cancer risk (11). However, some SGLT-2i can increase or decrease certain cancers' risk, such as dapagliflozin, which may increase the risk of bladder cancer and breast cancer in T2D patients (12), and canagliflozin may reduce the risk of gastrointestinal cancers (11). Given the low incidence of malignant tumors and the long incubation period, a longer follow-up time is mandatory. Our meta-analysis was conducted to investigate SGLT-2i impact on malignancy incidence in T2D patients.
Methods
Search Strategy
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (13). The included randomized controlled trials (RCTs) were SGLT-2i in T2D patients. SGLT-2i such as dapagliflozin, canagliflozin, empagliflozin, tofogliflozin, ertugliflozin, luseogliflozin, and bexagliflozin were compared with placebo or other active hypoglycemic drugs to explore malignancy incidence in patients during follow-up.
After conducting a comprehensive and systematic search in PubMed, ScienceDirect, EMBASE, Cochrane Central Register of Controlled Trials and Web of Science databases, only articles published in English by August 2020 and before are retrieved. The search formula was as follows: (type 2 diabetes OR type 2 diabetes mellitus OR T2DM OR T2D) AND (sodium-glucose cotransporter 2 inhibitor OR SGLT-2i OR sotagliflozin OR janagliflozin OR dapagliflozin OR canagliflozin OR empagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR luseogliflozin OR sergliflozin OR licogliflozin OR remogliflozin OR bexagliflozin). Two researchers independently searched the articles, reviewed the title and abstract, viewed the full text, and selected the inclusion articles. To avoid missing negative results, the vocabulary related to malignant tumors was not limited. Instead, the full text (including Supplementary Materials) was scanned to extract relevant data.
Study Selection
Studies that fulfill the following criteria were encompassed in this meta-analysis: (1) participants were T2D patients; (2) RCTs compared the therapeutic efficacy of SGLT-2i with placebo or other hypoglycemic agents; (3) RCTs stated thorough information on malignancy occurrence; (4) the experimental group was provided SGLT-2i therapy (including single drug or combination drug), and the control group was supplied non-SGLT-2i therapy (placebo or other hypoglycemic drugs). Exclusion criteria: (1) non-RCTs, including review, observational research, cases; (2) patients with type 1 diabetes mellitus or healthy volunteers; (3) non-English language; (4) duplicate reports. When articles were repeatedly updated, the most recent or data-complete one was involved herein. After a systematic search, the two authors evaluated all chosen works, and the questionable studies were further discussed to resolve various opinions.
Data Extraction and Quality Assessment
Data extraction for studies included was performed independently by two researchers and reviewed by a third one. The extracted data comprise (1) study characteristics, such as author, region, year of publication, and follow-up time; (2) participant characteristics, including age, gender and subject inclusion criteria; (3) total number of malignant neoplasms, including primary, recurrent and metastatic cancers and classification of different types of tumors; (4) drug dose utilized by the experimental and control groups.
RCTs were assessed utilizing the Cochrane Collaboration's tool. The evaluation criteria include review and judgment of “low risk,” “high risk,” or “unclear risk” in terms of sequence generation, allocation concealment, blinding, incomplete outcome data, as well as selective outcome reporting and free of other bias. Any differences between the two researchers were resolved by discussion or by a third person review.
Statistical Analysis
The Review Manager 5.3 statistical analysis software was employed for the above analysis. The risk ratio (RR) and 95% confidence interval (CI) were deployed to evaluate the results. Heterogeneity of included studies was assessed employing I2 statistics, where I2 < 50%, indicating low heterogeneity, and a fixed-effect model was used; otherwise, a random-effect model was utilized. P ≤ 0.05 was statistically significant, and P < 0.10 within the suspected influence scope.
Results
Eligible Studies and Characteristics
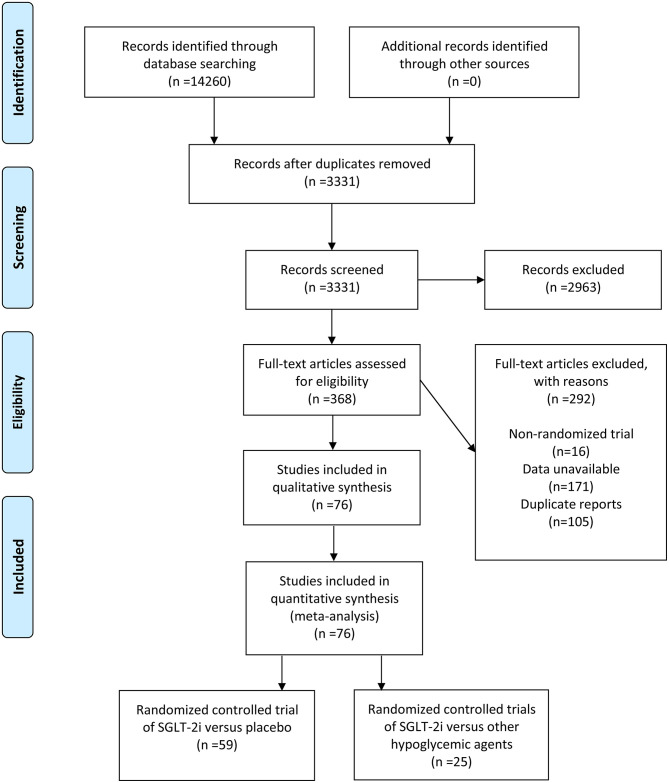
A total of 14,260 articles were initially searched, leaving 3,331 articles after deletion of duplicates. By reviewing title and abstract information, 2,963 articles were excluded. By evaluating 368 full-text articles, 292 articles were excluded, comprising 16 non-RCTs, 171 articles provided data without malignant tumors, and 105 repeated reports. Finally, 76 articles between 2012 and 2020 were chosen for this meta-analysis (14–89), with 77 RCTs. Typically, 59 articles existed on SGLT-2i vs. placebo [60 RCTs in total, one article containing 2 RCTs (64)], and 25 articles existed on SGLT-2i vs. other hypoglycemic agents (Figure 1).
Figure 1.
A schematic flow for selecting the articles included in this meta-analysis.
A total of 88,973 participants were included in 77 trials, of which 45,162 were randomly assigned to an intervention group comprising SGLT-2i, such as ertugliflozin, bexagliflozin, dapagliflozin, empagliflozin, canagliflozin, tofogliflozin, and luseogliflozin. A total of 43,811 patients were randomly assigned to a control group, including glucagon-like peptide-1 receptor agonist (GLP-1RA), dipeptidyl-peptidase-4 inhibitor (DPP-4i), sulfonylurea, thiazolidinedione, metformin, or placebo (part of the study was drug combination therapy). The RCTs were conducted in different countries, including Japan (13 RCTs), the United Kingdom (2 RCTs), and the United States (1 RCT). The remaining 61 were multi-national multicenter studies. Follow-up time ranged from 10 to 416 weeks, with 30 short-term studies (<52 weeks), 31 mid-term studies (52–104 weeks), and 16 long-term studies (≥104 weeks) (Table 1).
Table 1.
Characteristics of all the studies included in the meta-analysis.
| Author | Country | ClinicalTrials.gov identifier | Inclusion criteria | Follow-up time (week) | Therapeutic regimen | |
|---|---|---|---|---|---|---|
| Experiment | Control | |||||
| Allegretti et al. (14) | Multicenter | NCT02836873 | eGFR 30–59 ml/min/1.73 m2; no change treatment (≥8 weeks) | 26 | Bexagliflozin 20 mg | Placebo |
| Araki et al. (15) | Japan | NCT01368081 | HbA1c 7.0–10.0%; diet and exercise and monotherapy with an SU, biguanide, TZD, AGI, DPP-4i, or glinide; no change background antidiabetes therapies for 10 weeks; receive antihypertensive therapy for 4 weeks before randomization | 53 | Empagliflozin 10 mg/25 mg (SU) | MET (SU) |
| Empagliflozin 10 mg/25 mg (Biguanide) | ||||||
| Empagliflozin 10 mg/25 mg (TZD) | ||||||
| Empagliflozin 10 mg/25 mg (AGI) | ||||||
| Empagliflozin 10 mg/25 mg (DPP-4i) | ||||||
| Empagliflozin 10 mg/25 mg (Glinide) | ||||||
| Aronson et al. (16) | Multicenter | NCT01958671 | HbA1c 7.0–10.5%; not use OAD (≥8 weeks) or use OAD only once | 54 | Ertugliflozin 5 mg/15 mg | Placebo/MET |
| Bailey et al. (17) | Multicenter | NCT00528879 | MET ≥1,500 mg/day (≥8 weeks); C-peptide ≥1.0 ng/ml; Scr <1.50 mg/dl for men or <1.40 mg/dl for women | 102 | Dapagliflozin 2.5 mg/5 mg/10 mg + MET | Placebo + MET |
| Bailey et al. (18) | Multicenter | NCT00528372 | peptide ≥1.0 ng/ml; drug naive; Group 1: HbA1c ≥7% and ≤ 10%; Group 2: HbA1c ≥10.1% and ≤ 12.0% | 106 | Group 1: Dapagliflozin 2.5 mg/5 mg/10 mg AM | Group 1: Placebo AM & PM |
| Group 1: Dapagliflozin 2.5 mg/5 mg/10 mg PM | ||||||
| Group 2: Dapagliflozin 5 mg/10 mg AM | ||||||
| Barnett et al. (19) | Multicenter | NCT01164501 | HbA1c 7.0–10.0%; eGFR <90 ml/min; diet and exercise; pre-treated with any antidiabetic therapy and no change for 12 weeks | 52 | Empagliflozin 10 mg/25 mg | Placebo |
| Bolinder et al. (20) | Multicenter | NCT00855166 | HbA1c 6.5–8.5%; women 55–75 years (post-menopausal ≥5 years); men 30–75 years; FPG ≤ 13.2 mmol/l; body weight ≤ 120 kg; treatment with MET ≥1,500 mg/day (≥12 weeks) | 102 | Dapagliflozin 10 mg + MET | Placebo + MET |
| Brown et al. (21) | UK | NCT02956811 | HbA1c 6.5–10.0%; BP <145/90 mmHg; echocardiographic LV hypertrophy | 52 | Dapagliflozin 10 mg | Placebo |
| Böhm et al. (22) | Multicenter | NCT01131676 | CVD; eGFR of at least 30 ml/min/1.73 m2 | 260 | Empagliflozin 10 mg/25 mg | Placebo |
| Cahn et al. (23) | Multicenter | NCT01730534 | high CV risk; HbA1c 6.5–12.0%; creatinine clearance rate 60 ml/min | 270 | Dapagliflozin 10 mg | Placebo |
| Cefalu et al. (24) | Multicenter | NCT01031680 | cerebrovascular disease; hypertension | 52 | Dapagliflozin 10 mg | Placebo |
| Dagogo-Jack et al. (25) | Multicenter | NCT02036515 | HbA1c 7.0–10.5%; MET 1,500 mg/day and sitagliptin 100 mg/day for 8 weeks | 54 | Ertugliflozin 5 mg/15 mg | Placebo |
| Ferdinand et al. (26) | US | NCT02182830 | HbA1c 7.0–11.0%; hypertension; SBP 140–180 mmHg | 25 | Empagliflozin 10–25 mg | Placebo |
| Ferrannini et al. (27) | Multicenter | NCT00881530 | HbA1c 7.0–10.0%; drug naive or MET ≥1,500 mg/day or maximum tolerated dose ≥10 weeks | 79 | Empagliflozin 10 mg/25 mg | MET |
| Empagliflozin 10 mg/25 mg + MET | Sitagliptin 100 mg + MET | |||||
| Fioretto et al. (28) | Multicenter | NCT02413398 | HbA1c 7.0–11%; stable antidiabetic treatment; renal impairment: CKD 3A | 28 | Dapagliflozin 10 mg | Placebo |
| Forst et al. (29) | Multicenter | NCT01106690 | HbA1c 7–10.5%; pioglitazone or rosiglitazone and another AHA (MET); FPG <15 mmol/l | 52 | Canagliflozin 100 mg/300 mg | Placebo/Sitagliptin 100 mg |
| Fuchigami et al. (30) | Japan | NA | HbA1c 7.1–10.0%; not use any AHA within 8 weeks or only use MET | 24 | Dapaglifozin 5–10 mg | Sitagliptin 50–100 mg |
| Gallo et al. (31) | Multicenter | NCT02033889 | HbA1c 7.0–10.5%; MET (<8 weeks) or change diabetes regimen | 106 | Ertugliflozin 5 mg/15 mg | Placebo/Glimepiride |
| Grunberger et al. (32) | Multicenter | NCT01986855 | CKD 3; eGFR 30–60 ml/min/1.73 m2; HbA1c 7.0–10.5%; diet and exercise or with AHA monotherapy or combination therapy using other AHAs (INS and SU) | 54 | Ertugliflozin 5 mg/15 mg | Placebo |
| Hadjadj et al. (33) | Multicenter | NCT01719003 | HbA1c 7.5–12%; diet and exercise; drug-naive | 25 | Empagliflozin 12.5 mg/5 mg BID + MET 1,000 mg BID | MET 1,000 mg BID |
| Empagliflozin 12.5 mg/5 mg BID + MET 500 mg BID | MET 500 mg BID | |||||
| Empagliflozin 10 mg/25 mg QD | ||||||
| Halvorsen et al. (34) | Multicenter | NCT01377844 | HbA1c 7–10%; not treat with OAD: FPG <13.9 mmol/l; treat with OAD: FPG <13.3 mmol/l; antidiabetic or antihypertensive or antihyperlipidemic regimen must be stable (≥3 month);capillary blood glucose <13.9 mmol/l | 96 | Bexagliflozin 20 mg | Placebo |
| Halvorsen et al. (35) | Multicenter | NCT03115112 | MET ≥1,500 mg/day no change at 8 weeks; hypertension or hyperlipidemia medications must be stable (≥1 month) (if applicable) | 24 | Bexagliflozin 20 mg | Sitagliptin 100 mg |
| Halvorsen et al. (36) | Multicenter | NCT02390050 | naive or take one OAD in combination with diet and exercise; naive: HbA1c 7.0–8.5%; one OAD: HbA1c 6.5–8.5%; hypertension or hyperlipidemia medications must be stable (≥1 month) | 14 | Bexagliflozin 5 mg/10 mg/20 mg | Placebo |
| Haneda et al. (37) | Japan | NA | HbA1c 6.5–10.0%; eGFR 30–60 ml/min/1.73 m2; diet and exercise only or treat with 1 or 2 OHAs at a fixed dose >8 weeks | 52 | Luseogliflozin 2.5 mg | Placebo/Luseogliflozin |
| Henry et al. (38) | Multicenter | NCT00643851 | drug naive or with AHA for <24 weeks; C-peptide ≥1.0 ng/ml; Scr <1.50 mg/dl for men or <1.40 mg/dl for women | 28 | Dapagliflozin 5 mg + MET XR | MET XR |
| Dapagliflozin 5 mg | ||||||
| Hollander et al. (39) | Multicenter | NCT01999218 | HbA1c 7.0–9.0%; MET monotherapy 1,500 mg/day for 8 weeks or with an AHA | 106 | Ertugliflozin 5 mg/15 mg | Glimepiride |
| Ikeda et al. (40) | Multicenter | NCT00800176 | HbA1c 7.0–10.0%; diet and exercise or with stable MET (≥3 month) | 12 | Tofogliflozin 2.5 mg/5 mg/10 mg/20 mg/40 mg | Placebo |
| Inagaki et al. (41) | Japan | NCT01022112 | HbA1c 6.9–9.9%; diet and exercise; no change regimen for ≥8 weeks | 14 | Canagliflozin 50 mg/100 mg/200 mg/300 mg | Placebo |
| Inagaki et al. (42) | Japan | NCT01413204 | HbA1c 7.0–10.0%; diet and exercise for 55 days | 26 | Canagliflozin 100 mg/200 mg | Placebo |
| Jabbour et al. (43) | Multicenter | NCT00984867 | Not receive treatment, or receive MET, sitagliptin or vildagliptin or the combination of these; blood test: need additional therapy | 48 | Dapagliflozin 10 mg | Placebo |
| Jabbour et al. (44) | Multicenter | NCT02229396 | HbA1c 8.0–12.0%; MET ≥1,500 mg/day (≥2 months) | 104 | Dapagliflozin 10 mg + Placebo | Exenatide 2 mg + Placebo |
| Dapagliflozin 10 mg + Exenatide 2 mg | ||||||
| Januzzi et al. (45) | Multicenter | NCT01106651 | HbA1c 7–10.0%; no AHA or on a stable regimen of monotherapy or combination therapy; FPG <15 mmol/l; eGFR ≥50 ml/min/1.73 m2 | 104 | Canagliflozin 100 mg/300 mg | Placebo |
| Ji et al. (46) | Multicenter | NCT01095653 | HbA1c 7.5–10.5%; C-peptide level ≥1.0 ng/ml; drug naive | 28 | Dapagliflozin 5 mg/10 mg | Placebo |
| Ji et al. (47) | Multicenter | NCT02630706 | MET (≥1,500 mg/day): HbA1c 7.0–10.5%; MET <1,500 mg/day: HbA1c 7.5–11.0%; dual combination therapy with MET + SU, DDP-4i, meglitinide, or AGI: HbA1c 6.5–9.5% | 28 | Ertugliflozin 5 mg/15 mg | Placebo |
| Kadowaki et al. (48) | Japan | NCT01193218 | diet and exercise; drug naive HbA1c 7.0–10.0%; one AHA: HbA1c 6.5–9.0%; Visit 2: HbA1c 7.0–10% | 52 | Empagliflozin 5 mg/10 mg/25 mg/50 mg | Placebo |
| Kadowaki et al. (49) | Japan | NCT02354235 | HbA1c 7.0–10.5%; FPG ≤ 15 mmol/l; diet and exercise; teneligliptin 20 mg monotherapy once daily (≥8 weeks) | 26 | Canagliflozin 100 mg + Teneligliptin 20 mg | Placebo + Teneligliptin 20mg |
| Kaku et al. (50) | Japan | NCT00972244 | strictly/relatively treatment naive: HbA1c 7.0–10%; with single or two AHA: HbA1c ≤ 8%; FPG ≤ 13.3 mmol/l; C-peptide >1.0 ng/ml; Scr <1.5 mg/dl for men and <1.4 mg/dl for women; eGFR >60 ml/min/1.73 m2 | 16 | Dapagliflozin 1 mg/2.5 mg/ 5 mg/10 mg | Placebo |
| Kaku et al. (51) | Japan | NA | HbA1c 7.3–10.3%; diet and exercise only ≥8 weeks; percent change: HbA1c ≤ 10% and body weight <5% from the provisional registration visit to the final registration visit; no changes in antihypertensive medications; stop other AHAs ≥8 weeks | 26 | Tofogliflozin 10 mg/20 mg/40 mg | Placebo |
| Katakami et al. (52) | Japan | NA | HbA1c 6–9% with diet and exercise without being on drugs or on SGLT-2i in the past but without them ≥12 weeks; no change in the antidiabetic, antithrombotic, antihypertensive, anti-dyslipidemia medication ≥12 weeks | 104 | Tofoglifozin 20 mg | Conventional |
| Kawamori et al. (53) | Japan | NCT02453555 | diet and exercise and either treatment-naive or use one OAD for ≥12 weeks; treatment-naive: HbA1c 8.0–10.5%; OAD-pretreated (except linagliptin): HbA1c 7.5–10.5%; linagliptin-pretreated: HbA1c 7.5–10.0% | 53 | Empagliflozin 10 mg/25 mg + Linagliptin 5 mg | Placebo + Linagliptin 5 mg |
| Kohan et al. (54) | Multicenter | NCT00663260 | HbA1c 7.0–11.0%; eGFR 30–59 ml/min/1.73 m2; diet and exercise or with a regimen of any approved AHAs, no change for 6 weeks | 104 | Dapagliflozin 5 mg/10 mg | Placebo |
| Lavalle-González et al. (55) | Multicenter | NCT01106677 | HbA1c 7–10.5%; MET therapy ≥2,000 mg/day or ≥1,500 mg/day for ≥8 weeks; FPG <15 mmol/l at week −2 and fasting fingerstick glucose ≥6.1 mmol/l and <15 mmol/l on day 1 | 52 | Canagliflozin 100 mg | Placebo/Sitagliptin 100 mg |
| Canagliflozin 300 mg | Sitagliptin 100 mg | |||||
| Leiter et al. (56) | Multicenter | NCT01042977 | CVD; antidiabetic treatment (≥8 weeks); HbA1c 7.0–10.0% | 52 | Dapagliflozin 10 mg | Placebo |
| Leiter et al. (57) | Multicenter | NCT00968812 | MET ≥2,000 mg/day or ≥1,500 mg/day for ≥10 weeks; HbA1c 7.0–9.5%; FPG ≤ 15 mmol/l at week −2 | 104 | Canagliflozin 100 mg/300 mg | Glimepiride |
| Lewin et al. (58) | Multicenter | NCT01422876 | HbA1c 7.0–10.5%; diet and exercise with drug-naive or pre-treated with MET unchange for 12 weeks | 53 | Empagliflozin 10 mg/25 mg + Linagliptin 5 mg (MET) | Linagliptin 5 mg (MET) |
| Empagliflozin 10 mg/25 mg (MET) | ||||||
| Empagliflozin 10 mg/25 mg +Linagliptin 5 mg (Treatment Naive) | Linagliptin 5 mg (Treatment Naive) | |||||
| Empagliflozin 10 mg/25 mg (Treatment Naive) | ||||||
| Lingvay et al. (59) | Multicenter | NCT03136484 | HbA1c 7.0–10.5%; MET ≥1,500 mg/day or maximum tolerated dose for ≥90 days; eGFR ≥60 ml/min/1.73 m2 | 57 | Canagliflozin 300 mg | Semaglutide 1 mg |
| Mathieu et al. (60) | Multicenter | NCT01646320 | Stratum A: HbA1c 8.0–11.5%, MET ≥1,500 mg/day therapy alone (≥8 weeks) Stratum B: HbA1c 7.5–10.5%; MET ≥1,500 mg/day therapy and a DPP-4i (≥8 weeks); C-peptide ≥1.0 ng/ml | 52 | Dapagliflozin 10 mg + Saxagliptin 5 mg+ MET ≥1,500 mg | Placebo + Saxagliptin 5 mg + MET ≥1,500 mg |
| Matthaei et al. (61) | Multicenter | NCT01392677 | HbA1c 7.0–10.5%; MET ≥1,500 mg/day and a maximum tolerated dose of SU (≥8 weeks) | 52 | Dapagliflozin 10 mg + MET + SU | Placebo + MET + SU |
| Müller-Wieland et al. (62) | Multicenter | NCT02471404 | HbA1c 7.5–10.5%; MET ≥1,500 mg/day (≥8 weeks); C-peptide ≥1.0 ng/ml; FPG ≤ 15 mmol/l | 52 | Dapagliflozin 10 mg | Glimepiride 1 mg/2 mg/4 mg |
| Saxagliptin 5 mg + Dapagliflozin 10 mg | ||||||
| Nauck et al. (63) | Multicenter | NCT00660907 | HbA1c 6.5–10%; FPG ≤ 15 mmol/l; C-peptide ≥1.0 ng/ml; MET or MET plus one other OAD, administer up to half-maximal dose (≥8 weeks) | 208 | Dapagliflozin 2.5 mg/5 mg/ 10 mg + MET | Glipizide 5 mg/10 mg/20 mg+ MET |
| Oshima et al. (64) | Multicenter | NCT01032629 | HbA1c 7.0–10.5%; ≥30 years with history of CV event, or ≥50 years old with high risk of CV events; not on diabetes drug therapy or on therapy with any approved class of diabetes drugs | 416 | Canagliflozin 100 mg/300 mg | Placebo |
| Oshima et al. (64) | Multicenter | NCT01989754 | HbA1c 7.0–10.5%; ≥30 years with history of CV event, or ≥50 years old with high risk of CV events; not on AHA therapy, or on AHA monotherapy, or combination AHA therapy | 156 | Canagliflozin 100–300 mg | Placebo |
| Perkovic et al. (65) | Multicenter | NCT02065791 | HbA1c 6.5–12.0%; eGFR 30–90 ml/min/1.73 m2; maximum tolerated labeled daily dose of an ACEi or ARB (≥4 weeks); UACR >300 mg/g and ≤ 5,000 mg/g | 239 | Canagliflozin 100 mg | Placebo |
| Pratley et al. (66) | Multicenter | NCT02099110 | HbA1c 7.5–11.0%; MET ≥1,500 mg/day (≥8 weeks) | 54 | Ertugliflozin 5 mg/15 mg | Sitagliptin 100 mg |
| Ertugliflozin 5 mg/15 mg + Sitagliptin 100 mg | ||||||
| Qiu et al. (67) | Multicenter | NCT01340664 | HbA1c 7.0–10.5%; MET ≥2,000 mg/day or ≥1,500 mg/day (≥8 weeks); FPG <15 mmol/l at week −2; fasting fingerstick glucose 6.1–15 mmol/l on day 1 | 18 | Canagliflozin 50 mg/150 mg BID | Placebo |
| Ridderstråle et al. (68) | Multicenter | NCT01167881 | HbA1c 7.0–10.0%; MET IR ≥1,500 mg/day, maximum tolerated dose, or maximum dose according to the local label (≥3 months) | 208 | Empaglifozin 25 mg + MET | Glimepiride 1–4 mg + MET |
| Rodbard et al. (69) | Multicenter | NCT02863328 | HbA1c 7.0–10.5 %; MET ≥1,500 mg/day or maximum tolerated (≥3 months) | 57 | Empagliflozin 25 mg | Semaglutide 14 mg |
| Roden et al. (70) | Multicenter | NCT01289990 | HbA1c 7.0–11%; diet and exercise, drug-naive or pre-treated with pioglitazoneor with MET or pre-treated with MET or MET plus SU at 12 weeks | 77 | Empagliflozin 10 mg (Drug Naive) | Placebo (Drug Naive) |
| Empagliflozin 25 mg (Drug Naive) | Sitagliptin 100 mg (Drug Naive) | |||||
| Empagliflozin 10 mg/25 mg (Pioglitazone) | Placebo (Pioglitazone) | |||||
| Empagliflozin 10 mg/25 mg (MET) | Placebo (MET) | |||||
| Empagliflozin 10 mg/25 mg (MET + SU) | Placebo (MET + SU) | |||||
| Rosenstock et al. (75) | Multicenter | NCT00683878 | HbA1c 7.0–10.5%; C-peptide ≥1.0 ng/ml | 48 | Dapagliflozin 5 mg/10 mg + Pioglitazone | Placebo + Pioglitazone |
| Rosenstock et al. (74) | Multicenter | NCT00749190 | MET ≥1,500 mg/day or with one other OAD: HbA1c 6.5–9.0%; MET only: HbA1c 7.0–10%; HbA1c 7.0–10.0% at start of placebo run-in period | 13 | Empagliflozin 1 mg/5 mg/10 mg/25 mg/50 mg | Placebo |
| Sitagliptin 100 mg | ||||||
| Rosenstock et al. (72) | Multicenter | NCT01306214 | HbA1c 7.5–10%; diet and exercise; treatment with MDI of INS or with MET; MET ≥1,500 mg/day or maximum tolerated dose | 52 | Empagliflozin 10 mg/25 mg | Placebo |
| Rosenstock et al. (73) | Multicenter | NCT01011868 | HbA1c 7.0–10%; basal INS or with MET and/or SU | 82 | Empagliflozin 10 mg/25 mg | Placebo |
| Rosenstock et al. (71) | Multicenter | NCT01809327 | Diet and exercise; not on AHA therapy (≥3 months) fingerstick HbA1c 7–12.5%; HbA1c 7.5–12%; FPG ≤ 16.7 mmol/l; fasting fingerstick glucose ≥6.7 mmol/l | 30 | Canagliflozin 100 mg/300 mg | MET XR |
| Canagliflozin 100 mg/300 mg + MET XR | ||||||
| Ross et al. (76) | Multicenter | NCT01649297 | HbA1c 7.0–10%; diet and exercise; MET ≥1,500 mg/day (≥3 months) | 17 | Empagliflozin 12.5 mg BID/25 mg QD | Placebo |
| Empagliflozin 5 mg BID/10 mg QD | ||||||
| Schernthaner et al. (77) | Multicenter | NCT01137812 | HbA1c 7–10.5%; MET ≥2,000 mg/day or ≥1,500 mg/day and SU; FPG <16.7 mmol/l | 52 | Canagliflozin 300 mg | Sitagliptin 100 mg |
| Scott et al. (78) | Multicenter | NCT02532855 | HbA1c 7.0–9.5%; eGFR 60–90 ml/min/1.73 m2; MET (≥1,500 mg/day) or with a SU for ≥8 weeks; fasting fingerstick glucose: 6.1–14.4 mmol/l | 26 | Dapagliflozin 10 mg | Sitagliptin 100 mg |
| Seino et al. (79) | Japan | NA | HbA1c 6.9–10.5%, FPG ≥126 mg/dl at weeks −6 or −2; maximum change in body weight of 3.0% between weeks −6 and −2; diet therapy ≥6 weeks | 12 | Luseogliflozin 1 mg/2.5 mg/5 mg/10 mg | Placebo |
| Singh et al. (80) | UK | NCT02397421 | NYHA functional class I-III HF with prior echocardiographic evidence of LVSD; furosemide ≤ 80 mg daily or equivalent loop diuretic; eGFR ≥45 ml/min/1.73 m2; stable HF symptoms with therapy and no hospitalized for HF (≥3 months) | 52 | Dapagliflozin 10 mg | Placebo |
| Sone et al. (82) | Japan | NCT02589639 | diet and exercise; INS with or without 1 OAD (≥3 months); C-peptide >0.5 ng/ml; INS alone: HbA1c 7.5–10.0%; INS with 1 OAD: HbA1c 7.0–9.5%, placebo run-in period HbA1c 7.5–10.0% | 53 | Empagliflozin 10 mg/25 mg | Placebo |
| Stenlof et al. (83) | Multicenter | NCT01081834 | Main Study: HbA1c 7–10%, FPG <15 mmol/l; High Glycemic Cohort Sub-study: HbA1c 10–12%, FPG ≤ 19.44 mmol/l | 52 | Main Study: Canagliflozin 100 mg/300 mg | Main Study: Placebo/Sitagliptin 100 mg |
| High Glycemic Sub-study: Canagliflozin 100 mg/300 mg | ||||||
| Strojek et al. (84) | Multicenter | NCT00680745 | HbA1c 7.0–10%; SU monotherapy dose at least half the maximal recommended dose (≥8 weeks) | 48 | Dapagliflozin 2.5 mg/5 mg/10 mg + Glimepiride | Placebo + Glimepiride |
| Søfteland et al. (81) | Multicenter | NCT01734785 | HbA1c 8.0–10.5%; diet and exercise; MET IR ≥1,500 mg/day, maximum tolerated dose, or maximum dose according to the local label (≥3 months) | 25 | Empagliflozin 10 mg/25 mg | Placebo |
| Townsend et al. (85) | Multicenter | NCT01939496 | HbA1c 7.0–10%; use 1–3 anti-hyperglycemic agents (no INS); seated office SBP: 130–160 mmHg, seated office DBP ≥70 mmHg; use 1–3 anti-hypertensive agents (no loop diuretics) ≥5 weeks | 10 | Canagliflozin 100 mg/300 mg | Placebo |
| Wilding et al. (86) | Multicenter | NCT01106625 | HbA1c 7.0–10.5%; MET and SU; FPG <15 mmol/l | 52 | Canagliflozin 100 mg/300 mg | Placebo |
| Wilding et al. (87) | Multicenter | NCT00673231 | HbA1c 7.5–10.5%; INS ≥30 units/day (≥8 weeks) or with up to 2 OADs; MET ≥1,500 mg/day or maximum tolerated dose and other OADs on at least half the daily maximum dose; diet and exercise | 104 | Dapagliflozin 2.5 mg/5 mg/10 mg | Placebo |
| Yale et al. (88) | Multicenter | NCT01064414 | HbA1c 7.0–10.5%; eGFR 30–50 ml/min/1.73 m2; not on AHA therapy or AHA monotherapy or combination therapy; CKD 3, have generally stable renal function | 52 | Canagliflozin 100 mg/300 mg | Placebo |
| Yang et al. (89) | Multicenter | NCT02096705 | HbA1c 7.5–11.0% during screening/enrolment; HbA1c 7.5–10.5% 14 days prior to randomization; injectable INS ≥20 IU (≥8 weeks) | 28 | Dapagliflozin 10 mg | Placebo |
NA, not available; AGI, α-glucosidase inhibitor; DPP-4i, dipeptidyl-peptidase-4 inhibitor; SU, sulphonylurea; TZD, thiazolidinedione; SGLT-2i, sodium-glucose co-transporter 2 inhibitor; MET, metformin; INS, insulin; CKD, chronic kidney disease; AHA, anti-hyperglycemic agent; OHA, oral hypoglycemic agent; OAD, oral anti-diabetic drug; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; QD, once daily; BID, twice daily; XR, extended release; IR, immediate release; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; UACR, urine albumin to creatinine ratio; Scr, serum creatinine; MDI, multiple daily injection; NYHA, New York Heart Association; HF, heart failure; CV, cardiovascular; CVD, cardiovascular disease; LV, left ventricular; LVSD, left ventricular systolic dysfunction.
Risk of Bias Assessment
In most trials, the sequence generation and allocation concealment were low risk of bias, while only one research was unclear for allocation concealment. Five trials had a high risk of bias in the blind method. All the included trials possess low risk of bias for incomplete outcome data. Regarding selective outcome reporting, 11 studies had low risk of bias, while the rest were each unclear risk. Finally, all studies were judged as unclear risk for free of other bias (Supplementary Table 1).
SGLT-2i vs. Control
The control analysis combined placebo and other hypoglycemic drug trials, and 77 RCTs with 88,973 participants indicated that SGLT-2i had no increase in malignancy overall risk compared to non-SGLT-2i (RR = 1.05, P = 0.20). The statistical analysis of SGLT-2i for different types of drugs demonstrated statistical significance in seven studies related to ertugliflozin, which could increase malignant tumor risk in T2D patients (RR = 1.80, 95% CI = 1.02–3.17, P = 0.04). For other drugs, including bexagliflozin (RR = 1.25, P = 0.69), dapagliflozin (RR = 0.98, P = 0.71), empagliflozin (RR = 1.13, P = 0.13), canagliflozin (RR = 1.06, P = 0.45), tofogliflozin (RR = 1.23, P = 0.65) had no significant association with malignant tumors occurrence. Compared with non-SGLT-2i, SGLT-2i was also not linked to tumor incidence in hematological malignancy, digestive system malignancy, malignant breast tumor, malignant skin tumor, malignant tumor of urinary system, malignant tumor of respiratory system, gynecologic malignant tumor, malignant brain tumor, and thyroid malignancy. However, in various SGLT-2i types, dapagliflozin may reduce the incidence of respiratory malignancies, but the difference was not statistically significant (RR = 0.75, 95% CI = 0.54–1.05, P = 0.09), while other SGLT-2i types had no impact on the incidence of particular types of tumors. Regarding drug use duration, no significant difference existed in malignant tumor risk between short-, medium-, and long-term drug use (Table 2).
Table 2.
The incidence of malignant tumors between SGLT-2i and control.
| SGLT-2i vs. control | No. of studies | Participants | RR | 95% CI | p | Heterogeneity (I2) (%) |
|---|---|---|---|---|---|---|
| All | 77 | 88,973 | 1.05 | 0.97–1.14 | 0.20 | 0 |
| Type of malignant tumor | ||||||
| Hematological malignancy | 20 | 50,817 | 1.16 | 0.85–1.60 | 0.35 | 0 |
| Ertugliflozin | 4 | 2,093 | 3.50 | 0.73–16.83 | 0.12 | 0 |
| Dapagliflozin | 4 | 19,464 | 1.27 | 0.80–2.00 | 0.31 | 0 |
| Empagliflozin | 5 | 11,964 | 1.29 | 0.64–2.63 | 0.48 | 0 |
| Canagliflozin | 5 | 16,670 | 0.67 | 0.35–1.31 | 0.24 | 3 |
| Digestive system malignancy | 43 | 68,586 | 1.05 | 0.90–1.23 | 0.54 | 0 |
| Ertugliflozin | 5 | 4,049 | 1.83 | 0.68–4.96 | 0.23 | 0 |
| Dapagliflozin | 10 | 22,041 | 0.94 | 0.74–1.19 | 0.59 | 0 |
| Empagliflozin | 12 | 18,517 | 1.31 | 0.92–1.87 | 0.13 | 0 |
| Canagliflozin | 11 | 22,476 | 0.99 | 0.73–1.33 | 0.93 | 0 |
| Breast malignant tumor | 31 | 59,991 | 1.11 | 0.85–1.46 | 0.45 | 0 |
| Ertugliflozin | 3 | 2,673 | 1.17 | 0.38–3.64 | 0.78 | 0 |
| Dapagliflozin | 11 | 20,948 | 1.10 | 0.73–1.67 | 0.64 | 0 |
| Canagliflozin | 8 | 19,705 | 1.52 | 0.89–2.60 | 0.13 | 0 |
| Skin malignant tumor | 23 | 56,961 | 1.08 | 0.87–1.33 | 0.49 | 0 |
| Ertugliflozin | 3 | 2,787 | 1.13 | 0.41–3.11 | 0.81 | 0 |
| Dapagliflozin | 11 | 23,031 | 1.06 | 0.77–1.44 | 0.74 | 0 |
| Empagliflozin | 6 | 15,171 | 0.96 | 0.68–1.36 | 0.82 | 0 |
| Canagliflozin | 3 | 15,972 | 1.71 | 0.89–3.31 | 0.11 | 0 |
| Malignant tumor of urinary system | 41 | 66,633 | 1.08 | 0.92–1.27 | 0.33 | 0 |
| Ertugliflozin | 4 | 2,661 | 1.39 | 0.44–4.38 | 0.57 | 0 |
| Dapagliflozin | 13 | 23,721 | 1.07 | 0.85–1.33 | 0.57 | 0 |
| Empagliflozin | 13 | 18,943 | 1.00 | 0.71–1.40 | 0.99 | 0 |
| Canagliflozin | 9 | 20,836 | 1.17 | 0.85–1.61 | 0.35 | 0 |
| Malignant tumor of the respiratory system | 26 | 57,358 | 0.86 | 0.69–1.07 | 0.17 | 0 |
| Dapagliflozin | 6 | 20,890 | 0.75 | 0.54–1.05 | 0.09 | 0 |
| Empagliflozin | 9 | 16,390 | 1.07 | 0.70–1.64 | 0.76 | 0 |
| Canagliflozin | 8 | 19,370 | 0.82 | 0.54–1.23 | 0.33 | 0 |
| Gynecologic malignant tumor | 15 | 51,139 | 0.79 | 0.54–1.16 | 0.24 | 0 |
| Dapagliflozin | 3 | 18,068 | 0.72 | 0.40–1.32 | 0.29 | 0 |
| Empagliflozin | 5 | 12,210 | 1.42 | 0.59–3.43 | 0.44 | 15 |
| Canagliflozin | 6 | 20,556 | 0.63 | 0.33–1.19 | 0.16 | 0 |
| Brain malignant tumor | 5 | 29,269 | 1.10 | 0.47–2.59 | 0.83 | 0 |
| Thyroid malignancy | 10 | 46,115 | 1.17 | 0.63–2.20 | 0.62 | 0 |
| Canagliflozin | 5 | 16,919 | 1.89 | 0.60–5.91 | 0.27 | 0 |
| Types of SGLT-2i | ||||||
| Ertugliflozin | 7 | 6,084 | 1.80 | 1.02–3.17 | 0.04 | 0 |
| Bexagliflozin | 4 | 1,274 | 1.25 | 0.42–3.73 | 0.69 | 0 |
| Dapagliflozin | 25 | 28,994 | 0.98 | 0.88–1.10 | 0.71 | 0 |
| Empagliflozin | 18 | 24,933 | 1.13 | 0.96–1.32 | 0.13 | 0 |
| Canagliflozin | 18 | 26,618 | 1.06 | 0.91–1.24 | 0.45 | 0 |
| Tofogliflozin | 3 | 814 | 1.23 | 0.50–3.00 | 0.65 | 0 |
| Follow-up time | ||||||
| <52 weeks | 30 | 11,610 | 1.19 | 0.80–1.76 | 0.40 | 0 |
| 52–104 weeks | 31 | 23,804 | 0.94 | 0.73–1.21 | 0.63 | 0 |
| ≥104 weeks | 16 | 53,559 | 1.06 | 0.97–1.15 | 0.18 | 1 |
The values in italics represent statistical differences in the results (i.e., P < 0.05).
SGLT-2i vs. Other Hypoglycemic Drugs
Compared with other hypoglycemic drugs, 25 studies were involved, with 19,703 participants. Among them, 9,917 were SGLT-2i participants, with a total of 100 malignant tumors, and 9,786 were other hypoglycemic drugs, with a total of 94 malignant tumors. Compared with other hypoglycemic drugs, SGLT-2i had no overall risk increase of a malignant tumor (RR = 1.01, P = 0.95). Nevertheless, based on SGLT-2i types, empagliflozin (7 studies) was associated with reduced malignant tumor risk (RR = 0.67, 95% CI = 0.45–0.98, P = 0.04), while dapagliflozin (6 studies) can upsurge malignant tumor risk (RR = 2.71, 95% CI = 1.46–6.43, P = 0.02). Ertugliflozin (RR = 1.74, P = 0.12) and canagliflozin (RR = 0.79, P = 0.45) were not significantly associated with the malignant tumor risk. By analyzing particular malignant tumor types, empagliflozin data may propose a possible reduction in urinary malignancy risk (RR = 0.48, 95% CI = 0.21–1.10, P = 0.08), and dapagliflozin may augment digestive system malignancy occurrence risk (RR = 3.98, 95% CI = 0.85–18.69, P = 0.08), but the above differences have no statistical significance. However, compared with other hypoglycemic agents, SGLT-2i did not correlate with malignant tumors incidence in the rest of body. Additionally, follow-up time (short, medium, and long term) had no significant effect on carcinogenic rate of SGLT-2i and had no impact on reducing or increasing malignancy risk compared with different types of active drugs (Table 3).
Table 3.
The incidence of malignant tumors between SGLT-2i and other hypoglycemic drugs.
| SGLT-2i vs. other hypoglycemic drugs | No. of studies | Participants | RR | 95% CI | p | Heterogeneity (I2) (%) |
|---|---|---|---|---|---|---|
| All | 25 | 19,703 | 1.01 | 0.77–1.31 | 0.95 | 0 |
| Type of malignant tumor | ||||||
| Hematological malignancy | 5 | 3,321 | 1.76 | 0.51–6.01 | 0.37 | 0 |
| Digestive system malignancy | 16 | 13,423 | 1.36 | 0.82–2.27 | 0.23 | 0 |
| Ertugliflozin | 3 | 2,885 | 1.45 | 0.43–4.82 | 0.55 | 0 |
| Dapagliflozin | 4 | 2,225 | 3.98 | 0.85–18.69 | 0.08 | 0 |
| Empagliflozin | 5 | 3,824 | 0.78 | 0.36–1.73 | 0.55 | 7 |
| Breast malignant tumor | 12 | 10,137 | 1.01 | 0.54–1.90 | 0.98 | 0 |
| Empagliflozin | 4 | 3,138 | 0.81 | 0.27–2.44 | 0.71 | 0 |
| Canagliflozin | 3 | 3,077 | 1.10 | 0.29–4.22 | 0.89 | 0 |
| Skin malignant tumor | 9 | 7,572 | 1.12 | 0.58–2.18 | 0.73 | 0 |
| Dapagliflozin | 3 | 2,052 | 1.80 | 0.38–8.45 | 0.45 | 0 |
| Empagliflozin | 4 | 3,354 | 0.89 | 0.35–2.32 | 0.82 | 0 |
| Malignant tumor of urinary system | 14 | 11,616 | 1.04 | 0.60–1.81 | 0.88 | 0 |
| Empagliflozin | 5 | 4,106 | 0.48 | 0.21–1.10 | 0.08 | 0 |
| Canagliflozin | 4 | 4,021 | 1.86 | 0.55–6.26 | 0.32 | 0 |
| Malignant tumor of the respiratory system | 8 | 7,012 | 0.73 | 0.33–1.62 | 0.44 | 0 |
| Empagliflozin | 4 | 3,305 | 0.51 | 0.18–1.48 | 0.22 | 0 |
| Gynecologic malignant tumor | 8 | 7,098 | 0.56 | 0.26–1.23 | 0.15 | 0 |
| Empagliflozin | 3 | 2,532 | 0.50 | 0.15–1.71 | 0.27 | 2 |
| Canagliflozin | 3 | 3,636 | 0.41 | 0.12–1.42 | 0.16 | 0 |
| Types of SGLT-2i | ||||||
| Ertugliflozin | 3 | 3,194 | 1.74 | 0.86–3.53 | 0.12 | 0 |
| Dapagliflozin | 6 | 3,254 | 2.71 | 1.14–6.43 | 0.02 | 0 |
| Empagliflozin | 7 | 5,660 | 0.67 | 0.45–0.98 | 0.04 | 7 |
| Canagliflozin | 7 | 6,871 | 0.79 | 0.44–1.45 | 0.45 | 0 |
| Follow-up time | ||||||
| <52 weeks | 6 | 2,827 | 1.72 | 0.60–4.91 | 0.31 | 0 |
| 52–104 weeks | 12 | 9,202 | 0.80 | 0.53–1.20 | 0.28 | 6 |
| ≥104 weeks | 7 | 7,674 | 1.12 | 0.78–1.63 | 0.54 | 0 |
| Types of hypoglycemic drugs in the control group | ||||||
| GLP-1RA | 3 | 2,068 | 0.71 | 0.28–1.81 | 0.48 | 27 |
| DPP-4i | 11 | 7,106 | 1.13 | 0.66–1.92 | 0.66 | 0 |
| Sulfonylurea | 6 | 7,496 | 1.11 | 0.76–1.62 | 0.59 | 0 |
| Metformin | 5 | 2,693 | 0.55 | 0.26–1.17 | 0.12 | 0 |
The values in italics represent statistical differences in the results (i.e., P < 0.05).
SGLT-2i vs. Placebo
A total of 60 SGLT-2i and placebo-controlled studies were included, having 70,600 participants: 36,094 in SGLT-2i group, with 1,150 malignant tumors, and 34,506 in placebo group, with 1,079 malignant tumors. Compared with placebo, SGLT-2i had no overall risk increase of malignancies (RR = 1.05, P = 0.20). However, according to different SGLT-2i types, empagliflozin (15 studies) was significantly linked to increased risk of malignancies (RR = 1.25, 95% CI = 1.05–1.49, P = 0.01), and the rest of ertugliflozin (RR = 1.91, P = 0.18), bexagliflozin (RR = 1.08, P = 0.90), dapagliflozin (RR = 0.96, P = 0.46), canagliflozin (RR = 1.08, P = 0.35), and tofogliflozin (RR = 0.76, P = 0.70) were not significantly associated with increased malignancies risk. By analyzing specific types of malignancies, SGLT-2i population and each type did not correlate with the incidence of hematological malignancy, malignant skin tumor, malignant tumor of urinary system, malignant tumor of respiratory system, gynecologic malignant tumor, malignant brain tumor, and thyroid malignancy compared with placebo.
Compared with placebo, SGLT-2i overall was not associated with breast cancer incidence (RR = 1.15, P = 0.36). Canagliflozin potentially increased breast cancer risk, but the difference was not statistically significant (RR = 1.64, 95% CI = 0.93–2.90, P = 0.09). Besides, SGLT-2i population revealed no statistically significant difference in the incidence of digestive system malignancies (RR = 1.01, P = 0.92), but empagliflozin (8 studies) was associated with increased risk of digestive system malignancies (RR = 1.48, 95% CI = 0.99–2.21, P = 0.05). The data revealed that the follow-up time had no significant impact on malignancy incidence of SGLT-2i (Table 4).
Table 4.
The incidence of malignant tumors between SGLT-2i and placebo.
| SGLT-2i vs. placebo | No. of studies | Participants | RR | 95% CI | p | Heterogeneity (I2) (%) |
|---|---|---|---|---|---|---|
| All | 60 | 70,600 | 1.05 | 0.97–1.14 | 0.20 | 0 |
| Type of malignant tumor | ||||||
| Hematological malignancy | 16 | 47,496 | 1.13 | 0.81–1.57 | 0.47 | 0 |
| Dapagliflozin | 4 | 19,464 | 1.27 | 0.80–2.00 | 0.31 | 0 |
| Empagliflozin | 4 | 10,277 | 1.34 | 0.63–2.87 | 0.45 | 0 |
| Canagliflozin | 5 | 16,670 | 0.67 | 0.35–1.31 | 0.24 | 3 |
| Digestive system malignancy | 30 | 56,264 | 1.01 | 0.85–1.19 | 0.92 | 0 |
| Dapagliflozin | 7 | 19,816 | 0.89 | 0.70–1.14 | 0.35 | 0 |
| Empagliflozin | 8 | 14,693 | 1.48 | 0.99–2.21 | 0.05 | 0 |
| Canagliflozin | 10 | 19,812 | 0.96 | 0.71–1.30 | 0.80 | 0 |
| Breast malignant tumor | 23 | 50,633 | 1.15 | 0.85–1.55 | 0.36 | 0 |
| Dapagliflozin | 9 | 19,730 | 1.06 | 0.69–1.62 | 0.79 | 0 |
| Empagliflozin | 6 | 13,187 | 0.78 | 0.40–1.54 | 0.48 | 0 |
| Canagliflozin | 7 | 17,407 | 1.64 | 0.93–2.90 | 0.09 | 0 |
| Skin malignant tumor | 16 | 49,389 | 1.07 | 0.86–1.34 | 0.54 | 0 |
| Dapagliflozin | 8 | 20,979 | 1.03 | 0.75–1.42 | 0.86 | 0 |
| Empagliflozin | 4 | 11,817 | 0.97 | 0.67–1.40 | 0.88 | 0 |
| Canagliflozin | 3 | 15,972 | 1.71 | 0.89–3.31 | 0.11 | 0 |
| Malignant tumor of urinary system | 29 | 55,017 | 1.09 | 0.92–1.28 | 0.33 | 0 |
| Dapagliflozin | 11 | 22,282 | 1.05 | 0.84–1.31 | 0.70 | 0 |
| Empagliflozin | 10 | 14,837 | 1.17 | 0.80–1.70 | 0.43 | 0 |
| Canagliflozin | 5 | 16,815 | 1.13 | 0.80–1.58 | 0.49 | 9 |
| Malignant tumor of the respiratory system | 19 | 50,346 | 0.87 | 0.69–1.09 | 0.22 | 0 |
| Dapagliflozin | 5 | 20,076 | 0.76 | 0.55–1.06 | 0.11 | 0 |
| Empagliflozin | 6 | 13,085 | 1.23 | 0.77–1.98 | 0.39 | 0 |
| Canagliflozin | 6 | 16,782 | 0.79 | 0.52–1.21 | 0.28 | 0 |
| Gynecologic malignant tumor | 8 | 44,041 | 0.88 | 0.57–1.37 | 0.57 | 0 |
| Canagliflozin | 4 | 16,920 | 0.75 | 0.35–1.58 | 0.45 | 0 |
| Brain malignant tumor | 4 | 27,724 | 1.23 | 0.50–3.05 | 0.65 | 0 |
| Thyroid malignancy | 8 | 43,740 | 1.45 | 0.73–2.90 | 0.29 | 0 |
| Canagliflozin | 5 | 16,919 | 1.89 | 0.60–5.91 | 0.27 | 0 |
| Types of SGLT-2i | ||||||
| Ertugliflozin | 4 | 2,890 | 1.91 | 0.74–4.91 | 0.18 | 0 |
| Bexagliflozin | 3 | 890 | 1.08 | 0.33–3.54 | 0.90 | 0 |
| Dapagliflozin | 20 | 25,740 | 0.96 | 0.86–1.07 | 0.46 | 0 |
| Empagliflozin | 15 | 19,273 | 1.25 | 1.05–1.49 | 0.01 | 0 |
| Canagliflozin | 14 | 21,077 | 1.08 | 0.92–1.26 | 0.35 | 0 |
| Tofogliflozin | 2 | 474 | 0.76 | 0.19–3.02 | 0.70 | 0 |
| Follow-up time | ||||||
| <52 weeks | 28 | 10,113 | 1.07 | 0.71–1.61 | 0.74 | 0 |
| 52–104 weeks | 22 | 14,602 | 1.03 | 0.75–1.42 | 0.85 | 0 |
| ≥104 weeks | 10 | 45,885 | 1.06 | 0.97–1.15 | 0.21 | 14 |
Discussion
SGLT-2i possesses good benefits in lowering blood glucose, but some safety problems may result in urogenital infection, bone fractures, ketoacidosis, etc. (90), so its clinical use requires to be considered comprehensively. Epidemiological studies have manifested a link between T2D and cancer, and one of the reasons may be hyperglycemia itself (91). While SGLT-2i may affect malignant tumors occurrence by lowering blood glucose, its comprehensive impact is still uncertain. Tang et al. analyzed 46 RCTs from 24 to 160 weeks and stated that empagliflozin might correlate with increased risk of bladder cancer (11). Nevertheless, the data involved in this analysis were challenged, and the corrected data showcased that empagliflozin might not be linked to bladder cancer (92). Beyond that, Tang et al. also concluded that canagliflozin might have a protective effect on gastrointestinal cancer (11). A meta-analysis of 27 trials displayed that SGLT-2i were not statistically associated with any cancer type (93). The abovementioned studies may be due to the low incidence of malignant tumors, small statistical sample size and short follow-up time, making it challenging to get clear and unified results.
This meta-analysis showed neither a significant association between SGLT-2i and the overall risk of malignancy in T2D patients nor with medication duration, consistent with the results of previous analysis (11, 93, 94). In different types of SGLT-2i analyses, we found that ertugliflozin significantly increased overall malignancy incidence, had no statistically significant difference compared to other hypoglycemic drugs or placebo alone, and had no great risk of a specific malignant tumor. The above could be due to lack of test sample size. A pooled analysis of 7 RCTs concluded that ertugliflozin had no significant difference in malignancies incidence compared with placebo or other active hypoglycemic agents (95).
Moreover, we observed that dapagliflozin might reduce the risk of respiratory system malignancies compared with the control group, but without statistical significance. Sodium-glucose cotransporter 2 (SGLT2) expression increased at the lung premalignancy and early-stage lung adenocarcinoma (96), and dapagliflozin may be able to reduce cancer cells proliferation by inhibiting glucose transport. Villani et al. also found that canagliflozin could prevent lung cancer cells' proliferation by precluding respiration supported by mitochondrial complex-I (97). It is worth noting that our data also indicated that compared with other hypoglycemic drugs, dapagliflozin could increase the overall risk of malignant tumors, and the most likely one was the digestive system malignancy, but without statistically significant difference. According to preceding studies, some active antidiabetic drugs can impede tumors. For example, a meta-analysis by Dicembrini et al. indicates that DPP-4i may have a potential inhibitory effect on colorectal cancer (98). A meta-analysis of 21 studies showed that metformin might be beneficial for survival in patients with pancreatic cancer and diabetes (99). Wu et al. found that metformin had no effect on the overall esophageal cancer risk, but it may reduce esophageal cancer risk in T2D Asian patients (100). Du et al.'s research reported in diabetics of colorectal cancer patients that taking metformin can improve overall survival and cancer specific survival, especially the overall survival of patients with stage II and III (101). A study has indicated that GLP-1RA may not be associated with increased risk of pancreatic cancer (102). In conclusion, other hypoglycemic drugs may be connected with inhibiting digestive system malignancies, making dapagliflozin seemed to be linked to increased incidence of digestive system malignancies. Notably, research has shown that dapagliflozin may have a potential inhibition impact on colon cancer cells expressing SGLT2 but without UDP glucuronosyltransferase family 1 member A9 (UGT1A9) (103). Besides, Okada et al. suggested that dapagliflozin may inhibit tumor growth by inhibiting glucose entry into cancer cells and producing cytotoxic effects in non-metabolized dapagliflozin (104). Canagliflozin may directly reduce liver cancer growth by inhibiting glycolysis and angiogenic activity (105). Tang et al.'s meta-analysis of 35 trials displayed no significant association between SGLT-2i and pancreatic cancer incidence (106). Since our research records the digestive system malignant tumor sample size too small (includes only 4 RCTs, dapagliflozin group 6 cases, and other active hypoglycemic drugs group 0 cases), difference did not reach statistical significance. Accordingly, it is uncertain whether the dapagliflozin increase of digestive system malignancy arising from the overall risk is high.
Simultaneously, our data revealed that compared with other hypoglycemic drugs, empagliflozin could reduce the overall risk of malignant tumors and may reduce the incidence of urinary system malignant tumors, but the latter without statistically significant difference. As previous studies reported, in prostate cancer, SGLT2 is actively expressed and actively participates in glucose uptake, so SGLT-2i may inhibit tumor growth by reducing glucose uptake and disrupting glycolysis (107). Kuang et al. stated that dapagliflozin could induce apoptosis of renal carcinoma cells (108). Data analysis from 20 empagliflozin and placebo-controlled trials revealed no significant association between empagliflozin and the incidence of bladder and renal malignancies (109). An observational, prospective follow-up study has reported that empagliflozin can enhance anti-inflammatory and antioxidant effects in T2D patients, including cardiovascular advantages (110). Anti-inflammatory and antioxidant impacts may also be linked to reduced incidence of malignancies. However, due to the limited number of included RCTs and the difference was not statistically significant, it cannot be confirmed that the overall risk reduction of malignant tumors is ascribed to urinary malignancies reduction. Furthermore, compared with placebo, we stated that empagliflozin significantly increased malignant tumor risk, mainly those of the digestive system. Nevertheless, one summarizes 15 randomized phase I-III trials plus four extension studies of empagliflozin and a placebo-controlled study indicating that empagliflozin safety data had no linkage to T2D patients with malignant tumor (111). The conclusion is still out on whether empagliflozin increases the risk of malignancies.
Our study showcased that canagliflozin might potentially increase breast cancer risk compared with placebo, but no statistically significant difference existed. Earlier data submitted to the Food and Drug Administration (FDA) suggested that dapagliflozin might upsurge breast cancer risk, but subsequent studies have suggested that it may be increased risk due to early cancer diagnosis rather than actual increase in incidence (112). Studies have shown that breast cancer incidence in canagliflozin intervention groups was similar to that in non-canagliflozin groups, and both were lower (113). A large population cohort study with a median of 2.6 years of follow-up showed that SGLT-2i utilization was not associated with increased overall breast cancer risk than DPP-4i (114). Interestingly, a study has implied that canagliflozin holds anti-proliferation effect on breast cancer cells by increasing phosphorylation of adenosine monophosphate activated protein kinase (AMPK) and reducing the phosphorylation of 70 kilodalton (kDa) ribosomal protein S6 kinase 1, thereby blocking cell cycle and inducing apoptosis (115). Besides, a study has manifested that ipragliflozin can inhibit the proliferation of breast cancer cells (116).
The strength of our study lies in the large study scale and sample size. However, our limitations are also obvious. Few studies we referred primarily aimed at assessing the risk of malignancy, and the incidence of malignant tumors closely related to age, diabetes, gender and many other factors. However, due to limited data availability, we can not adjust these parameters. In summary, it is not sufficient to discuss or conclude the relationship between SGLT-2i and malignancy risk in this meta-analysis. More RCTs related to long-term use of SGLT-2i are required in the future to provide more evidence for the safety of such drugs in long-term use.
Conclusion
In summary, current data from RCTs had no significant association between SGLT-2i and overall malignant tumor risk. Our evidence proposes that ertugliflozin may increase the overall risk of malignancy. Compared with active hypoglycemic agents, dapagliflozin may increase the overall risk of malignant tumor, while empagliflozin may reduce its risk. But compared with placebo, empagliflozin may increase the overall malignancy risk, mainly in the digestive system. However, the follow-up time of the RCTs analyzed in our study were relatively short, and the data of various factors were incomplete, which could insufficient to account for the long-term effects of SGLT-2i on malignant tumors, and more data are required for comprehensive analysis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
FX designed the research process. NS and YShi searched the database for corresponding articles. JX and YSi extracted useful information from the articles above. TY and MZ used statistical software for analysis. NS and XL drafted the meta-analysis. DN polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- SGLT-2i
sodium-glucose cotransporter 2 inhibitor
- RCT
randomized controlled trial
- RR
risk ratio
- CI
confidence interval
- T2DM
type 2 diabetes mellitus
- T2D
type 2 diabetes
- GLP-1RA
glucagon-like peptide-1 receptor agonist
- DPP-4i
dipeptidyl-peptidase-4 inhibitor
- SGLT2
sodium-glucose cotransporter 2
- UGT1A9
UDP glucuronosyltransferase family 1 member A9
- AMPK
adenosine monophosphate activated protein kinase
- kDa
kilodalton.
Footnotes
Funding. This study was funded by the Hangzhou Health Science and Technology Project of Hangzhou Municipal Health Commission (No. 2018A08).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.668368/full#supplementary-material
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. (2019) 157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MP. Screening and treatment by the primary care provider of common diabetes complications. Med. Clin North Am. (2015) 99:201–19. 10.1016/j.mcna.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2020) 63:1671–93. 10.1007/s00125-020-05181-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Cancer (2018) . Available online at: https://www.who.int/news-room/fact-sheets/detail/cancer
- 5.Carstensen B, Jørgensen ME, Friis S. The epidemiology of diabetes and cancer. Curr Diabetes Rep. (2014) 14:535. 10.1007/s11892-014-0535-8 [DOI] [PubMed] [Google Scholar]
- 6.Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. (2012) 55:1607–18. 10.1007/s00125-012-2525-1 [DOI] [PubMed] [Google Scholar]
- 7.Onitilo AA, Engel JM, Glurich I, Stankowski RV, Williams GM, Doi SA. Diabetes and cancer II: role of diabetes medications and influence of shared risk factors. Cancer Causes Control. (2012) 23:991–1008. 10.1007/s10552-012-9971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig. (2014) 5:265–75. 10.1111/jdi.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novikov A, Vallon V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: an update. Curr Opin Nephrol Hypertens. (2016) 25:50–8. 10.1097/MNH.0000000000000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. (2017) 60:1862–72. 10.1007/s00125-017-4370-8 [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther. (2012) 5:313–27. 10.2147/DMSO.S22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Int Med. (2009) 151:W65–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 14.Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis. (2019) 74:328–37. 10.1053/j.ajkd.2019.03.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki E, Tanizawa Y, Tanaka Y, Taniguchi A, Koiwai K, Kim G, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. (2015) 17:665–74. 10.1111/dom.12464 [DOI] [PubMed] [Google Scholar]
- 16.Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab. (2018) 20:1453–60. 10.1111/dom.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. (2013) 11:43. 10.1186/1741-7015-11-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. (2015) 32:531–41. 10.1111/dme.12624 [DOI] [PubMed] [Google Scholar]
- 19.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2014) 2:369–84. 10.1016/S2213-8587(13)70208-0 [DOI] [PubMed] [Google Scholar]
- 20.Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. (2014) 16:159–69. 10.1111/dom.12189 [DOI] [PubMed] [Google Scholar]
- 21.Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. (2020) 41:3421–32. 10.1093/eurheartj/ehaa419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohm M, Fitchett D, Ofstad AP, Brueckmann M, Kaspers S, George JT, et al. Heart failure and renal outcomes according to baseline and achieved blood pressure in patients with type 2 diabetes: results from EMPA-REG OUTCOME. J Hypertens. (2020) 38:1829–40. 10.1097/HJH.0000000000002492 [DOI] [PubMed] [Google Scholar]
- 23.Cahn A, Raz I, Bonaca M, Mosenzon O, Murphy SA, Yanuv I, et al. Safety of dapagliflozin in a broad population of patients with type 2 diabetes: analyses from the DECLARE-TIMI 58 study. Diabetes Obes Metab. (2020) 22:1357–68. 10.1111/dom.14041 [DOI] [PubMed] [Google Scholar]
- 24.Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. (2015) 38:1218–27. 10.2337/dc14-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagogo-Jack S, Liu J, Eldor R, Amorin G, Johnson J, Hille D, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. (2018) 20:530–40. 10.1111/dom.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdinand KC, Izzo JL, Lee J, Meng L, George J, Salsali A, et al. Antihyperglycemic and blood pressure effects of empagliflozin in black patients with type 2 diabetes mellitus and hypertension. Circulation. (2019) 139:2098–109. 10.1161/CIRCULATIONAHA.118.036568 [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. (2013) 36:4015–21. 10.2337/dc13-0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab. (2018) 20:2532–40. 10.1111/dom.13413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. (2014) 16:467–77. 10.1111/dom.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchigami A, Shigiyama F, Kitazawa T, Okada Y, Ichijo T, Higa M, et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR). Cardiovasc Diabetol. (2020) 19:1. 10.1186/s12933-019-0977-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, et al. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab. (2019) 21:1027–36. 10.1111/dom.13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. (2018) 9:49–66. 10.1007/s13300-017-0337-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadjadj S, Rosenstock J, Meinicke T, Woerle HJ, Broedl UC. Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care. (2016) 39:1718–28. 10.2337/dc16-0522 [DOI] [PubMed] [Google Scholar]
- 34.Halvorsen YC, Walford GA, Massaro J, Aftring RP, Freeman MW. A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. (2019) 21:2496–504. 10.1111/dom.13833 [DOI] [PubMed] [Google Scholar]
- 35.Halvorsen YD, Lock JP, Zhou W, Zhu F, Freeman MW. A 24-week, randomized, double-blind, active-controlled clinical trial comparing bexagliflozin with sitagliptin as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab. (2019) 21:2248–56. 10.1111/dom.13801 [DOI] [PubMed] [Google Scholar]
- 36.Halvorsen YD, Walford G, Thurber T, Russell H, Massaro M, Freeman MW. A 12-week, randomized, double-blind, placebo-controlled, four-arm dose-finding phase 2 study evaluating bexagliflozin as monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. (2020) 22:566–73. 10.1111/dom.13928 [DOI] [PubMed] [Google Scholar]
- 37.Haneda M, Seino Y, Inagaki N, Kaku K, Sasaki T, Fukatsu A, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. (2016) 38:66–88.e20. 10.1016/j.clinthera.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 38.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. (2012) 66:446–56. 10.1111/j.1742-1241.2012.02911.x [DOI] [PubMed] [Google Scholar]
- 39.Hollander P, Hill J, Johnson J, Wei Jiang Z, Golm G, Huyck S, et al. Results of VERTIS SU extension study: safety and efficacy of ertugliflozin treatment over 104 weeks compared to glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin. Curr Med Res Opin. (2019) 35:1335–43. 10.1080/03007995.2019.1583450 [DOI] [PubMed] [Google Scholar]
- 40.Ikeda S, Takano Y, Cynshi O, Tanaka R, Christ AD, Boerlin V, et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. (2015) 17:984–93. 10.1111/dom.12538 [DOI] [PubMed] [Google Scholar]
- 41.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. (2013) 15:1136–45. 10.1111/dom.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother. (2014) 15:1501–15. 10.1517/14656566.2014.935764 [DOI] [PubMed] [Google Scholar]
- 43.Jabbour SA, Frias JP, Ahmed A, Hardy E, Choi J, Sjostrom CD, et al. Efficacy and safety over 2 Years of exenatide plus dapagliflozin in the DURATION-8 study: a multicenter, double-blind, phase 3, randomized controlled trial. Diabetes Care. (2020) 43:2528–36. 10.2337/dc19-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. (2014) 37:740–50. 10.2337/dc13-0467 [DOI] [PubMed] [Google Scholar]
- 45.Januzzi JL, Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. (2017) 70:704–12. 10.1016/j.jacc.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 46.Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. (2019) 21:1474–82. 10.1111/dom.13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji L, Ma J, Li H, Mansfield TA, T'Joen CL, Iqbal N, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. (2014) 36:84–100.e9. 10.1016/j.clinthera.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 48.Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther. (2015) 32:306–18. 10.1007/s12325-015-0198-0 [DOI] [PubMed] [Google Scholar]
- 49.Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2017) 19:874–82. 10.1111/dom.12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2013) 15:432–40. 10.1111/dom.12047 [DOI] [PubMed] [Google Scholar]
- 51.Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. (2014) 13:65. 10.1186/1475-2840-13-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol. (2020) 19:110. 10.1186/s12933-020-01079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, Miyamoto Y, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. (2018) 20:2200–9. 10.1111/dom.13352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. (2014) 85:962–71. 10.1038/ki.2013.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. (2013) 56:2582–92. 10.1007/s00125-013-3039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. (2014) 62:1252–62. 10.1111/jgs.12881 [DOI] [PubMed] [Google Scholar]
- 57.Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. (2015) 38:355–64. 10.2337/dc13-2762 [DOI] [PubMed] [Google Scholar]
- 58.Lewin A, DeFronzo RA, Patel S, Liu D, Kaste R, Woerle HJ, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. (2015) 38:394–402. 10.2337/dc14-2365 [DOI] [PubMed] [Google Scholar]
- 59.Lingvay I, Catarig A-M, Frias JP, Kumar H, Lausvig NL, le Roux CW, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diab Endocrinol. (2019) 7:834–44. 10.1016/S2213-8587(19)30311-0 [DOI] [PubMed] [Google Scholar]
- 60.Mathieu C, Herrera Marmolejo M, González González JG, Hansen L, Chen H, Johnsson E, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. (2016) 18:1134–7. 10.1111/dom.12737 [DOI] [PubMed] [Google Scholar]
- 61.Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E. Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. (2015) 17:1075–84. 10.1111/dom.12543 [DOI] [PubMed] [Google Scholar]
- 62.Muller-Wieland D, Kellerer M, Cypryk K, Skripova D, Rohwedder K, Johnsson E, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. (2018) 20:2598–607. 10.1111/dom.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nauck MA, Del Prato S, Durán-García S, Rohwedder K, Langkilde AM, Sugg J, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. (2014) 16:1111–20. 10.1111/dom.12327 [DOI] [PubMed] [Google Scholar]
- 64.Oshima M, Neal B, Toyama T, Ohkuma T, Li Q, de Zeeuw D, et al. Different eGFR decline thresholds and renal effects of canagliflozin: data from the CANVAS program. J Am Soc Nephrol. (2020) 31:2446–56. 10.1681/ASN.2019121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 66.Pratley RE, Eldor R, Raji A, Golm G, Huyck SB, Qiu Y, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. (2018) 20:1111–20. 10.1111/dom.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu R, Capuano G, Meininger G. Efficacy and safety of twice-daily treatment with canagliflozin, a sodium glucose co-transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. (2014) 1:54–60. 10.1016/j.jcte.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridderstrale M, Rosenstock J, Andersen KR, Woerle HJ, Salsali A, Investigators E-RHHSt. Empagliflozin compared with glimepiride in metformin-treated patients with type 2 diabetes: 208-week data from a masked randomized controlled trial. Diabetes Obes Metab. (2018) 20:2768–77. 10.1111/dom.13457 [DOI] [PubMed] [Google Scholar]
- 69.Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg S, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. (2019) 42:2272–81. 10.2337/dc19-0883 [DOI] [PubMed] [Google Scholar]
- 70.Roden M, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: a double-blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol. (2015) 14:154. 10.1186/s12933-015-0314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenstock J, Chuck L, González-Ortiz M, Merton K, Craig J, Capuano G, et al. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naïve type 2 diabetes. Diabetes Care. (2016) 39:353–62. 10.2337/dc15-1736 [DOI] [PubMed] [Google Scholar]
- 72.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. (2014) 37:1815–23. 10.2337/dc13-3055 [DOI] [PubMed] [Google Scholar]
- 73.Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2015) 17:936–48. 10.1111/dom.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. (2013) 15:1154–60. 10.1111/dom.12185 [DOI] [PubMed] [Google Scholar]
- 75.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. (2012) 35:1473–8. 10.2337/dc11-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross S, Thamer C, Cescutti J, Meinicke T, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes Obes Metab. (2015) 17:699–702. 10.1111/dom.12469 [DOI] [PubMed] [Google Scholar]
- 77.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. (2013) 36:2508–15. 10.2337/dc12-2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott R, Morgan J, Zimmer Z, Lam RLH, O'Neill EA, Kaufman KD, et al. A randomized clinical trial of the efficacy and safety of sitagliptin compared with dapagliflozin in patients with type 2 diabetes mellitus and mild renal insufficiency: the CompoSIT-R study. Diabetes Obes Metab. (2018) 20:2876–84. 10.1111/dom.13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Dose-finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, double-blind, placebo-controlled, phase II study. Curr Med Res Opin. (2014) 30:1231–44. 10.1185/03007995.2014.909390 [DOI] [PubMed] [Google Scholar]
- 80.Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. (2020) 43:1356–9. 10.2337/dc19-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. (2017) 40:201–9. 10.2337/dc16-1347 [DOI] [PubMed] [Google Scholar]
- 82.Sone H, Kaneko T, Shiki K, Tachibana Y, Pfarr E, Lee J, et al. Efficacy and safety of empagliflozin as add-on to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2020) 22:417–26. 10.1111/dom.13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenlof K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. (2014) 30:163–75. 10.1185/03007995.2013.850066 [DOI] [PubMed] [Google Scholar]
- 84.Strojek K, Yoon KH, Hruba V, Sugg J, Langkilde AM, Parikh S. Dapagliflozin added to glimepiride in patients with type 2 diabetes mellitus sustains glycemic control and weight loss over 48 weeks: a randomized, double-blind, parallel-group, placebo-controlled trial. Diabetes Ther. (2014) 5:267–83. 10.1007/s13300-014-0072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Townsend RR, Machin I, Ren J, Trujillo A, Kawaguchi M, Vijapurkar U, et al. Reductions in mean 24-hour ambulatory blood pressure after 6-week treatment with canagliflozin in patients with type 2 diabetes mellitus and hypertension. J Clin Hypertens. (2016) 18:43–52. 10.1111/jch.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. (2013) 67:1267–82. 10.1111/ijcp.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S, Dapagliflozin 006 Study G. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. (2014) 16:124–36. 10.1111/dom.12187 [DOI] [PubMed] [Google Scholar]
- 88.Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. (2014) 16:1016–27. 10.1111/dom.12348 [DOI] [PubMed] [Google Scholar]
- 89.Yang W, Ma J, Li Y, Li Y, Zhou Z, Kim JH, et al. Dapagliflozin as add-on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: a randomized controlled trial. J Diabetes. (2018) 10:589–99. 10.1111/1753-0407.12634 [DOI] [PubMed] [Google Scholar]
- 90.Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia. (2018) 61:2118–25. 10.1007/s00125-018-4663-6 [DOI] [PubMed] [Google Scholar]
- 91.Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. (2013) 104:9–14. 10.1111/cas.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaikh AMY. SGLT2 inhibitors and cancer: why further evidence is required. Diabetologia. (2017) 60:2536–7. 10.1007/s00125-017-4434-9 [DOI] [PubMed] [Google Scholar]
- 93.Dicembrini I, Nreu B, Mannucci E, Monami M. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and cancer: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2019) 21:1871–7. 10.1111/dom.13745 [DOI] [PubMed] [Google Scholar]
- 94.Pelletier R, Ng K, Alkabbani W, Labib Y, Mourad N, Gamble JM. The association of sodium-glucose cotransporter 2 inhibitors with cancer: an overview of quantitative systematic reviews. Endocrinol Diabetes Metab. (2020) 3:e00145. 10.1002/edm2.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel S, Hickman A, Frederich R, Johnson S, Huyck S, Mancuso JP, et al. Safety of ertugliflozin in patients with type 2 diabetes mellitus: pooled analysis of seven phase 3 randomized controlled trials. Diabetes Ther. (2020) 11:1347–67. 10.1007/s13300-020-00803-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scafoglio CR, Villegas B, Abdelhady G, Bailey ST, Liu J, Shirali AS, et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Trans Med. (2018) 10:eaat5933. 10.1126/scitranslmed.aat5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE, et al. The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metab. (2016) 5:1048–56. 10.1016/j.molmet.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dicembrini I, Nreu B, Montereggi C, Mannucci E, Monami M. Risk of cancer in patients treated with dipeptidyl peptidase-4 inhibitors: an extensive meta-analysis of randomized controlled trials. Acta Diabetol. (2020) 57:689–96. 10.1007/s00592-020-01479-8 [DOI] [PubMed] [Google Scholar]
- 99.Shi YQ, Zhou XC, Du P, Yin MY, Xu L, Chen WJ, et al. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine. (2020) 99:e21687. 10.1097/MD.0000000000021687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu HD, Zhang JJ, Zhou BJ. The effect of metformin on esophageal cancer risk in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Clin Transl Oncol. (2020) 23:275–82. 10.1007/s12094-020-02415-6 [DOI] [PubMed] [Google Scholar]
- 101.Du L, Wang M, Kang Y, Li B, Guo M, Cheng Z, et al. Prognostic role of metformin intake in diabetic patients with colorectal cancer: an updated qualitative evidence of cohort studies. Oncotarget. (2017) 8:26448–59. 10.18632/oncotarget.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. (2014) 11:202–30. 10.1900/RDS.2014.11.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okada J, Yamada E, Saito T, Yokoo H, Osaki A, Shimoda Y, et al. Dapagliflozin inhibits cell adhesion to collagen I and IV and increases ectodomain proteolytic cleavage of DDR1 by increasing ADAM10 activity. Molecules. (2020) 25:495. 10.3390/molecules25030495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okada J, Matsumoto S, Kaira K, Saito T, Yamada E, Yokoo H, et al. Sodium glucose cotransporter 2 inhibition combined with cetuximab significantly reduced tumor size and carcinoembryonic antigen level in colon cancer metastatic to liver. Clin Colorectal Cancer. (2018) 17:e45–8. 10.1016/j.clcc.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 105.Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. (2018) 142:1712–22. 10.1002/ijc.31193 [DOI] [PubMed] [Google Scholar]
- 106.Tang H, Yang K, Li X, Song Y, Han J. Pancreatic safety of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Pharmacoepidemiol Drug Safety. (2020) 29:161–72. 10.1002/pds.4943 [DOI] [PubMed] [Google Scholar]
- 107.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl. Acad Sci USA. (2015) 112:E4111–9. 10.1073/pnas.1511698112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monitor. (2017) 23:3737–45. 10.12659/MSM.902530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinduryte Schorling O, Clark D, Zwiener I, Kaspers S, Lee J, Iliev H. Pooled safety and tolerability analysis of empagliflozin in patients with type 2 diabetes mellitus. Adv Ther. (2020) 37:3463–84. 10.1007/s12325-020-01329-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iannantuoni F, de Marañon AM, Diaz-Morales N, Falcon R, Bañuls C, Abad-Jimenez Z, et al. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med. (2019) 8:1814. 10.3390/jcm8111814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. (2017) 34:1707–26. 10.1007/s12325-017-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin HW, Tseng CH. A review on the relationship between sglt2 inhibitors and cancer. Int J Endocrinol. (2014) 2014:719578. 10.1155/2014/719578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dandona P, Chaudhuri A. Sodium-glucose co-transporter 2 inhibitors for type 2 diabetes mellitus: an overview for the primary care physician. Int J Clin Pract. (2017) 71:e12937. 10.1111/ijcp.12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suissa M, Yin H, Yu OHY, Wong SM, Azoulay L. Sodium-glucose cotransporter 2 inhibitors and the short-term risk of breast cancer among women with type 2 diabetes. Diabetes Care. (2020) 44:e9–11. 10.2337/dc20-1073 [DOI] [PubMed] [Google Scholar]
- 115.Zhou J, Zhu J, Yu SJ, Ma HL, Chen J, Ding XF, et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother Biomed Pharmacother. (2020) 132:110821. 10.1016/j.biopha.2020.110821 [DOI] [PubMed] [Google Scholar]
- 116.Komatsu S, Nomiyama T, Numata T, Kawanami T, Hamaguchi Y, Iwaya C, et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocrine journal. (2020) 67:99–106. 10.1507/endocrj.EJ19-0428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.