Abstract
Objective
Prediction models have become frequent in the medical literature, but most published studies are conducted in a single setting. Heterogeneity between development and validation samples has been posited as a major obstacle for the generalization of models. We aimed to develop a multivariable prognostic model using sociodemographic variables easily obtainable from adolescents at age 15 to predict a depressive disorder diagnosis at age 18 and to evaluate its generalizability in 2 samples from diverse socioeconomic and cultural settings.
Method
Data from the 1993 Pelotas Birth Cohort were used to develop the prediction model, and its generalizability was evaluated in 2 representative cohort studies: the Environmental Risk (E-Risk) Longitudinal Twin Study and the Dunedin Multidisciplinary Health and Development Study.
Results
At age 15, 2,192 adolescents with no evidence of current or previous depression were included (44.6% male). The apparent C-statistic of the models derived in Pelotas ranged from 0.76 to 0.79, and the model obtained from a penalized logistic regression was selected for subsequent external evaluation. Major discrepancies between the samples were identified, impacting the external prognostic performance of the model (Dunedin and E-Risk C-statistics of 0.63 and 0.59, respectively). The implementation of recommended strategies to account for this heterogeneity among samples improved the model’s calibration in both samples.
Conclusion
An adolescent depression risk score comprising easily obtainable predictors was developed with good prognostic performance in a Brazilian sample. Heterogeneity among settings was not trivial, but strategies to deal with sample diversity were identified as pivotal for providing better risk stratification across samples. Future efforts should focus on developing better methodological approaches for incorporating heterogeneity in prognostic research.
Key words: adolescent, cohort studies, depression, prognosis, risk assessment
The field of prognostic research has seen a substantial rise in publications of prediction modeling studies in the last decade.1 This increase prompted significant advances in several medical specialties.2,3 However, most published prognostic models have been assessed in a single setting.4,5 Performance results obtained from model-development studies are frequently not achieved in validation trials when evaluated. This inconsistency can be explained either by an overoptimistic prognostic performance from an overfitted model or by significant discrepancies between development and validation samples.6
When assessing external validation across datasets, heterogeneity among prognostic studies is the norm rather than the exception.7 Differences in assessment strategies, frequency of outcome and/or studied factors, or availability of variables of interest could impose considerable difficulties for comparison purposes, impairing model generalizability. Current methodological guidelines recommend a set of careful development steps from derivation to external validation and ultimately use in clinical practice.8 In this process, understanding the similarities and differences between samples is essential,9 as guidelines suggest that a model with poor external performance should be updated before being discarded.6,10 This procedure integrates information obtained from new data to the developed model, potentially improving its prognostic ability.4,11 Even consolidated prediction models, such as the Framingham score for cardiovascular outcomes, face important drawbacks when applied in samples somewhat diverse from the original,12 demanding model adjustments to enhance generalizability to different settings.4,6
Up to now, the majority of psychiatric composite prognostic models studies have focused on model development, with very few being adequately validated in independent samples.13, 14, 15 In contrast to other areas of medicine, where hard outcomes are more easily defined, imprecise characterization of psychiatric outcomes imposes additional barriers for accurate prognostic model development and validation, as reliability of common mental disorders such as depression has been shown to be low.16 Substantial heterogeneity in clinical presentation and high rate of comorbidity produce additional obstacles for prediction of psychiatric disorders, as different assessment strategies influence the likelihood of endorsing a diagnosis.17
Prediction of psychosis, the most prolific and consolidated area in prognostic psychiatry, has greatly advanced at group level. However, it still faces challenges in prediction at the individual subject level.18 Prediction of major depressive disorder (MDD), the leading cause of mental health–related disease burden globally, is still in its infancy, relying mainly on single predictors for definition of at-risk people, with only a few studies combining risk factors.19 Following recently published standards for appropriate development and validation of psychiatric prediction models,20 using the most recent methodological recommendations1,6 and state-of-the-art statistical strategies,21,22 the present study aimed to derive and evaluate the generalizability of a psychiatric prediction model across samples from different sociocultural backgrounds.
Using data obtained from globally relevant longitudinal population-based cohorts, our first goal was to develop a multivariable prognostic model to evaluate the risk of developing a depressive episode by late adolescence in a Brazilian sample of adolescents with no evidence of previous depression, using a priori selected, easily obtainable sociodemographic variables collected directly from adolescents. Our second goal was to evaluate the impact of heterogeneity on its generalization to 2 diverse sociocultural contexts as well as to assess strategies to overcome these limitations.
Method
Samples and Participants
We derived our prediction model using data exclusively from the largest cohort available, the 1993 Pelotas Birth Cohort, a prospective study set in Brazil, and then evaluated the generalizability of findings in 2 diverse samples: the Environmental Risk (E-Risk) Longitudinal Twin Study, from the United Kingdom, and the Dunedin Multidisciplinary Health and Development Study, from New Zealand. Details about the 3 cohorts are reported elsewhere23, 24, 25 and in Supplement 1, available online. Briefly, in the Pelotas study, all 5,249 children born in the city of Pelotas in 1993 were enrolled in the study. The original goals of the 1993 Cohort were to evaluate trends in maternal and child health indicators to assess associations between early life variables and later outcomes. At the wave for ages 18–19 years old, the retention rate was 81.3% of the original sample. The Environmental Risk (E-Risk) Longitudinal Twin study tracks the development of a nationally representative birth cohort of 2,232 British twin children born in England and Wales in 1994–1995.20 The sample was constructed in 1999–2000, when 1,116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments. The Dunedin Study is a longitudinal investigation of health and behavior in a complete birth cohort. All study participants (N = 1,037; 91% of eligible births; 52% male) were born between April 1972 and March 1973 in Dunedin, New Zealand.
To be included in the final analysis, an evaluation for a depressive episode in late adolescence (18–19 years old) was required. Exclusionary criteria were applied, filtering out youths with intelligence quotient <70 and/or no signs of puberty by 15 years of age. Additionally, as our intention was to provide an alternative risk screening strategy beyond using previous depressive episodes or subthreshold depressive symptoms, participants with any suggestive evidence of a current or previous MDD diagnosis by the age of risk ascertainment were excluded from the final sample (see Table S1, available online). As the E-Risk sample was not evaluated at age 15, we selected the most comparable assessment wave, namely, age 12. Given the age difference at baseline between the E-Risk sample and the other samples, puberty was not considered an exclusionary criterion for this sample.
Assessment and Definition of Predictor Variables
Selection of predictors was based on scientific literature review and authors’ clinical expertise,26 but constrained to their availability in the Pelotas dataset. As we aimed for real-world implementation, following a pragmatic approach,27 we included variables readily available, not too costly to obtain, and simple to evaluate.20,22 We adopted an a priori defined criterion to use only variables directly obtained from the adolescents in the Pelotas study at the age 15 assessment wave to mirror the reality in routine practice, selecting 11 variables related to inherent characteristics (biological sex, skin color), problematic behavior indicators (drug use, school failure, social isolation, fight involvement), and markers of household dysfunction (poor relationship with mother, poor relationship with father, poor relationship between parents, childhood maltreatment, ran away from home). For comparison purposes, the harmonization of selected variables among cohorts was performed a priori by consensus among investigators from each site. Further details on variables’ assessment strategies are provided in Table S1, available online.
Assessment and Definition of the Outcome Variable
In each sample, the outcome of interest was a categorical diagnosis of depression in late adolescence. In the Pelotas cohort, trained psychologists interviewed the participants at ages 18–19 years in 2011–2012 with a structured interview for current MDD diagnosis using the Mini-International Neuropsychiatric Interview (MINI) based on DSM-IV-TR criteria, MDD section, assessing symptoms in the previous 2 weeks. For the E-Risk sample, MDD diagnosis in the previous 12 months was assessed using the Diagnostic Interview Schedule (DIS) at age 18 based on DSM-IV criteria in 2012–2014. In the Dunedin cohort, past-year MDD diagnosis was evaluated using the DIS at age 18 following DSM-III-R criteria in 1990–1991.
Statistical Analysis
A detailed description of statistical procedures used can be found in Supplement 2, available online. In an effort to enhance the reproducibility of our model, we transparently described the process of model development and validation. Using data from the Pelotas cohort, we developed a baseline model using binary logistic regression (LR) analysis—the most common statistical strategy in prognostic research. As overfitting is a major reason for irreproducibility, we derived 6 new models from the same dataset introducing different strategies of model penalization—1 penalized LR model using penalized maximum likelihood estimation (PMLE) and 5 models with increasing degrees of penalization using the Elastic-Net machine learning algorithm.21 Comparing parameters of penalized models with our baseline model, we selected for validation the one with more balanced performance measures.
To evaluate the performance of the selected model in new observations, we first internally validated it using standard bootstrapping procedures to measure undue optimism in the model’s performance metrics, which happens when the model is evaluated directly in the derivation cohort (apparent performance). Second, we quantified the model’s prognostic performance in independent observations in 2 prospective cohorts from diverse contexts.
When assessing a given model’s prediction in independent samples, its performance may be influenced by differences between derivation and validation cohorts.6 Differences not only can be related to distribution of participant characteristics (case mix), but also can be true differences in predictor effects. To take this into account, we adopted a sequence of recommended approaches.6,22 We calculated a case mix–corrected and a refitted model for each sample, and the obtained metrics were used as performance parameters for each sample. Additionally, some of the originally selected variables were not available in all the cohorts, a likely situation in real-world model application. Instead of excluding these variables, we evaluated the amount of the original model’s information lost by this mismatch.21 Finally, we evaluated the impact of between-study heterogeneity by aggregating all cohorts into an overall sample to model cohort differences either in baseline risk or in predictor effects (see Supplement 3, available online).28
All statistical analyses were performed using R 3.4.4 software (R Foundation for Statistical Computing, Vienna, Austria). A complete-case analysis strategy was used, excluding participants with any missing data. A multiple imputation procedure using R package mice (R Foundation for Statistical Computing) was applied to assess missing data impact (see Table S2 and Figure S1, available online).
Results
Sample Characteristics
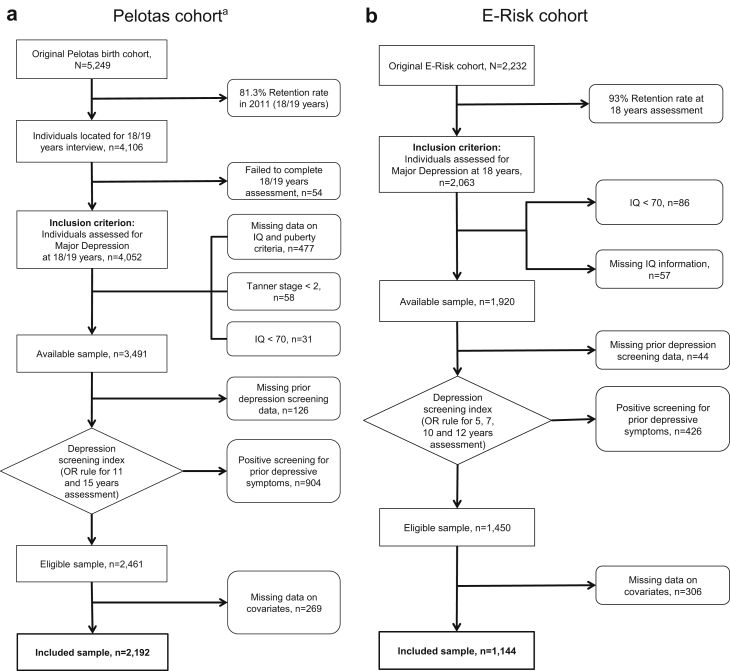
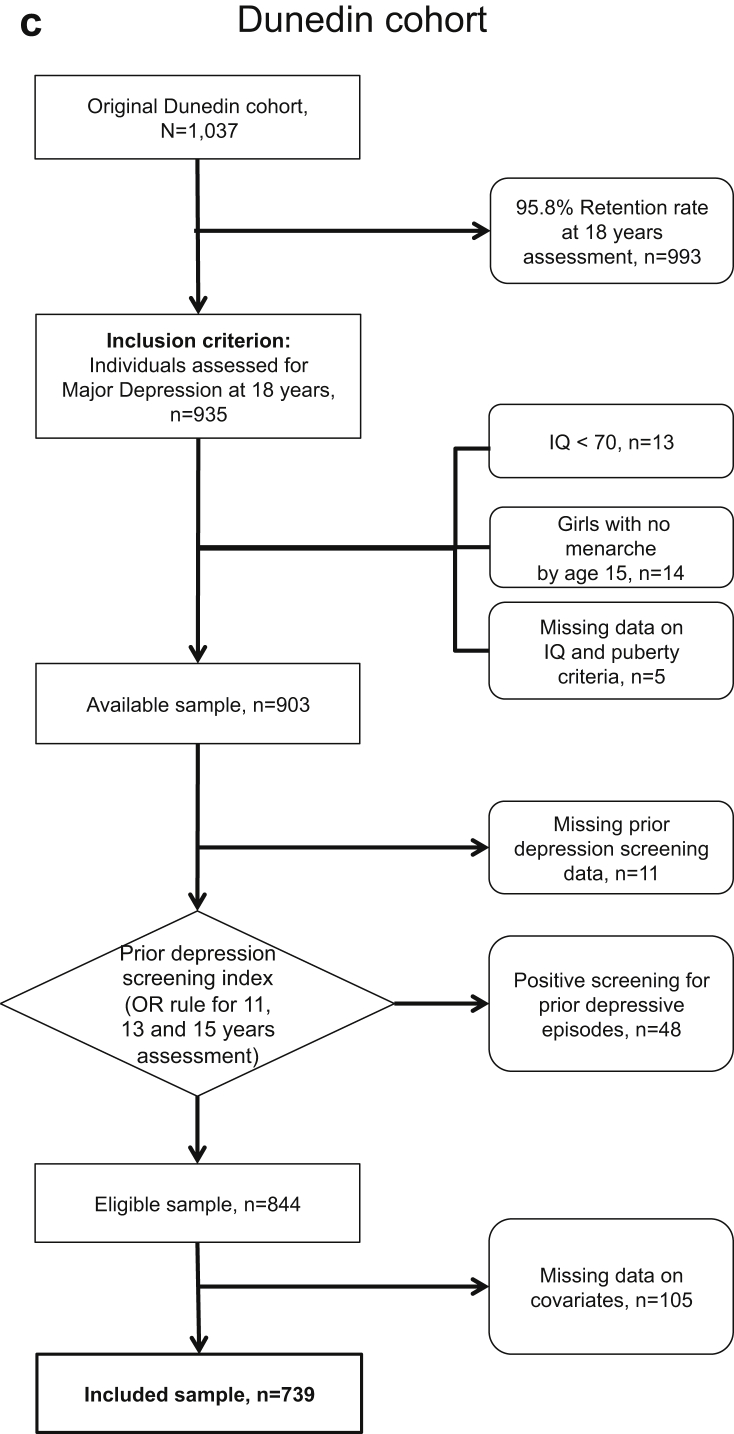
A flowchart for each cohort is shown in Figure 1a–c. From the original sample size of 5,249 adolescents in the Pelotas cohort, 81.3% were retained up to the 18–19 years old assessment, and 2,192 were included for final analyses after applying exclusion criteria. For the E-Risk and Dunedin samples, from the 2,232 and 1,037 initially assessed adolescents, 1,144 (51.3%) and 739 (71.3%) were available for assessment after exclusion criteria were applied, respectively. Comparisons on key characteristics between retained and excluded samples for the Pelotas cohort are provided in Table S3, available online.
Figure 1.
Flowcharts for Each Included Cohort Study
Note:(a) Pelotas cohort. (b) E-Risk cohort. (c) Dunedin cohort.
aIn the Pelotas dataset, 5 excluded participants had both Tanner < 2 and IQ <70.
Table 1 presents descriptive variables for both depression outcome and selected predictors in each sample. Noteworthy disparities were identified regarding rates of school failure, social isolation, fight involvement, and running away. Additionally, family relationships were not assessed in the E-Risk Study. MDD prevalence in Pelotas, E-Risk, and Dunedin samples was 3.1%, 17.7%, and 16.8%, respectively. Differences in outcome prevalence among cohorts may have reflected differences in timeframe for outcome assessment (2 weeks versus 12 months).
Table 1.
Sample Description for Each Cohorta
| Pelotas (Brazil) | E-Risk (United Kingdom) | Dunedin (New Zealand) | |
|---|---|---|---|
| Included sample | 2,192 | 1,144 | 739 |
| Assessment age, years | 15 | 12 | 15 |
| Male sex | 977 (44.6)b | 520 (45.5)b | 375 (50.7)c |
| White skin color | 1,478 (67.4)b | 1,040 (90.9)c | NAe |
| Childhood maltreatment | |||
| None | 1,539 (70.2)b | 963 (84.2)c | 489 (66.2)d |
| Probable | 390 (17.8) | 139 (12.2) | 187 (25.3) |
| Severe | 263 (12.0) | 42 (3.7) | 63 (8.5) |
| School failure | 1,127 (51.4)b | 212 (18.5)c | 80 (10.8)d |
| Social isolation | 231 (10.5)b | 63 (5.5)c | 70 (9.5)b |
| Fights | 211 (9.6)b | 130 (11.4)b | 12 (1.6)c |
| Ran away from home | 80 (3.6)b | 9 (0.8)c | 49 (6.6)d |
| Any drug use | 1,367 (62.4)b | 569 (49.7)c | 592 (80.1)d |
| Relationship with mother | NA | ||
| Great | 1,417 (64.6) | ||
| Very good | 430 (19.6) | ||
| Good | 264 (12.0) | ||
| Regular | 68 (3.1) | ||
| Bad | 13 (0.6) | ||
| Relationship with father | NA | 22.0 ± 5.4f | |
| Great | 1,019 (46.5) | ||
| Very good | 434 (19.8) | ||
| Good | 370 (16.9) | ||
| Regular | 237 (10.8) | ||
| Bad | 132 (6.0) | ||
| Relationship between parents | NA | ||
| Great | 886 (40.4)b | 345 (46.7)c | |
| Very good | 421 (19.2) | 278 (37.6) | |
| Good | 404 (18.4) | 91 (12.3) | |
| Regular | 301 (13.7) | 23 (3.1) | |
| Bad | 180 (8.2) | 2 (0.3) | |
| Depression prevalence | 69 (3.1)b,g | 202 (17.7)c,h | 124 (16.8)d,h |
Note: Results are shown as number of participants (percentage) for categorical variables and as mean ± SD for continuous variables for participants included in the final analyses. NA = Data not available in the cohort.
See Table S1, available online, for assessment strategies applied to each cohort.
Superscript letters b, c, and d denote column differences among the samples: different letters show significant differences and the same letters indicate nonsignificant differences from each other, assessed by χ2 test at .05 level. For variables with more than 2 categories, the superscript letters were placed in the first row of the variable and represent the assessment of the variable as a group, not per row.
Skin color was not assessed in the cohort. Less than 7% of the cohort had any nonwhite ancestry.
Parent Attachment Scale score (range, −6 to 28)—adolescent assessment about the relationship with both parents.
Presence of symptoms reaching diagnostic criteria within a 2-week period before assessment.
Presence of symptoms reaching diagnostic criteria within a 12-month period before assessment.
Model Development and Validation
Performance measures showed better results for models using LR strategies compared with machine learning Elastic-Net approaches. In the Pelotas sample, discriminative capacity to parse between adolescents who later developed depression at age 18 and those who did not, assessed by the C-statistic, ranged from 0.76 to 0.79, indicating overall good discrimination, as shown in Table 2.
Table 2.
Apparent Performance Parameters Obtained From the Models Derived From the Pelotas Dataset
| Model parameters |
|||||||
|---|---|---|---|---|---|---|---|
| LR | PMLEa | Ridgeb | .25b | .50b | .75b | LASSOb | |
| R2 | 0.15 | 0.12 | 0.12 | 0.10 | 0.10 | 0.10 | 0.10 |
| LR χ2c | 81.90 | 66.17 | 63.30 | 54.40 | 54.32 | 54.71 | 54.10 |
| Brier scored | 2.88 | 2.93 | 2.93 | 2.95 | 2.95 | 2.95 | 2.95 |
| C-statistice | 0.79 | 0.78 | 0.78 | 0.76 | 0.76 | 0.76 | 0.76 |
| Calibration slope | 1.00 | 1.26 | 1.35 | 1.47 | 1.42 | 1.38 | 1.39 |
Note: Higher results for R2, LR χ2, and C-statistic; lower results for Brier score; and results closer to 1 for calibration slope indicate better model performance. .25 = Elastic-Net with alpha = .25; .50 = Elastic-Net with α = .50; .75 = Elastic-Net with α = .75; Brier score = quadratic scoring rule that combines calibration and discrimination; C-statistic = concordance statistic, or area under the curve of the receiver operating characteristic; Calibration slope = measure of agreement between observed and predicted risk of the event (outcome) across the whole range of predicted values; LASSO = least absolute shrinkage and selection operator; LR = logistic regression; LR χ2 = likelihood ratio χ2; PMLE = penalized maximum likelihood estimation; R2 = Nagelkerke’s R2; Ridge = Ridge regression.
The penalty factor used in the PMLE was empirically obtained from our data.
For the Elastic-Net approach, we have a priori defined a grid of values for the hyperparameter α, ranging from 0 (full Ridge) to 1 (full LASSO), with increments of 0.25. For each α value, a 10-fold cross-validation was used to select the penalty coefficient (λ) that minimized the mean squared prediction error, which was then used for shrinkage of coefficients and/or variable selection. See Table S4, available online, for model’s coefficients.
All LR χ2p values < .001.
Multiplied by 102.
The C-statistic ranges from 0.5 for noninformative models to 1.0 for perfect models.
Predictably, the baseline model showed the best combination of performance metrics. Among penalized models, the PMLE model demonstrated better performance compared with all Elastic-Net models. As nonpenalized models face a greater risk of overfitting, we proceeded to the next step with both LR models for comparison. We internally validated each using bootstrapping evaluation with 1,000 iterations. As expected, measurement of optimism—difference between apparent and bias-corrected performance metrics—was lower for the PMLE model compared with the LR model (ΔC-statistic: 0.067 versus 0.098; Δslope: −0.004 versus 0.548; ΔR2: 0.034 versus 0.149), suggesting lower overfitting and higher probability of reliable results when applied to independent samples. Additionally, as shown in Figure S2a–b, the PMLE model was also more calibrated, with a 60% reduction in mean square error compared with the LR model. Therefore, the PMLE model was selected as the Pelotas final model, with a C-statistic of 0.78 (bootstrap-corrected 95% CI: 0.73–0.82).
Using the most common external validation strategy, the linear predictor derived from the selected Pelotas model (Table S4, available online) was applied to the other samples. There was an expected decrease in the performance metrics in both independent cohorts (E-Risk: C-statistic 0.59 [bootstrap-corrected 95% CI: 0.55–0.63]; Dunedin: C-statistic 0.63 [bootstrap-corrected 95% CI: 0.59–0.67]). The performance results for each step of the validation process are presented in Table 3.
Table 3.
Comparative Results for Each Step of Model Performance in the 3 Cohorts
| Performance parameter | Description | Pelotas |
E-risk |
Dunedin |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Apparent validation | Internal validation | External validation | Case mix–corrected modela | Refitted modelb | External validation | Case mix–corrected modela | Refitted modelb | ||
| C-statistic | Concordance statistic, equal to area under the curve of receiver operating characteristic in binary endpoints | 0.78 | 0.71 | 0.59 | 0.66 | 0.62 | 0.63 | 0.68 | 0.67 |
| Calibration-in-the-large | Overall measure of calibration, compares mean observed with mean predicted in validation dataset | 0.00 | 0.02 | 2.37 | 0.02 | 0.00 | 2.26 | −0.06 | 0.00 |
| Calibration slope | Measure of agreement between observed and predicted risk of event (outcome) across whole range of predicted values | 1.26 | 1.00 | 0.58 | 0.99 | 1.20 | 0.77 | 0.98 | 1.24 |
| R2 | Measure of overall goodness-of-fit of model | 0.12 | 0.06 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.09 |
| Brier score | Quadratic scoring rule that combines calibration and discrimination | 0.03 | 0.03 | 0.17 | 0.02 | 0.14 | 0.16 | 0.02 | 0.13 |
| Emax | Maximum absolute error in predicted probabilities | 0.19 | 0.03 | 0.29 | 0.01 | 0.09 | 0.38 | 0.01 | 0.11 |
| Available information for assessment of model performance | 100% | 86.9% | 93.1% | ||||||
Note: Higher results for C-statistic and R2, lower results for Brier score and Emax, results closer to 0 for calibration-in-the-large, and results closer to 1 for calibration slope indicate better model performance.
Reference values indicating the model’s performance under the assumption that Pelotas model’s coefficients are fully correct for the validation setting, simulating similar case mix between samples.22
Reference values indicating the model’s performance after refitting predictors’ coefficients that would be optimal for the validation sample.22 (See Supplement 2, available online, for further details.)
Model Updating
As variables from both independent datasets did not perfectly pair with the set selected from the Pelotas study, we calculated the amount of information lost owing to this mismatch.21 In the E-Risk dataset, 13.1% of original model information was unavailable, mainly from the household dysfunction indicators. In the Dunedin dataset, this percentage was lower, at around 6.9%.
Considering the relevant heterogeneity among cohorts, we evaluated whether the integration of information from the external cohorts could produce improvement in model performance, in line with current methodological recommendations.4 As differences in outcome prevalence were not trivial, we updated the Pelotas model by correcting its intercept for each cohort. In both validation samples, the updated model produced better calibration, reducing all measures of calibration error (Supplement 2 and Figure S3a–d, available online).
Exploratory Analyses
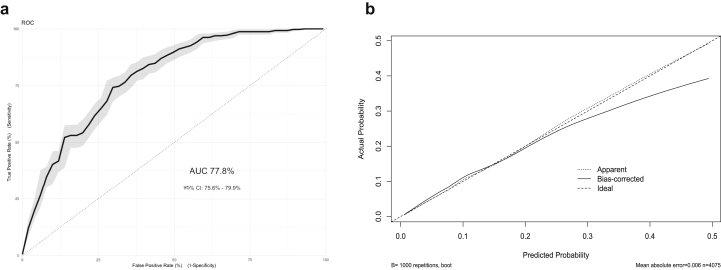
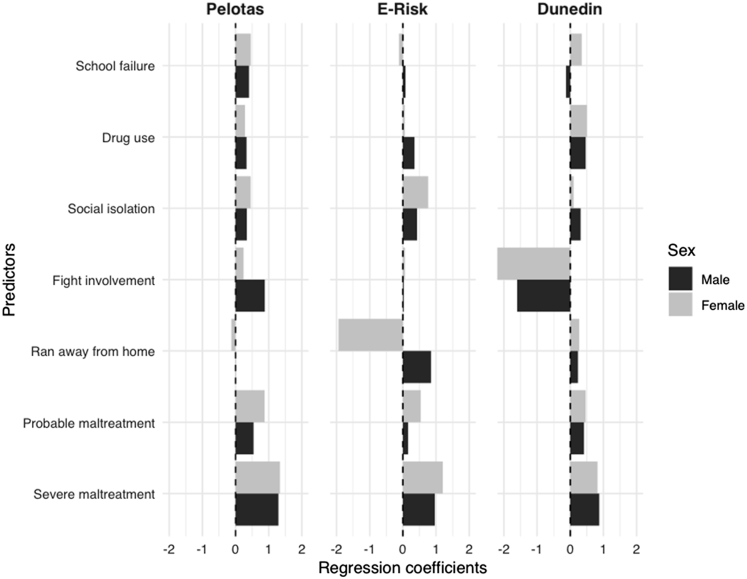
The merger of all 3 cohorts into an aggregated sample to assess between-cohort heterogeneity increased the total number of participants to 4,075, of which 395 (9.7%) demonstrated a positive outcome. Given that most of the participants were from the Pelotas cohort (53.8%), the C-statistic was also 0.78 (bootstrap-corrected 95% CI: 0.75–0.80), but showed lower overfitting after internal validation using bootstrapping (Figure 2a–b). Inclusion of each cohort’s main effects and their interaction terms with all predictors into a PMLE model suggested that not only disparities in case mix, as shown in Table 1, but also between-cohort differences in predictor effects might have influenced external validation results, particularly considering the difference in the ran-away and fight involvement variables (Figure 3).
Figure 2.
Performance Measures of the Aggregated Sample Model
Note:(a) The area under the curve (AUC) of the receiver operating characteristic (ROC) curve and the bootstrapped 95% CI (indicated by gray shading) of the C-statistic, and (b) calibration plot after internal validation using 1,000 iterations bootstrapping. Apparent and bias-corrected results were plotted as a nonparametric calibration curve, estimated over a sequence of predicted values versus observed values using a smoothing technique.
Figure 3.
Prognostic Contribution of Each Included Variable to the Aggregated Sample Prediction Model of Adolescent Depression
Note:Comparison of the prognostic contribution of each included variable in each cohort to the aggregated sample prediction model of adolescent depression, stratified by sex for Brazil, United Kingdom, and New Zealand cohorts. Predictors’ β coefficients from penalized logistic regression are shown as bars in the x-axis. Positive values represent greater risk and negative values represent lower risk of the outcome. The results shown are derived from values presented inTable S5, available online. Some of the variables previously included in the Pelotas model were excluded for comparability among datasets.
Discussion
Following current standards for psychiatric prognostic research,20 our study proposes a multivariable model developed in a Brazilian cohort to predict among adolescents with no evidence of previous depression the risk of developing a depressive episode in late adolescence. Our model showed beyond chance results of discrimination and calibration, with metrics comparable to established prognostic models from other areas of medicine,3,29 and could be viewed as a promising aid to adolescent depression risk stratification.30
Evaluation in independent samples is deemed essential for generalization of findings. Disparities among samples are frequently seen as major obstacles for model validation, replication, and generalizability. However, as the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement emphasizes, the term validation can be misleading, recommending that an external validation should quantify the model’s prognostic performance in a new sample, not simply classifying it as a positive or negative validation.4,31,32 This broader validation approach not only promotes the assessment of the model’s performance in the new sample but also facilitates understanding of why the results differ.
For this study, we assessed the validation performance of the model developed in our Brazilian sample in 2 population-based longitudinal cohorts from 2 different continents. The development of a model in 1 middle-income country and its external validation in samples representing diverse sociocultural and economic contexts, using different assessment strategies for data collection at different time periods among them, may help evaluate if and where its results can be generalized. Our results suggest that, albeit adaptations should be applied to the original model to enhance external clinical utility, the original prognostic model could be applied in multiple other contexts despite major differences in assessment strategies, socioeconomic characteristics, and cultural influences. Given such profound differences, it was expected that the developed model could not be easily transported to new settings.9 Even though lower in degree, our model kept a valid and beyond chance prognostic capacity in parsing future risk of depression among the adolescents in the independent cohorts, especially when heterogeneity among samples was accounted for (Supplement 3 and Figure S3a–d, available online).
Early identification of people at higher risk for psychiatric disorders could potentially lessen the massive burden imposed by these conditions. Positive family history of depression and the presence of subthreshold depressive symptoms have been the most commonly used criteria for identifying at-risk children and adolescents.33 Although these strategies have been replicated, reliance on single predictors restricts their prognostic contribution, not accounting for a wider range of risk. Additionally, from a pragmatic perspective, the requirement of trained staff for proper evaluation of such predictors limits their potential implementation, given that access to treatment has been systematically highlighted as a major barrier for child and adolescent mental health care.34
Our study has several strengths. We developed a prognostic model for MDD according to most recent guidelines in prognostic research and transparent reporting6,20 using modern, state-of-the-art statistical strategies21,22 with broad external validation assessment. Comprising only 11 predictors, all easily obtainable, quick to assess, and collected directly from the adolescent, with no need for highly specialized training, external informants, or laboratory analyses, our results could be seen as promising if further replicated. Additionally, consistent with the evidence-based pragmatic psychiatry initiative,27 we opted to prioritize simplicity over accuracy, selecting predictors that could be more easily and broadly implemented, enhancing probability of future clinical use and patient acceptance.
Significant limitations of our study also need to be considered. Having based the development of our prognostic model on the Pelotas cohort, an ongoing study not primarily focused on mental health, availability of variables of interest was limited to those previously collected, precluding the use of some potentially relevant factors. MDD diagnosis was assessed at the age 18–19 years wave by evaluating symptoms in the 2 weeks before the interview, limiting comparability to other epidemiological cohort studies as well as reducing the prevalence of the outcome of interest. Consequently, the number of outcome events per selected variable was lower in the Pelotas sample (events per variable = 6.27), increasing the risk of overfitting.20, 21, 22 Strategies such as machine learning regularization methods, with shrinkage and selection of predictors as well as measurement of performance optimism, were implemented to constrain the impact of this limitation. The proposed model is also not necessarily prognostic of earlier or later onsets of depression.35 Furthermore, as we were analyzing participants at higher risk of MDD diagnosis, we could not discard the chance that all self-report assessments were biased by this risk. Additionally, as our goal was to provide a risk stratification tool that could be supplementary to current strategies of risk evaluation, we opted to exclude participants with any evidence of previous or current depressive episodes because the occurrence of a depressive episode already heightens the risk of subsequent depression. This strategy resulted in a significant number of exclusions that could have biased our findings; therefore, we compared the covariates between included and excluded samples (Table S3, available online), with anticipated differences between them, and performed sensitivity analyses (see Table S6 and Figure S4, available online) in which similar performance results were identified.
The differences in predictors’ availability and assessment strategies among cohorts are another relevant shortcoming, which could have influenced results obtained in the external validations. The unavailability of assessment data at age 15 in the E-Risk sample could have impacted the comparability among the samples, as puberty is a well-known risk contributor for depression,36 and could therefore have contributed to the performance result of the model in that sample. A priori harmonization of variables and measurement of information lost as a result of mismatching variables were applied to minimize the effect of these limitations. Also, we were constrained to variables assessed in each cohort study, which precluded important predictors being included in our model, and the included variables could be carrying prognostic information from uncollected predictors, which could have contributed to discrepancies in predictor effects shown in Figure 3. Finally, in the present study, we could not evaluate the potential impact of the developed model on clinical decision making.20
Exploratory analyses suggested that information generated by our model increased prognostic ability above and beyond established risk factors, such as subsyndromal symptoms and a positive family history of depression (Supplement 4 and Table S7, available online). At the same time, the risk score was also associated, to a lesser degree, with other diagnostic outcomes (C-statistic range: 0.64–0.70) (Table S8, available online). In line with the current literature on the early detection of psychopathology in youth,37 we believe that a transdiagnostic approach could be considered, despite its limitations,38 as specificity of psychiatric prognostic models is likely to be low and as less specific preventive interventions could promote meaningful changes in psychiatric burden, either from individual or public health perspectives.9,39
In conclusion, we present the development of a prognostic model for MDD among Brazilian adolescents, externally evaluated in 2 samples from diverse sociocultural contexts using different strategies for data collection than the original cohort. Heterogeneity among studies was high and possibly accounted for major discrepancies in prognostic performance, probably related not only to different case mix but also to weight of coefficients.6 Future studies should pursue methodological strategies for embracing heterogeneity among samples, instead of avoiding it, thus producing results that are more likely to be translated into clinical practice across a range of contexts.
Footnotes
This work is supported by research grants from Brazilian public funding agencies to Drs. Kieling and Rohde: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). This article is based on data from the study “Pelotas Birth Cohort, 1993” conducted by Postgraduate Program in Epidemiology at Universidade Federal de Pelotas, currently supported by the Wellcome Trust through the program entitled Major Awards for Latin America on Health Consequences of Population Change. The E-Risk Study is funded by the UK Medical Research Council (G1002190). Additional support was provided by the National Institute of Child Health and Human Development (HD077482) and by the Jacobs Foundation. Dr. Arseneault is the Mental Health Leadership Fellow for the UK Economic and Social Research Council. The Dunedin Study is supported by the New Zealand Health Research Council, New Zealand Ministry of Business, Innovation, and Employment, National Institute on Aging Grant R01AG032282 and UK Medical Research Council Grant MR/P005918/1. The Identifying Depression Early in Adolescence (IDEA) project is funded by an MQ Brighter Futures grant (MQBF/1 IDEA). Additional support was provided by the UK Medical Research Council (MC_PC_MR/R019460/1) and the Academy of Medical Sciences (GCRFNG/100281) under the Global Challenges Research Fund. The views expressed are those of the authors. None of the funders played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
Conceptualization: Rohde, Kieling
Data curation: Rocha, Anselmi, Arseneault, Barros, Caspi, Danese, Gonçalves, Harrington, Menezes, Moffitt, Poulton, Wehrmeister, Kieling
Formal analysis: Rocha, Fisher, Caye, Harrington, Houts, Menezes, Moffitt, Mondelli, Rohde, Wehrmeister, Kieling
Funding acquisition: Fisher, Arseneault, Barros, Caspi, Mondelli, Kieling
Investigation: Fisher, Caye, Anselmi, Arseneault, Barros, Caspi, Danese, Gonçalves, Menezes, Moffitt, Poulton, Rohde, Kieling
Methodology: Rocha, Fisher, Caye, Arseneault, Barros, Caspi, Danese, Gonçalves, Harrington, Houts, Menezes, Moffitt, Mondelli, Poulton, Rohde, Wehrmeister, Kieling
Project administration: Arseneault, Mondelli, Kieling
Resources: Kieling
Supervision: Fisher, Kieling
Validation: Caye
Writing – original draft: Rocha, Kieling
Writing – review and editing: Rocha, Fisher, Caye, Anselmi, Arseneault, Barros, Caspi, Danese, Gonçalves, Harrington, Houts, Menezes, Moffitt, Mondelli, Poulton, Rohde, Wehrmeister, Kieling
ORCID
Thiago Botter-Maio Rocha, MD, PhD: https://orcid.org/0000-0002-3080-6078
Helen L. Fisher, PhD: https://orcid.org/0000-0003-4174-2126
Louise Arseneault, PhD: https://orcid.org/0000-0002-2938-2191
Fernando C. Barros, MD, PhD: https://orcid.org/0000-0001-5973-1746
Andrea Danese, MD, PhD: https://orcid.org/0000-0001-8718-5412
Helen Gonçalves, PhD: https://orcid.org/0000-0001-6470-3352
Hona Lee Harrington, BA: https://orcid.org/0000-0002-0225-0607
Valeria Mondelli, MD, PhD: https://orcid.org/0000-0001-8690-6839
Richie Poulton, PhD: https://orcid.org/0000-0002-1052-4583
Fernando Wehrmeister, PhD: https://orcid.org/0000-0001-7137-1747
Christian Kieling, MD, PhD: https://orcid.org/0000-0001-7691-4149
The authors are extremely grateful to the individuals who participated in the studies at each of the sites and to all members of the IDEA consortium and the study teams for their dedication, hard work, and insights. The authors thank all members of the ProDIA group for their assistance in the development of this work. The authors would like to especially thank João Ricardo Sato, PhD, of the Universidade Federal do ABC for his thoughtful insights into the initial version of this study and Rachel Latham, PhD, of King’s College London for assistance with checking the statistical analysis for the E-Risk study.
Disclosure: Dr. Mondelli has received research funding from Johnson and Johnson, a pharmaceutical company interested in the development of anti-inflammatory strategies for depression, but the research described in this article is unrelated to this funding. Dr. Rohde has been on the speakers’ bureau/advisory board and/or has acted as a consultant for Eli Lilly and Company, Janssen-Cilag, Novartis, and Shire (a Takeda company) in the last 3 years. He has received authorship royalties from Oxford University Press and ArtMed. He has received travel awards from Shire for taking part in the 2014 American Psychiatric Association 2014 Annual Meeting. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by him have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Eli Lilly and Company, Janssen-Cilag, Novartis, and Shire. Dr. Kieling is an Academy of Medical Sciences Newton Advanced Fellow and has received grant or research support from Brazilian governmental research funding agencies (Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [CAPES], and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul [Fapergs]) and United Kingdom funding agencies (MQ, Medical Research Council, and Academy of Medical Sciences). He has served on the editorial boards of Archives of Clinical Psychiatry, Global Mental Health, and Jornal Brasileiro de Psiquiatria. He has received authorship royalties from Brazilian publishers ArtMed and Editora Manole. Drs. Rocha, Fisher, Caye, Anselmi, Arseneault, Barros, Caspi, Danese, Gonçalves, Houts, Menezes, Moffitt, Poulton, and Wehrmeister and Ms. Harrington have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Steyerberg E.W., Moons K.G., van der Windt D.A. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.D’Agostino R.B., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 4.Moons K.G., Kengne A.P., Grobbee D.E. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98:691–698. doi: 10.1136/heartjnl-2011-301247. [DOI] [PubMed] [Google Scholar]
- 5.Noble D., Mathur R., Dent T., Meads C., Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ. 2011;343:d7163. doi: 10.1136/bmj.d7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debray T.P., Vergouwe Y., Koffijberg H., Nieboer D., Steyerberg E.W., Moons K.G. A new framework to enhance the interpretation of external validation studies of clinical prediction models. J Clin Epidemiol. 2015;68:279–289. doi: 10.1016/j.jclinepi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Debray T.P., Moons K.G., Ahmed I., Koffijberg H., Riley R.D. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med. 2013;32:3158–3180. doi: 10.1002/sim.5732. [DOI] [PubMed] [Google Scholar]
- 8.Siontis G.C., Tzoulaki I., Castaldi P.J., Ioannidis J.P. External validation of new risk prediction models is infrequent and reveals worse prognostic discrimination. J Clin Epidemiol. 2015;68:25–34. doi: 10.1016/j.jclinepi.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Fusar-Poli P., Werbeloff N., Rutigliano G. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45:562–570. doi: 10.1093/schbul/sby070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley R.D., Ensor J., Snell K.I. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ. 2016;353:i3140. doi: 10.1136/bmj.i3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snell K.I., Hua H., Debray T.P. Multivariate meta-analysis of individual participant data helped externally validate the performance and implementation of a prediction model. J Clin Epidemiol. 2016;69:40–50. doi: 10.1016/j.jclinepi.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agostino R.B., Grundy S., Sullivan L.M., Wilson P., CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 13.Fusar-Poli P., Rutigliano G., Stahl D. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74:493–500. doi: 10.1001/jamapsychiatry.2017.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birmaher B., Merranko J.A., Goldstein T.R. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry. 2018;57:755–763.e754. doi: 10.1016/j.jaac.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler R.C., Warner C.H., Ivany C. Predicting suicides after psychiatric hospitalization in US Army soldiers: the Army Study To Assess Risk and Resilience in Servicemembers (Army STARRS) JAMA Psychiatry. 2015;72:49–57. doi: 10.1001/jamapsychiatry.2014.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regier D.A., Narrow W.E., Clarke D.E. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman M., Ellison W., Young D., Chelminski I., Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry. 2015;56:29–34. doi: 10.1016/j.comppsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Studerus E., Ramyead A., Riecher-Rössler A. Prediction of transition to psychosis in patients with a clinical high risk for psychosis: a systematic review of methodology and reporting. Psychol Med. 2017;47:1163–1178. doi: 10.1017/S0033291716003494. [DOI] [PubMed] [Google Scholar]
- 19.King M., Walker C., Levy G. Development and validation of an international risk prediction algorithm for episodes of major depression in general practice attendees: the PredictD study. Arch Gen Psychiatry. 2008;65:1368–1376. doi: 10.1001/archpsyc.65.12.1368. [DOI] [PubMed] [Google Scholar]
- 20.Fusar-Poli P., Hijazi Z., Stahl D., Steyerberg E.W. The science of prognosis in psychiatry: a review. JAMA Psychiatry. 2018;75:1289–1297. doi: 10.1001/jamapsychiatry.2018.2530. [DOI] [PubMed] [Google Scholar]
- 21.Harrell F.E. Springer; New York: 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 22.Steyerberg E.W. Springer; New York: 2009. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. [Google Scholar]
- 23.Moffitt T.E., E-Risk Study Team Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 24.Poulton R., Moffitt T.E., Silva P.A. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50:679–693. doi: 10.1007/s00127-015-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Victora C.G., Hallal P.C., Araújo C.L., Menezes A.M., Wells J.C., Barros F.C. Cohort profile: the 1993 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2008;37:704–709. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 26.Newton S., Docter S., Reddin E., Merlin T., Hiller J. Depression in adolescents and young adults: evidence review. Adelaide: Adelaide Health Technology Assessment (AHTA), University of Adelaide. 2010. https://www.adelaide.edu.au/ahta/pubs/depression-in-adolescents-and-young-adults.pdf
- 27.Paulus M.P. Evidence-based pragmatic psychiatry—a call to action. JAMA Psychiatry. 2017;74:1185–1186. doi: 10.1001/jamapsychiatry.2017.2439. [DOI] [PubMed] [Google Scholar]
- 28.Steyerberg E.W., Harrell F.E. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–247. doi: 10.1016/j.jclinepi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambertini M., Pinto A.C., Ameye L. The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br J Cancer. 2016;115:1471–1478. doi: 10.1038/bjc.2016.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieling C., Adewuya A., Fisher H.L. Identifying depression early in adolescence. Lancet. 2019;3:211–212. doi: 10.1016/S2352-4642(19)30059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 32.Moons K.G., Altman D.G., Reitsma J.B. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 33.Hetrick S.E., Cox G.R., Witt K.G., Bir J.J., Merry S.N. Cognitive behavioural therapy (CBT), third-wave CBT and interpersonal therapy (IPT) based interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev. 2016;8:CD003380. doi: 10.1002/14651858.CD003380.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costello E.J., He J.P., Sampson N.A., Kessler R.C., Merikangas K.R. Services for adolescents with psychiatric disorders: 12-month data from the National Comorbidity Survey-Adolescent. Psychiatr Serv. 2014;65:359–366. doi: 10.1176/appi.ps.201100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birmaher B., Williamson D.E., Dahl R.E. Clinical presentation and course of depression in youth: does onset in childhood differ from onset in adolescence? J Am Acad Child Adolesc Psychiatry. 2004;43:63–70. doi: 10.1097/00004583-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Thapar A., Collishaw S., Pine D.S., Thapar A.K. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGorry P.D., Hartmann J.A., Spooner R., Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. 2018;17:133–142. doi: 10.1002/wps.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fusar-Poli P., Solmi M., Brondino N. Transdiagnostic psychiatry: a systematic review. World Psychiatry. 2019;18:192–207. doi: 10.1002/wps.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caspi A., Moffitt T.E. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175:831–844. doi: 10.1176/appi.ajp.2018.17121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.