Abstract
Maintaining strict temperature control during the maintenance phase of targeted temperature management (TTM) after cardiac arrest may be an important component of clinical care. Temperature variability outside of the goal temperature range may lessen the benefit of TTM and worsen neurologic outcomes. The purpose of this retrospective study of 186 adult patients (70.4% males, mean age 53.8 ± 15.7 years) was to investigate the relationship between body temperature variability (at least one body temperature measurement outside of 36°C ± 0.5°C) during the maintenance phase of TTM at 36°C after cardiac arrest and neurologic outcome at hospital discharge. Patients with temperature variability (n = 124 [66.7%]) did not have significantly higher odds of poor neurologic outcome compared with those with no temperature variability (odds ratio [OR] = 1.01, 95% confidence interval [CI] = 0.36–2.82). Use of neuromuscular blocking agents (NMBAs) and having an initial shockable rhythm were associated with both higher odds of good neurologic outcome (shockable rhythm: OR = 10.77, 95% CI = 4.30–26.98; NMBA use: OR = 4.54, 95% CI = 1.34–15.40) and survival to hospital discharge (shockable rhythm: OR = 5.90, 95% CI = 2.65–13.13; NMBA use: OR = 3.03, 95% CI = 1.16–7.90). In this cohort of postcardiac arrest comatose survivors undergoing TTM at 36°C, having temperature variability during maintenance phase did not significantly impact neurologic outcome or survival.
Keywords: cardiac arrest, induced hypothermia, induced mild hypothermia, therapeutic hypothermia
Introduction
Targeted temperature management (TTM) is a neuroprotective strategy that has largely become a standard of care for comatose survivors of cardiac arrest, particularly for those with out-of-hospital cardiac arrest and a shockable rhythm (Bernard et al., 2002; HACA, 2002; Callaway et al., 2015; Geocadin et al., 2017). TTM comprises three main phases; (1) induction, which is the rapid lowering of core body temperature to the targeted temperature, (2) maintenance, in which the targeted goal temperature is maintained for a specific period of time (usually 24 hours), and (3) rewarming, in which body temperature is slowly increased to normothermia (Geocadin et al., 2017; Madden et al., 2017).
National guidelines recommend targeting a goal temperature between 32°C and 36°C (Callaway et al., 2015), with many institutions choosing either 33°C (TTM33) or 36°C (TTM36) (Deye et al., 2016; Bray et al., 2017; Johnson et al., 2020). Targeting TTM36 for postresuscitative care has become increasingly utilized following a large clinical trial demonstrating no difference in neurologic outcomes compared with TTM33 (Nielsen et al., 2013). Despite the increase in utilization of TTM36, many facets related to implementation of this relatively recent targeted temperature remain uncertain.
One aspect of clinical management during TTM is the ability to maintain control of body temperature at the specified goal temperature. The Emergency Neurological Life Support (ENLS) guidelines recommend a variation in goal temperature of less than ±0.5°C during the maintenance phase of TTM (Rittenberger et al., 2015; Elmer and Polderman, 2017). It has been hypothesized that strict temperature control and avoiding fever during TTM are necessary to achieve the maximum neuroprotective benefit (Neumar et al., 2008; Polderman and Herold, 2009). However, several studies have demonstrated no difference in neurologic outcomes or survival for patients with temperature variability compared with those without (Nobile et al., 2015; Nayeri et al., 2017). Notably, these studies only included patients with TTM33.
Strict adherence to maintaining goal temperature may be more challenging when targeting 36°C compared with 33°C (Casamento et al., 2016; Bray et al., 2017). Low compliance with maintaining TTM36 results in patients spending more time outside of the goal temperature range. As TTM36 is just below normothermia, it is potentially more critical to maintain strict temperature control to avoid hyperthermia.
Although limited evidence has not shown low compliance with TTM36 to be associated with worse neurologic outcomes relative to TTM33, Bray et al. (2017) reported a trend toward worsening patient outcomes. However, the impact of temperature variability on neurologic outcomes specifically at TTM36 has not been widely studied. The purpose of this retrospective cohort study was to investigate the association between body temperature variability during the maintenance phase of TTM and neurologic outcomes for patients receiving TTM at 36°C after cardiac arrest.
Methods
Population and study design
This was a retrospective study of adult postcardiac arrest patients at Harborview Medical Center, a Level-1 Trauma Center and Postcardiac Arrest Receiving Center with 413 beds in Seattle, WA. Patients from November 2014 to June 2017 were included in the study if they were ≥18 years old, received TTM at 36°C using surface cooling following a cardiac arrest, and completed at least the maintenance phase of TTM. Patients were excluded if there were <10 body temperatures recorded, or if they did not complete 24 hours of TTM. Patients were identified using cardiac arrest ICD-9 diagnosis codes from the electronic health record and from an internal cardiac arrest database of all patients treated with TTM. This study was approved by the local institutional review board with a waiver of informed consent.
The primary outcome of this study was neurologic outcome at hospital discharge. Good neurologic outcome was defined as a Cerebral Performance Category (CPC) scale score of ≤2. Poor neurologic outcome was defined as a CPC score >2. CPC score was determined following a comprehensive review of the electronic health record, including physician, nursing, physical and occupational therapy notes, cognitive evaluations, and the discharge summary by the primary author before knowledge of specific cardiac arrest details. If the primary author was unsure of the appropriate CPC score, the case was discussed with other authors (also blinded to cardiac arrest details) for consensus.
TTM protocol
Patient management during TTM was guided by hospital protocol and the patient care team. In general, TTM was initiated using surface cooling gel pads with a temperature management system (Arctic Sun®; Bard Medical, Colorado, United States) for 24 hours after goal temperature was reached. Gel pads were selected based on patient's size according to the manufacturer's recommendations. The Arctic Sun temperature management system uses a proportional, integral derivative formula to systematically adjust the water temperature using a preprogrammed algorithm and adjusts water temperature in response to body temperature every 1 minute to maintain the programmed goal temperature (Ang et al., 2005; Badjatia et al., 2007). Temperature measurement accuracy is reported to be ±0.2°C, with precision of 0.1°C and a maximum water flow of 5 L/min. Water bath range is from 3°C to 45°C.
Core body temperature was monitored using an esophageal temperature sensing probe (Level 1® Esophageal Stethoscope with Temperature Sensor; Smiths Medical, St. Paul, MN). Sedation included propofol or midazolam infusion, and pain was managed using fentanyl intravenous (IV) boluses. Rewarming occurred following ∼24 hours at 36°C at a rate of 0.3°C/h to a temperature of 37°C. After rewarming, surface cooling gel pads were set to maintain normothermia at 37°C for an additional 48 hours for all patients regardless of neurologic status (i.e., for patients who remained comatose and for patients who regained consciousness).
Shivering was documented in the medical record using a binary code (present or absent). As per the TTM protocol, all patients received acetaminophen 650 mg via a nasogastric tube every 6 hours and counterwarming using a forced air warming blanket (3M™ Bair Hugger™ Normothermia System). If shivering was detected, a sedation bolus was given with an increase in the continuous infusion. If shivering continued, an IV bolus or continuous infusion of vecuronium or cisatracurium was used to stop shivering. Other medications used to manage shivering included magnesium and buspirone as ordered by the medical team.
Data management
Data were obtained from the electronic health record and included the following: age, sex, race, body mass index (BMI) (using weight on admission to the intensive care unit), location of arrest (in-hospital or out-of-hospital), initial rhythm (shockable or nonshockable), presence of a witness to the arrest, bystander cardiopulmonary resuscitation (CPR), length of cardiac arrest (from time of emergency services arrival [or time of call if available] to return of spontaneous circulation, or from inpatient code report), cause of cardiac arrest (cardiac, respiratory, or unknown), length of hospitalization, survival to hospital discharge, discharge disposition, body temperature, medications administered during TTM, seizure activity diagnosed by electroencephalogram (EEG) (EEG data only available for patients when specifically ordered by the provider, most often for patients with suspected seizure activity identified from clinical observations), and presence of shivering.
For the purposes of this study, the maintenance phase of TTM was defined as the period from the first time a body temperature of 36°C ± 0.2°C was recorded after induction of TTM until the start of rewarming. Patients with temperature variability were defined as having at least one body temperature outside of 36°C ± 0.5°C (either >36.5°C or <35.5°C) during the maintenance phase. Data were inspected for accuracy, and values falling outside of a plausible physiologic range were excluded (e.g., likely to be entry errors).
Statistical analysis
Patient characteristics were presented as counts, relative frequencies, means, and standard deviations (SDs), as appropriate. Multivariate logistic regression models were used to evaluate the relationship between neurologic outcome and temperature variability. Covariates included age, sex, race, BMI, location of cardiac arrest, initial rhythm, initiation of bystander CPR, cause of cardiac arrest, presence of shivering, and use of neuromuscular blocking agents (NMBAs). Time to return of spontaneous circulation was not included given >40% missing data.
As the total length of time spent outside of goal temperature may affect neurologic recovery, the proportion of time with temperature variability with respect to total time of maintenance phase was estimated. This was accomplished by first organizing the maintenance phase into 15-minute intervals, and then aligning the intervals with documented body temperatures. In the case where more than one value for body temperature occurred during a 15-minute interval, the mean of recorded values was used. To estimate body temperature during 15-minute intervals when no body temperature was recorded, interpolation using functions “na.approx” and “na.spline” from the package “zoo” (version zoo v1.8–0) in the R programming language (version 1.0.136) was applied. This approach uses weighted sums of linear and cubic splines (2/3 linear, 1/3 spline) to simultaneously maximize monotonicity of the interpolating function between the known data points, while minimizing the slope discontinuity of the interpolant at each known data point (Micula and Micula, 1999).
Percent of time with temperature variability was then calculated as a proportion of number of minutes with temperature variability (number of 15-minute intervals with body temperature outside of 36°C ± 0.5°C multiplied by 15) out of total minutes of the maintenance phase (count of all 15-minute intervals multiplied by 15). Statistical analysis was performed using STATA (version 14; StataCorp LP, College Station, TX). All analyses were 2-sided, with a significance level of α < 0.05.
Results
Out of 237 postcardiac arrest patients during the study period, 186 met the inclusion criteria. Reasons for exclusion were death or awakening before completion of the maintenance phase, too few recorded temperatures, or targeting a temperature other than 36°C. Table 1 summarizes sample characteristics and features of the cardiac arrest event. Notably, patients with temperature variability were significantly more likely to have had a shockable rhythm (temperature variability: 43.5% vs. no temperature variability 19.4%; p = 0.002), with less bystander CPR (temperature variability: 55.6% vs. no temperature variability 77.0%; p = 0.01), a longer arrest time (temperature variability: 21.4 ± 14.5 minutes vs. no temperature variability 14.6 ± 10.4 minutes; p = 0.01), and more shivering (temperature variability: 66.9% vs. no temperature variability 45.2%; p = 0.01).
Table 1.
Patient and Event Characteristics, Including Differences by Presence of Temperature Variability
| Characteristic | Total (n = 186) | Temperature variability (n = 124) | No temperature variability (n = 62) | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 131 (70.4) | 86 (69.4) | 45 (72.6) | 0.78 |
| Female | 55 (29.6) | 38 (30.6) | 17 (27.4) | |
| Age (years), mean ± SD | 53.8 ± 15.7 | 53.7 ± 13.7 | 54.3 ± 19.3 | 0.80 |
| BMI, mean ± SD | 27.2 ± 7.5 | 27.9 ± 7.1 | 25.8 ± 8.1 | 0.08 |
| Race | ||||
| Caucasian | 127 (68.3) | 82 (66.1) | 45 (72.6) | 0.13 |
| African American | 27 (14.5) | 18 (14.5) | 9 (14.5) | |
| Asian | 13 (67.0) | 7 (5.6) | 6 (9.7) | |
| Other/unknown | 19 (10.2) | 17 (13.7) | 2 (3.2) | |
| Location of cardiac arrest, | ||||
| Out of hospital | 167 (89.8) | 114 (91.9) | 50 (80.6) | 0.10 |
| In-hospital | 19 (10.2) | 9 (7.3) | 10 (16.1) | |
| Initial rhythm | ||||
| Shockable | 66 (35.5) | 54 (43.5) | 12 (19.4) | 0.002* |
| Nonshockable | 120 (64.5) | 70 (56.5) | 50 (80.6) | |
| Cause of cardiac arrest | ||||
| Cardiac | 82 (44.1) | 59 (47.6) | 23 (37.1) | 0.39 |
| Respiratory | 76 (40.9) | 47 (37.9) | 29 (46.8) | |
| Unknown | 28 (15.0) | 18 (14.5) | 10 (16.1) | |
| Witnessed | 103 (55.4) | 66 (53.7) | 37 (60.7) | 0.46 |
| Bystander CPR | 116 (62.4) | 69 (55.6) | 47 (77.0) | 0.01* |
| Length of CA from EMS arrival** (minutes) mean ± SD | 19.2 ± 13.7 | 21.4 ± 14.5 | 14.6 ± 10.4 | 0.01* |
| Shivering | 111 (59.7) | 83 (66.9) | 28 (45.2) | 0.01* |
| Paralytic use | 130 (69.9) | 91 (73.4) | 39 (62.9) | 0.19 |
| EEGa | 148 (79.6) | 98 (79.0) | 50 (80.6) | 0.95 |
| Epileptic seizuresb | 33 (17.7) | 20 (16.1) | 13 (21.0) | 0.54 |
| Length of hospitalization, mean ± SD | 12.7 ± 20.1 | 12.6 ± 21.9 | 13.0 ± 16.2 | 0.90 |
| Discharge disposition | ||||
| Home | 26 (14.0) | 21 (16.9) | 5 (8.1) | 0.54 |
| SNF/rehabilitation | 23 (12.4) | 14 (11.3) | 9 (14.5) | |
| AMA | 5 (2.7) | 3 (2.4) | 2 (3.2) | |
| Outside hospital | 4 (2.2) | 3 (2.4) | 1 (1.6) | |
| Deceased | 128 (68.8) | 83 (66.9) | 45 (72.6) | |
| CPCc at discharge | ||||
| 1–2 (good) | 47 (25.3) | 35 (28.2) | 12 (19.4) | 0.26 |
| 3–5 (poor) | 139 (74.7) | 89 (71.8) | 50 (80.6) | |
| Survival | 58 (31.2) | 41 (33.1) | 17 (27.4) | 0.54 |
Data are reported as n (%) unless otherwise noted.
Denotes group difference p < 0.05.
40% missing data.
EEG.
Seizures diagnosed by EEG.
Cerebral Performance Category scale.
AMA, against medical advice; BMI, body mass index; CPC, Cerebral Performance Category; CPR, cardiopulmonary resuscitation; EEG, electroencephalogram; SD, standard deviation; SNF, skilled nursing facility.
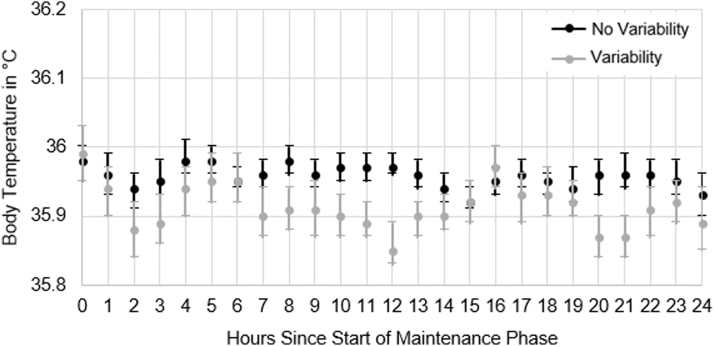
Figure 1 shows the average hourly body temperature for patients with and without temperature variability. Temperature variability was not significantly associated with odds of poor neurologic outcome both for the univariate model (odds ratio [OR] = 0.61, p = 0.19, 95% confidence interval [CI] = 0.29–1.28), and after adjustment for covariates (OR = 1.01, p = 0.98, 95% CI = 0.36–2.82). Similarly, percent of time spent outside of goal temperature range relative to total length of maintenance phase was not significantly associated with odds of poor neurologic outcome (OR = 1.00, p = 0.83, 95% CI = 0.95–1.04).
FIG. 1.
Differences in average hourly body temperature for patients with and without temperature variability data are graphed as the mean hourly body temperature with 95% confidence intervals from hour 0 to 24 of the TTM maintenance phase. The black points are patients with no temperature variability, and gray points are patients with temperature variability. TTM, targeted temperature management.
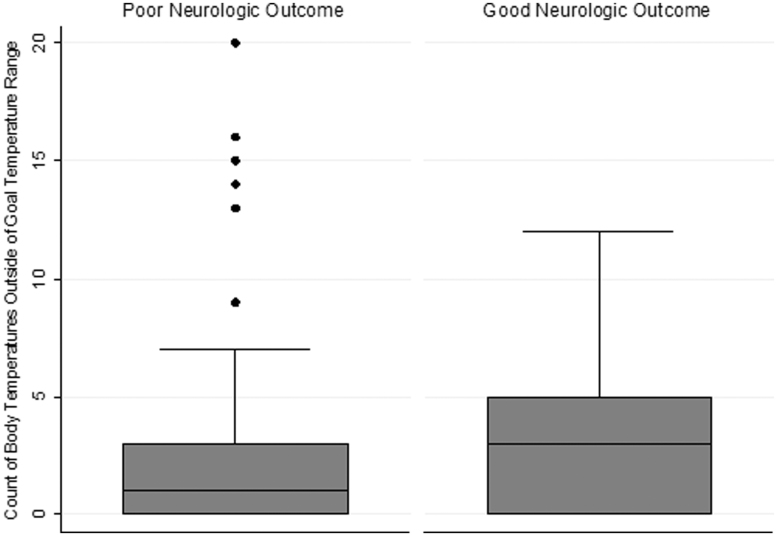
Figure 2 shows the number of recorded body temperatures outside of the goal temperature range comparing patients with good neurologic recovery to poor neurologic recovery. Among those with temperature variability, there were 353 (6.0%) documented body temperatures <35.5°C and 173 (2.9%) documented body temperatures >36.5°C (range: 31.8°C–37.6°C).
FIG. 2.
Box plot of the number of body temperatures outside of 36°C ± 0.5°C for patients with good neurologic outcome compared with patients with poor neurologic outcome. Good neurologic outcome is defined as a CPC scale score of ≤2. Poor neurologic outcome is defined as a CPC score >2. CPC, Cerebral Performance Category.
Having an initial shockable rhythm and use of NMBAs were significantly associated with odds of a good neurologic outcome (shockable rhythm: OR = 10.77, p < 0.001, 95% CI = 4.30–26.98; NMBA use: OR = 4.54, p = 0.02, 95% CI = 1.34–15.40) (Table 2). Similarly, an initial shockable rhythm and use of NMBAs were significantly associated with odds of survival to hospital discharge, but not temperature variability (shockable rhythm: OR = 5.90, p < 0.001, 95% CI = 2.65–13.13; NMBA use: OR = 3.03, p = 0.02, 95% CI = 1.16–7.90; temperature variability: OR = 0.69, p = 0.41, 95% CI = 0.28–1.67).
Table 2.
Results of Multivariate Logistic Regression Model for Patients with Good Neurologic Outcome
| Variable |
OR |
SE |
p |
95% CI for OR |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 0.99 | 0.01 | 0.50 | 0.96 | 1.02 |
| Female | 1.39 | 0.63 | 0.47 | 0.57 | 3.38 |
| Race | |||||
| Caucasian | Reference | — | — | — | — |
| African American | 1.18 | 0.70 | 0.78 | 0.37 | 3.77 |
| Asian/Pacific Islander | 0.95 | 0.87 | 0.96 | 0.16 | 5.68 |
| Other/unknown | 0.47 | 0.37 | 0.34 | 0.10 | 2.18 |
| BMI | 1.01 | 0.03 | 0.83 | 0.95 | 1.06 |
| In-hospital cardiac arrest | 3.81 | 2.75 | 0.06 | 0.93 | 15.64 |
| Initial shockable rhythm | 10.77 | 5.05 | <0.001 | 4.30 | 26.98 |
| Bystander CPR | 0.90 | 0.42 | 0.82 | 0.35 | 2.27 |
| Shivering | 1.21 | 0.57 | 0.68 | 0.48 | 3.03 |
| NMBA use | 4.54 | 2.83 | 0.02 | 1.34 | 15.40 |
CI, confidence interval; NMBA, neuromuscular blocking agent; OR, odds ratio.
Discussion
This was a retrospective study evaluating the association between temperature variability during the maintenance phase of TTM36 and neurologic outcome at hospital discharge. In this study, having temperature variability was not significantly associated with odds of poor neurologic outcome. Patients with good neurologic outcome were more likely to have an initial shockable rhythm and receive NMBAs during TTM. Likewise, temperature variability was not associated with odds of survival to hospital discharge, but patients who survived to discharge were more likely to have a shockable rhythm and receive NMBAs during TTM.
The present study is one of the first to assess the relationship between temperature variability and neurologic outcome specifically at TTM36. Nevertheless, our findings are in alignment with others who found that having temperature variability was not associated with poor neurologic outcomes after cardiac arrest when targeting TTM33 (Nobile et al., 2015; Nayeri et al., 2017). In a retrospective study of 229 comatose survivors of cardiac arrest who underwent TTM33, Nobile et al. (2015) found that having high temperature variability was not associated with worse neurologic outcome 3 months postarrest. Similarly, in a cohort of 242 comatose survivors of cardiac arrest treated with TTM33, Nayeri et al. (2017) did not find that temperature variability was associated with higher odds of poor neurologic outcome. It should be noted that both Nobile and Nayeri defined temperature variability using the variance or SD of all recorded body temperatures of subjects in their respective studies either by using a cutoff score to determine temperature variability or by using variance as a continuous scale. Whereas in the present study, we used the ENLS recommendation to maintain goal temperature within ±0.5°C of the targeted temperature to a priori define temperature variability as having at least one body temperature outside of ±0.5°C of the targeted temperature.
We also attempted to capture the total percentage of time with temperature variability relative to the total TTM maintenance period to determine if a greater amount of time with temperature variability negatively affected neurologic outcome; however, this was not the case. Although patients with temperature variability had temperatures >36.5°C (2.9% of temperatures), it was more frequently noted that body temperatures were <35.5°C (6% of temperatures), with a maximum recorded temperature of 37.6°C. Meaning that by and large, patients with temperature variability did not frequently experience elevated body temperatures during TTM. As hyperthermia is thought to worsen brain injury, the lack of elevated body temperatures in this cohort may explain why no difference in neurologic outcome was observed (Gebhardt et al., 2013; Geocadin et al., 2017).
A number of factors contribute to patients having temperature variability, which adds to the complexity in understanding the relationship between such variability and neurologic outcome. Characteristics of the cardiac arrest, such as initial rhythm, length of arrest, and bystander CPR, may play a larger role in patients having poor neurologic outcome than temperature variability (Daya et al., 2015; Malta Hansen et al., 2015; Fordyce et al., 2017; Yamaguchi et al., 2017). In addition, individual hospital protocols could impact the ability of patients to exhibit temperature variability. For example, protocols with aggressive antishivering management using NMBAs may prohibit dynamic temperature changes from a lack of heat generation due to skeletal muscle paralysis (Badjatia et al., 2008; Choi et al., 2011). The hospital protocol in this study did not call for immediate initiation of NMBAs, and there was similar NMBA utilization across temperature variability groups. Other hospital antishivering protocols may produce different results.
In the present study, use of NMBAs was associated both with higher odds of good neurologic outcome and higher odds of survival to hospital discharge compared with those with no NMBA use. This is similar to other studies that demonstrated a positive relationship between NMBA use and either good neurologic outcome or survival (Salciccioli et al., 2013; Lee et al., 2017; May et al., 2018).
We believe this is a notable finding of our study as few previous studies included patients with TTM36. However, a recent prospective study of 81 out-of-hospital cardiac arrest survivors treated with TTM33 or TTM36 did not find a significant difference in survival or neurologic outcome at hospital discharge when patients were randomly assigned to receive continuous NMBAs versus placebo (Lee et al., 2018). Importantly though, only six total subjects (7%) received TTM36. Other studies have similarly found no relationship between NMBA use and neurologic outcome or survival during TTM33 (Lascarrou et al., 2014; Stockl et al., 2017). Given the inconsistent findings and minimal inclusion of TTM36, larger prospective studies evaluating NMBA use, especially during TTM36, are warranted.
Having temperature variability was not associated with a significant difference in survival to hospital discharge compared with patients with no temperature variability. This may be, in part, because our sample had a high proportion of patients with a nonshockable rhythm (64.5%) and an overall high mortality (68.8%). Nonshockable rhythms have been associated with worse outcomes (Tian et al., 2010; Nielsen et al., 2013; Geocadin et al., 2017).
Surprisingly, we observed a significantly lower proportion of bystander CPR in patients with temperature variability, as well as longer arrest times, compared with those without temperature variability. We believe this is likely due to the way in which bystander CPR and length of cardiac arrest data were reported, and the quantity of missing data, and not necessarily reflective of a significant finding. We only included length of cardiac arrest if it was reported in the prehospital emergency services report, or in the hospital code report. We did not include information from the emergency room physician note, which often provided an estimate on arrest length.
There was no difference in the proportion of patients with a witnessed compared with an unwitnessed arrest, which suggests that a subset of patients received bystander CPR following an unwitnessed arrest. Although bystander CPR improves the likelihood of survival, the unknown length of downtime may have been a greater factor (Geri et al., 2017) that was not captured in our data.
Limitations
This was a retrospective observational study, which limits generalizability. The large proportion of patients presenting with nonshockable rhythm and overall high mortality may have biased results. In addition, differences in pre-TTM factors such as rate of bystander CPR and length of cardiac arrest between those with and without temperature variability may have significantly altered the true relationship between neurologic recovery and temperature variability. We also only included patients who completed the TTM maintenance phase to capture the greatest number of temperatures. This may have biased our sample to include patients who had to survive at least 24 hours, although this did allow us to compare patients who received a similar dose of TTM. A larger sample with a more heterogeneous population may produce different outcomes.
Finally, we used neurologic outcome at hospital discharge as the primary outcome, which may have biased our results toward classifying a higher proportion of survivors with poor neurologic outcome (19% [n = 11] of survivors) who may have actually recovered neurologically if followed over a longer time course. Given that the Harborview Medical Center is a regional receiving hospital and patients often do not return to the medical system for care, it was not possible to follow patients after discharge to determine longer term neurologic outcomes. Future prospective studies should evaluate neurologic status over a longer period of time, given the potential for delayed neurologic recovery.
Conclusion
In this sample of postcardiac arrest comatose survivors undergoing TTM at 36°C, there was no association between temperature variability and odds of poor neurologic outcome or survival to hospital discharge. Patients with good neurologic outcome were more likely to have an initial shockable rhythm and receive NMBAs. Future prospective studies are needed to determine the effect of temperature variability on neurologic recovery, especially when targeting TTM36.
Acknowledgments
This study was conducted at Harborview Medical Center and the University of Washington, Seattle, Washington.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the TL1 Translational Research Training Program, Institute of Translational Health Sciences (1TL1TR002318-01).
References
- Ang KH, Chong GCY, Li Y. PID control system analysis, design, and technology. IEEE Trans Control Syst Technol 2005;13:559–576 [Google Scholar]
- Badjatia N, Kowalski RG, Schmidt JM, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit Care 2007;6:186–191 [DOI] [PubMed] [Google Scholar]
- Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke 2008;39:3242–3247 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Bray JE, Stub D, Bloom JE, et al. Changing target temperature from 33 degrees C to 36 degrees C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation 2017;113:39–43 [DOI] [PubMed] [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 Suppl 2):S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamento A, Minson A, Radford S, et al. A comparison of therapeutic hypothermia and strict therapeutic normothermia after cardiac arrest. Resuscitation 2016;106:83–88 [DOI] [PubMed] [Google Scholar]
- Choi HA, Ko SB, Presciutti M, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care 2011;14:389–394 [DOI] [PubMed] [Google Scholar]
- Daya MR, Schmicker RH, Zive DM, et al. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC). Resuscitation 2015;91:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deye N, Vincent F, Michel P, et al. Changes in cardiac arrest patients' temperature management after the 2013 “TTM” trial: results from an international survey. Ann Intensive Care 2016;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J, Polderman KH. Emergency Neurological Life Support: resuscitation following cardiac arrest. Neurocrit Care 2017;27(Suppl 1):134–143 [DOI] [PubMed] [Google Scholar]
- Fordyce CB, Hansen CM, Kragholm K, et al. Association of public health initiatives with outcomes for out-of-hospital cardiac arrest at home and in public locations. JAMA Cardiol 2017;2:1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt K, Guyette FX, Doshi AA, et al. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation 2013;84:1062–1067 [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Wijdicks E, Armstrong MJ, et al. Practice guideline summary: reducing brain injury following cardiopulmonary resuscitation: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017;88:2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geri G, Fahrenbruch C, Meischke H, et al. Effects of bystander CPR following out-of-hospital cardiac arrest on hospital costs and long-term survival. Resuscitation 2017;115:129–134 [DOI] [PubMed] [Google Scholar]
- HACA Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Johnson NJ, Danielson KR, Counts CR, et al. Targeted temperature management at 33 versus 36 degrees: a retrospective cohort study. Crit Care Med 2020;48:362–369 [DOI] [PubMed] [Google Scholar]
- Lascarrou JB, Le Gouge A, Dimet J, et al. Neuromuscular blockade during therapeutic hypothermia after cardiac arrest: observational study of neurological and infectious outcomes. Resuscitation 2014;85:1257–1262 [DOI] [PubMed] [Google Scholar]
- Lee BK, Cho IS, Oh JS, et al. Continuous neuromuscular blockade infusion for out-of-hospital cardiac arrest patients treated with targeted temperature management: a multicenter randomized controlled trial. PLoS One 2018;13:e0209327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee BK, Jeung KW, et al. Neuromuscular blockade requirement is associated with good neurologic outcome in cardiac arrest survivors treated with targeted temperature management. J Crit Care 2017;40:218–224 [DOI] [PubMed] [Google Scholar]
- Madden LK, Hill M, May TL, et al. The implementation of targeted temperature management: an evidence-based guideline from the Neurocritical Care Society. Neurocrit Care 2017;27:468–487 [DOI] [PubMed] [Google Scholar]
- Malta Hansen C, Kragholm K, Pearson DA, et al. Association of bystander and first-responder intervention with survival after out-of-hospital cardiac arrest in North Carolina, 2010–2013. JAMA 2015;314:255–264 [DOI] [PubMed] [Google Scholar]
- May TL, Riker RR, Fraser GL, et al. Variation in sedation and neuromuscular blockade regimens on outcome after cardiac arrest. Crit Care Med 2018;46:e975–e980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micula G, Micula S. Handbook of Splines. Netherlands: Springer, 1999 [Google Scholar]
- Nayeri A, Bhatia N, Holmes B, et al. Temperature variability during targeted temperature management is not associated with neurological outcomes following cardiac arrest. Am J Emerg Med 2017;35:889–892 [DOI] [PubMed] [Google Scholar]
- Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–2483 [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–2206 [DOI] [PubMed] [Google Scholar]
- Nobile L, Lamanna I, Fontana V, et al. Greater temperature variability is not associated with a worse neurological outcome after cardiac arrest. Resuscitation 2015;96:268–274 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009;37:1101–1120 [DOI] [PubMed] [Google Scholar]
- Rittenberger JC, Friess S, Polderman KH. Emergency neurological life support: resuscitation following cardiac arrest. Neurocrit Care 2015;23 Suppl 2:S119–S128 [DOI] [PubMed] [Google Scholar]
- Salciccioli JD, Cocchi MN, Rittenberger JC, et al. Continuous neuromuscular blockade is associated with decreased mortality in post-cardiac arrest patients. Resuscitation 2013;84:1728–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockl M, Testori C, Sterz F, et al. Continuous versus intermittent neuromuscular blockade in patients during targeted temperature management after resuscitation from cardiac arrest-A randomized, double blinded, double dummy, clinical trial. Resuscitation 2017;120:14–19 [DOI] [PubMed] [Google Scholar]
- Tian J, Kaufman DA, Zarich S, et al. Outcomes of critically ill patients who received cardiopulmonary resuscitation. Am J Respir Crit Care Med 2010;182:501–506 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Woodin JA, Gibo K, et al. Improvements in out-of-hospital cardiac arrest survival from 1998 to 2013. Prehosp Emerg Care 2017;21:616–627 [DOI] [PubMed] [Google Scholar]