Abstract
Mild therapeutic hypothermia is protective against several cellular stresses, but the mechanisms underlying this protection are not completely resolved. In the present study, we used an in vitro model to investigate whether therapeutic hypothermia at 33°C applied following a peroxide-induced oxidative stress would protect PC12 cells. A 1-hour exposure to tert-butyl peroxide increased cell death measured 24 hours later. This cell death was dose-dependent in the range of 100–1000 μM tert-butyl peroxide with ∼50% cell death observed at 24 hours from 500 μM peroxide exposure. Cell survival/death was measured with an alamarBlue viability assay, and propidium iodide/Hoechst imaging for counts of living and dead cells. Therapeutic hypothermia at 33°C applied for 2 hours postperoxide exposure significantly increased cell survival measured 24 hours postperoxide-induced stress. This protection was present even when delayed hypothermia, 15 minutes after the peroxide washout, was applied. Addition of any of the three FDA-approved antioxidants (Tempol, EUK134, Edaravone at 100 μM) in combination with hypothermia improved cell survival. With the therapeutic hypothermia treatment, a significant downregulation of caspases-3 and -8 and tumor necrosis factor-α was observed at 3 and 24 hours poststress. Consistent with this, a cell-permeable pan-caspase inhibitor Z-VAD-FMK applied in combination with hypothermia significantly increased cell survival. Overall, these results suggest that the antioxidants quenching of reactive oxygen species likely works with hypothermia to reduce mitochondrial damage and/or apoptotic mechanisms. Further studies are required to confirm and extend these results to other cell types, including neuronal cells, and other forms of oxidative stress as well as to optimize the critical parameters of hypothermia treatment such as target temperature and duration.
Keywords: oxidative stress, hypothermia, therapeutic hypothermia, reactive oxygen, ROS, antioxidants, PC12, cell viability
Introduction
Hypothermia following ischemia-reperfusion enhances neuronal survival in the brain and several other tissues (Dietrich and Bramlett, 2007, 2017; Dash and Chavali, 2018) and its neuroprotective benefits have been shown against secondary injuries due to brain trauma, stroke, and spinal cord injury (Kawai et al., 2000; Dietrich et al., 2009, 2011; Cappuccino et al., 2010; Dietrich and Bramlett, 2010; Levi et al., 2010). Mild therapeutic hypothermia after a central nervous system injury has found promising applications as a neuroprotective intervention with significant results both in preclinical (Chatzipanteli et al., 2000; Truettner et al., 2005, 2005) and clinical models (Cappuccino et al., 2010; Levi et al., 2010). However, the mechanisms underlying protection carried out by hypothermia are multifactorial (Kawabori et al., 2013; Lee et al., 2016). Lowering the temperature might protect by slowing metabolism and thus preserving adenosine triphosphate (ATP) (Ziganshin et al., 2017). Hypothermia might also protect from conditions that generate reactive oxygen and nitrogen species (RONS) (Darwazeh and Yan, 2013; Lee et al., 2016), but it can decrease the activity of cellular antioxidant defenses, including both superoxide dismutase (SOD) and the glutathione antioxidant system (Biary et al., 2011). If so, then antioxidants might be complementary to hypothermia in providing protection from cellular stresses involving increased reactive oxygen species (ROS).
Previous studies have reported that combination therapies that involve hypothermia provide neuroprotection following hypoxic-ischemic brain injury (Green et al., 1995; Dietrich et al., 1999; Pazos et al., 1999; Gao et al., 2014; Amer and Oorschot, 2018). Identifying the most effective time and critical duration for application of hypothermia with additive, complementary treatments that target multiple mechanisms postinjury may improve its long-term neuroprotective efficacy (Schmid-Elsaesser et al., 1999; Scholler et al., 2004; Van Hemelrijck et al., 2005; Azzopardi et al., 2016; Baburamani and Arichi, 2020; Wassink et al., 2020).
Peroxide stress can increase Ca2+ in both cytoplasm and mitochondria causing mitochondrial Ca2+ overload and damage (Palee et al., 2016). Peroxides can also produce DNA damage (Zhang et al., 2014; Pronsato and Milanesi, 2016; Chwastek et al., 2017; Martín-Guerrero et al., 2017). This DNA damage can activate poly (ADP-ribose) polymerases (PARPs) and cause cell death by a pathway involving poly (ADP-ribose) (PAR) (Martín-Guerrero et al., 2017), mitochondrial damage, and release of apoptosis inducing factor (AIF) (Yu et al., 2006; Wang et al., 2009). Mitochondrial stress/dysfunction can be increased by PARP-dependent NAD+ depletion and PAR-dependent inhibition of glycolysis, cutting off the supply of pyruvate to the mitochondria (Andrabi et al., 2014). Peroxides can result in caspase activation, including the death receptor, caspase-8/caspases-3 pathway (Doti et al., 2014), but high levels of peroxides can also destroy caspases (Wu et al., 2011).
In the present study, we investigated the effects of mild therapeutic hypothermia, ∼33°C, with peroxide-induced stress in an attempt to resolve stress exacerbating and ameliorating effects of hypothermia. We further tested whether hypothermia applied after washout of peroxides would enhance cell survival and whether this survival might be enhanced with a combination therapy that involves antioxidants.
Experimental Procedures
Cell culture
Rat pheochromocytoma (PC12) cells are easy to culture and versatile for pharmacological studies (Halleck et al., 1992; Satoh et al., 1997; Jang and Surh, 2001; Tang et al., 2005) and were used here to elucidate the role of hypothermia in protecting from oxidative stress. PC12 cells (CRL-1721.1™, American Type Culture Collection, ATCC) were grown in F-12K Medium (Kaighn's Modification of Ham's F-12 Medium, ATCC 30-2004™) supplemented with 2.5% heat-inactivated fetal bovine serum, 15% horse serum, and 100U/ml penicillin/streptomycin (all from Millipore Sigma) and plated in 100 mm tissue culture plates. Cultures were maintained at 37°C in a 95% humidified incubator with 5% CO2. A total of 1 × 104 PC12 cells/well at passage 4–5 were seeded into a 96-well plate (Corning Costar 3904) for plate reader and imaging assays, or a total of 2 × 105 cells/plate seeded into 100 mm tissue culture plates for mRNA analysis. Cells were maintained in culture for 23 days before switching to differentiation medium containing the F-12K media, 1% nonheat inactivated horse serum, 50 U/mL penicillin/streptomycin, and 100 ng/mL nerve growth factor (NGF). The medium was switched to serum-free F-12K medium containing B27 (1 × from 50 × stock, Millipore Sigma) and NGF (100 ng/mL, Millipore Sigma) at least 24 hours before the stress experiments.
Tert-butyl Peroxide (TbH2O2) exposure and treatments
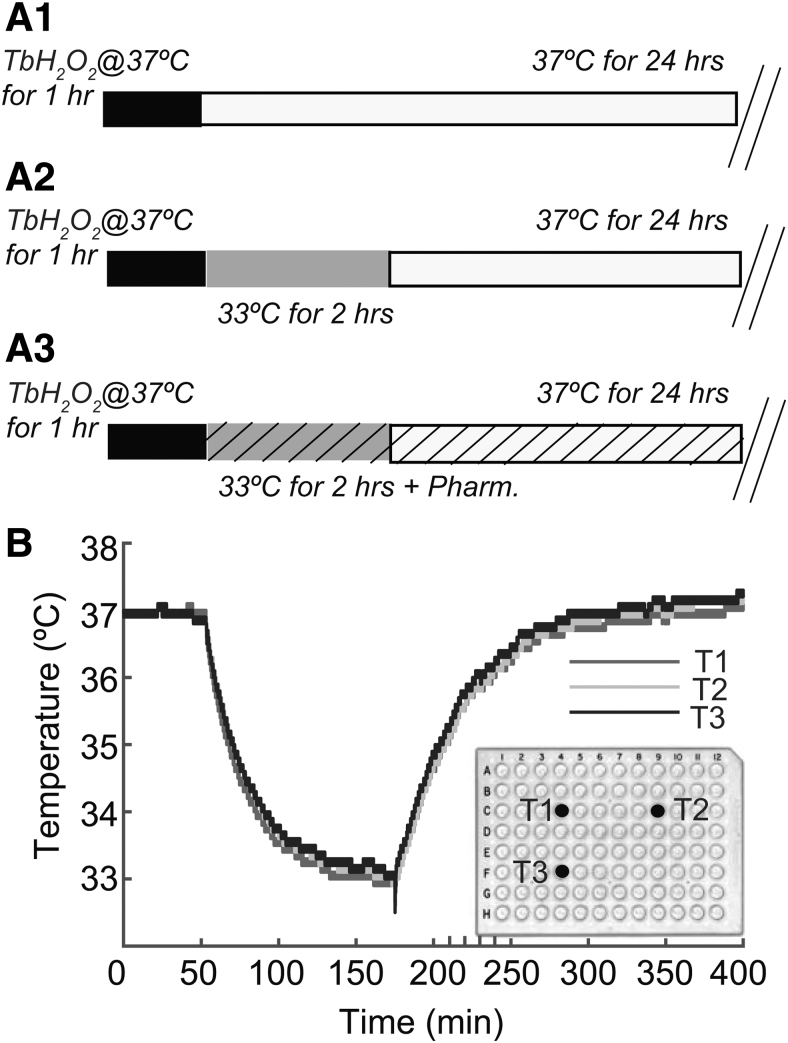
PC12 cells were exposed to 50–1000 μM peroxide (tert-butyl hydroperoxide, TbH2O2) for 60 minutes at 37°C in a tissue culture incubator. This peroxide stress model has been used previously to study mechanisms of oxidative stress (Chanvitayapongs et al., 1997; Lin et al., 2014; Wu et al., 2018). The peroxide was then washed out three times with the F-12K medium equilibrated at 37°C. Following peroxide washout, cocultures (seeded with cells at the same time) were either kept at 37°C (normothermia) or exposed for a 2-hour treatment at 33°C (hypothermia). After the 2 hours incubation at 33°C, the hypothermic cultures were transferred back to 37°C. All cell viability assays were performed 24 hours later. Figure 1 shows a schematic representation of the protocols used in this study (Fig. 1A1–A3) and temperature changes over time measured in culture wells of 96-well plates transferred from normothermic (37°C) to hypothermic (33°C), and then again to normothermic condition (37°C, Fig. 1B). Figure 1A1–A3 shows the protocol for normothermic samples undergoing oxidative stress with 1-hour exposure to TbH2O2, the protocol for poststress hypothermia and for poststress combination strategy. Cooling and rewarming followed exponential time courses, with temperature changing ∼3.5°C within 50 minutes (Fig. 1B).
FIG. 1.
(A1–A3) show schematic representation of the protocols for oxidative stress and hypothermia application used in the present study. (A1) Normothermia: cells were exposed to TbH2O2 for 1 hour at 37°C, and then kept at 37°C for 24 hours after TbH2O2 washout. (A2) Hypothermia: cells were exposed to TbH2PO2 for the 1 hour at 37°C, and after TbH2O2 washout given hypothermia treatment at 33°C for 2 hours. (A3) Hypothermia and antioxidants treatments: antioxidants were added to the cell cultures poststress (TbH2O2 for 1 hour at 37°C) and postwashout, in combination with hypothermic condition (33°C) for 2 hours. After treatments cells were always maintained at 37°C for a total of 24 hours. (B) In a pilot experiment, temperature measurements were obtaining during an experiment with three micro thermistors placed at different sites in the 96-well culture plate. Measurement of the temperature (°C) of the media over time (min) from three different wells (T1: blue, T2: red, T3: black) are shown. The plate was kept at 37°C for an hour, maintained at 33°C for 2 hours and then returned to 37°C. The graph shows a consistent decrease of ∼4°C with similar rates for cooling and slow rewarming. TbH2O2, tert-butyl peroxide.
Dose–response effects of peroxide on viability
Cell viability against TbH2O2-induced oxidative stress in PC12 was evaluated. To optimize the concentration of TbH2O2 stimuli used for the study, the PC12 cells were treated with increasing doses of TbH2O2 from 0.05 to 1 mM. As shown in Figure 2, the 1hour exposure to TbH2O2 decreased cell viability measured 24 hours later in a dose-dependent manner. At 500 μM TbH2O2 stimuli produced a consistent stress with cell viability around 62.5%. This concentration was used in all of the subsequent experiments to measure effects of treatments designed to improve cell viability.
FIG. 2.
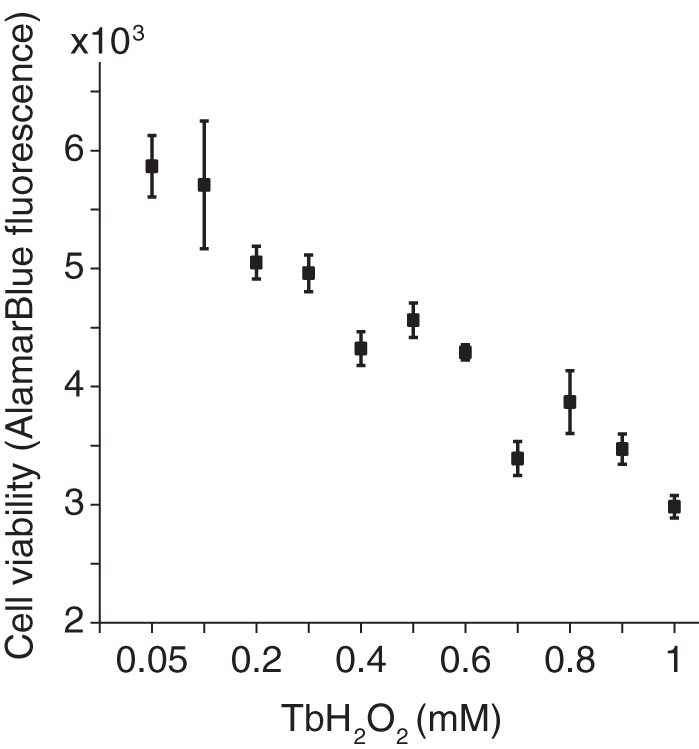
Effect of TbH2O2 on cell survival: PC12 cells were incubated with 0.05–1 mM of TbH2O2 for 1 hour at 37°C. The toxicity of TbH2O2 on cell survival was assessed with the alamarBlue assay. Data are expressed as mean ± SEM. n = 7 per dose of TbH2O2, three independent experiments.
Combination therapy
We investigated effects of hypothermia combined with one of three antioxidants: EUK-134 (100 μM), tempol (100 μM) or edaravone (Edr, 100 μM). All the drugs were purchased from Millipore Sigma. Antioxidants were added post-TbH2O2 stress, as mild hypothermia treatment was initiated, and left in the F-12K media for 24 hours. In a separate comparison, a caspase inhibitor Z-VAD-FMK (InvivoGen) was added at 50 or 100 μM after the TbH2O2 stress and combined with the hypothermia treatment.
Cell viability assays
The cultures were assayed for cell viability using alamarBlue or propidium iodide and Hoechst fluorescence imaging (both obtained from Bio-Rad Laboratories Inc.). For the alamarBlue assay, 5 μL from a secondary stock diluted 1/10 in phosphate-buffered saline (PBS) from the commercial stock was added to each well of the 96-well plates with PC12 cells (100 μL media total volume in each well). Five minutes after adding the alamarBlue, the fluorescence was read using a plate reader (Wallack 1450, excitation 535 nm, emission >590 nm). After 1-hour incubation, the same wells were read again at the same wavelengths. The first reading was subtracted from the second reading to give the change in fluorescence during the hour of incubation. This change (an increase) in fluorescence was used as a measure of the viability of the cells in each culture well. The alamarBlue assay measures the ability of the cells to reduce nonfluorescent resazurin to fluorescent resorufin, a process that reflects the electron donating reductive capacity of cells, including that of the mitochondria. Thus, this assay gives a measure of the “health” of the cells usually correlating with cell survival (Nonner et al., 1996; Al-Nasiry et al., 2007).
Hoechst DNA-binding dye (PureBlu™ 33342, 5 μM; Bio-Rad Laboratories, Inc.) was used in combination with propidium iodide (5 μM) to assay the number of living cells by imaging the Hoechst fluorescence and propidium iodide fluorescence. After a 20 minutes incubation, the cells in each well were imaged using 535/ > 590 nm excitation/emission for propidium iodide and 430/450 nm excitation/emission for Hoechst. The coordinates of images were selected randomly in the center of the well with one field per culture well. The images were analyzed by two researchers (J.S. and J.B.) using the object counting algorithm of ImageJ (Rueden et al., 2017) following conversion to binary images with the thresholding feature of ImageJ. Superposition of images indicated that cells positive for propidium iodide had very low fluorescence at the Hoechst wavelengths and were below the threshold used for detection of Hoechst staining. In contrast, cells with no fluorescence at the propidium iodide wavelengths showed bright Hoechst fluorescence. The low Hoechst fluorescence in propidium iodide positive cells is likely due to interference by propidium iodide either by competition for DNA-binding sites or quenching of the Hoechst fluorescence. Thus, cells were scored as living if they had above threshold Hoechst staining, but were propidium iodide negative. Threshold was set at 2 × the background fluorescence, but most cells showed Hoechst fluorescence at least 5 × the background level.
RNA extraction and qRT-PCR
The PC12 cultures were washed with 1 × PBS before RNA isolation. RNA was extracted using mirVana PARIS Kit (Invitrogen AM1556). Following disruption with Cell Disruption Buffer, the lysate was mixed with 2 × denaturing solution and subjected to acid-phenol:chloroform extraction, which provides a robust RNA purification that also removes most DNA. For purification of total RNA, 100% ethanol is added to samples, and they are filtered through a glass-fiber filter. The filter is then washed three times and the RNA is eluted with 50–100 μL of warm sterile water (Ultrapure DEPC-Treated; Invitrogen). Extracted RNA samples were used for cDNA synthesis using High-capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific 4368814), and gene expression assays were quantified by qPCR using TaqMan assays. Samples were run for tumor necrosis factor α (TNFα), Caspase-3, and Caspase-8 (Applied BioSystems). Data were normalized to GAPDH mRNA as the endogenous reference. Quantitation of data was performed using the normalized cycle threshold method and converting this data to the fold-change relative to the control group.
Statistics
All statistical analyses were conducted in Origin (OriginLab Corp.) and confirmed by four researchers (J.S., J.B., S.R., and R.S.). ANOVA followed by post hoc tests (Duncan multiple comparison test) was used. The number of culture wells for each group was from 6 to 12 and each experiment was repeated at least three times.
Results
Mild hypothermia following peroxide stress increases PC12 cell viability
The effect of mild hypothermia applied following the TbH2O2 stimuli was tested using the cell viability protocol. As shown in Figure 1, 500 μM TbH2O2 was added to cell cultures for 1 hour and then washed out at 37°C. Following the oxidative stress, the cultures were placed in hypothermic conditions at 33°C for 2 hours. Hypothermia was provided at different times following TbH2O2 stress—either immediately following the stress or up to an hour later. Cell viability was measured 24 hours later using the alamarBlue assay with normothermic and hypothermia-treated cultures and compared to control cultures (no TbH2O2, 37°C) and hypothermia-controls (no TbH2O2, hypothermia treatment for 2 hours at 33°C). As shown in Figure 3, the cell viability was reduced to 25% (±2%) in the normothermic cultures following the oxidative stress, however, therapeutic hypothermia gave more than a 100% increase in cell viability (44% ± 1.6%). The time interval between the TbH2O2 washout and the beginning of the 2-hour hypothermia treatment period varied between 2–3 minutes and 60 minutes. Compared with normothermic cultures, viability was significantly increased with the therapeutic hypothermia at each of the intervals. We observed a trend toward higher cell viability with a delayed hypothermia applied at 15 minutes (52%), but data at this delay were not statistically significantly different from other time points following the peroxide washout. However, hypothermia applied when the TbH2O2 was present in the media (before washout) reduced cell survival (Fig. 3B). This decrease is consistent with the hypothermia-induced decrease in cellular antioxidant systems reported for heart cells (Biary et al., 2011). The cell viability of cultures that received hypothermia alone without the TbH2O2 stress was not significantly different from controls.
FIG. 3.
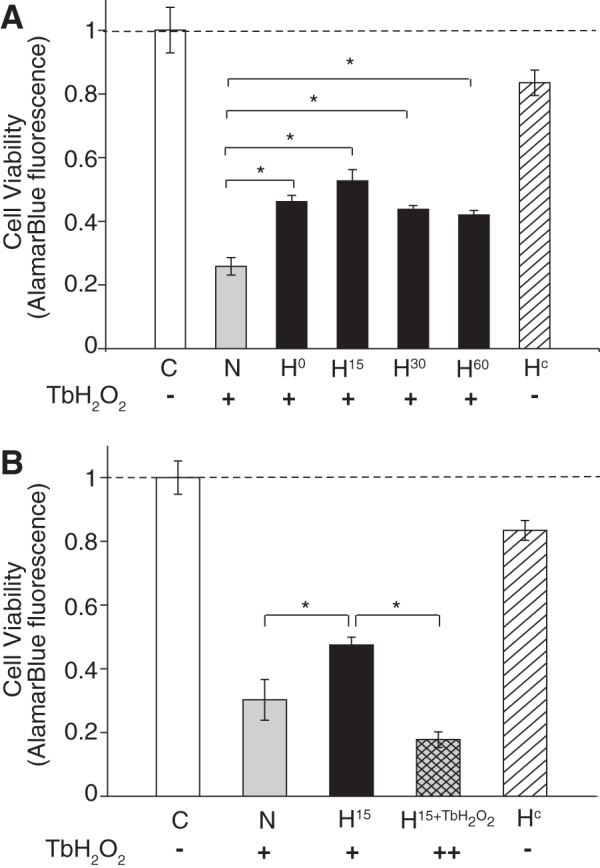
Delayed hypothermia reduced cell death after TbH2O2 stress: (A) cells were exposed to 0.5 mM TbH2O2 for 1 hour, followed by hypothermia treatment (33°C) at 0 (H0), 15 (H15), 30 (H30), or 60 (H60) min after TbH2O2 washout. (B) Cells were exposed to 0.5 mM TbH2O2 for 1 hour, followed by hypothermia treatment (33°C) at 15 (H15) or exposed to 0.5 mM TbH2O2 in combination with hypothermia treatment (33°C) (H15+TbH2O2) before washout. Cell survival was assessed with the alamarBlue assay. Data are expressed as the mean ± SEM (n = 6 per group, three independent experiments). *p < 0.01. C, control; N, normothermia; ++ (H15+TbH2O2), hypothermia treatment initiated in the presence of TbH2O2 stress; Hc, hypothermia alone (no TbH2O2).
Delayed hypothermia increases the number of living cells measured 24 hours following peroxide stress
With a delayed hypothermia (15 minutes post-TbH2O2 washout), we observed an increased number of cells positive for Hoechst DNA staining, but negative for propidium iodide staining (Fig. 4). Cells positive for propidium iodide had very weak Hoechst staining likely due to interference with Hoechst binding or quenching of Hoechst fluorescence by propidium iodide (see Experimental Procedures section). Hypothermia increased the number of Hoechst-positive cells (p < 0.01). There was a 54% reduction in Hoechst-positive cells in the normothermic cultures when compared to nonstressed controls. In comparison, hypothermia protected a significantly higher number of cells from the TbH2O2 stress. The decrease in number of living cells was <19% compared to a decrease of 54% with normothermia. The hypothermia-only treatment did not significantly affect the cells. These changes in the number of Hoechst-stained cells are in approximate agreement with the percentage decrease in the alamarBlue viability signal seen in similar experiments (e.g., Fig. 3).
FIG. 4.
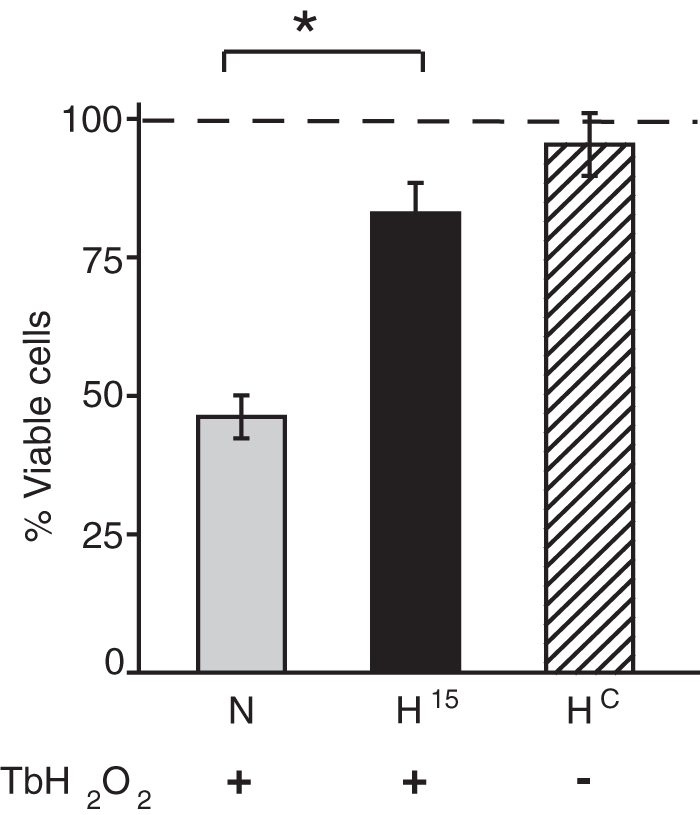
Mild hypothermia promotes cell survival following TbH2O2 stress: Cells were incubated at 33°C after 15 minutes (H15) post-TbH2O2 washout and kept for 2 hours. Cell viability was assessed by Hoechst-Propidium staining. Data are expressed as mean ± SEM (n = 6 per group, three independent experiments). *p < 0.01. N, normothermia; Hc, hypothermia alone (no TbH2O2).
Antioxidants provide additional protection to hypothermia alone treatment
Hypothermia may result in the accumulation of reducing equivalents in the mitochondrial electron transport chain, enhancing the production of RONS and the resulting oxidative stress (Gutteridge and Halliwell, 2000; Alva et al., 2009). Furthermore, rewarming (or return to physiological conditions) may also lead to an increased production of free radicals. Finally, hypothermia has been shown to reduce SOD or SOD2 expression. It has been reported that the protective effect of hypothermia against hypoxia-induced damage could be improved with the addition of catalase, for example, to the cellular medium (Zitta et al., 2010). Other enzymatic antioxidants such as SOD, glutathione peroxidase, as well as molecular antioxidants such as reduced glutathione can all scavenge RONS. In this study, we addressed whether a therapeutic agent may be combined with hypothermia to enhance cell viability when compared with hypothermia treatment alone. We hypothesized that a concurrent treatment with antioxidants to scavenge RONS may add to the efficacy of hypothermia. We tested three novel FDA-approved candidates for combination therapy with hypothermia. Tempol, which can be administered concurrently or following MTH-treatment, is a particularly attractive antioxidant molecule. It is considered a SOD mimetic and promotes the metabolism of superoxides at similar rates to SOD. Tempol facilitates breakdown of a wide range of RONS and exhibits catalase activity that limits generation of hydroxyl radicals (OH−) and H2O2 by Fenton reactions. It is considered a general-purpose redox-cycling agent and has been shown to reduce superoxide-related injury in ischemia/reperfusion, inflammation, and radiation-induced damage (Thiemermann, 2003). Edaravone, another free radical scavenger, quenches active oxygen and traps hydroxyl radicals and has been used in clinical studies (Watanabe et al., 2008; Ikeda and Iwasaki, 2015; Bhandari et al., 2018; Ishii et al., 2018). Also, EUK-134 has been used to investigate the role of oxidative stress in neurodegenerative disease (Anderson et al., 2001).
As shown in Figure 5, hypothermia treatment and the three antioxidants applied individually improved the cell viability over normothermic conditions. However, each of the three antioxidants tested, tempol, edaverone, and EUK-134, in the presence of hypothermia increased viability over hypothermia treatment alone (p < 0.05). Hypothermia without the oxidative stress (Hc) served as another control and did not significantly affect the cell viability. The statistical analyses (one-way ANOVA with posttests that included Bonferroni corrections) were limited to wells in the same plate–either normothermic or hypothermic.
FIG. 5.
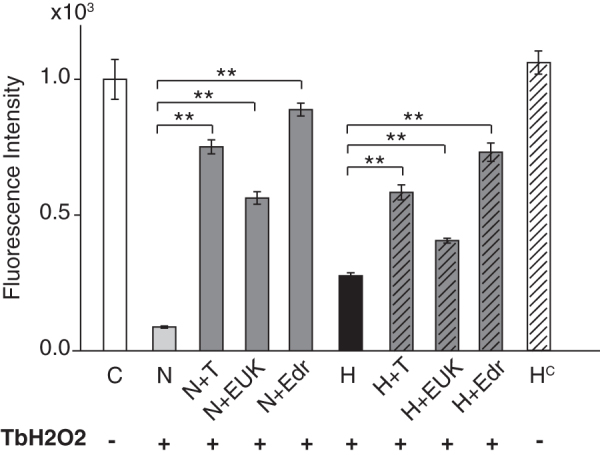
Additive protection of mild hypothermia and antioxidants on cell viability against TbH2O2 stress: antioxidants (tempol, EUK134, and edaravone, each 100 μM) were added to the cells after the oxidative stress, either alone or in combination with hypothermia (33°C). Cell viability was detected by alamarBlue assay. Data are expressed as mean ± SEM (n = 10 per group, three independent experiments). **p < 0.0001. C, control; N, normothermia; H, hypothermia; T, tempol; EUK, EUK134; EDR, edaravone; H+T, hypothermia+tempol; H+EUK, hypothermia+EUK134; H+Edr, hypothermia+edaravone; Hc, hypothermia alone (no TbH2O2).
Hypothermia and antioxidants reduced proinflammatory cytokine mRNA
Figure 6 shows the changes in proinflammatory cytokine TNFα with TbH2O2 as well as with hypothermia and combined antioxidant treatments at 3 hours and 24 hours. Data were normalized to controls (n = 3 plates per group minimum). While TbH2O2 slightly elevated the expression of TNFα mRNA in the normothermic cells above control levels, mild hypothermia significantly decreased its expression in the peroxide-stressed cells (0.25 ± 0.06, p < 0.01). Addition of antioxidants, tempol, EUK-134, or edaravone, during hypothermia treatment produced a similar decrease in TNFα mRNA, well below the levels observed in the TbH2O2-treated normothermia cells.
FIG. 6.
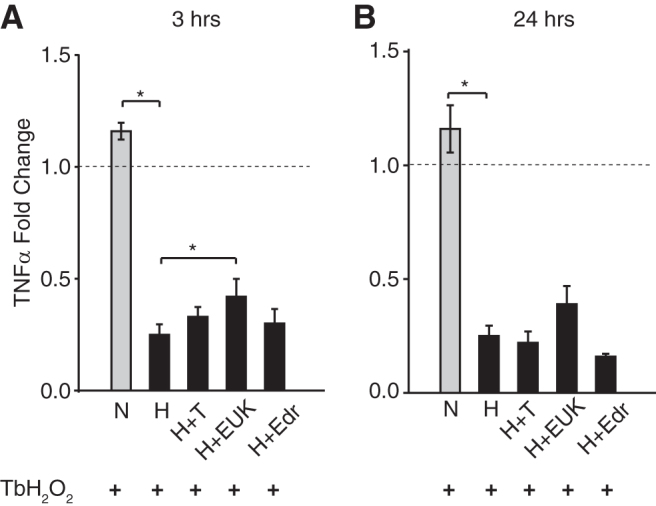
Combination of mild hypothermia and antioxidants reduces tumor necrosis factor expression level poststress. The mRNA level of TNFα was measured at 3 hours (A) and 24 hours (B) posttreatments. Data presented as mean ± SEM, fold change, n = 3 per group, three independent experiments. *p < 0.01. N, normothermia; H, hypothermia; T, tempol; EUK, EUK134; EDR, edaravone; H+T, hypothermia+ tempol; H+EUK, hypothermia+ EUK134; H+Edr, hypothermia+edaravone.
Hypothermia and a general caspase inhibitor reduced apoptosis
We also measured the mRNA levels of two intracellular proteases, caspase-3 and caspase-8, which when activated carry out disassembly of cells into apoptotic bodies during apoptosis. The mRNA levels of both these caspases following the TbH2O2 stress were significantly lower in the hypothermia-treated cultures than in the normothermia cultures at 3 hours (Fig. 7A, B) and at 24 hours (Fig. 7C, D). These results suggest that hypothermia might protect the cells against oxidative stress-induced apoptosis by decreasing caspase expression and/or activation. While the peroxide stress produced only a moderate increase in caspase-3 and caspase-8 message (Fig. 7), this stress reduced cell viability by 50% (Fig. 8) suggesting that increased caspase activity likely contributed to cell death. Addition of a cell-permeable pan-caspase inhibitor Z-VAD-FMK at 50 and 100 μM under both the normothermic and hypothermic conditions enhanced cell survival in a dose-dependent manner (Fig. 8). We observed a significant additive effect of Z-VAD-FMK at 100 μM with hypothermia (H+ZVAD100) over hypothermia alone. This increase in viability with the caspase inhibitor is consistent with hypothermia acting at least, in part, by decreasing caspases.
FIG. 7.
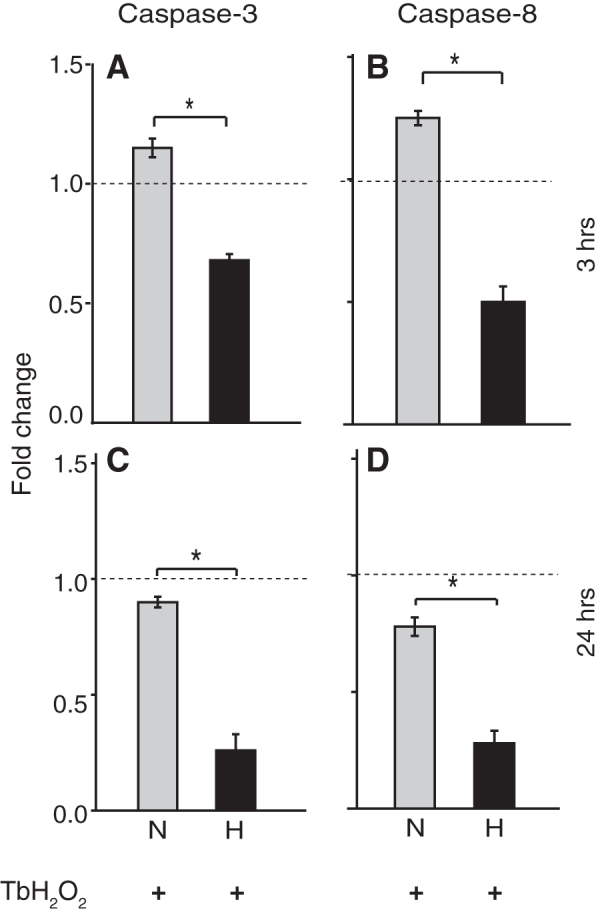
Mild hypothermia reduces apoptosis in PC12 cells: Caspase-3 and Caspase-8 expression levels were measured at 3 hours (A, B) and 24 hours (C, D) after TbH2O2 and hypothermia treatment by qRT-PCR. Data presented as mean ± SEM, fold change, n = 3 per group, three independent experiments. *p < 0.01. N, normothermia; H, hypothermia.
FIG. 8.
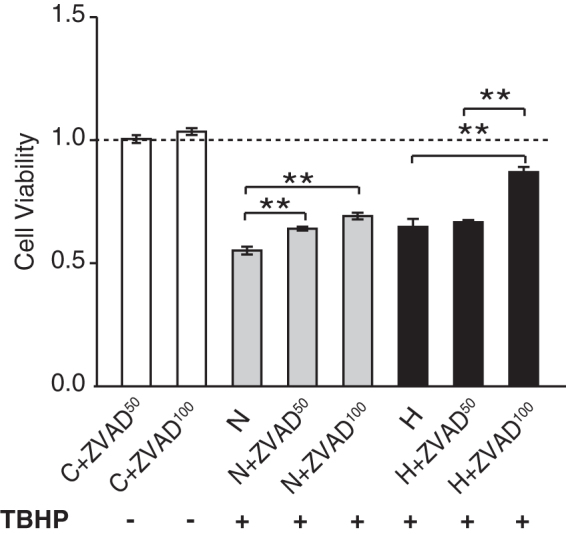
Combination of hypothermia and the caspase inhibitor Z-VAD-FMK protects from TbH2O2-induced cell death: Z-VAD-FMK (50 and 100 μM) was added to the cell cultures alone or in combination with hypothermia after treatment with TbH2O2. Cell viability was measured using alamarBlue assay. Data are expressed as mean ± SEM, n = 4 per group, three individual experiments. **p < 0.01. C+ ZVAD50, control +50 μm Z-VAD-FMK; C+ZVAD100, control +100 μm Z-VAD-FMK; N, normothermia; N+ZVAD50, normothermia +50 μm Z-VAD-FMK; N+ZVAD100, normothermia +100 μm Z-VAD-FMK; H, hypothermia; H+ZVAD50, hypothermia +50 μm Z-VAD-FMK; H+ZVAD100, hypothermia +100 μm Z-VAD-FMK.
Discussion
Oxidative stress-induced increases in ROS levels above a physiological threshold can trigger cell death by interfering with normal cellular operations. ROS-induced cellular death or dysfunction in neurons is a common factor in many stresses, injuries, and/or neurodegenerative diseases (Anderson et al., 2001; Henderson et al., 2006; Hroudova et al., 2014; Cao and Fang, 2015). Factors such as hydrogen peroxide (H2O2), hydroxyl radicals (OH−), or superoxides (O2−), through oxidization and/or damage to cellular components, play important roles in determining the fate of neuronal cells (Globus et al., 1995; Halliwell, 2001). These effects of free radicals can overwhelm endogenous antioxidant mechanisms requiring exogenous therapeutic approaches for neuroprotection. It has been well established that therapeutic hypothermia can significantly reduce the number of free radicals (Dede et al., 2002; Maier et al., 2002; Polderman, 2009; Dietrich and Bramlett, 2010; Antonic et al., 2014), but the mechanisms involved are multifactorial and not totally resolved. The present study evaluated these protective effects of mild therapeutic hypothermia at 33°C in an in vitro model of peroxide-induced stress. The results showed that hypothermia increased PC12 cell viability following oxidative stress from peroxide (Fig. 3). In addition, it was observed that exogenous antioxidants were complementary and additive to hypothermia in combating cell damage (Figs. 5 and 6). This may be particularly relevant for stresses such as ischemia, excitotoxicity, and other forms of injury, which involve an increased ROS activity (Matsushita et al., 2001; Rego and Oliveira, 2003; Henderson et al., 2006; Zhou et al., 2018). Hypothermia might protect cells by decreasing mitochondrial damage, and antioxidants might further increase survival by quenching ROS produced by damaged mitochondria. Our results suggest that protection is likely to be upstream of apoptotic processes such as caspase activation (Figs. 7 and 8).
Protection by delayed hypothermia
Hypothermia can affect multiple intracellular mechanisms, including excitotoxicity, inflammation, apoptosis, and free radical production (Polderman, 2009; Eroglu et al., 2017). Previous studies of mechanisms underlying therapeutic hypothermia have demonstrated that the timing of the hypothermia treatment and duration are key factors in its neuroprotective mechanisms. In the present study, hypothermia had a protective effect when applied after the peroxide was washed out with medium at 37°C. This protective effect was present even when the hypothermia was applied 15–60 minutes after washout of the peroxide. We note that while the degree of free radical production is temperature-dependent (Novack et al., 1996; Schaible et al., 2018), we only tested the effects of mild to moderate hypothermia (33°C, or 4°C lowering of temperature), which avoids potential side-effects of deep hypothermia (below 30°C). These results are consistent with the hypothesis that hypothermia decreases quenching of ROS by cellular mechanisms as in heart cells (Biary et al., 2011) and does not completely prevent free radical production. Thus, therapeutic hypothermia may allow complementary endogenous and/or other exogenous antioxidative and protective mechanisms to further mitigate oxidative damage to cells.
Additive effects of antioxidants with therapeutic hypothermia
Hypothermia has been reported to both increase (Dede et al., 2002; Gamez et al., 2008) and decrease (Maier et al., 1998, 2002; Ferrigno et al., 2009; Hasegawa et al., 2009; Vairetti et al., 2009) oxidative stress, but these effects strongly correlate with the parameters of cooling such as the temperature applied and duration of cooling. It has been reported that the protective effect of hypothermia against hypoxia-induced damage could be improved with the addition of catalase to the cellular medium (Zitta et al., 2010). Other enzymatic antioxidants such as SOD, glutathione peroxidase, as well as molecular antioxidants such as glutathione can all scavenge ROS. In this study, we tested the potential complementary effects of hypothermia and exogenous antioxidants poststress. Indeed, the survival of PC12 cells following washout of the peroxides with the hypothermia treatment was further increased by addition of FDA-approved antioxidants (tempol, EU134, or edaravone, Figs. 5–7). These antioxidants likely acted by quenching superoxide produced by damaged mitochondria (Zorov et al., 2000, 2014) and/or quenching remaining exogenous peroxides despite the extensive washing. Hypothermia can decrease mitochondrial ROS production, but hypothermia likely also decreases scavenging of the ROS already present (Biary et al., 2011). Thus, antioxidants are likely to be complementary to hypothermia. Consistent with our results with the three antioxidants and hypothermia, edaravone was found to be additive to hypothermia in a rat model of intracerebral hemorrhage (Zhu et al., 2015). The damaging effects of hypothermia in the presence of TbH2O2 and the additive protective effect of antioxidants when combined with hypothermia implies that ROS continued to be present intracellularly during stress and following washout of the exogenous TbH2O2. This is likely due to ROS-induced ROS caused by mitochondrial damage persisting following the exogenous peroxide washout (Zorov et al., 2014).
The additive effect of the antioxidants and hypothermia is consistent with the hypothesis that hypothermia decreases cellular antioxidant defence, whereas antioxidants could compensate for this by directly quenching ROS. The fact that hypothermia appeared to enhance protection in the presence of the antioxidants might reflect damaging mechanisms that are inhibited by hypothermia. For example, hypothermia might protect mitochondria from calcium overloading and/or energy depletion. Multiple previous studies have attempted to combine hypothermia with N-acetyl-aspartyl-glutamate (Wassink et al., 2020), magnesium (calcium and glutamate antagonist) and tirilazad (antioxidant) (Zausinger et al., 2003), Xenon gas which readily crosses the blood–brain barrier (Azzopardi et al., 2016), or erythropoietin therapy (Wassink et al., 2020). However, not all combination treatments produce additive neuroprotective effects (Scholler et al., 2004; Azzopardi et al., 2016; Wassink et al., 2020). Since hypothermia acts as a global inhibitor of many processes, additional studies are needed to find combinatorial strategies that address nonoverlapping mechanisms and augment neuroprotection with therapeutic hypothermia.
Mechanisms of peroxide-induced cell death and protective effects of hypothermia
Peroxide-induced cellular stress might lead to cell death by several mechanisms, including direct mitochondrial damage (Martín-Guerrero et al., 2017), those involving PARP activation by DNA damage (Andrabi et al., 2014). This initial damage likely activates downstream cell death mechanisms, including AIF translocation from mitochondrial to the nucleus and caspase activation (Yu et al., 2002, 2006; Doti et al., 2014). Presumably hypothermia inhibits this death cascade at one or more steps and so increases cell viability. Hypothermia reduces caspase-3 activation in hippocampal neurons following ischemia (Lu et al., 2014) consistent with the decrease in caspase-3 and -8 mRNA in the PC12 cells observed in our study (Fig. 7). Indeed, the protective effect of Z-VAD-FMK indicates that caspases contribute to peroxide-induced PC12 cell death under our experimental conditions (Fig. 8). Similar activation of caspase -3 via caspase-8 by TbH2O2 has been observed in HeLa cells (Wu et al., 2011). Hypothermia appears to protect hippocampal neurons from oxygen and glucose deprivation by inhibiting caspase-3 activation by an intrinsic mitochondrial mechanism (Zhou et al., 2018). Thus, hypothermia might lead to inhibition of the extrinsic (nonmitochondrial) caspase pathway and/or the intrinsic pathway possibly acting upstream of caspases in a death cascade. One indication that it acts upstream of caspases is that it also decreased TNFα expression in our study (Fig. 6). These protective actions of hypothermia likely involve the mitochondria since oxidative stress often acts via damage to mitochondria either directly or downstream from DNA damage from alkylating agents or peroxides (Yu et al., 2002; Zorov et al., 2014). One possibility is that hypothermia decreases stress on mitochondria by decreasing cellular energy demand and ameliorating ATP depletion (Yager and Asselin, 1996). Hypothermia might also decrease mitochondrial Ca2+ overload since uptake of Ca2+ into mitochondria is temperature dependent and decreases with decreasing temperature (David and Barrett, 2000).
Overall, this study provides evidence supporting a novel combinatorial application of delayed therapeutic hypothermia and exogenous antioxidants to prevent oxidative stress-induced cell death. Future studies are needed to confirm and extend the results to other cell types as well as additional forms of oxidative stress insult for a broader applicability of our results. The experimental paradigm discussed here also suffers from several sources of variability since present study was limited to comparing cell viability between two different plates, either placed in hypothermic or normothermic conditions. A Peltier system that can provide cooling to half of the wells on one plate, while maintaining the rest at normothermic conditions, may enable a robust and direct comparison of multiple combinatorial treatments. While it has been established that therapeutic hypothermia should be induced as early as possible posttrauma, time taken to induce cooling and the rewarming rates are challenging factors under clinical situations (Levi et al., 2009; Finkelstein and Alam, 2010; Crossley et al., 2014; Dunkley and McLeod, 2017). Hence, additional studies are needed to determine critical parameters related to hypothermia such as duration (up to 24 hours), target temperature (32–36°C), and timing of cooling (post stress) as well as timing and dosage of the antioxidants with respect to the stress. With a more comprehensive understanding of mechanisms underlying the hypothermia-induced protection of neurons, other molecular targets may be identified for similar temporally additive, combinatorial interventions.
Authors' Contributions
J.S., J.B., and S.M.R. conceptualized and designed the experiments, J.S., J.B., and R.S. carried out analysis, and all authors contributed to article preparation.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
NIH NIDCD 1R01DC013798 and Cochlear (Research Grants to SMR).
References
- Al-Nasiry S, Geusens N, Hanssens M, et al. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod 2007;22:1304–1309 [DOI] [PubMed] [Google Scholar]
- Alva N, Carbonell T, Palomeque J. Deep hypothermia impact on acid-base parameters and liver antioxidant status in an in vivo rat model. Can J Physiol Pharmacol 2009;87:471–478 [DOI] [PubMed] [Google Scholar]
- Amer AR, Oorschot DE. Xenon combined with hypothermia in perinatal hypoxic-ischemic encephalopathy: a Noble Gas, a Noble Mission. Pediatr Neurol 2018;84:5–10 [DOI] [PubMed] [Google Scholar]
- Anderson I, Adinolfi C, Doctrow S, et al. Oxidative signalling and inflammatory pathways in Alzheimer's disease. Biochem Soc Symp 2001;67:141–149 [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Umanah GKE, Chang C, et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A 2014;111:10209–10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonic A, Dottori M, Leung J, et al. Hypothermia protects human neurons. Int J Stroke 2014;9:544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi D, Robertson NJ,. Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol 2016;15145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburamani AA, Arichi T. Complementing cooling: the ongoing search for an effective adjunct to therapeutic hypothermia. J Physiol 2020;598:905–906 [DOI] [PubMed] [Google Scholar]
- Bhandari R, Kuhad A, Kuhad A. Edaravone: a new hope for deadly amyotrophic lateral sclerosis. Drugs Today (Barc) 2018;54:349–360 [DOI] [PubMed] [Google Scholar]
- Biary N, Xie C, Kauffman J. et al. Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J Physiol 2011;589(Pt 21):5167–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Fang Y. Transducing oxidative stress to death signals in neurons. J Cell Biol 2015;211:741–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccino A, Bisson LJ, Carpenter B, et al. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine (Phila Pa 1976) 2010;35:E57–62 [DOI] [PubMed] [Google Scholar]
- Chanvitayapongs S, Draczynska-Lusiak B, Sun AY. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport 1997;8:1499–1502 [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K, Alonso OF, Kraydieh S. et al. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab 2000;20:531–542 [DOI] [PubMed] [Google Scholar]
- Chwastek J, Jantas D, Lasoń W. The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a γH2AX/p-p53/caspase-3-independent mechanism: inhibition of calpain and cathepsin D. Int J Biochem Cell Biol 2017;87:38–53 [DOI] [PubMed] [Google Scholar]
- Crossley S, Reid J, McLatchie R, et al. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care 2014;18:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: positive or negative effects. Neural Regen Res 2013;8:2677–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash HH, Chavali S. Management of traumatic brain injury patients. Korean J Anesthesiol 2018;71:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca(2+)] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci 2000;20:7290–7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dede S, Deger Y, Meral I. Effect of short-term hypothermia on lipid peroxidation and antioxidant enzyme activity in rats. J Vet Med A Physiol Pathol Clin Med 2002;49:286–288 [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma 2009;26:301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res 2007;162:201–217 [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 2010;7:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: experimental and clinical experience. Brain Circ 2017;3:186–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol 1999;158:444–450 [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Levi AD, Wang M, et al. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics 2011;8:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doti N, Reuther C, Scognamiglio PL, et al. Inhibition of the AIF/CypA complex protects against intrinsic death pathways induced by oxidative stress. Cell Death Dis 2014;5:e993–e993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley S, McLeod A. Therapeutic hypothermia in patients following traumatic brain injury: a systematic review. Nurs Crit Care 2017;22:150–160 [DOI] [PubMed] [Google Scholar]
- Eroglu O, Deniz T, Kisa U, et al. Effect of hypothermia on apoptosis in traumatic brain injury and hemorrhagic shock model. Injury 2017;48:2675–2682 [DOI] [PubMed] [Google Scholar]
- Ferrigno A, Carlucci F, Tabucchi A, et al. Different susceptibility of liver grafts from lean and obese Zucker rats to preservation injury. Cryobiology 2009;59:327–334 [DOI] [PubMed] [Google Scholar]
- Finkelstein RA, Alam HB. Induced hypothermia for trauma: current research and practice. J Intensive Care Med 2010;25:205–226 [DOI] [PubMed] [Google Scholar]
- Gamez A, Alva N, Roig T, et al. Beneficial effects of fructose 1,6-biphosphate on hypothermia-induced reactive oxygen species injury in rats. Eur J Pharmacol 2008;590:115–119 [DOI] [PubMed] [Google Scholar]
- Gao XY, Huang JO, Hu YF, et al. Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury. Sci Rep 2014;4:7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus MY, Alonso O, Dietrich WD, et al. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995;65:1704–1711 [DOI] [PubMed] [Google Scholar]
- Green EJ, Pazos AJ, Dietrich WD, et al. Combined postischemic hypothermia and delayed MK-801 treatment attenuates neurobehavioral deficits associated with transient global ischemia in rats. Brain Res 1995;702:145–152 [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 2000;899:136–147 [DOI] [PubMed] [Google Scholar]
- Halleck MM, Richburg JH, Kauffman FC. Reversible and irreversible oxidant injury to PC12 cells by hydrogen peroxide. Free Radic Biol Med 1992;12:137–144 [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 2001;18:685–716 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Ogihara T, Tamai H, et al. Hypothermic inhibition of apoptotic pathways for combined neurotoxicity of iron and ascorbic acid in differentiated PC12 cells: reduction of oxidative stress and maintenance of the glutathione redox state. Brain Res 2009;1283:1–13 [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006;27:1–19 [DOI] [PubMed] [Google Scholar]
- Hroudova J, Singh N, Fisar Z. Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer's disease. Biomed Res Int 2014;2014:175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Iwasaki Y. Edaravone, a free radical scavenger, delayed symptomatic and pathological progression of motor neuron disease in the wobbler mouse. PLoS One 2015;10:e0140316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Petrenko AB, Sasaki M, et al. Free radical scavenger edaravone produces robust neuroprotection in a rat model of spinal cord injury. Brain Res 2018;1682:24–35 [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res 2001;496:181–190 [DOI] [PubMed] [Google Scholar]
- Kawabori M, Hokari M, Zheng Z, et al. Triggering receptor expressed on myeloid cells-2 correlates to hypothermic neuroprotection in ischemic stroke. Ther Hypothermia Temp Manag 2013;3:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Okauchi M, Morisaki K, et al. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke 2000;31:1982–1989; Discussion 1989. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wei ZZ, Cao W, et al. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis 2016;96:248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery 2010;66:670–677 [DOI] [PubMed] [Google Scholar]
- Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma 2009;26:407–415 [DOI] [PubMed] [Google Scholar]
- Lin WL, Wang SM, Ho YJ, et al. Ethyl acetate extract of Wedelia chinensis inhibits tert-butyl hydroperoxide-induced damage in PC12 cells and D-galactose-induced neuronal cell loss in mice. BMC Complement Altern Med 2014;14:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Shen Y, Qian HY, et al. Effects of mild hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of hippocampus nerve cells in rats after cardiopulmonary resuscitation. World J Emerg Med 2014;5:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, et al. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke 1998;29:2171–2180 [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Cheng D, et al. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis 2002;11:28–42 [DOI] [PubMed] [Google Scholar]
- Martín-Guerrero SM, Muñoz-Gámez JA, Carrasco M-C, et al. Poly(ADP-ribose)polymerases inhibitors prevent early mitochondrial fragmentation and hepatocyte cell death induced by H2O2. PLoS One 2017;12:e0187130–e0187130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Bramlett HM, Alonso O, et al. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit Care Med 2001;29:2060–2066 [DOI] [PubMed] [Google Scholar]
- Nonner D, Barrett EF, Barrett JN. Neurotrophin effects on survival and expression of cholinergic properties in cultured rat septal neurons under normal and stress conditions. J Neurosci 1996;16:6665–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack TA, Dillon MC, Jackson WT. Neurochemical mechanisms in brain injury and treatment: a review. J Clin Exp Neuropsychol 1996;18:685–706 [DOI] [PubMed] [Google Scholar]
- Palee S, Apaijai N, Shinlapawittayatorn K, et al. Acetylcholine attenuates hydrogen peroxide-induced intracellular calcium dyshomeostasis through both muscarinic and nicotinic receptors in cardiomyocytes. Cell Physiol Biochem 2016;39:341–349 [DOI] [PubMed] [Google Scholar]
- Pazos AJ, Green EJ, Busto R, et al. Effects of combined postischemic hypothermia and delayed N-tert-butyl-alpha-pheylnitrone (PBN) administration on histopathologicaland behavioral deficits associated with transient global ischemia in rats. Brain Res 1999;846:186–195 [DOI] [PubMed] [Google Scholar]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37(7 Suppl):S186–202 [DOI] [PubMed] [Google Scholar]
- Pronsato L, Milanesi L. Effect of testosterone on the regulation of p53 and p66Shc during oxidative stress damage in C2C12 cells. Steroids 2016;106:41–54 [DOI] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 2003;28:1563–1574 [DOI] [PubMed] [Google Scholar]
- Rueden CT,. Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 2017;18:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Enokido Y, Aoshima H, et al. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res 1997;50:413–420 [DOI] [PubMed] [Google Scholar]
- Schaible N, Han YS, Tveita T, et al. Role of superoxide ion formation in hypothermia/rewarming induced contractile dysfunction in cardiomyocytes. Cryobiology 2018;81:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Hungerhuber E, Zausinger S, et al. Combination drug therapy and mild hypothermia: a promising treatment strategy for reversible, focal cerebral ischemia. Stroke 1999;30:1891–1899 [DOI] [PubMed] [Google Scholar]
- Scholler K, Zausinger S, Baethmann A, et al. Neuroprotection in ischemic stroke—combination drug therapy and mild hypothermia in a rat model of permanent focal cerebral ischemia. Brain Res 2004;1023:272–278 [DOI] [PubMed] [Google Scholar]
- Tang XQ, Feng JQ, Chen J, et al. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: mechanisms via MMP, ROS, and Bcl-2. Brain Res 2005;1057:57–64 [DOI] [PubMed] [Google Scholar]
- Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med 2003;31(1 Suppl):S76–84 [DOI] [PubMed] [Google Scholar]
- Truettner JS, Alonso OF, Dietrich WD. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab 2005;25:1505–1516 [DOI] [PubMed] [Google Scholar]
- Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res 2005;138:124–134 [DOI] [PubMed] [Google Scholar]
- Vairetti M, Ferrigno A, Carlucci F, et al. Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl 2009;15:20–29 [DOI] [PubMed] [Google Scholar]
- Van Hemelrijck A, Hachimi-Idrissi S, Sarre S, et al. Neuroprotective effect of N-acetyl-aspartyl-glutamate in combination with mild hypothermia in the endothelin-1 rat model of focal cerebral ischaemia. J Neurochem 2005;95:1287–1297 [DOI] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol 2009;218:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink G, Davidson JO, Fraser M, et al. Non-additive effects of adjunct erythropoietin therapy with therapeutic hypothermia after global cerebral ischaemia in near-term fetal sheep. J Physiol 2020;598:999–1015 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tahara M, Todo S. “The novel antioxidant edaravone: from bench to bedside.” Cardiovasc Ther 2008;26:101–114 [DOI] [PubMed] [Google Scholar]
- Wu C, Zhao W, Yu J, et al. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci Rep 2018;8:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang D, Wang X, et al. Caspase 3 is activated through caspase 8 instead of caspase 9 during H2O2-induced apoptosis in HeLa cells. Cell Physiol Biochem 2011;27:539–546 [DOI] [PubMed] [Google Scholar]
- Yager JY, Asselin J. Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke 1996;27:919–925; Discussion 926. [DOI] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, et al. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 2006;103:18314–18319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002;297:259–263 [DOI] [PubMed] [Google Scholar]
- Zausinger S, Scholler K, Plesnila N, et al. Combination drug therapy and mild hypothermia after transient focal cerebral ischemia in rats. Stroke 2003;34:2246–2251 [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao G, Chen L, et al. Hydrogen peroxide/ATR-Chk2 activation mediates p53 protein stabilization and anti-cancer activity of cheliensisin A in human cancer cells. Oncotarget 2014;5:841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lin H, Jiang L, et al. Mild hypothermia protects hippocampal neurons from oxygen-glucose deprivation injury through inhibiting caspase-3 activation. Cryobiology 2018;80:55–61 [DOI] [PubMed] [Google Scholar]
- Zhou T, Prather ER, Garrison DE, et al. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int J Mol Sci 2018;19: E417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu C, Sun Z. Early combined therapy with pharmacologically induced hypothermia and edaravone exerts neuroprotective effects in a rat model of intracerebral hemorrhage. Cell Biochem Biophys 2015;73:581–587 [DOI] [PubMed] [Google Scholar]
- Ziganshin AU, Khairullin AE, Zobov VV, et al. Effects of ATP and adenosine on contraction amplitude of rat soleus muscle at different temperatures. Muscle Nerve 2017;55:417–423 [DOI] [PubMed] [Google Scholar]
- Zitta K, Meybohm P, Bein B, et al. Hypoxia-induced cell damage is reduced by mild hypothermia and postconditioning with catalase in-vitro: application of an enzyme based oxygen deficiency system. Eur J Pharmacol 2010;628:11–18 [DOI] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 2000;192:1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014;94:909–950 [DOI] [PMC free article] [PubMed] [Google Scholar]