Abstract
Japanese honewort (Cryptotaenia japonica) is consumed as a traditional vegetable and has medicinal applications. In Japan, C. japonica is mainly produced using hydroponic culture systems; however, damping-off is often caused by the adherence of pathogens to its seeds. Therefore, the use of sterile artificial seeds in hydroponic culture is likely to be effective for preventing disease. In this study, we established methods for stress-induced somatic embryogenesis and artificial seed production in Japanese honewort. Shoot apex explants from seedlings were treated with 0.7 M sucrose as a hyperosmotic stress for 3 or 6 weeks, and then transferred to stress-free conditions. Somatic embryos were formed after culture in stress-free conditions for 7 weeks. Stress-treated shoot apex explants that formed somatic embryos were cultured in Murashige and Skoog liquid medium with shaking. After 2 weeks of culture, approximately 800 somatic embryos were formed from each explant. Somatic embryos were formed continuously during 37 weeks under the same culture conditions. Thus, somatic embryogenesis was effectively induced in Japanese honewort via hyperosmotic stress, and embryogenic competence was maintained under stress- and phytohormone-free conditions. The somatic embryos produced by liquid culture were used to produce artificial seeds by enveloping the embryos in whipped alginate gel to avoid hypoxic conditions. The artificial seeds had a high germination rate (72%). This system is suitable for the sterile, highly productive hydroponic culture of Japanese honewort.
Keywords: artificial seed, hydroponic culture, hyperosmotic stress, Japanese honewort, somatic embryogenesis
Introduction
Japanese honewort (Cryptotaenia japonica; Apiaceae) is a perennial plant that inhabits forests in hilly areas of East Asia (Spalik and Downie 2007). In many Asian countries, Japanese honewort is used as a medicinal plant due to its hypotensive, hypolipidemic, and anti-obesity effects (Cheng et al. 2008). Japanese honewort is also consumed in Japan as a traditional vegetable or spice for the distinctive flavors of its leaves and petioles (Okuno et al. 2017).
In Japan, large quantities of Japanese honewort are produced from seeds in hydroponic culture systems (Abe et al. 2003; Fujime 2012); however, plants grown in these systems are affected by damping-off, caused by the adherence of pathogens to seeds (Neergaard 1977; Nishioka et al. 2014; Qu et al. 2008). Since this disease causes enormous agricultural production losses, seeds are commonly treated with pesticides or high temperature to eliminate pathogens (Lamichhane et al. 2017). However, these methods cannot completely prevent the disease, and pesticide residues are a source of concern for consumer health.
The use of artificial seeds is likely to be effective for preventing disease caused by the adherence of pathogens to natural seeds in hydroponic culture. Artificial seeds are not contaminated by pathogens because they are produced under sterile conditions (Rihan et al. 2017). Adequate moisture and clean conditions are required for the preservation and germination of artificial seeds (Bapat and Rao 1990; Mandel et al. 2000). Therefore, artificial seeds are generally suitable for hydroponic culture systems, which are characterized by controlled moisture and temperature conditions.
Artificial seeds are typically made as soft beads in which somatic embryos or adventitious shoots are encapsulated (Rihan et al. 2017). Somatic embryos, which were first reported in carrot, are produced by plant tissue culture methods (Steward et al. 1958). In carrot somatic embryo production, callus-like embryogenic cells are induced from explants cultured in medium containing phytohormones such as 2,4-dichlorophenoxyacetic acid as an artificial auxin (Kamada and Harada 1979). The embryogenic cells are then transferred to a phytohormone-free medium and used to induce somatic embryos. Large numbers of somatic embryos can be obtained by large-scale embryogenic cell culture (Paek et al. 2005).
Alternatively, stress-induced somatic embryogenesis has been performed for several plant species (Choi et al. 1998; Ikeda-Iwai et al. 2003; Kamada et al. 1989; Kiyosue et al. 1989; Patnaik et al. 2005). For example, carrot explants are treated with stress factors (e.g., osmotic pressure, salt, heavy metals, heat, or high concentrations of chemicals) for 1–4 weeks, followed by direct induction of somatic embryos from explants cultured under stress-free conditions (Kamada et al. 1989, 1993, 1994; Kiyosue et al. 1989, 1990; Tokuji and Masuda 1996). Stress-induced somatic embryos germinate and grow normally because they are not affected by exogenously applied phytohormones (Kamada et al. 1989). However, protocols for stress-induced somatic embryogenesis have been established for only a few plant species, and methods for large-scale stress-induced somatic embryogenesis culture are insufficient.
Therefore, in this study, we established methods for stress-induced somatic embryogenesis and artificial seed production from somatic embryos in Japanese honewort.
Materials and methods
Plant materials
In this study, we used seeds of Japanese honewort (Cryptotaenia japonica cv. Kantoshirokuki-Mitsuba; Takii Seed, Kyoto, Japan) and carrot (Daucus carota cv. US-Harumakigosun; Yokohama Nursery, Yokohama, Japan). On a daily basis, we collected 2-week-old seedlings grown on vermiculite at 25°C under a 16-h light/8-h dark cycle (approximately 40 µmol photon m−2 s−1). The seedlings were then surface-sterilized with hypochlorite solution (1% active chlorine) and rinsed five to six times with sterilized distilled water.
Stress-induced somatic embryogenesis
Shoot apex explants (length, 10 mm) were excised from sterilized seedlings and cultured at 25°C under continuous light (approximately 40 µmol photon m−2 s−1) on Murashige and Skoog (MS) semi-solid medium containing 0.7 M sucrose (MS+SUC medium) as a treatment for hyperosmotic stress (Kamada et al. 1993). After 3 or 6 weeks of hyperosmotic stress treatment, explants were transferred onto MS medium containing 0.09 M sucrose (stress-free condition) and cultured for 6–7 weeks (Kamada et al. 1993) until somatic embryos formed on the explant surface. The rate of somatic embryo formation (%) was calculated as [(number of explants forming embryos)/(number of survived explants)×100]. Explants that did not show chlorosis and bacterial contamination were defined as survived.
Each shoot apex explant that formed somatic embryos was cultured in 30–40 ml of MS liquid medium at 25°C in the dark with shaking (70 rpm) and passaged every 2 weeks. Newly formed somatic embryos were released from the explant. Embryos were counted using the ImageJ software (NIH, Bethesda, MS, USA).
To confirm germination, somatic embryos were placed on or immersed in MS semi-solid medium and cultured at 25°C under continuous light for 2 weeks. Embryo germination and growth were then observed.
Artificial seeds
Conventional artificial seeds were formed as alginate beads following the method described by Sharma et al. (2013) with modifications (bead-type artificial seeds; Figure 1A). MS medium containing 1% (w/v) sodium alginate with a somatic embryo was dropped into MS medium containing 1% (w/v) CaCl2. After 1 min of incubation, the alginate bead including the embryo was washed with MS medium.
Figure 1. Two types of artificial seeds. (A) Bead type: Murashige and Skoog (MS) medium containing 1% sodium alginate and a somatic embryo was dropped into MS medium containing 1% CaCl2. The alginate bead with an embryo was formed during incubation in 1% CaCl2. (B) Sponge type: MS medium containing 1% sodium alginate and 4% gelatin was whipped and dropped into MS medium containing 1% CaCl2 and a somatic embryo was placed in the floating whipped medium. The whipped alginate gel with an embryo was formed by immersing the whipped medium in 1% CaCl2. Finally, both seeds were washed with MS medium.
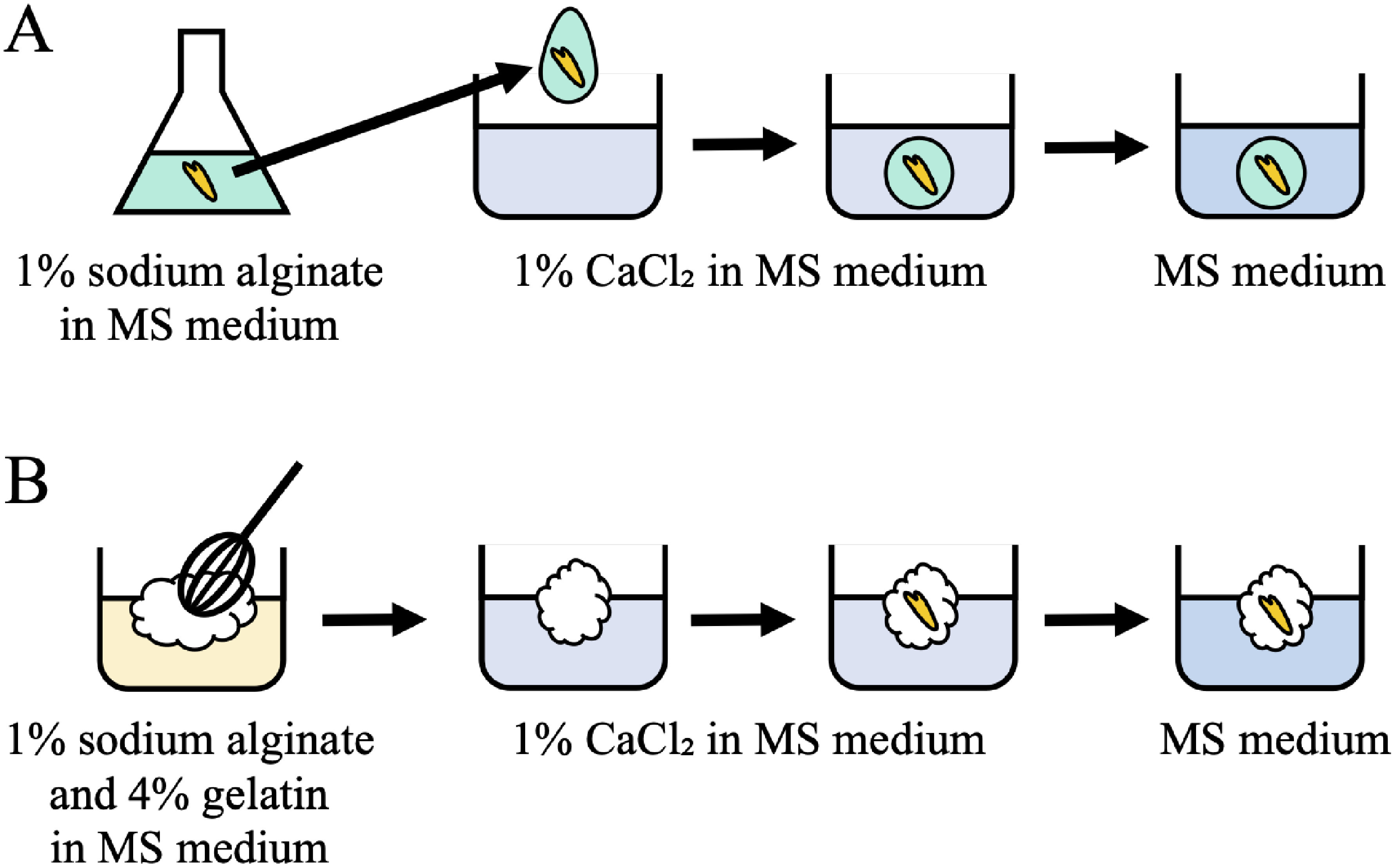
Modified artificial seeds were made as whipped alginate gels (sponge-type artificial seeds; Figure 1B). MS medium containing 1% (w/v) sodium alginate and 4% (w/v) gelatin was whipped manually using a whisk. The whipped medium was dropped into MS medium containing 1% (w/v) CaCl2, and the embryo was placed in the floating whipped medium. The whipped medium containing a somatic embryo was immediately immersed in medium for 1 min, thereby forming a whipped alginate gel in which the embryo was enveloped. The enveloped embryo was then washed with MS medium.
Both types of artificial seeds were placed on polyurethane sponges (UH-ZK200, UING, Osaka, Japan) containing MS liquid medium and cultured at 25°C for 14 days under continuous light. Survival and germination were defined as the de-etiolation of somatic embryos and formation of true leaves and radicles, respectively. Viability (%) was calculated as [(number of survived artificial seeds)/(number of total artificial seeds)×100], and the germination rate (%) was calculated as [(number of germinated artificial seeds)/(number of total artificial seeds)×100].
Statistical analyses
Data were analyzed using Student’s t-test or Welch’s t-test. Significance was evaluated at a level of p<0.05. Statistical analyses were performed using the Excel software (Microsoft, Redmond, WA, USA) with the Statcel 4 add-in (OMS, Tokyo, Japan).
Results and discussion
Hyperosmotic stress-induced somatic embryogenesis in Japanese honewort
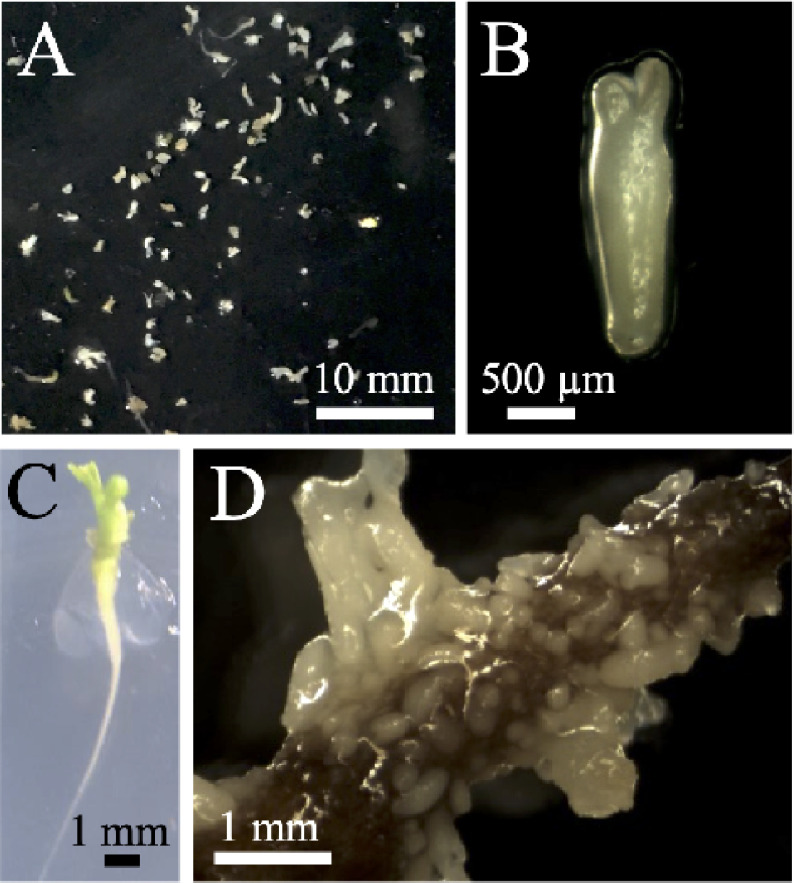
In this study, Japanese honewort seedling shoot apex explants were treated with hyperosmotic stress for 3 or 6 weeks. Somatic embryos were formed after culture under stress-free conditions for 7 weeks (Figure 2A, B); they germinated and grew normally (Figure 2C). At most, 160 somatic embryos were formed from each shoot apex explant treated with stress for 6 weeks. The somatic embryos exhibited normal morphology, suggesting that they were not affected by exogenously applied phytohormones (Figure 2A). Somatic embryo formation rates were 14% and 39% following stress treatment for 3 and 6 weeks, respectively, indicating that longer stress treatment enhances somatic embryogenesis (Table 1).
Figure 2. Somatic embryos of Japanese honewort. Somatic embryos were formed on shoot apex explants treated with hyperosmotic stress for (A) 3 or (B) 6 weeks and then cultured for 7 weeks without stress. (C) Somatic embryos germinated and grew to mature plants.
Table 1. Hyperosmotic stress-induced somatic embryogenesis.
| Plant species | Stress treatment (weeks) | Somatic embryo formation (%) | |
|---|---|---|---|
| Japanese honewort | 3 | 14±3 | * |
| 6 | 39±9 | ||
| Carrot | 3 | 28±5 | |
| 6 | 35±10 | ||
Rates of somatic embryo formation are expressed as the means of four individual experiments±standard error. Significant differences were evaluated using Welch’s t-test (* p<0.05).
Carrot somatic embryo formation rates were 28% and 35% after stress treatment for 3 and 6 weeks, respectively (Table 1), supporting the results of Kikuchi et al. (2006). Carrot somatic embryo formation rates were similar to those of Japanese honewort (Table 1). Therefore, Japanese honewort somatic embryo formation was as efficient as that of carrot.
The carrot somatic embryo formation rate by 6 weeks of hyperosmotic stress treatment is 81% in Kikuchi et al. (2006), whereas our value was 35%. It is difficult to compare both values because somatic embryo formation are affected by varieties of cell lines, seedling growth, and culture conditions. It is considered that improving seedling growth and culture conditions can increase somatic embryo formation rates in carrot and Japanese honewort.
In some plant species, phytohormone treatments are required for stress-induced somatic embryogenesis with or after stress treatment, and it is necessary to consider the phytohormone treatment processes (e.g., molecular species, concentration, and timing of treatment). (Ikeda-Iwai et al. 2003; Patnaik et al. 2005). We successfully established a simple method for stress-induced somatic embryogenesis in Japanese honewort without phytohormone application.
Molecular mechanisms for somatic embryogenesis have been suggested in previous studies. Consistent with the role of stress, the ratio of abscisic acid to gibberellins may affect somatic embryogenesis in carrot and Arabidopsis thaliana (Braybrook and Harada 2008). Somatic embryogenesis is induced by stress through DNA methylation in carrot and Cucurbita pepo (Leljak-Levanić et al. 2004; Yamamoto et al. 2005). Chromatin reorganization also plays an important role in somatic embryogenesis (Fehér 2015). Therefore, stress-induced somatic embryogenesis may be facilitated by endogenous phytohormones and epigenetic systems in Japanese honewort.
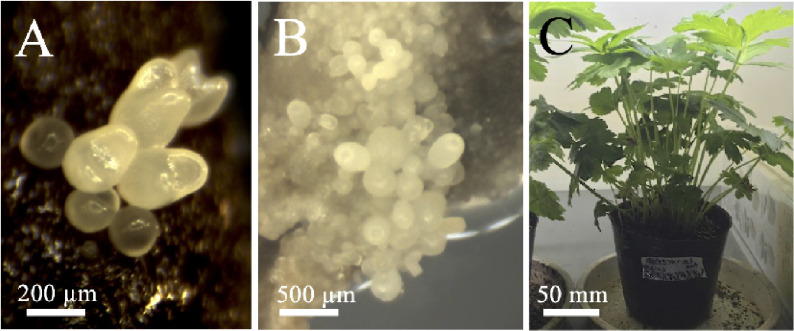
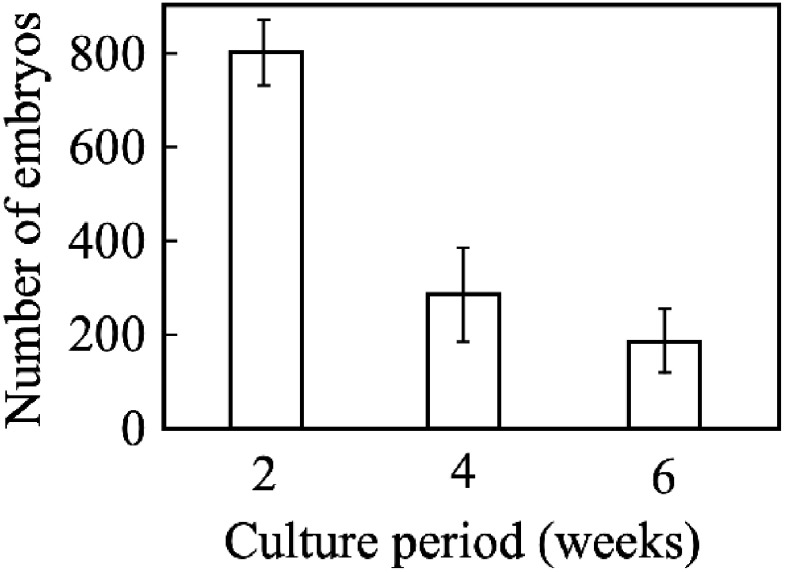
Hyperosmotic stress-treated shoot apex explants that formed somatic embryos were cultured in MS liquid medium with shaking and passaged every 2 weeks. After the culture, several hundreds of somatic embryos were newly formed and released from each explant (Figure 3A, B), which germinated and grew normally (Figure 3C). Thus, large numbers of somatic embryos were formed from stress-treated explants after culturing in liquid medium. The explants were passaged every 2 weeks, and approximately 800, 300, and 200 somatic embryos were formed and released from each explant after 2, 4, and 6 weeks of culture, respectively (Figure 4). In some explants, somatic embryos formed continuously for over 37 weeks (Figure 3D).
Figure 3. Somatic embryos in liquid culture. Shoot apex explants treated with hyperosmotic stress for 6 weeks were cultured in Murashige and Skoog liquid medium with shaking and passaged every 2 weeks. (A, B) Somatic embryos formed and released from explants. (C) Seedling from a somatic embryo. (D) Somatic embryos on an explant cultured with shaking for 37 weeks.
Figure 4. Somatic embryo formation through passage culture. Shoot apex explants treated with hyperosmotic stress for 6 weeks were cultured in Murashige and Skoog liquid medium with shaking. Somatic embryos were counted every 2 weeks during passage culture. Bar: standard error (n=5).
This is the first report of long-term somatic embryogenesis under stress- and phytohormone-free conditions. Previous studies have reported the formation of carrot somatic embryos from stress-treated explants through culture in MS liquid medium with shaking (Kamada et al. 1989); however, similar results have not been reported for long-term culture.
Artificial seeds
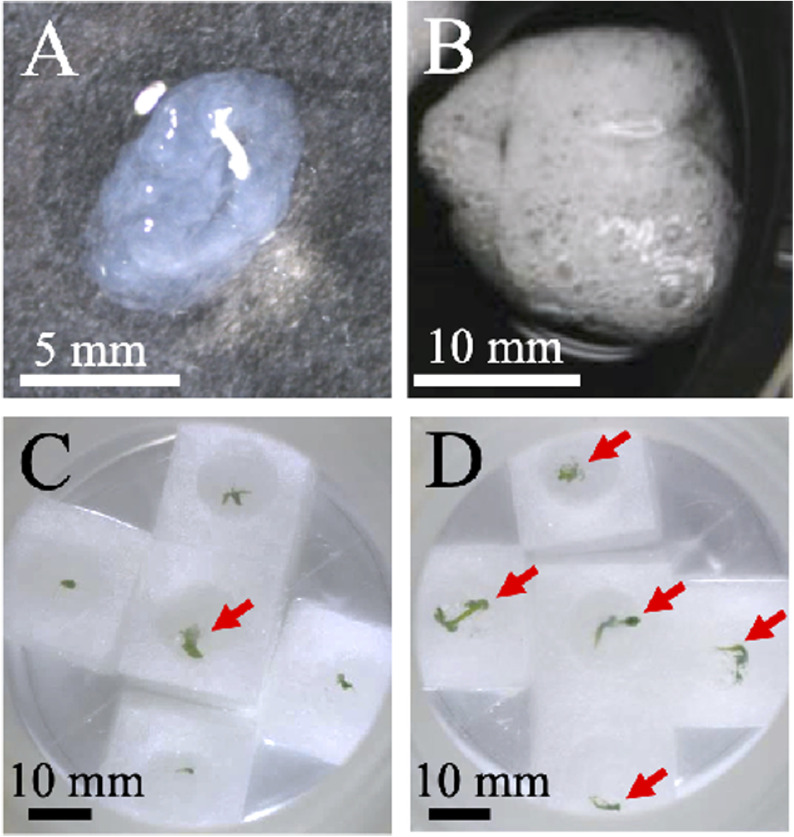
We produced two types (bead and sponge) of artificial seeds. Generally, bead-type artificial seeds are produced using 3% (w/v) sodium alginate (Sharma et al. 2013). Because 3% (w/v) alginate beads are hard, which might prevent embryo germination, we also prepared artificial seeds using 1% (w/v) sodium alginate (Figure 5A) (Ahmad and Anis 2010). Since the alginate beads were translucent, somatic embryos in the beads were not clearly observed (Figure 5A). All artificial seeds survived; however, only 26% geminated (Figure 5C, Table 2). Therefore, embryo germination was likely prevented by encapsulation within alginate beads.
Figure 5. Germination of artificial seeds. (A, C) Bead-type and (B, D) sponge-type artificial seeds were cultured on polyurethane sponges containing liquid Murashige and Skoog medium for 14 days (C, D). Arrows indicate germinated plants.
Table 2. Germination rates of two types of artificial seeds.
| Artificial seed type | Viability (%) | Germination (%) | |
|---|---|---|---|
| Bead | 100 | 26±4 | * |
| Sponge | 100 | 72±5 | |
Viability and germination rates after 14 days of incubation are expressed as the means of five individual experiments±standard error. Significant differences were evaluated using Student’s t-test (* p<0.05).
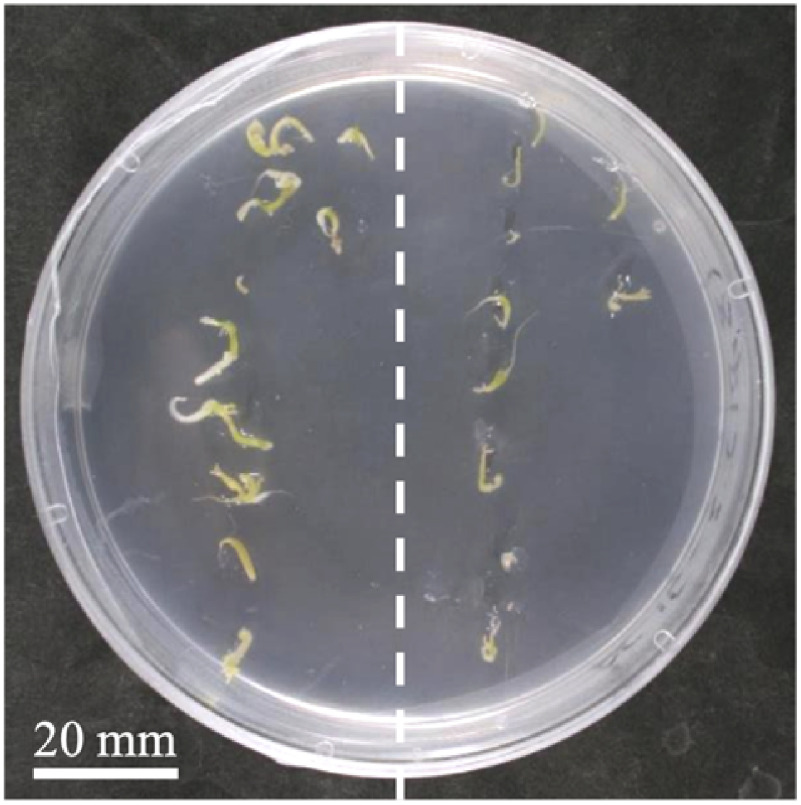
Very few of the somatic embryos immersed in MS semi-solid medium germinated (Figure 6, right). In contrast, those cultured on the surface of MS semi-solid medium geminated and grew normally (Figure 6, left). Previous studies have shown that oxygen influx to apple artificial seeds is limited by gel coating (Hulst et al. 1989; Piccioni 1997). Therefore, it is likely that somatic embryo germination was inhibited under hypoxic conditions in bead-type artificial Japanese honewort seeds.
Figure 6. Germination of somatic embryos on or within semi-solid medium. Somatic embryos were cultured on (left) or within (right) semi-solid Murashige and Skoog medium for 7 days.
We also prepared sponge-type artificial seeds, in which somatic embryos were enveloped in whipped alginate gels (Figure 5B). Somatic embryos covered with whipped gels were not observed because of air bubbles (Figure 5B). These artificial seeds contained many air bubbles, thus avoiding hypoxia; 72% of these seeds geminated within 2 weeks of seeding (Figure 5D, Table 2). Therefore, sponge-type artificial seeds showed much higher germination rates than bead-type artificial seeds.
Sponge-type artificial seeds, which are soft and porous, may be useful under hydroponic conditions because sufficient moisture and sterility can be maintained. Thus, sponge-type artificial seeds are promising candidates for the effective prevention of damping-off disease in the production of Japanese honewort in hydroponic culture systems.
Conclusion
In this study, we induced Japanese honewort and carrot somatic embryos via hyperosmotic stress. In Japanese honewort, somatic embryos formed continuously from shoot apex explants treated with hyperosmotic stress. Further analysis of continuous somatic embryogenesis may be a new approach for elucidating the mechanisms by which somatic embryogenesis competence is acquired. It is difficult for genetical and molecular biological analyses in Japanese honewort, in which genomic information has not been clarified. On the other hand, in the model plant Arabidopsis, higher-frequency of stress-induced somatic embryogenesis and continuation of somatic embryo formation such as in Japanese honewort and carrot have not been known. Therefore, it is desirable to establish continuous somatic embryogenesis in Arabidopsis system.
We also applied stress-induced somatic embryogenesis to develop sponge-type artificial Japanese honewort seeds. The quantity of the artificial seeds varied, since they were produced manually. For application in commercial hydroponic culture systems, automated operations are required to mass produce artificial seeds with consistent quality. The results of this study are expected to contribute to the development of artificial seed systems of various crops; such systems are expected to be more widely applied in future crop production.
Acknowledgments
This study was funded by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (nos. 18780149, 22580207, and 26450247) and the Science and Mathematics Program of Yokohama City University.
Abbreviations
- MS
Murashige and Skoog
- SUC
sucrose
References
- Abe K, Okada C, Iwade N, Shima S, Kusakari S, Achiwa N (2003) Effects of solution concentration control on yield, physiological and chemical properties, and quality characteristic of solution cultured mitsuba (Japanese honewort, Cryptotaenia japonica Hassl.). Food Preserv Sci 29: 281–289 [Google Scholar]
- Ahmad N, Anis M (2010) Direct plant regeneration from encapsulated nodal segments of Vitex negundo. Biol Plant 54: 748–752 [Google Scholar]
- Bapat AV, Rao SP (1990) In vivo growth of encapsulated axillary buds of mulberry (Morus indica L.). Plant Cell Tissue Organ Cult 20: 69–70 [Google Scholar]
- Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13: 624–630 [DOI] [PubMed] [Google Scholar]
- Cheng M, Lin L, Yu T, Peng YR (2008) Hypolipidemic and antioxidant activity of mountain celery (Cryptotaenia japonica Hassk.) sees essential oils. J Agric Food Chem 56: 3997–4003 [DOI] [PubMed] [Google Scholar]
- Choi YE, Yang DC, Choi KT (1998) Induction of somatic embryos by macrosalt stress from mature zygotic embryos of Panax ginseng. Plant Cell Tissue Organ Cult 52: 177–181 [Google Scholar]
- Fehér A (2015) Somatic embryogenesis: Stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849: 385–402 [DOI] [PubMed] [Google Scholar]
- Fujime Y (2012) Introduction to some indigenous vegetables in Japan. Hort Sci 47: 831–834 [Google Scholar]
- Hulst CA, Hens HJH, Buitelaar MR, Tramper J (1989) Determination of the effective diffusion coefficient of oxygen in gel materials in relation to gel concentration. Biotechnol Tech 3: 199–204 [Google Scholar]
- Ikeda-Iwai M, Umehara M, Satoh S, Kamada H (2003) Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J 34: 107–114 [DOI] [PubMed] [Google Scholar]
- Kamada H, Harada H (1979) Studies on the organogenesis in carrot tissue cultures: I. Effects of growth regulators on somatic embryogenesis and root formation. Z Pflanzenphysiol 91: 255–266 [Google Scholar]
- Kamada H, Ishikawa K, Saga H, Harada H (1993) Induction of somatic embryogenesis in carrot by osmotic stress. Plant Tiss Cult Lett 10: 38–44 [Google Scholar]
- Kamada H, Kobayashi K, Kiyosue T, Harada H (1989) Stress induced somatic embryogenesis in carrot and its application to synthetic seed production. In Vitro Cell Dev Biol 25: 1163–1166 [Google Scholar]
- Kamada H, Tachikawa Y, Saitou T, Harada H (1994) Heat stress induction of carrot somatic embryogenesis. Plant Tiss Cult Lett 11: 229–232 [Google Scholar]
- Kikuchi A, Sanuki N, Higashi K, Koshiba T, Kamada H (2006) Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223: 637–645 [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Kamada H, Harada H (1989) Induction of somatic embryogenesis by salt stress in carrot. Plant Tiss Cult Lett 6: 162–164 [Google Scholar]
- Kiyosue T, Takano K, Kamada H, Harada H (1990) Induction of somatic embryogenesis in carrot by heavy metals ions. Can J Bot 68: 2301–2303 [Google Scholar]
- Lamichhane RJ, Carolyne D, Schwanck AA, Robin M, Sarthou J, Cellier V, Messèan A, Aubertot J (2017) Integrated management of damping-off diseases. Agron Sustain Dev 37: 10 [Google Scholar]
- Leljak-Levanić D, Bauer N, Mihaljević S, Jelaska S (2004) Changes in DNA methylation during somatic embryogenesis in Cucurbita pepo L. Plant Cell Rep 23: 120–127 [DOI] [PubMed] [Google Scholar]
- Mandel J, Pattnaik S, Chand KP (2000) Alginate encapsulation of axillary buds of Ocimum americanum L. (Hoary basil), O. basilicum L. (Sweet basil), O. gratissimum L. (Shrubby basil) and O. Sanctum L. (Scared basil). In Vitro Cell Dev Biol Plant 36: 287–292 [Google Scholar]
- Neergaard P (1977) Seed Pathology. Macmillan Press, London
- Nishioka T, Takai Y, Kawaradani M, Okada K, Tanimoto H, Misawa T, Kusakari S (2014) Seed disinfection effect of atmospheric pressure plasma and low pressure plasma on Rhizoctonia solani. Biocontrol Sci 19: 99–102 [DOI] [PubMed] [Google Scholar]
- Okuno Y, Marumoto S, Miyazawa M (2017) Comparison of essential oils from three kinds of Cryptotaenia japonica Hassk (Kirimitsuba, Nemitsuba, and Itomitsuba) used in Japanese food. J Oleo Sci 66: 1273–1276 [DOI] [PubMed] [Google Scholar]
- Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. In: Hvoslef AK, Preil W (eds) Liquid Culture Systems for in vitro Plant Propagation. Springer, Dordrecht, pp 95–116
- Patnaik D, Mahalakshmi A, Khurana P (2005) Effect of water stress and heavy metals on induction of somatic embryogenesis in wheat leaf base cultures. Indian J Exp Biol 43: 740–745 [PubMed] [Google Scholar]
- Piccioni E (1997) Plantlets from encapsulated micropropagated buds of M.26 apple rootstock. Plant Cell Tissue Organ Cult 47: 255–260 [Google Scholar]
- Qu P, Yamashita K, Toda T, Priyatmojo A, Kubota M, Hyakumachi M (2008) Heterokaryon formation in Thanatephorus cucumeris (Rhizoctonia solani) AG-1 IC. Mycol Res 112: 1088–1100 [DOI] [PubMed] [Google Scholar]
- Rihan HZ, Kareem F, El-Mahrouk ME, Fuller MP (2017) Artificial seeds (principle, aspects and applications). Agronomy (Basel) 7: 71 [Google Scholar]
- Sharma S, Shazad A, Teixeria da Silva SJ (2013) Synseed technology: A complete synthesis. Biotechnol Adv 31: 186–207 [DOI] [PubMed] [Google Scholar]
- Spalik K, Downie SR (2007) Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): An appraisal using molecular data. J Biogeogr 34: 2039–2054 [Google Scholar]
- Steward FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells: II. Organization in cultures grown from freely suspended cells. Am J Bot 45: 705–708 [Google Scholar]
- Tokuji Y, Masuda H (1996) Duration of treatment of carrot hypocotyl explants with 2,4-dichlorophenoxyacetic acid for direct somatic embryogenesis. Biosci Biotechnol Biochem 60: 891–892 [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Kobayashi H, Togashi T, Mori Y, Kikuchi K, Kuriyama K, Tokuji Y (2005) Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J Plant Physiol 162: 47–54 [DOI] [PubMed] [Google Scholar]