Abstract
To date, regulatory pesticide risk assessments have relied on the honey bee (Apis mellifera L.) as a surrogate test species for estimating the risk of pesticide exposure to all bee species. However, honey bees and non-Apis bees may differ in their susceptibility and exposure to pesticides. In 2017, a workshop (“Pesticide Exposure Assessment Paradigm for Non-Apis Bees”) was held to assess if honey bee risk assessment frameworks are reflective of non-Apis bee pesticide exposure. In this paper, we summarize the workshop discussions on bumble bees (Bombus spp.). We review the life history and foraging behavior of bumble bees and honey bees and discuss how these traits may influence routes and levels of exposure for both taxa. Overall, the major pesticide exposure routes for bumble bees and honey bees are similar; however, bumble bees face additional exposure routes (direct exposure of foraging queens and exposure of larvae and adults to soil residues). Furthermore, bumble bees may receive comparatively higher pesticide doses via contact or oral exposure. We conclude that honey bee pesticide risk assessments may not always be protective of bumble bees, especially queens, in terms of exposure. Data needed to reliably quantify pesticide exposure for bumble bees (e.g., food consumption rates, soil residue levels) are lacking. Addressing these knowledge gaps will be crucial before bumble bee exposure can be incorporated into the pesticide risk assessment process. Because bumble bees exhibit appreciable interspecific variation in colony and behavioral characteristics, data relevant to pesticide exposure should be generated for multiple species.
Keywords: bumble bee, honey bee, pesticide exposure, risk assessment
Introduction
Bees provide an invaluable ecosystem service globally as the primary animal pollinators of many wild and agricultural plants. Historically, the honey bee (Apis mellifera L.) has been considered the most ecologically and economically significant bee pollinator (Allen-Wardell et al. 1998, Kevan 1999, Delaplane and Mayer 2000). However, non-Apis bees (i.e., all bees other than honey bees) are now recognized for their equally, or more so for some plants, important role as pollinators (Klein et al. 2007, Breeze et al. 2011, Garibaldi et al. 2013, Klatt et al. 2014).
Recently, honey bee and some non-Apis bee population declines have been documented globally (Biesmeijer et al. 2006, Potts et al. 2010, Cameron et al. 2011, Colla et al. 2012, Bartomeus et al. 2013, Burkle et al. 2013). These declines have been attributed to many factors, including exposure to pesticides (Potts et al. 2010, Vanbergen et al. 2013, Goulson et al. 2015). Depending on the mode of action, dose, and exposure route, pesticides can have lethal or sub-lethal effects on bees (Johansen et al. 1983, Thompson 2003, Brittain and Potts 2011, Godfray et al. 2015), which ultimately can impair their pollination services (Stanley et al. 2015). Because of these potential effects, a risk assessment for bees is required by many regulatory agencies for the registration and re-registration of pesticides, including those in North America and the European Union. These assessments have traditionally focused primarily on the honey bee, with the assumption that estimates of risk for honey bees were protective of all bees or that honey bee data could be used to estimate or model effects in other bees. However, honey bees and non-Apis bees differ significantly in terms of life history, morphology, and behavior, and these differences can translate to differences in pesticide susceptibility and exposure (Scott-Dupree et al. 2009, Brittain and Potts 2011, Cresswell et al. 2012, Arena and Sgolastra 2014, Heard et al. 2016, Thompson 2016). Recognition of these potential differences, along with concern for the accelerated loss of some non-Apis bees, has led to a global re-evaluation of regulatory risk assessment processes for pesticides to determine if the honey bee truly serves as a suitable surrogate for estimating the risk of pesticide exposure to all bees (EFSA 2012, 2013; USEPA 2012, USEPA et al. 2014, Stoner 2016).
In January 2017, a US Environmental Protection Agency-hosted workshop titled “Pesticide Exposure Paradigm for Non-Apis Bees” was held in Washington, DC. The workshop gathered 40 international participants together to determine if the current honey bee risk assessment framework adequately accounts for potential routes and levels of pesticide exposure experienced by solitary (Megachile rotundata Fabricius and Osmia spp. [Hymenoptera: Megachilidae], and Nomia melanderi Cockerell [Hymenoptera: Halictidae]), stingless (Tribe: Meliponini), and bumble bees (Bombus spp.). In this paper, we summarize the workshop outcomes for bumble bees, the proposed surrogate taxon for estimating the risk of pesticides to social non-Apis bees. Bumble bees are key wild pollinators of many plants, especially in northern temperate regions. Additionally, Bombus impatiens (Cresson), B. terrestris (L.), and B. ignitus (Smith) are available commercially for managed pollination of agricultural crops in North America, Europe, and Asia, respectively. A growing body of research suggests that bumble bees and honey bees can differ in pesticide susceptibility and exposure (Scott-Dupree et al. 2009, Cresswell et al. 2012, Stoner 2016), and in response, an international effort to develop a pesticide risk assessment framework for bumble bees is underway. Standardized protocols for laboratory acute LD50 tests for bumble bees were recently published by the Organisation for Economic Co-operation and Development (OECD 2017a, b). Recommendations for semi-field and field study test designs and assessment endpoints for bumble bees also have been published (e.g., Cabrera et al. 2016, Gradish et al. 2016), and the International Commission for Plant-Pollinator Relationships (ICPPR) non-Apis working group currently is ring-testing semi-field study protocols, with the aim of producing an OECD guidance document. Despite these efforts, uncertainties remain about potential routes and levels of pesticide exposure to bumble bees and how they compare to those experienced by honey bees.
Here we compare and contrast bumble bee and honey bee life history and behavioral traits and review how those traits may result in similar or different routes and levels of exposure for both taxa. Based on this review, we then discuss considerations for incorporating bumble bee pesticide exposure into the risk assessment process, highlighting critical knowledge gaps that will need to be addressed. Ultimately, this information will provide further insight into whether the honey bee is an adequate surrogate for estimating bumble bee pesticide exposure and aid in the development of risk assessment frameworks for bumble bees.
Bumble Bee and Honey Bee Life History and Behavior
There are 250 species of bumble bees (including ca. 45 cuckoo bumble bee species) found globally (Williams et al. 1994, 1998, 2008; Pedersen 1996), and among these species, there exists substantial variation in reproductive, developmental, behavioral, and ecological traits. Thus, we have provided only a basic overview of the colony cycle and foraging behavior of bumble bees, with a focus on general traits that are most relevant to understanding bumble bee pesticide exposure and how it compares to honey bees.
Colony Structure and Developmental Cycle
Bumble bee colonies consist of a queen, female workers, and males. There is significant intra- and interspecific variation in the number of individuals per colony, but the largest colonies typically contain no more than 350–450 workers (Lopez-Vaamonde et al. 2003, Goulson 2010). Adult body size also varies considerably within and between species and castes, with queen, worker, and male weight ranging from 298–1160 (Beekman et al. 1998, Owen 1988, Lopez-Vaamonde et al. 2009), 50–400 (Owen 1989, Goulson et al. 2002), and 90–317 mg (Owen 1989, Lopez-Vaamonde et al. 2009), respectively.
Bumble bees have an annual colony cycle, with queens single-handedly founding nests. The colony cycle begins in late winter or early spring when mated queens emerge from hibernation to search for suitable nest sites, which include abandoned rodent burrows, hollow logs, rock piles, or dense ground vegetation (Heinrich 2004, Goulson 2010). Once she locates a site, the queen creates a mass of pollen mixed with nectar. She then builds a small wax cup on the pollen mass in which she lays a first batch of eggs. She covers the cup in wax and the larvae develop within these closed wax cells. Bumble bee species can be grouped according to how their larvae are fed. In ”pocket-making” species, pollen is placed underneath the larvae, on which they collectively feed (Heinrich 2004, Goulson 2010). Later in their development, larvae are fed regurgitated nectar and pollen by the queen via a hole in their wax cell. Conversely, the larvae of ”pollen-storing” species are fed directly by the queen or workers throughout their development (Heinrich 2004, Goulson 2010). Initially, the larvae develop and are fed together as a group, but they separate into individual cells for the latter half of their development (Heinrich 2004). The queen incubates the brood and forages regularly to provide pollen and nectar to the developing brood. After 10–14 days, each larva spins a silk cocoon and pupates for ca. 14 days (Alford 1975). The adults that emerge following pupation are almost invariably female workers, and shortly after their emergence, the queen ceases to leave the colony. Foraging duty is assumed by some of the new workers, while others help the queen tend to the developing brood. The queen continues laying eggs destined to become workers, and colony growth rapidly accelerates (Heinrich 2004, Lopez-Vaamonde et al. 2009).
Later in the season, the colony switches from producing workers to producing males and new queens. Soon after emerging, males leave the colony permanently to feed and mate. During the day, new queens leave to forage and usually return to the colony at night. At this stage, they consume large quantities of pollen and nectar to build up substantial fat reserves for hibernation. Eventually, each new queen mates with a male and subsequently finds a hibernation site in the soil. The growth of the colony then decreases, and the foundress queen, workers, and males die before winter (Alford 1975, Heinrich 2004). The duration of the colony cycle varies with bumble bee species and is related to the length of the season. In temperate regions, colonies may live for 12–25 weeks, depending on the species (Goodwin 1995, Heinrich 2004).
Honey bee colonies also consist of a single queen but typically contain up to tens of thousands of workers and a few thousand drones (males) (Danka et al. 1986, Michener 2007, Wharton et al. 2007). Adult honey bee queens, workers, and drones weigh ca. 200, 100, and 200 mg, respectively. The majority of honey bee colonies are managed and reside in human-made boxes and frames. In contrast to bumble bees, honey bee colonies last multiple years, with the entire colony entering a period of dormancy every winter and surviving on honey and pollen stores. Workers are responsible for all non-reproductive tasks (e.g., foraging, brood care, wax and comb production, colony defense), while drones exist solely to mate with queens. Queens lay eggs, and, in managed colonies, they never leave the colony except to swarm (in feral/wild colonies, queens leave the colony to mate, and may leave a second time if the colony swarms). Queens are fed royal jelly—processed hypopharyngeal and mandibular gland secretions produced by nurse bees—for their entire juvenile and adult lives (Winston 1987). Honey bee larvae develop and are fed individually in wax comb cells. Worker and drone larvae are fed brood food and royal jelly for the first 3 days of their development and are provisioned pollen and nectar for the remainder of their development (Winston 1987).
Foraging Behavior
Bumble bees and honey bees are generalist foragers and visit a wide variety of plant types for nectar and pollen. In both taxa, workers are responsible for foraging and providing food to the colony; however, unlike honey bee queens, bumble bee queens also forage in the spring during colony establishment and in late summer and fall after emerging from pupation. Although the foraging range of bumble bees is variable and species-dependent, workers typically forage within 1.5 km of their colony (Knight et al. 2005, Osborne et al. 2008). Honey bees usually forage within 3 km of their colony but may travel up to 15 km if resources are scarce (Winston 1987, Beekman and Ratnieks 2000, Greenleaf et al. 2007).
Bumble bees are large-bodied compared to other bees and most are covered with dense hair. Furthermore, bumble bees are capable of raising their body temperature by rapidly contracting their flight muscles. These traits enable bumble bees to fly and forage under cool temperatures (Heinrich 1993, Heinrich 2004). They also are able to forage under cloudy or low light conditions. Therefore, bumble bees are typically active earlier and later in the day and season than honey bees (Heinrich 2004).
Potential Routes of Pesticide Exposure for Bumble Bees vs. Honey Bees
Figure 1 depicts a conceptual model of the primary pesticide exposure routes for bumble bees (for an analogous exposure model for honey bees, see USEPA 2012). In Table 1, we list the potential routes of pesticide exposure for bumble bees and honey bees and rank the relative importance of each exposure route for each taxon based on their respective colony cycles and foraging behaviors. We do not explicitly consider adult male bumble bees or drone honey bees in our exposure assessments, as pesticide exposure for adult males is expected to be similar to that experienced by foraging workers.
Figure 1.
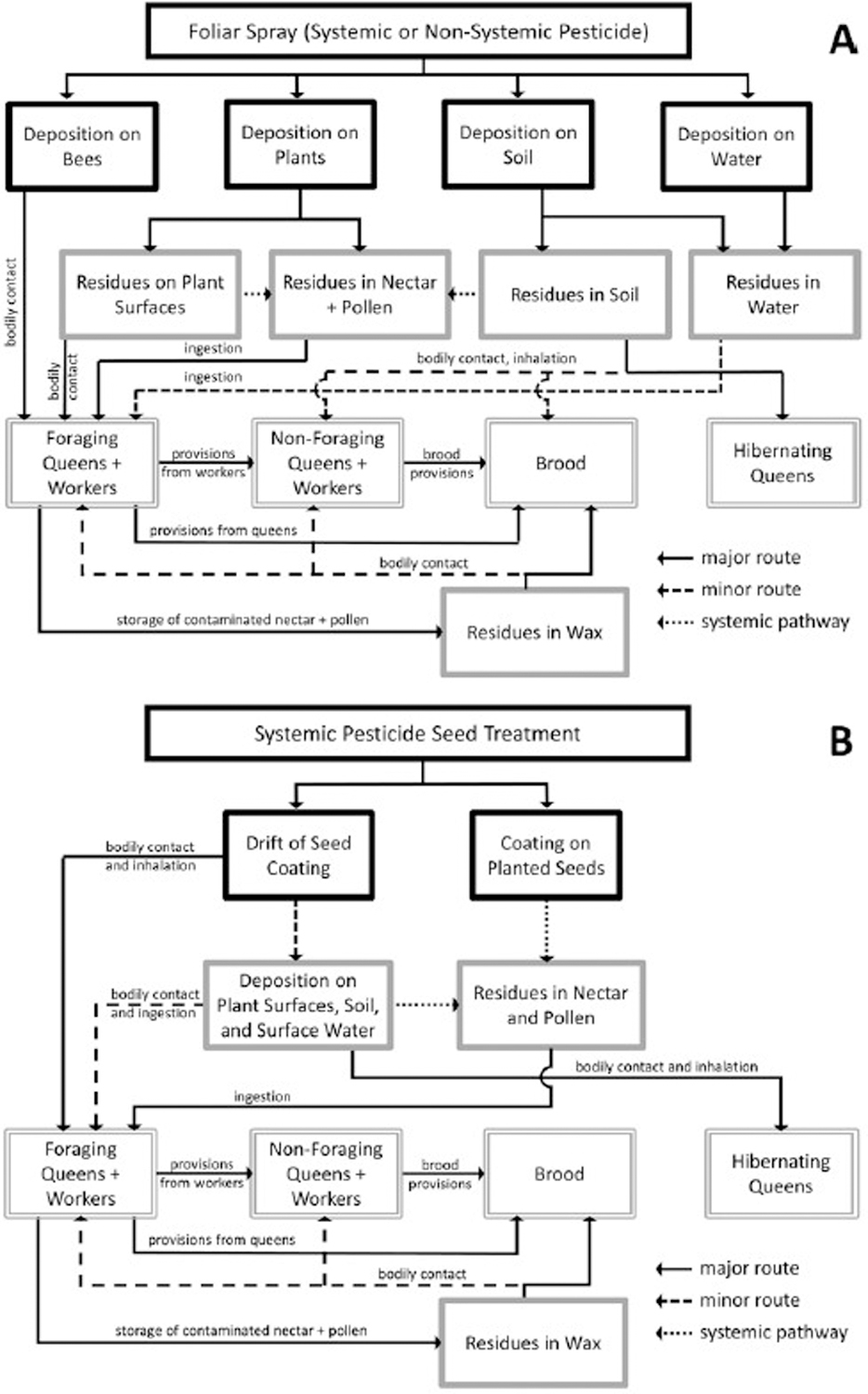
Conceptual model of pesticide exposure to bumble bees (Bombus spp.) from foliar-applied systemic or non-systemic pesticides (A) and systemic pesticide seed treatments (B). Black boxes = stressors and residue sources, solid gray boxes = exposure matrices, and double-lined gray boxes = receptors.
Table 1.
Potential routes of pesticide exposure and the expected probability of exposure via those routes (0 = no probability, 1 = low probability, 2 = moderate probability, 3 = high probability, 4 = very high probability) for different life stages and castes of bumble bees (Bombus spp.) and honey bees (Apis mellifera L.).
| Probability of Pesticide Exposure* |
||||
|---|---|---|---|---|
| Exposure Route | Substrate | Life Stage/Caste | Bumble Bees | Honey Bees |
| Bodily Contact | air particles (dust or spray) | foraging worker | 4 | 4 |
| non-foraging worker | 0 | 0 | ||
| queen | 4 | 1 | ||
| larva | 0 | 0 | ||
| plant surface residues | foraging worker | 4 | 4 | |
| non-foraging worker | 0 | 0 | ||
| queen | 4 | 0 | ||
| larva | 0 | 0 | ||
| wax residues | foraging worker | 1 | 1 | |
| non-foraging worker | 1 | 3 | ||
| queen | 1 | 3 | ||
| larva | 3 | 4 | ||
| soil residues | foraging worker | 1 | 0 | |
| non-foraging worker | 1 | 0 | ||
| queen | 2 | 0 | ||
| larva | 1 | 0 | ||
| Oral | nectar | foraging worker | 4 | 4 |
| non-foraging worker | 3 | 3 | ||
| queen | 4 | 1 | ||
| larva | 4 | 4 | ||
| pollen | foraging worker | 4 | 1 | |
| non-foraging worker | 3 | 3 | ||
| queen | 4 | 1 | ||
| larva | 4 | 4 | ||
| water | foraging worker | 2 | 4 | |
| non-foraging worker | 0 | 1 | ||
| queen | 1 | 1 | ||
| larva | 0 | 0 | ||
| guttation fluid | foraging worker | 0 | 1 | |
| non-foraging worker | 0 | 1 | ||
| queen | 0 | 1 | ||
| larva | 0 | 0 | ||
| honey dew | forager | 1 | 4 | |
| non-forager adult | 0 | 2 | ||
| queen | 1 | 2 | ||
| larva | 0 | 0 | ||
Rankings were assigned by bee experts based on the developmental, reproductive, and behavioral traits of each taxon.
Contact and Inhalation Exposure
Bumble bees and honey bees may be exposed to pesticides via bodily contact with dust generated during the planting of treated seeds, foliar sprays, or residues on various substrates (Table 1, Figure 1). For both taxa, direct contact with sprays, dust, or plant surface residues is an important exposure route for workers that leave the colony to forage but not for larvae or adult bees (e.g., nurse bees) that remain inside the colony (however, the probability of direct exposure to sprays or drift is higher for non-forager adults from bumble bee colonies found under vegetation on the ground) (Table 1, Figure 1). Larvae and adults of both taxa also may come into contact with pesticide residues that have accumulated in wax colony structures via the storage of contaminated nectar and pollen (Table 1, Figure 1).
Compared to honey bee queens, the probability of bodily contact with pesticides is higher for bumble bee queens (Table 1). While honey bee queens remain protected within the colony, bumble bee queens may be directly exposed to sprays and residues on plants while foraging in late summer and fall in preparation for hibernation and in spring while establishing colonies. Unlike honey bees, which do not rely on soil for nesting or as structural material, bumble bee larvae and adults from underground colonies may come into contact with residues in the surrounding soil, although the likelihood of such exposure is considered low overall (Table 1, Figure 1). However, bumble bee queens may also hibernate in the soil, which comparatively increases their probabilty of exposure to soil residues (Table 1).
Bumble bees and honey bees also may be exposed via the inhalation of pesticide spray or dust particles or fumes generated from the volatilization of residues from plant surfaces, wax, or soil. However, inhalation as an exposure route for bees has not been well-described or investigated.
Oral Exposure
Another major route of exposure for both taxa is the consumption of food or water resources that contain pesticide residues (Table 1). Pesticides may enter the nectar or pollen of plants via foliar spray or dust deposition on open flowers or the translocation of foliar-, soil-, or seed-applied systemic compounds (Figure 1). Bumble bee and honey bee foragers may be exposed directly while consuming contaminated pollen and nectar from flowers, while larvae and non-forager adults may be exposed when these contaminated resources are brought back to the colony by foraging workers (Table 1, Figure 1).
Finally, honey bee foragers collect water and guttation fluid to drink and bring back to the colony, and therefore foragers and non-forager adults may be exposed to pesticides if these fluids contain residues. As bumble bees are not known to collect water or guttation fluid, these exposure routes are of no or comparatively minor importance to bumble bees of all life stages and castes (Table 1).
Potential Levels of Pesticide Exposure for Bumble Bees vs. Honey Bees
Although the potential routes of pesticide exposure are similar for bumble bees and honey bees, the level of exposure (i.e., dose received) via any one of those routes may vary due to several morphological and behavioral differences.
Bumble bee adults are generally larger than honey bees. Consequently, bumble bees exhibit a lower surface area to volume ratio and, thus, at a given contact exposure, will receive a smaller dose of pesticide per unit body mass. Bumble bees also are covered in dense hair, which impedes pesticides from making contact with their cuticle. These two characteristics mean that exposure, and hence dose, via direct contact may be lower for individual adult bumble bees compared to honey bees.
For oral exposure routes, the dose of pesticide received by an individual bee will depend on how much food it consumes and the concentration of pesticide the food contains. Based on the limited data available for bumble bees, rates of daily nectar and pollen consumption by individual adult bumble bee and honey bee workers are similar (Table 2). Although individual bumble bee queens consume more total nectar and pollen than honey bee or bumble bee workers (Table 2), the amount of food consumed per mg of body mass is similar for all three groups (Table 3). Therefore, levels of oral pesticide exposure would be similar for adult bumble bees and honey bees based on rates of food consumption. However, adult honey bee queens consume only royal jelly, which, due to processing by nurse bees, contains lower pesticide residues than unprocessed nectar or pollen (USEPA et al. 2012, Lucchetti et al. 2018). Bumble bee queens, in contrast, consume only unprocessed nectar and pollen, and thus may receive higher oral pesticide doses than honey bee queens (but not workers) on a per weight basis.
Table 2.
Total amount (mg/bee) of nectar and pollen consumed per day for different life stages and castes of bumble bees (Bombus spp.) and honey bees (Apis mellifera L.).
| Amount Consumed Per Day (mg/bee)* |
|||
|---|---|---|---|
| Food | Life Stage/Caste | Bumble Bees | Honey Bees |
| sucrose/nectar† | adult worker | 73–4001–5 | 32–4995,6 |
| adult queen | 163–13107,8 | 09 | |
| larva (non-queen) | 24–605,10,11 | 12–1175,6,9 | |
| larva (queen) | unknown | 09 | |
| pollen | adult worker | 20–302,5,6,12,13 | 0–125,6 |
| adult queen | 37–608,14 | 09 | |
| larva (non-queen) | 10–405,11,13 | 0.3–2.76,15–17 | |
| larva (queen) | 75–1358 | 09 | |
Depending on the source, values may reflect direct measurements or calculated estimates. For comparison purposes, only data that were reported as or could be converted to mg/bee/day were included.
Depending on the source, values may reflect direct measurements of nectar or sucrose solution consumption, or estimates of nectar consumption.
1Tasei et al. 1994, 2Tasei et al. 2000, 3Cresswell et al. 2012, 4Laycock et al. 2012, 5USEPA et al. 2012, 6EFSA 2013, 7Heinrich 1972, 8Pomeroy 1979, 9USEPA et al. 2014, 10Řehoř et al. 2014, 11Pereboom 2000, 12Smeets and Duchateau 2001, 13Tasei and Aupinel 2008, 15Přidal and Hofbauer 1996, 16Simpson 1955, 17Babendreier et al. 2004
Table 3.
Total amount (mg/mg body mass) of nectar and pollen consumed per day for different life stages and castes of bumble bees (Bombus spp.) and honey bees (Apis mellifera L.).
| Amount Consumed Per Day (mg/mg body mass)* |
|||
|---|---|---|---|
| Taxon | Caste | Sucrose/Nectar | Pollen |
| honey bee | worker | 0.32–5.0 | 0–0.12 |
| bumble bee | worker | 0.40–2.2 | 0.11–0.16 |
| bumble bee | queen | 0.30–2.4 | 0.067–0.11 |
Values were calculated using consumption values from Table 2 and the median body mass for bumble bees (queens: 550 mg, workers: 184 mg) and mean body mass for honey bees (workers: 100 mg).
While larvae of both taxa consume a similar amount of nectar per day, bumble bee larvae may consume upwards of 130x more pollen per day than honey bee larvae (Table 2). Furthermore, for most of their development, honey bee larvae are fed brood food and royal jelly, which contain lower pesticide residues compared to unprocessed nectar or pollen (USEPA 2012, Lucchetti et al. 2018). Conversely, bumble bee larvae feed exclusively on unprocessed nectar and pollen for their entire development. Finally, the duration of development, and hence feeding period, for bumble bee larvae is approximately twice as long as for honey bee larvae. These three factors (i.e., increased consumption of pollen by larvae, consumption of unprocessed nectar and pollen by larvae, and longer larval development time) may substantially increase oral exposure of bumble bee larvae to pesticides compared to honey bees.
To the best of our knowledge, measurements of the quantity of nectar consumed by bumble bee queen larvae are currently not available. However, in one laboratory study, B. ruderatus queen larvae consumed 75–135 mg pollen/day (Pomeroy 1979), which is considerably higher than pollen consumption measures for non-queen bumble bee and honey bee larvae (Table 2). Furthermore, in bumble bees, queen larvae are fed for a longer duration and consume larger quantities of food than worker larvae (Duchateau and Velthuis 1988, Goulson 2010). Therefore, larval bumble bee queens may experience higher oral pesticide exposure compared to both honey bee and bumble bee non-queen larvae.
Finally, differences in bumble bee and honey bee foraging behavior may influence the contact and oral exposure of foraging adults. For instance, although bumble bee and honey bee foragers make a similar number of daily foraging trips (honey bees: 10 [Winston 1987]; bumble bees: 1–32 [Spaethe and Weidenmüller 2002, Gill and Raine 2014, Stanley et al. 2016, Evans et al. 2017]), bumble bee foragers visit 2–3x more flowers per trip. Bumble bees are also typically active in cooler temperatures and poorer weather conditions (e.g., overcast, precipitation), which can result in increased pesticide exposure compared to honey bees in three additional ways. First, bumble bees are active earlier and later in the day than honey bees, and bumble bee foragers thus may be exposed to early morning or late evening spray applications that are timed to avoid foraging honey bees. Second, because bumble bees will forage during inclement weather or overcast conditions, they will be active, and hence may be exposed to pesticides, for more hours or days within a season. Third, bumble bees are active earlier and later in the season, and therefore, bumble bee species with long colony cycles within a treated area may be exposed for a longer duration within a season (Wisk et al. 2014). Finally, the foraging range of bumble bees generally is smaller than honey bees, and therefore, for colonies in agricultural landscapes, bumble bees may encounter pesticide residues on plants over a larger proportion of their foraging range (USEPA et al. 2012, Raine and Gill 2015). All of these factors may comparatively increase the contact and oral exposure of foraging workers and the amount of contaminated nectar or pollen brought back to the colony.
Implications and Considerations for Pesticide Risk Assessment
Our comparison of bumble bee and honey bee life history and behavior suggests that each taxon may experience differential pesticide exposure. Although the major routes of pesticide exposure are similar for honey bees and bumble bees, bumble bees face an increased likelihood of exposure via the direct contact and oral exposure of foraging queens and a differential route of exposure via the contact or inhalation exposure of adults (especially queens and non-foraging workers) and larvae via soil residues (Figure 1). These exposure scenarios are not considered in current honey bee risk assessments. Bumble bees may also experience higher pesticide exposure than honey bees under some circumstances. Available data suggest that adult bumble bee and honey bee nectar and pollen consumption rates are similar, and current protocols for Tier I, acute honey bee oral exposure estimates are conservative and assume bees consume only unprocessed nectar and pollen (USEPA et al. 2012). Based on these factors, honey bee acute oral exposure estimates are considered protective of adult bumble bees (USEPA et al. 2012). However, limited data on bumble bee food consumption are available, and some of those data suggest that bumble bee larvae consume more pollen than honey bee larvae. Furthermore, in some cases on a per individual basis, bumble bee foragers may experience higher pesticide doses via contact or oral exposure compared to honey bees. Therefore, oral pesticide doses received by larval and adult bumble bees, especially queens, may be underestimated by honey bee risk assessment estimates. Finally, pesticide residue levels in matrices relevant to bumble bee exposure, such as soil around nest or hibernation sites, have not been well-quantified, making it difficult to compare exposure levels with those of honey bees.
Because of the apparent differences in pesticide exposure for bumble bees and honey bees, current risk assessment frameworks may need to be expanded to include bumble bees. It is particularly important to incorporate exposure to bumble bee queens as their loss to pesticide exposure during their solitary life phases (hibernation, colony establishment, and post-emergence foraging) translates into the loss of whole colonies (Baron et al. 2017a, b, Fauser et al. 2017, Wu-Smart and Spivak 2017, Leza et al. 2018). The incorporation of bumble bees into a risk assessment process necessitates exposure estimates for all life stages and castes of bumble bees, which in turn requires quantifiable data on bumble bee traits relevant to pesticide exposure. Currently, such data are generally lacking for bumble bees and available data are heavily biased towards B. terrestris. Therefore, generating more baseline data related to pesticide exposure for bumble bees should be a research priority. As bumble bee species differ in traits that may determine pesticide exposure, this research should be conducted on multiple species, and in particular should focus on B. impatiens and B. terrestris, the proposed surrogate species for bumble bee risk assessments in North America and Europe, respectively.
Estimates of oral pesticide exposure require knowledge of food consumption rates. Bumble bee nectar and pollen consumption data are scarce and difficult to summarize due to differences in the methods used to quantify consumption rates and units of data reported. This is especially true for bumble bee queens: To date, only five studies have investigated adult queen food consumption, and each focused on a different species and/or time point in the colony cycle (Cumber 1949, Alford 1969, Heinrich 1972, Pomeroy 1979, Přidal and Hofbauer 1996). Furthermore, most consumption data for bumble bee adults have been collected under laboratory conditions from non-foraging workers or queens (e.g., Tasei et al. 1994, Tasei et al. 2000, Cresswell et al. 2012, Laycock et al. 2012, Rotheray et al. 2017), and these measures may underestimate consumption by actively foraging adults with comparatively higher energy demands. To generate reliable oral exposure estimates for bumble bees, more data on pollen and nectar consumption by larvae and adults under natural foraging conditions are needed.
Food consumption by bees can be measured directly by providing individuals with radiolabeled glucose and tracking its movement (Nixon and Ribbands 1952, Řehoř et al. 2014). Alternatively, consumption can be estimated based on factors related to foraging energetics. For instance, estimates for the maximum nectar consumption rate by foraging honey bee workers can be obtained using the following equation (Rortais et al. 2005):
T = the average number of foraging trips made per day
SF = the quantity of sugar required for flight
D = the duration of each foraging trip
F = the fraction of the foraging trip spent flying
P = the percentage of sugar in nectar (accepted average = 30%)
For bumble bees, the number and duration of daily foraging trips has been studied exclusively with B. terrestris (Spaethe and Weidenmüller 2002, Peat and Goulson 2005, Gill and Raine 2014, Stanley et al. 2016, Evans et al. 2017), and to our knowledge, the remaining variables have not been measured for any bumble bee species. These data will be needed before estimates of nectar consumption can be determined for bumble bee foragers.
Measurements of food consumption specific to adult bumble bee queens also are needed. Data on food consumption by bumble bee workers should not necessarily be scaled up to estimate consumption by adult queens, because the energy demands, and hence nectar and pollen consumption, of bumble bee queens change during three distinct periods of their adult lives and may be overall higher than that of workers. Following emergence from pupation, queens forage and consume large quantities of pollen and nectar to build up fat reserves for hibernation. In the spring, the resource requirements of queens again are high, as they expend significant energy alternating between daily foraging trips and brood incubation, while also developing new eggs. Once foraging is assumed by workers, queens remain in the colony incubating brood and consuming food brought back to the colony by foragers. Therefore, to estimate lifetime pesticide exposure for bumble bee queens, pollen and nectar consumption data will be required for each of these stages.
Pesticide residue data also are necessary for estimating exposure via all routes, and such data are generally lacking for matrices relevant to bumble bee exposure. Of particular importance will be generating pesticide residue data for soil, from which estimates of exposure to bumble bee queens during hibernation (and, to a lesser extent, brood and adults in underground nests) can be derived. Generating such estimates will be challenging as the concentration of pesticides in soil, and how they dissipate and partition over time, is influenced by many factors, including pesticide chemistry and application method, soil type and moisture level, agricultural practices (e.g., tilling, irrigation), and environmental conditions (e.g., precipitation). Nevertheless, the potential hazard to bumble bee queens makes the development of soil exposure estimates a high priority. Soil residue data are often collected from within agricultural fields; however, bumble bees are unlikely to nest or hibernate directly within fields. Therefore, soils adjacent to or near agricultural fields should be sampled, and the resulting residue data can be used to develop models to predict soil contamination levels in areas where bumble bees are likely to nest or hibernate.
Contact with residues in wax also is an important potential exposure route for developing larvae and adult queens and non-foraging workers that make contact with wax while incubating the brood. Although pesticide residues in honey bee wax have been quantified (e.g., Chauzat and Faucon 2007, Mullin et al. 2010), it is unclear if comparable concentrations are found in bumble bee colony wax. There also have been few attempts to quantify the concentrations of pesticides found in stored nectar and pollen in bumble bee colonies (but see Thompson et al. 2013, David et al. 2016, Botías et al. 2017, Nicholls et al. 2018). Such data will be important for estimating oral exposure, particularly for larvae and non-foraging adults.
Finally, several aspects of bumble bee foraging behavior (e.g., daily and seasonal activity, foraging range) theoretically may influence the exposure of foraging adults and whole colonies. However, the impact of these behaviors on bumble bee pesticide exposure has not been quantified. This will need to be considered when developing bumble bee-specific exposure estimates, particularly for contact exposure for foraging workers and queens.
Conclusions
Because of their pronounced biological and ecological differences, there has been increasing concern that the routes of exposure currently used for honey bee risk assessment are not conservative or broad enough to represent the major exposure routes for bumble bees. Our comparison of the life histories and behaviors of bumble bees and honey bees indicates that certain life stages/castes of bumble bees may experience differential or higher pesticide exposure compared to honey bees, and therefore, in terms of exposure, honey bees may not be an adequate risk assessment surrogate for all life stages and castes of bumble bees. Of particular concern is that current honey bee exposure scenarios cannot account for exposure to bumble bee queens. Our findings reiterate the need for the inclusion of bumble bees in the risk assessment process, which will require quantifiable data from which to estimate bumble bee pesticide exposure and susceptibility. Although the major pesticide exposure routes for bumble bees are recognized, a lack of data makes it difficult to reliably quantify or estimate the level of exposure they may experience via those routes. Therefore, research efforts directed towards developing bumble bee risk assessment frameworks should prioritize quantifying individual- and colony-level traits relevant to bumble bee pesticide exposure.
Acknowledgements
We thank all of the participants of the “Pesticide Exposure Assessment Paradigm for non-Apis Bees” workshop for their insight and expertise, and Graham Ansell and Dr. Andrew Frewin for technical and editorial assistance during preparation of the manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer: This article has been reviewed by the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency or of the US Federal Government, nor does the mention of trade names or commercial products constitute endorsement or recommendations for use of those products. The authors report no financial or other conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alford DV 1969. A study of the hibernation of bumblebees (Hymenoptera: Bombidae) in southern England. J. Anim. Ecol. 38: 149–170. [Google Scholar]
- Alford DV 1975. Bumblebees. Davis-Poynter, London, UK. [Google Scholar]
- Allen-Wardell G, Bernhardt P, Bitner R, Burquez A, Buchmann S, Cane J, Cox PA, Dalton V, Feinsinger P, Ingram M, Inouye D, Jones CE, Kennedy K, Kevan P, Koopowitz H, Medellin R, Medellin-Morales S, Nabhan GP, Pavlik B, Tepedino V, Torchio P, and Walker S 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv. Biol 12: 8–17. [Google Scholar]
- Arena M, and Sgolastra F 2014. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23: 324–334. [DOI] [PubMed] [Google Scholar]
- Babendreier D, Kalberer N, Romeis J, Fluri P, and Bigler F 2004. Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie 35: 293–300. [Google Scholar]
- Baron GL, Jansen VAA, Brown MJF, and Raine NE 2017a. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nature Ecol. Evol 1: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron GL, Raine NE, and Brown MJF 2017b. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. Roy. Soc. B. Biol. Sci 284: 20170123. 10.1098/rspb.2017.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, and Winfree R 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. U. S. A 110: 4656–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, and Ratnieks FLW 2000. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol 14: 490–496. [Google Scholar]
- Beekman M, van Stratum P, and Lingeman R 1998. Diapause survival and post-diapause performance in bumblebee queens (Bombus terrestris). Entomol. Exp. App 89: 207–214. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, and Kunin WE 2006. Parallel declines in pollinators in insect-pollinated plants in Britain and the Netherlands. Science 313: 351–354. [DOI] [PubMed] [Google Scholar]
- Botías C, David A, Hill EM, and Goulson D 2017. Quantifying exposure of wild bumblebees to mixtures of agrochemicals in agricultural and urban landscapes. Environ. Pollut 222: 73–82. [DOI] [PubMed] [Google Scholar]
- Breeze TD, Bailey AP, Balcombe KG, and Potts SG 2011. Pollination service in the UK: How important are honeybees? Agr. Ecosyst. Environ 142: 137–143. [Google Scholar]
- Brittain C, and Potts SG 2011. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol 12: 321–331. [Google Scholar]
- Burkle LA, Marlin JC, and Knight TM 2013. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Cabrera AR, Almanza MT, Cutler GC, Fischer DL, Hinarejos S, Lewis G, Negra P, Olmstead J, Overmyer J, Potter DA, Raine NE, Stanley-Stahr C, Thompson H, and van der Steen JJM 2016. Initial recommendations for higher-tier risk assessment protocols for bumble bees, Bombus spp. (Hymenoptera: Apidae). Integr. Environ. Assess. Manag 12: 222–229. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, and Griswold TL 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U. S. A 108: 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauzat M-P, and Faucon J-P 2007. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest. Manag. Sci 63: 1100–1106. [DOI] [PubMed] [Google Scholar]
- Colla SR, Gadallah F, Richardson L, Wagner D, and Gall L 2012. Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodivers. Conserv 21: 3585–3595. [Google Scholar]
- Cresswell JE, Page CJ, Uygun MB, Holmbergh M, Li Y, Wheeler JG, Laycock I, Pook CJ, Hempel de Ibarra N, Smirnoff N, and Tyler CR 2012. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365–371. [DOI] [PubMed] [Google Scholar]
- Cumber RA 1949. The biology of humble bees, with special reference to the production of the worker caste. Trans. R. Entomol. Soc. Lond 100: 1–45. [Google Scholar]
- Danka RG, Rinderer TE, Hellmich RL, and Collins AM 1986. Foraging population sizes of Africanized and European honey bees (A. mellifera L.) colonies. Apidologie 17: 193–202. [Google Scholar]
- David A, Botías C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM, and Goulson D 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Internat 88: 169–178. [DOI] [PubMed] [Google Scholar]
- Delaplane KS, and Mayer DF 2000. Crop pollination by bees. CABI Publishing, Wallingford, UK. [Google Scholar]
- Duchateau MJ, and Velthuis H 1988. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 107: 186–207. [Google Scholar]
- (EFSA) European Food Safety Authority. 2012. Scientific opinion on the science behind the development of a risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 10: 2668. 10.2903/j.efsa.2012.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (EFSA) European Food Safety Authority. 2013. EFSA guidance document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11: 3295. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LJ, Smith KE, and Raine NE 2017. Fast learning in free-foraging bumble bees is negatively correlated with lifetime resource collection. Sci. Rep 7: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A, Sandrock C, Neumann P, and Sadd BM 2017. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol. Entomol 42: 306–314. [Google Scholar]
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O, Bartomeus I, Benjamin F, Boreux V, Cariveau D, Chacoff NP, Dudenhoffer JH, Freitas BM, Ghazoul J, Greenleaf S, Hipolito J, Holzschuh A, Howlett B, Isaacs R, Javorek SK, Kennedy CM, Krewenka K, Krishman S, Mandelik Y, Mayfield MM, Motzke I, Munyuli T, Nault BA, Otieno M, Peterson J, Pisanty J, Potts SG, Rader R, Ricketts TH, Rundlöf M, Seymour CL, Schuepp C, Szentgyörgyi H, Taki H, Tscharntke T, Vergara CH, Viana BF, Wanger TC, Westphal C, Williams NM, and Klein AM 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339: 1608–1611. [DOI] [PubMed] [Google Scholar]
- Gill RJ, and Raine NE 2014. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol 28: 1459–1471. [Google Scholar]
- Goodwin SG 1995. Seasonal phenology and abundance of early-, mid- and long-season bumble bees in southern England, 1985–1989. J. Apicult. Res 34: 79–87. [Google Scholar]
- Godfray HCJ, Blacquiere T, Field LM, Hails RS, Potts SG, Raine NE, Vanbergen AJ, and McLean AR 2015. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B. Biol. Sci 282: 20151821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D 2010. Bumblebees: behaviour, ecology, and conservation. Oxford University Press, Oxford, UK. [Google Scholar]
- Goulson D, Nicholls E, Botías C, and Rotheray EL 2015. Bee declines driven by combined stress from parasites, pesticides and lack of flowers. Science 347: 1255957. 10.1126/science.1255957 [DOI] [PubMed] [Google Scholar]
- Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, and Hughes WOH 2002. Can alloethism in workers of the bumblebee Bombus terrestris be explained in terms of foraging efficiency? Anim. Behav 64: 123–130. [Google Scholar]
- Gradish AE, Cutler GC, Frewin AJ, and Scott-Dupree CD 2016. Comparison of buckwheat, red clover, and purple tansy as potential surrogate plants for use in semi-field pesticide risk assessments with Bombus impatiens. PeerJ. 4: e2228. 10.7717/peerj.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf SS, Williams NE, Winfree R, and Kremen C 2007. Bee foraging ranges and their relationship to body size. Oecologia 153: 589–596. [DOI] [PubMed] [Google Scholar]
- Heard MS, Baas J, Dome J-L, Lehive E, Robinson AG, Rortais A, Spurgeon J, Svendsen C, and Hesketh H 2016. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Tot. Environ 578: 357–365. [DOI] [PubMed] [Google Scholar]
- Heinrich B 1972. Physiology of brood incubation in the bumblebee queen, Bombus vosnesenskii. Nature 239: 223–225. [Google Scholar]
- Heinrich B 2004. Bumblebee economics. Harvard University Press, Cambridge, UK. [Google Scholar]
- Heinrich B, and Vogt FD 1993. Abdominal temperature regulation by arctic bumblebees. Physiol. Zool 66: 257–269. [Google Scholar]
- Johansen CA, Mayer DF, Eves JD, and Kious CW 1983. Pesticides and bees. Environ. Entomol 5: 1513–1518. [Google Scholar]
- Kevan PG 1999. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric. Ecosyst. Environ 74: 373–393. [Google Scholar]
- Klatt BK, Holzschuh A, Westphal C, Clough Y, Smit I, Pawelzik E, and Tscharntke T 2014. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B. Biol. Sci. 281: 20132440. 10.1098/rspb.2013.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, and Tscharntke T 2007. Importance of pollinators in changing landscapes for world crops. Proc. Roy. Soc. B. Biol. Sci 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, Sanderson RA, and Goulson D 2005. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Mol. Ecol 14: 1811–1820. [DOI] [PubMed] [Google Scholar]
- Laycock I, Lenthall KM, Barratt AT, Cresswell JE 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21: 1937–1945. [DOI] [PubMed] [Google Scholar]
- Leza M, Watrous KM, Bratu J, and Woodard SH 2018. Effects of neonicotinoid insecticide exposure and monofloral diet on nest-founding bumblebee queens. Proc. Roy. Soc. B. Biol. Sci 285: 20180761. 10.1098/rspb.2018.0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vaamonde C, Koning JW, Jordan WC, and Bourke AFG 2003. No evidence that reproductive bumblebee workers reduce the production of new queens. Anim. Behav 66: 577–584. [Google Scholar]
- Lopez-Vaamonde C, Raine NE, Koning JW, Brown RM, Pereboom JJM, Ings TC, Ramos-Rodriguez O, Jordan WC, and Bourke AFG 2009. Lifetime reproductive success and longevity of queens in an annual social insect. J. Evol. Biol 22: 983–996. [DOI] [PubMed] [Google Scholar]
- Lucchetti MA, Kilchenmann V, Glauser G, Praz C, and Kast C 2018. Nursing protects honeybee larvae from secondary metabolites of pollen. Proc. R. Soc. B. Biol. Sci 285: 20172849. 10.1098/rspb.2017.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD 2007. Bees of the world, 2nd Edition. John Hopkins Press, Baltimore, MD. [Google Scholar]
- Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, vanEngelsdorp D, and Pettis JS 2010. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 5: e9754. 10.1371/journal.pone.0009754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls E, Botías C, Rotheray E, Whitehorn P, David A, Fowler R, David T, Feltham H, Swain J, Wells P, Hill E, Osborne J, and Goulson D 2018. Monitoring neonicotinoid exposure for bees in rural and peri-urban areas of the UK during the transition from pre- to post-moratorium. Envir. Sci. Tech In press. [DOI] [PubMed]
- Nixon HL, and Ribbands CR 1952. Food transmission within the honey bee community. Proc. R. Soc. B. Biol. Sci 140: 43–50. [DOI] [PubMed] [Google Scholar]
- (OECD) Organisation for Economic and Co-operation Development. 2017a. OECD guideline for the testing of chemicals: bumblebee, acute contact toxicity test. No. 246. Available at: https://www.oecd-ilibrary.org/environment/test-no-246-bumblebee-acute-contact-toxicity-test_9789264284104-en
- (OECD) Organisation for Economic and Co-operation Development. 2017b. OECD guideline for the testing of chemicals: bumblebee, acute oral toxicity test. No. 247. Available at: https://www.oecd-ilibrary.org/environment/test-no-247-bumblebee-acute-oral-toxicity-test_9789264284128-en
- Osborne JL, Martin AP, Carreck NL, Swain JL, Knight ME, Goulson D, Hale RJ, and Sanderson RA 2008. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol 77: 401–415. [DOI] [PubMed] [Google Scholar]
- Owen RE 1988. Body size variation and optimal body size of bumble bee queens (Hymenoptera: Apidae). Can. Entomol 120: 19–27. [Google Scholar]
- Owen RE 1989. Differential size variation of male and female bumblebees. J. Hered 80: 39–43. [Google Scholar]
- Peat J, and Goulson D 2005. Effects of experience and weather on foraging efficiency and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol 58: 152–156. [Google Scholar]
- Pedersen BV 1996. A phylogenetic analysis of cuckoo bumblebees (Psithyrus, Lepeletier) and bumblebees (Bombus, Latreille) inferred from sequences of the mitochondrial gene cytochrome oxidase I. Mol. Phylogen. Evol 5: 289–297. [DOI] [PubMed] [Google Scholar]
- Pereboom JJM 2000. The composition of larval food and the significance of exocrine secretion in the bumblebee Bombus terrestris. Insectes Soc 47: 11–20. [Google Scholar]
- Pomeroy N 1979. Brood bionomics of Bombus ruderatus in New Zealand (Hymenoptera: Apidae). Can. Entomol 111: 865–874. [Google Scholar]
- Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R, Neumann P, and Settele J 2010. Declines of managed honey bees and beekeepers in Europe. J. Apicult. Res 49: 15–22. [Google Scholar]
- Přidal A, and Hofbauer J 1996. Laboratory rearing and nutrition of young queens of bumblebee (Bombus terrestris L.) from emergence to diapause. Sci. Stud. Res. Inst. Fodder Plants Troubsko 14: 125–131. [Google Scholar]
- Raine NE, and Gill RJ 2015. Tasteless pesticides affect bees in the field. Nature 521: 38–40. [DOI] [PubMed] [Google Scholar]
- Řehoř I, Macháčková L, Bučánková A, Matějková S, Černá K, and Straka J 2014. Measuring the sugar consumption of larvae in bumblebee micro-colonies: a promising new method for tracking food economics in bees. Apidologie 45: 116–128. [Google Scholar]
- Rortais A, Arnold G, Halm M-P, and Touffet-Briens F 2005. Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36: 71–83. [Google Scholar]
- Rotheray EL, Osborne JL, and Goulson D 2017. Quantifying the food requirements and effects of food stress on bumble bee colony development. J. Apicult. Res 56: 288–299. [Google Scholar]
- Scott-Dupree CD, Conroy L, and Harris CR 2009. Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol 102: 177–182. [DOI] [PubMed] [Google Scholar]
- Simpson J 1955. The significance of the presence of pollen in the food of worker larvae of the honey bee. Q. J. Microsc. Sci 96: 117–120. [Google Scholar]
- Smeets P, and Duchateau MJ 2001. Feeding behaviour in the bumblebee Bombus terrestris. Belgian J. Zool 131: 119–126. [Google Scholar]
- Spaethe J, and Weidenmüller A 2002. Size variation and foraging rate in bumblebees (Bombus terrestris). Insectes Soc 49: 142–146. [Google Scholar]
- Stanley DA, Garratt MPD, Wickens JB, Wickens VJ, Potts SG, and Raine NE 2015. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528: 548–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DA, Russell AL, Morrison SJ, Rogers C, and Raine NE 2016. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Ecol 53: 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner KA 2016. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.). Front. Environ. Sci 4: 79. 10.3389/fenvs.2016.00079 [DOI] [Google Scholar]
- Tasei JN, Sabik H, Pirastru L, Langiu E, Blanché JM, Fournier F, and Taglioni JP 1994. Effects of sublethal doses of deltamethrin (Decis Ce) on Bombus terrestris. J. Apic. Res 33: 129–135. [Google Scholar]
- Tasei JN, Lerin J, and Ripault G 2000. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manag. Sci 56: 189–191. [Google Scholar]
- Tasei JN, and Aupinel P 2008. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39: 397–409. [Google Scholar]
- Thompson H 2003. Behavioural effects of pesticides in bees—their potential for use in risk assessment. Ecotoxicology 12: 317–330. [DOI] [PubMed] [Google Scholar]
- Thompson H 2016. Extrapolation of acute toxicity across bee species. Integ. Environ. Assess. Manag 12: 622–626. [DOI] [PubMed] [Google Scholar]
- Thompson H, Harrington P, Wilkins S, Pietravalle S, Sweet D, and Jones A 2013. Effects of neonicotinoid seed treatments on bumble bee colonies under field conditions Food Environment Research Agency Report. York, UK. Available at: http://www.ambienteterritorio.coldiretti.it/tematiche/Ogm/Documents/DEFRA%20report%20neonicotinoids%20-Mar13.pdf [Google Scholar]
- (USEPA) US Environmental Protection Agency, (PMRA) Pest Management Regulatory Agency Health Canada, and (CDPR) California Department of Pesticide Regulation. 2012. White paper in support of the proposed risk assessment process for bees. Submitted to the FIFRA Scientific Advisory Panel for Review and Comment. Available at: https://www.cdpr.ca.gov/docs/emon/surfwtr/presentations/epa_whitepaper.pdf
- (USEPA) Environmental Protection Agency, (PMRA) Health Canada Pest Management Regulatory Agency Health Canada, and (CDPR) California Department of Pesticide Regulation. 2014. Guidance for assessing pesticide risks to bees. Available at: https://www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf
- Vanbergen AJ, Baude M, Biesmeijer JC, Britton NF, Brown MJF, Brown M, Bryden J, Budge GE, Bull JC, Carvell C, Challinor AJ, Connolly CN, Evans DJ, Feil EJ, Garratt MP, Greco MK, Heard MS, Jansen VAA, Keeling MJ, Kunin WE, Marris GC, Memmott J, Murray JT, Nicolson SW, Osborne JL, Paxton RJ, Pirk CWW, Polce C, Potts SG, Priest NK, Raine NE, Roberts S, Ryabov EV, Shafir S, Shirley MDF, Simpson SJ, Stevenson PC, Stone GN, Termansen M, and Wright GA 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ 11: 251–259. [Google Scholar]
- Wharton KE, Dyer FC, Huang ZY, and Getty T 2007. The honeybee queen influences the regulation of colony drone production. Behav. Ecol 18: 1092–1099. [Google Scholar]
- Williams PH 1994. Phylogenetic relationships among bumble bees (Bombus Latr.): a reappraisal of morphological evidence. Sys. Entomol 19: 327–344. [Google Scholar]
- Williams PH 1998. An annotated checklist of bumble bees with an analysis of patterns of description (Hymenoptera: Apidae, Bombini). Bull. Nat. Hist. Mus 67: 79–152. [Google Scholar]
- Williams PH, Cameron SA, Hines HM, Cederberg B, and Rasmont P 2008. A simplified subgeneric classification of the bumblebees (genus Bombus). Apidologie 39: 46–74. [Google Scholar]
- Winston ML 1987. The biology of the honey bee. Harvard University Press, Cambridge, MA. [Google Scholar]
- Wisk JD, Pistorius J, Beevers M, Bireley R, Browning Z, Chauzat MP, Nikolakis A, Overmyer J, Rose R, Sebastien R, Vaissière BE, Maynard G, Kasina M, Nocelli RCF, Scott-Dupree C, Johansen E, Brittain C, Coulson M, Dinter A, and Vaughn M 2014. Assessing exposure of pesticides to bees, pp. 45–74. In Fischer D and Moriarty T (eds.), Pesticide risk assessment for pollinators. Society of Environmental Toxicology and Chemistry (SETAC), Ames, IA. [Google Scholar]
- Wu-Smart J, and Spivak M 2017. Effects of neonicotinoid imidacloprid exposure on bumble bee (Hymenoptera: Apidae) queen survival and nest initiation. Environ. Entomol 47: 55–62. [DOI] [PubMed] [Google Scholar]


