Abstract
Air pollution is defined as the presence of noxious substances in the air at levels that impose a health hazard. Thus, there has been long-standing interest in the possible role of indoor and outdoor air pollutants on the development of respiratory disease. In this regard, asthma has been of particular interest but many studies have also been conducted to explore the relationship between air pollution, allergic rhinitis, and atopic dermatitis. Traffic-related air pollutants or TRAP refers to a broad group of pollutants including elemental carbon, black soot, nitrogen dioxide (NO2), nitric oxide (NO), sulfur dioxide (SO2), particulate matter (PM2.5 and PM10), carbon monoxide (CO), and carbon dioxide (CO2). In this review, we aim to examine the current literature regarding the impact of early childhood exposure to TRAP on the development of asthma, allergic rhinitis, and atopic dermatitis. Although there is growing evidence suggesting significant associations, definitive conclusions cannot be made with regard to the effect of TRAP on these diseases. This conundrum may be due to a variety of factors, including different definitions used to define TRAP, case definitions under consideration, a limited number of studies, variation in study designs, and disparities between studies in consideration of confounding factors. Regardless, this review highlights the need for future studies to be conducted, particularly with birth cohorts that explore this relationship further. Such studies may assist in understanding more clearly the pathogenesis of these diseases, as well as other methods by which these diseases could be treated.
Keywords: Asthma, Incident asthma, Allergic rhinitis, Atopic dermatitis, Air pollution, TRAP, Childhood
Introduction
Air pollution is defined as the presence of noxious substances in the air at levels that impose a health hazard [1]. Recognition of air pollutants as a hazard dates back to thirteenth-century London when the use of coal was prohibited due to recognized detrimental health effects. In the nineteenth century, major cities in Great Britain where covered with a dense fog of smoke coined as “smog.” Recognition of smog led to the creation of the Public Health (Smoke Abatement) Act in 1926. Although this act aimed to reduce smoke emissions from industrial sources of air pollution, it did not tackle domestic sources [2]. This was followed by a series of laws to develop a national air quality strategy imposing limit values for the different outdoor air pollutants emitted into the atmosphere [2].
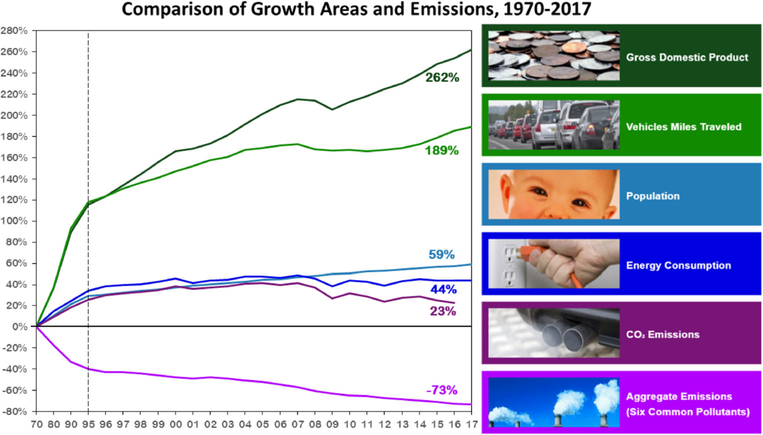
In the USA, the first federal air pollution law was enacted in 1955 [3]. Currently, the United States Environmental Protection Agency (EPA) regulates air quality standards through the National Ambient Air Quality Standards (NAAQS). These standards determine the concentration thresholds for both indoor and outdoor air pollutants. Regulated pollutants are carbon monoxide (CO), lead, nitric dioxide (NO2), ozone (O3), sulfur dioxide (SO2), and particulate matter [1, 4]. These pollutants are produced by vehicles, greenhouse gas emissions from gross domestic products, and population growth [5]. Regulation of these pollutants have led to a decrease in aggregate emissions of these six common pollutants, despite population growth and increased traffic distances, as shown in Fig. 1 [5]. However, the actual exposure levels determining the risk for asthma development during infancy and childhood remain uncertain.
Fig. 1.
Comparison of growth measurement and aggregate emissions from all sources, 1990–2007. Adapted from the US Environmental Protection Agency, 2008 [5]
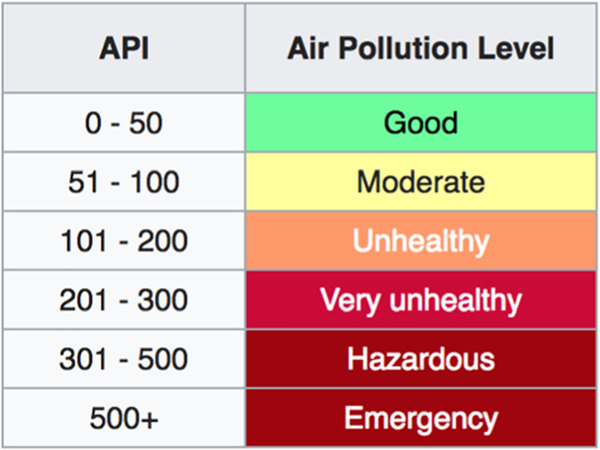
Relevant air pollutants are measured daily as part of the Air Pollution Index (API) and include particulate matter, NOx, and O3. Ranges of acceptable exposure limits have been defined and updated, as noted in Fig. 2. Air pollutants are emitted in two forms: gaseous compounds, such as nitric oxide (NO), sulfur oxides (SOx), carbon monoxide (CO), and carbon dioxide (CO2), and solid particulate matter primarily from traffic diesel exhaust particles (DEP) [1]. Particulate matter is classified by the size of the particles—the two most commonly referenced sizes are particles < 10 mm, referred to as PM10, and particles < 2.5 mm, referred to as PM2.5.
Fig. 2.
Air pollution index [3]
Among the recent epidemiologic studies examining air pollutants and asthma, nearly all have focused on traffic pollutants. The term traffic-related air pollution (TRAP) is a comprehensive term to describe all components produced by fuel combustion sources, including elemental carbon, black soot, NO2, NO, SO2, particulate matter (PM2.5 and PM10), ultrafine particulate matter, benzene, CO, and CO2 [5]. Of these, certain pollutants have been chosen as surrogates to measure pollutant exposure from traffic based on four criteria from the EPA [5]: production mainly by traffic, ability to be measured at low concentrations, ability to be measured by inexpensive and accurate methods, and no independent association with health effects [5]. Nitrous oxides (NOx), sulfur oxides (SOx), carbon monoxide (CO), particulate matter (PM2.5 and PM10), and black carbon are the most cited pollutants in the literature as surrogates for traffic-related air pollution (TRAP).
Although the incidence of asthma has significantly increased in recent decades, the rate of increase has been declining in recent years [6, 7]. Multiple environmental exposures may contribute to development of asthma: indoor and outdoor aeroallergens, viral infections, environmental tobacco smoke, the respiratory microbiome, and other airborne contaminants. There has been a long-standing interest in the possible role of indoor and outdoor air pollutants on the development of obstructive lung disease. By defining the health risks of air pollutants on respiratory health, this has enabled public health policy decisions that define safe and harmful exposure levels of outdoor air pollutants [4].
While a majority of the studies on TRAP have focused on respiratory disease, the effects of air pollutants on allergic rhinitis and eczema have also been examined. At this time, the literature remains inconclusive about a definitive relationship between the latter, perhaps due to the fact that clinical data from these studies are not comparable with regard to significant variability in the pollutants studied, models used to quantify exposure, defined outcomes, and adjustments for confounding factors are not consistent.
The Impact of Air Pollutants on Respiratory Health
Multiple cohort studies have evaluated the role of DEP (PM2.5, PM10, and black carbon) and/or gaseous air pollutants (NO2, NOx, O3, and SO2) on the development of asthma. Many studies have assessed multiple air pollutant exposures but NO2 is the most commonly assessed individual pollutant [8].
Most studies were conducted in the pediatric age group, though a few studies examined adult/late-onset asthma. A variety of exposure models have been used for the assessment of air pollutant concentrations; the most commonly utilized land use regression (LUR) models. LUR models utilize monitoring data of air pollutant collected at multiple site locations throughout a geographic area and infer TRAP exposure concentrations in individual subjects based on geographic information systems (GIS) [9]. LUR models consider the pollutant of interest as the dependent variable and test it against other independent variables including traffic, topography, and other geographic variables, through regression analysis [10]. This allows prediction of the level of air pollutant exposure for any location [10]. These models have successfully been used to estimate annual average concentrations of traffic-related air pollutants in North American and European cities [9]. Dispersion modeling, traffic density, and distance to major roads were also used as surrogates for the concentrations of traffic-related air pollutants.
Pathogenesis
In humans, diesel exhaust particles (DEP), specifically PM 2.5, PM10, and ultrafine particulate matter, have been extensively investigated for their capacity to enhance Th2-directed immune responses. Intranasal exposure to DEP during allergen exposure (e.g., ragweed) was shown to increase local Th2 cytokine and specific IgE production, suggesting a role for DEP as an immune adjuvant [11–14]. Diaz-Sanchez et al. first demonstrated that nasal mucosal specific IgE was produced after exposure to a neoantigen, keyhole limpet hemocyanin (KLH), but only with preceding exposure to DEP [15]. These findings prompted longitudinal epidemiologic and cross-sectional studies evaluating the risk of aeroallergen sensitization associated with high TRAP exposure early in life, as listed in Table 1. Although unproven, gestational exposure to TRAP during critical periods of fetal development could elicit maternal oxidative stress, thereby impacting the fetal lungs [20, 21]. BMI status may be associated with enhanced risk of asthma associated with exposure to air pollutants. When comparing long-term exposure to TRAP on in children with high BMI versus normal BMI, TRAP was associated with a twofold greater likelihood of asthma in children with normal BMI but not high BMI [22]. The investigators speculated that children with lower BMI could spend more time outdoors and hence encounter greater exposure to TRAP compared with those with high BMIs. Dong et al., however, found an opposing effect to BMI in children age 2–15 years in seven northeastern cities in China [23]. Obese or overweight children had higher odds of respiratory symptoms defined by wheeze, phlegm, and sputum production compared with their normal BMI counterparts; furthermore, the odds ratio for respiratory symptoms increased with exposure to increasing concentrations of PM10, SO2, NO2, and O327.
Table 1.
Effects of DEP on healthy volunteers
| Study | Exposures | Studies | Results |
|---|---|---|---|
| Salvi et al. [16] | 15 volunteers exposed to DEP for 1 h with intermittent exercise. | • Spirometry • Blood sampling • BAL • Bronchial biopsy |
• No change in lung function test • BAL showed increase in neutrophils, B lymphocytes, histamine, and fibronectin • Bronchial biopsies showed high levels of neutrophils, mast cells, CD4+ and CD8+ T lymphocytes, and upregulation of ICAM-1 and VCAM-1 at the endothelium, along with increases in the numbers of LFA-1+ cells in the bronchial tissue. • Peripheral blood showed a significant increase in neutrophil and platelet numbers |
| Salvi et al. [17] | 15 volunteers exposed to DEP on two separate occasions for 1 h each | • BAL | • Increase gene transcription of interleukin-8 (IL-8) in bronchial tissue. • Increased expression of growth-regulated oncogene-alpha (GRO-alpha) protein • Increase in IL-5 mRNA gene transcripts • No significant changes in the levels of gene transcription of interleukin-1B (IL-1beta), tumor necrosis factor-alpha (TNF-alpha), interferon gamma (IFN-gamma), and granulocyte macrophage colony-stimulating factor (GM-CSF) |
| Nightingale et al. [18] | Ten participants exposed for 2 h to DEP or air in a double-blind, randomized, crossover study | • Serial spirometry • Exhaled carbon monoxide (CO), and methacholine reactivity • Sputum induction • Blood sampling |
• Spirometry and Methacholine reactivity showed no significant difference between both groups. • Increased levels of exhaled CO in the DEP group compared with those exposed to air at 1 h (air, 2.9 ± 0.2 ppm [mean ± SEM]; DEP, 4.4 ± 0.3 ppm; p <0.001). • Sputum induction showed increased neutrophils and myeloperoxidase (MPO) at 4 h in the DEP group compared with air (neutrophils, 41 ± 4% versus 32 ± 4%; MPO, 151 ng/ml versus 115 ng/ml, p < 0.01) • Sputum induction showed no significant difference in the levels of IL-8 or TNF-α • No significant difference in levels of IL-6, TNF-α and P-selectin |
| Sehlstedt et al. [19] | 15 volunteers were exposed to DEP and air for 1 h on two separate occasions 3 weeks apart. | • Spirometry • BAL • Bronchial biopsy |
• No change in lung function test • BAL showed increase in eosinophil number (p = 0.017) but no significant change in number of inflammatory cells • Bronchial biopsy showed increased expression of P-selectin (p = 0.036) and VCAM-1 (p = 0.030). No significant increase in ICAM-1 expression as compared with filtered air |
Brunst et al. evaluated the effect of TRAP exposure on the development of asthma in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) cohort [24]. Early exposure to TRAP was associated with transient and persistent wheeze (aOR = 2.31; 95% CI, 1.28–4.15) in childhood. Children with high average TRAP exposure from birth through age 7 were at significantly increased risk for asthma at age 7 (aOR 1.71; 95% CI, 1.01–2.88). They concluded that although early exposure predicts persistent childhood wheezing, only persistent lifetime TRAP exposure significantly increased risk of asthma at age 7 [24].
A large retrospective study suggested that exposure to TRAP in the first year of life was associated with an increased likelihood for development of childhood asthma [25]. Hsu et al. further evaluated critical windows during the prenatal period and found that higher levels of exposure to PM2.5 in the mid-gestation period, particularly at 16 to 25 weeks of gestation, carried an increased risk for asthma development in boys at 6 years of age [26].
Gehring et al. studied the effects of exposure to NO2, PM2.5, and “soot” in children age 0–8 years in a birth cohort study and found an increased odds ratio for asthma development for all three pollutants [27]. This relationship remained significant at age 12 years with persistent exposure to TRAP [28]. The Swedish BAMSE birth cohort determined that exposure to NOx and PM10 during the first year of life was associated with increased asthma prevalence and incidence at ages 8 to 12 but not at younger ages [29].
Studies by Air Pollutant
The evidence regarding the association between TRAP and asthma development, or incidence of asthma, in the pediatric population has been conflicting (Table 2). Multiple studies have demonstrated this association [22, 24, 27, 29, 30, 32, 33, 36, 39, 40, 42], including NO2 [27, 30, 32, 33, 39], PM2.5 [27, 30], NO [30], black carbon [22, 27, 30, 32, 40], NOx [29], and PM10 [29, 42]. However, other studies have not shown a correlation between these environmental exposures and asthma [25, 31, 34, 35, 37–41]. In examining the literature, we could identify four variables that could account for this heterogeneity: duration of exposure to pollutants, a critical period of exposure, exposure to a certain cut-off level of the pollutant, and populations that are biologically at inherently increased risk.
Table 2.
Study evaluating asthma in the pediatric population on exposure to air pollution
| Study | Location | Exposure assessment | Age group | Pollutants | Results |
|---|---|---|---|---|---|
| Brunst et al., [24] | Cincinnati, USA | LUR | 1–7 years | Black carbon | Children who had high levels of TRAP exposure at birth has twofold increased risk of persistent wheeze at age 7. Those with persistent exposure from birth through 7 years of age at levels of TRAP >75 percentile had an increased OR for development of asthma that was statistically significant (OR = 1.71; 95% CI, 1.01–2.88). |
| Carlsten et al. [30] | Vancouver, Canada, | LUR model | Birth, 1 and 7 years | NO, NO2, black carbon, and PM2.5 | PM2.5 had the highest OR for the development of asthma (OR 3.1; 95 %CI of 1.3–7.4) compared with NO2 (OR 1.5; 95% CI 0.9–2.5), NO (OR 1.2, 95% CI 0.9–1.7), and black carbon (OR 1.1, 95% CI of 0.7–1.9). This did not hold true for bronchial reactivity, as the OR was not significant. |
| Clark et al. [25] | British Columbia, Canada | IDW, LUR | In utero, 1, 3–4 years | BC, CO, NO, NO2, PM10, and PM2.5 | NO was found to have an increased OR for exposure in utero and of borderline statistical significance at year 1 of life. NO2 exposure was only statistically significant during the first year of life. Black carbon was borderline statistically significant for both periods. IDW was used to assess effects of NO, NO2, CO, PM10, PM2.5, SO2, and 3. Exposure to NO, NO2, CO, PM10, and SO2 was found to have an increased OR for exposure in utero and during first year of life but not to O3, SO2, and PM2.5. |
| Fuertes et al. [31] | Germany | LUR | Birth, 6, 10 years | Black carbon, NO2, and PM2.5 | No association was found between exposure to TRAP and development of asthma |
| Gehring et al. [27] | Netherlands | LUR | Birth–8 years | NO2, PM2.5, and black carbon | Statistically significant increase in incidence of asthma (OR, 1.26; 95% CI, 1.04–1.51) and wheezing across all phenotypes (OR, 1.15; 95% CI, 1.02–1.28) for the first of year of life (6%) as well as years 2–8 but at lower prevalence (1–2%) on exposure to PM2.5. Similar associations were seen with NO2 and soot. Exposure to any was not associated with a significantly increased risk for bronchial hyper responsiveness. |
| Gehring et al. [32] | Netherlands | LUR | Birth–16 years | Black carbon, NO2, PM2.5, and PM10 | Significantly increased risk of asthma at age 14–16 with increasing exposure to NO2 ([OR] 1.13 per 10 μg/m3 [95% CI 1.02–1.25]) and black carbon at birth (1.29 per 1 unit [1.00–1.66]) but not for PM2.5 and PM10. Incidence of asthma was apparent at age 4 years and older. |
| Gruzieva et al. [29] | Sweden | Dispersion modeling | Birth–12 years | NOx and PM 10 | Exposure to PM10 and NOx in the post natal period was found to be significantly associated with occurrence of non-allergic asthma (negative IgE to aeroallergens) at 4 and 8 years of age. |
| Jerrett et al. [33] | CA, USA | LUR | 10–12 years | NO2 | Significantly increased risk of asthma on exposure to NO2 at an average concentration of 6.2 ppb annually (HR 1.29; 95% CI 1.07–1.56). The risk increased with exposure to higher concentrations of 28.9 ppb annually (HR 3.25; 95% CI, 1.35–7.85). |
| Kramer et al. [34] | Germany | LUR | 0–6 years | PM2.5 and NO2 | No association found between exposure to TRAP and development of asthma. |
| Le Masters et al. [22] | USA | LUR | 0–7 years | Black carbon | Higher levels of eNO in normal-weight vs obese children in those exposed to TRAP. Similarly high lifetime exposure to TRAP was associated with a twofold increase in asthma in normal BMI compared with children with high BMI. |
| Lindgren et al. [35] | Sweden | Dispersion modeling and traffic intensity | 0–6 years | NOx | No relation between development of asthma and exposure to NOx. No association between living close to high-traffic roads (≥8640 cars/day) and asthma. |
| McConnell et al. [36] | Southern CA, USA | Dispersion modeling for NOx. Average annual concentrations for NO2, PM2.5, and PM10 were estimated based on the distances to the nearest freeway or other highways or arterial roads and traffic density within 150 m of each participant’s residence and school and at central community sites | Kindergarten and first grade to fourth grade | NOx, NO2, PM2.5, and PM10 | Increased risk of asthma on exposure to traffic-related air pollution at home or school. Increased association was seen when exposure to TRAP was at both locations. |
| Molter et al. [37] | England | Monitored levels of TRAP at participant’s “microenvironments”. LUR was used for outdoor exposures and INDAIR model for indoor exposures | 0–11 years | PM 10 and NO2 | No association found between the development of wheeze and asthma and exposure to PM10 and NO2. |
| Molter et al. [38] | England, Germany, Sweden, Netherlands | LUR LUR models used were based on the measurement campaigns carried out in 2009, but the exposure timepoints specified were between 1994 and 2008 |
0–10 years | NO2, NOx, PM10, PM2.5, and black carbon | No association found between the development of wheeze and asthma and exposure to NO2, NOx, PM10, PM2.5, and black carbon. |
| Morgenstern et al. [39] | Munich, Germany | LUR | 0–2 years | Black carbon, NO2, and PM2.5 | Statistically significant association was found between wheezing and exposure to NO2 for the first and second years of life but not for PM2.5 or black carbon. |
| Morgenstern et al. [40] | Munich, Germany | LUR and distance to major roads. | 4–6 years | Black carbon, NO2, and PM2.5 | Positive associations were found for exposure to black carbon and asthma (OR, 1.56; 95% confidence interval [CI], 1.03–2.37). Living within a 50-m range from a major road was also associated with both asthma OR 1.66; 95% CI (1.01–2.59) and wheeze OR 1.24 95% CI (1.01–1.52). No associations were found for NO2 and PM2.5. |
| Oftedal et al. [41] | Oslo, Norway | Dispersion modeling and distance to major roads. | 0–10 years | NO2 | No association was found between TRAP exposure and development of asthma. An interquartile range increase in the concentration of NO2 was associated with an adjusted risk ratio of 0.82; 95% CI 0.67–1.02 for the development of asthma. |
| Wu et al. [42] | Southern Taiwan | Kriging method to estimate individual exposure to air pollution | School-age children | NO2, SO2, PM10, and CO | Positive association found between high level of exposure to PM10 and asthma onset at > 10 years. Associations were not significant for NO2, SO2, or CO. |
DEP, diesel exhaust particles; TRAP, traffic-related air pollution; LUR, land use regression model; IDW, inverse distance weighting; NOx, nitrogen oxides
Studies Focusing on One Pollutant
Nitrous oxide (NOx) and nitrous dioxide (NO2) were the most common pollutants evaluated individually. An increased risk for asthma development was noted when pediatric subjects aged 10–12 years were exposed to high annual concentrations of NO2 [33]. More specifically, those who experienced a concentration of 29 parts per billion (ppb) had a hazard ratio of 3.25 in comparison with subjects exposed to 6.29 ppb annually (HR 1.29). However, Oftedal et al. did not find positive associations between traffic-related exposure and the onset of physician-diagnosed asthma in children [41]. Although associations for late asthma onset (defined as greater than or equal to 4 years of age) were positive, these findings were not statistically significant. Interestingly, while NO2 did not seem to be associated with the general development of asthma, those who carried the NQO1 rs2917666 genotype demonstrated a significant association between nitrous dioxide exposure and asthma development [43].
The correlation between asthma incidence and NOx seemed to be dependent on the cohort age. Two studies conducted by Lindgren et al. focused on NOx exposure by evaluating adults aged 18–77 years old who lived within 100 m of a road with at least ten cars per minute [44, 45]. Participants with at least > 19 μg/m3 NOx exposure had an increased odds ratio for asthma symptoms but not necessarily an objectively confirmed diagnosis [44], though an increased prevalence of allergic asthma versus non-allergic asthma was noted among those with high NOx exposure [43]. Unlike the aforementioned adult subjects, no relationship between NOx and asthma development could be determined when looking at similar parameters in pediatric patients [38].
One study that focused on black carbon (BC) showed an increased odds ratio for asthma development based on pulmonary function testing in a subgroup of children persistently exposed to TRAP from birth through 7 years of age [24], and Morgenstern et al. also found positive associations between exposure to black carbon and asthma (OR, 1.56; 95% confidence interval [CI], 1.03–2.37) [39]. Another study showed that exposure to BC was significantly associated with asthma among normal-weight children but not overweight children (adjusted odds ratio of 1.8 versus 0.7), whereas the reverse was true when looking at exposure to secondhand smoke [22]. It was postulated that this could be due to the patterns of activity between both groups, particularly when comparing the amount of time these groups of children spent indoors versus outdoors.
Studies Examining Multiple Pollutants
Generally, studies that have been conducted to examine the relationship between TRAP and respiratory disease have considered multiple pollutants at the same time, as much of these pollutants are mixed together. Again, however, the results have been conflicting. While some found no association [25, 31, 34, 38], others identified a potential culprit pollutant or group of pollutants [32, 39, 40]. Of note, most of these studies focused on pediatric populations, but those done in adults are presented in Table 3.
Table 3.
Studies evaluating adult onset asthma on exposure to air pollution
| Castro-Giner et al. [43] | Sweden, UK, Spain Barcelona, Germany, France, Paris, Belgium | Exposures to NO2 at each of the participants’ addresses were determined using air pollution maps using focal sum techniques in a global information system model. | 20–44 years | NO2 | No significant association between NO2 exposure and development of asthma. Significant association between development of asthma and NO2 exposure in those carrying NQO1 rs2917666 genotype. |
| Lindgren et al. [44] | Sweden | Self-reported exposure to traffic, traffic intensity within 100 m, modeled exposure to NOxusing modified Gaussian dispersion model. | 18–77 years | NOx | Asthma diagnosis was associated with living within 100 m of a road with greater than 10 cars/min. Participants with high exposure to NOx (> 19 μg/m3) had increased odds ratio for asthma symptoms but not diagnosis. |
| Lindgren et al. [45] | Sweden | Self-reported exposure to traffic, traffic intensity within 100 m, modeled exposure to NOx using modified Gaussian dispersion model. | 18–77 years | NOx | Increased prevalence of allergic asthma was seen for participants living within 100 m of a road with greater than 10 cars/min but not for non-allergic asthma. No statistical significance of allergic and non-allergic asthma prevalence on exposure to NOx at various concentrations. |
The evidence to support asthma development among birth cohorts exposed to TRAP has not been clear-cut. When monitoring children at birth and at subsequent ages, the effect of pollutants seemed to depend on the age at which the children are exposed [14, 26, 36, 39]. One study that focused on PM2.5 and NO2 found that the odds ratio for asthma diagnosis was statistically significant when comparing children aged 0–12 months versus those diagnosed at 12–24 months [14]. Similar findings were noted when considering incidence of asthma or wheezing [36, 39]; however, in these cohorts, PM2.5 seemed to have more effect at the first year of life and years 2–8 [21] versus NO2 and BC at age 14–16 years [39]. The latter group showed a significantly increased risk of asthma with at least 1.13 per 10 μg/m3 NO2 and 1.29 per 1 unit of black carbon at birth.
As mentioned previously, the etiology of these associations could be partially attributed to potential genetic differences among these children. Positive association with development of asthma on exposure to NOx and PM10 at older age groups has also been reported without finding associations with younger age groups [29], and asthma onset at greater than 10 years of age also seemed to correlate with high levels of PM10 exposure [31]. However, no significant associations were noted for NO2, SO2, or CO in the aforementioned study. Another study, though, found that PM10 and NO2 did not have an effect of asthma development or wheeze in pediatric subjects [41].
Role of Indoor Air Pollution on the Development of Asthma
The majority of studies have focused on the effects of outdoor air pollution on the development of respiratory disease. However, indoor air pollutants have also been implicated in the development and exacerbation of asthma, and as such, there is growing interest in their role on this disease.
Indoor air pollution occurs as a product of multiple sources: transfer from outdoor sources, chemicals used in cleaning or renovation, tobacco smoking and use of gas, kerosene wood, or coal in the home. Inhaled indoor pollutants that have been studied include nitrogen dioxide, particulate matter, volatile organic compounds (VOCs), formaldehyde, and phthalates. Nitrogen dioxide and particulate matter can be found in homes as a product of outdoor pollution transferred indoors, as well as from tobacco smoking and heating devices running on gas, kerosene, wood, or coal [46].
Prior studies have shown that a drop in FEV1 in asthmatics occurs upon exposure to domestic NO2. A drop in FEV1 was seen on exposure to 100 ppb over a duration of 1 h, and higher concentrations of NO2 (400 ppb) were shown to cause a more profound drop in FEV1 compared with those inhaling lower concentrations [47, 48]. A meta-analysis conducted by Gehring et al. included 41 studies assessing the incidence of asthma in children exposed to NO2 from exposure to gas cooking. Based on their analysis, there was a significant association between the two variables [49]. Nineteen of the included studies showed an increased odds ratio for current asthma as well as lifetime prevalence of asthma. However, four other studies in the meta-analysis did not show a statistically significant association.
Volatile organic compounds (VOCs) are another category of emitted gases contributing to indoor air pollution. VOCs arise from a multitude of household products that include, but are not limited to, paints, varnishes and wax, cleaning and cosmetic products, aerosol spray, pesticides, building material, glues and adhesives, and office equipment, such as copiers and printers [46]. The National Health and Nutrition Examination Survey (NHANES) conducted over a 1-year period in the USA showed higher odds of physician-diagnosed asthma in patients known to have exposure to VOCs [50]. The study also reported increased odds of wheezing attacks [50].
The role of VOCs on the incidence of asthma remains unclear due to the conflicting data produced by previously conducted studies. Norback et al. demonstrated that exposure to VOCs produced asthma-like symptoms; however, concrete data confirming an association with a diagnosis of asthma through pulmonary function tests and methacholine challenge testing was not seen [51]. A population-based case-control study by Rumchev et al. with children 6 months to 3 years of age found that exposure to VOCs was an independent risk factor for the development of asthma [52]. They also showed that for every ten unit increase of toluene and benzene, both of which fall under the umbrella of VOCs, children had an increased risk of two- and threefold, respectively, for the development of asthma [52]. Other studies, however, did not report statistically significant associations between the development of asthma and indoor exposure to VOCs [53, 54].
Formaldehyde is primarily an indoor air pollutant emitted as a gas from urea. Formaldehyde resins are used as preservatives in decorative laminates, adhesives, foam in building material and household products, cotton and fiber blends, and electrical equipment [55]. The World Health Organization limits indoor levels of formaldehyde to 100 μg/m3 due to its effects on human health. The Occupational Safety and Health Administration (OSHA) cautions against the respiratory effect of formaldehyde since concentrations above 1 ppm can lead to upper airway irritation, and when above 50 ppm, can lead to bronchial irritation and pulmonary edema [56]. Studies have shown that indoor concentration of formaldehyde can range between 9.6 and 90 μg/m3, and some cities in Hong Kong have recorded levels greater than the recommended 100 μg/m3 threshold [57, 58].
Multiple studies have been carried out in order to determine the effects of formaldehyde on respiratory health. In children, formaldehyde levels > 0.06 mg/m3 have been associated with an increased risk of asthma development, but at levels of 0.1 mg/m3, this association was not seen [59]. This study showed that even at low levels of formaldehyde, i.e., less than 100 μg/m3, those with asthma and known dust mite allergy had increased bronchial responsiveness to mite allergen [59].
Phthalates are used to make plastic materials for improved malleability and can be found in some polyvinyl chloride products. They are also used as solvents in vinyl flooring, adhesives, detergents, plastic clothes, and personal-care products [60]. Indoor concentration of phthalates has been reported to be ten times higher indoors than they are outdoors [61]. A number of case reports have described the incidence of occupational asthma in people exposed to phthalates. An association has also been seen in children exposed to indoor phthalates, as reported by multiple studies linking high levels of fractional exhaled nitric oxide, a marker of pulmonary inflammation associated with asthma, with the presence of high levels of urinary phthalate metabolites [62–64]. It is unclear, however, if the pulmonary inflammation can be linked immediately to phthalates, or if exposure to phthalates is an independent factor in the development of asthma.
Role of Air Pollution on the Development of Aeroallergen Sensitization and Atopic Dermatitis
In addition to asthma, a number of studies have looked into the relationship between TRAP exposure and the development of atopic dermatitis and aeroallergen sensitization. Gruzieva et al. found that children in the BAMSE cohort exposed to nitrogen oxides and PM10 in the first year of life had an increased risk of pollen sensitization at 4 years of age (odds ratio, 1.83; 95% confidence interval 1.02–3.28), though this did not hold true at 8 years of age [65]. Codispoti et al. showed similar findings in that the risk of early aeroallergen sensitization was enhanced by DEP exposure, and the aeroallergen wheal area noted at 2 and 3 years of age was associated with allergic rhinitis by the age of 4 [66]. Another study explored the relationship between an increased risk for atopic disease and living near a main road [67]. It was found that children who lived within 75 m of a main road were at an increased risk of lifetime allergic rhinitis. Also, the distance to the main road, the length of the main road, and the proportion of the main road area were associated with allergic sensitization.
Along with allergic rhinitis, recent evidence has suggested that air pollutants may be risk factors for the development of atopic dermatitis due to their possible ability to induce oxidative stress in the skin and cause barrier dysfunction. Lee et al. found that following adjustment for possible confounders, flexural eczema was associated with nitrogen oxides and carbon monoxide in school-aged Taiwanese children going to school within 2 km of 55 stations [68]. Another study that utilized a dispersion model determined that eczema symptoms in 9–11-year-old children were associated with exposure to 3-year averaged concentrations of CO, NOx, PM10, and benzene [69]. Two other studies done in birth cohorts in Munich demonstrated strong positive associations between eczema and distance to main roads [70].
It should be noted that while there seems to be a great amount of evidence supporting the development or aggravation of atopic disease with air pollutants, definitive statements cannot necessarily be made. The small number of studies, inherent challenges in study design, varying definitions of TRAP and the diseases in question, differing methods of exposure assessment and health outcomes, the presence of confounding variables (e.g., obesity, genetics, and comorbidities), and other factors all present limitations in making a firm conclusion about the causative link between air pollution and atopic disease.
Current Recommendations Regarding Travel to Areas of High Pollution
The current literature does not support a definitive causative role for traffic-related air pollution on the development of asthma; however, potential hazards of high air pollution levels on health remain a concern. The most notable indication of public concern regarding this issue was in relation to athletes and their performance levels during the 2008 Beijing Olympics. Beijing’s environment authorities took swift action prior to the opening ceremony to decrease the levels of air pollution in order to meet national air quality standards. They focused their efforts on decreasing vehicle emissions and industrial emissions, as they are the major contributors to poor air quality recorded in the city. Pressure from international communities and athletes forced authorities to impose a 30% reduction in industrial emissions by closing down major industries and pulling 1.3 million cars off the roads every day prior to the event [71].
The CDC advises travelers to familiarize themselves with the air quality of their destination, especially those with preexisting pulmonary or cardiovascular disease, children, and the elderly, as these groups are the most susceptible to poor air quality [72]. The CDC recommends limiting exertion and time spent outdoors in cities with high levels of air pollution. The use of face masks is also encouraged, though they do not make final recommendations on the matter due to lack of evidence that the use of regular face masks will provide protection against traffic-related air pollution [72]. The use of valved dust respirators has been shown to decrease the effects of air pollution on heart rate and blood pressure in a small study conducted in Beijing [72]. The US Environmental Protection Agency recommends checking the level of air pollution at travel destinations, as well as the day-to-day levels of air pollution in hometowns using the airnow.gov website. The quality of air can be assessed using Fig. 2 [73]. They deem an air pollution index of 150 as unhealthy. At that level, the EPA concurs with the CDC’s recommendation to limit excessive and prolonged outdoor activity [73].
Conclusion
The current state of literature indicates that the development of asthma, allergic rhinitis, and atopic dermatitis from exposure to traffic-related air pollutants remains inconclusive. The reason for this may be attributed to a number of factors related to study design. In some of the reported studies, participants were assessed for brief periods of time that were not sufficient to observe the development of these conditions. An absence of correlation could also be secondary to low levels of pollutant concentrations in the areas assessed, thus not reaching critical levels as defined by the World Health Organization. It should also be noted that in younger age groups, it is often difficult to accurately diagnose asthma, as case definitions may vary among researchers. Also, the number of studies looking specifically at allergic rhinitis and atopic dermatitis is much smaller. This review highlights the need for further research to understand the potential role of air pollutants on atopic disease.
Acknowledgments
Funding This study was funded by the NIAID Allergy Training Grant T32 AI060515 and NIEHS Training Grant T32 ES010957-16.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical Approval This article does not contain any studies with human participants or animals performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.USEPA (2016) NArAQS table. Usepa https://www.epa.gov/criteria-air-pollutants/naaqs
- 2.Air quality | History of air pollution in the UK. http://www.air-quality.org.uk/02.php. Accessed 7 Feb 2018
- 3.Stern AC History of air pollution legislation in the United States. J Air Pollut Control Assoc 32(1):44–61 [DOI] [PubMed] [Google Scholar]
- 4.Safety and health topics | Indoor air quality | Occupational safety and health administration. https://www.osha.gov/SLTC/indoorairquality/. Accessed 7 Feb 2018
- 5.Greenbaum D. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Presented at the Clean Air Act Advisory Committee. 2009. Crystal City, VA [Google Scholar]
- 6.Products - Data briefs - Number 10 - October 2008. https://www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed 5 Dec 2017
- 7.Peterson B, Saxon A (1996) Global increases in allergic respiratory disease: the possible role of diesel exhaust particles. Ann Allergy Asthma Immunol 77:263–270. 10.1016/S10811206(10)63318-2 [DOI] [PubMed] [Google Scholar]
- 8.Favarato G, Anderson HR, Atkinson R, Fuller G, Mills I, Walton H (2014) Traffic-related pollution and asthma prevalence in children. Quantification of associations with nitrogen dioxide. Air Qual Atmos Health 7:459–166. 10.1007/sll869-014-0265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoek G, Beelen R, de Hoogh K, Vienneau D, Gulliver J, Fischer P, Briggs D (2008) A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos Environ 42:7561–7578. 10.1016/J.ATMOSENV.2008.05.057 [DOI] [Google Scholar]
- 10.Ryan PH, LeMasters GK (2007) A review of land-use regression models for characterizing intraurban air pollution exposure. Inhal Toxicol 19(Suppl 1): 127–133. 10.1080/08958370701495998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki S, Takizawa H, Takami K, Desaki M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka M, Sagai M, Ohtoshi T (2001) Benzene-extracted components are important for the major activity of diesel exhaust particles. Am J Respir Cell Mol Biol 24:419–126. 10.1165/ajrcmb.24.4.4085 [DOI] [PubMed] [Google Scholar]
- 12.Takano H, Yoshikawa T, Ichinose T et al. (1997) Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med 156:36–42. 10.1164/ajrccm.156.1.9610054 [DOI] [PubMed] [Google Scholar]
- 13.Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A (1995) Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J Allergy Clin Immunol 95:103–115 [DOI] [PubMed] [Google Scholar]
- 14.Fernvik E, Scharnweber T, Knopp D, Niessner R, Vargaftig BB, Peltre G (2002) Effects of fractions of traffic particulate matter on Th2-cytokines, IgE levels, and bronchial hyperresponsiveness in mice. J Toxicol Environ Health A 65:1025–1045. 10.1080/152873902760125200 [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A (1999) Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 104:1183–1188. 10.1016/S0091-6749(99)70011-4 [DOI] [PubMed] [Google Scholar]
- 16.Salvi S, Blomberg A, Rudell B et al. (1999) Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 159:702–709. 10.1164/ajrccm.159.3.9709083 [DOI] [PubMed] [Google Scholar]
- 17.Salvi SS, Nordenhall C, Blomberg A et al. (2000) Acute exposure to diesel exhaust increases IL-8 and GRO-alpha production in healthy human airways. Am J Respir Crit Care Med 161:550–557. 10.1164/ajrccm.161.2.9905052 [DOI] [PubMed] [Google Scholar]
- 18.Nightingale JA, Maggs R, Cullinan P et al. (2000) Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med 162:161–166. 10.1164/ajrccm.162.1.9908092 [DOI] [PubMed] [Google Scholar]
- 19.Sehlstedt M, Behndig AF, Boman C, Blomberg A, Sandström T, Pourazar J (2010) Airway inflammatory response to diesel exhaust generated at urban cycle running conditions. Inhal Toxicol 22: 1144–1150. 10.3109/08958378.2010.529181 [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Deng F, Guo X, Lv P, Zhong M, Liu C, Wang A, Tzan K, Jiang SY, Lippmann M, Rajagopalan S, Qu Q, Chen LC, Sun Q (2012) Association of systemic inflammation with marked changes in particulate air pollution in Beijing in 2008. Toxicol Lett 212:147–156. 10.1016/j.toxlet.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajekar R (2007) Environmental factors and developmental outcomes in the lung. Pharmacol Ther 114:129–145. 10.1016/j.pharmthera.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 22.LeMasters G, Levin L, Bernstein DI, Lockey SDIV, Lockey JE, Burkle J, Khurana Hershey GK, Brunst K, Ryan PH (2015) Secondhand smoke and traffic exhaust confer opposing risks for asthma in normal and overweight children. Obesity 23:32–36. 10.1002/oby.20941 [DOI] [PubMed] [Google Scholar]
- 23.Dong GH, Qian Z, Liu M-M, Wang D, Ren WH, Fu Q, Wang J, Simckes M, Ferguson TF, Trevathan E (2013) Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the seven northeastern cities study. Int J Obes 37:94–100. 10.1038/ijo.2012.125 [DOI] [PubMed] [Google Scholar]
- 24.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, Khurana Hershey GK, Levin L, Grinshpun SA, LeMasters G (2015) Timing and duration of traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am J Respir Crit Care Med 192:421–427. 10.1164/rccm.201407-1314OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M (2010) Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect 118: 284–290. 10.1289/ehp.0900916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon Hsu H-H, Mathilda Chiu Y-H, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ (2015) Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med 192:1052–1059. 10.1164/rccm.201504-0658OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B (2010) Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med 181:596–603. 10.1164/rccm.200906-0858OC [DOI] [PubMed] [Google Scholar]
- 28.Gehring U, Beelen R, Eeftens M, Hoek G, de Hoogh K, de Jongste JC, Keuken M, Koppelman GH, Meliefste K, Oldenwening M, Postma DS, van Rossem L, Wang M, Smit HA, Brunekreef B (2015) Particulate matter composition and respiratory health: the PIAMA birth cohort study. Epidemiology 26:300–309. 10.1097/EDE.0000000000000264 [DOI] [PubMed] [Google Scholar]
- 29.Gruzieva O, Bergström A, Hulchiy O, Kull I, Lind T, Melén E, Moskalenko V, Pershagen G, Bellander T (2013) Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology 24:54–61. 10.1097/EDE.0b013e318276c1ea [DOI] [PubMed] [Google Scholar]
- 30.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M (2011) Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med 68:291–295. 10.1136/oem.2010.055152 [DOI] [PubMed] [Google Scholar]
- 31.Fuertes E, Standl M, Cyrys J, Berdel D, von Berg A, Bauer CP, Krà ¤mer U, Sugiri D, Lehmann I, Koletzko S, Carlsten C, Brauer M, Heinrich J (2013) A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ 1:e193. 10.7717/peerj.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, Fuertes E, Gruzieva O, Heinrich J, Hoffmann B, de Jongste JC, Klümper C, Koppelman GH, Korek M, Krämer U, Maier D, Melén E, Pershagen G, Postma DS, Standl M, von Berg A, Anto JM, Bousquet J, Keil T, Smit HA, Brunekreef B (2015) Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 3:933–942. 10.1016/S2213-2600(15)00426-9 [DOI] [PubMed] [Google Scholar]
- 33.Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Künzli N, Avol E, Gilliland F, Lurmann F, Molitor JN, Molitor JT, Thomas DC, Peters J, McConnell R (2008) Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect 116:1433–1438. 10.1289/ehp.10968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krämer U, Sugiri D, Ranft U, Krutmann J, von Berg A, Berdel D, Behrendt H, Kuhlbusch T, Hochadel M, Wichmann HE, Heinrich J, GINIplus and LISAplus study groups (2009) Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J Dermatol Sci 56:99–105. 10.1016/j.jdermsci.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 35.Lindgren A, Stroh E, Björk J, Jakobsson K (2013) Asthma incidence in children growing up close to traffic: a registry-based birth cohort. Environ Health 12:91. 10.1186/1476-069X-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, Avol E, Künzli N, Yao L, Peters J, Berhane K (2010) Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 118: 1021–1026. 10.1289/ehp.0901232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mölter A, Agius R, de Vocht F, Lindley S, Gerrard W, Custovic A, Simpson A (2014) Effects of long-term exposure to PM10 and NO2 on asthma and wheeze in a prospective birth cohort. J Epidemiol Community Health 68:21–28. 10.1136/jech-2013-202681 [DOI] [PubMed] [Google Scholar]
- 38.Mölter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, de Jongste J, de Vocht F, Fuertes E, Gehring U, Gruzieva O, Heinrich J, Hoek G, Hoffmann B, Klümper C, Korek M, Kuhlbusch TAJ, Lindley S, Postma D, Tischer C, Wijga A, Pershagen G, Agius R (2015) A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J 45:610–624. 10.1183/09031936.00083614 [DOI] [PubMed] [Google Scholar]
- 39.Morgenstern V, Zutavem A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J (2007) Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med 64:8–16. 10.1136/oem.2006.028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J (2008) Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 177:1331–1337. 10.1164/rccm.200701-036OC [DOI] [PubMed] [Google Scholar]
- 41.Oftedal B, Nystad W, Brunekreef B, Nafstad P (2009) Long-term traffic-related exposures and asthma onset in schoolchildren in Oslo, Norway. Environ Health Perspect 117:839–844. 10.1289/ehp.11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T-J, Wu C-F, Chen B-Y, Lee YL, Guo YL (2016) Age of asthma onset and vulnerability to ambient air pollution: an observational population-based study of adults from Southern Taiwan. BMC PulmMed 16:54. 10.1186/s12890-016-02180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro-Giner F, Künzli N, Jacquemin B, Forsberg B, de Cid R, Sunyer J, Jarvis D, Briggs D, Vienneau D, Norback D, González JR, Guerra S, Janson C, Antó JM, Wjst M, Heinrich J, Estivill X, Kogevinas M (2009) Traffic-related air pollution, oxidative stress genes, and asthma (ECHRS). Environ Health Perspect 117:1919–1924. 10.1289/ehp.0900589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindgren A, Stroh E, Montnémery P, Nihlén U, Jakobsson K, Axmon A (2009) Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int J Health Geogr 8:2. 10.1186/1476-072X-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindgren A, Stroh E, Nihlén U, Montnémery P, Axmon A, Jakobsson K (2009) Traffic exposure associated with allergic asthma and allergic rhinitis in adults. A cross-sectional study in southern Sweden. Int J Health Geogr 8:25. 10.1186/1476072X-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Environmental Protection Agency (2015) Volatile organic compounds' impact on indoor air quality, https://www.epa.gov/indoorair-quality-iaq/volatile-organic-compounds-impact-indoor-airquality#Sources. Accessed 7 Jun 2018
- 47.Tunniclifie WS, Burge PS, Ayres JG (1994) Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet 344:1733–1736. 10.1016/S0140-6736(94)92886-X [DOI] [PubMed] [Google Scholar]
- 48.Van Winkle MR, Scheff PA (2001) INDOOR AIR Volatile organic compounds, polycyclic aromatic hydrocarbons and elements in the air of ten urban homes. Indoor Air C Indoor Air 11:49–64. 10.1034/j.1600-0668.2001.011001049.x [DOI] [PubMed] [Google Scholar]
- 49.Lin W, Bnmkeef B, Gehring U (2013) Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheezing in children. Int J Epidemiol 42(6):1724–1737. 10.1093/ije/dyt150 [DOI] [PubMed] [Google Scholar]
- 50.Arif AA, Shah SM (2007) Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health 80:711–719. 10.1007/s00420-007-0183-2 [DOI] [PubMed] [Google Scholar]
- 51.Norbäck D, Björnsson E, Janson C et al. (1995) Asthmatic symptoms and volatile organic compounds, formaldehyde, and carbon dioxide in dwellings. Occup Environ Med 52:388–395. 10.1136/OEM.52.6.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S (2004) Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 59:746–751. 10.1136/thx.2003.013680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nurmatov UB, Tagiyeva N, Semple S, Devereux G, Sheikh A (2015) Volatile organic compounds and risk of asthma and allergy: a systematic review. Eur Respir Rev 24:92–101. 10.1183/09059180.00000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smedje G, Norbäck D (2001) Incidence of asthma diagnosis and self-reported allergy in relation to the school environment–a four-year follow-up study in schoolchildren. Int J Tuberc Lung Dis 5:1059–1066 [PubMed] [Google Scholar]
- 55.US EPA, OAR,ORIA I Lead’s impact on indoor air quality https://www.epa.gov/indoor-air-quality-iaq/formaldehydes-impactindoor-air-quality. Accessed 13 Jun 2018
- 56.Medical surveillance - formaldehyde - 1910.1048 App C | Occupational safety and health administration. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10078. Accessed 13 Jun 2018 [Google Scholar]
- 57.Gilbert NL, Gauvin D, Guay M, Héroux MÈ, Dupuis G, Legris M, Chan CC, Dietz RN, Lévesque B (2006) Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ Res 102:1–8. 10.1016/j.envres.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 58.Guo H, Kwok NH, Cheng HR, Lee SC, Hung WT, Li YS (2009) Formaldehyde and volatile organic compounds in Hong Kong homes: concentrations and impact factors. Indoor Air 19:206–217. 10.1111/j.1600-0668.2008.00580.x [DOI] [PubMed] [Google Scholar]
- 59.Casset A, Marchand C, Purohit A, le Calve S, Uring-Lambert B, Donnay C, Meyer P, de Blay F (2006) Inhaled formaldehyde exposure: effect on bronchial response to mite allergen in sensitized asthma patients. Allergy Eur J Allergy Clin Immunol 61:1344–1350. 10.1111/j.1398-9995.2006.01174.x [DOI] [PubMed] [Google Scholar]
- 60.CDC (2009) Phthalates. Cent Dis Control:1–3
- 61.Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43:170–181. 10.1016/j.atmosenv.2008.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu NY, Lee CC, Wang JY, Li YC, Chang HW, Bornehag CG, Wu PC, Sundell J, Su HJ (2012) Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 22:186–199. 10.1111/j.1600-0668.2011.00753.x [DOI] [PubMed] [Google Scholar]
- 63.Bertelsen RJ, LØdrup Carlsen KC, Calafat AM et al. (2013) Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ Health Perspect 121:251–256. 10.1289/ehp.1205256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Just AC, Whyatt RM, Miller RL, Rundle AG, Chen Q, Calafat AM, Divjan A, Rosa MJ, Zhang H, Perera FP, Goldstein IF, Perzanowski MS (2012) Children’s urinary phthalate metabolites and fractional exhaled nitric oxide in an urban cohort. Am J Respir Crit Care Med 186:830–837. 10.1164/rccm.201203-0398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruzieva O, Bellander T, Eneroth K, Kull I, Melén E, Nordling E, van Hage M, Wickman M, Moskalenko V, Hulchiy O, Pershagen G (2012) Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol 129(1):240–246 [DOI] [PubMed] [Google Scholar]
- 66.Codispoti CD, LeMaster GK, Levin L et al. (2015) Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol 114(2):126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung D, Leem J, Kim H et al. (2015) Effect of traffic-related air pollution on allergic disease: results of the children’s health and environmental research. Allergy Asthma Immunol Res 7(4):359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YL, Su HJ, Sheu HM, Yu HS, Guo YL (2008) Traffic-related air pollution, climate and prevalence of eczema in Taiwanese school children. J Invest Dermatol 128(10):2412–2420 [DOI] [PubMed] [Google Scholar]
- 69.Penard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, Annesi-maesano I (2010) Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J 36:33–40 [DOI] [PubMed] [Google Scholar]
- 70.Morganstern V, Zutavern A, Cyrys J et al. (2008) Atopic diseases, allergic sensitizations, exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 177:1331–1337 [DOI] [PubMed] [Google Scholar]
- 71.China’s Olympian efforts to tackle air pollution - SciDevNet https://www.scidev.net/global/pollution/feature/china-s-olympian-effortsto-tackle-air-pollution.html. Accessed 7 Jun 2018
- 72.Environmental hazards - Chapter 2 – 2018 Yellow book | Travelers’ Chen CY,health | CDC. https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/environmental-hazards. Accessed 7 Jun 2018 [Google Scholar]
- 73.USEPA (2014) Air quality index (AQI). A guide to air quality your health 12