Abstract
Background:
The increasing incidence of pediatric food allergy results in significant health care burden and family stress. Oral immunotherapy (OIT) can induce tolerance to peanut, milk, and egg. OIT for other foods, particularly multiple foods simultaneously, has not been thoroughly studied.
Objective:
To summarize our experience with OIT for multiple foods in a pediatric allergy clinic setting.
Methods:
Medical records were reviewed for patients undergoing OIT for multiple foods. Methods and outcomes of OIT were summarized. Outcomes were analyzed for correlation with baseline food allergen skin prick tests (SPTs) and specific IgE (sIgE) test results.
Results:
Forty-five patients aged 1.5 to 18 years undertook OIT for up to 12 foods, including peanut, tree nuts, seeds, legumes, and egg. At the time of review, 35 patients were receiving daily maintenance dosing, 4 had completed OIT and were continuing to eat their foods 3 times weekly, and 6 had stopped OIT because of anxiety, inconvenience, or allergy symptoms. A total of 49% of patients had reactions during the up-dosing process, mostly oral itching (33%), perioral hives (40%), and abdominal pain (35%). There was no correlation of baseline skin prick test (SPT) and sIgE test results with reaction threshold for baseline food challenge, lowest dose causing reactions during up-dosing, or time to reach maintenance. Higher baseline sIgE level but not baseline SPT result was associated with an increased number of allergic reactions during OIT. Baseline SPT correlated with stopping OIT.
Conclusion:
A similar approach to that used for peanut OIT can be taken for nonpeanut foods and for multiple foods simultaneously. High baseline allergy test results are not a contraindication to OIT.
Introduction
The incidence of food allergy has increased in recent years.1,2 Until we have effective strategies for primary prevention, the role of the allergist is to help patients live safely with their food allergies. Avoidance is not always successful; 66% of patients reported a history of more than 5 reactions to their peanut or tree nut allergens/3,4 Food-induced anaphylaxis increases health care burden,5 and the stress of living with food allergies has a major effect on patient and family quality of life.6 The health-related quality of life is lower with multiple vs single food allergy and improves when suspected allergies are cleared through oral food challenges (OFCs).7 However, many patients have multiple confirmed food allergies.8,9 and the burden of avoidance is significant.
There is much interest among allergists, patients, and their families in finding safe and effective methods for food allergen desensitization. Studies have found that oral immunotherapy (OIT) for peanut is effective in increasing the threshold for reaction10 and, with long-term maintenance dosing, can lead to sustained unresponsiveness to peanut in a minority of patients.11,12 However, for patients who are allergic to multiple foods, OIT with only one food is not as helpful; they still need to restrict their diets. Epstein-Rigbi et al13 found lower health-related quality of life improvement with single-food OIT in patients with multiple food allergies. One effective approach for these patients may be to include multiple food allergens in their OIT.
To date, only 1 trial of multiple-food OIT without omalizumab pretreatment has been published: Bégin et al14 included up to 5 foods simultaneously for OIT in 25 patients, of whom 22 were able to reach daily doses of 10 times their original reaction thresholds for each food. Additional successful trials of multiple-food OIT have been performed in conjunction with omalizumab treatment.15–18 This article reports our clinical experience with multiple-food OIT without omalizumab treatment in a nonresearch pediatric allergy clinic setting during a 2-year period.
Methods
Electronic Medical Record Review
All patients of one of the authors (M.B.L.) undergoing OIT for multiple foods were identified by electronic medical record review, which was approved by the Cincinnati Children’s Hospital Institutional Review Board. Patients who were receiving daily maintenance dosing, patients who had successfully completed OIT, and those who had stopped OIT were included in this analysis. Patients who were still up-dosing were not included. Data extracted from the electronic medical record included food allergy history, other allergic conditions, baseline allergy test results, OFC results, OIT visits, and documented communications with the families between visits.
Emergency department (ED) visits for OIT-related allergic reactions were recorded for the patients undergoing OIT. Medical records of a control group of patients with food allergy who were accepted for the OIT program and were waiting for appointments to start OIT were also reviewed for the number of ED visits for food allergy reactions during the preceding 18 months, which is the mean time patients spent up-dosing and on daily maintenance.
Inclusion Criteria
The diagnosis of each food allergy was based on 1 or more of the following: (1) convincing clinical history of immediate-type hypersensitivity reaction to the food, with positive skin prick test (SPT) and/or sIgE test results for the food; (2) reaction to OFC with the food; and (3) inclusion of additional foods in OIT based on allergy test results alone if the patient met criteria 1 and/or 2 for some foods. All patients had at least 1 food allergy confirmed by clinical reaction or OFC or by reaction during up-dosing.
Patients were selected for OIT based on the following criteria: (1) food allergies that were unlikely to resolve spontaneously, (2) patient and family motivated to participate in OIT and well organized enough to adhere to the protocol, and (3) no history of eosinophilic esophagitis (EoE) or symptoms suggestive of EoE (dysphagia, heartburn, vomiting or abdominal pain). Patients were not excluded from OIT based on large SPTs, high sIgE levels, or severity of initial allergic reactions to foods. Asthma control was optimized before initiating OIT.
OIT Initiation
The starting dose for OIT was based on the highest tolerated dose at OFC, if available. In cases with clinical history suggestive of a higher threshold for reaction and no OFC, a graded series of doubling test doses starting from tsp was given initially; the patient’s daily home dose started with the highest tolerated dose in clinic. Patients with a history of reacting to trace amounts of the food had initial test dosing starting at tsp of a 1:10 dilution of the food mixture (or in the case of peanut, with peanut powder diluted in water to give 1 mg/mL of peanut protein).
The parents purchased the foods and processed them at home if needed, per our instructions (eAppendix 1). Foods were brought to clinic the first OIT day and measured by allergy nurses with instructions to parents on how to measure the doses at home. Parents were provided with measuring spoon sets with increments down to tsp and/or syringes for measurement of liquid doses. Measurements were taken by volume (milliliters for liquids or fractions of teaspoons) rather than by weight because this was more practical for the parents to reproduce at home. eAppendix 2 gives the approximate milligram protein content for each food at a given volume measurement.
The OIT test doses were given in clinic, with injectable epinephrine and diphenhydramine oral suspension available; patients were monitored for 1 hour after the final dose. Once a safe starting dose was established, the patient was sent home with instructions to take this dose daily until the next visit. Up-dosing visits were at least 2 weeks and up to 4 weeks apart. At each visit, the dose was doubled, and the patient was observed in clinic for 1 hour after the dose and then sent home with the new dose.
Parent Instruction
Parents were advised to give OIT doses with a meal or snack at a time when (1) they would be available to monitor the child for 1 hour after the dose, (2) the child would not exercise or be in a hot environment (including hot bath or shower) just before or for 2 hours after the dose, and (3) the child was not sick with fever, malaise, or significant respiratory symptoms. Parents were instructed in management of OIT reactions at home, with appropriate doses of diphenhydramine (for mild itching or perioral hives) and ranitidine (for post-OIT abdominal pain or nausea) included in postvisit instructions. They were instructed to inject epinephrine if the patient had allergy symptoms that involved at least 2 organ systems, generalized hives, respiratory distress, or protracted abdominal symptoms. If epinephrine was administered, the patient was taken to the nearest ED immediately.
Adverse Events Monitoring
Adverse events were documented by parent report or observation during clinic visits and by telephone call or encrypted e-mail between visits. Parents were provided with the senior author’s e-mail address and cell phone number and were asked to report any reactions. If allergic symptoms occurred with a dose, the patient was treated with appropriate medications, and the dose was reduced to the previously tolerated dose, then advanced more slowly as tolerated. All dose escalations were done in the clinic.
OIT Maintenance
The daily maintenance dose was determined based on the number of foods included. For peanut, the most common maintenance dose was 2 tsp of peanut butter (2332 mg of peanut protein) or 10 peanuts daily (for preschool-aged children and those with strong aversion, 1 tsp peanut butter [1166 mg of peanut protein] or 5 peanuts daily). Peanut was often dosed separately. For multiple other foods, the maintenance dose was between 2 to 4 tsp of the mixture, determined by the patient’s willingness for daily ingestion.
At the first 6-month maintenance visit, patients were challenged with 2 to 4 times the maintenance dose to test their tolerance to larger amounts of the foods. Daily dosing was then continued with at least the original maintenance dose, but the patient could eat more of their OIT foods (up to the amount they had tolerated in clinic), if desired. At the 12-month maintenance visit, patients were challenged up to full servings of each food. SPTs and sIgE tests were repeated every 6 months after the maintenance dose was reached, until the SPT wheal was less than 8 mm and the sIgE level was less than 1 kUA/L and the patient was able to tolerate full servings of the food allergens; at this point, patients were advised they could decrease dosing of their allergenic foods to 2 to 3 times weekly. It was emphasized that the food allergies could recur if patients stopped eating the foods regularly.
Statistical Analysis
Descriptive analysis was reported as number (percentage) for categorical variables and median and (interquartile range) for continuous variables. Bivariate analysis (Spearman correlation) was used to assess ρ (correlation coefficient) and P value for baseline sIgE and baseline SPT diameter in millimeters with reaction threshold, lowest dose, and weeks to reach maintenance. Continuous outcomes (baseline sIgE test and SPT results) were compared with reaction ever and antihistamines used (yes/no) variables using nonparametric (Mann-Whitney U and Kruskal Wallis) tests. Paired t tests were used to assess differences in serum IgE levels and SPT results from baseline to 6 months, 6 to 12 months, and baseline to 12 months. The Fisher exact test was used to compare incidence of ED visits between patients undergoing OIT and wait-listed patients.
Statistical significance was determined at P < .05. Analysis was performed using SPSS software, version 21.0 (IBM Corp).
Results
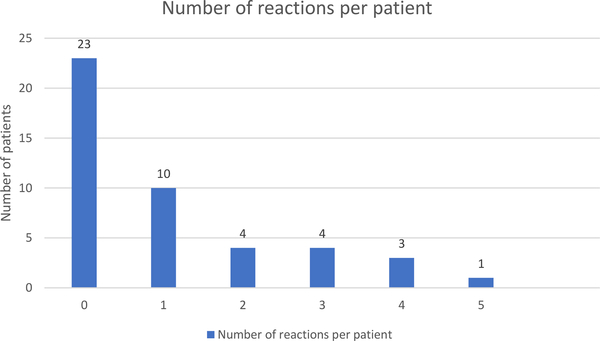
Forty-five patients were receiving daily maintenance or 2 to 3 times weekly ingestion of their food allergens or had stopped OIT at the time of this report. Demographic characteristics are given in Table 1. The foods included most often were peanut, tree nuts, and seeds (Table 2). Most patients (76%) were receiving OIT for 4 or fewer foods, although a few patients have more foods (up to 12) in their OIT mixture (Fig. 1). Thirty-five patients started OIT based on positive OFC results to some of or all their food allergens; 10 patients started therapy based on a history of clinical reaction to the food (ranging from 1 to 12 years before starting OIT) and still had positive allergy test results. Three of these 10 patients had allergic reactions during up-dosing. At the time of this submission, 35 patients are undergoing daily maintenance dosing; the mean time to reach maintenance was 24 weeks (range, 9–54 weeks). Four patients met the criteria for 3 times weekly maintenance dosing (after 6, 8, 10, and 24 months of daily maintenance for each patient, respectively). Six patients (13%) stopped OIT: 1 because of scheduling difficulty, 2 for anxiety and aversion to the foods, 1 for persistent oral allergy symptoms, and 2 because of reactions to the doses (recurrent abdominal pain in 1 and a single generalized urticarial reaction in 1).
Table 1.
Demographic Characteristics and Diagnoses
| Variable | Findinga |
|---|---|
| Age at start of OIT, median (range), y | 9.8 (1.5–18.7) |
| Sex | |
| Female | 20 (44) |
| Male | 25 (56) |
| Race/ethnicityb | |
| White | 41 (91) |
| Black | 3 (7) |
| Hispanic | 1 (2) |
| Asthma | |
| Mild intermittentc | 15 (33) |
| Mild persistent | 11 |
| Moderate | 3 |
| Severe | 1 |
| Allergic rhinitis | 37 (82) |
| Atopic dermatitis | 12 (27) |
Abbreviation: OIT, oral immunotherapy.
Data are presented as number (percentage) of patients unless otherwise indicated.
The racial mix in our area is 79% white, 12% black, 3% Hispanic, and 3% Asian (2017 US Census Report).
Asthma severity based on National Heart, Lung, and Blood Institute scoring.30
Table 2.
Foods Included in OIT and Baseline Allergy Test Results
| Food | No. of patients receiving OIT for each food | Baseline sIgE, median (range), kUA/La | Baseline SPT result, median (range) wheal diameter, mm |
|---|---|---|---|
| Cashew | 34 | 11.3 (0.2–75) | 17(5–32) |
| Pistachio | 34 | 13.9 (0.34–64.4) | 15 (5–41) |
| Walnut | 25 | 20.3 (0.2–64.5) | 11 (0–20) |
| Pecan | 25 | 7.6 (0b–42) | 10 (0–23) |
| Peanut | 20 | 35.8 (0.8–100b) | 16 (8–28) |
| Hazelnut | 13 | 13.1 (0.3–79.5) | 9(2–18) |
| Almond | 8 | 1.8 (0.31–6.64) | 5(0–13) |
| Brazil nut | 8 | 2.6 (0–8.8) | 5(0–16) |
| Sesame seed | 4 | 27.1 (1.2–71) | 11 (2–22) |
| Macadamia nut | 4 | 7.5 (2.7–21.1) | NA |
| Pine nut | 3 | 1.3 (0.7–2.5) | NA |
| Sunflower seed | 2 | 1.9 (0.7–3.2) | 7(3–12) |
| Soy | 1 | 2.5 | 0 |
| Coconut | 1 | 2.8 | NA |
| Pumpkin seed | 1 | 0.2 | NA |
| Poppyseed | 1 | 2.1 | NA |
| Egg | 1 | 20.7 | 15 |
| Flaxseed | 1 | 10 | NA |
| Fish (mix of cod, tilapia, and salmon) | 1 | Tilapia: 12.3; cod: 10.3; salmon: 8.12 | Cod: 20 |
Abbreviations: NA, not applicable; OIT, oral immunotherapy; sIgE, specific IgE; SPT, skin prick test.
Serum sIgE for the food (Phadia ImmunoCAP).
For analysis purposes, sIgE results reported as less than 0.09 kUA/L were assigned the value of 0, and results reported as greater than 100 kUA/L were assigned the value of 100.
Figure 1.
Number of foods included in oral immunotherapy per patient.
Allergic Reactions During OIT
Reactions occurred in 22 patients (49%) during up-dosing or in the first 3 months of maintenance dosing (Table 3) during clinic visits or with a home dose. No patients reported reactions after the first 3 months of daily maintenance. Twenty-three patients (51%) had no reaction during the OIT process,10 (22%) had 1 reaction, and 12 (27%) had 2 or more reactions (Fig. 2). Reactions were scored per eAppendix 3: 91% were mild (grade 1), 9% were moderate (grade 2), and there were no severe reactions (grade 3). The most common reactions were mouth and throat pruritus in 12 patients (27%) and hives or abdominal pain in 10 patients each (22%). Most of these reactions resolved with H1 antihistamines for rash/oral pruritus and/or H2 antihistamine for abdominal pain. Three reactions were treated with injected epinephrine and 1 with albuterol. These medications were given at home, and the patients were sent to the emergency department. Twenty-nine patients (64%) required no medication for allergy symptoms, 11 (24%) took an H1 antihistamine, 8 (18%) took an H2 antihistamine, and 3 (7%) took both.
Table 3.
Number and Type of Reactions at Each Oral Immunotherapy Dosea
| Dose, tsp | No. of patients receiving each dose | No. of reactions |
No. of patients reactingb | Grade 1 reactionc | Grade 2 reaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Skin | Mouth and throat | Abdominal | Cardiovascular | Chest | |||||
| 7 | 3 | 0 | 1 | 0 | 0 | 3 | 3 | 0 | |
| 31 | 5 | 1 | 3 | 0 | 0 | 9 | 8 | 0 | |
| 33 | 2 | 2 | 1 | 0 | 0 | 5 | 4 | 1 | |
| 38 | 0 | 1 | 5 | 0 | 0 | 5 | 5 | 0 | |
| ⅛ | 41 | 2 | 1 | 0 | 0 | 0 | 3 | 2 | 1 |
| ¼ | 41 | 0 | 1 | 2 | 1 | 0 | 4 | 4 | 0 |
| ½ | 41 | 1 | 2 | 1 | 0 | 0 | 3 | 3 | 0 |
| 1 tsp | 41 | 2 | 4 | 3 | 0 | 0 | 10 | 9 | 1 |
| 1.5 tsp | 4 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 2 tsp | 38 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 0 |
| 3 tsp | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 4 tsp | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total reactions | 18 | 15 | 16 | 1 | 1 | 46d | 41 | 4 | |
Only doses that were associated with reactions are shown; there were several additional doses of diluted peanut, tree nuts, or sesame to which none of the patients had reactions.
Not every patient received every dose (some started with higher doses based on tolerance at oral food challenge, some stopped oral immunotherapy before reaching higher doses, and many stopped with 2 tsp as their maintenance dose).
Grade 1 reactions were of mild severity; grade 2 were moderate. The scoring system used for grading reaction severity is given in eAppendix 3.
Some patients reacted to more than one dose; some reactions involved more than one symptom. Only 22 patients had any reaction to oral immunotherapy; 23 patients had no reactions.
Figure 2.
Number of food allergy reactions during oral immunotherapy per patient.
Oral allergy symptoms improved when the food allergens were taken with other foods and/or with drinking fluids immediately after the dose. Urticarial reactions were noted to occur when the patient exercised or took a hot shower within 2 hours of dosing and rarely occurred when exercise and heat were avoided. Mild perioral rashes were often seen in young children who had perioral skin contact with foods and could be prevented by careful feeding and immediate wiping of the mouth.
All patients with abdominal pain had resolution of this problem by the time they reached their maintenance dose, except for 1 patient who stopped OIT because of ongoing problems with nausea and abdominal pain at low doses.
ED Visits During OIT
Our 45 patients had 4 ED visits for food allergy reactions during OIT compared with 7 in the 44 wait-listed patients during a similar period of 18 months (Table 4 and Table 5). In each case of the 4 patients undergoing OIT, their parents gave appropriate treatment at home. All patients experienced symptom resolution by the time of arrival to the ED, although 1 patient had a relapse of mild urticaria 2 hours later and was observed in the ED for 6 hours.
Table 4.
Characteristics of ED Patients Undergoing OIT vs a Control Group of Patients on the Waiting List to Start OIT During a Similar Period of 18 Months
| Characteristic | OIT patients (n = 45) | Wait-list patients (n = 44) |
|---|---|---|
| Age, mean (range), y | 9.8 (1.5–18.7) | 8.3 (1.2–18.6) |
| Male sex, % | 58 | 43 |
| Race, %a | ||
| White | 91 | 84 |
| Black | 7 | 11 |
| Hispanic | 2 | 2 |
| Asian | 2 | |
| Food allergy reactions with ED visit, No. (%) | 4(11) | 7 (16)b |
| Epinephrine doses given, No. (%) | 3(7) | 5(11) |
Abbreviations: ED, emergency department; OIT, oral immunotherapy.
The racial mix in our area is 79% white, 12% black, 3% Hispanic, and 3% Asian (2017 US Census Report).
P = .35 (Fisher’s exact test).
Table 5.
ED Visits for Food Allergy During OIT
| Patient No. | No. of ED visits during OIT | Dose/food | Symptoms | Treatment at home | Treatment in ED | Status on arrival in ED |
|---|---|---|---|---|---|---|
| 13 | 1 | 1 tsp mixed C/Pi | OAS | Epi, DP | Pred | Symptoms resolved |
| 15 | 1 | 8 PN, 8 Pi, 4 C | OAS, N, W, U | Alb, DP | SM | Symptoms resolved |
| 28 | 1 | 1/8 tsp mixed A, B, C, Coc, PineN, Mac, Pi, H, WN, Pe | N, U | Epi, DP | DP | Symptoms resolved on arrival; relapse of mild hives 2 hours later |
| 36 | 1 | 1/32 tsp C, Pi | OAS, U | Epi, DP | None | Symptoms resolved |
Abbreviations: A, almond; Alb, albuterol aerosol; B, Brazil nut; C, cashew; Coc, coconut; DP, diphenhydramine orally; ED, emergency department; Epi, epinephrine injection subcutaneously; H, hazelnut; Mac, macadamia nut; N, nausea or abdominal pain; OAS, oral allergy symptoms (includes itching of mouth or throat, perioral rash); OIT, oral immunotherapy; Pe, pecan; Pi, pistachio; PineN, pine nut; PN, peanut; Pred, prednisone or prednisolone orally; SM, Solu-Medrol intravenously; WN, walnut; W, wheezing; U, urticaria.
Changes in Allergy Test Results During OIT
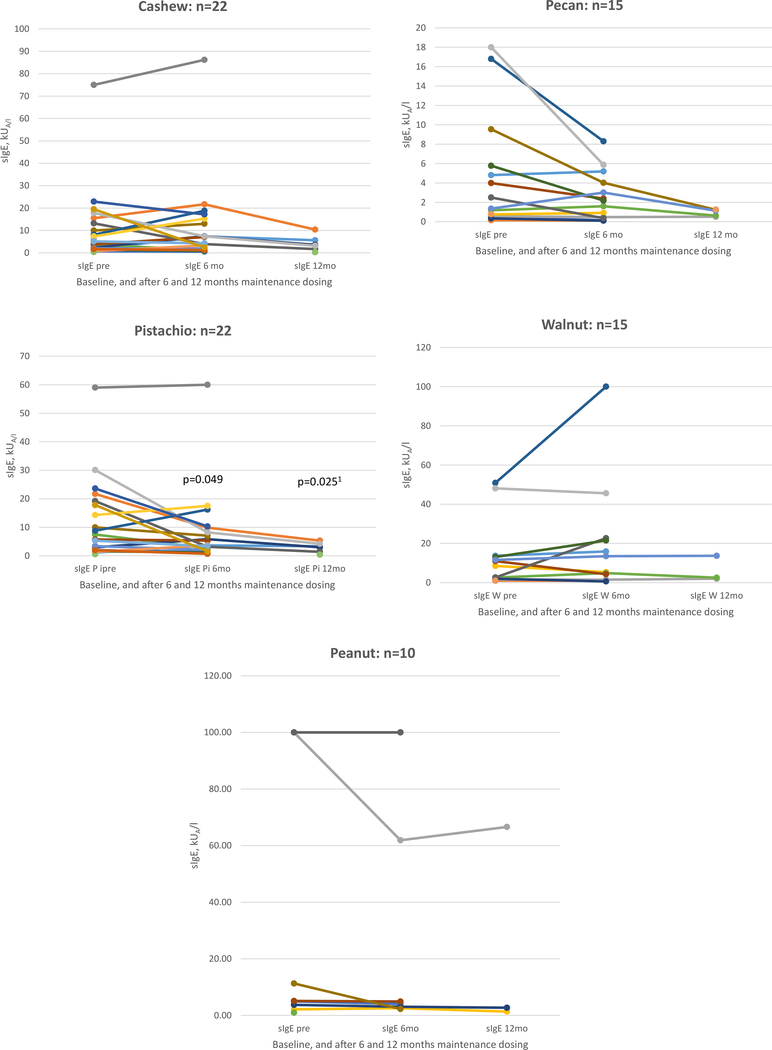
Additional sIgE test and SPT results after 6 and 12 months of maintenance dosing were available for a subset of the patients (Fig. 3 and Fig. 4). These test results indicate a decrease in SPT size after the first 6 months of maintenance dosing; the sIgE levels typically decreased after 12 months of maintenance dosing.
Figure 3.
Changes in serum specific IgE (sIgE) levels during oral immunotherapy. Data are shown only for those patients who had data available from baseline through 6 or 12 months of maintenance dosing for the most commonly included foods. Changes in sIgE are not statistically significant except as marked. 1P value is shown for the change in pistachio sIgE from 6 months to 12 months; the change from baseline to 12 months was not statistically significant.
Figure 4.
Changes in skin prick test (SPT) results during oral immunotherapy. Data are shown only for those patients who had data available from baseline through 6 or 12 months of maintenance dosing for the most commonly used foods. Changes are not statistically significant except as marked. 1P value is shown for comparison of baseline and 12 months; P value for comparison of 6 months to 12 months was not significant.
Correlations of Baseline Allergy Test Results With Outcomes of OIT
Table 6 gives the correlations between baseline SPT and sIgE test results and OIT outcomes for the most commonly included foods. Correlations were found for some foods between baseline allergy test results and threshold for reacting to OFC, lowest dose causing an allergic reaction during up-dosing, time to reach maintenance dose, and need for antihistamine treatment. The results of baseline sIgE tests and SPTs for pecan, pistachio, and hazelnut (data not shown) indicated no significant correlations with clinical outcomes, except for correlation of pecan sIgE and SPT with OFC and OIT reaction thresholds.
Table 6.
Correlations Between OIT Outcomes: Baseline sIgE Test and SPT Resultsa
| Variable | R or pre-OIT sIgE, median (IQR), kUA/L | P value | No. of patients | R or pre-OIT SPT result, mean wheal diameter, mm | P value | No. of patients |
|---|---|---|---|---|---|---|
| Peanut (n = 20) | ||||||
| OFC reaction thresholda | −0.18 | .62 | 10 | −0.873 | .005 | 8 |
| OIT lowest reaction doseb | −0.416 | .31 | 8 | −0.395 | .33 | 8 |
| Weeks to reach maintenance | −0.093 | .75 | 14 | −0.168 | .60 | 12 |
| Reaction ever, yes | 36.3 (2.7–98.1) | 1 | 9 | 18 (9.7–26.5) | 1 | 8 |
| Reaction ever, no | 31.8 (4.6–92.5) | 11 | 16(12.1–20.0) | 10 | ||
| Medication needed for OIT, yes | 60.5 (l1.7–100) | 0.237 | 7 | 20(18–26) | 0.046 | 5 |
| Medication needed for OIT, no | 11.3 (2.2–90.1) | 15 | 13 (10–19.5) | 13 | ||
| Cashew (n = 34) | ||||||
| OFC reaction threshold | −0.62 | 0.004 | 20 | −0.147 | 0.537 | 20 |
| OIT lowest reaction dose | −0.381 | 0.146 | 16 | 0.161 | 0.55 | 16 |
| Weeks to reach maintenance | 0.247 | 0.224 | 26 | 0.435 | 0.034 | 24 |
| Reaction ever, yes | 7.2 (2.2–14.9) | 0.347 | 16 | 19.5 (12.5–25) | 0.11 | 16 |
| Reaction ever, no | 3.0(1.2–17.9) | 18 | 15 (9–17) | 17 | ||
| Medication needed for OIT, yes | 7.0 (1.3–9.9) | 0.77 | 11 | 18 (14–23) | 0.269 | 12 |
| Medication needed for OIT, no | 3.8 (1.6–17.8) | 23 | 15 (10–23) | 21 | ||
| Walnut (n = 25) | ||||||
| OFC reaction threshold | −0.813 | <0.001 | 16 | −0.747 | 0.001 | 16 |
| OIT lowest reaction dose | −0.694 | 0.009 | 13 | −0.612 | 0.026 | 13 |
| Weeks to reach maintenance | 0.137 | 0.576 | 19 | 0.163 | 0.517 | 18 |
| Reaction ever, yes | 13.7 (2.0–44.0) | 0.65 | 13 | 13 (8.0–18.0) | 0.034 | 13 |
| Reaction ever, no | 7.53 (1.9–33.8) | 12 | 5.0 (3.5–10) | 12 | ||
| Medication needed for OIT, yes | 15.3 (1.8–42.7) | 0.537 | 10 | 13 (7.7–17.7) | 0.053 | 10 |
| Medication needed for OIT, no | 11 (1.8–38.3) | 17 | 6.5 (3.2–12.5) | 16 |
Abbreviations: IQR, interquartile range; OIT, oral immunotherapy; sIgE, specific IgE; SPT, skin prick test.
Analysis was performed for those foods with the largest number of OIT participants; only one each of the cross-reactive nuts (cashew representing cashew and pistachio; walnut representing walnut and pecan) is shown.
Discussion
OIT is an emerging treatment for food allergies, and there is much to be learned about this process. Our experience with multiple-food OIT in a clinical setting demonstrates that it is feasible and reasonably safe compared with multiple-food avoidance. Most patients can reach a maintenance dose that gives them a safety margin against reactions to unintentional ingestion of the food. After 6 to 12 months of this maintenance dose, patients tolerate full servings of the foods and can have an unrestricted diet, although they are still at risk for relapse of the allergy if they stop eating the foods daily. Four of our patients have reached the point of 3 times weekly ingestion of the food to maintain long-term tolerance. This recommendation is based on the patient tolerating full servings of the food for several months, a skin test wheal smaller than 8 mm, and an sIgE level of less than 1 kUA/l. These parameters are arbitrary, but of the 4 patients described in this report and others undergoing single-food OIT in our practice who have met these criteria, none have had relapse of their food allergies.
The OIT regimen described here was tailored to the individual patient for practical reasons. Starting doses could be higher for patients who had demonstrated tolerance to the higher dose at OFC, thus decreasing the number of up-dosing visits required to reach maintenance dosing. The final maintenance dose was also individually determined based on the number of food allergens included in the OIT and the child’s tolerance for daily ingestion of the foods. Dose-response studies have not been performed for most foods. Bègin et al14 used a target maintenance dose of 4000 mg of each food protein, roughly equivalent to 3.5 tsp of peanut butter or other nut butter, which would be challenging as a daily maintenance dose for a child taking several foods. Nachson et al12 compared adherence and efficacy of 3000 mg and 1200 mg of peanut protein as a maintenance dose for peanut OIT and found that adherence was significantly better with the lower dose, whereas efficacy was similar. Vickery et al19 also found that a lower maintenance dose of 300 mg of peanut protein was as effective as 3000 mg for achieving peanut desensitization in preschool children. In our practice, we aim for the highest dose of each food that the child is willing to eat daily on a long-term basis. As we gather more long-term data, comparisons of outcomes with different maintenance doses of each food will become possible.
Some nuts, such as cashew/pistachio and walnut/pecan, are cross-reactive and may be cross-desensitizing. We elected to include all nuts indicated by each patient’s sensitization and reaction history because complete cross-desensitization is not guaranteed.
Most of the allergic reactions experienced by our patients were enteric local reactions (oral itching, perioral rashes, or transient abdominal pain). Systemic allergic reactions were rare, and none were severe. Only 1 episode of wheezing occurred among our patients; however, our patients’ asthma was mostly mild (Table 1), and their asthma was well controlled. Mild local reactions usually responded well to administration of H1 antihistamines for oral or skin symptoms, and/or H2 antihistamines for stomach symptoms. None of our patients reported ongoing problems with allergic reactions after the first 3 months of daily maintenance dosing. Other studies have also noted that reactions to OIT are more common during up-dosing than during maintenance.19,20–22 In our cohort, frequency of ED visits during OIT was not significantly different than the frequency of ED visits for unintentional food allergen ingestion in a similar cohort of patients on the waiting list to start OIT during a similar timeframe. High positive sIgE levels or large SPT reactions do not preclude a patient participating in OIT, although patients with higher baseline allergy test results might have lower reaction thresholds and take longer to reach maintenance dosing than patients with lower test results.
There are several limitations to this study. These data were collected from a clinical practice not a controlled clinical trial. There was less rigorous standardization compared with a research trial. There was no blinding or control group. Our results cannot be directly compared with those of published research trials of OIT.
OFCs were not done for all the foods included in the OIT mixes, so we do not know the baseline reaction threshold for each food. Every patient had a positive OFC result or a convincing clinical reaction history for at least some of the foods, although in some cases the clinical reaction was up to 12 years before the onset of OIT; it is possible that some of these food allergies had resolved spontaneously. Multiple OFCs are expensive and time consuming, so some families preferred to include additional foods in the OIT without OFC confirmation of all food allergens.
There is selection bias in our patient population. Our patients and families self-selected by their level of motivation and desire for OIT and were also informally screened by the medical staff to determine that they were reliable and well organized. This regimen is demanding: it places significant responsibility on the caregivers and the allergic patient to maintain the regular daily dosing, prepare the food allergen doses correctly, and keep the up-dosing appointments. OIT is not appropriate or necessary for all patients with food allergy. Some patients are comfortable living with their food allergies and do not feel the need to undergo OIT. In other families, the caregivers are too busy or not sufficiently competent to safely and consistently administer the OIT doses at home.
There is also a question of how consistent the doses are because families prepare doses themselves. However, use of a compounding pharmacy to prepare doses adds significant costs, inconvenience, and complications. We had similar rates of adverse reactions compared with OIT in studies using standardized food doses prepared by research pharmacies14,19–22; thus, the potential dosing variability did not appear to be clinically detrimental for our patients.
The risk of developing EoE is a concern in the implementation of OIT. Studies have documented development or exacerbation of previously unrecognized EoE in patients undergoing OIT for peanut23,24 or other foods.25 The typical symptom pattern for these patients is abdominal pain, nausea, or vomiting unrelated to the timing of the food allergen dosing, occurring randomly throughout the day. Typically, the gastrointestinal symptoms resolve when OIT is stopped. This symptom pattern is different from that reported by 22% of our patients, who had onset of abdominal discomfort within an hour of the dose and in most cases had quick resolution of the symptoms with administration of ranitidine. Patients should be warned about the development of symptoms suggestive of EoE, and if these symptoms develop, the patient should undergo endoscopy or stop OIT.
Because of the low but real risk for systemic allergic reactions, it is important that the patients and their caregivers have excellent support during the process of OIT. The signs and symptoms of anaphylaxis, treatment plan for anaphylaxis, treatment for milder adverse reactions with doses of H1 and H2 antihistamines (and bronchodilator if indicated), and means of contacting the allergy physician with questions or concerns are provided verbally and in writing at each OIT visit. In our experience, most patients and their families are anxious in the early stages of OIT but are greatly reassured when they can quickly reach the physician to discuss their concerns. Thus, embarking on an OIT treatment protocol represents a significant commitment for the physician as well as the patients and families.
Despite the challenges, providing the option of OIT to patients with food allergies is very rewarding for the families and medical practitioners. The constant fear of severe food allergy reaction can dominate the lives of parents and children with food allergy. The relief of stress for these families can be tremendous, and families anecdotally report that OIT has led to a significant improvement in their life, although we did not prospectively gather quality of life data on our patients. Studies have found significant improvements in quality-of-life scores once patients reach the maintenance dose of OIT,13,26 although some patients have decreased quality of life during the up-dosing process.13,27 Vasquez-Ortiz et al27 also noted more improvement in quality-of-life scores before and after egg OIT reported by the participating children vs their parents.
The sequence of immunologic responses leading to sustained unresponsiveness to OIT is still being studied.28,29 We do not have reliable immunological markers to tell us when a patient has achieved sustained unresponsiveness or to predict food allergy resolution. For the foreseeable future, patients who achieve long-term food desensitization by OIT cannot be considered to have resolution of their food allergies. To maintain their tolerance to the foods long term, the foods should be kept in their diet regularly. There are insufficient data to know how often the food should be ingested, and in what quantity, to maintain desensitization. Andorf et al,20 reporting on up to 6 years of long-term follow-up of 45 patients undergoing multiple-food OIT, note that a low-dose (300 mg) vs high-dose (2000 mg of each food) regimen and food doses every other day vs daily were equally effective.
In conclusion, our clinical experience with multiple-food OIT indicates that it is reasonably safe and effective. Reactions were mostly mild, and 87% of patients were successful in reaching a maintenance dose that increases their threshold for food allergy reactions.
Supplementary Material
Acknowledgments
We appreciate the tireless work of Christa Mills, RN, food allergy nurse coordinator, in organizing and scheduling the oral immunotherapy program and providing educational materials and telephone support to the families. We thank the allergy clinic nurses for their assistance in monitoring, teaching, and supporting the food allergy patients and their families.
Footnotes
Disclosures: The author(s) has/have no conflicts of interest to report.
References
- 1.Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:350–50. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;121:1–8. [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112: 1203–1207. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001;108(1):128–132. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostou A Anaphylaxis in children: epidemiology, risk factors and management. Curr Pediatr. 2018;14:180–186. [DOI] [PubMed] [Google Scholar]
- 6.Feng C, Kim JH. Beyond avoidance: the psychosocial impact of food allergies. Clin Rev Allergy Immunol. 2019;57(1):74–82. [DOI] [PubMed] [Google Scholar]
- 7.Kansen HM, Le TM, Meijer Y, et al. The impact of oral food challenges for food allergy on quality of life: a systematic review. Pediatr Allergy Immunol. 2018; 29:527–537. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011; 128:e9–e17. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger T, Sicherer S. Current perspectives on tree nut allergy: a review. J Asthma Allergy. 2018;11:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nachshon L, Goldberg MR, Katz Y, Levy MB, Elizur A. Long-term outcome of peanut oral immunotherapy: real life experience. Pediatr Allergy Immunol. 2018;29:519–526. [DOI] [PubMed] [Google Scholar]
- 13.Epstein-Rigbi N, Goldberg MR, Levy MB, Nachshon L, Elizur A. Quality of life of food-allergic patients before, during, and after oral immunotherapy. J Allergy Clin Immunol Pract. 2019;7:429–436. [DOI] [PubMed] [Google Scholar]
- 14.Bégin P, Winterroth LC, Dominguez T, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bégin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol. 2014;10(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrosse R, Graham F, Des Roches A, Bégin P. The use of omalizumab in food oral immunotherapy. Arch Immunol Ther Exp (Warsz). 2017;65(3): 189–199. [DOI] [PubMed] [Google Scholar]
- 17.Andorf S, Manohar M, Dominguez T, et al. Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol. 2017;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andorf S, Purington N, Block WM, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018;3(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickery BP, Berglund JP, Burk CM, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andorf S, Manohar M, Dominguez T, et al. Feasibility of sustained response through long-term dosing in food allergy immunotherapy. Allergy Asthma Clin Immunol. 2017;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varshney P, Steele PH, Vickery BP, et al. Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol. 2009;124(6):1351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virkud YV, Burks AW, Steele PH, et al. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol. 2017;139:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burk CM, Dellon ES, Steele PH, et al. Eosinophilic esophagitis during peanut oral immunotherapy with omalizumab. Allergy Clin Immunol Pract. 2017;5(2): 498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semancik E, Sayej W. Oral immunotherapy for peanut allergy induces eosinophilic esophagitis: three pediatric case reports. Pediatric Allergy Immunol. 2016;27(5):539–541. [DOI] [PubMed] [Google Scholar]
- 25.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113(6):624–629. [DOI] [PubMed] [Google Scholar]
- 26.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasquez-Ortiz M, Alvaro M, Piquer M, et al. Impact of oral immunotherapy on quality of life in egg-allergic children. Pediatr Allergy Immunol. 2015;26: 291–294. [DOI] [PubMed] [Google Scholar]
- 28.Kulis M, Yue X, Guo R, et al. High- and low-dose oral immunotherapy similarly suppress pro-allergic cytokines and basophil activation in young children. Clin Exp Allergy. 2019;49(2):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sindher SB, Long A, Acharya S, Sampath V, Nadeau KC. The use of biomarkers to predict aero-allergen and food immunotherapy responses. Clin Rev Allergy Immunol. 2018;55(2):190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Heart, Blood, and Lung Institute.. National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007. NIH publication 08–5846. [Google Scholar]
- 31.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.