Abstract
Background:
Workplace exposure to trimellitic anhydride (TMA) can elicit TMA-specific IgE (sIgE), which may lead to occupational asthma (OA). An occupational immunosurveillance program (OISP) has been implemented to monitor TMA exposure and immunologic outcomes. The purpose of this study was to determine whether TMA-specific IgG (sIgG) responses can discriminate between TMA-exposed workers with and without sIgE responses.
Methods:
Serum TMA-specific antibody (IgG, IgG4, and IgE) levels were estimated longitudinally (years 2006 to 2014) in TMA-exposed workers recruited in low, medium, and high exposure areas. sIgG and sIgE titers plotted against exposure duration were compared between workers with (a) sIgG only and (b) with sIgG who developed sIgE.
Results:
Among 92 TMA-exposed workers continuously monitored for sIgG and sIgE, 38 developed sIgG; 11 developed a sIgE response 342.38 ± 186.03 days posthire and were removed from exposure. The average detection time of sIgG in removed workers (159 ± 92 days) was significantly shorter than for actively exposed workers with only sIgG (346 ± 187 days). Workers with earlier sIgG responses of higher titer (mean value 42.25 μg/mL) compared to delayed responders with lower sIgG titers (mean value 14.79 μg/mL) more frequently developed sIgE responses. Hierarchical clustering showed the initial magnitude and exposure time required for detectable sIgG production discriminated between workers with only sIgG from workers who subsequently produced sIgE.
Conclusions:
This study demonstrates the utility of longitudinally monitoring TMA-specific antibodies in an OISP as exposed workers with early sIgG responses and of higher magnitude are more likely to develop TMA sIgE sensitization.
Keywords: hapten, occupational asthma, serum antibodies, trimellitic anhydride
1 |. INTRODUCTION
An estimated 15% of chronic obstructive pulmonary disease and asthma cases are work related, costing the healthcare system in the United States over $7 billion due to lost work productivity, worker’s compensation, and disability.1 Workplace exposure to chemicals used in the formulation of paints, plastics, dyes, and adhesives constitutes increasingly recognized causes of occupational asthma. Industries producing these chemicals have proactively established occupational immunosurveillance programs (OISPs) in an attempt to mitigate or prevent work-related respiratory conditions upon exposure to specific agents that are well known to be sensitizing or highly irritating to the upper and lower respiratory tracts. These programs are designed to promote the health, safety, and quality of life of exposed workers by collecting, analyzing, interpreting health data, and designing rational intervention strategies.
A prototypic OISP established to longitudinally monitor workers exposed to trimellitic anhydride (TMA), a low molecular weight chemical used as a plasticizer and hardener, has been in use for several decades.2,3 Inhaled free TMA can be converted to trimellitic acid, which acts as a respiratory irritant. However, once systemically absorbed, it can also bind to endogenous carrier proteins (eg, human serum albumin) leading to a trimellityl-protein conjugate capable of eliciting TMA-specific IgG (sIgG) and TMA-specific IgE (sIgE) antibody responses, which can result in several types of workplace-related respiratory diseases including occupational rhinitis (OR) and asthma (OA).3–6 Workers in high TMA exposure areas remain resistant or tolerant (negative for TMA-specific serum antibody) or become TMA-sensitized (serum TMA-specific IgG and/or IgE positive) over time. TMA-specific antibodies can be measured using standard assays such as ImmunoCAP and ELISA. In addition, we have described the synthesis and usefulness of a TMA-carrier protein conjugate, which can be used as a skin test reagent to screen workers for TMA sIgE sensitization and that there is a good correlation between ImmunoCAP and skin test results showing cutaneous reactivity.6
Grammer et al previously demonstrated that workers who develop serum sIgE antibody to TMA are more likely to develop respiratory disease with continued exposure.7 Despite the implementation of state-of-the-art engineering controls and personal protective measures to limit exposure, susceptible workers with TMA exposure continue to become TMA sensitized. Once a worker has become sensitized, early diagnosis and medical management, which involves exposure reduction or complete removal from high exposure areas, is imperative. Thus, immunosurveillance using TMA-specific serum antibodies as markers for exposure and sensitization has been very successful in preventing TMA-induced occupational lung disease.6
Several studies have confirmed a strong association between TMA sIgG and sIgE serum antibodies and subsequent development of occupational respiratory diseases.3,7–10 Particularly, TMA sIgE, demonstrated through positive serologic or skin tests, has been shown to be an independent risk factor for developing work-related bronchial hyper-responsiveness, while other factors such as smoking history, atopic status (ie, sensitization to common inhalant allergens), and age were not significant after controlling for FEV1 (forced expiratory volume in 1s).11 Current practice is to remove workers from high TMA exposure areas only after developing sIgE, as present data are insufficient to predict early on whether a worker who develops TMA serum sIgG will go on to develop TMA sIgE, or remain tolerant. Furthermore, limited data are available concerning long-term outcomes after sIgE-sensitized workers are removed from further exposure. Grammer and colleagues retrospectively investigated 29 TMA-exposed workers diagnosed with TMA-induced immunologic lung diseases who had been moved to low exposure jobs for more than 1 year.12 About half of the symptomatic workers showed significant improvement in their symptoms. For those workers who did not improve, their sIgE levels were higher lending to speculation that a higher sIgE could be a marker for poorer outcomes even after complete removal from further TMA exposure.
Longitudinal assessments of specific antibody (IgG, IgE, IgG4) responses have been performed for relevant environmental allergens.13 Animal models of TMA exposure demonstrated a robust TMA sIgG along with sIgE response.14,15 In this study, we asked the question whether the time from first TMA exposure to development of TMA sIgG is associated with subsequent sIgE response in TMA-exposed workers. Our hypothesis was that TMA-exposed workers who develop a more rapid onset of TMA sIgG are more likely to develop TMA sIgE compared to workers who have a delayed or no TMA sIgG antibody responses.
2 |. MATERIALS AND METHODS
2.1 |. Subject population
De-identified data on TMA-exposed workers for this study were longitudinally collected between 2006 through 2014 as part of an ongoing OISP. As the database used was retrospective and de-identified, the University of Cincinnati IRB ruled that this was not human research, and therefore, an informed consent was not required. All TMA-exposed workers are required to participate in the OISP as part of their employment agreement.
2.2 |. Trimellitic anhydride exposure levels
The TMA production facility has been divided into four areas depending on the TMA exposure levels: L1—undetectable TMA exposure, for example, front office; L2—very low TMA exposure, for example, research laboratory; L3—high potential for TMA exposure (TMA production area); and L4—very high potential for TMA exposure (TMA-packaging/warehouse). Workers with intermittent exposure included maintenance, fire fighters, and emergency medical personnel as well as supervisors. The high and very high TMA exposure areas (L3 and L4) have the potential for exposure higher than the recommended PEL (permissible exposure limit =0.04 mg/m3 of air). Workers in high exposure areas (L3 and L4) included packaging stuff, quality control engineers, and warehouse and production workers who had frequent or consistent exposure to TMA dust or fumes. All workers in high exposure areas are also required to wear personal protective exposure equipment (Tyvek suits and respirators).
2.3 |. Occupational immunosurveillance protocol
To monitor immune responses over time, a database including the worker’s age, gender, hire date, exposure status and TMA sIgG, sIgG4, sIgE levels, and total IgE levels is maintained. If TMA-exposed workers develop sIgG after hire, blood samples to measure specific antibody responses are collected every 3 months; otherwise, samples are collected every 6 months. In general, workers with TMA sIgG are not removed from TMA exposure. However, if workers develop TMA sIgE and/or clinical symptoms consistent with asthma, they are removed from further TMA exposure and monitored for resolution or decline of specific antibody responses and/or clinical symptoms.
2.4 |. Study design
Only TMA-naïve exposed workers who were hired to work directly with TMA between 2006 and 2014 were included in this analysis as the OISP was restructured at that time to capture TMA sIgG, IgE, and IgG4 longitudinally over consistent time-points. As the purpose of this study was to understand the kinetics of TMA sIgG production and subsequent TMA sIgE production, TMA-exposed workers with TMA sIgG and/or sIgE prior to 2006 were not included in this analysis.
2.5 |. Antibody measurements
Trimellitic anhydride -specific IgG, IgG4, and IgE antibody measurements were performed commercially using Phadia ImmunoCAP 1000 platform (Uppsala, Sweden). Briefly, this system measures antibody responses to a TMA-HSA conjugate covalently coupled to an encapsulated hydrophilic carrier polymer (cellulose). The binding of sIgE to the TMA-HSA conjugate was detected using either anti-IgG, IgE, or IgG4 b-galactosidase-labeled secondary antibody. Signals were developed using MUG (4-methylumbelliferyl-ß-Dgalactoside) per manufacturer’s protocol. A high fluorogenic signal correlates with higher levels of TMA antibody responses. Undiluted samples were used for sIgE detection, whereas for sIgG and sIgG4 testing, samples were diluted 1:100 with diluent. Each immunoglobulin assay used a 6-point calibration curve using manufacture-provided calibration strips (corresponding to IgE concentrations 0, 0.35, 0.7, 3.5, 17.5, and 100 kU/L). The measured fluorescence units were evaluated against the calibration curve and expressed as concentration of allergen-specific units (kU/L). For the sIgG and sIgG4 assays, results are expressed in mass units (μg/mL). The range of detection for TMA sIgE is 0.10 to 100 kU/L, for TMA sIgG is 2.0 to 200 μg/mL, and for TMA sIgG4 is 0.15 to 30 μg/mL (Viracor Eurofins, Lee’s Summit, ML, USA).
2.6 |. Data collection and analysis
To determine the kinetics of TMA-specific antibody responses, all data points for TMA sIgG, IgE, and IgG4 and total IgE for all workers assigned to areas with high TMA exposure from the time of hire in or after 2006 (negative TMA-specific antibody) until the last available data point in 2014 were included in the data analysis. The titer of TMA sIgG response at the first time of detection and at the peak level prior to removal of the worker from TMA exposure was compared between those groups of workers with TMA sIgG only and those with both TMA sIgG and sIgE. In addition, the mean, median, standard deviation (SD), and standard error of the means (SEM) for all TMA sIgG and sIgG4 responses were calculated and compared between actively TMA-exposed workers and those removed because of TMA sIgE. For calculating the magnitude of specific antibody responses, when a worker was moved from a higher (level 3 or 4) to a lower (level 1 or 2) exposure level, the values prior to relocation were included in the final analysis. Antibody profiles of workers with TMA sIgG vs those with sIgG and sIgE (removed workers) were plotted.
2.7 |. Statistical analysis
All descriptive statistics and between-group comparisons were performed using SAS 12 (Statistical Analysis Systems, Cary, NC, USA). Workers with TMA-specific IgE > 0.34 kU/L and/or TMA-specific IgG > 7.0 μg/mL anytime during the surveillance time period were included for analysis, and a P value < .05 was considered significant. Workers were clustered by time to detectable TMA sIgG and the magnitude of TMA sIgG to determine whether these parameters could discriminate subjects who developed TMA sIgE from those who only produced TMA sIgG using a hierarchical clustering approach.16,17 Kinetic profiles (expressed as days since hire) and magnitudes (expressed as kU/L) for serum antibodies over the duration of exposure for the TMA-exposed workers with sIgG and removed workers with sIgG and sIgE were generated using JMP SAS software package (Statistical Analysis Systems)
3 |. RESULTS
3.1 |. Trimellitic anhydride exposure and specific antibody responses
Distribution of workers in different TMA exposure levels and associated antibody responses are summarized in Table 1. Ninety-two workers were hired between 2006 and 2014, of which 18 were recruited to work in L3 and 31 were recruited to work in L4 exposure areas of the factory, while others were hired to work in an L2 exposure level or in office/maintenance jobs which involved none or short-term intermittent TMA exposure. Of 92 workers, a total of 25 TMA-exposed workers (2 from L3 and 23 from L4 exposure areas) developed sIgG, while two additional workers who were assigned to L4 exposure produced sIgG, transiently and marginally with detectable sIgE (1.42 kU/L). Out of this workforce, a total of 11 workers who primarily developed sIgG subsequently developed sIgE response and were removed from the high exposure areas.
TABLE 1.
Number of workers recruited in different trimellitic anhydride (TMA) exposure areas according to their antibody responses. Ten of 18 actively exposure workers in L4 areas developed sIgG; two subjects with sIgG (marked with asterisk) transiently produced borderline sIgE (sIgE 0.11 kU/L to 0.27 kU/L). Only 2 of 18 workers in L3 exposure developed sIgG. In contrast, 11 of 13 removed workers in an L4 exposure area developed sIgG and sIgE and two developed sIgG only
| Active workers (N = 77) |
Removed workers (N = 15) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 | Total number | |
| No TMA-specific Ab | 22 | 19 | 16 | 6 | 0 | 2 | 0 | 0 | 65 |
| With only slgG | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 2 | 14 |
| With slgG + slgE | 0 | 0 | 0 | 2* | 0 | 0 | 0 | 11 | 11 |
| Total number of workers recruited | 22 | 19 | 18 | 18 | 0 | 2 | 0 | 13 | 92 |
Of the 77 actively TMA-exposed workers, 12 developed sIgG, 10 of which did so within this study period and thus were able to be included in the longitudinal analysis of TMA-specific antibody responses. The similar longitudinal analysis could be performed for 9 of 11 workers who developed sIgE and were subsequently removed from further TMA exposure. None of the TMA-exposed workers who developed TMA sIgG or sIgE developed any symptoms or changes in lung function indicative of occupational asthma or other respiratory diseases, which suggests that the OISP was effective.
3.2 |. Longitudinal analysis of workers with TMA sIgG vs sIgG and sIgE between 2006 and 2014
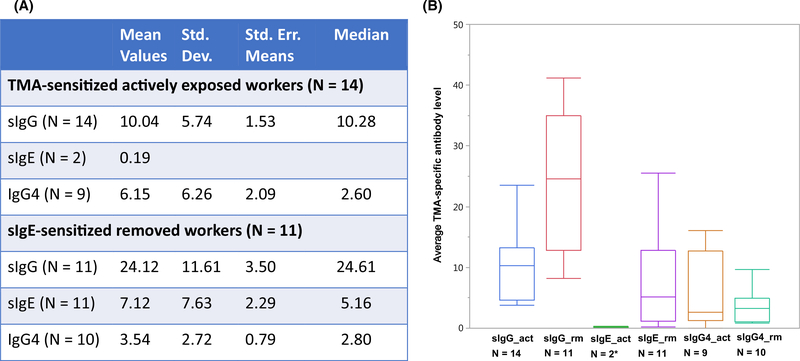
Figure 1A summarizes the magnitudes of TMA-specific antibody (sIgG, sIgE, and sIgG4) responses for workers in the high TMA exposure areas (levels 3 and 4). All workers were male. There were no significant age differences between actively exposed and removed workers or those with or without TMA sIgG, sIgG4, or sIgE responses (according to age requirements in the hiring policy; data not shown). The mean and median sIgG, sIgG4, and sIgE antibody levels for all actively exposed workers (sIgG and sIgG4 only) and removed workers (sIgG and sIgE) expressed as kU/L were calculated. Actively exposed workers had a higher in magnitude sIgG4, whereas removed workers with high sIgE had a higher in magnitude sIgG. In addition, the overall antibody status of removed and actively exposed workers is illustrated in Figure 1B. Removed workers with both TMA sIgG and sIgE had a significantly higher average level of sIgG and sIgE (P < .05), with a modest decrease in IgG4 (P = .245), compared to actively exposed workers. The decreased sIgG4 value in the removed group compared to the actively exposed group supports the notion that sIgG4, rather than sIgG may be a good marker of persistent high TMA exposure levels, as reported by previous investigators.18–20 Tomee et al suggested that IgG4 responses may be associated with chronic exposure.18 This was also evident in the wheat allergen exposure-response model, where an increase in wheat allergen concentration was significantly associated with IgG4 production in both wheat-sensitized and nonsensitized bakery workers.21 A similar result was observed for allergen-specific IgG4 responses among laboratory animal handlers demonstrating that IgG4 is a marker of exposure.19,22
FIGURE 1.
A, Titers of of trimellitic anhydride (TMA)-specific antibody classes (sIgG, sIgE, sIgG4) in TMA-exposed workers in high exposure areas (levels 3 and 4). B, Diagrammatic representation of average TMA-specific antibody profiles of active and removed workers. Removed workers manifest significantly higher average sIgG and sIgE values compared to actively exposed workers. sIgE values were expressed in kU/L, while specific IgG and specific IgG4 values were expressed in lg/mL. *Two workers in the actively exposed area with borderline sIgE were reassigned to lower exposure areas
3.3 |. Kinetic assessment in high TMA-exposed workers who developed sIgG with or without sIgE
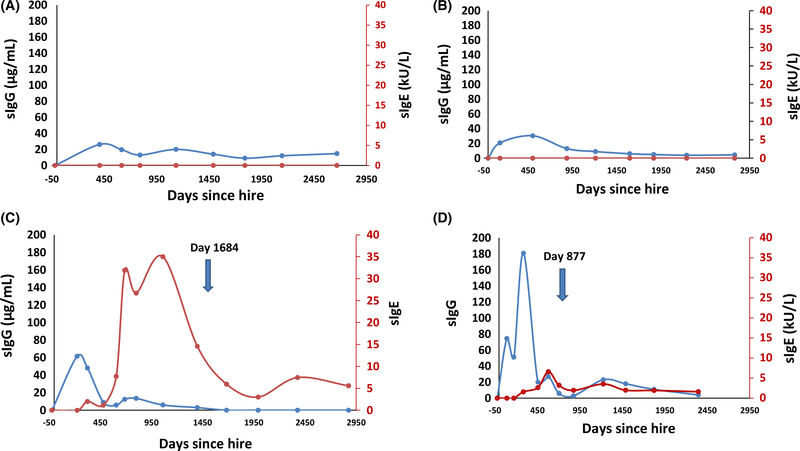
To characterize the sIgG response in workers who developed sIgG with or without sIgE, sIgG levels were plotted against duration of exposure for both groups. Figure 2 illustrates TMA sIgG and sIgE titers plotted against time for representative workers who produced sIgG alone (a and b) or with sIgE (c and d).
FIGURE 2.
Representative curves showing trimellitic anhydride (TMA) sIgG and TMA sIgE titers plotted against time (days after hire) for actively exposed workers (with sIgG alone; A and B, and removed workers (with sIgG and sIgE; C and D, Each removed worker produced sIgG prior to producing sIgE. Days after hire when workers were removed are depicted for 2C (1684 days) and 2D (877 days) are depicted
We subsequently compared the exposure time required to produce detectable sIgG, between worker groups who developed sIgG alone vs those who developed sIgG and sIgE.
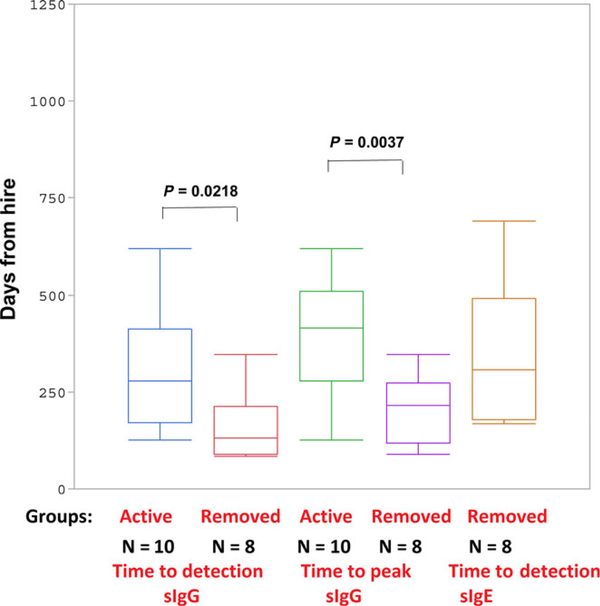
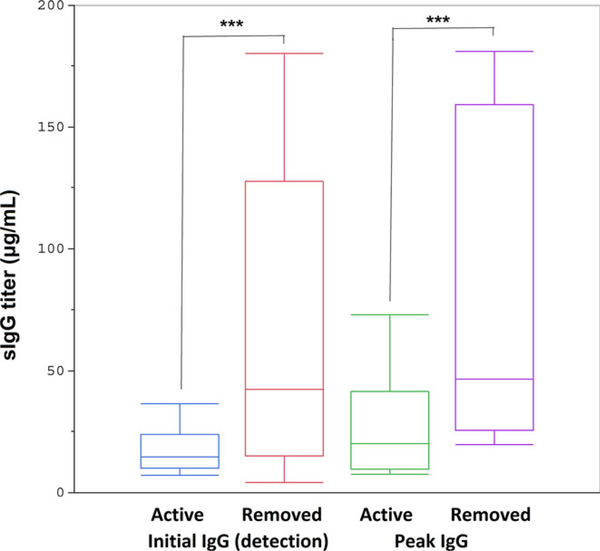
Figure 3 illustrates the average time (in days) for detectable and peak sIgG as well as time to detectable sIgE in removed workers. It shows that the initial and peak sIgG levels occurred earlier in those workers with sIgG and sIgE who were ultimately removed from TMA exposure areas compared to active workers with sIgG only. Table S1 summarizes the descriptive statistics of specific antibody kinetics in exposed workers. In the actively exposed worker group, the median time to sIgG was 278 days after hire. This timeframe was approximately 131 days for workers who subsequently developed sIgE. Serum sIgG reached peak concentration in 415 days in the actively exposed worker group compared to 215 days in the removed group (Figure 3). The median and mean times for detectable sIgE were 306 and 342 ± 186 days, respectively, for removed workers. The peak TMA sIgG level is higher in workers with sIgG and sIgE compared to those with only sIgG (Figure 4; Table S2). Interestingly, this level of sIgG is also higher at the time of detection in the workers who went on to produce sIgE, which can be potentially used as an early biomarker for future sIgE response.
FIGURE 3.
The average time (days) required for detectable sIgG and peak sIgG in workers with sIgG only (Group: active) vs sIgG and sIgE (Group: removed) is shown. The average time to detection for sIgG in removed workersof 159 ± 92 days was significantly shorter than actively exposed workers with only sIgG of 346 ± 187 days. The average time of sIgE detection in the removed group was 342 ± 186 days
FIGURE 4.
The initial and peak sIgG titers of active and removed workers demonstrate that both initial and peak sIgG levels were significantly greater for removed workers with sIgG and sIgE (P-values .03 and .02., respectively)
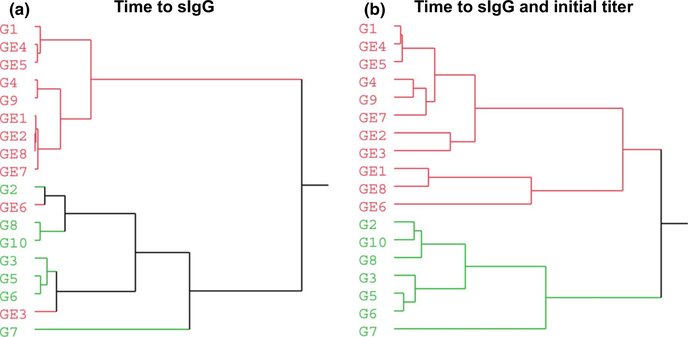
A hierarchical clustering (Ward’s minimum variance application) approach was used to determine whether the (a) time to detectable TMA sIgG or (b) the time to detectable TMA sIgG and magnitude of sIgG at the time of detection can discriminate workers with TMA sIgG only from workers with both sIgG and sIgE serum antibodies (Figure 5).16,17 When we used these two parameters, we found that the algorithm could cluster the workers into two distinct groups (Figure 5B). In one cluster (red), three workers with sIgG (G1, G4, and G9) were misclassified with eight workers with sIgG and sIgE (GE1, GE2, GE3, GE6, GE7, and GE8), while all other workers with only sIgG (G2, G3, G5, G6, G7, G8, and G10) were clustered together in a separate group (green). Assuming that there are three misclassified workers in the red cluster and no misclassified worker in the green cluster (homogeneous cluster), the number of correctly classified workers was 15 of a total of 18 workers (83%). Thus, although the population sizes of both groups were small, our preliminary analysis indicated that the time to development of sIgG in combination with the magnitude of the initial sIgG at the time of detection can differentiate Group 1 (sIgG only) from Group 2 (sIgG and sIgE) with more than 80% accuracy.
FIGURE 5.
Samples clustered by either A, time to detectable sIgG only or B, in combination with initial sIgG titer at the time of detection. Workers with trimellitic anhydride (TMA) sIgG were designated as G1 through G10, while those who later developed sIgE in addition to sIgG were designated as GE1 through GE8. Results indicate that the time to detectable sIgG can discriminate between Group 1 and Group 2 with about 72% accuracy. However, this parameter when combined with the time of the initial sIgG titer can discriminate between these two groups with approximately 80% accuracy. The two clusters are depicted in red and green colors
4 |. DISCUSSION
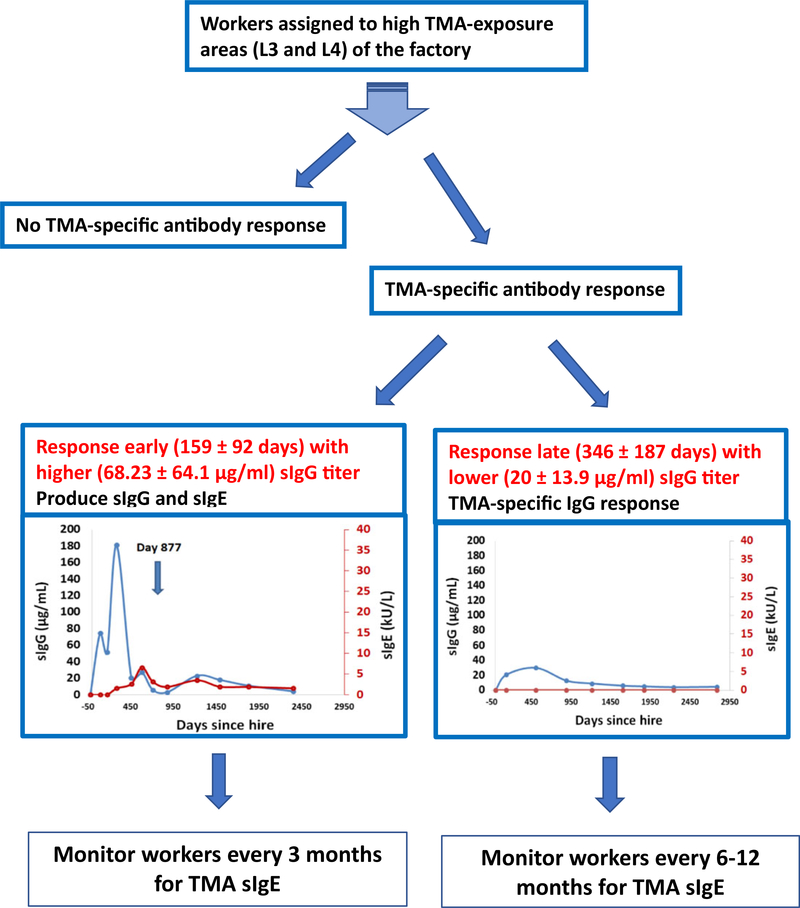
While TMA sIgE has been found to be highly associated with the development of TMA-induced occupational rhinitis and asthma in TMA-exposed workers,5,10 the role of sIgG in occupational asthma has been less clear and is usually regarded as a marker of TMA exposure.23,24 The results of our study suggest an alternative role for serial monitoring of sIgG as earlier detection of sIgG after initial high TMA exposure appears to be predictive of subsequent sIgE production. This information is very useful to the ongoing OISP as it will help identify much earlier the new workers assigned to high TMA exposure areas at risk for developing TMA sIgE responses. Thus, workers who develop TMA sIgG within 6 months after hire would be required to have more frequent sIgE monitoring every 3 months for at least a year which is the approximate time-point at which TMA sIgE was detected in removed workers. In contrast, workers with TMA sIgG responses that develop after approximately 1 year would require less frequent monitoring for TMA sIgE development (eg, once or twice a year) based on our findings (Figure 6).
FIGURE 6.
Flowchart illustrating the course of trimellitic anhydride (TMA)-associated antibody responses. Workers recruited to high exposure areas produce no TMA-specific antibody, produce sIgG alone, or produce sIgG in combination with sIgE. Workers who produce sIgG sooner (after 159 ± 92 days of exposure) with a higher magnitude of sIgG (68.23 ± 64.1 kU/L) are more likely to produce sIgG and sIgE (after 342 ± 186 days of hire) and will require reassignment to lower exposure area. In contrast, those who produced sIgG later into their employment hire period (after 346 ± 187 days of exposure) with a lower magnitude of response (serum sIgG level 20.15 ± 13.9 kU/L) continued to produce only sIgG
Although many studies have already established the relationship between specific antibody responses and health effects for TMA,25 there has been no clear elucidation of predisposing risk factors for developing TMA sIgE.26 Thus, there is an ongoing need for an OISP to ensure the safety of TMA-exposed workers. However, based on our data, it may now be possible to screen and remove TMA-exposed workers who produce high levels of TMA sIgG earlier after initial exposure which will eventually lead to a workforce that is either tolerant or resistant to TMA sensitization. It should be emphasized that although monitoring for specific antibody responses can prevent the development of immunologic mediated occupational lung disorders, it cannot prevent irritant-induced asthma (a.k.a. reactive airway dysfunction syndrome or RADS), which occurs without a latency period after a large chemical exposure. Therefore, an OISP does not negate the need for continuous monitoring for TMA exposure and training of personnel to follow standard operating procedures and wear personal protective equipment to prevent TMA exposure as much as possible. This OISP has been extremely successful as there have been no reported cases of TMA-induced occupational asthma or other lung diseases over the course of this observational period and to the present day.
Interestingly, once workers developed TMA sIgE and were removed from further exposure, we observed a more rapid decline of sIgG4 compared to sIgG and sIgE over time. In fact, sIgG and sIgE persisted much longer than anticipated after complete TMA avoidance. The sustained levels of TMA sIgG and sIgE may reflect the persistence of memory B lymphocytes which requires further investigation. The role of sIgG4 in development of sensitization and allergic diseases remains highly debated as to whether it represents a protective blocking antibody or a marker of high antigen exposure.27–29,19,30 In our OISP, the finding that removed workers with high TMA sIgG and sIgE had a more rapid decline in TMA sIgG4, in contrast to actively exposed workers who had persistently higher IgG4 levels, supports the notion that sIgG4 could be a marker of persistent TMA exposure more so than TMA sIgG, which requires further investigation.19,22,31 Additionally, we noticeda decrease in sIgG titers after an initial peak in about 60% of sIgG-positive workers even with no change in their exposure level. Although the mechanism behind the antibody kinetics with sIgG titers reaching a peak before reaching a stable level, even without change in exposure level, is poorly elucidated, this observation supports the notion that IgG4 may be a better marker than sIgG for chronic TMA exposure. Longitudinal measurements of sIgG subclasses (eg, sIgG1) in addition to sIgG4 and sIgG could be performed in the future to better address this point. However, as sIgG always preceded sIgE response in removed workers, early sIgG response might be used to predict future IgE response.
Our study has several potential strengths that are noteworthy. First, although TMA-workers who develop sIgE are removed from high TMA exposure areas, until now there has been no standard way to predict which worker(s) are more likely to produce sIgE. The results of this study now suggest that an earlier onset and higher magnitude of TMA sIgG might be useful in predicting TMA-exposed workers at risk for sIgE sensitization. Secondly, longitudinal monitoring of TMA-naïve new hires assigned to high TMA exposure areas provides a unique opportunity to characterize the relationship between TMA exposure levels and specific antibody response outcomes. Finally, this OISP confirms that careful monitoring of TMA exposure and specific TMA antibody responses prevents the development of OA and other lung diseases.
There are limitations to this study that require mentioning. First, this was a retrospective study and was limited by a small number of workers with complete data starting from date of hire in 2006 through 2014. However, despite the relatively small population size, our results were highly statistically significant suggesting that they are clinically relevant. Ongoing surveillance of existing and newly hired workers assigned to high TMA-exposed areas will allow us to test and confirm the findings of this study over time. Finally, the interval between two time-points of serologic data collection for workers was considerably varied for some workers due to scheduling for blood draws. Collecting data more frequently and at regular intervals may have improved the quality of our data.
In summary, adopting primary prevention strategies such as the use of personal protective equipment, improved production, and packaging engineering procedures are effective at decreasing TMA exposure, but despite these measures, some exposed workers still develop TMA sIgE sensitization. Serial monitoring of TMA sIgG and sIgE has been found effective at identifying workers who develop TMA sIgE early on so they can be removed from further TMA exposure. We now have identified specific timelines for TMA sIgG detection that can be used as a means for stratifying workers at risk for developing TMA sIgE. This information has practical implication for this OISP as it can be used to determine the need for more or less frequent specific antibody monitoring. The earlier appearance of TMA sIgG with higher serum titers might indicate a genetic susceptibility by some workers for hapten sensitization which certainly warrants further investigation.
Supplementary Material
Footnotes
CONFLICTS OF INTEREST
DDG has no disclosures JB is the medical director of Flint Hills Resources immunosurveillance program.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Balmes J, Becklake M, Blanc P, et al. American thoracic society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–797. [DOI] [PubMed] [Google Scholar]
- 2.Baur X, Czuppon AB, Rauluk I, et al. A clinical and immunological study on 92 workers occupationally exposed to anhydrides. Int Arch Occup Environ Health. 1995;67:395–403. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DI, Patterson R, Zeiss CR. Clinical and immunologic evaluation of trimellitic anhydride-and phthalic anhydride-exposed workers using a questionnaire with comparative analysis of enzyme-linked immunosorbent and radioimmunoassay studies. J Allergy Clin Immunol. 1982;69:311–318. [DOI] [PubMed] [Google Scholar]
- 4.Zeiss CR, Patterson R, Pruzansky JJ, Miller MM, Rosenberg M, Levitz D. Trimellitic anhydride-induced airway syndromes: clinical and immunologic studies. J Allergy Clin Immunol. 1977;60:96–103. [DOI] [PubMed] [Google Scholar]
- 5.Zeiss CR, Wolkonsky P, Pruzansky JJ, Patterson R. Clinical and immunologic evaluation of trimellitic anhydride workers in multiple industrial settings. J Allergy Clin Immunol. 1982;70:15–18. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein JA, Ghosh D, Sublett WJ, Wells H, Levin L. Is trimellitic anhydride skin testing a sufficient screening tool for selectively identifying TMA-exposed workers with TMA-specific serum IgE antibodies? J Occup Environ Med. 2011;53:1122–1127. [DOI] [PubMed] [Google Scholar]
- 7.Grammer L, Shaughnessy M, Kenamore B. Utility of antibody in identifying individuals who have or will develop anhydride-induced respiratory disease. Chest. 1998;114:1199–1202. [DOI] [PubMed] [Google Scholar]
- 8.Grammer LC, Shaughnessy MA, Kenamore BD, Yarnold PR. A clinical and immunologic study to assess risk of TMA-induced lung disease as related to exposure. J Occup Environ Med. 1999;41:1048–1051. [DOI] [PubMed] [Google Scholar]
- 9.Zeiss CR, Mitchell JH, Van Peenen PF, Harris J, Levitz D. A twelve-year clinical and immunologic evaluation of workers involved in the manufacture of trimellitic anhydride (TMA). Allergy Proc. 1990;11:71–77. [DOI] [PubMed] [Google Scholar]
- 10.Zeiss CR, Mitchell JH, Van Peenen PF, et al. A clinical and immunologic study of employees in a facility manufacturing trimellitic anhydride. Allergy Proc. 1992;13:193–198. [DOI] [PubMed] [Google Scholar]
- 11.Barker RD, van Tongeren MJ, Harris JM, Gardiner K, Venables KM, Newman Taylor AJ. Risk factors for bronchial hyperresponsiveness in workers exposed to acid anhydrides. Eur Respir J. 2000;15:710–715. [DOI] [PubMed] [Google Scholar]
- 12.Grammer LC, Shaughnessy MA, Henderson J, et al. A clinical and immunologic study of workers with trimellitic-anhydride-induced immunologic lung disease after transfer to low exposure jobs. Am Rev Respir Dis. 1993;148:54–57. [DOI] [PubMed] [Google Scholar]
- 13.Salonen EM, Hovi T, Meurman O, Vesikari T, Vaheri A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J Med Virol. 1985;16:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Warbrick EV, Dearman RJ, Kimber I. Induced changes in total serum IgE concentration in the Brown Norway rat: potential for identification of chemical respiratory allergens. J Appl Toxicol. 2002;22:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Warbrick EV, Dearman RJ, Kimber I. IgG and IgE antibody responses following exposure of Brown Norway rats to trimellitic anhydride: comparison of inhalation and topical exposure. Toxicology. 2002;172:157–168. [DOI] [PubMed] [Google Scholar]
- 16.Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. [Google Scholar]
- 17.Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering method: which algorithms implement ward’s criterion? J Classif. 2014;31:274–295. [Google Scholar]
- 18.Tomee JF, Dubois AE, Koeter GH, Beaumont F, van der Werf TS, Kauffman HF. Specific IgG4 responses during chronic and transient antigen exposure in aspergillosis. Am J Respir Crit Care Med. 1996;153:1952–1957. [DOI] [PubMed] [Google Scholar]
- 19.Van der Zee JS, Aalberse RC. The role of IgG in immediate-type hypersensitivity. Eur Respir J Suppl. 1991;13:91s–96s. [PubMed] [Google Scholar]
- 20.Baatjies R, Meijster T, Heederik D, Jeebhay MF. Exposure-response relationships for inhalant wheat allergen exposure and asthma. Occup Environ Med. 2015;72:200–207. [DOI] [PubMed] [Google Scholar]
- 21.Stobnicka A, Gorny RL. Exposure to flour dust in the occupational environment. Int J Occup Saf Ergon. 2015;21:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krop EJ, Doekes G, Heederik DJ, Aalberse RC, Van Der Zee JS. IgG4 antibodies against rodents in laboratory animal workers do not protect against allergic sensitization. Allergy. 2011;66:517–522. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein DI, Roach DE, McGrath KG, Larsen RS, Zeiss CR, Patterson R. The relationship of airborne trimellitic anhydride concentrations to trimellitic anhydride–induced symptoms and immune responses. J Allergy Clin Immunol. 1983;72:709–713. [DOI] [PubMed] [Google Scholar]
- 24.Grammer LC, Patterson R. Immunological evaluation of occupational asthma. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI, editors. Asthma in the workplace. 2nd ed. New York: Marcel Dekker Inc.; 1999: pp 159–172. [Google Scholar]
- 25.Bernstein JA. Occupational asthma. In: Mahmoudi M, ed. Allergy and Asthma: Practical Diagnosis and Management. Cham: Springer International Publishing; 2016:253–270. [Google Scholar]
- 26.Malo JL, Lemiere C, Gautrin D, Labrecque M. Occupational asthma. Curr Opin Pulm Med. 2004;10:57–61. [DOI] [PubMed] [Google Scholar]
- 27.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–1125. [DOI] [PubMed] [Google Scholar]
- 28.Savilahti EM, Rantanen V, Lin JS, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geroldinger-Simic M, Zelniker T, Aberer W, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011;127:616–622. [DOI] [PubMed] [Google Scholar]
- 30.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokota K, Yamaguchi K, Takeshita T, Morimoto K. The significance of specific IgG4 antibodies to methyltetrahydrophthalic anhydride in occupationally exposed subjects. Clin Exp Allergy. 1998;28:694–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.