Abstract
Background:
The HIV treatment cascade is a crucial tool to guide HIV prevention and treatment strategies. The extent to which opioid agonist treatments (OATs) such as methadone and buprenorphine influence this cascade was examined in a nationwide study of people who inject drugs (PWID) in Ukraine.
Setting:
Cross-sectional stratified survey of PWID followed by HIV and hepatitis C virus testing in 5 Ukrainian cities.
Methods:
Opioid-dependent PWID (N = 1613) were sampled from January 2014 to March 2015. Analysis was confined to 520 participants with HIV, with 184 (35.4%) prescribed OAT. Weighted logistic regression models were used to assess independent factors associated with the 5 steps in the HIV treatment cascade.
Results:
Compared with PWID not on OAT (N = 336), participants who prescribed OAT (N = 184) were significantly more likely to be diagnosed (91% vs. 71%), linked (81% vs. 52%), and retained (69% vs. 35%) in HIV care, and prescribed (56% vs. 31%) and optimally (>95% of doses) adherent to antiretroviral therapy (41% vs. 22%). Receiving OAT contributed most as an independent factor with every step of the cascade. Other steps in the HIV treatment cascade were influenced by age, depression, and geographical variability.
Conclusions:
OAT remains an essential and effective strategy to not only treat patients with opioid use disorder, but also a crucial strategy to engage PWID in care to meet UNAIDS 90–90-90 targets. Geographical differences suggest local structural impediments. With low OAT coverage prescribed for 2.9% of the estimated 347,000 PWID in Ukraine, OAT expansion requires strategic interventions that target the individual, clinical care settings, policies, and funding.
Keywords: HIV treatment cascade, PWID, Ukraine, opioid agonist therapies, methadone, buprenorphine, depression, HIV prevention
INTRODUCTION
Eastern Europe and Central Asia (EECA), where HIV is concentrated among people who inject drugs (PWID), remains the only region globally where both HIV incidence and mortality continue to increase.1 In Ukraine, one of the 6 countries that account for half of the global epidemic of PWID with HIV,2 HIV prevalence (mean: 21.5%; range: 2.5%–43.8%) among the country’s estimated 347,000 PWID remains high.3–5 Ukraine has one of the highest HIV prevalence (1.2%) among adults and the second highest number of people living with HIV (PLWH) in the EECA region.1 Among the 220,000 PLWH in Ukraine, antiretroviral therapy (ART) coverage remains under 30% overall and under 5% in PWID.6
The Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90-90 strategy sets ambitious goals for engaging PLWH into the HIV treatment continuum.7 The HIV treatment cascade, or continuum of care, provides a framework for clinicians and policymakers to identify critical gaps in addressing HIV prevention and treatment8 and generally includes the following steps: diagnosed with HIV, linked to care, retained in care, prescribed ART, and treatment success (ART adherence and viral suppression).8 Numerous individual and structural factors restrict access to and utilization of ART, which are often more salient in resource-constrained settings.9 Individual factors include competing stigma, individual myths and beliefs about HIV care, and comorbidities. Social factors, and structural factors also influence everyday life for PWID,9 whereas clinical factors include physicians withholding ART from PWID.10 Unfortunately, few interventions have successfully optimized the HIV cascade at every step, especially in low-/middle-income countries (LMICs). One systematic review and meta-analysis found that opioid agonist therapies (OATs) with methadone (MMT) or buprenorphine maintenance treatment improve outcomes along a few steps of the HIV treatment cascade for PWID.11 This review, however, included only 2 studies from LMIC, and none of these studies assessed the influence of OAT across the entire cascade.
Nonetheless, one mathematical modeling study conducted for Ukraine suggests that MMT scale-up for 25% of PWID would be the most cost-effective HIV prevention strategy,12 whereas another study found that MMT scale-up in prisons with MMT continuation after release would markedly reduce new HIV infections in PWID.4 In Ukraine, buprenorphine was introduced in 2004, followed by MMT in 2008.13 OAT was introduced in Ukraine initially for HIV prevention because the HIV epidemic was concentrated in PWID.13 At that time, OAT programs prioritized PWID with HIV, but integrated care that included a combination of OAT, HIV, and tuberculosis services were introduced in 2008 and then expanded nationally.14 Ukraine, with funding from international donors, had planned OAT for 20,000 PWID by 2014; yet, in 2018, a little over 10,000 are successfully enrolled,15 a number that has not substantially increased and represents 2.9% of Ukraine’s 347,000 PWID.16
Despite the importance of assessing outcomes along the entire HIV treatment cascade, there is a lack of published data that explores the HIV care continuum in Ukraine, the EECA region, and other resource-poor settings. We, therefore, examined data to explore the association between enrollment in OAT and stages of the HIV care continuum: HIV status awareness, linkage to and retention in care, ART prescription, and treatment adherence using the sample from a large survey of PWID with opioid use disorders in Ukraine.
METHODS
Population
One thousand six hundred thirteen individuals with opioid user disorder were recruited into the study in 5 cities: Kyiv, Mykolaiv, Odesa, Dnipro, and Lviv. These cities bear a substantial burden of HIV and opioid use. The analysis was restricted to a subsample of 520 PWID who tested positive for HIV. One thousand ninety-three PWID without HIV were excluded from analysis. Recruitment occurred between January 2014 and March 2015. Study recruitment and procedures have been described previously,17 and eligibility criteria included the following: aged 18 years and older; meeting ICD-10 criteria for opioid dependence; lived/worked in the city where the survey was conducted; and willingness to undergo HIV and hepatitis C virus (HCV) testing. Participants were paid 100 Ukrainian hryvnia (UAH) (~US$4–10) for their time. Recruitment targeted PWID who were currently on OAT and those who had never received it. The sampling frame consists of a roster of current OAT participants who after de-identification, was taken from every treatment site in each city. Once the roster list was compiled, the study participants were selected at random from the roster using a random number generator. OAT personnel contacted randomly selected participants and referred them for study participation; 99% of selected participants agreed to take part in the study. PWID with no OAT experience were recruited using standardized respondent-driven sampling (RDS) procedures.18 Initial RDS seeds were selected through community outreach and harm reduction agencies. Diversity was set as a priority to include hard to reach subgroups of PWID, including women, youths (aged 18–25 years), and individuals who recently started injecting (<2 years of experience). RDS participants earned 20 UAH (~US$1–2) for each additional social and injection network contact they recruited, up to 3. Study participation was voluntary, and a medical professional performed HIV pre-test/post-test counseling. All HIV patients not in care were referred to treatment services. Institutional review boards at Yale University and the Gromashevskiy Institute at the National Academy of Medical Sciences in Ukraine approved the study procedures.
Measures
Participants completed a computer-assisted, self-administered instrument using Qualtrics. The survey included substantive sections related to OAT attitudes, beliefs, and experiences; HIV risk behaviors; as well as validated instruments from previous research conducted in Ukraine to assess demographic characteristics, drug use, and treatment experiences and to measure alcohol use disorders (WHO Alcohol Use Disorders Identification Test), depression (10-item Center for Epidemiological Studies of Depression Scale: CES-D scale), and addiction severity (10-item Drug Abuse Severity Test).17 Rapid HIV testing and HCV testing were performed using CITO TEST HIV 1/2/0 and CITO TEST HCV (Abon Biopharm Hangzhou Co., Ltd., Hangzhou, China).
The independent correlates for achieving the primary outcome for each stage of the cascade were derived from self-reported data. Awareness of HIV status was based on self-reported HIV testing results; not returning for HIV results was included as not being diagnosed. Linkage to care was defined as being a registered patient at the AIDS center because HIV must be confirmed by an HIV specialist using confirmatory testing accompanied by official governmental registration. Retention in care was defined as having CD4 count tested within the previous 6 months (governmental guidelines recommend CD4 testing every 6 month), which must be performed in conjunction with a visit to see the HIV specialist. Being on ART was defined as currently taking ART medication. ART adherence levels (past 30 days) were measured using a validated visual analogue scale,19 with >95% defined as optimally adherent.
Statistical Analysis
Continuous variables included age, monthly income (in UAH), number of days injecting drugs during the past 30 days, and years of drug injection. Relationship status was dichotomously defined as “in a relationship” if they were married or had a steady partner, whereas “single” was defined as single, widowed, or separated. Housing was defined using 3 categories: (1) living in their own place, including renting their own apartment, (2) living with family or friends, and (3) having unstable housing, which included being homeless, living at a shelter, or any other temporary housing. Using standardized cutoffs, alcohol use disorders were defined as ≥8 for men and ≥4 for women using the Alcohol Use Disorders Identification Test.20 Moderate to severe depression was dichotomized as scores ≥10 on the CES-D scale,21,22 and addiction severity was dichotomously reported as low/moderate for scores ≤5 and substantial/severe if >5 on the 10-item Drug Abuse Severity Test.23
The Fisher exact test was used to compare the frequencies of categorical variables, whereas a t test was used to compare the mean values of continuous variables. We then compared the proportion of PLWH who were on OAT with that of those not on OAT at each stage of the HIV cascade using a χ2 test with application of sampling weights based on population estimates in each OAT strata by city. The population estimates for the OAT groups were derived from administrative records of OAT programs using medical records, whereas population size for those not on OAT was adjusted based on the estimated number of PWID.24 Weighted multivariable logistic regression models were then used to analyze the independent correlates for each step in the HIV treatment cascade. The final model for each of the stages of the cascade was selected based on the best subset variable selection according to the Akaike information criterion. The final model fit was checked using goodness-of-fit Hosmer–Lemeshow test modified for survey data. We verified that the models were robust, and the results were not sensitive to the exclusion of influential observations, which were identified using (1) Pearson residual and (2) deviance residual. Statistical analyses were performed using STATA v.14.25
RESULTS
The characteristics of the sample of 520 HIV-infected PWID, stratified by OAT status, are provided in Table 1. Briefly, the majority of the sample was men (71%), single (64%), and in their late 30s (mean = 38 years). Most men were long-term injectors (mean = 20 years), completed high school (79%), with 43% being unemployed and 4% having unstable housing. Nearly all (93%) met criteria for substantial/severe addiction severity with a mean of 15 days of injecting in the past 30 days, and nearly 40% had an underlying alcohol use disorder. Two-thirds (67%) met screening criteria for moderate/severe depression. Compared with those without OAT experience, individuals on OAT had significantly lower mean days of injection, prevalence of depressive symptoms or alcohol use disorders, and lower addiction severity (Table 1).
Table 1.
Characteristics of Study Participants, Stratified by Receipt of Opioid Agonist Treatments (N = 520)
| On OAT | ||||
|---|---|---|---|---|
| Variables | Total Sample (N = 520), N (%) | Yes (N = 184), N (%) | No (N = 336), N (%) | P |
| Mean age, yr (SD) | 38 (6.96) | 38 (6.86) | 39 (7.02) | 0.940 |
| Mean monthly income UAH (SD) | 1500 (2001) | 1535 (2186) | 1500 (1895) | 0.750 |
| Mean years from injection initiation (SD) | 20 (7.9) | 21 (6.9) | 20 (8.3) | 0.186 |
| Mean number of injection days within past 30 d (SD) | 15 (15.3) | 0 (6.7) | 28 (9.9) | <0.001* |
| Female | 151 (29) | 46 (25) | 105 (31.3) | 0.157 |
| Education | ||||
| Incomplete secondary | 112 (21.5) | 28 (15.2) | 84 (25) | 0.032† |
| Completed secondary | 136 (26.2) | 52 (28.3) | 84 (25) | |
| Postsecondary education | 272 (52.3) | 104 (56.5) | 168 (50) | |
| In relationships | 194 (37.3) | 72 (39.1) | 122 (36.3) | 0.570 |
| Housing | ||||
| Own place | 166 (31.9) | 59 (32) | 107 (31.9) | 0.369 |
| Friends’ or family’s place | 334 (64.2) | 121 (65.8) | 213 (63.4) | |
| Unstable housing | 20 (3.9) | 4 (2.2) | 16 (4.7) | |
| Employment | ||||
| Full-time | 66 (12.7) | 26 (14.1) | 40 (11.9) | 0.831 |
| Part-time | 140 (26.9) | 48 (26.1) | 92 (27.4) | |
| Seasonal | 91 (17.5) | 34 (18.5) | 57 (16.9) | |
| Unemployed | 223 (42.9) | 76 (41.3) | 147 (43.8) | |
| City | ||||
| Kyiv | 100 (19.2) | 59 (32) | 41 (12.2) | <0.001* |
| Mykolaiv | 98 (16.4) | 36 (19.6) | 62 (18.5) | |
| Odesa | 85 (18.9) | 17 (9.2) | 68 (20.2) | |
| Dnipro | 174 (33.5) | 54 (29.4) | 120 (35.7) | |
| Lviv | 63 (12.1) | 18 (9.8) | 45 (13.4) | |
| Moderate to severe depression | 350 (67.3) | 109 (59.2) | 241 (71.7) | 0.005‡ |
| Alcohol use disorders | 206 (39.6) | 49 (26.6) | 157 (46.7) | <0.001* |
| Addiction severity (substantial to severe) | 483 (92.9) | 163 (88.6) | 320 (95.2) | 0.007‡ |
P < 0.01.
P < 0.05.
P < 0.1.
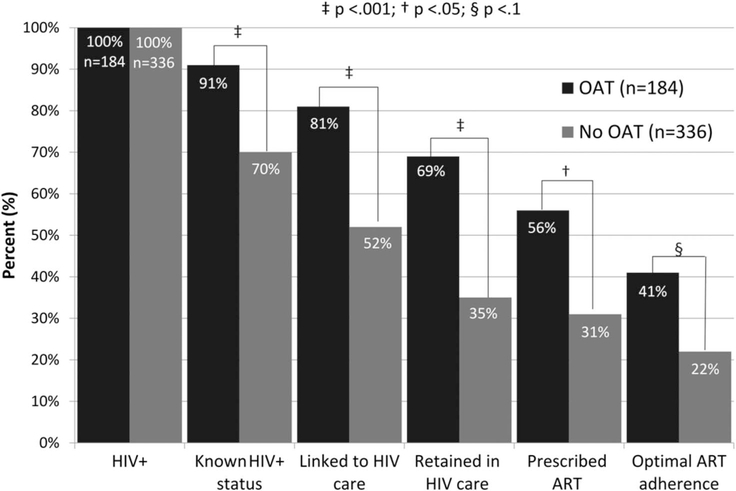
Figure 1 depicts the HIV treatment cascade, stratified by OAT status. The 2 groups differed significantly at each stage of the cascade, including being aware of their HIV diagnosis (91% on OAT vs. 71% not on OAT), linked (81% vs. 52%) and retained (69% vs. 35%) in HIV care, prescribed ART (56% vs. 31%), and optimal ART adherence (41% vs. 22%). The independent correlates of achieving the outcome for each step in the HIV treatment cascade are presented in Table 2. City of residence, being on OAT, and increasing age remained significant in each of the models, with receiving OAT being the strongest correlate of engagement in each stage of the continuum. Importantly, receiving OAT was associated with a 9.58-fold increased odds for being diagnosed, 7.13-fold for being linked to HIV care, 7.48-fold for being retained in HIV care, 7.42-fold for being prescribed ART, and 4.29-fold for optimal ART adherence.
FIGURE 1.
HIV continuum of care for PWID prescribed and not prescribed opioid agonist treatments (N = 520).
Table 2.
Results of Multivariate Analysis for Each Stage of the HIV Continuum of Care (N = 520)
| Aware of HIV Status | Linked to Care | Retained in Care | Prescribed ART | ART Adherence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AOR | 95% CI | P | AOR | 95% CI | P | AOR | 95% CI | P | AOR | 95% CI | P | AOR | 95% CI | P |
| City | |||||||||||||||
| Kyiv | Ref. | ||||||||||||||
| Odesa | 1.68* | 1.53–1.84 | <0.001 | 1.78* | 1.57–2.03 | <0.001 | 2.47* | 2.13–2.87 | <0.001 | 5.41* | 4.39–6.67 | <0.001 | 2.43* | 1.82–3.26 | <0.001 |
| Mykolaiv | 1.50* | 1.24–1.81 | 0.001 | 1.62* | 1.32–1.99 | 0.001 | 2.09* | 1.84–2.37 | <0.001 | 6.26* | 5.22–7.50 | <0.001 | 6.73* | 5.18–8.73 | <0.001 |
| Dnipro | 2.81* | 2.34–3.36 | <0.001 | 1.76* | 1.32–2.35 | 0.002 | 2.02* | 1.73–2.35 | <0.001 | 7.62* | 6.21–9.35 | <0.001 | 7.03* | 4.89–10.10 | <0.001 |
| Lviv | 0.78† | 0.63–0.97 | 0.030 | 0.53* | 0.48–0.59 | <0.001 | 0.98 | 0.81–1.18 | 0.818 | 3.47* | 2.50–4.81 | <0.001 | 4.55* | 3.09–6.72 | <0.001 |
| Prescribed OAT | |||||||||||||||
| No | Ref. | ||||||||||||||
| Yes | 9.58* | 5.22–17.57 | <0.001 | 7.13* | 2.92–17.38 | 0.001 | 7.48* | 3.10–18.00 | 0.001 | 7.42† | 1.38–39.74 | 0.025 | 4.29‡ | 0.87–22.59 | 0.078 |
| Addiction severity | |||||||||||||||
| Low to moderate | Ref. | ||||||||||||||
| Substantial to severe | 7.28† | 1.32–40.11 | 0.028 | 6.74 | 0.57–79.85 | 0.113 | 4.4 | 0.62–31.34 | 0.120 | 2.55 | 0.33–19.55 | 0.321 | 3.36 | 0.67–16.79 | 0.121 |
| Alcohol use disorder | Excluded from the model | Excluded from the model | Excluded from the model | Excluded from the model | |||||||||||
| No | Ref. | ||||||||||||||
| Yes | 1.08 | 0.54–2.16 | 0.809 | ||||||||||||
| Moderate to severe depression | |||||||||||||||
| No | Ref. | ||||||||||||||
| Yes | 2.21† | 1.18–4.16 | 0.020 | 2.34* | 1.47–3.74 | 0.003 | 1.97* | 1.30–2.98 | 0.006 | 1.64‡ | 0.89–3.03 | 0.098 | Excluded from the model | ||
| Sex | |||||||||||||||
| Male | Ref. | ||||||||||||||
| Female | 2.07† | 1.09–3.91 | 0.031 | 1.85‡ | 0.87–3.93 | 0.098 | 1.56 | 0.76–3.20 | 0.189 | 1.88 | 0.78–4.54 | 0.139 | 1.9 | 0.83–4.32 | 0.111 |
| Age (continuous) | 1.08† | 1.02–1.14 | 0.016 | 1.09* | 1.04–1.15 | 0.002 | 1.08* | 1.05–1.10 | <0.001 | 1.07* | 1.04–1.11 | 0.001 | 1.07* | 1.03–1.12 | 0.003 |
| In relationships | Excluded from the model | Excluded from the model | Excluded from the model | Excluded from the model | |||||||||||
| No | Ref. | ||||||||||||||
| Yes | 1.79 | 0.86–3.69 | 0.103 | ||||||||||||
| Housing status | |||||||||||||||
| Own place | Ref. | Excluded from the model | Excluded from the model | ||||||||||||
| Family or friends’ place | 2.45‡ | 0.87–6.91 | 0.081 | 2.24† | 1.10–4.59 | 0.032 | |||||||||
| Unstable housing | 11.27 | 0.39–327.99 | 0.136 | 2.03 | 0.28–14.70 | 0.434 | |||||||||
| Employment | Excluded from the model | Excluded from the model | Excluded from the model | ||||||||||||
| Full-time | Ref. | ||||||||||||||
| Part-time | 0.45 | 0.08–2.47 | 0.312 | ||||||||||||
| Seasonal | 1.11 | 0.17–6.99 | 0.903 | ||||||||||||
| Unemployed | 0.9 | 0.08–10.21 | 0.925 | ||||||||||||
P < 0.01.
P < 0.05.
P < 0.1.
Ref, referent.
Geographic differences were noted among participants at each stage of the continuum with significant increases in likelihood for achieving the desired outcome among participants from Odesa, Mykolaiv, and Dnipro, when compared with Kyiv. Participants from Lviv had significantly lower odds of being aware of HIV status [adjusted odds ratio (AOR): 0.78, 95% confidence interval (CI): 0.63 to 0.97], linked to care (AOR: 0.53; 95% CI: 0.48 to 0.59), but higher odds of being prescribed ART (AOR: 3.47; 95% CI: 2.50 to 4.81) and being adherent to ART (AOR: 4.55; 95% CI: 3.09 to 6.72), when compared with Kyiv. Compared with men, women were significantly more likely to be diagnosed and linked to care. Moderate to severe depression was associated with an increased odds of being engaged in all steps of the treatment cascade except optimal adherence. PWID who lived with family or friends were twice as likely to be diagnosed and linked to HIV care. Higher addiction severity was significantly associated with being diagnosed but not with other stages of the continuum.
DISCUSSION
To the best of our knowledge, this is the largest study of PWID in Ukraine that incorporates extensive data on the HIV treatment cascade and directly assesses whether receiving OAT is associated with HIV treatment outcomes in a LMIC where the HIV epidemic is concentrated in PWID. This study builds on the systematic review and meta-analysis of 32 studies,11 where only 2 (China and Indonesia) LMICs were included and were restricted to PLWH already engaged in care. Here, the contribution of OAT was examined across the entire HIV care continuum and confirmed that OAT significantly and consistently is associated with improvements at each stage. Striking is the finding that although Ukraine is generally doing poorly regarding the HIV care continuum, especially in the latter stages of the cascade, PWID on OAT meet the first target of the UNAIDS 90–90-90 strategy: HIV diagnosis and status awareness.7 This finding stands out in contrast with the HIV diagnosis rate among the general population (44%)26 or among prisoners (49%)27 in Ukraine and is likely explained by the proliferation of integrated care sites that simultaneously provide OAT, HIV, and tuberculosis services, including routine HIV testing of OAT clients.14 For PWID not on OAT, 71% had been diagnosed, which is higher than national averages, but similar to that found in HIV prisoners who were PWID (74%).27 Routine HIV testing at OAT sites for previous OAT clients and through harm reduction efforts for clients who were never on OAT may have contributed to the higher HIV diagnosis in PWID not on OAT relative to the general population. Although OAT scale-up has not increased markedly over the past 5 years because of many individual and structural factors,28–30 strategies that further scale-up OAT coverage beyond the 2.9% of the 347,000 PWID would greatly align Ukraine with UNAIDS targets.
For all Ukrainians, but especially PWID, further steps in the HIV cascade are far below UNAIDS goals. UNAIDS calls for treatment engagement (linked and retained in care) for 82% of all PLWH; yet, the gaps for Ukrainian PWID, irrespective of OAT status, are among the highest (81% vs. 52% and 69% vs. 35%, respectively). In most settings, not being engaged in HIV care contributes to most of new HIV infections,31 weakening HIV treatment as prevention benefits. Although low ART prescription overall may reflect national standards that fail to incorporate universal ART coverage for PLWH, marked differences remain based on OAT status (56% vs. 31%). The low ART prescription in PWID not on OAT in Ukraine is similar to reports for PLWH who are not PWID from resource-constrained settings in sub-Saharan Africa with generalized HIV epidemics (32% vs. 36%).32 Yet, proximal cascade stages in the sub-Saharan generalized epidemic, such as HIV diagnosis (51% vs. 71%), are where the greatest need remains. Once diagnosed in sub-Saharan Africa, however, ART uptake is higher (63% vs. 51%). Although ART prescription in PWID remains low in Ukraine, higher rates in OAT clients might be explained by physicians being unwilling to prescribe ART to PWID, unless they are also prescribed OAT.10 Alternatively, that PWID who prescribed OAT received treatment in integrated care settings that provided OAT and ART medication free of charge, a strategy which have been associated with better HIV quality indicators, including ART prescription and monitoring.14 Suboptimal treatment engagement in PWID may also include the presence of multiple comorbidities, and various social, physical, political, and economic factors that may prevent PWID from effective treatment engagement.9,33
OAT is highly stigmatized in Ukraine, and findings from previous studies suggest that family and friends influence OAT uptake.17,29 The various forms of social capital, when supportive, should be harnessed to expand OAT; yet, in this study, we did not find evidence for the association between being in relationships (supportive or otherwise) and the HIV treatment cascade. Previous studies have found that social support was provided by “trusted others,”34 which might include others beyond their primary relationship, including case manager and adherence counselors who could also provide social support in Ukraine after patients are linked to care, but other types of support were not measured.
We noted 2 counterintuitive findings in this study; first, that depression was associated with the first 3 cascade steps (being diagnosed, linked, and retained in care), and, second, higher levels of addiction severity were associated with being diagnosed with HIV but not with the remaining stages of the continuum. In Ukraine, where resources are constrained, HIV services specifically target patients with the most advanced stages of the disease. For example, case management services may initially focus on PWID with the highest burden of disease, including those with higher addiction severity. Once linked to care, enabling resources such as psychological and adherence support are often provided to fully engage clients, including those prescribed ART. Elsewhere, both depression35 and higher addiction severity36 are often associated with poor treatment outcomes, but in Ukraine, it is possible that the enabling community resources available were sufficient in some settings to overcome these obstacles. Moreover, the heightened comorbidity from depression and higher addiction severity may have triggered increased provision of psychosocial, case management, and adherence support services, including antidepressant treatment, that may have improved the HIV cascade outcomes further along the continuum.37,38 An alternative direction of this association is also plausible. PWID aware of their diagnosis and going through the fragmented system of health care in Ukraine may suffer from more depressive symptoms compared with those who are not aware of their HIV status. This explanation is especially likely given that depressive symptoms are rarely addressed within the health care systems in Ukraine. Given the sheer magnitude of depression in PWID, routine screening and treatment is warranted to optimize HIV outcomes.9
Concerning here is the marked geographic differences in treatment engagement even after controlling for individual factors. Epidemiological and sociopolitical differences exist between regions (oblasts) in Ukraine. Policies by local health care authorities and their practices may contribute to treatment engagement differences that influence outcomes beyond the individual level. Half of officially registered PLWH live in 3 oblasts: Donetsk, Dnipro, and Odesa, and the highest HIV prevalence in PWID are in Odesa, Dnipro, Donetsk, and Mykolaiv. Nevertheless, significant time delays between diagnosis and registration also exist in Lviv, particularly for female PWID, documented previously using medical records data.39 Integrated care sites exist in all 5 surveyed regions but differ in size and scope. Clinic-based and structural factors may be partly responsible for such variation, including the extent to which integrated services have been expanded.14 Clinical resources may also influence HIV outcomes. For example, there are only 8 ART-prescribing physicians available for approximately 11,000 registered (ie, linked) PLWH in Kyiv, a city of almost 3 million, the highest patient-to-physician ratio (1375:1) in the country. Strategies that expand the capability of nonspecialty physicians to provide HIV treatment, such as through tele-educational collaborative environments as deployed in the Project ECHO, may address acute shortages of specialty clinicians.40 Such strategies may be especially beneficial, given the recent health care reform in Ukraine that emphasizes expansion of access to medical care through greater contact with primary health care physicians.41
Future research in Ukraine should concentrate efforts on assessing the HIV care continuum based on clinically confirmed data, especially suppressed viral load as an ultimate goal of the HIV care continuum. Expanding OAT in Ukraine is also crucial to address the HIV epidemic, especially given its relatively low cost. Evidence-based implementation strategies such as the Network for the Improvement of Addiction Treatment (NIATx) are now underway.42 NIATx is treatment improvement model for behavioral health using organizational change projects to improve entry and retention outcomes.43 NIATx strategies that incorporate change projects focused on PWID with comorbidities (eg, depression and higher addiction severity), build infrastructure to support integrated services, and build physician infrastructure in selected regions have the highest potential of meeting UNAIDS targets for HIV prevention and treatment.41
Despite these important new findings, this study is not without limitations. First, the cross-sectional nature of the study cannot confirm causality. Second, despite the use of stratified sampling and statistical weighting, the data may not be free from selection bias, but the larger sample size may in part overcome this concern. Third, self-reported information is prone to bias, but the use of computer-assisted, self-administered instrument markedly reduces underreporting of sensitive information. Fourth, we did not measure CD4 counts, which might have influenced ART prescription that was limited to CD4 thresholds under 350 cells per milliliter during data collection. Fifth, although we measured structural factors based on regions, this variable is insufficiently granular to fully assess differences related to staffing, convenience, ART availability, and physician attitudes toward PWID. Finally, despite a wide range of potential and unmeasured factors that might influence the HIV care continuum, residual confounding may exist; yet, the sheer magnitude of the association of OAT on each stage of the HIV continuum is unlikely to be reduced markedly.
CONCLUSIONS
To the best of our knowledge, this study provides one of the first epidemiologic estimates characterizing the HIV care continuum among PWID along the entire continuum of care globally, but especially in EECA and in Ukraine where HIV incidence and mortality continue to increase.1 Central to these findings is that after controlling for other covariates, the receipt of OAT portends the greatest achievement with every step in the HIV treatment cascade. Such strategies are crucial for Ukraine to meet UNAIDS 90–90-90 targets. Looming high against meeting this target in Ukraine is the challenge of expanding OAT beyond the current coverage level of 2.9%. OAT expansion efforts in Ukraine will require interventions that target the individual, the clinic setting, policies, and funding because multilevel factors have been documented to restrict OAT expansion.17,29 Finally, as international donors consider reducing funding for HIV services in Ukraine and primary financing shifts to Ukraine’s government, inadequate funding may undermine the many gains that have been achieved in HIV prevention and treatment, but central among the sustainability factors is scaling up OAT.
ACKNOWLEDGMENTS
The authors are thankful to our colleagues Tatiana Fomenko and Tatiana Prokhorova who were greatly involved during data collection and cleaning. The authors extend their gratitude to their local research assistants and all the survey participants in each city for their dedication and time.
Supported by the National Institute on Drug Abuse for funding for research (R01 DA029910, R01 DA043125, and R01 DA033679) and career development (K24DA017072 and K01DA037826) and the Global Health Equity Scholars Program funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases (Research Training Grant R25 TW009338) as well as New York State International Training and Research Program through an in-country training grant funded by the Fogarty International Center (D43TW000233).
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Update 2016. Geneva, Switzerland; 2016: Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. Accessed May 28, 2016. [Google Scholar]
- 2.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. [DOI] [PubMed] [Google Scholar]
- 3.Alliance for Public Health. Monitoring of Behavior and HIV Prevalence in People Who Inject Drugs and Their Sexual Partners in Ukraine: Alliance for Public Health; 2015. Available at: http://aph.org.ua/wp-content/uploads/2015/09/Monitoryng-povedinky-SIN_PROEKT.pdf. Accessed January 15, 2018. [Google Scholar]
- 4.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016; 388:1228–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bank TW. Prevalence of HIV, Total (% of Population Ages 15–49). UNAIDS Estimates. Overview Per Country 2014; 2016. Available at: http://data.worldbank.org/indicator/SH.DYN.AIDS.ZS?end=2014&start=2003. Accessed July 14, 2016. [Google Scholar]
- 6.Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Pol. 2014; 25:53–60. [DOI] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS. 90–90-90- an Ambitious Treatment Target to Help End the AIDS Epidemic. New York, NY; 2014. Available at: http://www.unaids.org/en/resources/documents/2014/2090-2090-2090. Accessed March 12, 2015. [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamarulzaman A, Altice FL. Challenges in managing HIV in people who use drugs. Curr Opin Infect Dis. 2015;28:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enrico G, Ferro EG, Culbert GJ, Wickersham JA, et al. Physician decisions to defer antiretroviral therapy in key populations: implications for reducing HIV incidence and mortality in Malaysia. Open Forum Infect Dis. 2016;3:ofw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis. 2016;63:1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8: e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaub M, Chtenguelov V, Subata E, et al. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Int J Drug Pol. 2010;21:229–233. [DOI] [PubMed] [Google Scholar]
- 14.Bachireddy C, Soule MC, Izenberg JM, et al. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Information in Quantitative and Qualitative Non-Personal Characteristics of SMT Patients as of April 1, 2018: Public Health Center Ukraine; 2018. Available at: https://phc.org.ua/en/pages/diseases/opioid_addiction/stat-docs. Accessed May 15, 2018. [Google Scholar]
- 16.Berleva G, Sazonova Y. Report for the Study “Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine”: ICF Alliance for Public Health; 2017. Available at: http://aph.org.ua/wp-content/uploads/2016/07/mio2016high.pdf. Accessed January 15, 2018. [Google Scholar]
- 17.Makarenko I, Mazhnaya A, Polonsky M, et al. Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend. 2016;165: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Social Probl. 1997;44:174–199. [Google Scholar]
- 19.Giordano TP, Guzman D, Clark R, et al. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74–79. [DOI] [PubMed] [Google Scholar]
- 20.Babor TF, Higgins-Biddle JC, Saunders JB, et al. The Alcohol Use Disorders Identification Test (AUDIT). Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Zhang W, O’Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7:e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner H The Drug Abuse Screening Test (DAST): Guidelines for Administration and Scoring. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- 24.Berleva G, Dumchev K, Kasianchuk M, et al. Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine as of 2012. Kyiv, Ukraine: ICF International HIV/AIDS Alliance in Ukraine; 2012. [Google Scholar]
- 25.StataCorp. Stata: Release 14: Statistical Software. College Station, TX: StataCorp LP; 2014. [Google Scholar]
- 26.Levi J, Raymond A, Pozniak A, et al. Can the UNAIDS 90–90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health. 2016;1:e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azbel L, Wickersham JA, Grishaev Y, et al. Correlates of HIV infection and being unaware of HIV status among soon-to-be-released Ukrainian prisoners. J Int AIDS Soc. 2014;17:19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bojko MJ, Mazhnaya A, Makarenko I, et al. “Bureaucracy & Beliefs”: assessing the barriers to accessing opioid substitution therapy by people who inject drugs in Ukraine. Drugs Educ Prev Policy. 2015;22: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bojko MJ, Mazhnaya A, Marcus R, et al. The future of opioid agonist therapies in Ukraine: a qualitative assessment of multilevel barriers and ways forward to promote retention in treatment. J substance abuse Treat. 2016;66:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelenev A, Shea P, Mazhnaya A, et al. Assessment of barrier severity and willingness to enter opioid agonist treatment among people who inject drugs in Ukraine. Drug Alcohol Depend. 2018;190:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588–596. [DOI] [PubMed] [Google Scholar]
- 32.UNAIDS. Global Report: UNAIDS report on the Global AIDS Epidemic 2013. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [Google Scholar]
- 33.Altice FL, Kamarulzaman A, Soriano VV, et al. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001; 28:47–58. [DOI] [PubMed] [Google Scholar]
- 35.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. [DOI] [PubMed] [Google Scholar]
- 36.Azar P, Wood E, Nguyen P, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infect Dis. 2015;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith-Rohrberg D, Mezger J, Walton M, et al. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S48–S53. [DOI] [PubMed] [Google Scholar]
- 38.Malta M, Magnanini MM, Strathdee SA, et al. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. [DOI] [PubMed] [Google Scholar]
- 39.Dethier D, Rybak N, Hirway P, et al. The changing face of women living with HIV in western Ukraine. Int J STD AIDS. 2018;29:318–323. [DOI] [PubMed] [Google Scholar]
- 40.Arora S, Kalishman S, Dion D, et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff (Project Hope). 2011;30:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morozova O, Dvoriak S, Pykalo I, et al. Primary healthcare-based integrated care with opioid agonist treatment: first experience from Ukraine. Drug Alcohol Depend. 2017;173:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarty D, Gustafson DH, Wisdom JP, et al. The Network for the Improvement of Addiction Treatment (NIATx): enhancing access and retention. Drug Alcohol Depend. 2007;88:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madden L, Bojko MJ, Farnum S, et al. Using nominal group technique among clinical providers to identify barriers and prioritize solutions to scaling up opioid agonist therapies in Ukraine. Int J Drug Pol. 2017;49: 48–53. [DOI] [PubMed] [Google Scholar]