Abstract
The high incidence of degenerative tears and prevalence of retears (20–95%) after surgical repair makes rotator cuff injuries a significant health problem. This high retear rate is attributed to the failure of the repaired tissue to regenerate the native tendon-to-bone insertion (enthesis). Biological augmentation of surgical repair such as autografts, allografts, and xenografts are confounded by donor site morbidity, immunogenicity, and disease transmission, respectively. In contrast, these risks may be alleviated via growth factor therapy, which can actively influence the healing environment to promote functional repair. Several challenges have to be overcome before growth factor delivery can translate into clinical practice such as the selection of optimal growth factor(s) or combination, identification of the most efficient stage and duration of delivery, and the design considerations for the delivery device. Emerging insight into the injury-repair microenvironment and our understanding of growth factor mechanisms in healing are informing the design of advanced delivery scaffolds to effectively treat rotator cuff tears. Here, we review potential growth factor candidates, design parameters and material selection for growth factor delivery, innovative and dynamic delivery scaffolds, and novel therapeutic targets from tendon and developmental biology for the structural and functional healing of rotator cuff repair.
Keywords: Rotator cuff, tendon, enthesis, growth factor, delivery, scaffold
Graphical abstract
1. Introduction
Rotator cuff tears are one of the most leading musculoskeletal injuries in the United States with over 200,000 repair procedures done annually and an estimated $474 million dollars in health care costs (Novakova et al., 2017; Pedowitz et al., 2011). The rotator cuff is a set of muscle-tendon units that stabilize the shoulder joint. It includes the supraspinatus, infraspinatus, subscapularis, and the teres minor and major. These tendons attach to the bone via a specialized tissue called the enthesis, which is a structurally continuous tissue with four transitional zones, fibrous tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bony attachment (Apostolakos et al., 2014) as illustrated in Figure 1. The chronic degeneration of the enthesis with age is the primary cause of rotator cuff tears but acute tears also occur because of injury.
Figure 1. Structure and constitution of the native fibrocartilaginous enthesis.
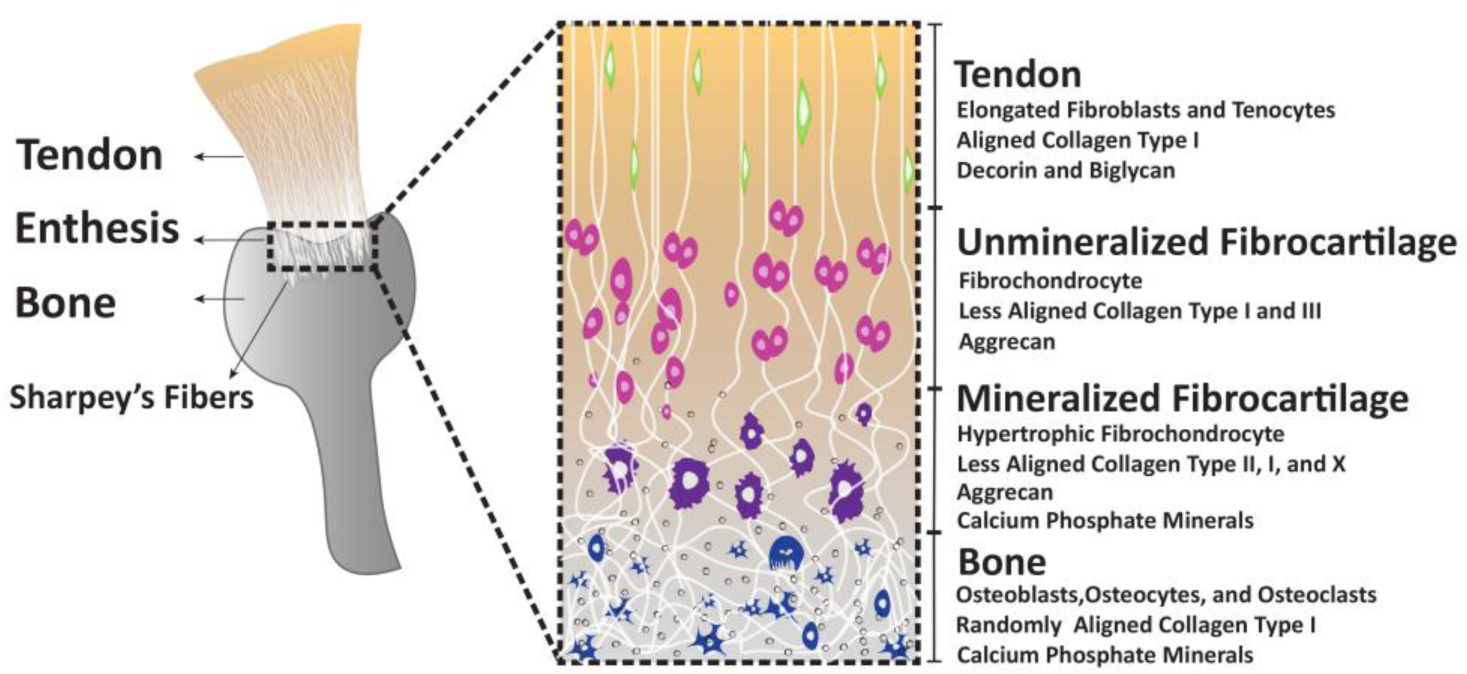
Tendon anchors into the bone via a transitional fibrocartilaginous enthesis that constitutes tendon, fibrocartilage, unmineralized fibrocartilage, and bone. The transitions in cellular and ECM constitution are illustrated in the inset.
When a tear occurs, physicians manage the injury both surgically and non-surgically (Harrison and Flatow, 2011) depending on the severity, size, and pattern of the tear. For large and painful tears, or when non-surgical therapy fails to improve painful symptoms in smaller tears, surgical repair is considered. The current method for surgical repair of rotator cuff tears utilizes a single/double row suture technique to re-approximate the torn tendon back to its insertion site on the bone. However, the surgically repaired tendon insertion tissue (enthesis) is prone to high rate of retear between 20–95%, depending upon the extend of tears (Derwin et al., 2010b; Galatz et al., 2004). This high retear rate is attributed to several factors such as age, chronicity of tears, poor vascularization, increased fibrosis, musculo-tendinous retraction, fatty infiltration, peritendinous adhesions, and increased stress concentration at the insertion site (Galatz et al., 2004; Melis et al., 2009; Meyer et al., 2012; Saadat et al., 2016). These factors constitute to the formation of a highly disorganized scar tissue that has poor biomechanical properties. Clinical repair strategies seek to recreate the native enthesis tissue organization (Figure 1.) by reapproximating the torn tendon to its anatomic footprint, providing adequate initial fixation strength for the repair, minimizing potential gap formation, and maintaining mechanical support until sufficient tissue formation.
Surgical repair of rotator cuff tears is confounded by musculo-tendinous retraction and tendon retears that tend to occur within 12 weeks after surgery (McCarron et al., 2013). To prevent tendon retraction during this early rehabilitative phase, suture protection from intra-tendon movement and sub-acromial bursa friction is desirable. Augmentation of surgical repairs using a patch/scaffold has the potential to protect the suture from this movement and friction, mechanically support the repair, and facilitate biological healing. Patch augmentation is recommended for grade II-V tears, which includes small and medium full thickness tears (< 3 cm involving one tendon) to irreparable large massive tears (3–5 cm involving 2–3 tendons) that are unable to reapproximate to the tuberosity with low tension (Derwin et al., 2010a). Tissue-based and engineered materials have been investigated for patch augmentation.
Tissue-based scaffolds such as autografts, xenografts, and allografts can reinforce the repair by acting as a bridge for cellular infiltration, collagen assembly, and mechanical support. These matrices offer natural porosity, 3-dimensional extracellular matrix cues, and growth signals to promote host tissue integration. Autografts have excellent biocompatibility and graft integration but have limited availability and cause donor-site morbidity. Advances in decellularization techniques have led to improved xenografts (Conexa®) (Lederman et al., 2016) and allografts (Graftjacket®) (Barber et al. 2012), which are more accessible, but they still carry the risk of infection and severe inflammatory reaction from retained source DNA (Walton et al. 2007). These scaffolds also suffer from unpredictable degradation rate, inadequate mechanical properties, and non-specific induction ability (Chen et al., 2009). Such concerns have generated considerable interest in the development of engineered grafts/scaffolds for rotator cuff repair augmentation.
Engineered scaffolds using both natural and synthetic biomaterials and their combinations can provide defined properties for rotator cuff repair. Natural biomaterials such as fibrin, collagen, elastin, and hyaluronic acid present extracellular cues that support cell infiltration and tissue repair, but degrade rapidly and lack the mechanical characteristics necessary for load bearing applications. On the other hand, synthetic non-/biodegradable polymers can be designed to have optimum mechanical properties to support the repaired tissue. Non-biodegradable polymers, although mechanically robust (Ciampi et al. 2014) generally have poor biocompatibility because of frustrated phagocytosis and inflammation. In contrast, biodegradable polymers may provide better biocompatibility. The predictable degradation characteristics of these polymers can be tailored to provide initial mechanical support during the critical rehabilitative phase and slow degradation to permit drug delivery and tissue integration. However, these synthetic scaffolds lack the necessary bioactive cues instructive for tissue repair (Longo et al., 2012). Creation of an instructive environment is significant in the repair of the rotator cuff, which is characterized by a poorly vascularized space, with a majority of degenerative tears seen in the aged population with compromised tissue repair capability. Therefore, tissue repair strategies have sought to incorporate instructive bioactive agents such as cells and/or signaling molecules in biomaterials to augment repair.
Both stem and tissue-typic cell delivery via biomaterials has shown regenerative benefits in rotator cuff healing (Peach et al., 2017) (Funakoshi et al., 2005). Cell delivery faces the challenges of availability, seeding, survival, and specificity, with stem cells facing additional regulatory and ethical barriers. Stem cells and tissue-typic cells are thought to contribute to healing via autocrine/paracrine signaling through growth factors and cytokines. Growth factors delivery from scaffolds; therefore, may be a more simple and direct approach to improve healing outcomes in rotator cuff repair.
Growth factors have the ability to enhance healing response by increasing cell recruitment, proliferation, differentiation, ECM synthesis, and remodeling at the repair site. Several studies have investigated the regenerative potential of growth factors in small animal models and a handful of large animal models (Hee et al., 2011; Novakova et al., 2017; Tokunaga et al., 2015b; Uggen et al., 2010; Zhao et al., 2014). However, growth factor augmented repair, often with better mechanical properties than the control repair groups are still far from achieving the same biomechanical properties as the native enthesis. Several questions need to be addressed to achieve effective growth factor delivery for rotator cuff repair as follows:
Which growth factor(s) or combinations of growth factors are needed?
What is the optimum dose and duration of growth factor delivery?
How to deliver these growth factors over a therapeutic time window while maintaining their bioactivity?
Here, we review innovative growth factor delivery strategies to promote rotator cuff repair focusing on promising growth factor candidates, design characteristics and material selection for growth factor delivery scaffolds, and novel therapeutic targets from tendon and developmental biology for rotator cuff repair.
2. Mechanisms of Enthesis Healing and the Role of Growth Factors
Enthesis healing takes place in three phases: inflammatory, reparative, and remodeling (Yang et al., 2013). The inflammatory phase (day 0 to day 7) is characterized by the release of growth factors and pro-inflammatory cytokines needed for the recruitment of inflammatory cells at the site of injury (Reddy et al., 1999). These inflammatory cells phagocytize necrotic tissue and induces further growth factor and cytokine release that is needed for the recruitment and proliferation of cells for repair (Hays et al., 2008). Induction of angiogenic growth factors such as VEGF stimulates blood vessel formation in the repair tissue. In the reparative/formative phase (week 1 to week 8), there is active proliferation of fibroblasts and differentiation of stem cells to relevant tissue-typic cells such as osteoblasts, chondrocytes, and tenocytes (Aicale et al., 2017; Andarawis-Puri et al., 2015; Koike et al., 2005). The proliferated fibroblasts differentially synthesize collagen type I, II, III and X matrix over the following 8-week period. This improves the mechanical strength of the tissue because of the large collagen-based callus formation (Aicale et al., 2017; Andarawis-Puri et al., 2015). In the final remodeling phase (8 week to 6 months), cellularity and matrix synthesis is reduced. The disorganized collagen is gradually resorbed and replaced by a more aligned and cross-linked collagen. Over the following months, continuous remodeling of the injured site leads to a decrease in the area of the callus and improvement in mechanical strength. Nevertheless, complete regeneration of the tissue does not take place (Maffulli et al., 2002).
Growth factors are expressed during all stages of enthesis healing. They influence multiple pathways by signaling with different cell types in a complex manner to stimulate a response. Studies have sought to understand the temporal expression profile of the main growth factors involved during enthesis repair and their targets (Dahlgren et al., 2005; Kobayashi et al., 2006; Würgler-Hauri et al., 2007). Wurgler Hauri et al. studied the expression of BMP-12,13,14, b-FGF, CTGF, PDGF, TGF-β1, and COMP-1 in tendon-to-bone healing in a rat supraspinatus tendon insertion defect over a 16 week period (Würgler-Hauri et al., 2007). They showed that all growth factors were upregulated during the inflammatory stage, followed by a return to control or undetectable levels by 16 weeks. PDGF-B expression was detectable only at early time points at the insertion site and it coincides with early collagen I synthesis (Ojima et al., 2003) and activation of other growth factors such as TGF-β1 (Ojima et al., 2003; Porsch et al., 2014). Coinciding with the early upregulation of PDGF-B, the TGF-β1 expression was upregulated again at week 2, and independently at week 8. The delayed upregulation of TGF-β1 at week 8 can be correlated to the scarring process (Dahlgren et al., 2005; Galatz et al., 2006). BMP-12 was moderately expressed during all time points, with a significant increase around 8 weeks during the remodeling phase (Würgler-Hauri et al., 2007). BMP-12 has previously been reported to be expressed more in active fibroblasts (non-terminally differentiated, high-growth factor expressing) than in the resident tenocytes showing its active involvement in tissue remodeling (Fu et al., 2003; Wolfman et al., 1997). Thus, growth factors exhibit unique temporal expression profiles that correlate to specific stages in the enthesis repair process (Dahlgren et al., 2005; Würgler-Hauri et al., 2007). Although much information is known about the temporal expression of growth factors during rotator cuff healing, the mechanisms through which these growth factors influence healing are not completely understood. Such insight can be used to develop growth factor delivery devices that can spatio-temporally regulate therapeutic targets in rotator cuff repairs.
2.1. Mechanism of Scar Tissue Formation in Rotator Cuff Healing
The surgically repaired rotator cuff tendon insertion lacks the organized fibrocartilage structure of the native enthesis; instead, they heal via disorganized fibrovascular scar formation. TGF-β signaling plays an important role in pathological scarring across multiple organ systems (Penn et al., 2012) and has also been indicated in the formation of fibrovascular scar tissue in the rotator cuff (Liu et al., 2014). Of the three individual isoforms, TGF-β1 expression is positively correlated to scar tissue formation in adult tissue repairs (Wang et al., 2000). A gradual increase in TGF-β1 expression is seen in adult acute rotator cuff tears with a peak at day 10. In massive rotator cuff tears involving more than two tendons and a sub-scapular nerve damage, the scar tissue formation in the rotator cuff muscles was concomitant to the increase in TGF-β1 (Galatz et al., 2006). In another study, treatment of rotator cuff explants with TGF-β1 resulted in increased expression of alpha smooth muscle actin (α-SMA) characteristic of scar forming myofibroblasts (Premdas et al., 2001). On the other hand, fetal wounds heal without scarring, do not express TGF-β1, but TGF-β3 instead (Galatz et al., 2007). Thus, the levels of individual TGF-β isoforms can significantly influence the process of scar tissue formation in rotator cuff tendon healing (Pryce et al., 2009).
The TGF-β1 isoform inflicts scar tissue formation via a number of mechanisms. The key cellular mechanisms involved in the scarring process are as follows: TGF-β1 induces differentiation of fibroblasts into myofibroblasts via expression of α-smooth muscle actin (Desmoulière, 1995). Myofibroblasts have been identified as the primary source of disorganized collagen III matrix synthesis in many fibrotic disorders (Todd et al., 2012). These myofibroblasts promote the induction of more TGF-β1 and other fibrogenic chemokines to the repair site (Zhang et al., 1995). TGF-β1 in turn ensures the survival of the differentiated myofibroblast population by preventing them from undergoing apoptosis (Zhang and Phan, 1999). The consequent increased callus size as a result of exaggerated matrix production and myofibroblast hyperplasia is further promoted by an inhibition of matrix metalloprotease activity (MMPs) (Farhat et al., 2015; Fenwick et al., 2008). Since an extensive discussion of the TGF-β mediated scarring phenomenon is beyond the scope of this review, we recommend the reader to the following comprehensive reviews on the subject: (Beanes et al., 2003; Penn et al., 2012).
3. Growth Factor Delivery
Growth factor delivery devices for rotator cuff repair include fibrous mats, braided constructs, sponges, and hydrogels made from synthetic polymers, extracellular matrix components, and inorganic materials. To meet the challenge of repairing a load bearing tissue, growth factor delivery devices are also designed to mechanically support the tissue during repair. These devices are superimposed on the tendon from the bursal-side in the sub-acromial space (Rodeo et al., 2007) or placed between the tendon and bone as an interpositional graft (Hee et al., 2011) and seek to promote the following targeted repair outcomes:
The gap formed between the tendon and the bone is closed with healed tissue.
The collagen fibers in the newly formed fibrocartilaginous tissue insert and anchor into the bone.
The repaired tissue possesses the biomechanical properties necessary to support loading at the insertion site.
3.1. Design Characteristics of Growth Factor Delivery Devices
Regenerating the native structure of the rotator cuff enthesis would require recreation of the specialized structure and mechanical properties of this tissue. For effective regeneration, growth factor delivery devices for enthesis repair must fulfil following design criteria some of which have been reviewed elsewhere (Chainani and Little, 2016; Cheung et al., 2010):
Support surgical handling, pliability, and suturability in mini-open and arthroscopic repairs.
Be sterilizable without affecting growth factor activity and mechanical properties of the scaffold.
Maintain bioactivity of the growth factor through the shelf life.
Be recumbent to the tendon-to-bone insertion with dimensions tailored to the sub-acromial space to prevent impingement.
Withstand the complex multiaxial loading of the tissue at the re-attachment of the tendon-to-bone through the duration of repair.
Promote endogenous recruitment of stem and progenitor cells to augment tendon and fibrocartilage formation.
Minimize fibro-vascular scar formation.
Degrade slowly with non-toxic degradation products.
Be resistant to infection.
Growth factors are incorporated in scaffolds via the following three main approaches: (1) physical adsorption onto scaffolds by simple dip coating (this method has poor control over the release rate with most of the growth factor being released within the first day (Uggen et al., 2010) and up to a period of 2 weeks (Tokunaga et al., 2015a), (2) encapsulation within polymeric or ceramic nano/micro-sphere scaffolds that can be injected or implanted at the defect site via incorporation inside hydrogels or scaffolds providing controlled release kinetics in the tissue microenvironment and enhanced growth factor protection (Lee et al., 2004; Yilgor et al., 2009), (3) Direct 3-D dispersion in a matrix or immobilization to a scaffold via electrostatic interactions or covalent bonding ensures controlled release and/or presentation of target growth factors (Park et al., 2009; Wang et al., 2017). Recently, supercritical fluid technology has been innovatively used to ensure three-dimensional encapsulation and controlled release of selected growth factors from a scaffold (Diaz-Gomez et al., 2016; Govoni et al., 2017). The reader is directed to the following excellent reviews for further reading on different growth factor delivery strategies (Santo et al., 2013a, 2013b; Wang et al., 2017).
3.2. Material Selection for Growth Factor Delivery Device
Direct delivery of growth factors by local injection at the site of injury has shown to promote tendon healing (Lamplot et al., 2014; Shah et al., 2013). Such delivery faces the limitations of growth factor loss via circulation and degradation, and need multiple injections that reduces patient compliance. The short half-life of growth factors reduce the efficiency of direct injection. To overcome these limitations, growth factor impregnated surgical sutures were applied (Dines et al., 2007b). These sutures are physician friendly, provide mechanical support, and can simultaneously deliver growth factors locally to the site of injury. However, burst release of growth factors within hours of coming in contact with body fluids and the limited loading capacity of sutures minimize their efficiency for rotator cuff enthesis repair that takes place over a period of weeks to months (Cummings et al., 2012; Fuchs et al., 2012). Therefore, the development of effective growth factor delivery devices that extend the delivery over the therapeutic repair window is of great interest.
Engineered synthetic biodegradable and natural extracellular matrix-based scaffolds loaded with growth factors have shown promise in regenerating the enthesis (Smith and Grande, 2015); (Peterson et al., 2015). Synthetic biodegradable polyesters such as polylactic acid (PLA), polyglycolic acid (PGA), polylactic-co-glycolic acid (PLGA), and polycaprolactone (PCL) have been used as scaffolds for mechanical reinforcement and growth factor delivery in rotator cuff repair (Chainani and Little, 2016; Derwin et al., 2009; Hakimi et al., 2013; MacGillivray et al., 2006). These polymers can be tailored to control the degradation properties, which can influence tissue- mechanics, infiltration, and remodeling, and enable controlled growth factor delivery. In an ACL reconstruction model, controlled release of bFGF from a poly-L-lactic acid (PLLA) scaffold enhanced ligament-like tissue formation and osseointegration, which compensated for the degradation and loss of mechanical support of the scaffold (Kimura et al., 2008). PLGA offers excellent pliability as well as the ability to tune the release rate by modifying the lactide/glycolide content and molecular weight (Babensee et al., 2000; Sahoo et al., 2010). However, PLGA exudes a majority of the growth factor payload via burst release within hours of implantation (Gombotz and Pettit, 1995). Furthermore, the accumulation of acidic polymeric breakdown products leads to synovial inflammation, denaturation and degradation of the growth factors encapsulated in the matrix, and increased matrix metalloprotease (MMP) activity. The combination of elevated acid levels and MMP activity can cause osteolysis of the cortical bone and failure of the repair tissue to integrate (Lipner et al., 2015). To overcome this limitation, Zhao et al. developed a PLGA electrospun core-sheath membrane loaded with b-FGF, which slowed down the burst release, and maintained b-FGF activity during the first 3 weeks in vitro (Zhao et al., 2014). This scaffold showed minimum inflammation and significantly improved collagen organization and ultimate load to failure of the healing enthesis in a rat rotator cuff chronic repair model. Although with a lesser elastic modulus than PLGA, PCL degrades slower and has greater failure strain, making it an excellent material for sustained delivery of growth factors in the poorly vascularized space of the rotator cuff (Lam et al., 2009; Woodruff and Hutmacher, 2010). Moreover, the hydrolytic degradation product of PCL, 6-hydroxylcaproic acid, has shown no osteolysis in long-term implantation studies in animal models (Woodruff and Hutmacher, 2010).
Natural biodegradable polymer chitin was assessed for scaffold augmentation in a rabbit model for rotator cuff insertion repair (Funakoshi et al., 2006). A 10 mm surgical defect was created at the humeral insertion of the infraspinatus tendon and the defect was covered with the chitin patch. Tendon-to-bone interphase covered with the chitin patch demonstrated greater cell number, better collagen fiber alignment, and greater mechanical strength than the unrepaired control. Although chitin faces batch-to-batch variability based on source it has been successfully applied for macromolecular delivery in other orthopedic applications (Jayakumar et al., 2011). We believe this material shows great potential in growth factor delivery for rotator cuff repair.
Extracellular matrix-derived materials, such as collagen, gelatin, heparin, and blood-derived fibrin, have been used to load growth factors for tendon-to-bone insertion repairs (Hee et al., 2011; Ide et al., 2009; Manning et al., 2011; Tokunaga et al., 2015a; Zhang et al., 2016). However, these polymers lack the mechanical properties necessary to support the complex loading at the rotator cuff insertions (Kosuge et al., 2013). To overcome this challenge, advanced composites that combine the defined mechanical properties of synthetic polymers with the bioactive cues from ECM proteins have been investigated (Leong et al., 2015; Manning et al., 2011). A bioresorbable poly-4 hydroxy butyrate (P4HB) mesh embedded in a porous type I collagen sponge (Biofiber-CM) and loaded with fibroblast growth factor mimicking peptide (F2A) was tested in an ovine rotator cuff repair model (Peterson et al., 2015). P4HB is more pliable than traditional biodegradable polymers and the scaffold made from this material is easily inserted through an arthroscopic cannula. Its monofilament fibers resorb completely within a year, and has excellent biocompatibility (Martin and Williams, 2003). This scaffold-augmented repair showed improved tendon-like tissue coverage at the foot print, new bone formation at the interface, and lesser tears at the insertion on the humeral head (Peterson et al., 2015). Such combination strategies using existing FDA-approved materials to advance rotator cuff therapy can ensure faster translatability of promising materials.
3.3. Growth Factor Selection
Growth factors offer a simple and direct approach to improve healing outcomes in rotator cuff repair. Repair of rotator cuff entails the repair of the enthesis, which encompasses tendon, fibrocartilage, and bone. Interventions to repair such a complex tissue must factor in the heterogeneity of the tissue, and particularly the challenges posed by older patients, who have advanced degenerative tissue loss and diminished healing potential.
When a supraspinatus tendon tears, there is increased growth factor production at the injury site (Würgler-Hauri et al., 2007). The repair of the tendon-to-bone insertion involves growth factor-mediated migration of undifferentiated cells from the sub-acromial bursa and the underlying bone marrow (Koike Y, 2005 J Orthop Res). The growth factors involved include bone morphogenetic protein (BMP) 2,4,7; growth differentiation factor (GDF) 5,7; basic fibroblast growth factor (b-FGF); platelet-derived growth factor (PDGF); transforming growth factor (TGF)- β1-3; and connective tissue growth factor (CTGF). All these growth factors return to near physiological levels 16 weeks post-injury, establishing a connection between their in vivo upregulation and tissue repair (Chen et al., 2008; Würgler-Hauri et al., 2007). Despite this response by the body to heal, gaps often form between the tendon and bone after surgical repair of large rotator cuff tears (Reilly et al., 2004), which impair tendon structural and mechanical outcomes (Killian et al., 2014). Growth factors, being potent morphogens, can reduce the gap formation by promoting new tissue formation at the interphase by increasing cellular migration, proliferation, differentiation, and matrix synthesis (Oliva et al., 2011). In the following sections, we discuss interesting findings from studies applying growth factors to augment tendon-to-bone healing in animal models.
3.3.1. Bone Morphogenetic Protein-2, 3, and 7
Rotator cuff tendon tears result in unloading, and; therefore, loss of physical stimuli at the tendon-to-bone insertion. This unloading results in bone loss at the insertion site (Cadet et al., 2008; Waldorff et al., 2011). For formation of a mechanically functional enthesis, the tendon collagen fibers must be anchored into the bone via formation of a mineralized fibrocartilage (Dyment et al. 2015) and positive remodeling of the underlying epiphyseal bone. This understanding led to the use of osteoinductive factors such as BMP-2 through 7 for rotator cuff enthesis healing. Osteoinductive bone protein extract containing BMPs- 2 through 7, TGFβ- 1 through 3, and b-FGF was delivered though a collagen sponge in a sheep infraspinatus tendon detachment model (Rodeo et al., 2007). New bone and soft tissue formation with increased load to failure was observed at 6 and 12 weeks following this treatment. However, when the data was normalized to tissue volume there was no difference between groups, suggesting that the growth factor treatment resulted in a poor-quality scar tissue. Perhaps the confounding limitations of gap formation and suture failure in the sheep model, along with the fast degradation and loss of growth factors from the collagen sponge used for delivery may have obscured the benefits of this growth factor cocktail.
BMP 2 and 7 have also been reported to induce chondrogenic differentiation (Dorman et al., 2012), and stimulate synthesis of fibrocartilage ECM components, proteoglycans and type II collagen (Flechtenmacher J. 1996, Kabuto et al 2015). In line with this, BMP-7 released from gelatin hydrogel sheets over a 3-week period post-operatively, significantly improved enthesis matrix production, and, as a result, tendon-to-bone maturing scores (Kabuto et al 2015).
In an indirect approach, BMP-2 was used to promote tendon-to-bone insertion healing by first injecting it into the flexor digitorum communis tendon in a rabbit hind limb to induce ectopic ossicle formation (Hashimoto et al., 2007). The resultant tendon/ossicle complex was surgically transferred onto the surface of the rabbit tibia to generate a stable tendon-to-bone junction. Failure loads were significantly higher in this treatment group compared to the control group without BMP-2 treatment. This shows that osteoinductive growth factors successfully promote enthesis healing when the engineered construct has pre-existing functionalities such as structural and mechanical compliance to the native tissue and biologically instructive ECM signals.
3.3.2. Growth Differentiation Factor GDFs 5, 6, and 7 (Bone Morphogenetic Proteins BMP 12, 13 and 14)
GDFs 5,6,7 are expressed in developing tendons and ligaments and are shown to promote tendon healing (Clark et al., 2001; Mikic et al., 2006). Within this family, GDF-7/BMP-12 shows greatest potential for tendon specific cell differentiation by expressing early phase tenogenic markers such as scleraxis (Scx) and tenomodulin (Tnmd) (Hoffmann et al., 2006; Shen et al., 2013). BMP-12 gene transfer in a complete tendon laceration model in the chicken resulted in a two-fold increase of tensile strength and stiffness of repaired tendons along with increase in collagen type I expression (Lou et al., 2001). Lamplot et al showed that direct delivery of adenovirus-mediated BMP 13 (AdBMP 13) into the rat supraspinatus tendon insertion defect demonstrated higher stress to failure than the delivery of platelet rich plasma that is routinely used in the clinic (Lamplot et al., 2014). This improvement in mechanical strength is positively correlated to the upregulation of tenocyte (tenascin and scleraxis) and ECM-associated (fibronectin and collagen type III and I) genes that are key in the early stages of tendon healing. Since the histopathological changes associated with rotator cuff tears are not localized only to the insertion but also in the macroscopic intact tendon portion (Longo et al., 2011), these growth factors that improve tendon morphology may be essential for functional healing of the rotator cuff.
In the developing tendon enthesis, GDF5/BMP-14 expressing progenitor cells proliferate and contribute to the linear growth of the tissue (Dyment et al., 2015). GDF5/BMP14 is also associated with normal and pathological fibrocartilage differentiation during fracture healing in anatomically similar sites such as the intervertebral disc enthesis (Bostrom et al. 1995; Takae et al. 1999; Nakase et al. 2001). Therefore, this growth factor may be an interesting target to investigate for recapitulating developmental and injury-mediated processes in fibrocartilaginous tissues for the purpose of repair.
BMP-12 delivered in a type I/III collagen sponge improved tissue formation and mechanical properties in an ovine model compared to a hyaluronan paste carrier (Seeherman et al., 2008). The increased efficacy of BMP-12 when delivered through collagen sponge carriers may be because of its local retention at the repair site compared to the hyaluronan paste carrier. However, the healed tissue had a scar-like morphology and a higher cross-sectional area (Kovacevic and Rodeo, 2008). This fibrotic response may be due to the inhibition of MMP activity by GDFs (Enochson et al., 2014). Although increased MMP levels have been associated with tendinopathy and degenerative rotator cuff tears, and their inhibition shown improved collagen organization and fibrocartilage formation in acute rotator cuff tears (Bedi et al., 2010), global inhibition may disrupt later-stage remodeling of the repaired tissue..
3.3.3. Basic Fibroblasts Growth Factor (b-FGF/FGF-2)
Basic fibroblast growth factor (b-FGF) stimulates tendon fibroblast proliferation and migration (Chan et al., 1997) and induces differentiation of MSCs into tenocytes (Cai et al., 2013). Various models have suggested that the addition of b-FGF may increase the strength of the repair and accelerate tendon-to-bone remodeling (Ide et al., 2009; Peterson et al., 2015; Zhang et al., 2016; Zhao et al., 2014). In a rotator cuff supraspinatus injury model, b-FGF showed peak expression at day 7 (Würgler-Hauri et al., 2007). This early upregulation of FGF may promote gap closure between the tendon and the bone by increasing the proliferation of fibroblasts that synthesize collagen matrix.
More recently, FGF-2 has been used in rotator cuff tears because of anti-scarring properties. FGF-2 has been shown to block TGF-β1 mediated myofibroblast activation (Cushing et al., 2008) and induce apoptosis in the granulation tissue, thereby minimizing scar tissue formation (Akasaka et al., 2004). In line with these properties, decreased fibro-vascular scarring and improved biomechanical strength was seen within 6 weeks following FGF-2 delivery via gelatin hydrogels implanted as an interpositional graft between the injured supraspinatus tendon and bone (Tokunaga et al., 2015b). In another study, early delivery of FGF-2 from a fibrin sealant accelerated bone ingrowth and biomechanical strength at 2 weeks following acute rotator cuff repairs, but failed to show significant differences at later time points (Ide et al., 2009). This response may be because fibrin sealants release >50% of the payload within 24 hours of implantation (Ishii et al., 2007). In contrast, b-FGF released at a sustained rate over a 3 week period from a PLGA fibrous membrane significantly increased the collagen organization and failure load (Zhao et al., 2014). Prolonging the release of FGF may promote tissue formation and bone ingrowth at the early stage and controlled remodeling at a later stage.
3.3.4. Platelet Derive Growth Factor (PDGF)
PDGF is highly upregulated during the early phase of tendon healing (Kobayashi et al., 2006). This early upregulation of PDGF promotes the activation of other growth factors (Porsch et al., 2014) and stimulates cell migration, proliferation, and matrix synthesis at the healing site (Lynch et al., 1989; Tsuzaki et al., 2000). In rat rotator cuff insertion tears, PDGF-BB, a homo-dimeric isoform released from gelatin hydrogel sheets, showed significantly higher cell proliferation, greater collagen fiber orientation, and ultimate load to failure at the insertion site (Tokunaga et al., 2015a). PDGF-BB delivered from collagen scaffolds enhanced cell proliferation and angiogenesis at the enthesis site during the early phase of healing (day 2), but failed to have any effect on fibrocartilage formation or collagen fiber maturity in the late phase (day 28) (Kovacevic et al., 2015). This may be because the accelerated diffusion of the PDGF from the collagen sponge leaves little to no growth factor during the later stages of repair in vivo; approximately 50% was released within the first hour in vitro (Bhargava et al., 2005). This begs the need for the development of sustained growth factor delivery devices to improve repair outcomes.
To promote sustained delivery of PDGF-BB in rotator cuff repairs, Min et al. developed a PCL/Pluronic F127 membrane that immobilized PDGF-BB via heparin (Min et al., 2016). This asymmetrically porous membrane facilitated sustained release of the growth factor and selective permeability (allowing permeation of oxygen/nutrients but preventing scar tissue invasion into defect region) thereby improving the repair of the fibrocartilaginous enthesis. The heparin-bound PDGF-BB immobilized on the membrane underwent sustained release over 42 days in vitro. However, this response may be altered in vivo as release of the growth factor from heparin would depend on a more complex dynamics involving multiple growth factor binding affinity and stability, cross receptor binding, and enzymatic and proteolytic degradation (Forsten-Williams et al., 2008). Nevertheless, these results support the efficacy of sustained PDGF delivery in regenerative healing.
3.3.5. Insulin-Like Growth Factor (IGF)
The IGFs have structural similarity to insulin, giving them the ability to bind to insulin receptors. IGF-1 improves functional outcomes of healing in rotator cuff tears (Dines et al., 2007a). In a chronic rotator cuff repair model, IGF-1 transfected tendon fibroblasts improved both toughness and maximal load to failure compared to untreated controls (Dines et al., 2007a). IGF-1 also acts in synergy with other growth factors such as PDGF-BB to increase cell proliferation and collagen production (Tsuzaki et al., 2000). Likewise, engineered ligament constructs treated with a combination of IGF-1 and TGF-β had higher maximal tensile load capacity compared to the ones treated with a single growth factor (Hagerty et al., 2012).
IGF-1 is known to prevent swelling and modulate inflammation in tendon and muscle injuries. The presence of IGF-1 in muscle regeneration is correlated with upregulation of inflammation-resolving macrophage M2; the conditional knock out of the IGF gene has also showed erratic healing response (Tonkin et al., 2015). Since inflammation and pro-inflammatory macrophages (M1) are exaggerated in tendon healing (Kawamura et al., 2005; Manning et al., 2014), growth factor therapies such as IGF-1 that can modulate macrophage polarization to promote inflammation resolution will be highly valuable in rotator cuff healing.
3.3.6. Transforming Growth Factor Beta (TGF-β)
TGF-β plays a critical role in pathological fibrosis in adult tissue repair (Samarakoon et al., 2013). TGF-β signaling, involving isoforms 1 and 2, is highly upregulated during adult rotator cuff healing and this overexpression was concomitant to increased fibrosis in the rotator cuff muscles in massive tears (Liu et al., 2014). TGF-β1 significantly increases α-smooth muscle actin (α-SMA) in rotator cuff tears and has been suggested to contribute to retraction of the torn tendon (Premdas et al., 2001). Selective inhibition of TGF-β1 by a small molecule inhibitor SB431542 has shown significant reduction in fibrosis, fatty infiltration, and muscle atrophy in a murine rotator cuff tear model (Davies et al., 2016). This study demonstrates that TGF-β1 inhibition results in improved tissue quality in rotator cuff repair.
On the other hand, fetal wounds that heal without scar tissue show an upregulation of TGF-β3 isoform instead of 1 and 2 (Liu et al., 2014; Soo et al., 2003). The scar-free healing potential of TGF-β3 was tested by delivering it via an injectable calcium phosphate matrix in rat rotator cuff tears. However, the healing outcomes as measured by increased fibrocartilage area, improved collagen organization and bone formation were significantly better with the calcium phosphate matrix by itself than with TGF-β3 + calcium phosphate carrier group at the concentrations tested. Likewise, TGF-β3 delivery showed no reduction in scar tissue formation in a similar animal model (Manning et al., 2011). The positive effect of TGF-β3 in adult rotator cuff repairs are yet to be demonstrated.
3.3.7. Vascular Endothelial Growth Factor (VEGF)
Tendons are highly vascularized during development; however, this level of vascularization is not sustained as the tendon matures (Fenwick et al., 2002; Ferrara and Gerber, 2001; Peacock, 1959; Takasugi et al., 1978). Vascularization spikes after the inflammatory phase following injury of the mature tendon, with an increased expression of VEGF receptors on the endothelial cells inside the tendon (Pufe et al., 2005). Although, this vascularization leads to repair and remodeling of the injured tendon, it may also cause proteolysis of the ECM by the invading endothelial cells, thereby weakening the healing tendon (Savitskaya et al., 2011). Furthermore, VEGF expression and increased vessel density have been positively correlated with fatty infiltration (FI) and muscle atrophy (MA), two signature endpoints of degeneration (Lakemeier et al., 2010). In addition, VEGF was found to be responsible for the development of shoulder joint contracture: a condition in which the movements of the shoulder joint become markedly limited in patients undergoing rotator cuff repair (Handa et al., 2003). This could be because of motion pain or impingement in patients resulting from VEGF-induced increased synovial proliferation in the sub-acromial bursa (Handa et al., 2003).
Overall, it appears that the detriments of VEGF overweigh the benefits induced by this class of growth factor in rotator cuff healing. A thorough understanding of the association between vascularization and regeneration of the hypo-vascular rotator cuff tendon can better inform the application of this growth factor.
3.3.8. Platelet Rich Plasma
Individual growth factors may not provide conditions necessary for rotator cuff repair. Platelet rich plasma provides a rich source of growth factors and cytokines such as PDGF, TGF-β, FGF, VEGF, and IGF (Randelli et al., 2014). Although PRP therapy has many benefits in easy surgical implementation and patient safety, current results are unclear regarding its benefit on structural healing or patient outcome of pain compared to surgery alone in rotator cuff repair (Randelli et al., 2015).
Platelet rich fibrin clot (PRFM), a variant of PRP with a fibrin matrix was tested in a randomized controlled trial on 79 patients undergoing arthroscopic rotator cuff repair. A suture anchor was placed in the tuberosity, the suture was passed through the PRFM and the tendon, with the PRFM secured between the tendon and bone at the interface. Tendon-to-bone insertion healing evaluated at 12 weeks post-operatively showed no difference between the PRFM and the control group. In a similar study using PRFM for augmentation of small and medium rotator cuff tear double row repair showed no significant differences in tendon thickness and coverage of greater tuberosity between PRFM and control group (Castricini et al., 2011). Based on these studies, the use of PRP for rotator cuff tendon has shown no benefit. This may be a result of variable PRP preparations used, undefined nature of PRP, as well as the level of platelet activation not being accounted for in each clinical study (Mazzocca et al., 2012). Further research with controlled PRP formulations are needed to assess accurately the benefits of this therapy.
3.4. Growth Factor Dose
Tailoring growth factor dose and availability to the coordinated tissue repair processes, spatio-temporally and safely, are critical factors limiting the use of growth factor delivery systems in the clinic. In pre-clinical models of rotator cuff repair, growth factors have been used in a broad range of concentrations; as presented in Table 1, PDGF-BB and FGF-2 have been used in a range of 0.5 to 5 μg and 0.5 to 50 μg per implant, respectively, in rat rotator cuff repair models. Likewise, as can be seen in Table 1, even within the same species, the dose difference is mediated by the type of model (acute or chronic repair); the placement of the scaffold (sutured on the bursal surface, or interpositional to the repaired tendon-to-bone insertion, or simply secured in a bony trough at the footprint); and the loading method (adsorbed, encapsulated, or presented in a controlled manner on the scaffold surface). High doses of growth factor can be detrimental to the tendon-to-bone healing. Improved tendon-to-bone interdigitation and biomechanical strength was observed with the application of 75 μg and 150 μg PDGF-BB incorporated in a type I collagen matrix, compared to 500 μg PDGF-BB, which was found to be detrimental to healing in an ovine rotator cuff repair augmentation model (Hee et al., 2011) (see Figure 3). Similarly, uncontrolled long-term presence of growth factors at the repair site can also led to poor tissue healing outcomes due to negative feedback at higher doses. For example, sustained expression of BMP-2 via adenoviral vector-based delivery lead to bone resorption and decreased mechanical properties of the healing tendon-to-bone insertion (Lipner et al., 2015). Controlled delivery of multiple growth factor doses that takes into consideration the spatiotemporal complexity of the injury microenvironment and acts in concert with the endogenous reparative processes via positive and negative feedback mechanisms will lead to significant improvements in growth factor therapy.
Table 1.
Summary of key pre-clinical studies using growth factor delivery to augment rotator cuff repair
| Growth Factor | Dose | Delivery Scaffold | Loading Method | Duration of Release | Animal Model | Scaffold Placement | Histological and Biomechanical Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 75, 150, 500 μg | Type I collagen matrix | Soaking | Sheep infraspinatus tendon detachment and acute repair | Interpositional to the repaired infraspinatus tendon-to-bone insertion | Better tendon-to-bone interdigitation and higher ultimate load-to-failure in the 75 μg and 150 μg PDGF-BB with type I collagen matrix group than the 500 μg group and suture only control at 12 weeks. Higher doses led to poor morphological appearance and biomechanics. | (Hee et al., 2011) | ||
| 0.5 μg | Gelatin hydrogel sheets | Soaking | 100% released within 2 weeks during subcutaneous implantation | Rat supraspinatus tendon detachment and acute repair | Lateral to the repaired supraspinatus tendon-to-bone insertion | Better organized collagen fiber at the insertion site and significantly greater ultimate load-to-failure and stiffness in the PDGF-BB treated group than that in the suture repair and PBS-soaked gelatin hydrogel sheet controls at 12 weeks. | (Tokunaga et al.,2015a) | |
| 0.6, 2, 6 μg | Type I collagen scaffold | Soaking | Rat supraspinatus tendon detachment and acute repair | Bursal to the repaired supraspinatus tendon-to-bone insertion | Dose-dependent response in cellular proliferation and angiogenesis in PDGF-BB delivery group at 5 days compared with repair only and scaffold only control. No significant difference between treatment and control groups on the area of fibrocartilage, collagen fiber orientation, or biomechanical properties at 28 days. | (Kovacevic et al., 2015) | ||
| 896 ng/cm | Fiberwire sutures | Dip coating | 80% released within 1 hour, and remaining released in 48 hours | Sheep infraspinatus tendon detachment and chronic repair | Significantly better organized gap tissue with extensive cartilage formation at the tendon-to-bone interface in the rhPDGF-BB coated sutures repair group compared with controls at 6 weeks, but ultimate load-to-failure was equivalent to uncoated controls. | (Uggen et al., 2010) | ||
| 0.5, 5 and 50 μg | Gelatin hydrogel sheet | Soaking | 100% released within 2 weeks | Rat supraspinatus tendon detachment and acute repair | Interpositional to the repaired infraspinatus tendon-to-bone insertion | Significant improvement in mechanical strength in the FGF-2-treated group at 6 and 12 weeks. No significant difference between different doses. | (Tokunaga et al.,2015b) | |
| 2 μg | Electrospun PLGA fibrous membrane | Emulsion encapsulation | 50% released in 2 weeks in a sustained manner | Rat supraspinatus tendon detachment and chronic repair | Bursal to the repaired supraspinatus tendon-to-bone insertion | Significantly improved collagen organization and highest ultimate load-to-failure and stiffness in the bFGF–PLGA scaffolds than control suture repair and PLGA scaffold alone groups at 8 weeks. | (Zhao et al., 2014) | |
| 3, 30 μg | Gelatin hydrogel sheet | Dip coating | 30% released within 1 day and the remaining released in a sustained manner in approximately 2 weeks | Rabbit supraspinatus tendon detachment and acute repair | Placed in a created bony trough interpositional to the repaired infraspinatus tendon-to-bone insertion | Histologically and biomechanically enhanced rotator cuff tendon-to-bone healing in both 3 and 30 μg of FGF-2 impregnated gelatin hydrogel sheets with no significant difference between the two doses at 6 weeks. | (Tokunaga et al.,2017) | |
| F2A (peptide mimetic of FGF-2) | 1, 8 mg | Collagen-coated P4HB fibrous scaffold | Dripping | Sheep infraspinatus tendon detachment and acute repair | Bursal to the repaired infraspinatus tendon-to-bone insertion | The extent of delamination decreased with increasing doses of F2A, and more tendon-like tissue and new bone formation was observed in the growth factor augmented group at 8 weeks. | (Peterson et al.,2015) | |
| 50 μg/ml | Acellular dermal patch | Coated on the surfaces and then freeze dried | 75% released in a sustained manner in 2 weeks | Rabbit supraspinatus tendon detachment and chronic repair | Interpositional to the repaired supraspinatus tendon-to-bone insertion | Greater new bone formation, cell penetration into the host bone, well connected interface with no gap formation, and significantly higher ultimate tensile load in the rhBMP-2 augmented repair group at 8 weeks. | (Lee et al., 2017) | |
| 0.5 μg | Gelatin hydrogel sheet | Coated on the gelatin hydrogel sheets | 90% released in a sustained manner within 2 weeks | Rat supraspinatus tendon detachment and acute repair | Bursal to the repaired supraspinatus tendon-to-bone insertion | Significantly higher tendon maturing score and highest ultimate load-to-failure in the BMP-7 delivery from gelatin hydrogel sheet group than the following controls: direct injection of BMP-7, gelatin hydrogel sheet alone, and the suture repair alone, respectively, at 8 weeks. | (Kabuto et al., 2015) | |
| BMP-12 | ||||||||
| 75*/30† μg | Type I/III collagen sponge | † Bursal to the repaired supraspinatus tendon-to-bone insertion | ||||||
| TGF-β3 | 2.75 μg | Calcium phosphate matrix | Injected into the calcium phosphate matrix | Rat supraspinatus tendon detachment and acute repair | Placed in a created bony trough interpositional to the repaired infraspinatus tendon-to-bone insertion | Improved fibrocartilage formation and collagen organization at the enthesis in the calcium phosphate matrix alone group than the calcium phosphate matrix with TGF-β3 at 2 weeks. | (Kovacevic et al., 2011) | |
| TGF-β1 | 0.1 μg | Gelatin hydrogel sheet | Soaking | Rat supraspinatus tendon detachment and acute repair | Interpositional to the repaired supraspinatus tendon-to-bone insertion | Tough fibrous tissues at the healing site with significantly higher ultimate load-to-failure and higher collagen content in the TGF-β1 gelatin hydrogel sheets group than saline control at 12 weeks. | (Arimura et al.,2017)) |
Figure 3. Polarized light images of hematoxylin and eosin–stained sections show growth factor dose-dependent changes in the tissue morphology (regions of interdigitation as noted by the arrows) at the repaired tendon-to-bone insertion site (Scale bar = 1 mm).
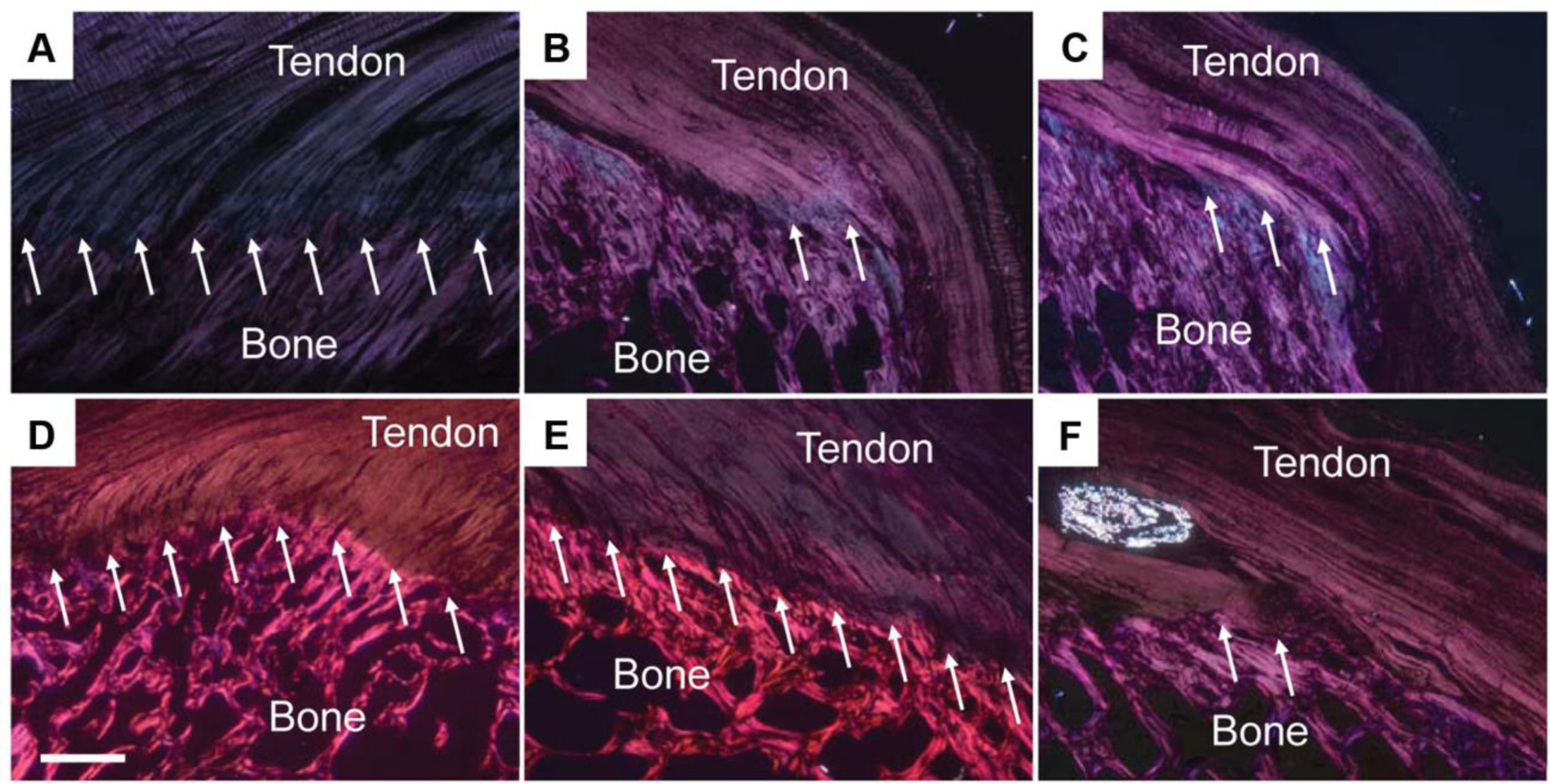
(A) Native tendon; (B) suture repair only; (C) collagen matrix+0 μg rhPDGF-BB; (D) collagen matrix+75 μg rhPDGF-BB; (E) collagen matrix+150 μg rhPDGF-BB; (F) collagen matrix+500 μg rhPDGF-BB. The morphological appearance was significantly improved in the 75 and 150 μg rhPDGF-BB augmented repairs when compared to the 0 and 500 μg rhPDGF-BB that showed poor morphology. Reprinted from Kee CH et al. Augmentation of a Rotator Cuff Suture Repair Using rhPDGF-BB and a Type I Bovine Collagen Matrix in an Ovine Model. The American Journal of Sports Medicine 2011; 39 (8):1630-39; with permission (SAGE publications).
4. Innovative Materials for Rotator Cuff Repair
Recent innovations in material design and scaffold fabrication have led to engineered constructs that mimic the ultrastructural organization and mechanics of the native enthesis tissue (Chainani et al., 2013; Zhang, 2017; Zhang et al., 2012). One such strategy is to develop ECM-like nanofibrous structures by electrospinning and simultaneously create a gradient mineralized matrix (Lipner et al., 2014; Smith et al., 2012). Such functionally graded transitions can dissipate stress concentrations and toughen attachments (Lipner et al., 2014).
Gradients of biochemical cues on scaffolds have also been investigated. Polycaprolactone (PCL)/ Pluronic F127 membranes with counter gradients of dual growth factors - PDGF and BMP 2 released in a spatiotemporal manner for 35 days promoted ADSC differentiation into osteoblasts and tenocytes at the two ends of the gradients, respectively (Min et al., 2014). However, the authors did not evaluate the chondrogenic differentiation in the middle of the construct, which is most equitably stimulated by both PDGF and BMP2. Nevertheless, this construct shows promise for tendon-to-bone insertion repair by spatio-temporally regulating morphogen gradients.
Sustained release of a growth factor was achieved in a cellular environment by a heparin-fibrin based drug delivery system (Sakiyama-Elbert and Hubbell, 2000). This system includes an antithrombin III-based bi-domain peptide that is covalently cross-linked to a fibrin matrix by the transglutaminase activity of Factor XIIIa on the N-terminus. The C-terminal heparin-binding domain on the other end immobilizes heparin electrostatically to the matrix. The heparin in turn sequesters the growth factors by the interaction of its anionic sulphate groups with cationic amino acid groups found on growth factors (Thomopoulos et al., 2010, 2009, 2007). The release of growth factor from this matrix may occur by 3 mechanisms: (i) passive diffusion by dissociation of the growth factor from the matrix-bound heparin, (ii) active diffusion by proteolytic degradation of the peptide, and (iii) active diffusion by enzymatic degradation of the matrix (Sakiyama-Elbert and Hubbell, 2000). This heparin-fibrin hydrogel was then incorporated into multiple layers of electrospun PLGA nanofibers to provide structural integrity to the scaffold for surgical handling (Manning et al., 2013). This matrix has been shown to also retain and deliver mesenchymal stem cells with minimum toxicity in animal models (Gelberman et al., 2016). Innovations such as the fabrication of ECM-mimicking nanostructures, growth factor and mineral presenting gradients, etc. by integrating novel materials and fabrication technologies will lead the development of the next-generation scaffolds.
5. Novel Therapeutic Targets from Tendon and Developmental Biology
The early responding cells that migrate to the enthesis repair site may play a crucial role in adult tissue regeneration (Yoshida et al., 2016). These cells, which migrate from the bursal side of the tendon in response to supraspinatus tendon injury, express myofibroblast markers (Yoshida et al., 2016), and may be responsible for the scar-like tissue seen in the later stages of healing.
Recent studies have investigated the role of mechanoactive signals such as the hedgehog signaling in enthesis regeneration (Carbone et al., 2016; Dyment et al., 2015; Schwartz et al., 2015). Hedgehog responsive cells (Gli+) act transiently to promote mineralization of the neonatal healing enthesis, and its expression goes down after mineralization to prevent excessive remodeling of the tissue (Schwartz et al., 2015). On the other hand, the adult healing enthesis has a very small population of early-stage hedgehog responsive cells and shows incomplete mineralization (Schwartz et al., 2017). The failure to close the gap between the tendon and bone with repair tissue may prevent loading and thereby the activation of mechanoactive signals that are necessary for mineralization. These studies open up exciting avenues for using signaling molecules and growth factors in a time-dependent manner for rotator cuff enthesis regeneration. Manipulating time-sensitive signaling targets such as the hedgehog would require controlled and programmable advanced drug delivery strategies.
6. Dynamic Matrices for Endogenous Cell Recruitment in Enthesis Regeneration
Growth factor interactions with the ECM facilitate localized and spatially regulated signaling. Enhancing these interactions in clinically used growth factor applications can boost their therapeutic efficacy (Martino et al., 2014). A domain in placenta growth factor-2 (PIGF-2123–144) binds exceptionally strongly and promiscuously to ECM proteins (Martino et al., 2014). By substituting the heparin-binding domain of growth factors such as VEGF, PDGF-BB, and BMP-2 with PIGF-2123–144, engineered GF variants were generated that had super-affinity to the ECM. These ECM super-affinity variants induced repair in critical sized bone defects in rodent models. Enhanced cell homing by the controlled retention of these growth factor in a scaffold improved regeneration in these defects.
Growth factor binding to transmembrane protein receptors initiates cell signaling. Receptor binding of growth factors is regulated by interactions with heparan sulfate proteoglycans (HSPGs) that are ubiquitously present in the extra cellular matrix. Growth factor binding to HSPGs is closely regulated by the patterns of sulfate groups that are distributed along the length of the glycosaminoglycan (GAG) chains (Esko et al., 2009).The binding of the growth factors to HSPGs allow their local retention (Sarrazin et al., 2011), protection from degradation (Sadir et al., 2004), activation by oligomerization (Proudfoot et al., 2003), and presentation to cells (Handel et al., 2005).These varied sulfation patterns facilitate the formation of morphogen gradients that are essential for selective cell recruitment in the tissue regeneration process (Sarrazin et al., 2011). The next generation growth factor delivery strategies in enthesis regeneration will incorporate our emerging understanding of the HSPG-controlled growth factor interactions during scaffold design.
7. Animal Models of Rotator Cuff Repair
Animal models in rotator cuff rely heavily on the similarity of the anatomical macrostructures and functions to mimic the pathophysiology of rotator cuff tears (Lebaschi et al., 2016). Rat is the most commonly used animal model in rotator cuff repair assessments because of it’s anatomical and kinematic similarity to humans, with the acromial arch, a supraspinatus tendon below the arch, and forward arm elevation. It is also cost effective. However, rat rotator cuff tears heal better with minimal fatty infiltration and have minimum post-operative tears unlike those seen in humans (Derwin et al., 2010b). To mimic fatty infiltration in the rotator cuff muscle as seen in humans, rodent rotator cuff tear models were created with simultaneous transection of the suprascapular nerve (Kim et al., 2012). Larger animals, in particular the sheep has been used to test the effect of new surgical scaffolds, and is an excellent model to study the chronic degenerative changes in the muscle following rotator cuff tear. However, the sheep lacks a coracoacromial arch and its weight bearing forelimb leads to failure of all repairs. The paucity of species-specific probes also limit the ability to conduct detailed histological analysis in this model. For more extensive literature on animal models in rotator cuff repair please refer to these excellent reviews (Deprés-Tremblay et al., 2016; Derwin et al., 2010b; Lebaschi et al., 2016).
8. Summary and Future Outlook
Rotator cuff tears are becoming increasingly common with more than half of the adults >65 years being affected. Retears after surgical repair are pushing for newer strategies in rotator cuff repair augmentation. Growth factor delivery from engineered scaffolds shows promise by enhancing the rate and quality of repair. Despite several growth factors showing improved healing in preclinical studies of rotator cuff repair, there are no growth factor formulations currently approved by the FDA for this indication. In fact, there are only two growth factor formulations currently approved by the FDA for clinical use in the Unites States mainly for the purpose of bone fracture healing: BMP2 (Infuse™, Medtronic Sofamor Danek, Inc.) and BMP7 (OP-1™, Stryker Biotech) (Santo et al., 2013b), both using Type I collagen as a carrier. To provide therapeutic benefit, supraphysiological doses of these growth factors have been used in the clinic. For example, the clinical doses of BMP-2 used in spinal fusion are associated with increased risk of cancer and ectopic bone formation (Carragee et al., 2013). The required excess of these growth factors may be explained by ineffective delivery systems that do not provide spatiotemporal regulation of the growth factor delivery. Overall, the safety, efficacy, and prohibitive cost of growth factor delivery limit their widespread clinical use.
Rotator cuff being a complex tissue, activation and mobilization of the heterogeneous cell population may require multiple complementary growth factor signals acting in concert. Therefore, finding the right combination of growth factors that can simultaneously promote tendon-enthesis tissue formation, mineralization, and integration of the repaired construct is of interest. Our evolving understanding of the role of growth factors during the different stages of healing will inform the design of sophisticated spatio-temporally controlled multiple growth factor delivery systems. These growth factor delivery systems must be designed to provide an active cell-instructive environment during the therapeutic healing period. Such a strategy would especially benefit the older population whose endogenous healing mechanism is affected by senescence and degeneration.
Cell-instructive environments are inherently present in natural ECM-based scaffolds. However, their use as growth factor delivery scaffolds is limited by their rapid degradation and uncontrolled release of growth factor in a short time period. Synthetic scaffolds can be engineered with controlled degradation properties to deliver the growth factor payload as and when needed, while simultaneously being mechanically tailored to withstand the loading demands of the tissue over the remodeling period. Composites of these synthetic biodegradable scaffolds with extracellular matrix proteins would augment the repair by providing tailorable growth factor delivery and mechanical properties, while retaining the biological cues. Innovations in material design and scaffold fabrication have led to engineered constructs that mimic the ultrastructural organization and mechanics of the native enthesis tissue. Scaffolds presenting gradient biochemical cues and having sustained growth factor delivery capability have also been proposed. Our recent understanding of enthesis development and regeneration biology is pointing to newer targets for growth factor delivery strategies. We are entering an exciting area of growth factor therapy in rotator cuff repair. The next-generation growth factor delivery scaffolds must undergo comprehensive evaluation of host and foreign body response. Added capabilities such as surgical handling and amenability to arthroscopic repairs can expedite clinical translation of such scaffolds.
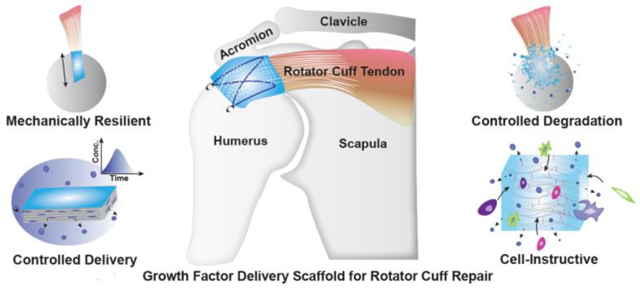
Figure 2. Desirable attributes of growth factor delivery scaffold for rotator cuff repair.

An example supraspinatus tendon-to-bone insertion tear at the humeral head is depicted in the center, with offsets (A), (B), (C) and (D) depicting the desirable traits of a growth factor delivery scaffold for this repair. Scaffolds must (A) mechanically support the repaired tissue and dissipate stress concentrations at the insertion; (B) deliver growth factor(s) in a controlled manner through the therapeutic time frame while being mechanically resilient to complex loading; (C) undergo degradation with minimal change in local pH with the degradation rate synchronized to the developing structure and function of the new tissue; (D) be cell-instructive to recruit stem and progenitor cells from the bursa and the underlying primary cartilage to the healing site to promote enthesis regeneration.
Acknowledgements
The authors gratefully acknowledge funding from Novartis, G600795, and the Office of the Vice President for Research, University of Connecticut, 401543-10301-20. Cato T. Laurencin, M.D., Ph.D. is a recipient of the NIH Director’s Pioneer Award 2014, 1DP1AR068147-01.
Abbreviations
- VEGF
vascular endothelial growth factor
- BMP
bone morphogenetic protein
- TGF-β
transforming growth factor beta
- b-FGF
basic fibroblast growth factor
- GDF
growth and differentiation factor
- PDGF
platelet derived growth factor
- MMP
matrix metalloprotease
- COMP
cartilage oligomeric matrix protein
- CTGF
connective tissue growth factor
- IGF
insulin-like growth factor
- α-SMA
alpha smooth muscle actin
- SCX
scleraxis
- SOX-9
SRY-box-9
- PRP
platelet rich plasma
- PRFM
platelet rich fiber matrix
- IHH
Indian hedgehog
- Gli+
glioma associate oncogene
- PlGF
placenta growth factor
- HSPG
heparin sulfate proteoglycans
- GAG
glycosaminoglycan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicale R, Tarantino D, Maffulli N, 2017. Basic science of tendons, in: Gobbi A, Espregueira-Mendes J, Lane JG, Karahan M (Eds.), Bio-orthopaedics. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 249–273. doi: 10.1007/978-3-662-54181-4_21 [DOI] [Google Scholar]
- Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T, 2004. Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol 203, 710–720. doi: 10.1002/path.1574 [DOI] [PubMed] [Google Scholar]
- Andarawis-Puri N, Flatow EL, Soslowsky LJ, 2015. Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res 33, 780–784. doi: 10.1002/jor.22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F, Beitzel K, McCarthy MB, Cote MP, Mazzocca AD, 2014. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J 4, 333–342. [PMC free article] [PubMed] [Google Scholar]
- Arimura H, Shukunami C, Tokunaga T, Karasugi T, Okamoto N, Taniwaki T, Sakamoto H, Mizuta H, Hiraki Y, 2017. TGF-β1 Improves Biomechanical Strength by Extracellular Matrix Accumulation Without Increasing the Number of Tenogenic Lineage Cells in a Rat Rotator Cuff Repair Model. Am J Sports Med 45, 2394–2404. doi: 10.1177/0363546517707940 [DOI] [PubMed] [Google Scholar]
- Babensee JE, McIntire LV, Mikos AG, 2000. Growth Factor Delivery for Tissue Engineering. Pharm Res. [DOI] [PubMed] [Google Scholar]
- Beanes SR, Dang C, Soo C, Ting K, 2003. Skin repair and scar formation: the central role of TGF-[beta]. Expert Rev Mol Med 5. doi: 10.1017/S1462399403005817 [DOI] [PubMed] [Google Scholar]
- Bedi A, Kovacevic D, Hettrich C, Gulotta LV, Ehteshami JR, Warren RF, Rodeo SA, 2010. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg 19, 384–391. doi: 10.1016/j.jse.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF, 2005. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In Vitro Cell Dev Biol Anim 41, 305–310. doi: 10.1290/0503018.1 [DOI] [PubMed] [Google Scholar]
- Cadet ER, Hsu JW, Levine WN, Bigliani LU, Ahmad CS, 2008. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg 17, 73–77. doi: 10.1016/j.jse.2007.04.017 [DOI] [PubMed] [Google Scholar]
- Cai T-Y, Zhu W, Chen X-S, Zhou S-Y, Jia L-S, Sun Y-Q, 2013. Fibroblast growth factor 2 induces mesenchymal stem cells to differentiate into tenocytes through the MAPK pathway. Mol Med Report 8, 1323–1328. doi: 10.3892/mmr.2013.1668 [DOI] [PubMed] [Google Scholar]
- Carbone A, Carballo C, Ma R, Wang H, Deng X, Dahia C, Rodeo S, 2016. Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: Evaluation in a rat ACL reconstruction model. J Orthop Res 34, 641–649. doi: 10.1002/jor.23066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, Comer G, Kopjar B, 2013. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 95, 1537–1545. doi: 10.2106/JBJS.L.01483 [DOI] [PubMed] [Google Scholar]
- Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, Maffulli N, Denaro V, 2011. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med 39, 258–265. doi: 10.1177/0363546510390780 [DOI] [PubMed] [Google Scholar]
- Chainani A, Hippensteel KJ, Kishan A, Garrigues NW, Ruch DS, Guilak F, Little D, 2013. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng Part A 19, 2594–2604. doi: 10.1089/ten.TEA.2013.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani A, Little D, 2016. Current Status of Tissue-Engineered Scaffolds for Rotator Cuff Repair. Tech Orthop 31, 91–97. doi: 10.1097/BTO.0000000000000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BP, Chan KM, Maffulli N, Webb S, Lee KK, 1997. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin Orthop Relat Res 239–247. [PubMed] [Google Scholar]
- Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB, 2008. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am 33, 1834–1842. doi: 10.1016/j.jhsa.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Chen J, Xu J, Wang A, Zheng M, 2009. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices 6, 61–73. doi: 10.1586/17434440.6.1.61 [DOI] [PubMed] [Google Scholar]
- Cheung EV, Silverio L, Yao J, 2010. Delivered growth factor therapy to improve healing after rotator cuff repair. Stem Cells Cloning 3, 135–144. doi: 10.2147/SCCAA.S7359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RT, Johnson TL, Schalet BJ, Davis L, Gaschen V, Hunziker EB, Oldberg A, Mikic B, 2001. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res 42, 175–186. [DOI] [PubMed] [Google Scholar]
- Cummings SH, Grande DA, Hee CK, Kestler HK, Roden CM, Shah NV, Razzano P, Dines DM, Chahine NO, Dines JS, 2012. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: A histological and biomechanical study. J Tissue Eng 3, 2041731412453577. doi: 10.1177/2041731412453577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing MC, Mariner PD, Liao J-T, Sims EA, Anseth KS, 2008. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J 22, 1769–1777. doi: 10.1096/fj.07-087627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren LA, Mohammed HO, Nixon AJ, 2005. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res 23, 84–92. doi: 10.1016/j.orthres.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT, Feeley BT, 2016. TGF-β Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis. PLoS ONE 11, e0155486. doi: 10.1371/journal.pone.0155486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprés-Tremblay G, Chevrier A, Snow M, Hurtig MB, Rodeo S, Buschmann MD, 2016. Rotator cuff repair: a review of surgical techniques, animal models, and new technologies under development. J Shoulder Elbow Surg 25, 2078–2085. doi: 10.1016/j.jse.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Derwin KA, Badylak SF, Steinmann SP, Iannotti JP, 2010a. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg 19, 467–476. doi: 10.1016/j.jse.2009.10.020 [DOI] [PubMed] [Google Scholar]
- Derwin KA, Baker AR, Iannotti JP, McCarron JA, 2010b. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev 16, 21–30. doi: 10.1089/ten.TEB.2009.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP, 2009. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am 91, 1159–1171. doi: 10.2106/JBJS.H.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A, 1995. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 19, 471–476. doi: 10.1006/cbir.1995.1090 [DOI] [PubMed] [Google Scholar]
- Diaz-Gomez L, Concheiro A, Alvarez-Lorenzo C, García-González CA, 2016. Growth factors delivery from hybrid PCL-starch scaffolds processed using supercritical fluid technology. Carbohydr Polym 142, 282–292. doi: 10.1016/j.carbpol.2016.01.051 [DOI] [PubMed] [Google Scholar]
- Dines JS, Grande DA, Dines DM, 2007a. Tissue engineering and rotator cuff tendon healing. J Shoulder Elbow Surg 16, S204–7. doi: 10.1016/j.jse.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Dines JS, Weber L, Razzano P, Prajapati R, Timmer M, Bowman S, Bonasser L, Dines DM, Grande DP, 2007b. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg 16, S215–21. doi: 10.1016/j.jse.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Dorman LJ, Tucci M, Benghuzzi H, 2012. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum 48, 81–87. [PubMed] [Google Scholar]
- Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, Butler DL, Rowe DW, 2015. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol 405, 96–107. doi: 10.1016/j.ydbio.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enochson L, Stenberg J, Brittberg M, Lindahl A, 2014. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthr Cartil 22, 566–577. doi: 10.1016/j.joca.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Esko JD, Kimata K, Lindahl U, 2009. Proteoglycans and Sulfated Glycosaminoglycans, in: Varki A, Cummings RD, Esko JD, (Eds.), Essentials of Glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY). [PubMed] [Google Scholar]
- Farhat YM, Al-Maliki AA, Easa A, O’Keefe RJ, Schwarz EM, Awad HA, 2015. TGF-β1 Suppresses Plasmin and MMP Activity in Flexor Tendon Cells via PAI-1: Implications for Scarless Flexor Tendon Repair. J Cell Physiol 230, 318–326. doi: 10.1002/jcp.24707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick, Hazelman, Harrall, Hackney, Riley, 2008. Transforming growth factor-β isoform expression in chronic achilles tendinopathy and their effects on tendon cell populations. Int J Exp Pathol 81, A11–A12. doi: 10.1046/j.1365-2613.2000.0145n.x [DOI] [Google Scholar]
- Fenwick SA, Hazleman BL, Riley GP, 2002. The vasculature and its role in the damaged and healing tendon. Arthritis Res 4, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, 2001. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol 106, 148–156. [DOI] [PubMed] [Google Scholar]
- Forsten-Williams K, Chu CL, Fannon M, Buczek-Thomas JA, Nugent MA, 2008. Control of growth factor networks by heparan sulfate proteoglycans. Ann Biomed Eng 36, 2134–2148. doi: 10.1007/s10439-008-9575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, Chan K-M, 2003. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci 72, 2965–2974. [DOI] [PubMed] [Google Scholar]
- Fuchs TF, Surke C, Stange R, Quandte S, Wildemann B, Raschke MJ, Schmidmaier G, 2012. Local delivery of growth factors using coated suture material. ScientificWorldJournal 2012, 109216. doi: 10.1100/2012/109216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Majima T, Iwasaki N, Suenaga N, Sawaguchi N, Shimode K, Minami A, Harada K, Nishimura S, 2005. Application of tissue engineering techniques for rotator cuff regeneration using a chitosan-based hyaluronan hybrid fiber scaffold. Am J Sports Med 33, 1193–1201. doi: 10.1177/0363546504272689 [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A, 2006. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg 15, 112–118. doi: 10.1016/j.jse.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S, 2007. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res 25, 1621–1628. doi: 10.1002/jor.20441 [DOI] [PubMed] [Google Scholar]
- Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K, 2004. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86-A, 219–224. [DOI] [PubMed] [Google Scholar]
- Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S, 2006. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res 24, 541–550. doi: 10.1002/jor.20067 [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Shen H, Kormpakis I, Rothrauff B, Yang G, Tuan RS, Xia Y, Sakiyama-Elbert S, Silva MJ, Thomopoulos S, 2016. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J Orthop Res 34, 630–640. doi: 10.1002/jor.23064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombotz WR, Pettit DK, 1995. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem 6, 332–351. [DOI] [PubMed] [Google Scholar]
- Govoni M, Berardi AC, Muscari C, Campardelli R, Bonafè F, Guarnieri C, Reverchon E, Giordano E, Maffulli N, Della Porta G, 2017. * An Engineered Multiphase Three-Dimensional Microenvironment to Ensure the Controlled Delivery of Cyclic Strain and Human Growth Differentiation Factor 5 for the Tenogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells. Tissue Eng Part A 23, 811–822. doi: 10.1089/ten.TEA.2016.0407 [DOI] [PubMed] [Google Scholar]
- Hagerty P, Lee A, Calve S, Lee CA, Vidal M, Baar K, 2012. The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials 33, 6355–6361. doi: 10.1016/j.biomaterials.2012.05.045 [DOI] [PubMed] [Google Scholar]
- Hakimi O, Mouthuy P-A, Carr A, 2013. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol 94, 287–292. doi: 10.1111/iep.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A, Gotoh M, Hamada K, Yanagisawa K, Yamazaki H, Nakamura M, Ueyama Y, Mochida J, Fukuda H, 2003. Vascular endothelial growth factor 121 and 165 in the subacromial bursa are involved in shoulder joint contracture in type II diabetics with rotator cuff disease. J Orthop Res 21, 1138–1144. doi: 10.1016/S0736-0266(03)00102-5 [DOI] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE, 2005. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu Rev Biochem 74, 385–410. doi: 10.1146/annurev.biochem.72.121801.161747 [DOI] [PubMed] [Google Scholar]
- Harrison AK, Flatow EL, 2011. Subacromial impingement syndrome. J Am Acad Orthop Surg 19, 701–708. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yoshida G, Toyoda H, Takaoka K, 2007. Generation of tendon-to-bone interface “enthesis” with use of recombinant BMP-2 in a rabbit model. J Orthop Res 25, 1415–1424. doi: 10.1002/jor.20447 [DOI] [PubMed] [Google Scholar]
- Hays PL, Kawamura S, Deng X-H, Dagher E, Mithoefer K, Ying L, Rodeo SA, 2008. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am 90, 565–579. doi: 10.2106/JBJS.F.00531 [DOI] [PubMed] [Google Scholar]
- Hee CK, Dines JS, Dines DM, Roden CM, Wisner-Lynch LA, Turner AS, McGilvray KC, Lyons AS, Puttlitz CM, Santoni BG, 2011. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med 39, 1630–1639. doi: 10.1177/0363546511404942 [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D, 2006. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest 116, 940–952. doi: 10.1172/JCI22689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Fujimoto T, Mizuta H, 2009. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elbow Surg 18, 391–398. doi: 10.1016/j.jse.2009.01.013 [DOI] [PubMed] [Google Scholar]
- Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y, 2007. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br 89, 693–700. doi: 10.1302/0301-620X.89B5.18450 [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Chennazhi KP, Srinivasan S, Nair SV, Furuike T, Tamura H, 2011. Chitin scaffolds in tissue engineering. Int J Mol Sci 12, 1876–1887. doi: 10.3390/ijms12031876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto Y, Morihara T, Sukenari T, Kida Y, Oda R, Arai Y, Sawada K, Matsuda K-I, Kawata M, Tabata Y, Fujiwara H, Kubo T, 2015. Stimulation of Rotator Cuff Repair by Sustained Release of Bone Morphogenetic Protein-7 Using a Gelatin Hydrogel Sheet. Tissue Eng Part A 21, 2025–2033. doi: 10.1089/ten.TEA.2014.0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Ying L, Kim H, Dynybil C, Rodeo S, 2005. Macrophages accumulate in the early phase of tendon–bone healing. J Orthop Res 23, 1425–1432. doi: 10.1016/j.orthres.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Killian ML, Cavinatto L, Shah SA, Sato EJ, Ward SR, Havlioglu N, Galatz LM, Thomopoulos S, 2014. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res 32, 439–447. doi: 10.1002/jor.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S, 2012. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg 21, 847–858. doi: 10.1016/j.jse.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Hokugo A, Takamoto T, Tabata Y, Kurosawa H, 2008. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue Eng Part C Methods 14, 47–57. doi: 10.1089/tec.2007.0286 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y, 2006. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg 15, 371–377. doi: 10.1016/j.jse.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Koike Y, Trudel G, Uhthoff HK, 2005. Formation of a new enthesis after attachment of the supraspinatus tendon: A quantitative histologic study in rabbits. J Orthop Res 23, 1433–1440. doi: 10.1016/j.orthres.2005.02.015.1100230628 [DOI] [PubMed] [Google Scholar]
- Kosuge D, Khan WS, Haddad B, Marsh D, 2013. Biomaterials and scaffolds in bone and musculoskeletal engineering. Curr Stem Cell Res Ther 8, 185–191. [DOI] [PubMed] [Google Scholar]
- Kovacevic D, Fox AJ, Bedi A, Ying L, Deng X-H, Warren RF, Rodeo SA, 2011. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med 39, 811–819. doi: 10.1177/0363546511399378 [DOI] [PubMed] [Google Scholar]
- Kovacevic D, Gulotta LV, Ying L, Ehteshami JR, Deng X-H, Rodeo SA, 2015. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res 473, 1644–1654. doi: 10.1007/s11999-014-4020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic D, Rodeo SA, 2008. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res 466, 622–633. doi: 10.1007/s11999-007-0112-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakemeier S, Reichelt JJA, Patzer T, Fuchs-Winkelmann S, Paletta JRJ, Schofer MD, 2010. The association between retraction of the torn rotator cuff and increasing expression of hypoxia inducible factor 1α and vascular endothelial growth factor expression: an immunohistological study. BMC Musculoskelet Disord 11, 230. doi: 10.1186/1471-2474-11-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CXF, Hutmacher DW, Schantz J-T, Woodruff MA, Teoh SH, 2009. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J Biomed Mater Res A 90, 906–919. doi: 10.1002/jbm.a.32052 [DOI] [PubMed] [Google Scholar]
- Lamplot JD, Angeline M, Angeles J, Beederman M, Wagner E, Rastegar F, Scott B, Skjong C, Mass D, Kang R, Ho S, Shi LL, 2014. Distinct effects of platelet-rich plasma and BMP13 on rotator cuff tendon injury healing in a rat model. Am J Sports Med 42, 2877–2887. doi: 10.1177/0363546514547171 [DOI] [PubMed] [Google Scholar]
- Lebaschi A, Deng X-H, Zong J, Cong G-T, Carballo CB, Album ZM, Camp C, Rodeo SA, 2016. Animal models for rotator cuff repair. Ann N Y Acad Sci 1383, 43–57. doi: 10.1111/nyas.13203 [DOI] [PubMed] [Google Scholar]
- Lederman ES, Toth AP, Nicholson GP, Nowinski RJ, Bal GK, Williams GR, Iannotti JP, 2016. A prospective, multicenter study to evaluate clinical and radiographic outcomes in primary rotator cuff repair reinforced with a xenograft dermal matrix. J Shoulder Elbow Surg 25, 1961–1970. doi: 10.1016/j.jse.2016.02.029 [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee S-H, Cho H, Kim HJ, Seong SC, Lee MC, 2004. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 25, 4163–4173. doi: 10.1016/j.biomaterials.2003.10.057 [DOI] [PubMed] [Google Scholar]
- Lee K-W, Lee J-S, Kim Y-S, Shim Y-B, Jang J-W, Lee K-I, 2017. Effective healing of chronic rotator cuff injury using recombinant bone morphogenetic protein-2 coated dermal patch in vivo. J Biomed Mater Res Part B Appl Biomater 105, 1840–1846. doi: 10.1002/jbm.b.33716 [DOI] [PubMed] [Google Scholar]
- Leong NL, Kabir N, Arshi A, Nazemi A, Wu B, Petrigliano FA, McAllister DR, 2015. Evaluation of polycaprolactone scaffold with basic fibroblast growth factor and fibroblasts in an athymic rat model for anterior cruciate ligament reconstruction. Tissue Eng Part A 21, 1859–1868. doi: 10.1089/ten.TEA.2014.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipner J, Liu W, Liu Y, Boyle J, Genin GM, Xia Y, Thomopoulos S, 2014. The mechanics of PLGA nanofiber scaffolds with biomimetic gradients in mineral for tendon-to-bone repair. J Mech Behav Biomed Mater 40, 59–68. doi: 10.1016/j.jmbbm.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipner J, Shen H, Cavinatto L, Liu W, Havlioglu N, Xia Y, Galatz LM, Thomopoulos S, 2015. In Vivo Evaluation of Adipose-Derived Stromal Cells Delivered with a Nanofiber Scaffold for Tendon-to-Bone Repair. Tissue Eng Part A 21, 2766–2774. doi: 10.1089/ten.TEA.2015.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Joshi SK, Ravishankar B, Laron D, Kim HT, Feeley BT, 2014. Upregulation of transforming growth factor-β signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg 23, 1709–1716. doi: 10.1016/j.jse.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo UG, Berton A, Khan WS, Maffulli N, Denaro V, 2011. Histopathology of rotator cuff tears. Sports Med Arthrosc 19, 227–236. doi: 10.1097/JSA.0b013e318213bccb [DOI] [PubMed] [Google Scholar]
- Longo UG, Lamberti A, Rizzello G, Maffulli N, Denaro V, 2012. Synthetic augmentation in massive rotator cuff tears. Med Sport Sci 57, 168–177. doi: 10.1159/000328891 [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Burns M, Silva MJ, Manske P, 2001. BMP-12 gene transfer augmentation of lacerated tendon repair. J. Orthop. Res 19, 1199–1202. doi: 10.1016/S0736-0266(01)00042-0 [DOI] [PubMed] [Google Scholar]
- Lynch SE, Colvin RB, Antoniades HN, 1989. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest 84, 640–646. doi: 10.1172/JCI114210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray JD, Fealy S, Terry MA, Koh JL, Nixon AJ, Warren RF, 2006. Biomechanical evaluation of a rotator cuff defect model augmented with a bioresorbable scaffold in goats. J Shoulder Elbow Surg 15, 639–644. doi: 10.1016/j.jse.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Maffulli N, Moller HD, Evans CH, 2002. Tendon healing: can it be optimised? Br J Sports Med 36, 315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CN, Havlioglu N, Knutsen E, Sakiyama-Elbert SE, Silva MJ, Thomopoulos S, Gelberman RH, 2014. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res 32, 645–652. doi: 10.1002/jor.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S, 2011. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res 29, 1099–1105. doi: 10.1002/jor.21301 [DOI] [PubMed] [Google Scholar]
- Manning CN, Schwartz AG, Liu W, Xie J, Havlioglu N, Sakiyama-Elbert SE, Silva MJ, Xia Y, Gelberman RH, Thomopoulos S, 2013. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater 9, 6905–6914. doi: 10.1016/j.actbio.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Williams SF, 2003. Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Eng J 16, 97–105. doi: 10.1016/S1369-703X(03)00040-8 [DOI] [Google Scholar]
- Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Müller R, Swartz MA, Hubbell JA, 2014. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885–888. doi: 10.1126/science.1247663 [DOI] [PubMed] [Google Scholar]
- Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, Arciero RA, Beitzel K, 2012. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am 94, 308–316. doi: 10.2106/JBJS.K.00430 [DOI] [PubMed] [Google Scholar]
- McCarron JA, Derwin KA, Bey MJ, Polster JM, Schils JP, Ricchetti ET, Iannotti JP, 2013. Failure with continuity in rotator cuff repair “healing”. Am J Sports Med 41, 134–141. doi: 10.1177/0363546512459477 [DOI] [PubMed] [Google Scholar]
- Melis B, Nemoz C, Walch G, 2009. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res 95, 319–324. doi: 10.1016/j.otsr.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K, 2012. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med 40, 606–610. doi: 10.1177/0363546511429778 [DOI] [PubMed] [Google Scholar]
- Mikic B, Bierwert L, Tsou D, 2006. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res 24, 831–841. doi: 10.1002/jor.20092 [DOI] [PubMed] [Google Scholar]
- Min HK, Kwon OS, Oh SH, Lee JH, 2016. Platelet-derived growth factor-BB-immobilized asymmetrically porous membrane for enhanced rotator cuff tendon healing. Tissue Engineering and Regenerative Medicine 13, 568–578. doi: 10.1007/s13770-016-9120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HK, Oh SH, Lee JM, Im GI, Lee JH, 2014. Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: in vitro evaluation on adipose-derived stem cell differentiation. Acta Biomater 10, 1272–1279. doi: 10.1016/j.actbio.2013.12.031 [DOI] [PubMed] [Google Scholar]
- Novakova SS, Mahalingam VD, Florida SE, Mendias CL, Allen A, Arruda EM, Bedi A, Larkin LM, 2017. Tissue-Engineered Tendon Constructs for Rotator Cuff Repair in Sheep. J Orthop Res. doi: 10.1002/jor.23642 [DOI] [PubMed] [Google Scholar]
- Ojima Y, Mizuno M, Kuboki Y, Komori T, 2003. In vitro effect of platelet-derived growth factor-BB on collagen synthesis and proliferation of human periodontal ligament cells. Oral Dis 9, 144–151. doi: 10.1034/j.1601-0825.2003.02906.x [DOI] [PubMed] [Google Scholar]
- Oliva F, Via AG, Maffulli N, 2011. Role of growth factors in rotator cuff healing. Sports Med Arthrosc 19, 218–226. doi: 10.1097/JSA.0b013e3182250c78 [DOI] [PubMed] [Google Scholar]
- Park JS, Woo DG, Yang HN, Na K, Park K-H, 2009. Transforming growth factor beta-3 bound with sulfate polysaccharide in synthetic extracellular matrix enhanced the biological activities for neocartilage formation in vivo. J Biomed Mater Res A 91, 408–415. doi: 10.1002/jbm.a.32271 [DOI] [PubMed] [Google Scholar]
- Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar SG, Laurencin CT, 2017. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLoS ONE 12, e0174789. doi: 10.1371/journal.pone.0174789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock EE, 1959. A study of the circulation in normal tendons and healing grafts. Ann Surg 149, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedowitz R, Yamaguchi K, Ahmad C, Burks R, Flatow E, Green A, Iannotti J, Miller B, Tashjian R, Watters W 3rd, Weber K, Turkelson T, Wies J, Anderson S, Andre J, Boyer K, Raymond L, Sluka P, McGowan R, 2011. Optimizing the Management of Rotator Cuff Problems. J Am Acad Orthop Surg 19, 368–79. [DOI] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ, 2012. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2, 18–28. [PMC free article] [PubMed] [Google Scholar]
- Peterson DR, Ohashi KL, Aberman HM, Piza PA, Crockett HC, Fernandez JI, Lund PJ, Funk KA, Hawes ML, Parks BG, Mattern R-H, 2015. Evaluation of a collagen-coated, resorbable fiber scaffold loaded with a peptide basic fibroblast growth factor mimetic in a sheep model of rotator cuff repair. J Shoulder Elbow Surg 24, 1764–1773. doi: 10.1016/j.jse.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Porsch H, Mehić M, Olofsson B, Heldin P, Heldin C-H, 2014. Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other’s signaling and stability. J Biol Chem 289, 19747–19757. doi: 10.1074/jbc.M114.547273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premdas J, Tang J-B, Warner JP, Murray MM, Spector M, 2001. The presence of smooth muscle actin in fibroblasts in the torn human rotator cuff. J. Orthop. Res 19, 221–228. doi: 10.1016/S0736-0266(00)90011-1 [DOI] [PubMed] [Google Scholar]
- Proudfoot AEI, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TNC, Kosco-Vilbois MH, 2003. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A 100, 1885–1890. doi: 10.1073/pnas.0334864100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R, 2009. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351–1361. doi: 10.1242/dev.027342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufe T, Petersen WJ, Mentlein R, Tillmann BN, 2005. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports 15, 211–222. doi: 10.1111/j.1600-0838.2005.00465.x [DOI] [PubMed] [Google Scholar]
- Randelli P, Cucchi D, Ragone V, de Girolamo L, Cabitza P, Randelli M, 2015. History of rotator cuff surgery. Knee Surg Sports Traumatol Arthrosc 23, 344–362. doi: 10.1007/s00167-014-3445-z [DOI] [PubMed] [Google Scholar]
- Randelli P, Randelli F, Ragone V, Menon A, D’Ambrosi R, Cucchi D, Cabitza P, Banfi G, 2014. Regenerative medicine in rotator cuff injuries. BioMed research international 2014, 129515. doi: 10.1155/2014/129515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK, Stehno-Bittel L, Enwemeka CS, 1999. Matrix remodeling in healing rabbit Achilles tendon. Wound Repair Regen 7, 518–527. [DOI] [PubMed] [Google Scholar]
- Reilly P, Bull AMJ, Amis AA, Wallace AL, Richards A, Hill AM, Emery RJH, 2004. Passive tension and gap formation of rotator cuff repairs. J Shoulder Elbow Surg 13, 664–667. doi: 10.1016/S1058274604001272 [DOI] [PubMed] [Google Scholar]
- Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL, 2007. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am 89, 2485–2497. doi: 10.2106/JBJS.C.01627 [DOI] [PubMed] [Google Scholar]
- Saadat F, Deymier AC, Birman V, Thomopoulos S, Genin GM, 2016. The concentration of stress at the rotator cuff tendon-to-bone attachment site is conserved across species. J Mech Behav Biomed Mater 62, 24–32. doi: 10.1016/j.jmbbm.2016.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadir R, Imberty A, Baleux F, Lortat-Jacob H, 2004. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem 279, 43854–43860. doi: 10.1074/jbc.M405392200 [DOI] [PubMed] [Google Scholar]
- Sahoo S, Ang LT, Goh JC-H, Toh S-L, 2010. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A 93, 1539–1550. doi: 10.1002/jbm.a.32645 [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA, 2000. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release 65, 389–402. doi: 10.1016/S0168-3659(99)00221-7 [DOI] [PubMed] [Google Scholar]
- Samarakoon R, Overstreet JM, Higgins PJ, 2013. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 25, 264–268. doi: 10.1016/j.cellsig.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF, Reis RL, 2013a. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev 19, 327–352. doi: 10.1089/ten.TEB.2012.0727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF, Reis RL, 2013b. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev 19, 308–326. doi: 10.1089/ten.TEB.2012.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD, 2011. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3. doi: 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitskaya YA, Izaguirre A, Sierra L, Perez F, Cruz F, Villalobos E, Almazan A, Ibarra C, 2011. Effect of angiogenesis-related cytokines on rotator cuff disease: the search for sensitive biomarkers of early tendon degeneration. Clinical medicine insights. Arthritis and musculoskeletal disorders 4, 43–53. doi: 10.4137/CMAMD.S7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AG, Galatz LM, Thomopoulos S, 2017. Enthesis regeneration: a role for Gli1+ progenitor cells. Development 144, 1159–1164. doi: 10.1242/dev.139303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AG, Long F, Thomopoulos S, 2015. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196–206. doi: 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D’Augusta D, Li XJ, Smith E, Wozney JM, 2008. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am 90, 2206–2219. doi: 10.2106/JBJS.G.00742 [DOI] [PubMed] [Google Scholar]
- Shah V, Bendele A, Dines JS, Kestler HK, Hollinger JO, Chahine NO, Hee CK, 2013. Dose-response effect of an intra-tendon application of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in a rat Achilles tendinopathy model. J Orthop Res 31, 413–420. doi: 10.1002/jor.22222 [DOI] [PubMed] [Google Scholar]
- Shen H, Gelberman RH, Silva MJ, Sakiyama-Elbert SE, Thomopoulos S, 2013. BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS ONE 8, e77613. doi: 10.1371/journal.pone.0077613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BD, Grande DA, 2015. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 11, 213–222. doi: 10.1038/nrrheum.2015.27 [DOI] [PubMed] [Google Scholar]
- Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S, 2012. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect Tissue Res 53, 95–105. doi: 10.3109/03008207.2011.650804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo C, Beanes SR, Hu F-Y, Zhang X, Dang C, Chang G, Wang Y, Nishimura I, Freymiller E, Longaker MT, Lorenz HP, Ting K, 2003. Ontogenetic Transition in Fetal Wound Transforming Growth Factor-β Regulation Correlates with Collagen Organization. The American journal of pathology 163, 2459–2476. doi: 10.1016/S0002-9440(10)63601-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi H, Akahori O, Nishihara K, Tada K, 1978. Three-dimensional architecture of blood vessels of tendons demonstrated by corrosion casts. Hand 10, 9–15. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH, 2010. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng 38, 225–234. doi: 10.1007/s10439-009-9844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH, 2009. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res 27, 1209–1215. doi: 10.1002/jor.20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, Sakiyama-Elbert S, Gelberman RH, 2007. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res 25, 1358–1368. doi: 10.1002/jor.20444 [DOI] [PubMed] [Google Scholar]
- Todd NW, Luzina IG, Atamas SP, 2012. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5, 11. doi: 10.1186/1755-1536-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T, Ide J, Arimura H, Nakamura T, Uehara Y, Sakamoto H, Mizuta H, 2015a. Local Application of Gelatin Hydrogel Sheets Impregnated With Platelet-Derived Growth Factor BB Promotes Tendon-to-Bone Healing After Rotator Cuff Repair in Rats. Arthroscopy 31, 1482–1491. doi: 10.1016/j.arthro.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Tokunaga T, Karasugi T, Arimura H, Yonemitsu R, Sakamoto H, Ide J, Mizuta H, 2017. Enhancement of rotator cuff tendon-bone healing with fibroblast growth factor 2 impregnated in gelatin hydrogel sheets in a rabbit model. J Shoulder Elbow Surg 26, 1708–1717. doi: 10.1016/j.jse.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Tokunaga T, Shukunami C, Okamoto N, Taniwaki T, Oka K, Sakamoto H, Ide J, Mizuta H, Hiraki Y, 2015b. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am J Sports Med 43, 2411–2422. doi: 10.1177/0363546515597488 [DOI] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N, 2015. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol Ther 23, 1189–1200. doi: 10.1038/mt.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki M, Brigman BE, Yamamoto J, Lawrence WT, Simmons JG, Mohapatra NK, Lund PK, Van Wyk J, Hannafin JA, Bhargava MM, Banes AJ, 2000. Insulin-like growth factor-I is expressed by avian flexor tendon cells. J Orthop Res 18, 546–556. doi: 10.1002/jor.1100180406 [DOI] [PubMed] [Google Scholar]
- Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O, 2010. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy 26, 1456–1462. doi: 10.1016/j.arthro.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Waldorff EI, Lindner J, Kijek TG, Downie BK, Hughes RE, Carpenter JE, Miller BS, 2011. Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J Shoulder Elbow Surg 20, 904–908. doi: 10.1016/j.jse.2010.12.009 [DOI] [PubMed] [Google Scholar]
- Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE, 2000. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen 8, 128–137. [DOI] [PubMed] [Google Scholar]
- Wang Zhenming, Wang Zhefeng, Lu WW, Zhen W, Yang D, Peng S, 2017. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials 9, e435. doi: 10.1038/am.2017.171 [DOI] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V, 1997. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 100, 321–330. doi: 10.1172/JCI119537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MA, Hutmacher DW, 2010. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci 35, 1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002 [DOI] [Google Scholar]
- Würgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ, 2007. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 16, S198–203. doi: 10.1016/j.jse.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Rothrauff BB, Tuan RS, 2013. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today 99, 203–222. doi: 10.1002/bdrc.21041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V, 2009. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 30, 3551–3559. doi: 10.1016/j.biomaterials.2009.03.024 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Alaee F, Dyrna F, Kronenberg MS, Maye P, Kalajzic I, Rowe DW, Mazzocca AD, Dyment NA, 2016. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res 57, 507–515. doi: 10.1080/03008207.2016.1189910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Q, Deng S, Fu W, Tang X, Chen G, Qin T, Li J, 2016. bFGF- and CaPP-Loaded Fibrin Clots Enhance the Bioactivity of the Tendon-Bone Interface to Augment Healing. Am J Sports Med 44, 1972–1982. doi: 10.1177/0363546516637603 [DOI] [PubMed] [Google Scholar]
- Zhang HY, Phan SH, 1999. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol 21, 658–665. doi: 10.1165/ajrcmb.21.6.3720 [DOI] [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH, 1995. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol 147, 352–361. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, 2017. Nanofiber-Based Scaffold for Integrative Rotator Cuff Repair - Academic Commons.
- Zhang X, Bogdanowicz D, Erisken C, Lee NM, Lu HH, 2012. Biomimetic scaffold design for functional and integrative tendon repair. J Shoulder Elbow Surg 21, 266–277. doi: 10.1016/j.jse.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhao Jingwen, Dong S, Huangfu X, Li B, Yang H, Zhao Jinzhong, Cui W, 2014. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int J Nanomedicine 9, 2373–2385. doi: 10.2147/IJN.S59536 [DOI] [PMC free article] [PubMed] [Google Scholar]


