Primary progressive aphasia (PPA) is one of the most difficult differential diagnostic challenges faced by the neurologist. Changing terminologies (1), evolving classification sces (1,2), rapidly evolving understanding of the neurobiology of frontotemporal dementia (3,4) --- one of the causes of PPA --- have all conspired to make this syndrome foreign to all but the most intrepid of behavioral neurologists. Recently, however, some order and logic has begun to emerge from the chaos and the contribution from Rabinvici and colleagues (5) in this issue of the Annals of Neurology, takes the next step in clarifying this complex area of differential diagnosis. The paper also demonstrates the growing importance of biomarkers in making accurate etiologic diagnoses and shows that the clinical phenotype may not always be the best guide to etiology. Etiologic diagnosis becomes increasingly important as we move toward pathologic process-specific disease-modifying therapies and the need to give these to patients accurately identified as harboring the target process. Etiologic diagnosis is also critically important in drug development where including only patients with the targeted disease is necessary for treatment efficacy evaluation.
Three phenotypes of PPA are now recognized: progressive nonfluent aphasia, semantic dementia, and logopenic aphasia (6,7)(Figure 1). Progressive nonfluent aphasia features reduced speech output, loss of grammar, appearance of effort in producing speech, and apraxia of vocal movements. Language comprehension at least at the word meaning level is typically preserved. Semantic dementia is a progressive fluent aphasic with semantic anomia (loss of word meaning). Semantic dementia often is combined with an associative visual agnosia (inability to name or recognize objects despite preserved ability to perceive and draw them). Logopenic aphasia is characterized by slow and hesitant speech with obvious word-finding deficits and disturbances of sentence repetition. The disorder resembles conduction aphasia. Unlike progressive nonfluent aphasia, patients with logopenic apahsia are not agrammatic; unlike semantia dementia, they have preserved word comprehension. While there is variability in the clinical presentations of PPA and related focal syndromes, these three variants appear to be the most common and to account for the majority of syndromes encountered in clinical practice.
Figure 1.
Classification of the primary progressive aphasias beginning with the clinical syndrome. (AD – Alzheimer’s disease; Apo – apoliprotein; FDG – fluorodeoxyglucose; PET – positon emission tomography; PIB – Pittsburgh Compound B; TDP – transactivation response element (TAR) deoxyribonucleic acid (DNA) binding protein)
Patients in the study by Rabinvci and coworkers (5) were studied with both fluorodexyglucose (FDG) positron emission tomography (PET) and Pittsburgh Compound B (PIB) amyloid-labeling PET (8). FDG PET faithfully corresponded to the clinical phentoype displayed by the patients. Patients with logopenic aphasia had left parietal and posteroloateral temporal lobar hypometabolism; those with progressive nonfluent aphasia had left frontal hypometabolism; and patients with semantic dementia had bilateral anterior temporal and hypometabolism more severe on the left. The FDG PET findings corroborate the proposed clinical classification based on phenotype. Each has a distinct anatomic correlate as demonstrated manifested by regional hypometabolism.
Etiologically, however, neither the phenotype nor the FDG PET studies provide certain guidance to causative diagnosis. Assuming the PIB PET identifies underlying neuritic plaques and fibrillar amyloid characteristic of AD, as seems to be increasingly well established (9), then PIB demonstrates that all cases of logopenic aphasia were due to AD. Surprisingly, however, 16% of progressive nonfluent aphasias and 20% of cases with semantic dementia also were caused by AD. Cases of progressive nonfluent aphasia and semantic dementia caused by AD could not be distinguished from those associated with frontotemporal lobar degeneration based on clinical features combined with FDG PET. Even when memory deficits --- the central abnormality of most cases of AD --- were carefully studied, the clinical assessment did not differentiate those with and without AD as the underlying etiology. Clinical and metabolic assessment would not lead to accurate etiologic diagnosis and would not identify candidates for anti-AD therapy (either cholinergic or anti-amyloid treatments).
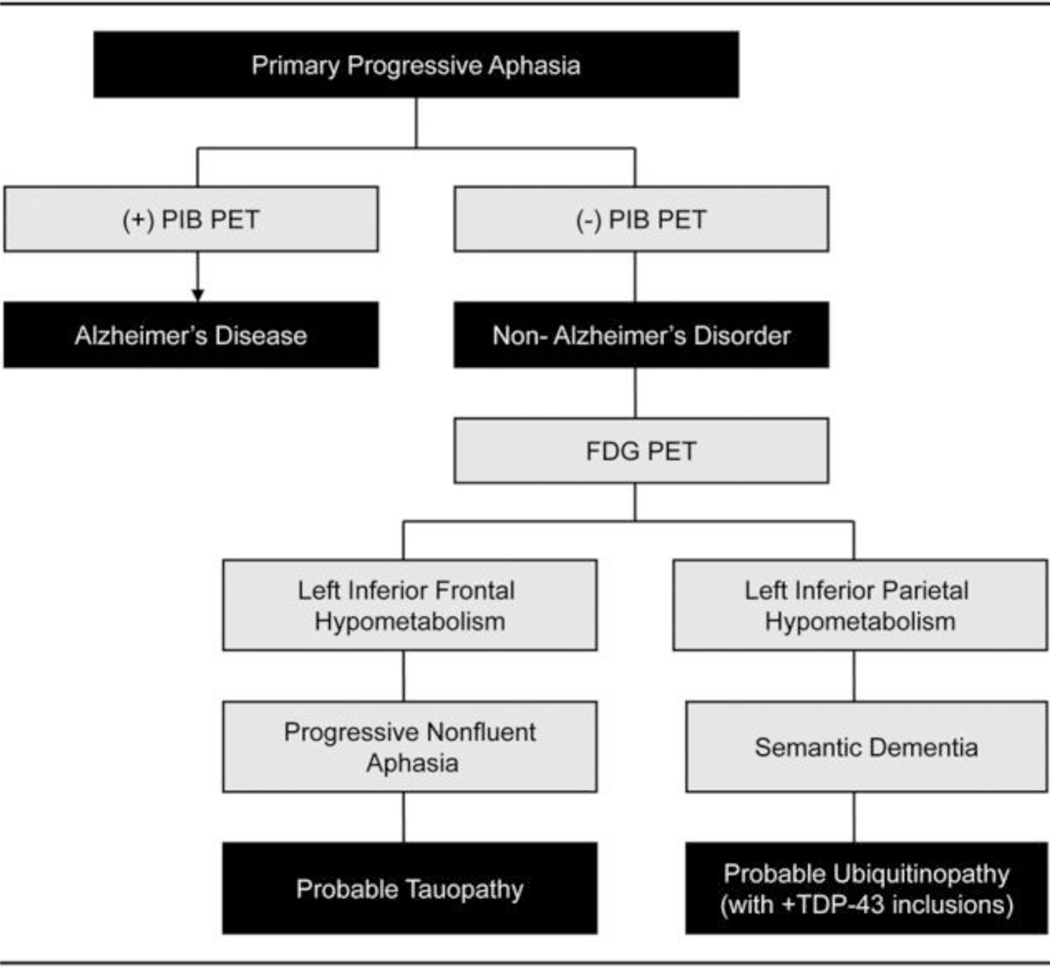
When the data from Rabinovici and colleagues (5) are reorganized to give primacy to the role of biomarkers (Figure 2), an accurate etiologic diagnosis can be achieved. In this case the biomarkers provide more etiologically specific information than the phenotype. This is a compelling demonstration of the increasing power of biomarkers to assist clinicians in identifying the etiology of complex neurologic syndromes. Biomarkers could also aid in identifying patients with etiologically homogeneous disorders for inclusion in clinical trials of agents targeting exploitable steps in pathophysiologic cascades. They may eventually play a role in clinical practice in identifying patients with specific pathophysiologies requiring mechanistically specific therapies.
Figure 2.
Classification of the primary progressive aphasias based on biomarker findings.
The young age of the AD patients in the study reported by Rabinvici and colleagues (5) stands out as a unique demographic feature. The average age at onset of the patients with AD presenting with logopenic aphasia was 54, approximately 15 years younger than the average age of onset of AD presenting with episodic memory abnormalities. This increases the likelihood of clinical misdiagnosis; the clinician presented with a young patient manifesting PPA may not consider AD as the most common diagnosis. The paper by Rabinvici et al (5) does a service by reinforcing this uncommon AD phenotype and clinical features that lead to its recognition and proper management.
Patients with logopenic aphasia in this study did not have the typical AD clinical syndrome plus aphasia, instead they had a unique clinical condition in which the episodic memory deficit typical of AD was absent. Memory was no more severely impaired in the logopenic syndrome associated with AD then in the non-AD forms of primary progressive aphasia. The young age of the logopenic aphasia presentation of AD and the absence of the typical type of memory deficit suggest that these patients had a unique pathophysiology in which the brain dysfunction was geographically constrained despite widespread fibrillar amyloid as shown by PIB. This observation adds to a growing body of evidence that suggests that amyloid may not itself be the principle cause of cell death in AD (10) but rather initiates a cascade of events that lead to synaptic compromise and neuronal dysfunction, which are responsible for the clinical syndrome. Where these secondary processes can be contained, neuronal function continues unabated (as shown by normal FDG PET activity in areas with a PIB signal of the presence of amyloid) and clinical manifestations are thwarted. The secondary processes may involve tau metabolism abnormalities or other secondary mechanisms and should be more seriously regarded as targets for drug development for the treatment of AD.
Acknowledgements:
JLC is supported by an National Institute on Aging Alzheimer’s Disease Research Center grant (AG P50 07650), an Alzheimer’s Disease Research Center grant from the State of California, the Sidell-Kagan Foundation and the Jim Easton gift.
Footnotes
Disclosures: none relevant to the content of this article
References
- 1).Cairns NJ, Bigio EH, Macxkenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontomeporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degneration. Acta Neuropath 2007: 114: 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings JL, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3).Kumar-Singh S, Van Broeckhaven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol 2007; 17: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Boeve B, Hutton M. Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN). Arch Neurol. 2008; 65:460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Rabinvici GD, Jagust WJ, Furst AJ, et al. Aß amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Mesulam MM. Slowlt orogressive aphasia without generalized dementia. Ann Neurol 1982; 11: 582–598. [DOI] [PubMed] [Google Scholar]
- 7).Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Klunk WE, Engler H, Norberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. [DOI] [PubMed] [Google Scholar]
- 9).Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(Pt 6):1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Roberson ED, Scearce-Levie K, Palop JJ et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007; 316:750–4. [DOI] [PubMed] [Google Scholar]