Abstract
The US Preventive Services Task Force (USPSTF) recently proposed to widen the current lung cancer screening guideline to include less-heavy smokers. We sought to incorporate both genetic and tobacco smoking data to evaluate the proposed new guideline in white smokers. We constructed a polygenic risk score (PRS) using lung cancer risk variants. Using data from 308 490 participants of European descent in the UK Biobank, a population-based cohort study, we estimated hazard ratios of lung cancer associated with both tobacco smoking and PRS to identify individuals at a similar or higher risk than the group of heavy smokers who are recommended for screening under the USPSTF-2014 guideline (≥30 pack-years, either current or former smokers who quit within 15 years). During a median follow-up of 5.8 years, 1449 incident cases of lung cancer were identified. We found a similar lung cancer risk for current smokers with 20–29 pack-years [hazard ratio = 20.7, 95% confidence interval: 16.3–26.4] and the ‘heavy smoker group’ defined above (hazard ratio = 19.9, 95% confidence interval: 16.8–23.6) compared with never smokers. Current smokers with 20–29 pack-years did not reach a 6-year absolute risk of 0.0151, a suggested risk threshold for using low-dose computed tomography screening, until the age of 55 years. However, these smokers at high genetic risk (PRS ≥ 80%) reached this risk level at the age of 50. Our findings support the USPSTF proposal to lower the smoking pack-year eligibility to 20 pack-years for current smokers and suggest that PRS for lung cancer could be considered to identify high-risk smokers for screening.
Incorporating genetic and tobacco smoking data, this study supports lowering the smoking pack-year eligibility for current smokers and suggests that genetic risk could be considered to further identify high-risk smokers to start screening at an early age.
Introduction
Globally, lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths. In 2018, there were approximately 2.09 million new cases diagnosed and 1.76 million individuals who died of lung cancer worldwide (1). Smoking, as the most important risk factor, accounts for >80% of the lung cancer cases and is used in the current screening guidelines to identify high-risk individuals (2,3). The current screening guideline for lung cancer was established in 2014 by the US Preventive Services Task Force (USPSTF), which recommends that adults aged 55–80 years who have a smoking history of ≥30 pack-years and currently smoke, or have quit within the past 15 years, should receive an annual lung cancer screening using low-dose computed tomography (USPSTF-2014 guideline) (4). However, only about one-third of lung cancer patients meet this criterion and are, thus, eligible for screening (5,6). Recently, the USPSTF proposed to expand the eligible age range to 50 years and lower the smoking pack-year eligibility to 20 pack-years (7). However, this proposed change has not yet properly evaluated in population-based studies.
Since 2007, genome-wide association studies (GWASs) have identified common genetic variants in 45 loci associated with lung cancer risk (8). Although each of these risk variants is associated with only a small to moderate increased risk of lung cancer, the cumulative risk [as measured using a polygenic risk score (PRS)] conferred by these variants could be substantial. In a recent study, we created a PRS using GWAS-identified risk variants for lung cancer and showed that this PRS was strongly associated with lung cancer risk following a dose–response pattern (9). However, genetic risk factors have not been taken into consideration in the screening guidelines for lung cancer. In this study, we sought to quantify the risk of lung cancer associated with both tobacco smoking and genetic risk variants in order to identify less-heavy smokers at a risk level that is comparable to heavy smokers, those who had a smoking history of ≥30 pack-years and currently smoked or had quit within the past 15 years and eligible for screening according to the USPSTF-2014 guideline.
Methods
Study subjects, genotype and imputation
In this study, we used data from the UK Biobank, a population-based cohort study that has recruited >500,000 adults aged 40–69 years across England, Scotland, and Wales. Details of the design and methods of the study have been described previously (10). Baseline characteristics and demographic information were collected via a touch-screen questionnaire and a computer-assisted interview at enrollment. Data and diagnoses of incident cancers were provided by the National Health Service Information Centre for participants from England and Wales (follow-up through 31 March 2016) and by the NHS Central Register Scotland for participants from Scotland (follow-up through 31 October 2015). Cancers were coded by the International Classification of Diseases, Ninth Revision (ICD-9) or the International Classification of Diseases, Tenth Revision (ICD-10). Study participants diagnosed with primary lung cancer (ICD9 = 162.2–162.9 or ICD10 = C34) were defined as case patients.
We acquired genotype imputation data from 488 377 participants from the UK Biobank. Samples were genotyped by two arrays, the UK BiLEVE Axiom (UKBL) and the UK Biobank Axiom (UKBB), which shared 95% marker content. Genotyping data were imputed using reference panels of the Haplotype Reference Consortium combined with the UK10K haplotype resource. Individuals marked as outliers for heterozygosity, low call rates and sex chromosome aneuploidy were excluded (n = 628). We identified participants of European descent by projecting the genotype data of all samples on the first two major principal components of four 1000 Genome populations (CEU, YRI, CHB and JPT) (11) and excluded individuals not falling in the neighborhood of the CEU cluster (n = 23 425). We also excluded participants who were younger than age 50 at baseline (n = 90 924), participants who were second-degree (or higher) relatives [determined by a KING-robust kinship estimator ≥ 0.0442 (n = 37 590)] (12), participants who had been diagnosed with cancer at baseline (n = 24 944), and those who were missing a value for smoking status (n = 1398). A total of 308 490 individuals (144 173 men and 164 317 women) remained after these exclusions (not mutually exclusive).
PRS building
Of the 45 lung cancer susceptibility loci identified to date for any types of lung cancer, 32 loci were associated with overall lung cancer at a P-value < 5.0 × 10−8 in previous studies (8). We excluded risk variants not replicated previous studies of populations of European descent (which excluded 18 loci). We also excluded the variants in linkage disequilibrium (r2 > 0.2) and variants with a minor allele frequency < 0.01. After all exclusions, 19 variants from 14 loci remained and were selected to construct the PRS for lung cancer. A list of the 19 single-nucleotide polymorphisms is shown in Supplementary Table 1, available at Carcinogenesis Online. We used regression coefficients reported by a previous GWAS (29 266 cases and 56 450 controls of European descent) as variant-specific weights (13). We calculated the PRS as the sum of the product of the weight and the number of risk alleles for each risk variant across all selected risk variants per individual (9). Since the PRS was pre-defined using external data, overfitting is not a concern in this study.
Assessment of tobacco smoking
Information on cigarette smoking, including smoking status (never, former, and current), pack-year of smoking and age at quitting for former smokers, was collected at the baseline survey. We defined heavy smokers as individuals who had a smoking history of ≥30 pack-years and currently smoked or had quit within the past 15 years and thus are eligible for lung cancer screening based on the USPSTF-2014 screening guideline. We also categorized current smokers by pack-year (20–29 pack-years and <20 pack-years) and former smokers by pack-year and quitting time (≥30 pack-years and quit-time = 16–20 years ago, 20–29 pack-years and quit-time ≤ 15 years ago, 20–29 pack-years and quit-time > 16 years and others).
Statistical analysis
Mann–Whitney–Wilcoxon tests and chi-square tests were used for univariate analyses of continuous and categorical baseline variables, respectively. We used Cox proportional hazard models, with age as the time scale left truncated at age at enrollment, to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of lung cancer risk associated with tobacco smoking (by groups defined previously) and PRS (by quintiles). Individuals with missing pack-year information were listed as separate groups. We also conducted analyses by types of major lung cancer subtypes including adenocarcinoma, squamous cell carcinoma and all non-small cell lung cancer combined. Possible interactions of smoking status with PRS or each of the risk variants were evaluated using likelihood ratio tests. We then estimated the risk of lung cancer for groups defined jointly by smoking status and PRS levels, compared with never smokers or heavy smokers. Covariates included in the models were sex, education (college or university degree, some professional qualifications, secondary education and none of the above), genotype array (UKBL or UKBB) and 10 principal components for ancestry. The assumption of proportional hazards was checked with the Schoenfeld residuals. Using the Cox regression model, we calculated the 6-year absolute risk of lung cancer for individuals in each group jointly defined by smoking status and PRS levels, which were adjusted to the mean of continuous covariates and the mode of categorical covariates in the model. We used a 6-year risk of 0.0151 as the threshold to assess eligibility for screening. This threshold was derived from a large study showing that compared with chest X-ray, low-dose computed tomography significantly reduced lung cancer mortality for those with a 6-year absolute risk of lung cancer at 0.0151 or higher (14). All tests were two sided at a significance level of P = 0.05.
Results
During a median follow-up of 5.8 years, 1449 incident lung cancer cases were identified in the cohort. Table 1 compares cases and non-cases by selected baseline characteristics. Cases were more likely to be smokers with higher pack-years of smoking, male, older, and have a lower education level than non-cases (Table 1).
Table 1.
Selected baseline characteristics for lung cancer cases and non-cases in the UK Biobank, 2006–2010
| Characteristics | Cases (n = 1449) | Non-cases (n = 307 041) | P-value |
|---|---|---|---|
| Age, mean (SD) | 62.5 (4.8) | 60.0 (5.4) | <0.001 |
| Sex, no. (%) | <0.001 | ||
| Female | 662 (45.7) | 163 665 (53.3) | |
| Male | 787 (54.3) | 143 386 (46.7) | |
| Education, no. (%) | <0.001 | ||
| College or university degree | 232 (16.0) | 97 266 (31.7) | |
| Some professional qualifications | 364 (25.1) | 82 897 (27.0) | |
| Secondary education | 259 (17.9) | 65 102 (21.2) | |
| None of the above | 594 (41.0) | 61 776 (20.1) | |
| Smoking status, no. (%) | <0.001 | ||
| Never smokers | 179 (12.4) | 160 908 (52.4) | |
| Former smokers | 668 (46.1) | 117 416 (38.2) | |
| Current smokers | 602 (41.5) | 28 717 (9.35) | |
| Pack-years for smokers, mean (SD)a | 42.1 (25.2) | 25.0 (19.6) | <0.001 |
| PRS for lung cancer, mean (SD) | 1.97 (0.4) | 1.90 (0.4) | <0.001 |
aParticipants who never smoked (n = 161 087) or had a missing value of pack-years (n = 50 093) were excluded.
Table 2 shows the association between lung cancer risk and smoking status. Compared with never smokers, an elevated risk of lung cancer was found in all smoking groups. Heavy smokers with 30 or more pack-years of smoking had an HR of 19.9 (95% CI: 16.8–23.6) compared with never smokers. A similar HR of 20.7 (95% CI: 16.3–26.4) was observed among current smokers with 20–29 pack-years of smoking. The risks of lung cancer estimated for other smoking groups were substantially lower than heavy smokers. Particularly, the former smokers who had 20–29 pack-years of smoking and quit within the past 15 years had a lower risk (HR = 7.45, 95% CI: 5.37–10.3) than former heavy smokers (≥30 pack-years and quit-time ≤ 15 years, HR = 14.8, 95% CI: 12.2–17.8). Similar trends were also found for all major lung cancer subtypes (Supplementary Table 2, available at Carcinogenesis Online).
Table 2.
Hazard ratios (95% CI) for lung cancer risk associated with smoking status, UK Biobank, 2006–2016
| Smoking status | No. of cases | HR (95% CI)a |
|---|---|---|
| Never smokers | 179 | 1 (reference) |
| Heavy smokersb | 654 | 19.9 (16.8–23.6) |
| Current smokers | 365 | 27.6 (23.0–33.1) |
| Former smokers, quit-time ≤ 15 years | 289 | 14.8 (12.2–17.8) |
| Other current smokers | ||
| 20–29 pack-years | 106 | 20.7 (16.3–26.4) |
| <20 pack-years | 58 | 9.83 (7.30–13.2) |
| Missing pack-year information | 73 | 8.88 (6.75–11.7) |
| Other former smokers | ||
| ≥30 pack-years, quit-time >15 years | 63 | 6.29 (4.70–8.42) |
| 20–29 pack-years, quit-time ≤ 15 years | 45 | 7.45 (5.37–10.3) |
| 20–29 pack-years, quit-time > 15 years | 50 | 4.23 (3.08–5.79) |
| <20 pack-years, quit-time ≤ 15 years | 18 | 3.40 (2.10–5.53) |
| <20 pack-years, quit-time > 15 years | 80 | 1.92 (1.47–2.50) |
| Missing pack-years information | 123 | 2.38 (1.89–3.00) |
aHRs were adjusted for sex and education.
bIndividuals who had a smoking history of ≥30 pack-years and currently smoked or had quit within the past 15 years.
The association of lung cancer risk with PRS percentile groups is shown in Table 3, stratified by smoking status. The risk of lung cancer was statistically significantly associated with the PRS, following a dose–response pattern within each smoking status. Similar patterns were also found for adenocarcinoma and all non-small cell lung cancers combined (Supplementary Table 3, available at Carcinogenesis Online). Interaction tests between smoking status and PRS based on the multiplicative scale were not statistically significant (P for interaction > 0.05). Two risk variants showed a significant interaction at P < 0.05, and the interaction was no longer significant after adjusting for multiple comparisons (Supplementary Table 4, available at Carcinogenesis Online).
Table 3.
Hazard ratios (95% CI) for lung cancer risk associated with the PRS of lung cancer, stratified by smoking status, UK Biobank, 2006–2016a
| PRS | Never smokers | Ever smokers | Current smokers | Former smokers |
|---|---|---|---|---|
| Q1 (lowest) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 | 1.12 (0.67–1.89) | 1.18 (0.95–1.45) | 1.32 (0.98–1.77) | 1.04 (0.77–1.41) |
| Q3 | 1.24 (0.74–2.06) | 1.34 (1.01–1.52) | 1.17 (0.87–1.58) | 1.31 (0.98–1.74) |
| Q4 | 1.37 (0.83–2.24) | 1.42 (1.16–1.73) | 1.44 (1.08–1.92) | 1.40 (1.06–1.85) |
| Q5 (highest) | 1.93 (1.21–3.07) | 1.57 (1.29–1.91) | 1.55 (1.17–2.06) | 1.58 (1.21–2.08) |
| P for trend | 0.003 | <0.001 | 0.002 | <0.001 |
aHRs adjusted for sex, education, first 10 principal components, genotype array, pack-years (ever smokers only), and quit-time (former smokers only); P = 0.815 for interaction between never smokers and ever smokers; P = 0.778 for interaction across never, current and former smokers.
In Table 4, we classified participants jointly by PRS levels and smoking status. Compared with never-smokers with a low genetic risk of lung cancer (the lowest quintile of PRS), the highest risk of lung cancer was observed among current smokers with 20–29 pack-years at the highest genetic risk (the highest quintile of PRS, HR = 45.5, 95% CI = 27.6–75.0). Current heavy smokers at the highest genetic risk group had an HR of 36.1 (95% CI = 23.2–55.9).
Table 4.
Estimates of lung cancer risk for groups defined jointly by smoking status and quintiles of PRS, UK Biobank, 2006–2016
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Smoking status | No. cases | HRa | No. cases | HRa | No. cases | HRa | No. cases | HRa | No. cases | HRa |
| Never smokers | 27 | 1 (reference) | 30 | 1.11 (0.66–1.87) | 33 | 1.23 (0.74–2.05) | 37 | 1.36 (0.83–2.24) | 52 | 1.90 (1.20–3.03) |
| Current heavy smokersb | 51 | 25.4 (15.9–40.6) | 75 | 36.1 (23.2–56.1) | 73 | 32.7 (21.0–51.0) | 84 | 36.9 (23.9–57.1) | 82 | 36.1 (23.3–55.9) |
| Former heavy smokersc | 45 | 15.1 (9.37–24.4) | 49 | 15.5 (9.67–24.8) | 55 | 17.6 (11.1–27.9) | 63 | 19.1 (12.2–30.1) | 77 | 22.4 (14.4–34.8) |
| Current less-heavy smokersd | 17 | 19.4 (10.5–35.6) | 17 | 19.8 (10.8–36.4) | 15 | 18.0 (9.57–33.9) | 21 | 27.0 (15.2–47.8) | 36 | 45.5 (27.6–75.0) |
| Other current smokers | 16 | 6.92 (3.73–12.9) | 21 | 9.51 (5.37–16.8) | 30 | 14.3 (8.48–24.0) | 33 | 15.8 (9.50–26.3) | 31 | 16.1 (9.62–27.0) |
| Other former smokers | 56 | 2.88 (1.82–4.55) | 67 | 3.47 (2.22–5.43) | 87 | 4.51 (2.93–6.95) | 82 | 4.33 (2.80–6.69) | 87 | 4.63 (3.00–7.13) |
aHRs were adjusted for sex, education, first 10 principal components and genotype array.
bCurrent heavy smokers: current smokers with a smoking history of ≥30 pack-years.
cFormer heavy smokers: former smokers with a smoking history of ≥30 pack-years and quit within 15 years.
dCurrent less-heavy smokers: current smokers with a smoking history of 20–29 pack-years.
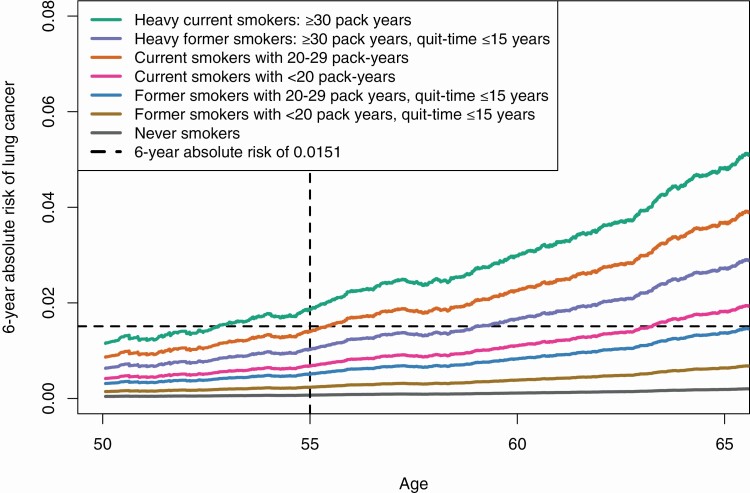
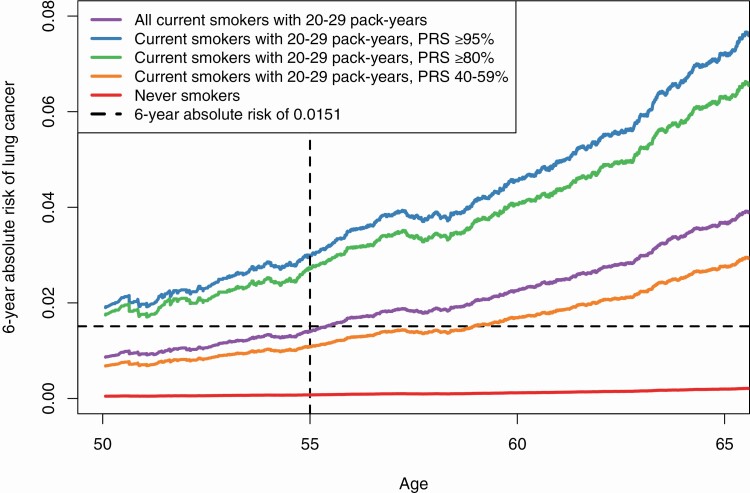
We calculated the 6-year absolute risk of lung cancer observed in the UK Biobank cohort. Figure 1 shows the absolute risk of lung cancer by smoking status. Heavy current smokers (≥30 pack-years) had the highest risk of developing lung cancer, followed by less-heavy current smokers (20–29 pack-years) and heavy former smokers. Smokers with <20 pack-years had low risks of lung cancer. The dashed horizontal line shows a 6-year risk level of 0.0151, a suggested threshold for using low-dose computed tomography screening (14). Current smokers with 20–29 pack-years reached this risk level around the age of 55 years. Figure 2 shows the absolute risk of lung cancer for current smokers with 20–29 pack-years stratified by their PRS levels. Those at high genetic risk (PRS ≥ 80%) exceeded the risk level of 0.0151 starting at age 50 years.
Figure 1.
Adjusted 6-year absolute lung cancer risks by smoking status. Risks were adjusted for age at baseline, sex, education, first 10 principal components and genotype arrays.
Figure 2.
Adjusted 6-year absolute lung cancer risks for current smokers with 20–29 pack-years by polygenetic risk score (PRS) level. Risks were adjusted for age at baseline, sex, education, first 10 principal components and genotype arrays.
Discussion
In this study, we evaluated the recently proposed change of lung cancer screening guideline by USPSTF in smokers of European descent. We found that current smokers with 20–29 pack-years of smoking had a risk of developing lung cancer similar to all heavy smokers who had a smoking history of ≥30 pack-years and currently smoked, or quit within the past 15 years and are thus eligible for lung cancer screening based on the USPSTF-2014 guideline. However, the 6-year absolute risk of lung cancer among current smokers with 20–29 pack-years did not reach 0.0151, a suggested threshold for screening with the use of low-dose computed tomography, until the age of 55. Using PRS derived from GWAS-identified risk variants for lung cancer, we showed that current smokers who had a 20–29 pack-year smoking history and a high genetic risk (a PRS at the highest quintile) reached the risk threshold at age 50. These results support, in general, the newly proposed USPSTF guideline to expand the eligibility to 20 pack-years for current smokers and suggest that lung cancer PRS could be considered in identifying high-risk smokers to start lung cancer screening at a younger age.
Our findings for a similar risk of current smokers who had a history of 20–29 pack-years with all heavy smokers combined (a history of 30 pack-years of smoking, either current or former smokers who quit within 15 years) are supported by a previous smaller study, in which similar relative risks were found for current smokers who had 20–29 pack-years (HR = 17.8, 95% CI: 12.2–26.0) and former heavy smokers (HR = 16.6, 95% CI: 13.6–20.3), compared with never smokers (15). In the UK Biobank cohort, there were 654 lung cancer case patients who were eligible for screening under the USPSTF-2014 guideline and 106 case patients who were current smokers with 20–29 pack-years. If the USPSTF-2014 screening guideline is expanded to include current smokers with a history of 20–29 pack-years of smoking, an additional 16.2% (106/654) of lung cancer cases would be screened and potentially detected. The proposal by USPSTF also recommended screenings for former smokers who had a 20–29 pack-year smoking history and quit within the past 15 years. However, we found that these former smokers had a substantially lower risk of lung cancer compared with the former heavy smokers who met the USPSTF-2014 screening guideline. Since the number of cases was small (n = 45) for these former smokers, further studies are needed to estimate their risk of lung cancer.
In order to evaluate the proposed change of lowering the eligible starting age from 55 to 50 years, we calculated the 6-year absolute lung cancer risks in the cohort by age. Tammemägi et al. reported that compared with chest X-ray, low-dose computed tomography significantly reduced lung cancer mortality for individuals with a 6-year absolute risk of lung cancer at 0.0151 or higher, and thus proposed to use this risk threshold to recommend lung cancer screening (14). This risk threshold has been validated in two more studies (16,17). In this study, we observed that current smokers with 20–29 pack-years did not reach this risk level until the age of 55 years, which does not support lowering the eligible starting age to 50 years for all of these less-heavy smokers.
While smoking is the leading risk factor for lung cancer, genetic factors also play an important role in the development of lung cancer (18,19). In this study, we constructed a PRS to measure genetic risk of lung cancer. To avoid overfitting in PRS construction, we selected risk variants that were identified by previous GWAS and used the regression coefficients reported in previous GWAS as weights to construct the PRS. Stratified by levels of PRS, the current smokers who had 20–29 pack-years and a high genetic risk (PRS ≥ 80%) reached the risk threshold of 0.0151 at the age of 50 years. These findings are supported by a recent study conducted in China, in which a lung cancer PRS was used to classify smokers into different risk groups, and smokers who smoked 20–29 pack-years and had a high genetic risk (top 5% PRS) reached the same cumulative risk of lung cancer earlier than the heavy smokers (who smoked ≥30 pack-years) at intermediate genetic risk (20).
There are some limitations in our study. First, the number of case patients in our study was relatively small for light smokers, such as current smokers with <20 pack-years of smoking history, especially in the analysis stratified by PRS, so we did not estimate their lung cancer risk by PRS level. However, it is unlikely for this group of smokers, even with a high PRS, to reach a risk comparable with the risk among heavy smokers who are eligible for screening, given the substantial difference in risk estimated from this study. Second, the number of lung cancer patients diagnosed from the ages of 50–55 years (n = 158) was relatively small, and the observed 6-year risk of lung cancer in this age group could be unstable. Future large studies are needed to provide more precise estimates of risk by smoking and PRS groups. In addition, the follow-up time of this study was relatively short and thus we could not calculate absolute risk for a longer-time period.
In summary, for smokers of European descent, our findings support lowering the smoking pack-year eligibility from 30 to 20 pack-years for current smokers, but not for former smokers. Our study also suggests that PRS of lung cancer could be considered to further identify high-risk smokers to start lung cancer screening at an early age.
Supplementary Material
Acknowledgement
This research has been conducted using the UK Biobank Resource under Application Number 40685. The authors would also like to thank Marshal Younger at Department of Epidemiology, Vanderbilt University Medical Center, for assistance with editing and manuscript preparation. He did not receive additional compensation besides his usual salary. The UK Biobank cohort study was approved by the UK Biobank Research Ethics Committee (approval number: 11/NW/0382). Our study has been conducted using the UK Biobank Resource under Application Number 40685. Only de-identified data were acquired in our study. An ethical approval for this was obtained from Vanderbilt University Medical Center.
Glossary
Abbreviations
- CI
confidence interval
- GWAS
genome-wide association study
- HR
hazard ratio
- PRS
polygenic risk score
- USPSTF
US Preventive Services Task Force
Funding
This research was supported in part by funds provided by National Institutes of Health grant U01 CA202979 as well as Anne Potter Wilson chair endowment at Vanderbilt University. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Data Availability
Our study has been conducted using the UK Biobank Resource under Application Number 40685. Data are publicly available, with the application approved by the UK Biobank.
Conflict of Interest Statement: None declared.
References
- 1. Bray, F., et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Pesch, B., et al. (2012) Cigarette smoking and lung cancer – relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int. J. Cancer, 131, 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walser, T., et al. (2008) Smoking and lung cancer. Proc. Am. Thorac. Soc., 5, 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moyer, V.A. U.S. Preventive Services Task Force . (2014) Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med., 160, 330–338. [DOI] [PubMed] [Google Scholar]
- 5. Wang, Y., et al. (2015) Trends in the proportion of patients with lung cancer meeting screening criteria. JAMA, 313, 853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang, P., et al. (2016) Trends in subpopulations at high risk for lung cancer. J. Thorac. Oncol., 11, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong, M.B. (2020) USPSTF broadens age range, risk threshold in new recommendation for lung cancer screening. Cancer Lett., 46, 10–12. [Google Scholar]
- 8. Bossé, Y., et al. (2018) A decade of GWAS results in lung cancer. Cancer Epidemiol. Biomarkers Prev., 27, 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia, G., et al. (2020) Evaluating the utility of polygenic risk scores in identifying high-risk individuals for eight common cancers. JNCI Cancer Spectr., 4, pkaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudlow, C., et al. (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med., 12, e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 1000 Genomes Project Consortium et al. (2015) A global reference for human genetic variation. Nature, 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manichaikul, A., et al. (2010) Robust relationship inference in genome-wide association studies. Bioinformatics, 26, 2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKay, J.D., et al. ; SpiroMeta Consortium. (2017) Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet., 49, 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tammemägi, M.C., et al. (2014) Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med., 11, e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinsky, P.F., et al. (2015) Lung cancer risk and demographic characteristics of current 20–29 pack-year smokers: implications for screening. J. Natl. Cancer Inst., 107, djv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crosbie, P.A., et al. (2019) Second round results from the Manchester ‘Lung Health Check’ community-based targeted lung cancer screening pilot. Thorax, 74, 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam, S., et al. (2019) PL02.02 Lung cancer screenee selection by USPSTF versus PLCOm2012 criteria – interim ILST findings. J. Thoracic Oncol., 14, S4–S5. [Google Scholar]
- 18. Schwartz, A.G., et al. (2016) Epidemiology of lung cancer. Adv. Exp. Med. Biol., 893, 21–41. [DOI] [PubMed] [Google Scholar]
- 19. de Alencar, V.T.L., et al. (2020) Inherited lung cancer: a review. Ecancermedicalscience, 14, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai, J., et al. (2019) Identification of risk loci and a polygenic risk score for lung cancer: a large-scale prospective cohort study in Chinese populations. Lancet Respir. Med., 7, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our study has been conducted using the UK Biobank Resource under Application Number 40685. Data are publicly available, with the application approved by the UK Biobank.
Conflict of Interest Statement: None declared.