Abstract
Colorectal cancer (CRC) ranks as the third leading cause of cancer-related deaths in the USA. 5-Fluorouracil (5FU)-based chemotherapeutic drug remains a mainstay of CRC treatment. Unfortunately, ~50–60% of patients eventually develop resistance to 5FU, leading to poor survival outcomes. Our previous work revealed that andrographis enhanced 5FU-induced anti-cancer activity, but the underlying mechanistic understanding largely remains unclear. In this study, we first established 5FU-resistant (5FUR) CRC cells and observed that combined treatment with andrographis-5FU in 5FUR cells exhibited superior effect on cell viability, proliferation, and colony formation capacity compared with individual treatments (P < 0.001). To identify key genes and pathways responsible for 5FU resistance, we analyzed genome-wide transcriptomic profiling data from CRC patients who either responded or did not respond to 5FU. Among a panel of differentially expressed genes, Dickkopf-1 (DKK1) overexpression was a critical event for 5FU resistance. Moreover, andrographis significantly downregulated 5FU-induced DKK1 overexpression, accompanied with enhanced anti-tumor effects by abrogating downstream Akt-phosphorylation. In line with in vitro findings, andrographis enhanced 5FU-induced anti-cancer activity in mice xenografts and patient-derived tumoroids (P < 0.01). In conclusion, our data provide novel evidence for andrographis-mediated reversal of 5FU resistance, highlighting its potential role as an adjunct to conventional chemotherapy in CRC.
We systematically demonstrated that andrographis overcomes 5FU-associated chemoresistance in 5FU-resiatant CRC cells by attenuating DKK1-dependent Akt-phosphorylation using multiple in vitro and in vivo models, revealing that andrographis might serve as potential adjunct to conventional chemotherapeutic drugs in patients with CRC.
Introduction
Colorectal cancer (CRC) ranks as the third leading cause of cancer-related deaths in both men and women in the USA (1). Even with advances in the screening and therapeutic strategies for the management of CRC in recent years, it is still estimated that 147 950 new cases and 53 200 deaths occurred from this malignancy in 2020 (2). Metastasis of cancer cells that lead to spread of cancer cells to surrounding tissues and distant organs is one of the leading causes for increased morbidity and mortality in patients with advanced-stage CRC (3). Chemotherapy remains the most common therapeutic approach for treating patients with advanced CRC. Currently the first-line chemotherapeutic strategy for CRC involves the use of FOLFOX/FOLFIRI regimen, which comprises 5-fluorouracil (5FU) plus oxaliplatin/irinotecan; unfortunately however, ~50–60% of patients treated with these drugs eventually develop acquired chemoresistance and become insensitive to 5FU-based chemotherapeutic drugs (4,5). Therefore, it is imperative to better understand the mechanisms underlying acquired chemoresistance in CRC and develop effective strategies to overcome the chemoresistance for patients who do not respond well.
Natural products and their derived phytochemical compounds exert their pharmacological roles through multitarget manners and multiple growth-regulatory pathways, thereby inhibiting the growth of many types of cancer cells (6–17). Numerous studies have confirmed that various natural compounds can also exert synergistic inhibitory effects combined with conventional chemotherapy by overcoming 5FU resistance (14,17–27). In particular, andrographolide, a diterpenoid lactone isolated from the leaves of Andrographis paniculata, has shown great promise as an anti-cancer compound against various types of cancers (28,29). Recent studies have reported that andrographis exerts its anti-cancer activity through the activation of proapoptotic signaling, cell cycle arrest, as well as inhibition of cellular pathways related to proliferation (30–32). In addition to its direct anti-cancer potential, evidence indicates that andrographis also functions as an adjunct to classical chemotherapeutic drugs such as 5FU, by inducing expression of the BAX gene (15), regulating p38 MAPK signaling pathway (33), and inhibiting c-MET signaling cascade (34).
In one of the previous studies from our group, we also reported an enhanced pro-apoptotic effect of andrographis with 5FU. In a more recent subsequent systematic analysis of genome-wide transcriptomic profiling analysis in CRC cells treated with andrographis and 5FU has revealed that Wnt/β-catenin signaling pathway is significantly involved for mediating anti-cancer activity of andrographis in CRC (20). However, the specific events underlying regulated Wnt/β-catenin pathway in the progression of such enhanced anti-cancer efficacy of andrographis with 5FU in cancer remain largely unclear. In this study, after a series of cell culture, animal model and tumor-derived organoid experiments, we have revealed that upregulation of Dickkopf-1 (DKK1) is a major event of 5FU resistance in CRC, and combined treatment of andrographis can conquer this problem. Taken together, we have identified the one of the underlying mechanisms for andrographis-mediated reversal of 5FU resistance and highlighted the ability of this compound to serve as a potential adjunct to conventional chemotherapy for CRC.
Materials and methods
Cell culture and materials
The human CRC cell lines, HCT116 and SW480, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in the complete medium containing DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C in 5% CO2. All cell lines were tested for mycoplasma on a regular basis and authenticated by a panel of genetic and epigenetic markers. 5FU (Sigma–Aldrich, St. Louis, MO) and andrographis (standardized to 20% andrographolide content; Andrographis EP80 was generously provided by EuroPharma USA, Green Bay, WI) were dissolved in dimethyl sulfoxide (DMSO) (Sigma–Aldrich) and diluted to appropriate experimental concentrations in complete medium before use. 5FU-resistant (5FUR) cell lines (HCT116 5FUR and SW480 5FUR) were established by continuous culturing of cells with increasing dose of 5FU for 9 months, as described in previous study (25).
Patient-derived CRC organoid culture
Human primary CRC tissues were obtained from patients with CRC at the Baylor University Medical Center, Dallas, TX. All experiments were approved by the institutional review board of the institution. Written informed consents from all patients were obtained in accordance with Declaration of Helsinki.
The colorectal segments were incubated in Gentle Cell Dissociation Reagent (STEMCELL Technologies, Vancouver, BC, Canada), centrifuged (290 × g, 4°C, 5 min), and resuspended in DMEM/F-12 with 15 mM HEPES (STEMCELL Technologies, Vancouver, BC, Canada) to obtain the colorectal crypts. Dissociated crypts were filtered with 70 µm strainers, counted, and cultured in 50 µl 3D matrix composed of IntestiCult Organoid Growth Medium (STEMCELL Technologies, Vancouver, BC, Canada) and Matrigel (Corning, Tehama County, CA) in 1:1 ratio, in a 24-well plate at 37°C, 5% CO2. Seven hundred and fifty microliters of IntestiCult Organoid Growth Medium each well was added to 24-well plate to immerse the matrix and refreshed every 3 days.
For treatments, organoids were randomly assigned into four groups, and appropriate concentrations of 5FU (10 µM), andrographis (30 ng/µl) and their combination were added to the culture medium of each group and then cultured for 10 days. The control cells were treated with very low concentration of DMSO, which was equivalent to the vehicle to dissolve 5FU. The organoids were observed and counted under a bright-field microscope and harvested by Gentle Cell Dissociation Reagent, followed by ice-cold phosphate-buffered saline (PBS) washes (290 × g, 4°C, 5 min). Harvested organoids were aliquoted for total RNA and protein extractions.
Xenograft animal experiments
For xenograft mice experiments, HCT116 5FUR cells (5 × 106 cells) were subcutaneously injected into 7-week-old male athymic nude mice (Envigo, Houston, TX) using a 27-gauge needle. After xenografts were visible, mice were randomly assigned into four groups: 5FU (30 mg/kg body weight), andrographis (125 mg/kg body weight), their combination and control group (equal amount of DMSO), n = 10 per group, and were given intraperitoneally dose of each compound every other day for up to 15 days. The tumor volume was calculated as 0.5 × length × width × width. All mice experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (Bethesda, MD).
Cell Counting Kit-8 assay
For Cell Counting Kit-8 (CCK-8) assay, CCK-8 kit (Dojindo, Kumamoto, Japan) was used to measure cell viability according to the instructions as follows: Cells were seeded in 96-well plates at a density of 3 × 103 cells/well in 100 µl of complete medium and grown for 24 h. Thereafter, the cells were treated with the appropriate concentration of 5FU (10 µM), andrographis (30 ng/µl) and their combination for 48 h. At the indicated time points, 10 µl CCK-8 solution was added into every well and incubated for 2 h. Subsequently the absorbance was measured at a wavelength of 450 nm (OD450) using a microplate reader (Molecular Devices). The experiments were performed in triplicate, and each experiment was repeated three times.
5-Ethynyl-20-deoxyuridine incorporation assay
For 5-ethynyl-20-deoxyuridine (EdU) incorporation assay, EdU Kit (Invitrogen, Carlsbad, CA) was used to examine cell proliferation following various treatments. Cells were seeded into 96-well plates (3 × 103 cells/well) and grown for 24 h and thereafter treated with appropriate concentrations of 5FU (10 µM), andrographis (30 ng/µl) and their combination for 48 h. Thereafter, the cells were incubated in complete medium supplemented with 10 µM EdU for 2 h at 37°C, washed using PBS three times, followed by fixation with 4% formaldehyde solution (Sigma–Aldrich) for 30 min and permeabilization by incubating them with 0.5 % Triton X-100 (Sigma–Aldrich) in PBS for 10 min. Cells were stained with Hoechst33342 reaction buffer for 10 min protected from light, followed by washings with PBS three times. After the staining, the cells were observed and counted microscopically using a 10× objective (Carl Zeiss, Dublin, CA). The experiments were performed in triplicate and each experiment was repeated three times.
Colony formation assay
For colony formation assay, cells were seeded into six-well plates (800 cells/well) and maintained in complete medium for 1 week. Thereafter, the cells were treated with appropriate concentration of 5FU (10 µM), andrographis (30 ng/µl) and their combination for 1 week, and the medium was refreshed every 3 days. The colonies were fixed with methanol and stained with 0.1% crystal violet (ACROS Organics, Gujarat, India), and then the numbers of stained colonies were determined by counting. The experiments were performed in triplicate and repeated three times.
Western blotting and antibodies
CRC cells, dissociated organoids and minced xenograft fragments (~1 mm3 in size) were lysed with RIPA Lysis and Extraction Buffer (ThermoFisher Scientific, Waltham, MA) supplied with protease inhibitor cocktail (ThermoFisher Scientific, Waltham, MA) on ice for 30 min. After centrifugation (12 000 × g, 4°C, 15 min), protein samples were boiled in Laemmli’s buffer (Bio-Rad, Hercules, CA) containing β-mercaptoethanol (Bio-Rad). Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to 0.45 µm polyvinylidene fluoride membranes (Cytiva, Marlborough, MA), followed by blocking in 5% bovine serum albumin (Sigma–Aldrich) and 1% Tween-20 (Sigma–Aldrich) in PBS for 1 h. Membranes were thereafter incubated with primary antibodies overnight at 4°C, which are listed as follows: anti-β-actin (#A2228, Sigma–Aldrich), anti-DKK1 (#ab109416, Abcam), anti-PARP (#sc-7150, Santa Cruz), anti-cleaved PARP (#5625, CST), anti-cleaved caspase-3 (#9661, CST), anti-caspase-3 (#9662, CST), anti-phospho-Akt (Ser473) (#4060, CST) and anti-Akt (#4691, CST). After washed by PBS with 1% Tween-20 three times, membranes were incubated with horseradish peroxidase conjugated secondary antibodies (#7074 or #7076, CST). The signals were detected by Gel Imaging Systems (Bio-Rad) using HRP-based chemiluminescence kit (ThermoFisher Scientific, Waltham, MA).
Real-time qPCR analysis and primers
Total RNA was extracted from CRC cells, dissociated organoids and minced xenograft fragments (~1 mm3 in size) by TRIzol (Invitrogen, Carlsbad, CA) and then reverse transcribed into cDNA by High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time PCR analyses were performed by SensiFAS SYBR Lo-ROX Kit (Bioline, London, UK). The expression for DKK1 was analyzed by the 2−ΔΔCT method normalized against housekeeping β-actin gene. The specific primers used are as follows: DKK1: forward, 5′-ATAGCACCTTGGATGGGTATTCC-3′ and reverse, 5′-CTGATGACCGGAGACAAACAG-3′; β-actin: forward, 5′-CACCATTGGCAATGAGCGGTTC-3′ and reverse, 5′-AGGTCTTTGCGGATGTCCACGT-3′.
Gene expression profiling using microarray
Total RNA was isolated from 2 × 106 HCT116 5FUR and SW480 5FUR cells treated by DMSO or andrographis 48 h using miRNeasy kit (Qiagen, Hilden, Germany). Gene expression microarray profiling was performed at the Genomics and Microarray Core at University of Texas Southwestern Medical Center. High-quality RNA (integrity number values > 9.5) were used for gene expression profiling studies by high-densitiy oligonucleotide arrays (Clariom S, Affymetrix, Santa Clara, CA). The raw data were preprocessed using robust multiarray average method by Transcriptome Analysis Console (TAC) software. Differential expression genes were selected using a threshold of P < 0.05 and |log2 fold changes| > 1.
Small interfering RNA transfection
To silence DKK1 expression, double-stranded small interfering RNA (siRNA) specific against DKK1 (Invitrogen, Carlsbad, CA) were transfected into HCT116 5FUR and SW480 5FUR cell lines by Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to their manufacturer’s instructions. Silence Select Negative Control #1 siRNA (Invitrogen) was used as a negative control. After transfection for 72 h, transfected cells were subsequently detached and frozen for RNA extraction and protein extraction or were re-plated for CCK-8 assay.
Statistical analysis
The statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA). Microarray data and comparison of two groups of data were analyzed by Student t-test to examine the differences, and multiple comparisons were analyzed by one-way ANOVA test. All results are expressed as the means ± standard deviation of three independent experiments and P < 0.05 was regarded as statistically significant.
Results
Establishment of 5FU-resistant CRC cells and toxicity characterization
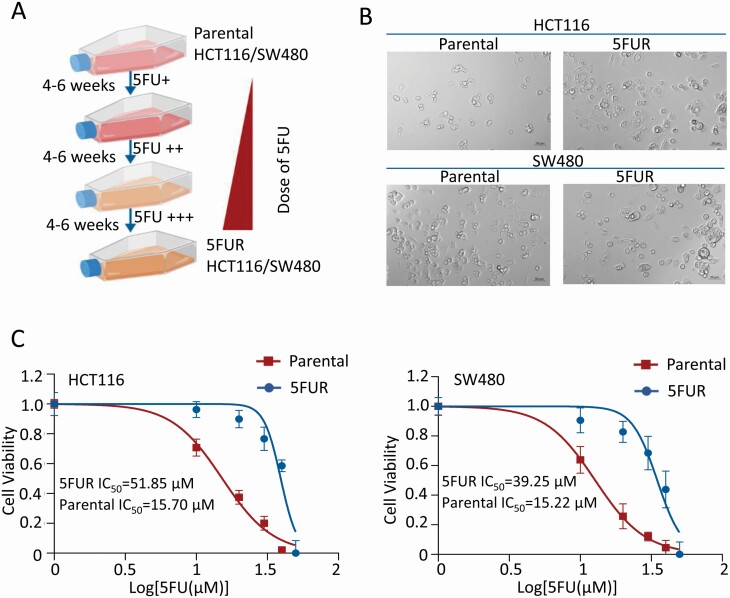
To obtain HCT116 5FUR and SW480 5FUR cell lines, parental HCT116 and SW480 cells were treated by continuously increasing the concentration of 5FU in the culture medium for 9 months (Figure 1A). A number of differences between 5FUR cells and parental cells can be observed. Morphologically, HCT116 and SW480 parental cells were more epithelial cell-like, while both 5FUR cell lines had a spindle shape resembling that of their mesenchymal origin (Figure 1B). Second, to confirm 5FU-resistant properties, the proliferation rates between 5FUR and their parental cell lines were compared by treating these cells with increasing doses of 5FU. As expected, the 50% inhibitory concentration (IC50) of 5FU in 5FUR cell lines was much higher than their parental counterparts: The IC50 value of HCT116 was calculated to be 15.70 µM, while that of HCT116 5FUR was 51.85 µM. The IC50 value of SW480 was calculated to be 15.22 µM, while that of SW480 5FUR was 39.25 µM, at 48 h, respectively (Figure 1C). These data confirmed successful establishment of 5FU-resistant cells lines (HCT116 5FUR and SW480 5FUR) for subsequent experiments.
Figure 1.
Establishment and toxicity identification of 5FUR CRC cells. (A) Schematic diagram for the establishment of 5FUR CRC cells. (B) Microscopic images illustrating the significant alteration in cellular morphology of HCT116 5FUR and SW480 5FUR cells (200× magnification). (C) Results of CCK-8 assay showing the 5FUR and parental cells proliferation rates after exposed to various concentrations of 5FU for 48 h.
Andrographis exerts anti-proliferative effects in 5FUR CRC cells
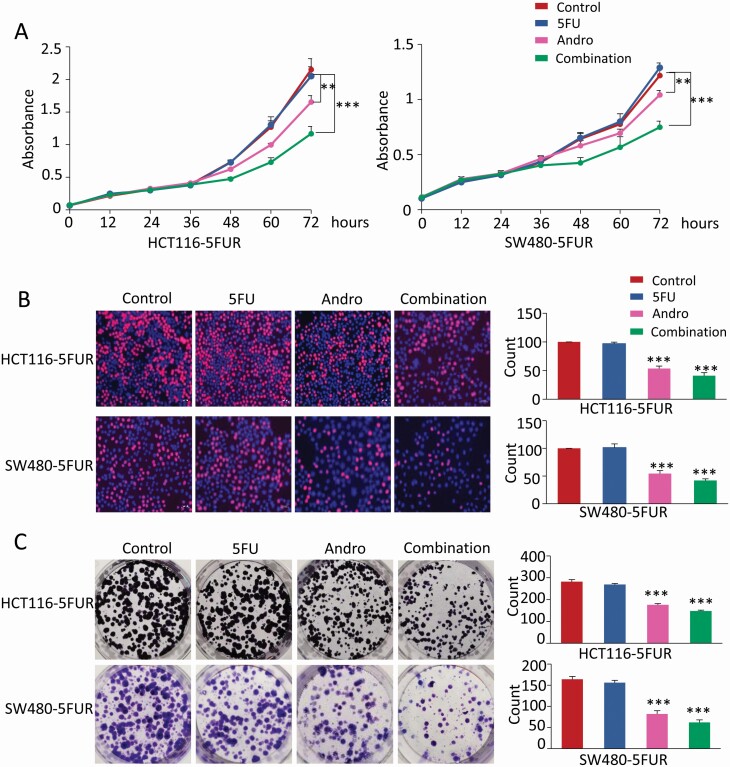
Our previous study demonstrated that andrographis sensitized the cytotoxicity of 5FU to 5FUR cells by enhancing apoptosis (20). To further evaluate whether andrographis can enhance 5FU-induced growth inhibition, we observed the differences in cellular growth in cells treated with andrographis and 5FU alone, and their combination in HCT116 5FUR and SW480 5FUR cells. Cell viability of both 5FUR cell lines were measured by CCK-8 assay after treatment with andrographis and 5FU alone, and in combination. The results showed that 5FU treatment did not significantly affect cell viability of both 5FUR cell lines, whereas andrographis could reverse 5FU resistance, and the combination treatment of 5FU and andrographis was significantly more effective in inhibiting cell viability (P < 0.01–0.001; Figure 2A). Next, we investigated the combinational effects of andrographis and 5FU on cell proliferation with an EdU assay. The percentage of EdU-positive cells was significantly lower in cells that were treated with a combination of andrographis and 5FU (P < 0.001; Figure 2B). In addition, we also found that combined treatment with andrographis and 5FU resulted in a significantly reduced colony formation capacity (P < 0.001; Figure 2C) compared with those of treatments alone. Collectively, these results indicated that andrographis could significantly improve the anti-cancer potency of 5FU and even reverse 5FU resistance in CRC cells.
Figure 2.
Andrographis exerts anti-proliferative effects in 5FUR CRC cells. (A) Results of CCK-8 assay showing the effects of andrographis and 5FU on 5FUR CRC cells viability. Complete medium containing 5FU, andrographis and their combination were added at 24 h and the absorbances at 450 nm were measured after culture for 0, 12, 24, 36, 48, 60 and 72 h. (B) Representative results of the EdU assay (EdU, red; Hoechst, blue) after treatment with andrographis and 5FU alone, and in combination in 5FUR CRC cells for 48 h. Quantitative analysis of EdU-positive cells is shown in the right panel. (C) Representative results of colony formation assay in 5FUR CRC cells after exposed to andrographis and 5FU alone, and in combination 7 days. Colonies count analysis is shown in the right panel. Statistical significance: **P < 0.01, ***P < 0.001. Andro, andrographis.
DKK1 expression correlates with 5FU resistance in primary CRC cancers and CRC cell lines
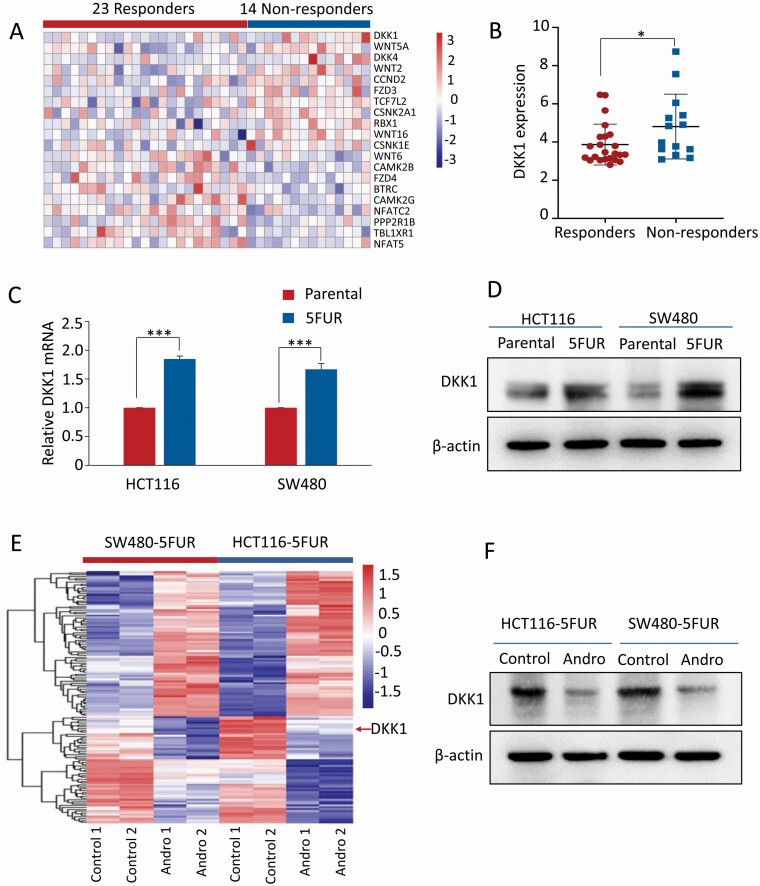
In order to identify the specific regulatory roles of Wnt/β-catenin signaling pathway in 5FU-based chemotherapeutic resistance in patients with advanced CRC, the gene expression profiling data set (GSE52735) was downloaded from the Gene Expression Omnibus database. This data set included transcriptomic profiling from 14 nonresponder and 23 responder patients with advanced-stage CRC treated with 5FU-based chemotherapy (5FU and capecitabine, an oral prodrug of 5FU). Based on the gene set of Wnt/β-catenin signaling pathway obtained from Gene Set Enrichment Analysis (https://www.gsea-msigdb.org/gsea/msigdb/cards/KEGG_WNT_SIGNALING_PATHWAY), we defined the 151 members of Wnt/β-catenin pathway-specific genes and performed differential expression analysis in CRC patients that were either responders or nonresponders to this chemotherapeutic drug regimen. Among these genes along the Wnt-signaling cascade, we noted a panel of 20 genes that were differentially expressed between the two groups (Figure 3A). However, a more systematic evaluation of each of these genes revealed that the expression of DKK1 was significantly up-regulated in patients that did not respond to 5FU-based therapy (P < 0.05; Figure 3B). For in vitro verification, the mRNA and protein levels of DKK1 were examined in HCT116 5FUR and SW480 5FUR cells, as well as their parental cell lines, which confirmed that DKK1 expression was indeed significantly elevated in both pairs of 5FUR cell lines (P < 0.001; Figure 3C and D).
Figure 3.
DKK1 expression correlates with 5FU resistance in primary CRC cancers and CRC cell lines. (A) Heatmap of the transcriptional expression of Wnt/β-catenin pathway-specific genes in GSE52735. (B) Scatter plots of DKK1 expression in 14 nonresponder and 23 responder patients treated with 5FU-based chemotherapy. (C) Bar graph showing relative mRNA expression of DKK1 in HCT116 5FUR cells and SW480 5FUR cells compared with their respective parental cells by qPCR analysis. (D) Representative images of DKK1 protein expression in HCT116 5FUR cells and SW480 5FUR cells compared with their respective parental cells by western blot analysis. (E) Heatmap of common differentially expressed genes in both SW480 5FUR and HCT116 5FUR cells (|log2 fold change | > 1, P < 0.05). (F) Representative images of DKK1 protein expression in HCT116 5FUR cells and SW480 5FUR with or without andrographis treatment. Statistical significance: *P < 0.05, ***P < 0.001. Andro, andrographis.
Next, to elucidate the role of this pathway for its roles in andrographis-mediated sensitization of 5FUR cells to 5FU, we conducted microarray-based genome-wide transcriptome profiling using HCT116 5FUR and SW480 5FUR cells treated by andrographis. Interestingly, we noted that the transcriptional expressions of DKK1 in both pairs of 5FUR cells were significantly downregulated by the treatment with andrographis versus controls (log2 fold change = −1.8; Figure 3E). Furthermore, validation by western blot analysis confirmed the downregulation of DKK1 at protein level after the andrographis treatment on both 5FUR cell lines (Figure 3F), highlighting the important role of Wnt/β-catenin pathway, but more importantly the role of DKK1 in overcoming chemoresistance to 5FU through treatment with andrographis.
Silencing DKK1 sensitizes 5FUR cells to 5FU via regulating Akt-phosphorylation
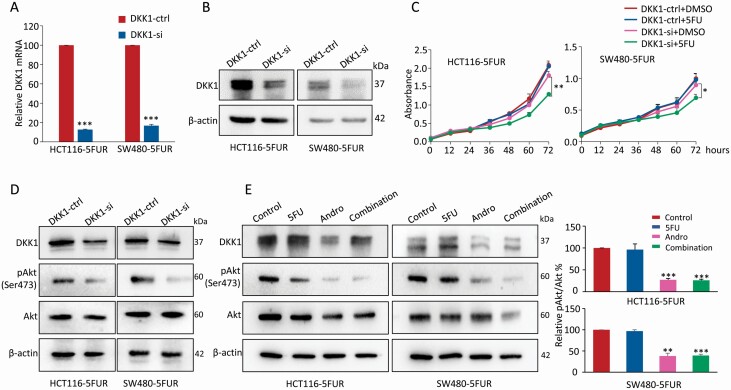
Next, in order to evaluate whether the observed overexpression of DKK1 in 5FUR cells is functionally relevant for 5FU resistance, siRNA-mediated DKK1 silencing was employed in both 5FUR cell lines, the HCT116 5FUR and SW480 5FUR cells. The qPCR analysis revealed that the mRNA expression of DKK1 in both 5FUR cell lines was downregulated by over 80% compared with those transfected with negative control siRNA (Figure 4A). Furthermore, western blot analysis confirmed that the protein levels of DKK1 were also significantly decreased when the 5FUR cell lines were transfected with DKK1 siRNA (Figure 4B). Thereafter, the 5FU cytotoxicity were assessed using the CCK-8 assay in both 5FUR cell lines transfected with DKK1 siRNA or negative control siRNA. The results showed that the growth curves of 5FUR cells transfected with negative control siRNA were not affected by 5FU. While in the 5FUR cells with DKK1 knockdown, 5FU treatment could significantly inhibit the proliferation (P < 0.05–0.01; Figure 4C), suggesting that silencing of DKK1 attenuated the 5FU resistance in HCT116 5FUR and SW480 5FUR cells.
Figure 4.
Silencing DKK1 sensitizes 5FUR cells to 5FU via regulating Akt-phosphorylation. (A) Bar graph showing relative DKK1 mRNA expression of 5FUR cells transfected with negative control siRNA or DKK1 siRNA by qPCR analysis. (B) Representative images of DKK1 protein expression of 5FUR cells transfected with negative control siRNA or DKK1 siRNA by western blot analysis. (C) Results of CCK-8 assay showing the effects of 5FU on 5FUR cells transfected with negative control siRNA or DKK1 siRNA. The complete medium containing 5FU (10 µM) was added at 24 h, and the absorbances at 450 nm were measured after culture for 0, 12, 24, 36, 48, 60 and 72 h. (D) Representative images of Akt and pAkt expression of 5FUR cells transfected with negative control siRNA or DKK1 siRNA. (E) Representative images of DKK1, Akt and pAkt expression of 5FUR cells in each treatment group, and the phosphorylation of Akt was analyzed as quotient of pAkt versus Akt expression in the right panel. Statistical significance: **P < 0.01, ***P < 0.001. Andro, andrographis; pAkt, phosphorylated-Serine473-Akt.
Recent reports have showed that DKK1-mediated tumor promotion was found to manifest via activation of Akt-mediated intracellular pathways (35,36). To better understand the underlying mechanism of DKK1-mediated 5FU sensitivity in 5FUR CRC cells, we examined how DKK1 affected Akt activation. As shown by western blot analysis, DKK1 knockdown by siRNA in 5FUR cells attenuated phosphorylation of Akt at Ser473 (Figure 4D). Furthermore, we observed that treatment of andrographis alone and in combination with 5FU in 5FUR cells significantly downregulated DKK1 expression and also inhibited Akt-phosphorylation (Figure 4E), highlighting that blocking the activation of Akt via DKK1 downregulation might be one of the primary mechanisms for andrographis-mediated sensitization of 5FUR cells to 5FU.
Andrographis enhances anti-tumor effect of 5FU in a xenograft animal model and downregulates DKK1 expression
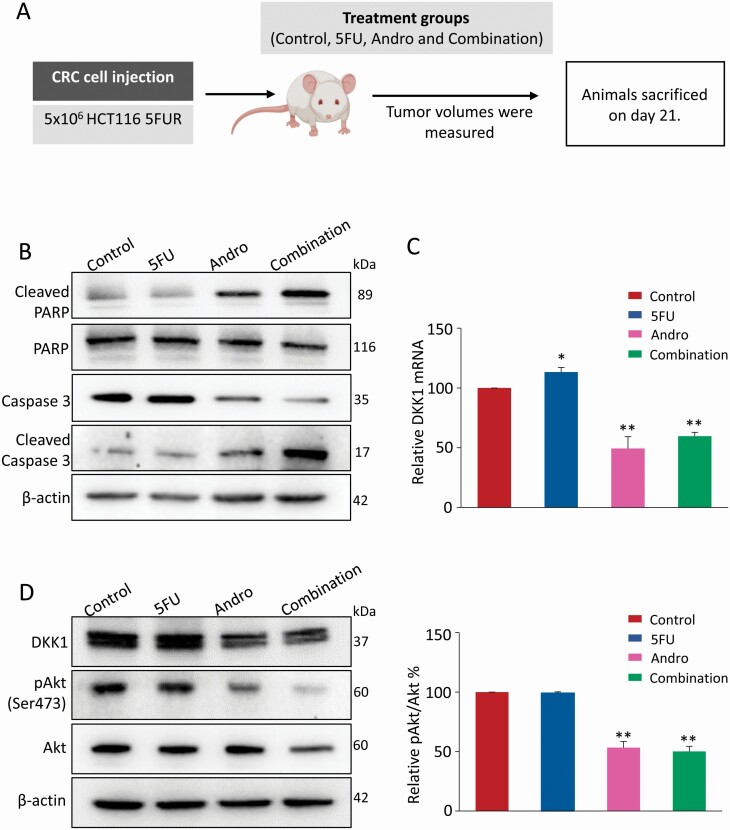
In order to confirm whether andrographis can also sensitize the tumor growth inhibitory effect of 5FU in 5FUR cells in a pre-clinical animal model, we next established a subcutaneous xenograft mice model as described previously (20). As shown in the study design (Figure 5A), HCT116 5FUR xenografts were generated by subcutaneous injection of 5 × 106 cells. Once the xenografts were visible (about 6 days), nude mice carrying HCT116 5FUR cells xenograft tumors were administered by appropriate concentration of 5FU, andrographis and their combination, every other day for up to 15 days. Finally, nude mice were sacrificed approximately 21 days after subcutaneous injection. RNA and protein were extracted from harvested xenografts. Subsequently, the expression of various markers, including apoptotic biomarkers (cleaved caspase-3 and cleaved PARP) and total caspase-3 and PARP, were assessed.
Figure 5.
Andrographis enhances anti-tumor effect of 5FU in a xenograft animal model and downregulates DKK1 expression. (A) Schematic diagram of HCT116 5FUR xenograft model in nude mice and the treatment schedule of andrographis and 5FU. (B) Representative images of cleaved PARP, PARP, cleaved caspase-3 and caspase-3 of xenograft extraction in each treatment group by western blot analysis. (C) Bar graph showing relative mRNA expression of DKK1 in each treatment group by qPCR analysis. (D) Representative images of DKK1, Akt and pAkt expression of xenografts extraction in each treatment group, and the phosphorylation of Akt was analyzed as quotient of pAkt versus Akt expression in the right panel. Statistical significance: *P < 0.05, **P < 0.01. Andro, andrographis; pAkt, phosphorylated-Serine473-Akt.
The results showed that 5FU treatment did not affect apoptotic biomarkers expression significantly, whereas andrographis treatment resulted in increased expression of apoptotic biomarkers (cleaved caspase-3 and cleaved PARP) and a corresponding decrease in the expression of total caspase-3 and PARP, and in each instance, the combined treatment with andrographis and 5FU was significantly more effective in promoting apoptosis (Figure 5B). In addition, qPCR and western blot analysis from xenograft tissues revealed that 5FU treatment resulted in increased expression of DKK1 at both mRNA and protein levels, while coadministration of andrographis along with 5FU reversed the 5FU-induced DKK1 overexpression in vivo (P < 0.05–0.01; Figure 5C and D). Meanwhile, the treatment of andrographis alone or along with 5FU in 5FUR cells significantly attenuated Akt-phosphorylation at Ser 473 (P < 0.01; Figure 5D), highlighting the role of DKK1 even in the pre-clinical animal model.
The enhanced anti-tumor effect of andrographis and 5FUR was further demonstrated in patient-derived organoids
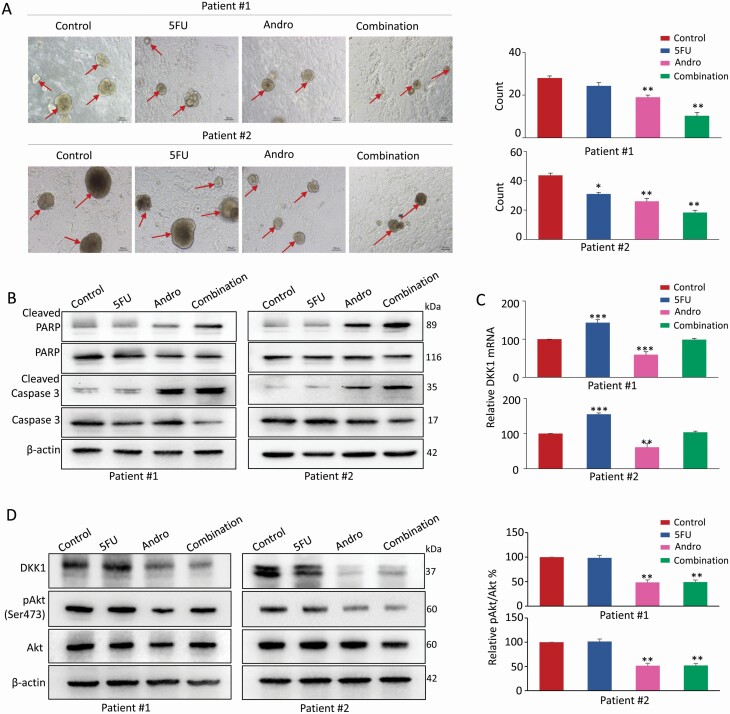
Tumor organoid model and 3D primary cultures are superior to conventional 2D monolayer of cultured cells in terms of studying anti-cancer agents, as they provide a physiologically more relevant microenvironment for cell-to-cell interactions and cellular responses (37). Thus, to further confirm these observations from in vitro and in vivo experiments, next we examined the anti-tumor effects of andrographis and 5FU using tumor organoid models derived from two CRC patients in advanced stage. The tumor organoids were cultured in medium containing appropriate concentrations of 5FU, andrographis and their combination for 10 days. Interestingly, consistent with the results in cell lines and mice xenografts, the andrographis and 5FU combination significantly decreased the number and size of patient-derived tumor organoids compared with treatments alone (P < 0.01; Figure 6A). Furthermore, the protein expression of apoptosis-related biomarkers, including cleaved caspase-3 and cleaved PARP, as well as total caspase-3 and PARP were analyzed. Western blot analysis showed that both andrographis and 5FU promoted apoptosis; however, andrographis-5FU combination group exhibited a significantly higher degree of apoptosis compared with treatments with individual compounds (Figure 6B). In addition, we also examined the expression of DKK1 at both mRNA and protein level and observed that coadministration of andrographis along with 5FU reversed the 5FU-induced DKK1 overexpression in tumor organoid model (Figure 6C and D), and also attenuated Akt-phosphorylation (Ser473) (P < 0.01; Figure 6D). The above data showed that combined treatment with andrographis enhanced the 5FU-induced anti-cancer activity in inhibiting organoid growth by downregulating the expression of DKK1 and abrogating the downstream Akt-phosphorylation.
Figure 6.
The enhanced anti-tumor effect of andrographis and 5FUR in patient-derived organoids. (A) Microscopic images of tumor organoids derived from two patients cultured in each treatment group (20× magnification), and the organoid count analysis is shown in the right panel. (B) Representative images of cleaved PARP, PARP, cleaved caspase-3 and caspase-3 of organoids extraction in each treatment group by western blot analysis. (C) Bar graph showing relative DKK1 mRNA expression of organoids extraction in each treatment group by qPCR analysis. (D) Representative images of DKK1, Akt and pAkt expression of organoids extraction in each treatment group, and the phosphorylation of Akt was analyzed as quotient of pAkt versus Akt expression in the right panel. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. Andro, andrographis; pAkt, phosphorylated-Serine473-Akt.
Discussion
Since the 1990s, 5FU-based chemotherapy has been the backbone of CRC treatment regimen, which has led to reduced rates for recurrence and mortality by 41 and 33%, respectively (38). To date, despite the increasing availability of new agents, the combination chemotherapy based on 5FU remains the preferred choice for patients with CRC. 5FU is a thymidylate synthase inhibitor, which can increase DNA damage by interfering with the folate pathway, cause cell growth arrest and apoptosis, thereby exerting its anti-tumor effects (39). Although the anti-cancer activity of 5FU is primarily is beneficial, several studies have shown that such treatments are also associated with increasing DNA damage, reducing DNA damage repair (40), altering drug targets (41), and activation of pro-survival pathways (42)—all of which ultimately contribute toward development of resistance to 5FU, which is a major obstacle to achieve effective anti-cancer treatment. As a consequence, more than 50% of patients eventually develop resistance to 5FU-based chemotherapy, especially in the patients with advanced and metastatic CRC, leading to frequent relapse (43). Not surprisingly, there is a great degree of interest in developing novel anti-cancer drugs that can serve as adjuncts to 5FU-based therapy and help mitigate or overcome the challenges with chemotherapeutic resistance. For subsequent experiments, we first established 5FU-resistant isogenic CRC cell lines of HCT116 and SW480 cells, and we demonstrated their 5FUR resistance by cell proliferation assays.
Over the past few decades, a deeper understanding of 5FU-mediated resistance mechanisms has led to the development of new strategies for improving anti-tumor activity. There is a growing evidence that phytochemicals from natural products lack systemic toxicity and have the potential to lower and alleviate the development of chemotherapeutic resistance, which has garnered widespread attention (44,45). Andrographis is a member of the Acanthaceae plant family (acanthus family), with a wide range of pharmacological effects, including antioxidant, anti-inflammatory, antiviral and anti-cancer activities (46,47). Pharmacological studies have suggested that andrographis has the ability to mitigate or alleviate a variety of adverse effects associated with cancer chemotherapy, and the major findings are as follows: (i) andrographis sensitized the cytotoxicity of CRC cells toward cisplatin by enhancing apoptotic pathways in cultured cells and animal models (48); (ii) andrographis inhibited the JAK-STAT3 signaling pathway and enhanced the sensitivity of cancer cells to doxorubicin (49); and (iii) andrographis enhanced recombinant human TNF-related apoptosis inducing ligand mediated cancer cell apoptosis (50). In the present study, we also found that combined treatment with andrographis and 5FU exhibited significantly superior effects on cell viability, proliferation and colony formation capacity, compared with treatment with individual treatments. In line with our in vitro findings, the enhanced anti-cancer effect of combined treatment with andrographis and 5FU could be observed in mice xenografts as well as patient-derived tumoroids. Taken together, the previous findings along with the existing evidence regarding the biological effect of andrographis indicate that andrographis might be a promising anti-cancer compound with multiple targets and has a strong potency for improving the cytotoxicity of cancer treatment drugs.
It has been reported that abnormal activation of Wnt/β-catenin pathway is an important mechanism for 5FU resistance, and the regulation of major genes in this pathway is a potential strategy for reversing 5FU resistance (51). For instance, there is a consensus that expression of TCF4, a Wnt transcription factor, correlated with chemoresistance in CRC cell lines (52). In this regard, treatment with cardamom extract, specifically inhibited the formation of β-catenin/TCF4 complex, leading to TCF4-mediated transcriptional activation in 5FUR cells, thereby enhancing the sensitivity of 5FUR cells to 5FU (53). In this study, to further explore the molecular mechanisms responsible for the 5FU resistance in CRC, we first analyzed the Wnt/β-catenin signaling pathway genes in an expression profiling dataset from CRC patients (GSE52735) who either responded or did not respond to 5FU, which led us to identify that overexpression of DKK1 gene was a critical event for 5FU resistance in CRC. In line with our preliminary results, another study demonstrated that the protein expression of DKK1 can be used as a predictive biomarker for chemotherapeutic resistance in CRC (54). Many studies on gastrointestinal tumors have indicated that DKK1 contributed to hepatocellular carcinoma tumorigenesis (55), promoted tumor growth in Barrett’s associated esophageal adenocarcinoma (36), and was associated with metastatic and low short survival time of a variety of malignancies (56,57), which support our findings. Moreover, qRT-PCR and western blotting data revealed that andrographis resulted in downregulation of 5FU-induced DKK1 overexpression accompanied with the enhanced anti-tumor effects in 5FUR cell lines, animal xenografts, as well as patient-derived tumor organoids. Furthermore, 5FU resistance in 5FUR cells could be reversed by following siRNA-mediated silencing of DKK1.
DKK1-mediated tumor promotion is demonstrated via the Akt signaling pathway activation, which is known as an intracellular signal transduction pathway that maybe an important driver of neoplastic progression (36,58). For example, the activated Akt could enhance cancer cell survival and resistance to apoptosis by positively regulating the nuclear factor-kappaB pathway (59). Similarly, we also demonstrated that the silencing of DKK1 attenuated phosphorylation of Akt at Ser473. In addition, the inhibition of Akt-phosphorylation could be observed after the treatment with andrographis alone or in combination with 5FU in 5FUR cells, animal xenografts, as well as patient-derived tumor organoids, accompanied by the downregulation of DKK1 expression. These findings suggest that Akt-phosphorylation may serve as an essential role in the development of chemotherapy resistance in CRC cells.
We acknowledge several limitations of our study. Since the current study focused on the potential adjunctive application of natural phytochemicals, we deliberately investigated the total andrographis extract instead of analyzing specific active principles, an approach that be believe needs further investigation. In addition, although we have demonstrated that andrographis facilitated reversal of chemoresistance in 5FUR CRC cells by attenuating DKK1-dependent Akt-phosphorylation, the detailed mechanisms by which andrographis suppressing DKK1 expression remains unknown. Therefore, further studies are necessary to research upstream targets of DKK1 regulated by andrographis.
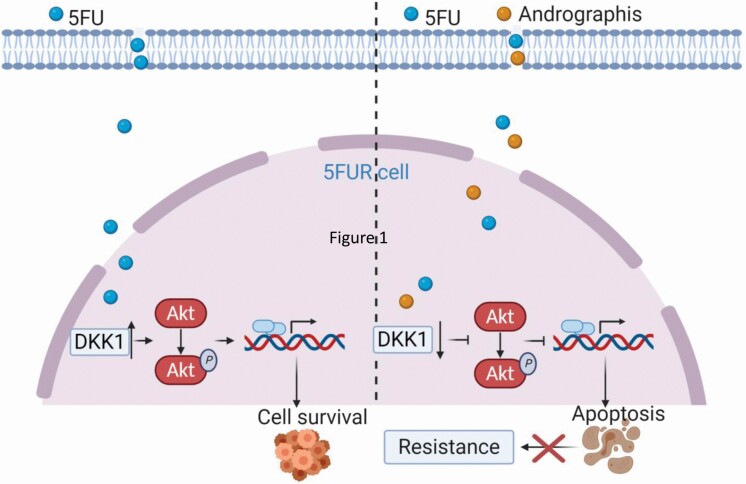
Collectively, this study systematically demonstrated the one of the important molecular mechanisms underlying andrographis-mediated reversal of chemoresistance to 5FU in CRC cells using multiple in vitro and in vivo models. In particular, the successful utilization of 3D patient-derived tumor organoids emphasizes the therapeutic importance of andrographis as an adjunctive therapy to 5FU in CRC patients. Mechanistically, DKK1 overexpression was a critical event for 5FU resistance in CRC, and andrographis could overcome 5FU resistance via inhibition of DKK1 and attenuating the downstream Akt-phosphorylation (Figure 7). Given the cost-effective and nontoxic characteristics of andrographis, it might be used as a potential adjunct to routine chemotherapy for CRC.
Figure 7.
A schematic illustration of andrographis-mediated chemosensitization in colorectal cancer. This illustration demonstrates 5-FU-mediated chemoresistance in CRC cells (left) and the role of andrographis in overcoming such chemoresistance through downregulation of DKK1 and downstream signaling pathways in CRC cells (right).
Acknowledgements
We thank Priyanka Sharma, Geeta Sharma, Yuma Wada, In Seob Lee and Souvick Roy for discussing the experiments and analysis. Yinghui Zhao is grateful to the Chinese Scholarship Council (201906220156) for a fellowship.
Glossary
Abbreviations
- 5FU
5-fluorouracil
- 5FUR
5-fluorouracil resistant
- CCK-8
Cell Counting Kit-8
- CRC
colorectal cancer
- DKK1
Dickkopf-1
- DMSO
dimethyl sulfoxide
- EdU
5-ethynyl-20-deoxyuridine
- IC50
the 50% inhibitory concentration
- PBS
phosphate-buffered saline
- siRNA
small interfering RNA
Funding
This work was supported by CA184792, CA187956, CA227602, CA072851 and CA202797 grants from the National Cancer Institute, National Institutes of Health.
Author contributions
A.G. and C.W. conceived and designed the experiments. Y.Z. performed the experiments and analyzed the data. Y.Z. and A.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest Statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1. Siegel, R.L., et al. (2020) Cancer statistics, 2020. CA. Cancer J. Clin., 70, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Siegel, R.L., et al. (2020) Colorectal cancer statistics, 2020. CA. Cancer J. Clin., 70, 145–164. [DOI] [PubMed] [Google Scholar]
- 3. Jiang, W.G., et al. (2015) Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol., 35 (Suppl), S244–S275. [DOI] [PubMed] [Google Scholar]
- 4. Van der Jeught, K., et al. (2018) Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol., 24, 3834–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cremolini, C., et al. ; GONO Foundation Investigators (2020) Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol., 21, 497–507. [DOI] [PubMed] [Google Scholar]
- 6. Miura, K., et al. (2015) The use of natural products in colorectal cancer drug discovery. Expert Opin. Drug Discov., 10, 411–426. [DOI] [PubMed] [Google Scholar]
- 7. Chandran, B., et al. (2012) A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res., 26, 1719–1725. [DOI] [PubMed] [Google Scholar]
- 8. Goel, A., et al. (2010) Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer, 62, 919–930. [DOI] [PubMed] [Google Scholar]
- 9. Goel, A., et al. (2001) Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett., 172, 111–118. [DOI] [PubMed] [Google Scholar]
- 10. Goel, A., et al. (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol., 75, 787–809. [DOI] [PubMed] [Google Scholar]
- 11. Ravindranathan, P., et al. (2018) A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep., 8, 13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toden, S., et al. (2017) The holy grail of curcumin and its efficacy in various diseases: is bioavailability truly a big concern? J. Restor. Med., 6, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toden, S., et al. (2015) Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. (Phila), 8, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toden, S., et al. (2015) Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis, 36, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang, W., et al. (2016) Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem. Pharmacol., 121, 8–17. [DOI] [PubMed] [Google Scholar]
- 16. Weng, W., et al. (2020) Curcumin and colorectal cancer: an update and current perspective on this natural medicine. Semin Cancer Biol. doi: 10.1016/j.semcancer.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida, K., et al. (2017) Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis, 38, 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caesar, L.K., et al. (2019) Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep., 36, 869–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alnuqaydan, A.M., et al. (2020) Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am. J. Cancer Res., 10, 799–815. [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma, P., et al. (2020) Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis, 41, 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahn, K.S., et al. (2008) Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res., 68, 4406–4415. [DOI] [PubMed] [Google Scholar]
- 22. Buhrmann, C., et al. (2018) Resveratrol chemosensitizes TNF-beta-induced survival of 5-FU-treated colorectal cancer cells. Nutrients, 10, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izumi, D., et al. (2017) Colorectal cancer stem cells acquire chemoresistance through the upregulation of F-Box/WD repeat-containing protein 7 and the consequent degradation of c-Myc. Stem Cells, 35, 2027–2036. [DOI] [PubMed] [Google Scholar]
- 24. Izumi, D., et al. (2019) TIAM1 promotes chemoresistance and tumor invasiveness in colorectal cancer. Cell Death Dis., 10, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ravindranathan, P., et al. (2019) Oligomeric proanthocyanidins (OPCs) from grape seed extract suppress the activity of ABC transporters in overcoming chemoresistance in colorectal cancer cells. Carcinogenesis, 40, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakatani, A., et al. (2019) Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis, 40, 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toden, S., et al. (2016) Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget, 7, 16158–16171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou, J., et al. (2012) Andrographolide sensitizes cisplatin-induced apoptosis via suppression of autophagosome-lysosome fusion in human cancer cells. Autophagy, 8, 338–349. [DOI] [PubMed] [Google Scholar]
- 29. Peng, Y., et al. (2018) Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J. Exp. Clin. Cancer Res., 37, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang, S., et al. (2010) Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin. Cancer Res., 16, 4755–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang, L., et al. (2009) Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett., 276, 180–188. [DOI] [PubMed] [Google Scholar]
- 32. Khan, I., et al. (2018) Andrographolide exhibits anticancer potential against human colon cancer cells by inducing cell cycle arrest and programmed cell death via augmentation of intracellular reactive oxygen species level. Nutr. Cancer, 70, 787–803. [DOI] [PubMed] [Google Scholar]
- 33. Xiang, D.C., et al. (2020) Protective effect of andrographolide on 5-fu induced intestinal mucositis by regulating p38 MAPK signaling pathway. Life Sci., 252, 117612. [DOI] [PubMed] [Google Scholar]
- 34. Su, M., et al. (2017) Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des. Devel. Ther., 11, 3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimura, H., et al. (2016) CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J. Clin. Invest., 126, 2689–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyros, O., et al. (2019) Dickkopf-1 (DKK1) promotes tumor growth via Akt-phosphorylation and independently of Wnt-axis in Barrett’s associated esophageal adenocarcinoma. Am. J. Cancer Res., 9, 330–346. [PMC free article] [PubMed] [Google Scholar]
- 37. Edmondson, R., et al. (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol., 12, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moertel, C.G., et al. (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med., 322, 352–358. [DOI] [PubMed] [Google Scholar]
- 39. Longley, D.B., et al. (2003) 5-Fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer, 3, 330–338. [DOI] [PubMed] [Google Scholar]
- 40. Pettersen, H.S., et al. (2011) UNG-initiated base excision repair is the major repair route for 5-fluorouracil in DNA, but 5-fluorouracil cytotoxicity depends mainly on RNA incorporation. Nucleic Acids Res., 39, 8430–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebert, M.P., et al. (2012) TFAP2E-DKK4 and chemoresistance in colorectal cancer. N. Engl. J. Med., 366, 44–53. [DOI] [PubMed] [Google Scholar]
- 42. Liu, C., et al. (2020) FoxO3 reverses 5-fluorouracil resistance in human colorectal cancer cells by inhibiting the Nrf2/TR1 signaling pathway. Cancer Lett., 470, 29–42. [DOI] [PubMed] [Google Scholar]
- 43. Panczyk, M. (2014) Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J. Gastroenterol., 20, 9775–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li, Y.H., et al. (2015) Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol., 21, 9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sak, K. (2012) Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract., 2012, 282570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Islam, M.T., et al. (2018) Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett., 420, 129–145. [DOI] [PubMed] [Google Scholar]
- 47. Jayakumar, T., et al. (2013) Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid. Based Complement. Alternat. Med., 2013, 846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin, H.H., et al. (2014) Andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells toward cisplatin via enhancing apoptosis pathways in vitro and in vivo. Toxicol. Sci., 139, 108–120. [DOI] [PubMed] [Google Scholar]
- 49. Zhou, J., et al. (2010) Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem. Pharmacol., 79, 1242–1250. [DOI] [PubMed] [Google Scholar]
- 50. Lim, S.C., et al. (2017) Andrographolide induces apoptotic and non-apoptotic death and enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in gastric cancer cells. Oncol. Lett., 13, 3837–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parker, T.W., et al. (2020) APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes. Sci. Rep., 10, 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun, S., et al. (2020) TCF4 promotes colorectal cancer drug resistance and stemness via regulating ZEB1/ZEB2 expression. Protoplasma, 257, 921–930. [DOI] [PubMed] [Google Scholar]
- 53. Hou, G., et al. (2020) Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Invest. New Drugs, 38, 329–339. [DOI] [PubMed] [Google Scholar]
- 54. Aguilera, O., et al. (2015) Nuclear DICKKOPF-1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancer. Oncotarget, 6, 5903–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang, R., et al. (2019) Dickkopf-1 contributes to hepatocellular carcinoma tumorigenesis by activating the Wnt/β-catenin signaling pathway. Signal Transduct. Target. Ther., 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Shen, Q., et al. (2012) Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol., 13, 817–826. [DOI] [PubMed] [Google Scholar]
- 57. Han, S.X., et al. (2015) Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget, 6, 19907–19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chin, Y.R., et al. (2009) Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal., 21, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang, B., et al. (2016) Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int. J. Nanomed., 11, 6401–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]