Abstract
Background:
Alterations in fibroblast growth factor receptor 3 (FGFR3) occur in ~15% of muscle-invasive bladder cancers (MIBCs) and metastatic urothelial carcinomas (mUCs).
Objective:
To determine the association between FGFR3 status and response to platinum-based chemotherapy in patients with MIBC or mUC.
Design, setting, and participants:
The authors conducted a retrospective review and comparison of patients having (1) MIBC treated with neoadjuvant chemotherapy (NAC), (2) mUC treated with first-line platinum-based chemotherapy (M1 cohort), and (3) MIBC who were from The Cancer Genome Atlas (TCGA).
Intervention:
Platinum-based chemotherapy.
Outcome measurements and statistical analysis:
Pathologic response, recurrence-free (RFS) or progression-free (PFS) survival, and overall survival (OS) were compared between patients with FGFR3 alteration (FGFR3alt) and those without it (FGFR3 wild type [FGFR3wt]) in the three cohorts.
Results and limitations:
Nine of 72 NAC patients (13%) had FGFR3alt, of whom none had pathologic complete response and three had residual non-MIBC (carcinoma in situ, n = 1; pT1, n = 2). FGFR3alt was associated with shorter RFS (hazard ratio, 2.74; p = 0.044) but not OS. Among TCGA patients who underwent adjuvant chemotherapy (n = 74), FGFR3alt patients had shorter RFS as well. Conversely, among chemotherapy-naive TCGA patients, FGFR3alt was associated with longer RFS and OS. In the M1 cohort (FGFR3alt, n = 27; FGFR3wt, n = 81), FGFR3alt was associated with higher rates of pulmonary metastases and nonregional lymphadenopathy. Despite lower response rates among FGFR3alt patients (37% vs 49%; p = 0.056), PFS and OS were not significantly different from FGFR3wt patients.
Conclusions:
FGFR3 status is associated with lower responses to platinum-based chemotherapy, which may prompt exploration of nonchemotherapeutic approaches for perioperative management of FGFR3alt urothelial cancers.
Patient summary:
Approximately 15% of bladder cancers harbor mutations in the fibroblast growth factor receptor 3 (FGFR3) gene. Our findings suggest that FGFR3 mutations might be associated with lower responses and shorter time to recurrence among patients with muscle-invasive bladder cancer who received perioperative platinum-based chemotherapy. FGFR3 status does not significantly impact response to chemotherapy among those with metastatic urothelial cancers.
Keywords: FGFR3, Urothelial cancer, Chemotherapy, Platinum
1. Introduction
Urothelial carcinoma of the bladder and urinary tract represents the sixth most commonly diagnosed cancer in the USA [1]. One-third of patients present with muscle-invasive bladder cancer (MIBC) and ~5% with de novo metastatic disease [2]. Platinum-based chemotherapy remains an essential therapeutic option, both perioperatively [3] and for metastatic disease [4]. However, response to platinum-based chemotherapy remains variable across patients.
The genomic landscape of urothelial cancers is characterized by a high mutational burden and recurrent mutations in several genes [5], including numerous DNA damage response and repair (DDR) genes and fibroblast growth factor receptor 3 (FGFR3). FGFR3 encodes for a tyrosine kinase receptor that activates the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K) pathways, resulting in promotion of cell proliferation and survival [6]. FGFR3 is altered in ~50% of non-MIBCs and up to 80% of low-grade and low-stage tumors [7]. Conversely, it is altered in only ~15% of muscle-invasive or metastatic urothelial carcinomas [5]. Most of these alterations are hotspot mutations that lead to constitutive receptor signaling, whereas <5% harbor FGFR3 fusions, with TACC3 being the most common fusion partner. While FGFR3 alterations are predictive of sensitivity to FGFR inhibitors, the association, if any, between FGFR3 alterations and platinum-based chemotherapy has not been explored significantly.
We sought to evaluate the association between FGFR3 alterations and response to platinum-based chemotherapy in the perioperative and metastatic settings, taking into account DDR gene mutation status, given the known association between alterations within these genes and chemosensitivity [8,9].
2. Patients and methods
2.1. Study design and patients
The following cohorts were included: (1) patients with MIBC treated with cisplatin-based neoadjuvant chemotherapy (NAC), (2) patients with metastatic urothelial carcinoma (mUC) treated with first-line platinum-based chemotherapy at Memorial Sloan Kettering Cancer Center (MSK; M1 cohort), and (3) patients with MIBC from The Cancer Genome Atlas (TCGA). Approval for this retrospective study was obtained from the institutional review board at MSK. Written informed consent was obtained before tumor sequencing as part of a genomic profiling protocol. Patients enrolled in ongoing clinical trials were excluded from this analysis.
The NAC cohort consisted of patients from a published phase II multicenter study of neoadjuvant dose-dense gemcitabine and cisplatin [8] as well as consecutive patients treated with standard-of-care NAC between April 2015 and June 2018 at MSK. All patients had T2 disease confirmed on transurethral resection of the bladder tumor and did not have radiographic evidence of nodal disease at presentation. Only patients who underwent cystectomy and in whom prechemotherapy tumor was sequenced were included. For the M1 cohort, we initially identified patients with mUC with FGFR3 alterations from our institutional database who received first-line platinum-based chemotherapy. Patients who received immunotherapy-based therapy, nonplatinum chemotherapy, or FGFR3 inhibitors were excluded from this analysis. A subset of these patients was part of a previously reported analysis associating DDR genes with response to platinum-based chemotherapy, including 67 FGFR3 wild-type (FGFR3wt) patients who served as a comparator group to evaluate clinical outcomes [9]. Clinical data from all patients in the TCGA cohort were downloaded from the cBioPortal for Cancer Genomics (www.cbioportal.org) on May 1, 2019.
2.2. Genomic analysis
Tumor sequencing was performed using the Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT) clinical sequencing assay. MSK-IMPACT is a hybridization capture–based next-generation sequencing platform that is Food and Drug Administration (FDA) approved and performed in a Clinical Laboratory Improvement Amendments–certified laboratory. Tumor and matched normal DNA are interrogated using an Illumina HiSeq platform for all exons and select introns of a panel of cancer-associated genes. All coding exons and select introns of FGFR3 are sequenced, allowing for the detection of mutations, copy number alterations, and fusions of this gene [10,11]. All coding exons of FGFR3 are included within the assay; DDR gene status was also evaluated using a panel of 34 genes involved in different DDR pathways on the MSK-IMPACT panel, which have been shown to be associated with platinum responsiveness [8,9]. Tumor mutational burden (TMB) was calculated by dividing the number of mutations by total genome coverage of the MSK-IMPACT assay.
2.3. Outcomes
For the NAC and TCGA cohorts, pathologic response and pathologic complete response (pCR) were defined as <ypT2 pN0 and ypT0 pN0, respectively, on pathologic evaluation of radical cystectomy specimens. Recurrence-free survival (RFS) and overall survival (OS) were calculated from the date of cystectomy to the date of the first radiographic evidence of recurrent disease or death, and to the date of death or last follow-up, respectively. In the absence of events, patients were censored at the last clinical follow-up. For the M1 cohort, response rates were determined by review of scan images and reports. Progression-free survival (PFS) and OS were calculated from the start of platinum-based chemotherapy to the date of progression or death and to the date of death, respectively.
2.4. Statistical analysis
Descriptive statistics were used to describe and summarize the baseline characteristics of each cohort. Chi-square tests or Fisher’s exact tests (if fewer than five cases) were used to determine co-occurrence or mutual exclusivity of genes within each sample. All time-to-event variables were compared between mutation groups using Kaplan-Meier curves, log-rank tests, and Cox proportional hazard models. The significance of interactions between gene mutations and DDR and between gene mutations and type of platinum treatment administered were tested in the NAC and M1 cohorts. For the M1 cohort, a multivariable Cox model incorporating other known baseline prognostic characteristics selected based on clinical relevance and univariable significance was built for PFS and OS. Significance was set at p < 0.05. Global p values were presented for variables with more than two levels, except in the case of mutation-related variables, where each level was individually tested against a reference level to better understand the differences in levels. No adjustments for multiple testing were made. All analyses were performed using R (version 3.5.2).
3. Results
3.1. Overall study population
Figure 1 depicts the CONSORT diagrams for the analyzed cohorts. A total of 163 unique patients were included in the NAC and M1 cohorts (NAC, n = 72; M1, n = 108), 17 of whom were included in both cohorts. Complete treatment data were available for 263 of 413 patients in the TCGA data set. Ten patients who received neoadjuvant chemotherapy were excluded (Fig. 1). Of these 253 patients, 74 underwent adjuvant chemotherapy and 179 did not receive any perioperative chemotherapy.
Fig. 1 –
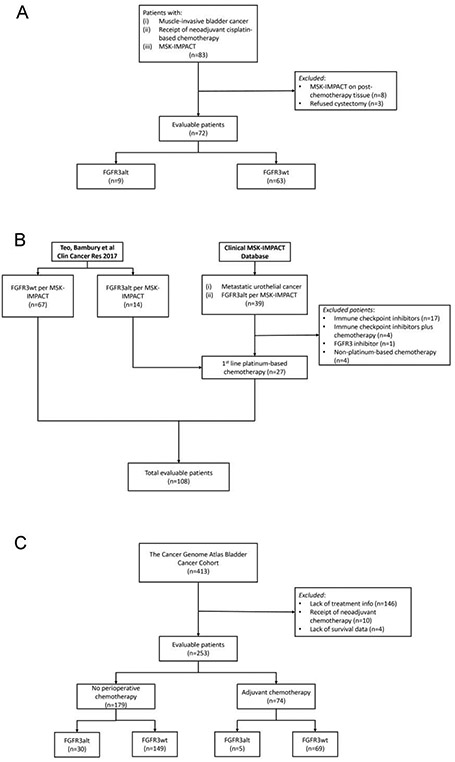
CONSORT diagrams for the analyzed cohorts: (A) patients with muscle-invasive bladder cancer treated with neoadjuvant cisplatin-based chemotherapy (NAC cohort); (B) patients with metastatic urothelial cancer treated with first-line platinum-based chemotherapy (M1 cohort); and (C) patients with muscle-invasive bladder cancer from The Cancer Genome Atlas (TCGA cohort). FGFR3 = fibroblast growth factor receptor 3; FGFR3alt = FGFR3 alteration; FGFR3wt = FGFR3 wild type; MSK-IMPACT = Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets; NAC = neoadjuvant chemotherapy; TCGA = The Cancer Genome Atlas.
S249C mutation was the most common FGFR3 alteration (NAC, n = 7 [78%]; TCGA, n = 29 [57%]; M1, n = 16 [59%]; Supplementary Table 1). Mutual exclusivity between TP53 and FGFR3 alterations was observed (p < 0.001), with 19% of tumors with FGFR3 alterations (6/31) also harboring TP53 alterations (Supplementary Table 2).
TMBs based on FGFR3 status were comparable. For the NAC cohort, the median TMB was 10.4 (range: 4.2–17.0) for patients with FGFR3 alteration (FGFR3alt) and 10.4 (4.2–54.7) for FGFR3wt patients (Wilcoxon rank sum test, p = 0.7); For M1 patients, the median TMB was 10.4 (4.7–43.7) and 9.1 (1.1–31.8), respectively (p = 0.3).
3.2. NAC cohort
Seventy-two patients received NAC (median age: 62 yr); 82% were male. Nine patients (13%) in this cohort were FGFR3alt. Age and sex distribution did not differ significantly by FGFR3 status (Table 1). All patients were treated with cisplatin and gemcitabine.
Table 1 –
Patient characteristics
| Characteristic | Neoadjuvant setting | Metastatic setting | ||
|---|---|---|---|---|
| FGFR3alt (n = 9) |
FGFR3wt (n = 63) |
FGFR3alt (n = 27) |
FGFR3wt (n = 81) |
|
| Age at diagnosis (yr), median (IQR) | 55 (54–59) | 63 (55–67) | 67 (58–72) | 65 (59–72) |
| Sex | ||||
| Male | 9 (100) | 50 (79) | 15 (56) | 64 (79) |
| Primary site of disease | ||||
| Bladder | 9 (100) | 63 (100) | 14 (52) | 62 (77) |
| Renal pelvis/ureter | 0 (0) | 0 (0) | 13 (48) | 11 (14) |
| Urethra | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 7 (8) |
| Pathologic T stage | ||||
| 0 | 0 (0) | 16 (25) | NA | NA |
| is | 1 (11) | 13 (21) | NA | NA |
| 1 | 2 (22) | 6 (10) | NA | NA |
| 2 | 2 (22) | 10 (16) | NA | NA |
| 3 | 4 (44) | 17 (27) | NA | NA |
| 4 | 0 (0) | 1 (2) | NA | NA |
| Pathologic N stage | ||||
| N+ | 1 (11) | 6 (10) | NA | NA |
| Bajorin score | ||||
| 0 | NA | NA | 8 (29) | 28 (35) |
| 1 | NA | NA | 18 (67) | 47 (58) |
| 2 | NA | NA | 1 (4) | 6 (7) |
| ECOG performance status | ||||
| 0 | NA | NA | 21 (78) | 74 (91) |
| 1 | NA | NA | 6 (22) | 7 (9) |
| Metastasis distribution | ||||
| Liver | NA | NA | 4 (15) | 11 (14) |
| Lung | NA | NA | 12 (44) | 22 (27) |
| Bone | NA | NA | 5 (19) | 22 (27) |
| Node | NA | NA | 19 (70) | 46 (57) |
| Visceral | NA | NA | 15 (56) | 47 (58) |
| DDR alterations | 3 (33) | 20 (31) | 4 (15) | 22 (27) |
| TP53 alterations | 2 (22) | 43 (68) | 5 (19) | 50 (62) |
alt = altered; DDR = DNA damage response and repair genes; ECOG = Eastern Cooperative Oncology Group; FGFR3 = fibroblast growth factor receptor 3; IQR = interquartile range; NA = not applicable; wt = wild type.
Data are no. (%), unless otherwise noted.
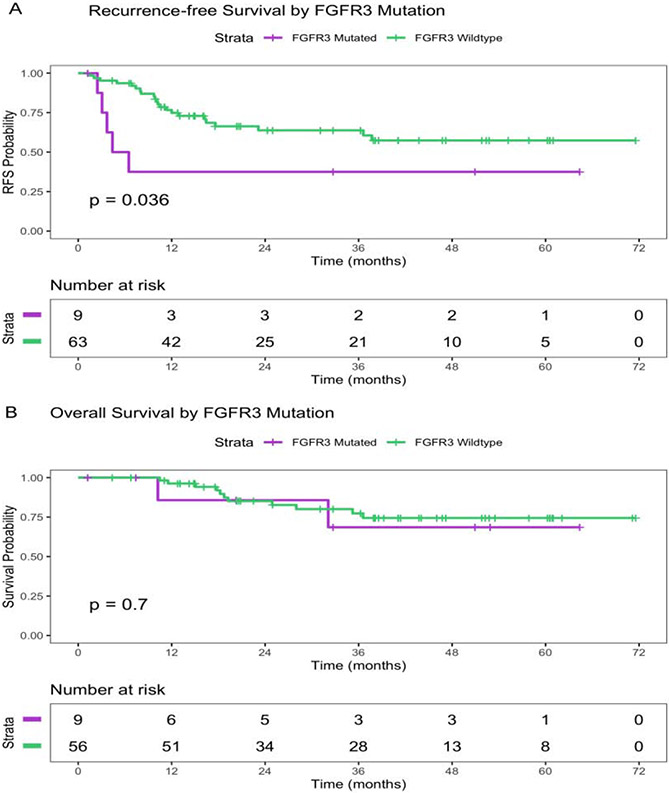
The pCR rate was 19%, and the <ypT2 rate was 54%. Of the nine patients with FGFR3alt, none had pCR, three (33%) exhibited downstaging (carcinoma in situ, n = 1; pT1, n = 2), and six (67%) had residual muscle-invasive disease (pT2, n = 1; pT3, n = 4) or nodal disease (n = 1). In contrast, of the 63 patients who were FGFR3wt, 14 (22%) had pCR and 19 (30%) had residual noninvasive disease. The difference in pCR rates between FGFR3wt and FGFR3alt was not statistically significant (p = 0.19; Table 2). The median follow-up period for the entire cohort was 38.4 mo. FGFR3alt was significantly associated with shorter RFS (6-mo survival probability, 50% [95% confidence interval {CI}, 25–100%] for FGFR3alt vs 94% [95% CI, 88–100%] for FGFR3wt; hazard ratio [HR], 2.74 [95% CI, 1.03–7.27]; p = 0.044; Fig. 2A and Table 3). No difference in OS was noted (p = 0.7, log-rank test; Fig. 2B).
Table 2 –
Univariable analysis for pathologic complete response (ypT0 pN0) in the neoadjuvant chemotherapy cohort
| Characteristic | Complete response (n = 14) |
Residual disease (n = 58) |
p value |
|---|---|---|---|
| Gender | 0.3 | ||
| Female | 4 (29) | 9 (16) | |
| Age | >0.9 | ||
| Median (interquartile range) | 64 (55, 69) | 62 (55,66) | |
| Mutation group | 0.054 | ||
| Neither mutation | 7 (35) | 13 (65) | |
| TP53alt only | 7 (16) | 36 (84) | |
| FGFR3alt or both | 0 (0) | 9 (100) | |
| TP53 alterations | 7 (16) | 38 (84) | 0.4 |
| FGFR3 alterations | 0 (0) | 9 (100) | 0.19 |
| DDR alterations | 7 (30) | 16 (70) | 0.12 |
alt = altered; DDR = DNA damage response and repair genes; FGFR3 = fibroblast growth factor receptor 3.
Data are no. (%).
Fig. 2 –
(A) Recurrence-free survival (RFS) and (B) overall survival by FGFR3 mutation status in the neoadjuvant cohort. FGFR3 = fibroblast growth factor receptor 3.
Table 3 –
Univariable analysis for recurrence-free survival in the neoadjuvant chemotherapy cohort
| Characteristic | HR | 95% CI | p value |
|---|---|---|---|
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.74 | 0.26–2.14 | 0.6 |
| Age, continuous variable | 1 | 0.96–1.04 | >0.9 |
| Mutation group | |||
| Neither | Reference | Reference | Reference |
| TP53alt only | 2.28 | 0.77–6.74 | 0.137 |
| FGFR3alt or both | 5.05 | 1.35–18.9 | 0.016 |
| FGFR3 status | |||
| FGFR3wt | Reference | Reference | Reference |
| FGFR3alt | 2.74 | 1.03–7.27 | 0.044 |
| TP53 status | |||
| TP53wt | Reference | Reference | Reference |
| TP53alt | 1.35 | 0.59–3.09 | 0.475 |
| DDR status | |||
| DDRwt | Reference | Reference | Reference |
| DDRalt | 0.65 | 0.26–1.60 | 0.348 |
| Mutations/Mb, continuous variable | 0.99 | 0.94–1.03 | 0.519 |
alt = altered; CI = confidence interval; DDR = DNA damage response and repair genes; HR = hazard ratio; wt = wild type.
Owing to significant mutual exclusivity of FGFR3 and TP53 gene alterations (p < 0.001), patients were also stratified using the following alteration groups to identify any differential clinical impact of FGFR3 and TP53 alterations: FGFR3alt (±TP53alt) versus TP53 alteration only versus FGFR3wt + TP53wt. The pCR rate was lower in the FGFR3alt (±TP53alt) group than in the other two groups (0%, 16%, and 35%, respectively; p = 0.054, Fisher’s exact test; Table 2). Similarly, RFS was shorter in the FGFR3alt ± TP53alt group, significantly so when compared with the FGFR3wt + TP53wt group (HR, 5.05 [95% CI, 1.35–18.9]; p = 0.016; Table 3 and Supplementary Fig. 1A). OS was not significantly different between the three groups (p = 0.4, log-rank test; Supplementary Fig. 1B). Other clinical and genomic parameters, including sex, age, and TMB, were not significantly associated with clinical outcomes (Table 3).
Of note, no statistical interactions were observed between FGFR3 alterations and DDR gene mutation status. Of the nine patients with FGFR3alt, three had DDR gene alterations (#1: NBN M17*, #2: ATR S1660*, and #3: ERCC2 N238S and CHEK2 Q330*), two of whom (cases #1 and #3) have relapsed (after 3.2 and 4.4 mo, respectively). None of these patients had a pathologic response. We also examined the association between genomic alterations with ≥10% prevalence and clinical outcomes. These genes included ARID1A, KDM6A, CDKN2A, RB1, PIK3CA, KMT2D, ERBB2, MDM2, and CREBBP, none of which demonstrated an association with clinical outcomes (Supplementary Table 3). We examined TMB as a variable for pCR and RFS. TMB was also not statistically significantly associated with pCR (p = 0.8) or RFS (p = 0.5).
3.3. TCGA cohort
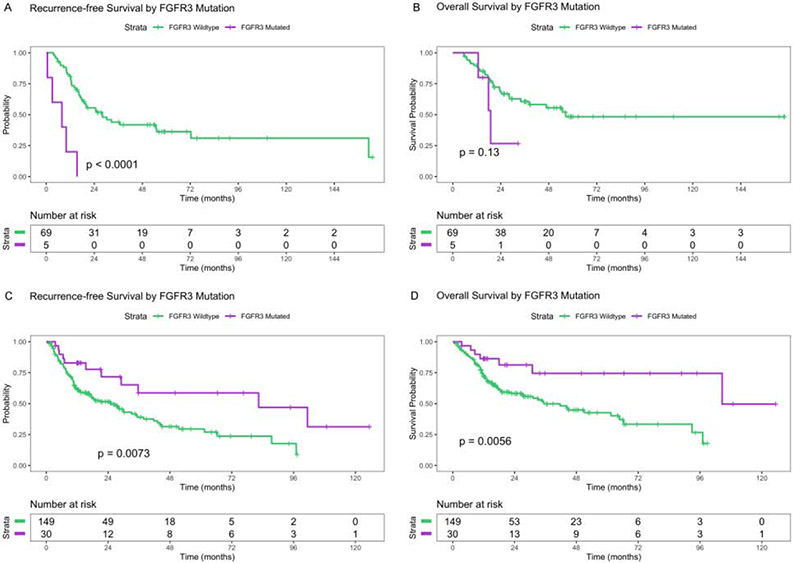
Seventy-four TCGA patients received adjuvant chemotherapy: five (7%) had alterations in FGFR3 and none had co-occurring TP53 alterations. The median follow-up period was 37.0 mo. RFS was significantly shorter among FGFR3alt patients (Fig. 3A). The difference remained significant after adjustment for pathologic T stage and age at diagnosis (median RFS, 7.7 vs 28.0 mo; HR, 7.27 [95% CI, 2.26–23.4]; p < 0.001; Supplementary Table 4). FGFR3alt tumors had inferior OS (Fig. 3B), although the association was not significant (median OS, 19.0 vs 56.4 mo; HR, 2.47 [95% CI, 0.73–8.32]; p = 0.15; Supplementary Table 5). When FGFR3 and TP53 mutation groups were considered (three groups: FGFR3alt [±TP53alt], TP53 alteration only, and no mutations), patients with FGFR3alt had a significantly higher risk of recurrence than those with neither mutation, after adjustment for stage and age at diagnosis. RFS in FGFR3wt patients with TP53 alterations did not differ significantly from that in patients with neither mutation (Supplementary Table 5).
Fig. 3 –
(A) Recurrence-free survival and (B) overall survival by FGFR3 mutation status in the TCGA bladder cancer subset who received adjuvant chemotherapy; (C) recurrence-free survival and (D) overall survival in the TCGA bladder cancer subset who did not receive perioperative chemotherapy. All analyses are log-rank tests and not adjusted for other variables. FGFR3 = fibroblast growth factor receptor 3; TCGA = The Cancer Genome Atlas.
Of the 179 TCGA patients who did not receive perioperative chemotherapy, 30 (17%) had FGFR3alt. Patients with FGFR3alt were younger (median age, 61 vs 73 yr; p < 0.001) and less likely to have pT3–4 disease (38% vs 68%; p < 0.01). The median follow-up time was 21.5 mo. RFS was significantly longer among FGFR3alt patients (Fig. 3C), a difference that remained significant after adjustment for pathologic T stage and age at diagnosis (median RFS, 82.4 vs 25.2 mo; HR, 0.40 [95% CI, 0.18–0.91]; p < 0.030; Supplementary Table 5). FGFR3alt patients also had superior unadjusted (Fig. 3D) and adjusted OS (median OS, 104.6 vs 35.4 mo; HR, 0.33 [95% CI, 0.12–0.94]; p = 0.038; Supplementary Table 5).
3.4. M1 cohort treated with platinum-based chemotherapy
Thirty-nine patients with mUC with FGFR3alt were identified from our genomic database, 13 of whom received platinum-based chemotherapy, along with 14 FGFR3alt cases from a previously published series (Fig. 1). FGFR3alt was associated with higher rates of pulmonary metastasis (47% vs 30%; p = 0.024) and extrapelvic lymphadenopathy (75% vs 62%; p = 0.038; Supplementary Table 6). The 27 FGFR3alt patients in this cohort were compared with the 81 FGFR3wt patients to determine clinical outcomes. The median age at the start of treatment was 66 yr, and 73% of patients were male. Of the patients, 42% (n = 45) were treated with cisplatin plus gemcitabine, while the remaining patients received carboplatin plus gemcitabine. No significant differences in baseline characteristics were noted by FGFR3 alteration status (Table 1).
Among evaluable patients, FGFR3alt was associated with a lower response rate, although the difference was not statistically significant (FGFR3alt vs FGFR3wt, 10/27 [37%] vs 36/74 [49%]; p = 0.056). With a median follow-up of 21.0 mo, no differences in PFS or OS were noted by FGFR3 status (median PFS: 7.0 mo [FGFR3wt] vs 6.7 mo [FGFR3alt], HR, 1.35 [95% CI, 0.84–2.18]; median OS: 16.8 mo [FGFR3wt] vs 28.7 mo [FGFR3alt], HR, 0.70 [95% CI, 0.39–1.26]; Supplementary Fig. 2A and 2B, and Supplementary Table 7).
When examined by individual mutations within FGFR3 (S249C [n = 16] vs Y373C [n = 6] vs others [n = 5] vs wild type), PFS and OS were not different (Supplementary Fig. 3A and 3B, and Supplementary Table 8). Commonly observed genomic alterations and TMB were not associated with PFS (p = 0.4).
4. Discussion
Accumulating evidence suggests that FGFR3alt urothelial carcinoma represents a unique biological disease entity with distinct molecular, histopathologic, and clinical features. FGFR3 activating alterations have been identified as occurring early in urothelial carcinoma development, with low-grade and noninvasive tumors being enriched for these alterations compared with high-grade, muscle-invasive, and metastatic tumors. Several groups have also shown that FGFR3 alterations are frequently found in urothelial carcinomas of the ureter and renal pelvis [12-14]. Additionally, using RNA expression profiling to define molecular subtypes of bladder cancer, the luminal papillary (or luminal I) subtype is characterized by papillary tumors that are enriched for FGFR3 mutations [5]. Correlative analyses from prospective trials of checkpoint blockade have shown an association between luminal papillary, FGFR3-altered bladder tumors, and both a non–T-cell-inflamed microenvironment and a lower response rate to immunotherapy, although it remains unclear whether FGFR3 plays a causal role in inducing this immune desert phenotype [15-17].
Our data indicate that FGFR3 alterations are associated with a distinct metastatic phenotype and possessed predictive and prognostic features, including lower rates of pathologic response following NAC and a higher likelihood of recurrence following perioperative chemotherapy (although not all associations met the threshold for statistical significance). Notably, alterations in FGFR3 were associated with superior clinical outcomes among patients who did not receive perioperative chemotherapy, even after adjustment for disease stage. Outcomes did not differ by FGFR3 status in patients with mUC treated with platinum-based chemotherapy. Preclinically, the FGFR3 mutant urothelial carcinoma cell line 97-7 exhibits resistance to cisplatin chemotherapy, possibly through activation of PI3K/Akt signaling [18]. Additionally, Sung and colleagues [19] examined 72 patients with MIBC, 42 of whom received adjuvant cisplatin and gemcitabine, and found that FGFR3 overexpression by immunohistochemistry independently correlated with shorter disease-free survival and OS following adjuvant chemotherapy, after adjustment for known risk factors. In a separate series of 112 patients with mUC treated with platinum-based chemotherapy, 20% of whom harbored FGFR3 alterations, no difference was observed in PFS and OS between FGFR3 altered and wild-type tumors [19]. In a recent retrospective analysis of patients enrolled in the phase II BLC2001 study of the pan-FGFR inhibitor erdafitinib, the overall response rate to previous first-line platinum-based chemotherapy was numerically lower than in historical controls, at 29%, but the median time to progression, at 7.6 mo, was similar [20].
The unique biological features associated with FGFR3 alterations could account for differences in clinical response to platinum-based chemotherapy among patients with MIBC. However, additional genomic events likely contribute to the development of metastatic disease, superseding FGFR3 as the determinant of the tumor’s biological behavior and, therefore, accounting for the lack of FGFR3-dependent differences in clinical outcomes among patients with metastatic disease.
The potentially detrimental effect of perioperative chemotherapy, if confirmed, might serve as an impetus to explore nonchemotherapeutic approaches for perioperative management of FGFR3 altered bladder cancers, including the incorporation of FGFR3 inhibitors into earlier disease settings. A trial of the FGFR inhibitor infigratinib in the adjuvant setting for patients with FGFR3alt tumors who are at high risk for metastatic recurrence is planned. Furthermore, incorporating prior observations on the predictive roles of DDR alterations [8,21], a molecularly informed stratification and treatment algorithm could be developed for MIBC.
Our study is retrospective in nature. However, our findings are similar to other series [19,20]. We also observed a differential impact of FGFR3 alterations on response to platinum-based chemotherapy across disease states, which could be an effect of sample size or biological differences. Finally, our analysis did not include RNA expression profiling, and therefore the association between platinum sensitivity and molecular subtypes as well as FGFR3 overexpression could not be explored in this cohort. Larger data sets will be required to elucidate the clinical impact of individual mutations.
5. Conclusions
In summary, our analysis indicated that patients with FGFR3 mutant tumors had inferior outcomes following perioperative chemotherapy. This relationship, however, was not observed in the metastatic setting. A planned multiplatform sequencing analysis of the recently reported S1314 study of cisplatin-based NAC for MIBC will help provide clarity on this interaction. Additionally, modern biomarker-driven neoadjuvant studies that incorporate next-generation sequencing into their workflow, such as Alliance A031701 (NCT03609216) and RETAIN (NCT02710734), will serve as prospective validation cohorts to confirm the above observations.
Supplementary Material
Acknowledgments:
David B. Sewell of the Memorial Sloan Kettering Department of Surgery provided editorial assistance.
Funding/Support and role of the sponsor:
This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Financial disclosures: Gopa Iyer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 2018;124:2785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66(6 Suppl 1):4–34. [DOI] [PubMed] [Google Scholar]
- [3].Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. [DOI] [PubMed] [Google Scholar]
- [4].von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8. [DOI] [PubMed] [Google Scholar]
- [5].Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017;171:540–556 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010;10:116–29. [DOI] [PubMed] [Google Scholar]
- [7].Pietzak EJ, Bagrodia A, Cha EK, et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol 2017;72:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Iyer G, Balar AV, Milowsky MI, et al. Multicenter prospective phase II trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J Clin Oncol 2018;36:1949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017;23:3610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Robinson BD, Vlachostergios PJ, Bhinder B, et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun 2019;10:2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Audenet F, Isharwal S, Cha EK, et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res 2019;25:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nassar AH, Umeton R, Kim J, et al. Mutational analysis of 472 urothelial carcinoma across grades and anatomic sites. Clin Cancer Res 2019;25:2458–70. [DOI] [PubMed] [Google Scholar]
- [15].Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48. [DOI] [PubMed] [Google Scholar]
- [16].Rose TL, Hayward MC, Salazar AH, et al. Fibroblast growth factor receptor status and response to immune checkpoint inhibition in metastatic urothelial cancer. J Clin Oncol 2019;37(7_suppl):458. [Google Scholar]
- [17].Wang L, Gong Y, Saci A, et al. Fibroblast Growth Factor Receptor 3 Alterations and Response to PD-1/PD-L1 Blockade in Patients with Metastatic Urothelial Cancer. Eur Urol 2019;76:599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xie X, Lin J, Zhong Y, Fu M, Tang A. FGFR(3S249C) mutation promotes chemoresistance by activating Akt signaling in bladder cancer cells. Exp Ther Med 2019;18:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sung JY, Sun JM, Chang Jeong B, et al. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy. Urol Oncol 2014;32:49 e23–31. [DOI] [PubMed] [Google Scholar]
- [20].Loriot Y, Necchi A, Park SH, et al. Erdafitinib (ERDA; JNJ-42756493), a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRa): phase 2 continuous versus intermittent dosing. J Clin Oncol 2018;36(6_suppl):411. [Google Scholar]
- [21].Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.