Abstract
Background:
Mutations in the serine-threonine kinase LKB1/STK11 have been implicated in mediating resistance to checkpoint blockade among patients with advanced lung adenocarcinoma. We sought to examine the associations between clinicopathologic characteristics, tumor LKB1 expression, features of the immune microenvironment, and postoperative prognosis among patients with early-stage lung adenocarcinoma undergoing surgical therapy.
Methods:
Formalin-fixed, paraffin-embedded specimens of patients undergoing resection of stage I-III, chemotherapy-naïve adenocarcinomas (1997–2008) were analyzed using tissue microarray sectioning. Sublobar resections were excluded. Intratumoral LKB1/STK11 expression was quantified as H-score. In a subset, tumor associated immune cell populations were quantified using whole tumor sections in peritumoral and intratumoral compartments.
Results:
104 patients met inclusion criteria. LKB1/STK11 expression (median H-score 102.9) was higher in women (median 123.3) than men (100.0, p=0.004) and in never-smokers (median 145.0) than former/current smokers (100.0, p=0.002). LKB1/STK11 expression was positively correlated with intratumoral infiltration of CD3+ (r=0.351, P=0.005), CD4+ (r=0.436, P<0.001), and CD8+ (r=0.263, P=0.049) cells. Patients with extrathoracic recurrence had lower tumor expression of LKB1/STK11 than did other patients with recurrent disease. On multivariate analysis, low LKB1/STK11 expression remained independently associated with poor disease-free survival and distant disease-free survival.
Conclusions:
Low LKB1/STK11 expression is associated with specific patient characteristics and poor postoperative prognosis in chemotherapy-naïve lung adenocarcinoma. Further investigation is warranted to delineate its clinical significance in the context of evaluating novel therapeutic agents in patients with resectable disease.
Keywords: non-small cell lung cancer, tumor microenvironment, LKB1, STK11, adenocarcinoma
The evolving characterization of the immunogenomic landscape in non-small cell lung cancer (NSCLC) has greatly enhanced understanding of the processes driving tumorigenesis, disease progression, and tumor evasion of immunosurveillance (1). The identification of novel therapeutic targets and the development of management strategies tailored to tumor bimolecular profiles have been aided by this knowledge and, in turn, have revolutionized the management approach to patients with this disease (1–7).
Serine/threonine kinase 11 (STK11)/liver kinase B1 (LKB1) is a protein that regulates cellular metabolic processes and growth via activation of enzymes in the 5’ adenosine monophosphate-activated protein kinase family (8). LKB1/STK11 additionally functions as a tumor suppressor, and inactivation is associated with an aggressive tumor phenotype and with prometastatic features (9–11). In the setting of advanced KRAS-mutant lung adenocarcinoma, coexisting LKB1/STK11 mutations have been identified as driving local immunosuppression within the tumor microenvironment (TME) (7, 12). Importantly, these mutations have been further shown to mediate resistance to inhibitors of the PD-1/PD-L1 axis (13). However, although there have been previous efforts to characterize the role of LKB1/STK11 in the context of advanced KRAS-mutant NSCLC, its associations with clinicopathologic characteristics and its prognostic significance in the context of resectable disease have not been fully elucidated. Because mutations in LKB1/STK11 are predominantly manifested by a loss of protein expression, precise quantification and delineation of the relationships between LKB1/STK11 expression, clinical features, and oncologic outcomes constitute a clinically salient and incompletely developed line of inquiry (11).
Antitumor immunity is marked by innate and adaptive responses that are mediated in large part by a complex interplay between T lymphocyte populations (CD3+, CD4+ [helper], CD8+ [cytotoxic], CD45RO+ [memory], FOXP3+ [regulatory]), natural killer cells (CD57+), tumor-associated macrophages (CD68+), and tumor evasion of the immune response by activation of the PD1/PD-L1 immunoinhibitory axis (1). In this study, we hypothesized that lung adenocarcinomas with low LKB1/STK11 expression would be characterized by poor immune cell infiltration, an increased propensity for systemic metastases, and adverse postoperative prognosis. To that end, we analyzed relationships between tumor LKB1/STK11 expression, tumor associated immune cell (TAIC) densities, and patterns of recurrence after curative-intent resection of lung adenocarcinoma.
PATIENTS AND METHODS
Patient Selection
Eligible patients for this study were those who underwent resection of primary lung adenocarcinoma at the University of Texas MD Anderson Cancer Center between 1997 and 2008 and had been previously included in a tissue microarray with LKB1/STK11 expression data available (n = 104, Supplementary Figure 1). Patients who received neoadjuvant therapy (to limit possible confounding effects on the tumor microenvironment) (14) and those who underwent sublobar resection were excluded from analysis to limit possible confounding effects on oncologic outcomes. This retrospective study was approved by MD Anderson’s Institutional Review Board with a waiver of individual patient consent. Tumors were retrospectively staged using the seventh edition of the American Joint Committee on Cancer’s staging system (15).
Immunohistochemical Staining and Analyses
Tissue microarrays had been previously constructed using formalin-fixed, paraffin-embedded tumor blocks using methods that have been described previously among a cohort of NSCLC patients who underwent primary resection (16, 17). Tissue microarray sections were prepared using three 1.0 mm tissue cores obtained from the center, middle, and periphery of formalin-fixed and paraffin-embedded histological sections. Cases with at least two TMA cores were included in the analysis. LKB1/STK11 expression in tumor cells was quantified as an H-score (range, 0 to 300) by multiplying the observed staining intensity (LKB1/clone D60C5F10, dilution 1:250; Cell Signaling Technology, Beverly, MA; range 0 [no staining], 1+ [weak staining], 2+ [moderate staining], 3+ [strong staining]) and the percentage of cells expressing the marker (range 0–100%) (representative examples of staining levels are provided in Figure 1; Figure 1A, no staining; 1B, weak staining [1+]; 1C, moderate staining [2+]; 1D, strong staining [3+]; 1E, biological negative control; 1F, biological positive control; stained sections of all available cases have been uploaded as Supplementary Figure 2). For a subset of cases (83/104, 80%; baseline clinical characteristics provided in Supplementary Table 1), whole tumor sections had been previously analyzed to quantify tumor-associated immune cell densities in the peritumoral and intratumoral (tumor nests and stroma of tumor) compartments using methods previously described (16). Briefly, four micrometer-thick tumor sections were stained for identification and quantification of cells expressing CD3 (polyclonal antibody, catalogue number A045201–2, dilution 1:100; Dako, Carpinteria, CA), CD4 (Novocastra, Leica Microsystems, Milton Keynes, UK; clone 4B12, dilution 1:80; Leica Biosystems, Buffalo Grove, IL), CD8 (CD8/144B, 1:20; ThermoFisher Scientific, Inc), CD45RO (UCHL1, ready to use; Leica Biosystems), CD57 (HNK-1, 1:40; BD Biosciences), CD68 (cloe PG-M1, 1:450; Dako), PD-1 (EPR4877–2, 1:250; Abcam), FOXP3 (206D, 1:50; BioLegend), and PD-L1 (E1L3N, 1:100; Cell Signaling Technology). Stained slides as well as positive and negative controls were scanned at x200 magnification using the Aperio AT2 Scanner (Leica Microsystems) and visualized and analyzed using ImageScope and Toolbox software (Leica Microsystems). As previously described, TAIC densities were examined in five 1 mm2 areas and quantified as the mean density of examined areas (16).
Figure 1:
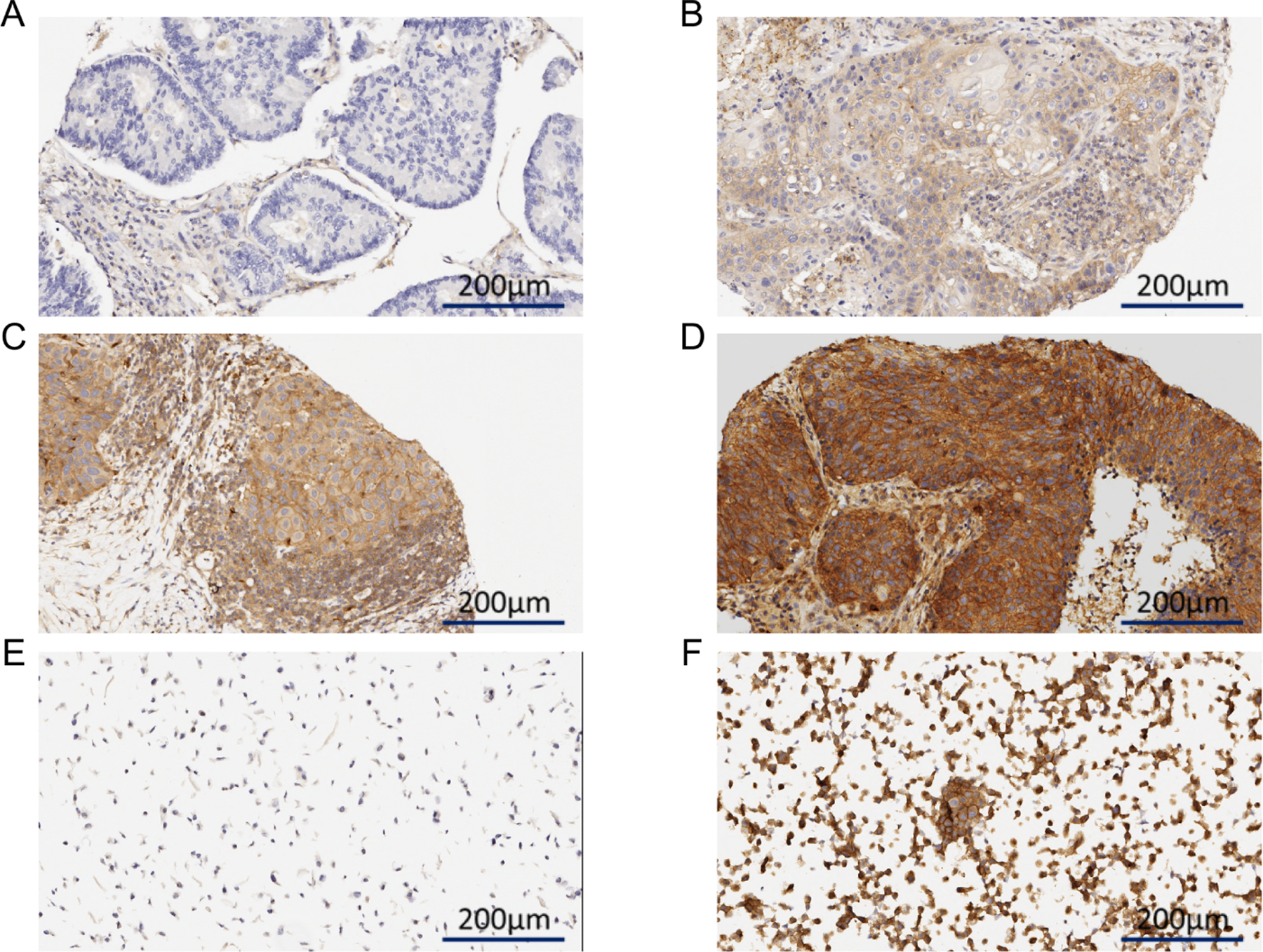
Representative examples of LKB1/STK11 staining: (A) absent, (B) 1+, (C) 2+, (D) 3+, as well as (E) negative and (F) positive controls.
Outcome Definitions and Statistical Analysis
Associations between tumor expression of LKB1/STK11 and clinicopathologic characteristics were analyzed using the Mann-Whitney U and Kruskal-Wallis tests. Pairwise correlations between LKB1/STK11 and TAIC densities were analyzed using Spearman’s correlations. For correlations between LKB1/STK11 expression and TAIC densities, correction for multiple comparisons (9 aforementioned TAIC populations) was performed using the Benjamini-Hochberg method (18). Overall survival (OS) was defined as the time from surgery to death from any cause; patients alive at the end of the study period were censored at the date of last follow-up. Disease-free survival (DFS) was defined as the time from resection to death or recurrence; patients without a DFS event at the end of the study period were censored at the date of last follow-up. Distant DFS was defined as the time to death or distant recurrence; patients without a distant DFS event at the end of the study period were censored at the date of last follow-up. Locoregional recurrence was defined as recurrence at resection margins or within N1/N2 nodal stations at the first diagnosis of recurrence, and distant recurrence was defined as recurrence elsewhere (19). Survival times were estimated using the Kaplan-Meier method, and differences in time-to-event outcomes were analyzed using the log-rank test. Univariate Cox proportional hazards regressions were performed to examine associations between LKB1/STK11 expression and relevant clinicopathologic features with survival. Variables with p<0.20 on univariate analysis were entered into a multivariate model; stepwise backwards selection was then performed with p<0.10 as the final selection criterion in order to optimize parsimony of the final multivariable model (parsimony assessed using Akaike’s Information Criterion). For survival analysis, LKB1/STK11 expression was as a continuous variable. All analyses were performed using R (version 3.3.0; http://www.r-project.org), SPSS (version 24.0.0; IBM, Armonk, NY), and STATA (version 14.2, StataCorp, College Station, TX). A two-tailed P < 0.05 was considered significant for all analyses, and a false discovery rate (FDR)-adjusted P value < 0.05 was considered significant for analyses in which FDR correction was performed.
RESULTS
Patient, Tumor, and Treatment Characteristics
Of 104 patients who met inclusion criteria (Supplementary Figure 1), most (62, 60%) had pathologic stage I disease (Table 1). The cohort was evenly distributed by sex, and a majority of patients were former or current smokers (89, 86%). Adjuvant chemotherapy and radiotherapy were used in 23 (22%) and 13 (13%), respectively.
Table 1:
Baseline patient, tumor, and treatment characteristics of the study cohort (n = 104).
| Variable | N (%) or Median (IQR) |
|---|---|
| Age, median (IQR) (years) | 64.0 (56.5–73.0) |
| Sex | |
| Female | 51 (49.0) |
| Male | 53 (51.0) |
| Smoking | |
| Never | 15 (14.4) |
| Former/Current | 89 (85.6) |
| FEV1 (% predicted)* | 87.0 (77.0–101.0) |
| Differentiation | |
| Poor | 43 (41.3) |
| Well/Moderate | 61 (58.7) |
| Zubrod | |
| 0 | 57 (54.8) |
| 1 | 47 (45.2) |
| Extent of resection | |
| Lobectomy/Bilobectomy | 100 (96.2) |
| Pneumonectomy | 4 (3.8) |
| Pathologic Margin | |
| R0 | 100 (96.2) |
| R1 | 4 (3.8) |
| Pathologic Stage | |
| I | 62 (59.6) |
| II | 24 (23.1) |
| III | 18 (17.3) |
| Adjuvant therapy | |
| Chemotherapy | 23 (22.1) |
| Radiotherapy | 13 (12.5) |
FEV1: forced expiratory volume in one second; IQR: interquartile range
available in 103/104 (99.0)
Associations with Clinicopathologic Features
The median expression of LKB1/STK11 for the entire cohort was 102.9 (H-score, interquartile range [IQR] 21.3–144.2). Higher LKB1/STK11 expression was observed among women (median H-score 123.3 [IQR 40.0–170.0] versus 100.0 [IQR 9.2–120.0], P=0.004) and never-smokers (median H-score 145.0 [IQR 121.7–190.0] versus 100.0 [IQR 14.2–135.8], P=0.002) (Figure 2A and B). Although there were no observed differences in expression according to tumor differentiation (well/moderate 113.3 [IQR 14.2–157.5] versus poor 100.0 [IQR 25.0–123.3], P=0.289) or pathologic stage (stage I 110.0 [IQR 31.3–155.8] versus stage II 100.0 [IQR 0.0–124.2] versus stage III 101.3 [IQR 32.5–136.7], P=0.334), LKB1/STK11 expression was inversely correlated with pathologic tumor size (r=−0.329, P<0.001) (Figure 2C and D). No statistically-significant difference was observed according to margin status (R0: median 105.0, IQR 22.5–147.5; R1: median 52.5, IQR 17.5–96.7; P=0.256).
Figure 2:
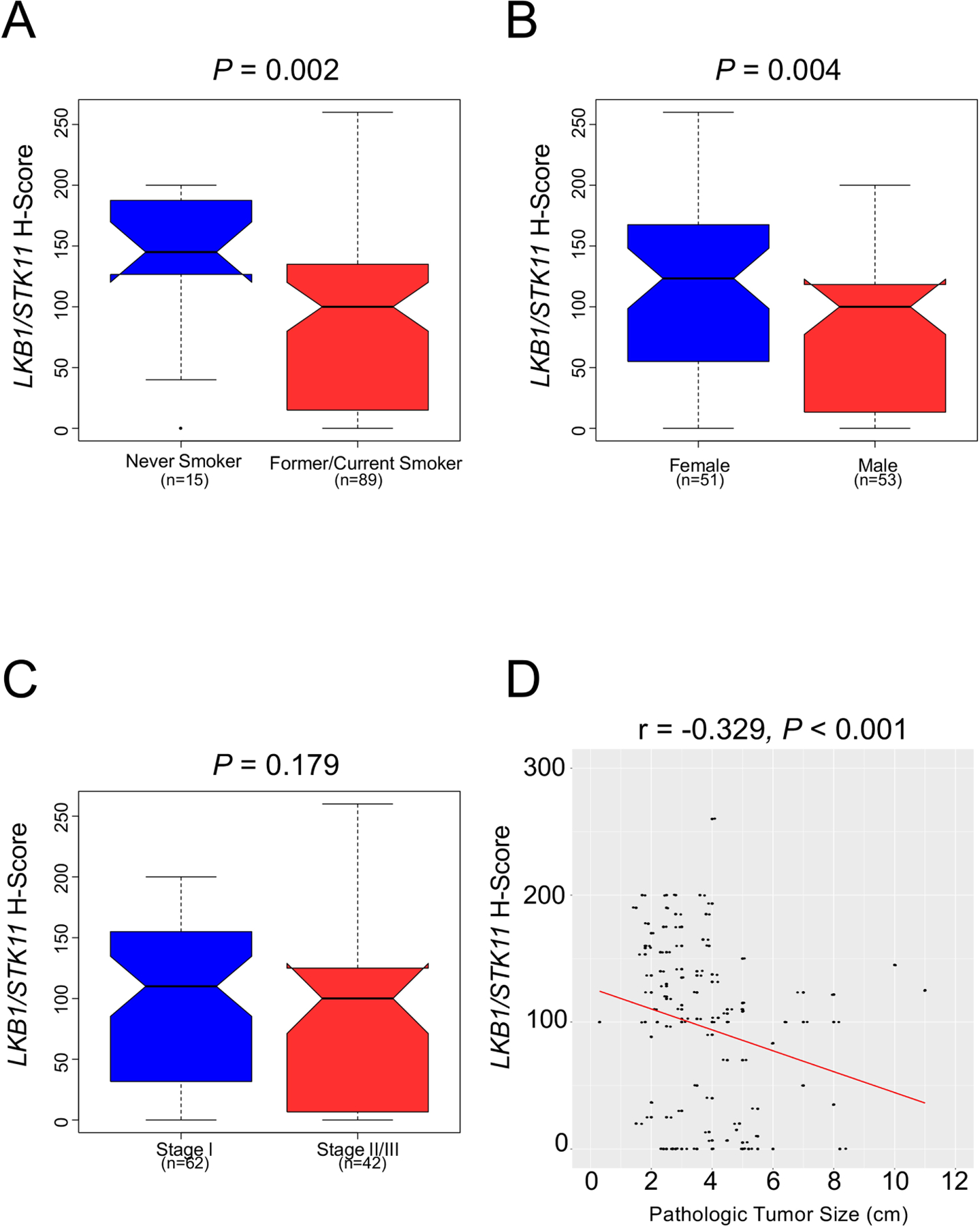
Associations between tumor LKB1/STK11 expression and clinicopathologic characteristics (n = 104). LKB1/STK11 expression was noted to be higher (A) among never smokers than former or current smokers, and (B) among women. Although (C) no association was identified between LKB1/STK11 expression and pathologic stage, its expression (D) was inversely correlated with pathologic tumor size. Tumor LKB1/STK11 expression is quantified as intratumoral H-score.
Associations with Densities of Tumor-Associated Immune Cells
LKB1/STK11 expression was positively correlated with intratumoral densities of CD3+ (r=0.351, P=0.005), CD4+ (r=0.436, P<0.001), and CD8+ (r=0.263, P=0.049) among the subset of cases with available TAIC IHC data in this cohort (N = 83/104) (Fig. 3A–C). After adjusting for multiple comparisons, no statistically-significant associations were identified with densities of CD45RO+, FOXP3+, CD57+, and CD68+ cell populations among the subgroup of study patients with IHC data available (N = 83/104; Supplementary Table 2).
Figure 3:
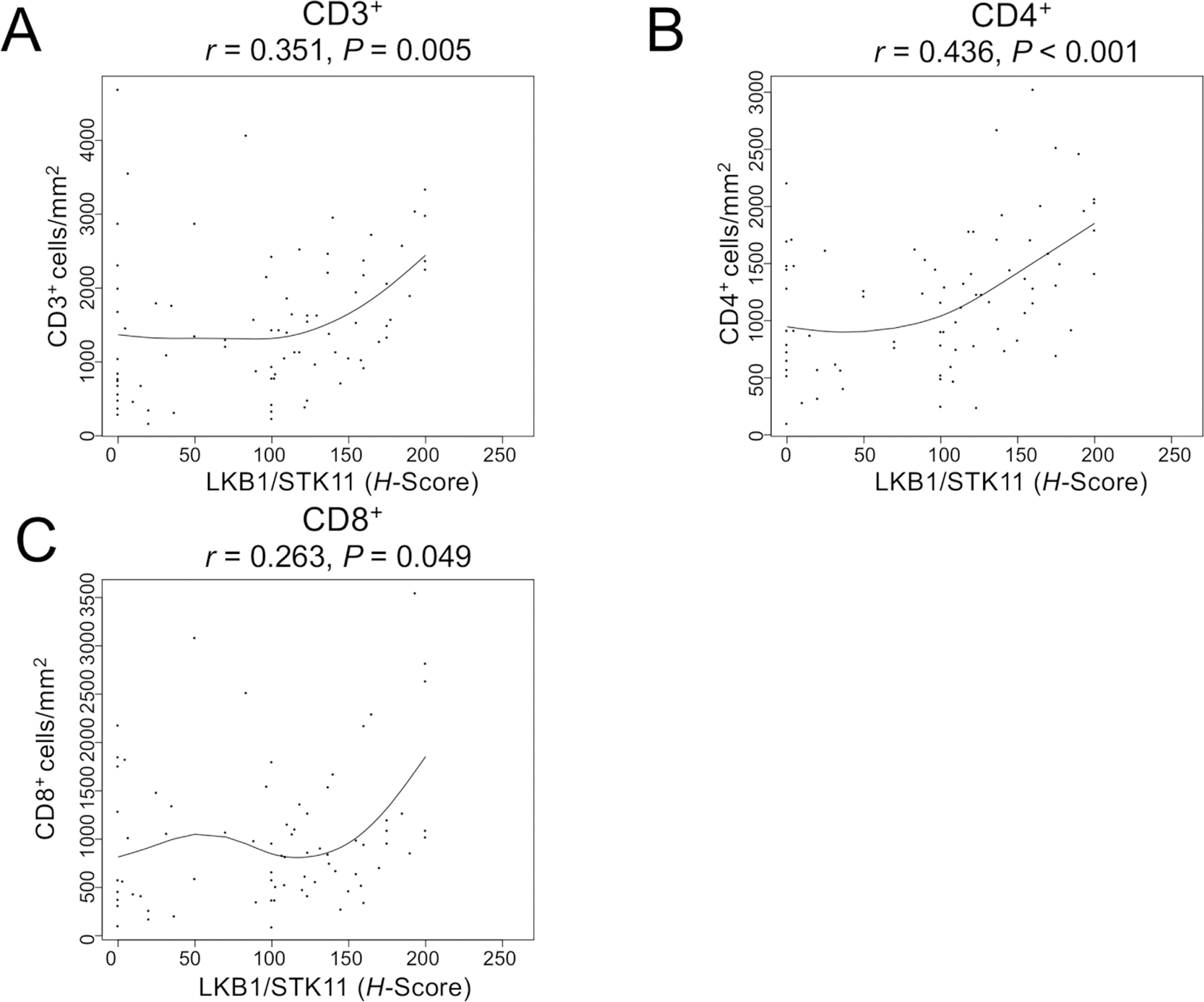
Relationships between tumor expression of LKB1/STK11 and intratumoral densities of cells expressing (A) CD3, (B) CD4, and (C) CD8. Tumor LKB1/STK11 expression is quantified as intratumoral H-score. A spline fit curve is indicated in each panel.
Associations with Postoperative Recurrence and Disease-Free Survival
After a median follow-up duration of 89.7 (IQR 34.4–135.8) months, there were 65 (63%) deaths and 66 (64%) DFS events. Median survival time (MST) and median disease-free survival time (MDFST) for the entire cohort were 87.0 (95% CI 61.3–112.8) months and 66.7 (95% CI 25.9–107.5) months, respectively. Whereas isolated locoregional recurrences were uncommon (1/104, 1%), isolated distant failure (27/104, 26%) and simultaneous locoregional and distant recurrence (11/104, 11%) were more frequently observed. Analysis of patients who had any disease recurrence (n = 39/104) identified LKB1/STK11 expression to be lower among patients who suffered extrathoracic failure (n = 19/39, median H-score 40.0 [IQR 0.0–110.0]) than those that did not (n = 20/39, median H-score 120.8 [IQR 91.7–139.4], P=0.024). Next, we examined whether intratumoral LKB1/STK11 expression retained prognostic significance in this cohort after controlling for other relevant clinicopathologic and treatment characteristics. On multivariate analysis, higher LKB1/STK11 expression was independently associated with a reduced hazard of DFS events and distant disease-free survival events (Tables 2 and 3, Supplementary Tables 3 and 4).
Table 2:
Clinicopathologic characteristics associated with disease-free survival, with LKB1/STK11 expression analyzed as a continuous variable (n = 104).
|
Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (≥65 years) | 49 (47.1) | 1.48 | 0.91–2.41 | 0.118 | 1.56 | 0.93–2.62 | 0.09 |
| Sex (Male) | 53 (51.0) | 1.86 | 1.13–3.06 | 0.015 | |||
| Smoker (Ever) | 89 (85.6) | 0.86 | 0.44–1.69 | 0.658 | |||
| Zubrod (1) | 47 (45.2) | 1.29 | 0.79–2.10 | 0.304 | |||
| FEV1 (% Predicted)* | n/a | 1.00 | 0.99–1.02 | 0.879 | |||
| Pathologic Stage | |||||||
| I | 62 (59.6) | Reference | Reference | ||||
| II | 24 (23.1) | 2.49 | 1.40–4.44 | 0.002 | 2.37 | 1.31–4.27 | 0.004 |
| III | 18 (17.3) | 3.43 | 1.86–6.33 | <0.001 | 2.83 | 1.40–5.73 | 0.004 |
| Differentiation (Poor) | 43 (41.4) | 1.11 | 0.68–1.83 | 0.668 | |||
| Extent of resection (Pneumonectomy) | 4 (3.8) | 1.41 | 0.44–4.49 | 0.564 | |||
| Margin (R1) | 4 (3.8) | 4.47 | 1.57–12.69 | 0.005 | 2.33 | 0.71–7.63 | 0.161 |
| LKB1/STK11 expression (H-score, per 10 unit increase) | n/a | 0.96 | 0.93–1.00 | 0.029 | 0.96 | 0.93–1.00 | 0.044 |
| Adjuvant Chemotherapy | 23 (22.1) | 1.06 | 0.60–1.90 | 0.838 | 0.74 | 0.39–1.42 | 0.367 |
| Adjuvant Radiotherapy | 13 (12.5) | 2.78 | 1.48–5.24 | 0.002 | 2.12 | 1.02–4.39 | 0.044 |
Data available for 103/104 (99.0%)
CI: confidence interval; FEV1: forced expiratory volume in one second; HR: hazard ratio
Table 3:
Clinicopathologic characteristics associated with distant disease-free survival, with LKB1/STK11 expression analyzed as a continuous variable (n = 104).
|
Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (≥65 years) | 49 (47.1) | 1.55 | 0.95–2.54 | 0.082 | 1.62 | 0.96–2.73 | 0.069 |
| Sex (Male) | 53 (51.0) | 1.96 | 1.18–3.25 | 0.009 | |||
| Smoker (Ever) | 89 (85.6) | 0.94 | 0.48–1.85 | 0.858 | |||
| Zubrod (1) | 47 (45.2) | 1.18 | 0.72–1.94 | 0.501 | |||
| FEV1 (% Predicted)* | n/a | 1.00 | 0.98–1.02 | 0.871 | |||
| Pathologic Stage | |||||||
| I | 62 (59.6) | Reference | Reference | ||||
| II | 24 (23.1) | 2.44 | 1.37–4.35 | 0.003 | 2.38 | 1.32–4.30 | 0.004 |
| III | 18 (17.3) | 3.59 | 1.92–6.73 | <0.001 | 3.41 | 1.69–6.85 | 0.001 |
| Differentiation (Poor) | 43 (41.4) | 1.12 | 0.68–1.84 | 0.665 | |||
| Extent of resection (Pneumonectomy) | 4 (3.8) | 1.51 | 0.47–4.82 | 0.488 | |||
| Margin (R1) | 4 (3.8) | 4.33 | 1.31–14.32 | 0.016 | 2.11 | 0.56–7.90 | 0.266 |
| LKB1/STK11 expression (H-score, per 10 unit increase) | n/a | 0.96 | 0.92–0.99 | 0.014 | 0.95 | 0.92–0.99 | 0.013 |
| Adjuvant Chemotherapy | 23 (22.1) | 0.86 | 0.47–1.56 | 0.617 | 0.59 | 0.31–1.14 | 0.118 |
| Adjuvant Radiotherapy | 13 (12.5) | 2.30 | 1.20–4.41 | 0.012 | 1.97 | 0.93–4.14 | 0.075 |
Data available for 103/104 (99.0%)
CI: confidence interval; FEV1: forced expiratory volume in one second; HR: hazard ratio
COMMENT
We report associations between low tumor expression of LKB1/STK11, baseline patient characteristics, increased distant metastases, and poor postoperative prognosis. Moreover, we identified modest associations between reduced LKB1/STK11 expression and reduced intratumoral infiltration by populations of immune cells with key roles in the antitumor immune response (CD3+ [T cells], CD4+ [helper T cells], and CD8+ [cytotoxic T cells]),. Considered together, our findings suggest that diminished expression of this tumor suppressor has important effects both locally, within the tumor microenvironment, and systemically.
The results of the present analysis identified differing expression of LKB1/STK11 according to patient clinical characteristics. Although associations with some clinicopathologic features have been consistently identified across previous studies, others have yet to be clearly defined. Previous tobacco exposure has been similarly reported to be associated with LBK1/STK11 status (11, 20, 21). In contrast, studies have come to conflicting conclusions regarding associations between LBK1/STK11 genomic alterations and sex (11, 20, 21). Additionally, though we did not identify lower expression among poorly differentiated tumors in the present report, previous analyses have suggested that LKB1/STK11 mutations are more common in this subgroup (21, 22). Given these discrepancies, further study is warranted to clarify associations between LKB1/STK11 status and patient and tumor clinicopathologic characteristics. In light of the metabolic regulatory functions of LKB1/STK11 and emerging evidence attesting to the ability of 18F-fluorodeoxyglucose positron emission tomography to characterize features of the tumor microenvironment, additional investigation of LKB1/STK11 status in the context of volumetric and metabolic radiographic assessment of glucose avidity remains an intriguing line of inquiry for further investigation (23, 24).
Mutations in and low expression of LKB1/STK11 have been previously reported as associated with poor prognosis in patients with solid tumors in several contexts, including advanced NSCLC, breast cancer, and colorectal carcinoma (25, 26). In concert with the findings of the present study, relative LKB1/STK11 deficiency has been previously identified as being associated with increased extrathoracic metastases (11). Taken in the context of work demonstrating that the antitumor immune response generates effects in the local microenvironment as well as sustained effects systemically, the associations in the present and previous reports of associations between LKB1/STK11 status and reduced immune cell infiltration could in part explain the associations with poor prognosis (7, 11, 12, 27, 28). We speculate that these observations may reflect effects of local immunosuppression and subsequent inhibition of immune priming that are manifested as an increased propensity for systemic failures. Although further investigation is needed to elucidate these findings, the use of pretreatment biopsy as a means of identification of patients with low tumor LKB1/STK11 expression who might be less likely to respond to novel therapeutic agents in the neoadjuvant setting is a potential avenue for future clinical application.
This study is limited by its retrospective nature, temporal and treatment heterogeneity among the study cohort, and the fact that microarray data were available in only a subset of the patients undergoing resection for primary lung adenocarcinoma during the study period. Additionally, although LKB1/STK11 expression was independently associated with poor DFS when modeled both as a continuous and categorical variable, validation in external cohorts is needed to delineate the threshold of expression that best identifies patients at increased risk of poor postoperative prognosis. Finally, the variety of genomic alterations in LKB1/STK11 and the complexities of intratumoral heterogeneity introduce further subtlety into the interpretation of these results. However, work by previous groups has suggested that LKB1/STK11 loss occurs relatively early in the progression of dysplastic lesions to carcinoma, and analysis of expression by immunohistochemistry has been validated as representative of tumor genomic status (7, 10, 11).
In summary, we identified differential expression of LKB1/STK11 according to sex and tobacco exposure, and further observed associations between low expression and an increased risk of disease recurrence. Further examination of tumor LKB1/STK11 expression in the context of trials of novel therapeutic agents is needed in order to identify the clinical relevance of these findings in patients with resectable disease as treatment paradigms continue to rapidly evolve.
Supplementary Material
Supplementary Figure 1: Consolidated Standards of Reporting Trials diagram depicting patient selection for the present study. IHC: immunohistochemistry; MDACC: MD Anderson Cancer Center; SCC: squamous cell carcinoma; TAIC: tumor-associated immune cell.
Supplementary Figure 2: Tissue microarray (TMA) sections for all patients with LKB1/STK11 staining performed.
Acknowledgment:
J.V. Heymach has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Spectrum. This work was supported in part by the Cancer Prevention Research Institute of Texas Multi-Investigator Research Awards (RP160668, IIW), the National Institutes of Health/National Cancer Institute through the University of Texas Lung Specialized Programs of Research Excellence grant (P50CA70907, IIW), by generous philanthropic donations by the Mason family and anonymous donors, and by departmental funding.
GLOSSARY OF ABBREVIATIONS
- CD
cluster of differentiation
- CI
confidence interval
- FOXP3
forkhead box P3
- HR
hazard ratio
- LKB1
liver kinase B1, see STK11
- NSCLC
non-small cell lung cancer
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- STK11
serine/threonine kinase 11, see LKB1
- TAIC
tumor-associated immune cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript was presented at the 55th Annual Meeting of the Society of Thoracic Surgeons; 28 January 2019; San Diego, California.
REFERENCES
- 1.Wellenstein MD, de Visser KE. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity 2018;48(3):399–416. [DOI] [PubMed] [Google Scholar]
- 2.Lopes G, Wu Y-L, Kudaba I et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic nsclc with a PD-L1 tumor proportion score (tps) ≥ 1%: Open-label, phase 3 Keynote-042 study. J Clin Oncol 2018;36(18_suppl):LBA4–LBA4. [Google Scholar]
- 3.Nilsson MB, Sun H, Diao L et al. Stress hormones promote EGFR inhibitor resistance in nsclc: Implications for combinations with beta-blockers. Sci Transl Med 2017;9(415). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang H, Xiao G, Behrens C et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non–small cell lung cancer patients. Clin Cancer Res 2013;19(6):1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 6.Forde PM, Chaft JE, Smith KN et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoulidis F, Byers LA, Diao L et al. Co-occurring genomic alterations define major subsets of kras-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5(8):860–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carretero J, Medina PP, Blanco R et al. Dysfunctional AMPK activity, signalling through mtor and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 2006;26:1616. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Cespedes M A role for lkb1 gene in human cancer beyond the Peutz–Jeghers syndrome. Oncogene 2007;26:7825. [DOI] [PubMed] [Google Scholar]
- 10.Ghaffar H, Sahin F, Sanchez-Cepedes M et al. Lkb1 protein expression in the evolution of glandular neoplasia of the lung. Clin Cancer Res 2003;9(8):2998–3003. [PubMed] [Google Scholar]
- 11.Calles A, Sholl LM, Rodig SJ et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS-mutant lung adenocarcinoma. Clin Cancer Res 2015;21(12):2851–2860. [DOI] [PubMed] [Google Scholar]
- 12.Koyama S, Akbay EA, Li YY et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress t-cell activity in the lung tumor microenvironment. Cancer Res 2016;76(5):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoulidis F, Goldberg ME, Greenawalt DM et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 2018;8(7):822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra ER, Villalobos P, Behrens C et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 2018;6(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K et al. The IASCLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2(8):706–714. [DOI] [PubMed] [Google Scholar]
- 16.Parra ER, Behrens C, Rodriguez-Canales J et al. Image analysis–based assessment of PD-L1 and tumor-associated immune cells density supports distinct intratumoral microenvironment groups in non–small cell lung carcinoma patients. Clin Cancer Res 2016;22(24):6278–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parra ER, Villalobos P, Zhang J et al. Immunohistochemical and image analysis-based study shows that several immune checkpoints are co-expressed in non-small cell lung carcinoma tumors. J Thorac Oncol 2018;13(6):779–791. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 19.Brandt WS, Yan W, Leeman JE et al. Postoperative radiotherapy for surgically resected ypN2 non-small cell lung cancer. Ann Thorac Surg 2018;106(3):848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koivunen JP, Kim J, Lee J et al. Mutations in the LKB1 tumour suppressor are frequently detected in tumours from caucasian but not asian lung cancer patients. Br J Cancer 2008;99:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto S, Iwakawa R, Takahashi K et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 2007;26:5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L, Liang X, Liu M et al. Reduced expression of liver kinase b1 and beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncol Rep 2014;32(5):1931–1938. [DOI] [PubMed] [Google Scholar]
- 23.Kaira K, Serizawa M, Koh Y et al. Biological significance of 18F-FDG uptake on PET in patients with non-small-cell lung cancer. Lung Cancer 2014;83(2):197–204. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Hu Z, Cai L et al. Cps1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature 2017;546:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He T-Y, Tsai L-H, Huang C-C, Chou M-C, Lee H. LKB1 loss at transcriptional level promotes tumor malignancy and poor patient outcomes in colorectal cancer. Ann Surg Oncol 2014;21(4):703–710. [DOI] [PubMed] [Google Scholar]
- 26.Xiao J, Zou Y, Chen X et al. The prognostic value of decreased LKB1 in solid tumors: A meta-analysis. PLoS One 2016;11(4):e0152674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 2012;12:298. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Coussens Lisa M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21(3):309–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Consolidated Standards of Reporting Trials diagram depicting patient selection for the present study. IHC: immunohistochemistry; MDACC: MD Anderson Cancer Center; SCC: squamous cell carcinoma; TAIC: tumor-associated immune cell.
Supplementary Figure 2: Tissue microarray (TMA) sections for all patients with LKB1/STK11 staining performed.


